Abstract
Terrestrial vertebrates have evolved two anatomically and mechanistically distinct chemosensory structures: the main olfactory epithelium (MOE) and the vomeronasal organ (VNO). Although it has been generally thought that pheromones are detected through the VNO, whereas other chemicals are sensed by the MOE, recent evidence suggests that some pheromones may be detected through the MOE. Odorant receptors in the MOE are coupled to the type 3 adenylyl cyclase (AC3), an enzyme not expressed in the VNO. Consequently, odorants and pheromones do not elicit electrophysiological responses in the MOE of AC3−/−mice, although VNO function is intact. Here we report that AC3−/−mice cannot detect mouse milk, urine, or mouse pheromones. Inter-male aggressiveness and male sexual behaviors are absent in AC3−/−mice. Furthermore, adenylyl cyclase activity in membranes prepared from the MOE of wild-type mice, but not AC3−/−mice, is stimulated by 2-heptanone, a mouse pheromone. We conclude that signaling through AC3 in the MOE is obligatory for male sexual behavior, male–male aggressiveness, and the detection of some pheromones.
Keywords: MOE, VNO, AC3, pheromone, male aggressiveness, sexual behavior
Introduction
Pheromones are “airborne chemical signals that are released by an individual into the environment and which affect the physiology and behavior of other members of the same species” (Karlson and Luscher, 1959). Until recently, it was thought that odorants are detected in the main olfactory epithelium (MOE), whereas pheromones are detected in the vomeronasal organ (VNO) (Sam et al., 2001) (but see Trinh and Storm, 2003; Mandiyan et al., 2005). The main components of the MOE signaling cascade, G-protein olfactory subunit (Golf) (Belluscio et al., 1998), type 3 adenylyl cyclase (AC3) (Wong et al., 2000), and cyclic nucleotide-gated channel (CNG) (Brunet et al., 1996), are not expressed in the VNO (Berghard and Buck, 1996; Berghard et al., 1996).
Although the VNO is implicated in the detection of pheromones as well as pheromone-based behaviors including inter-male aggression and male sexual activity (Del Punta et al., 2002; Leypold et al., 2002; Stowers et al., 2002; Halpern and Martinez-Marcos, 2003), the role of the MOE in these behaviors is unclear. Ablation of the VNO has no effect on the suckling behavior of rabbits (Hudson and Distel, 1986) or mating behavior of male hamsters (Pfeiffer and Johnston, 1994), indirectly suggesting that the MOE may mediate some pheromone responses. To evaluate the importance of AC3 for pheromone-mediated responses, we examined the effects of pheromones on the electro-olfactogram (EOG) responses to pheromones, pheromone-mediated behaviors, and adenylyl cyclase activity using AC3−/−mice, a mouse strain that exhibits no sensory signaling through the MOE (Wong et al., 2000).
Materials and Methods
AC3−/−mice.
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington. Mice were maintained on a 12 h light/dark cycle and had access to water and food ad libitum. Adult male AC3−/−mice and wild-type littermates were generated by breeding heterozygotes (Wong et al., 2000). After weaning, the males were not exposed to females before the behavioral tests and wild-type as well as AC3−/−mice were housed individually. Mice used in this study were 2–4 months of age.
Male–male aggressive and male–female sexual behavior.
Male–male aggression was observed by the resident/intruder assay (Leypold et al., 2002). Adult, sexually naive mice were housed individually for 10 d, and their bedding was not changed for 4 d before testing. Anogenital area investigation duration, attack number, and attack duration of the host males were observed during a 15 min period by introducing a group-housed, sexually inexperienced, unfamiliar, wild-type adult male into their home cage. Aggressive behavior is defined as biting, chasing, or wrestling/tumbling. Male–female sexual behaviors of adult mice were observed during a 15 min period by introducing an adult, sexually receptive, unfamiliar, wild-type female into the home cage of the resident male. Male sexual behaviors including anogenital investigating latency and duration, mounting latency, number and duration, as well as intromission were monitored. The receptive status of females was verified using sexual-experienced, male, wild-type mice. Male sexual behavior was also observed for a 4 h period (Pankevich et al., 2004). All reported behavioral data were obtained in the light phase. Identical results were obtained when mice were observed in the dark phase under red light. Each male mouse was tested for male–male aggressiveness and sexual behavior with three different intruders on different days.
Pheromone habituation test.
The pheromone habituation test of adult male mice was performed as described previously (Trinh and Storm, 2003). The data are presented as a ratio of the number of sniffs an animal took when the pheromone-laced cotton swab was first introduced to the number of sniffs observed when the water-laced swab was first introduced. Female and male mouse urine was collected into tubes by holding the mouse by the scruff of the neck. Urine was pooled and stored at −80°C until use. Mouse milk was collected manually from lactating females, pooled, and stored as aliquots at −80°C until use. All chemical odorants were diluted in mineral oil, except mouse milk and urine, which were diluted in water. The habituation test was repeated three times on different days.
Olfactory epithelium lesion.
The MOE was destroyed by applying 25 μl of 5% ZnSO4into each nasal vault using a syringe; controls were treated with the same volume of 0.9% NaCl (Harding et al., 1978; Trinh et al., 2003). Cellular structure was visualized by Hoechst nuclear counterstaining, and sensory neurons were immunostained with an antibody against β-tubulin III.
EOGs.
EOG recording from olfactory receptive neurons in the MOE was performed as described previously (Wong et al., 2000). The olfactory turbinate was exposed by dissecting the mouse head through the septum. The EOG was recorded with an agar- and saline-filled glass microelectrode in contact with the apical surface of the MOE in the open circuit configuration. Pheromone solutions were puffed onto the exposed epithelia for 1 s, followed by a stream of moisturized oxygen. Traces were captured and digitized using a Digidata 1200A (Molecular Devices, Union City, CA). The traces were low-filtered at 30 Hz and sampled at 100 Hz. We explored a minimum of three locations in the MOE of each animal with every substance. Recordings were made at least two times in each location, and the largest response was analyzed to minimize the effects of cross-adaptation. Vomeronasal recordings [electrovomeronasal olfactogram (EVG)] were made in the same manner. The VNOs were dissected out from an encapsulating bone, and the tubular structures were opened.
Adenylyl cyclase assay.
MOE membranes were collected and homogenized on ice in homogenization buffer that contained 50 mmTris-HCl, pH 7.4, 2 mmMgCl2, 1 mmEDTA, 0.5 mmDTT, and protease inhibitor cocktail tablets (1 tablet/10 ml; Roche, Indianapolis, IN). The homongenate was centrifuged at 750 × gfor 2 min at 4°C. The supernatant was centrifuged for 60 min at 100,000 × g, and the resulting pellet was resuspended in homogenization buffer for adenylyl cyclase assays (Chan et al., 2005). Protein concentrations were determined by a BCA assay (Pierce, Rockford, IL) according to the manufacturer’s instructions.
Testosterone analysis.
Blood was collected from the tails of AC3+/+and AC3−/−male mice. The serum testosterone level was determined by an ACE competitive enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) according to manufacturer’s instructions. The sensitivity of the testosterone assay was 3.9 pg/ml.
Statistical analysis.
All experimental data were analyzed by an unpaired, two-tailed Student’s ttest. Significance was defined as p< 0.05. All error values were presented as mean ± SEM. In behavioral tests, the investigator was blind to the genotype of the mice.
Results
No pheromone-stimulated EOG responses in AC3−/−mice
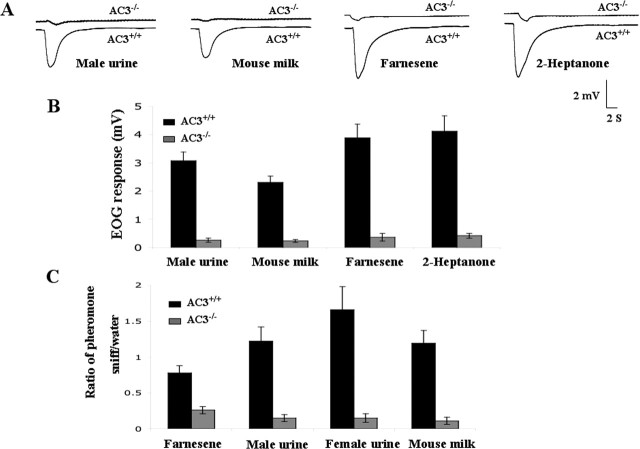
To determine whether milk, male urine, or pheromones elicit electrophysiological responses in the MOE of AC3−/−mice, EOG recordings were made in the MOE to monitor pheromone-evoked potentials (Ottoson, 1956). We tested farnesene because it is an aggression-evoking pheromone (Novotny et al., 1990), 2-heptanone because it exhibits puberty-delaying activity (Novotny et al., 1986), and male urine because it contains a pheromone that evokes male–male aggression (Mugford and Nowell, 1970). Although all of these agents evoked robust EOG responses in the MOE of AC3+/+mice, AC3−/−mice showed no EOG responses to milk, male urine, farnesene, or 2-heptanone (Fig. 1A,B). This suggests that these substances are detected in the MOE by receptors coupled to AC3.
Figure 1.
Pheromone detection defects in the MOE of AC3−/−mice. A, Pheromone-stimulated EOG responses from adult AC3+/+and AC3−/−mice. Male mouse urine was diluted 20-fold and mouse milk was diluted 50-fold in water; farnesene (500 μm) and heptanone (50 μm) were diluted in mineral oil. B, Summary of the mean EOG amplitudes in response to pheromones. AC3+/+mice (n= 10) exhibited significantly greater EOG responses to all agents compared with AC3−/−mice (n= 6): urine, p< 0.0001; milk, p< 0.0001; farnesene, p< 0.0001; heptanone, p< 0.0001. C, AC3−/−mice are unable to detect male urine, female urine, milk, or farnesene. Detection of pheromones was monitored using the odorant-habituation assay described in Materials and Methods. The ratio of the number of times the mouse sniffed a pheromone-soaked cotton swab compared with the number of times it sniffed a water-soaked cotton swab on initial exposure is an indication of the ability of the animal to detect a specific substance. Cotton swabs were laced with 50 μl of farnesene (500 μm), male urine (20-fold diluted), female urine (20-fold diluted), or mouse milk (50-fold diluted). There were significant differences in the ability of AC3−/−(n= 15) and AC3+/+(n= 13) mice to detect farnesene (p< 0.001), male urine (p< 0.001), female urine (p< 0.001), and mouse milk (p< 0.001). Error bars represent ±SEM.
AC3−/−mice do not detect pheromones
The odorant habituation test (Gregg and Thiessen, 1981) was used to determine whether AC3−/−mice can detect pheromones, milk, or urine. AC3+/+mice, but not AC3−/−mice, detected male urine, female urine, milk, and farnesene (Fig. 1C). AC3−/−mice were unable to detect these substances even when their concentrations were increased 10-fold (data not shown). These data suggest that farnesene and the pheromones present in milk and urine are detected by the MOE through receptors coupled to AC3.
AC3−/−male mice do not exhibit male–male aggression
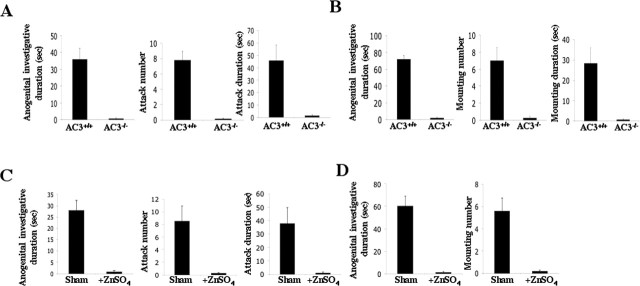
Because pheromones play an important role in aggressive and sexual behaviors, AC3−/−mice were assayed for male–male aggression and male–female sexual behaviors using the resident/intruder assay (Leypold et al., 2002). In one version of this paradigm, the behavior of a singly housed male mouse is observed when a sexually inexperienced male intruder is introduced into his cage. The difference in behavior between the AC3+/+and AC3−/−mice was striking. AC3+/+mice spent significantly more time examining the anogenital area of the intruder than AC3−/−mice (Fig. 2A). Although AC3+/+mice invariably attacked the intruder, AC3−/−mice never attacked and were nonaggressive toward intruder males. Because aggressive behavior is enhanced by cohabitation with females (Goyens and Noirot, 1975), we paired one female with each AC3−/−male and examined the males for male–male aggression. Still, the AC3−/−mice exhibited no aggressive behaviors toward other males even after cohabitation with females for 7 d (data not shown). Furthermore, AC3−/−male mice triggered attacks from AC3+/+male residents when they were introduced into the home cage of AC3+/+males, and they displayed defensive behavior when attacked by AC3+/+male residents. Endogenous testosterone is linked to aggressive and sexual behaviors in animals and humans (for review, see Brain and Haug, 1992; Bahrke et al., 1996; Giammanco et al., 2005). However, no significant testosterone differences were found between AC3+/+and AC3−/−male mice (AC3+/+: 3.2 ± 0.8 ng/ml, n= 6; AC3−/−: 2.9 ± 0.6 ng/ml, n= 6; p= 0.5). These data indicate that AC3 activity is required for male–male aggressive behavior and support the hypothesis that pheromones underlying these behaviors are sensed through the MOE by a mechanism that depends on AC3.
Figure 2.
Sexual behavioral defects in AC3−/−mice. A, AC3−/−mice display no male–male aggressive behavior. There were significant differences between AC3−/−(n= 17) and AC3+/+(n= 16) mice in anogenital area investigation (p< 0.0001), attack number (p< 0.0001), and attack duration (p< 0.0001). B, AC3−/−male mice do not exhibit sexual behavior toward females. There were significant differences between AC3+/+(n= 14) and AC3−/−(n= 15) mice in anogenital area investigation (p< 0.0001), mounting number (p< 0.001), and mounting duration (p< 0.001) assayed during a 15 min observation period. C, Male–male aggressive behaviors were ablated in ZnSO4-treated male mice. There were significant differences in anogenital area investigation (p< 0.001), attack number (p< 0.05), and attack duration (p< 0.01) between control (n= 5) and ZnSO4-treated (n= 7) mice. D, Male–female sexual behaviors were ablated in ZnSO4-treated male mice. There were significant differences in anogenital investigation duration (p< 0.05) and mounting frequency (p< 0.01) between control (n= 6) and ZnSO4-treated (n= 7) mice. Error bars represent ±SEM.
AC3−/−male mice do not exhibit mating behavior
Sensory activation of VNO neurons requires TRP2, an ion channel of the transient receptor potential family that is expressed exclusively in these neurons (Stowers et al., 2002). TRP2−/−male mice mate normally with females, indicating that signaling through the VNO is not required for initiating sexual behavior (Leypold et al., 2002; Stowers et al., 2002). This prompted us to investigate the role of AC3 for male-mating behavior. We monitored male sexual behavior by introducing a sexually receptive, unfamiliar, wild-type female into their home cage and monitored the male during a 15 min period. Wild-type male mice intensively investigated the females and displayed mounting as well as intromission behavior toward the females. In contrast, AC3−/−male mice did not investigate the female’s anogenital area, nor did they try to mount the females (Fig. 2B). Because it can be argued that a 15 min period is too short to accurately test male mouse sexual behavior, 4 h assays were also used to further confirm the lack of normal sexual behavior in AC3−/−male mice (Pankevich et al., 2004). AC3−/−male mice also did not exhibit sexual behavior toward females when they were observed for 4 h periods (data not shown). Furthermore, when we paired AC3−/−male mice with a wild-type female (n= 10 pairs), no plugs were found in the females in 10 consecutive days of observation and there were no pregnancies after 1 month.
Lesioning of the MOE with ZnSO4eliminates male aggressive and sexual behaviors
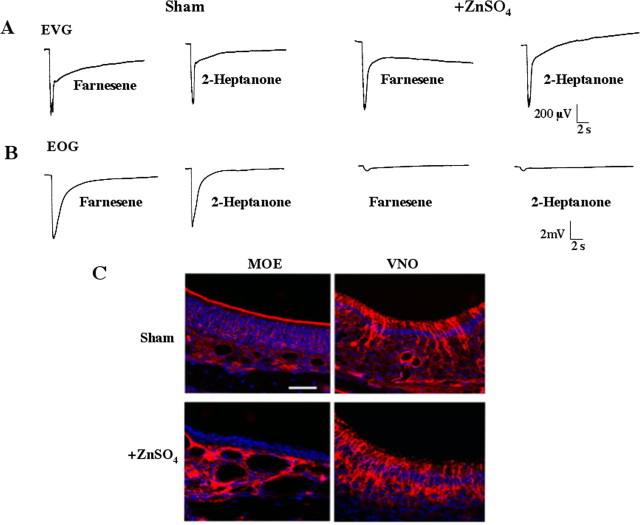
To confirm that the phenotypes described above for the AC3−/−mice were attributable to ablation of MOE function, ZnSO4was used to destroy the MOE of wild-type mice (Harding et al., 1978; Trinh and Storm, 2003). Treatment of the MOE with ZnSO4eliminated male–male aggressiveness and the mating behavior of male wild-type mice (Fig. 2C,D). It was important to confirm that the VNO was functional after ZnSO4treatment. Accordingly, we recorded pheromone-evoked EVG responses in the VNO of ZnSO4-treated mice. We found that 2-heptanone and farnesene elicit normal EVG responses in the VNO of ZnSO4-treated mice, although their EOG responses in the MOE are completely ablated (Fig. 3A,B). In addition, the VNO of ZnSO4-treated mice appeared normal (Fig. 3C), and Fluoro-Jade B staining and cleaved caspase-3 immunolabeling failed to reveal any neurodegeneration in the VNO of ZnSO4-treated mice (data not shown). These data indicate that the structure and function of the VNO in ZnSO4-treated mice is normal.
Figure 3.
ZnSO4treatment of the MOE destroys olfactory receptor neurons without affecting the function of the VNO. A, Pheromone-stimulated EVG responses from sham and ZnSO4-treated mice. B, Pheromone-stimulated EOG response in the MOE from sham and ZnSO4-treated mice. Farnesene (500 μm) and 2-heptanone (50 μm) were diluted in mineral oil. C, Cellular structure was visualized by Hoechst nuclear counterstaining (blue), and sensory neurons were immunostained using an antibody against β-tubulin III (red). Scale bar, 40 μm. The MOE was treated with ZnSO4as described in Materials and Methods.
Stimulation of adenylyl cyclase in the MOE by 2-heptanone
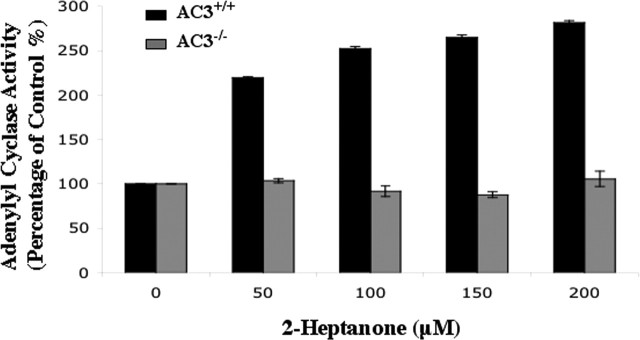
Activation of AC3 by odorants is the primary signaling event in the MOE (Wong et al., 2000). To test the role of adenylyl cyclase in pheromone detection in the MOE, the sensitivity of adenylyl cyclase activity to 2-heptanone was examined. Although 2-heptanone increased adenylyl cyclase activity 2.8-fold in MOE membrane preparations from wild-type mice, it had no effect on adenylyl cyclase activity in the MOE of AC3−/−male mice (Fig. 4). This indicates that receptors for this hormone are coupled to AC3 in the MOE and supports the idea that some pheromone receptors are coupled to cAMP signaling in the MOE through activation of AC3.
Figure 4.
Adenylyl cyclase activity is stimulated by 2-heptanone in membranes from the MOE of AC3+/+but not AC3−/−male mice. Stimulation of adenylyl cyclase activity by 2-heptanone in the MOE of AC3+/+but not AC3−/−male mice is shown. 2-Heptanone was diluted in DMSO. Data are presented as mean ± SEM of triplicates. The adenylyl cyclase activity is expressed as a percentage relative to controls in AC3+/+and AC3−/−male mice.
Discussion
Until recently, the VNO was thought to be specialized for the perception of pheromones (Stowers et al., 2002; Leypold et al., 2002; Trinh and Storm, 2003). However, male mice with surgically removed VNOs are still able to distinguish urine odors from males and estrous females, and from mice of both sexes that are in different endocrine states (Pankevich et al., 2004). Furthermore, TRP2−/−male mice mate normally with female mice, indicating that the VNO is not required for initiating sexual behavior (Stowers et al., 2002). The objective of this study was to determine whether detection of pheromones or pheromone-based behaviors depend on AC3 in the MOE.
Recently, it was reported that mutant mice lacking CNGA2 show deficits in sexual and aggressive behaviors (Mandiyan et al., 2005). On the basis of these data, it was concluded that these behaviors are mediated through pheromone signaling in the MOE. However, CNGA2 is expressed throughout the brain (Kingston et al., 1999), as well as other tissues (Cheng et al., 2003). Therefore, the observation that CNGA2−/−mice do not exhibit pheromone-mediated behavior does not prove that pheromones are detected in the MOE. Furthermore, because the CNG is activated by cAMP, cGMP, and various kinases, behavioral data obtained with the CNG knock-out mice do not indicate that pheromone receptors are coupled to adenylyl cyclase or any other specific effector system.
AC3 is not expressed exclusively in the MOE (Xia et al., 1992), and the observation that AC3−/−mice do not detect some pheromones cannot be taken as definitive evidence that pheromones are detected through the MOE. However, our data showing that pheromones produce EOG responses in the MOE and ZnSO4lesioning of the MOE disrupts pheromone-based behavior coupled with the behavior of AC3−/−mice strongly supports the hypothesis that some pheromone responses are mediated by the MOE. Moreover, data showing that 2-heptanone stimulates adenylyl cyclase activity in the MOE of wild-type mice also supports a role for the MOE in pheromone detection. This is unexpected because pheromone receptor signaling in the VNO is not mediated through AC3 or cAMP increases (Kroner et al., 1996). Collectively, our data indicate that male–male aggressiveness and male-mating behavior in mice depend on signaling through AC3 in the MOE but do not exclude contributions from other signaling mechanisms. The fact that the MOE is required for some pheromone responses does not exclude a role for the VNO, because perturbation of VNO function impairs some pheromone-mediated behaviors.
The discovery that the MOE plays a pivotal role in male sexual behavior in mice raises interesting questions about pheromone detection by humans. Although humans may have a VNO, reports that it is functional are controversial (Halpern and Martinez-Marcos, 2003). The data reported here introduces the interesting possibility that humans might be able to detect pheromones through the MOE. In conclusion, AC3 is required for pheromone-mediated responses of male mice, suggesting that pheromone-stimulated increases in cAMP may play a significant role in pheromone-based behaviors.
Footnotes
This work was supported by National Institutes of Health Grant DC04156. We thank members of the Storm laboratory for critical reading of this manuscript.
References
- Bahrke MS, Yesalis CE III, Wright JE (1996). Psychological and behavioural effects of endogenous testosterone and anabolic-androgenic steroids. An update. Sports Med 22:367–390. [DOI] [PubMed] [Google Scholar]
- Belluscio L, Gold GH, Nemes A, Axel R (1998). Mice deficient in G(olf) are anosmic. Neuron 20:69–81. [DOI] [PubMed] [Google Scholar]
- Berghard A, Buck LB (1996). Sensory transduction in vomeronasal neurons: evidence for Gαo, Gαi2, and adenylyl cyclase II as major components of a pheromone signaling cascade. J Neurosci 16:909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghard A, Buck LB, Liman ER (1996). Evidence for distinct signaling mechanisms in two mammalian olfactory sense organs. Proc Natl Acad Sci USA 93:2365–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain PF, Haug M (1992). Hormonal and neurochemical correlates of various forms of animal “aggression.”. Psychoneuroendocrinology 17:537–551. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, Gold GH, Ngai J (1996). General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron 17:681–693. [DOI] [PubMed] [Google Scholar]
- Chan GC, Tonegawa S, Storm DR (2005). Hippocampal neurons express a calcineurin-activated adenylyl cyclase. J Neurosci 25:9913–9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KT, Chan FL, Huang Y, Chan WY, Yao X (2003). Expression of olfactory-type cyclic nucleotide-gated channel (CNGA2) in vascular tissues. Histochem Cell Biol 120:475–481. [DOI] [PubMed] [Google Scholar]
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P (2002). Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature 419:70–74. [DOI] [PubMed] [Google Scholar]
- Giammanco M, Tabacchi G, Giammanco S, Di Majo D, La Guardia M (2005). Testosterone and aggressiveness. Med Sci Monit 11:RA136–RA145. [PubMed] [Google Scholar]
- Goyens J, Noirot E (1975). Effects of cohabitation with females on aggressive behavior between male mice. Dev Psychobiol 8:79–84. [DOI] [PubMed] [Google Scholar]
- Gregg B, Thiessen DD (1981). A simple method of olfactory discrimination of urines for the Mongolian gerbil, Meriones unguiculatus. Physiol Behav 26:1133–1136. [DOI] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A (2003). Structure and function of the vomeronasal system: an update. Prog Neurobiol 70:245–318. [DOI] [PubMed] [Google Scholar]
- Harding JW, Getchell TV, Margolis FL (1978). Denervation of the primary olfactory pathway in mice. V. Long-term effect of intranasal ZnSO4irrigation on behavior, biochemistry and morphology. Brain Res 140:271–285. [DOI] [PubMed] [Google Scholar]
- Hudson R, Distel H (1986). Pheromonal release of suckling in rabbits does not depend on the vomeronasal organ. Physiol Behav 37:123–128. [DOI] [PubMed] [Google Scholar]
- Karlson P, Luscher M (1959). “Pheromones”: a new term for a class of biologically active substances. Nature 183:55–56. [DOI] [PubMed] [Google Scholar]
- Kingston PA, Zufall F, Barnstable CJ (1999). Widespread expression of olfactory cyclic nucleotide-gated channel genes in rat brain: implications for neuronal signalling. Synapse 32:1–12. [DOI] [PubMed] [Google Scholar]
- Kroner C, Breer H, Singer AG, O’Connell RJ (1996). Pheromone-induced second messenger signaling in the hamster vomeronasal organ. NeuroReport 7:2989–2992. [DOI] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R (2002). Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA 99:6376–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM (2005). Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci 8:1660–1662. [DOI] [PubMed] [Google Scholar]
- Mugford RA, Nowell NW (1970). Pheromones and their effect on aggression in mice. Nature 226:967–968. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Jemiolo B, Harvey S, Wiesler D, Marchlewska-Koj A (1986). Adrenal-mediated endogenous metabolites inhibit puberty in female mice. Science 231:722–725. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Harvey S, Jemiolo B (1990). Chemistry of male dominance in the house mouse, Mus domesticus. Experientia 46:109–113. [DOI] [PubMed] [Google Scholar]
- Ottoson D (1956). Analysis of the electrical activity of the olfactory epithelium. Acta Physiol Scand 35:[Suppl 122], 1–83. [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA (2004). Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci 24:9451–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer CA, Johnston RE (1994). Hormonal and behavioral responses of male hamsters to females and female odors: roles of olfaction, the vomeronasal system, and sexual experience. Physiol Behav 55:129–138. [DOI] [PubMed] [Google Scholar]
- Sam M, Vora S, Malnic B, Ma W, Novotny MV, Buck LB (2001). Neuropharmacology. Odorants may arouse instinctive behaviours. Nature 412:142. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G (2002). Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295:1493–1500. [DOI] [PubMed] [Google Scholar]
- Trinh K, Storm DR (2003). Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci 6:519–525. [DOI] [PubMed] [Google Scholar]
- Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR (2000). Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27:487–497. [DOI] [PubMed] [Google Scholar]
- Xia Z, Choi EJ, Wang F, Storm DR (1992). The type III calcium/calmodulin-sensitive adenylyl cyclase is not specific to olfactory sensory neurons. Neurosci Lett 144:169–173. [DOI] [PubMed] [Google Scholar]