Abstract
Although studies in animals and patients have demonstrated that brain oscillations play a role in declarative memory encoding and retrieval, little has been done to investigate the temporal dynamics and sources of brain activity in healthy human subjects performing such tasks. In a magnetoencephalography study using pictorial stimuli, we have now identified oscillatory activity in the gamma (60–90 Hz) and theta (4.5–8.5 Hz) band during declarative memory operations in healthy participants. Both theta and gamma activity was stronger for the later remembered compared with the later forgotten items (the “subsequent memory effect”). In the retrieval session, theta and gamma activity was stronger for recognized items compared with correctly rejected new items (the “old/new effect”). The gamma activity was also stronger for recognized compared with forgotten old items (the “recognition effect”). The effects in the theta band were observed over right parietotemporal areas, whereas the sources of the effects in the gamma band were identified in Brodmann area 18/19. We propose that the theta activity is directly engaged in mnemonic operations. The increase in neuronal synchronization in the gamma band in occipital areas may result in a stronger drive to subsequent areas, thus facilitating both memory encoding and retrieval. Alternatively, the gamma synchronization might reflect representations being reinforced by top-down activity from higher-level memory areas. Our results provide additional insight on human declarative memory operations and oscillatory brain activity that complements previous electrophysiological and brain imaging studies.
Keywords: subsequent memory effect, old/new effect, MEG, episodic memory, working memory, EEG
Introduction
Declarative memory is a type of explicit memory for facts and events. Declarative memory formation has been studied by comparing brain activity during encoding of items that subsequently were retrieved versus those that were forgotten (“subsequent memory effect”) (Sanquist et al., 1980; Paller et al., 1987; Rugg, 1990). Declarative memory retrieval has been investigated by comparing the brain activity recorded during correctly recognized old versus correctly identified new items (the “old/new effect”) (for review, see Rugg, 1995).
Previous functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies have shown an increased activation in the medial temporal lobe (MTL) and inferior prefrontal areas associated with memory formation (Brewer et al., 1998; Wagner et al., 1998; Kirchhoff et al., 2000; Otten et al., 2001; Davachi and Wagner, 2002; Strange et al., 2002; Weis et al., 2004), whereas anterior prefrontal cortex, parietal cortex, and medial-frontal areas were activated during the old/new effect (Henson et al., 1999; Konishi et al., 2000; Donaldson et al., 2001; Weis et al., 2004) (for review, see Rugg and Henson, 2002; Wagner et al., 2005).
To investigate the brain dynamics on a faster timescale, electroencephalography (EEG) and magnetoencephalography (MEG) have been applied to characterize, respectively, event-related potentials and fields (ERPs and ERFs) (Rugg, 1995; Friedman and Johnson, 2000; Tendolkar et al., 2000; Takashima et al., 2006). These studies have shown that the differential effects with respect to encoding and retrieval start relatively late (∼0.3 s) after stimulus onset. Unfortunately, given that these ERP/ERF effects are spatially very distributed, reliable localization of the involved sources has been problematic.
Relatively late oscillatory responses (>0.2 s) that are induced by but not phase locked to the stimulus like ERPs/ERFs can reflect important cognitive processing as well (Tallon-Baudry and Bertrand, 1999). Strong arguments support the case that oscillatory neuronal synchronization plays an essential role in neuronal processing in general (for review, see Singer, 1999; Salinas and Sejnowski, 2001). Indeed, successful declarative encoding of words was first shown to be associated with changes in EEG theta (4–8 Hz) power (Klimesch et al., 1996) and coherence (Weiss et al., 2000); however, the sources of the theta activity in these studies were not identified. A study using intracranial EEG (iEEG) recordings in epileptic patients reported an increase in the frontal and right temporal theta activity and widely distributed gamma (30–100 Hz) activity (Sederberg et al., 2003) associated with successful encoding of words. Using depth-electrode recordings in epileptic patients, Fell et al. (2001) demonstrated an increase in rhinal-hippocampal gamma band synchronization during word encoding. This increase was subsequently shown to correlate with theta coherence over subjects (Fell et al., 2003).
These intracranial studies suggest that theta and gamma oscillations play an important role in memory formation. However, the electrode locations were defined by the surgical requirements, and some of the findings might be related to the pathology per se or to the administered drugs. In the present work, we investigated oscillatory activity during declarative memory encoding and retrieval of pictorial stimuli in adult healthy subjects using whole-head MEG. Because of its good temporal and spatial resolution, MEG allows us to monitor the temporal dynamics of oscillatory activity in various frequency bands and to identify the involved sources.
Materials and Methods
Subjects.
Thirteen healthy subjects (mean age, 25 ± 4.8 years; nine females) participated in the experiment. None of the subjects had a history of neurological or psychiatric disorders. All participants had normal or corrected-to-normal vision and were right-handed according to the Edinburgh Handedness Index. The study was approved by the local ethics committee, and a written informed consent was obtained from the subjects according to the Declaration of Helsinki.
Experimental paradigm.
Four hundred eighty real-life photographs of either buildings or landscapes (240 in each category) were used as stimuli. Pictures were obtained from websites and had resolutions exceeding 480 × 640 pixels. Pictures of well known buildings and landscapes were avoided. Each subject participated in an encoding session, followed by a retrieval session.
In the encoding session, 120 images of buildings were presented randomly intermixed with 120 images of landscapes. Stimuli were projected onto a screen with a visual angle of 8.5° vertically and 10.8° horizontally. Each trial started with a fixation cross displayed with a random duration of 1.2–1.8 s (mean, 1.5 s), followed by the actual stimulus presented for 1 s (Fig. 1). A question mark was displayed for 1 s after the stimulus offset, followed by the next fixation cross. During the presentation of the question mark, the subjects were instructed to make a building/landscape decision by a button press with the left or right index finger, respectively. Note that no motor responses were given during the presentation of pictures. The response time of the subject had no influence on the duration of each trial. The subjects were instructed to memorize the images for the subsequent memory test in the retrieval session.
Figure 1.
The experimental design used in the study. In the encoding session, subjects were shown photographs (e.g., S1, S2) and instructed to make a building/landscape decision by a button press. In the retrieval session, participants were shown pictures from the encoding session (e.g., S2) intermixed with the new stimuli (e.g., P1). They were instructed to respond to whether the picture had been shown in the previous session. Based on the answers, trials in the encoding session were classified as LR and LF. Trials in the retrieval session were labeled as HIT, CR, and MISS.
The retrieval session followed 5–10 min after the encoding session (Fig. 1). The 240 stimuli from the encoding session were randomly intermixed with 240 new pictures. The timing of the retrieval session was similar to the encoding session. Subjects were instructed to respond with a left index finger button press when they were highly confident that the presented picture had been shown previously in the encoding session. When subjects were highly confident that the picture was new, they were instructed to give a right index finger button press. To avoid guesses, subjects were asked not to respond when they were uncertain. The stimuli in the encoding session were counterbalanced across subjects such that the set of old pictures for one subject corresponded to the set of new pictures for another subject. All investigations were performed between 9:00 A.M. and 6:00 P.M.
MEG measurement.
Ongoing brain activity was recorded (low-pass filter, 150 Hz; sampling rate, 600 Hz) using a whole-head MEG system with 151 axial gradiometers (VSM/CTF Systems, Port Coquitlam, British Columbia, Canada). Head localization was done before and after the experiment using marker coils that were placed at the cardinal points of the head (nasion, left and right ear canal). The magnetic fields produced by these coils were used to measure the position of the subject’s head with respect to the MEG sensor array. In addition to the MEG, the electrooculogram was recorded from the supraorbital and infraorbital ridge of the left eye for the subsequent artifact rejection.
Data analysis.
All data analysis was performed using the FieldTrip toolbox developed at the F. C. Donders Centre for Cognitive Neuroimaging (http://www.ru.nl/fcdonders/fieldtrip) using Matlab 7.0.4 (MathWorks, Natick, MA). The trials from the first session were divided into two categories: later remembered (LR) and later forgotten (LF), depending on the response given in the recognition session. Trials in the recognition session were classified as correctly identified previously seen pictures (HIT), correctly rejected new stimuli (CR), not recognized old pictures (MISS), and new pictures incorrectly identified as old, i.e., false alarms (FA). The number of FA trials was too low for the analysis. The performance rates are presented at Table 1.
Table 1.
Means and SDs of recognition performance in the retrieval phase
| Old |
New |
|||
|---|---|---|---|---|
| HITS | MISS | CR | FA | |
| Buildings | 0.63 ± 0.07 | 0.37 ± 0.07 | 0.86 ± 0.11 | 0.14 ± 0.11 |
| Landscape | 0.57 ± 0.12 | 0.43 ± 0.12 | 0.79 ± 0.14 | 0.21 ± 0.14 |
| Buildings+Landscapes | 0.60 ± 0.07 | 0.40 ± 0.07 | 0.83 ± 0.11 | 0.17 ± 0.11 |
Old pictures were presented in the encoding phase, and new pictures correspond to a new set of pictures presented only in the retrieval phase. For buildings, d′ = 1.5 ± 0.5. For landscapes, d′ = 1.1 ± 0.5.
Partial artifact rejection was performed by rejecting segments of the trials containing eyeblinks, muscle, and superconducting quantum interference device (SQUID) sensor artifacts. By this procedure, smaller segments of a trial, rather than a whole trial, can be rejected. This is advantageous when calculating time–frequency representations (TFR) based on sliding time windows because fewer full trials have to be rejected (in the subsequent averaging, the number of segments applied is of course taken into account). To ensure that segments were sufficiently long for the subsequent analysis, segments shorter than 0.6 s were discarded as well. On average, the fraction of data segments rejected because of artifacts were 16.4 ± 9.2% (LR), 16.8 ± 10% (LF), 21.9 ± 13.1% (HIT), 20.0 ± 10.9% (CR), and 19.9 ± 10.4% (MISS). For the sensor-level analysis, an estimate of the planar gradient was calculated for each sensor using the signals from the neighboring sensors. The horizontal and vertical components of the planar gradients approximate the signal measured by MEG systems with planar gradiometers. The planar field gradient simplifies the interpretation of the sensor-level data because the maximal signal power is located above the source (Hämäläinen et al., 1993).
TFRs of power were calculated for each trial using a fast Fourier transform (multi)taper approach applied to short sliding time windows (Percival and Walden, 1993). The data in each time window were multiplied with a set of orthogonal Slepian tapers. The Fourier transforms of the tapered time windows were then calculated, and the resulting power estimates were averaged across tapers. The power values were calculated for the horizontal and vertical component of the estimated planar gradient and then summed. The planar gradient power estimates were subsequently averaged over multiple trials for a given condition. For the frequencies 5–34 Hz, we applied an adaptive time window of three cycles for each frequency (ΔT = 3/f) and an adaptive smoothing of Δf = 1/ΔT. This resulted in one taper being applied to the data from the sliding time window. In the higher-frequency bands (35–180 Hz), we used a fixed time window of ΔT = 0.2 s and a frequency smoothing of Δf = 10 Hz, resulting in three tapers being applied to the sliding time window. The change in power was calculated with respect to a baseline period −0.5 to −0.3 s before the presentation of the stimulus.
The significance of the difference between the two trial types in the encoding session (LR and LF) and the three trial types in the retrieval session (HIT, CR, and MISS) was established using a nonparametric randomization test (Nichols and Holmes, 2002) (E. Maris and R. Oostenveld, unpublished work). This test effectively controls the type I error rate in a situation involving multiple comparisons (such as 151 sensors) by clustering neighboring sensor pairs that exhibit the same effect. Time points were averaged within the 0.3–1 s interval, which is in agreement with the time course of memory effects indicated in ERP studies (for review, see Friedman and Johnson, 2000). This window of analysis also results in little interference from early visual evoked responses. Data points were also averaged within frequency bands. The frequency boundaries of theta, alpha, and beta bands were based on those widely accepted in EEG/MEG literature (Niedermeyer, 2005). The frequency boundaries of the gamma band were based on visual inspection of the TFRs for the averaged conditions. The randomization method identified sensors whose t statistics exceeded a critical value when comparing two conditions sensor by sensor (p < 0.05, two-sided). Note that the goal of this step is to identify sensors with effects exceeding a threshold for the subsequent cluster analysis, i.e., it is not required that the power values to be tested are normally distributed. To correct for multiple comparisons, the sensors that were exceeding the critical value and neighboring in the sensor array (separated by <5 cm) were grouped as a cluster. This approach is justified by the fact that a physiological source produces the strongest planar gradient field in a contiguous group of sensors right above the source (Hämäläinen et al., 1993). The cluster-level test statistic was defined from the sum of the t values of the sensors in a given cluster. The cluster with the maximum sum was used as the test statistics. The type I error rate for the complete set of 151 sensors was controlled by evaluating the cluster-level test statistic under the randomization null distribution of the maximum cluster-level test statistic. This was obtained by randomizing the data between the two conditions across multiple subjects calculating t statistics for the new set of clusters. A reference distribution of cluster-level t statistics was created from 3000 randomizations. The p value was estimated according to the proportion of the randomization null distribution exceeding the observed maximum cluster-level test statistic (the so-called Monte Carlo p value). It should be emphasized that we assessed significance using a nonparametric statistical test (and not a t test). This nonparametric statistical test uses a test statistic (the maximum of the cluster-level statistics) whose calculation is based on thresholded sample-specific t statistics. For a nonparametric test, it is irrelevant whether these sample-specific t statistics have a T distribution under the null hypothesis.
A frequency-domain beam-forming approach [Dynamic Imaging of Coherent Sources (DICS)] was used to identify sources of oscillatory activity. Note that, for source reconstruction, we used the data from the true axial sensors and not the planar gradient estimate. The DICS technique uses adaptive spatial filters to localize power in the entire brain (Gross et al., 2001; Liljeström et al., 2005). The filter uses the cross-spectral density matrix that is calculated separately in the prestimulus and poststimulus periods of the individual trials and averaged. The data length of the segmented trials for the theta sources was no less than 0.5 s and for the gamma sources no less than 0.2 s. Multisphere forward models were fitted to individual head shapes identified from the individual MRIs (Huang and Mosher, 1997) obtained for 12 of 13 subjects. The brain volume of each individual subject was discretized to a grid with a 0.5 cm resolution. Using the cross-spectral density matrices and the forward models, a spatial filter was constructed for each grid point, and the power was estimated for each condition in each subject. The individual subjects’ source estimates were overlaid on the corresponding anatomical MRI, and the anatomical and functional data were subsequently spatially normalized using SPM2 (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm) to the International Consortium for Brain Mapping template [Montreal Neurological Institute (MNI), Montreal, Quebec, Canada; http://www.bic.mni.mcgill.ca/brainweb]. After spatial normalization, the beam-former source reconstructions were averaged across subjects.
Results
Behavioral performance
In the encoding session, the building/landscape decision was performed with a mean accuracy of 96.5 ± 2.8%, demonstrating that subjects were attending to the stimuli. Incorrect (2.7 ± 1.8%) and no response (0.6 ± 1.0%) trials were excluded from the analysis. Notably, the d′ values were higher for buildings than landscapes. Recognition memory performance for buildings and landscapes separately is presented in Table 1. Accuracy of recognition was calculated as a difference in probabilities of a correct old judgment (HIT) and an incorrect old judgment for a new item (FA) (Pr = probability HIT − probability FA). Because of the low number of FA trials, the recognition performance substantially exceeded chance level (mean ± SD, Pr = 0.44 ± 0.12; t(12) = 12.48; p < 0.001).
Encoding session
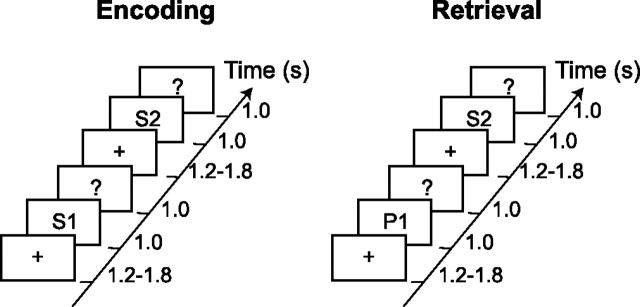
The TFRs of power for the combined planar gradients were calculated for different conditions. In the gamma band (60–90 Hz), we identified strong activity over posterior areas for both later remembered and forgotten items (Fig. 2). The TFRs revealed that the gamma activity was stronger for later remembered compared with later forgotten items during the 0.3–1 s time interval of the item presentations (Fig. 2B,C). This reflects the subsequent memory effect. A randomization routine correcting for the multiple comparisons between sensors was applied to access the significance between conditions (Maris and Oostenveld, unpublished work). This routine identified two clusters of occipital sensors corresponding to a significant difference in the gamma band between the two conditions (p < 0.01 and p < 0.05) (Fig. 2A). Furthermore, we performed a beam-former analysis to identify sources of gamma activity (Gross et al., 2001; Liljeström et al., 2005). As shown in Figure 2D, gamma sources in both conditions were located bilaterally in Brodmann areas (BA) 18 and 19. Comparison of source strength for the later remembered and later forgotten trials revealed that the source difference between the conditions was in the same areas. The location of these sources is consistent with the topographies of power in Figure 2A.
Figure 2.
Gamma activity during the encoding session: the subsequent memory effect. A, The grand average of the topography of gamma power for the LR and LF trials and their difference (LR − LF). Two adjacent clusters of occipital sensors showed significant increase in gamma power (*p < 0.01; +p < 0.05). B, Grand-averaged time–frequency representations of power from one significant sensor showing the time course of gamma oscillations for LR, LF, and their difference. C, Grand-averaged gamma power averaged between 60 and 90 Hz for both conditions for the same sensor as in B. D, Source reconstruction of gamma activity, averaged over subjects and overlaid on the MNI standard brain. The sources for LR and LF conditions were located bilaterally in BA18/19. The difference in power (LR − LF) revealed sources in BA18/19 as well. The power of the source representations was thresholded at half-maximum.
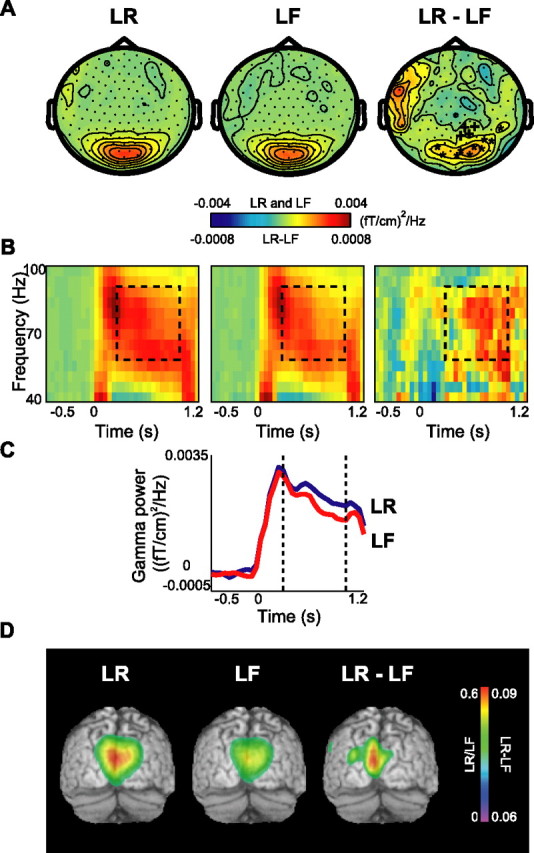
In the theta band (4.5–8.5 Hz), we observed a systematic difference in theta power when comparing the encoding period for later remembered with forgotten trials (Fig. 3). This difference was most pronounced over the right temporal areas (Fig. 3A). The TFRs over right temporal sensors showed that the difference was the strongest during the 0.3–1 s period of the presentation interval (Fig. 3B,C). In the theta band, statistics was done for 6.0 ± 2.2 Hz, in which the ±2.2 Hz smoothing was determined by our spectral estimation approach. The randomization approach identified a cluster of sensors over the right temporal cortex with a significant difference in the theta band (p < 0.05) (Fig. 3A). We attempted to identify the sources of the effect in the theta band using the beam-former approach. However, we were not successful, most likely because of an insufficient signal-to-noise ratio.
Figure 3.
Theta activity during the encoding session: the subsequent memory effect. A, The grand average of topography of theta power for the LR and LF trials and their difference (LR − LF). The cluster of right temporal sensors that showed significantly increased power in the LR compared with the LF condition is indicated by + signs (p < 0.05). B, Grand-averaged time–frequency representations of power from a significant representative sensor showing the time course of theta oscillations during LF, LR, and their difference. C, Grand average of theta power (4.5–8.5 Hz) for both conditions for the same sensor as in B.
Using the same approach, we also systematically investigated modulations in alpha (8–13 Hz) and beta (13–25 Hz) frequency bands during encoding but found no significant effects. We systematically compared the baselines period (−0.5 to −0.3 s) in the four frequency bands (theta, alpha, beta, and gamma) between each condition in the encoding session and each condition in the retrieval session (later remembered vs hits vs misses vs correct rejections; later forgotten vs hits vs misses vs correct rejections). As for the other tests presented in this paper, the statistics were done at the sensor level using a cluster-randomization routine controlling for multiple comparisons. No significant differences were found in the baseline intervals (p values for the tests were at least p > 0.15). Thus, the significant findings we reported are explained by differences in the encoding and recall intervals, not prestimulus effects.
To test whether the differences in the stimulation material (buildings vs landscapes) can account for the present results, we compared theta and gamma power for building and landscape stimuli with respect to encoding. When comparing buildings with landscapes during encoding collapsed over LR and LF, no differences were found. This shows that there are no perceptual differences reflected in the theta and gamma bands when comparing the landscapes with the building stimuli. Furthermore, we compared LR buildings with LF buildings and LR landscapes with LF landscapes in the theta and gamma bands. Statistically significant differences were observed only for the LR versus LF landscapes over the occipital area in the gamma band (p < 0.01). In other conditions (in the theta band or for LR vs LF buildings in the gamma band), the trends were observed, but the differences remained statistically insignificant; this is most likely attributable to a lower number of trials when dividing data into buildings and landscapes. In conclusion, although there were differences in behavioral performance between landscapes and buildings (Table 1), the oscillatory encoding effects in the theta and gamma band could not be attributable to differences between buildings and landscapes.
Retrieval session
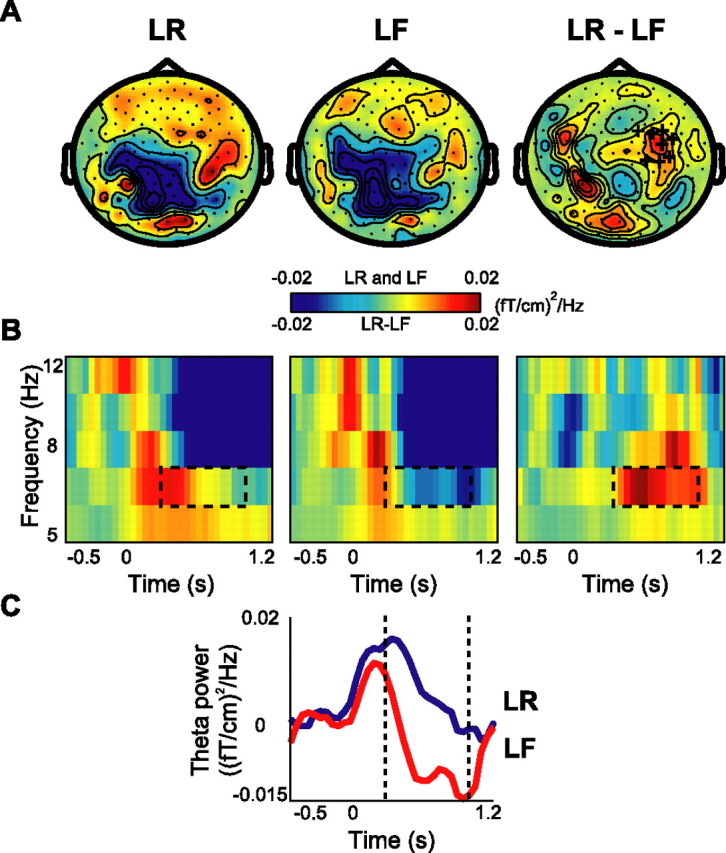
Old/new effects were identified by comparing hits with correct rejections. We observed strong gamma band activity over the posterior brain regions during retrieval (Fig. 4A). The frequency range was 60–90 Hz, similar to that observed during the encoding session. This gamma activity increased in the last part of the retrieval interval (0.3–1 s) over the occipital region for hits compared with correct rejections, reflecting the old/new effect (Fig. 4B,C). The increase was weak but statistically significant (p < 0.05). We localized the primary sources of the gamma activity to BA18 and BA19, similar to the ones in the encoding session (Fig. 4D). The difference in source strength involved parietal and occipital sources. However, because of the relatively low signal-to-noise ratio, the spatial distribution of this source power difference outside BA18/19 should be interpreted with caution.
Figure 4.
Gamma activity during the retrieval session: the old/new effect. A, The grand average of the topography of gamma power for HIT and CR trials and their difference (HIT − CR). A cluster of occipital sensors showed significant difference in gamma power, indicated by + signs (p < 0.05). B, Grand average of time–frequency representations of power from one significant representative sensor showing the time course of gamma oscillations in two conditions and their difference. C, Grand-averaged gamma power averaged between 60 and 90 Hz for both conditions for the same sensor as in B. D, Source reconstruction of gamma activity, averaged over subjects and overlaid on the MNI standard brain. The sources for HIT and CR conditions were located bilaterally in BA18/19. The difference in power (HIT − CR) revealed sources in BA18/19 as well. The power of the source representations was thresholded at half-maximum.
During retrieval, we also found a modulation in theta (4.5–8.5 Hz) band when comparing hits with correct rejections (Fig. 5). As seen in the topographical plots (Fig. 5A), the difference in theta band activity when comparing hits with correct rejections was lateralized over right posterior regions; this effect was statistically significant in the time window 0.3–1 s (p < 0.05) (Fig. 5C). The TFRs show the effect in the theta band (Fig. 5B). Source analysis for the theta band did not produce any convincing results, probably because of an insufficient signal-to-noise ratio.
Figure 5.
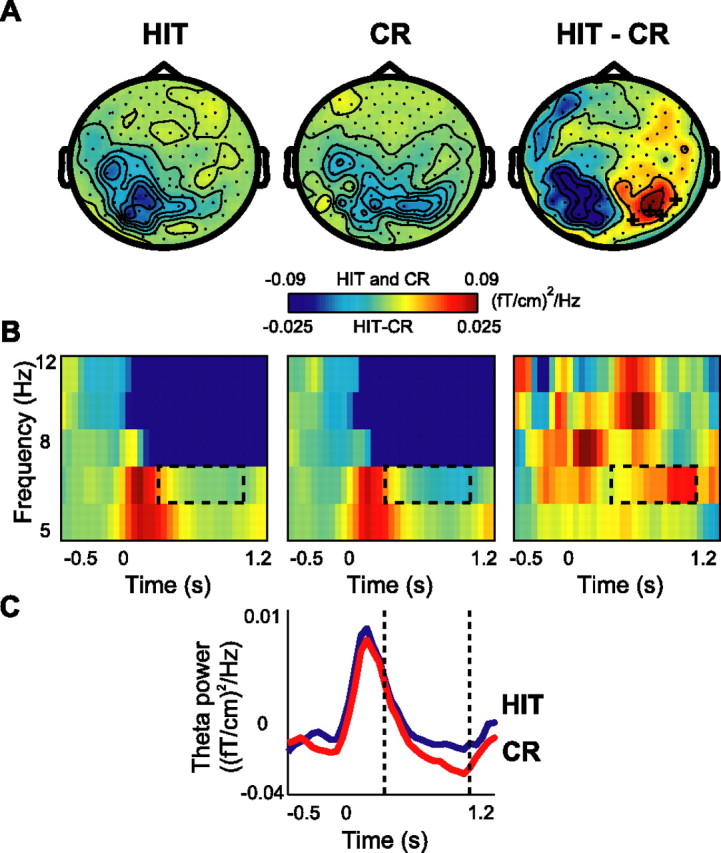
Theta activity during the retrieval session: the old/new effect. A, The grand average of the topography of theta power for HIT and CR trials and their difference (HIT − CR). A cluster of right occipital sensors showed significant increase in theta power, indicated by + signs (p < 0.05). B, Grand average of time–frequency representations of power from one significant representative sensor showing the time course of theta oscillations in two conditions and their difference. C, Grand average of theta power (4.5–8.5 Hz) for both conditions for the same sensor as in B.
Recognition effects were identified by comparing hits with misses. No significant effects were observed in the theta band; however, the gamma band modulations were highly robust (Fig. 6A). The difference in the gamma activity was also significant between hits and misses (two adjacent clusters of p < 0.01 and p < 0.05) (Fig. 6B,C). The sources of the gamma activity were located bilaterally in BA18 and BA19, likewise the difference in source strength (Fig. 6D).
Figure 6.
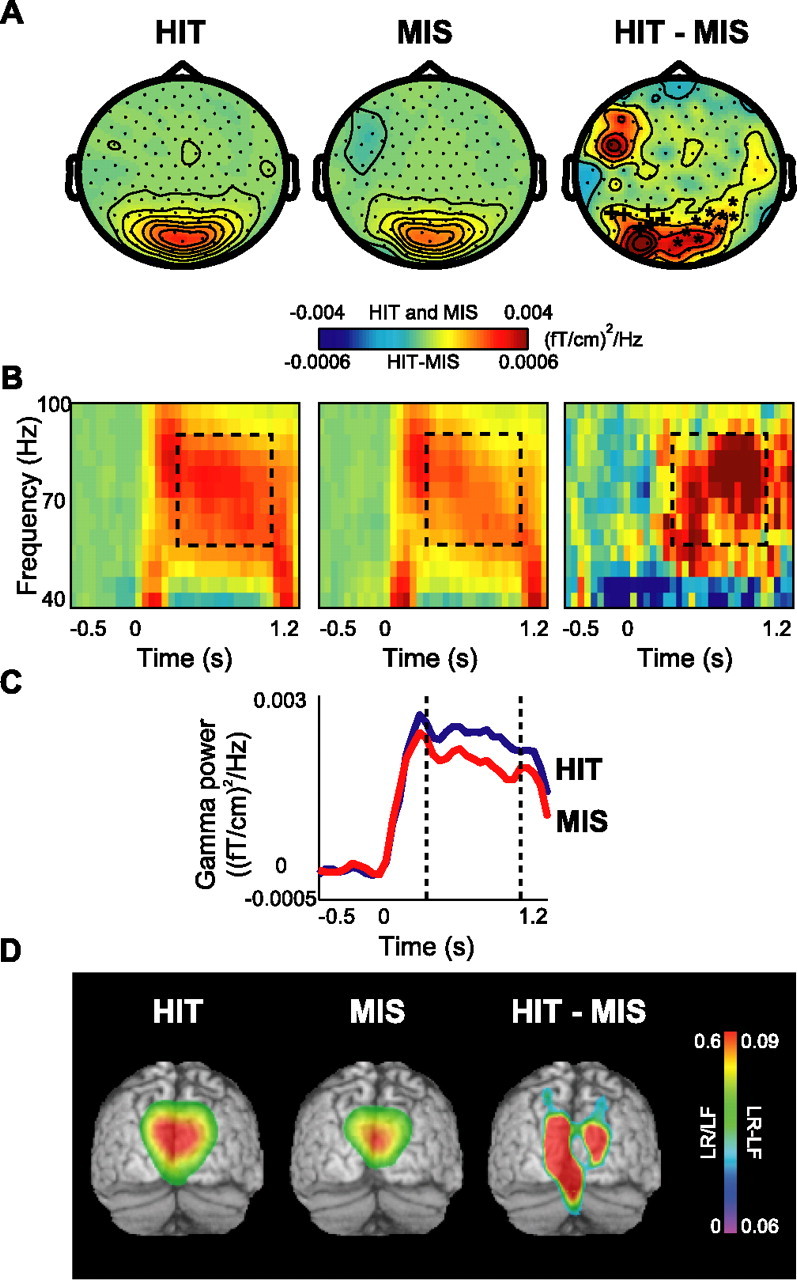
Gamma activity in the retrieval session: the recognition effect. A, The grand average of the topography of gamma power for HIT and MISS items and their difference (HIT − MISS). Two adjacent clusters of occipital sensors showed significant increase in gamma power (*p < 0.01; +p < 0.05). B, Grand-averaged time–frequency representations of power from one significant sensor associated with HIT versus MISS items and the subtraction of two conditions. C, Grand-averaged gamma power averaged between 60 and 90 Hz for both conditions for the same sensor as in B. D, Source reconstruction of gamma activity, averaged over subjects and overlaid on the MNI standard brain. The sources for HIT and MISS conditions were located bilaterally in BA18/19. The difference in power (HIT − MISS) revealed sources in BA18/19 as well. The power of the source representations was thresholded at half-maximum.
Beta power was bilaterally significantly decreased for the hits compared with correct rejections (p < 0.01; data not shown). For hits versus misses, beta power was also decreased bilaterally over wide areas (p < 0.03; data not shown). However, because hits responses were consistently given with the left index finger and correct rejections and miss responses with the right index finger, the lateralized effects in the beta band are most likely explained by motor preparation (Pfurtscheller et al., 1998). No effects were found in the alpha band in the retrieval session.
In the retrieval session, we also compared theta and gamma power for the trials divided among building and landscape stimuli. First, we compared HIT versus CR buildings and HIT versus CR landscapes. Statistically significant differences were observed only for the HIT versus CR landscapes over the occipital area in the gamma band (p < 0.05). In other conditions (in the theta band or for HIT vs CR buildings in the gamma band) only trends were observed, but the differences remained statistically insignificant. When comparing HIT with MISS buildings and HIT with MISS landscapes, we identified statistically significant differences for landscapes over the occipital area in the theta band (p < 0.05) and for both buildings and landscapes in the gamma band (p < 0.05 and p < 0.01, respectively). Although there was a small tendency for the retrieval effect in the theta and gamma band to be more robust for landscapes than buildings, the effects primarily resembled our main result.
Relationships to visual evoked fields
The EEG/MEG literature distinguishes between evoked and induced oscillatory activity. Evoked activity is phase locked to the stimuli and is thus also present in the ERPs/ERFs. Induced activity is not strictly phase locked to the stimuli and is not present in the ERPs/ERFs (Tallon-Baudry and Bertrand, 1999). To clarify whether the changes we observed are attributable to modulations in evoked activity, we calculated the TFRs for the ERFs. Only one cluster of six frontotemporal sensors in the gamma band was significant for HITs compared with CRs (p < 0.05). However, the location of this significant cluster was clearly different from the posterior cluster showing the increase in induced gamma activity (Fig. 4). No other significant memory-related changes in evoked activity were observed (data not shown). Notably, Takashima et al. (2006) found memory effects in the ERFs during the encoding session using the same paradigm. These ERF effects had very slow time courses and were sustained over several hundred milliseconds. Therefore, they would be present only in the low-frequency ranges (<2 Hz) and cannot be considered oscillatory phenomena. We conclude that the memory-related effects in the gamma and theta bands are explained by induced oscillatory activity rather than the difference in the ERFs.
Discussion
In this study, we applied MEG to systematically investigate oscillatory brain activity during encoding and retrieval of pictorial stimuli. Our results show that declarative memory encoding and retrieval are associated with modulations of oscillatory activity in the gamma and theta bands. Successful encoding, the subsequent memory effect, was accompanied by a large magnitude of gamma power produced in occipital areas (BA18/19). This gamma power was significantly stronger for the later remembered than for the later forgotten items. The effects in the gamma band occurred in the 0.3–1 s interval with respect to stimulus onset. We also found that theta power was increased over the right temporal areas with respect to subsequent memory, but we were unable to identify the sources. In the retrieval session, the old/new effect was expressed as gamma power being significantly greater for hits compared with correct rejections. The old/new effect was also associated with a significant increase in the theta power over right parietotemporal regions. The recognition effect was expressed as high power in the gamma band when comparing hits with misses. The gamma sources for both effects were identical to those in the encoding session. To the best of our knowledge, this is the first study demonstrating induced gamma activity in the occipital areas associated with declarative memory encoding and retrieval.
Physiological role of theta and gamma oscillations during declarative memory encoding
The increase in gamma band power during declarative memory encoding and retrieval most likely reflects enhanced neuronal synchronization. Experimental and theoretical work shows that a neuron receiving multiple inputs is more likely to fire if these inputs are synchronized (for review, see Salinas and Sejnowski, 2001). Thus, the increase in synchronized activity in BA18/19 is likely to lead to a stronger drive to downstream areas directly involved in memory encoding and retrieval.
Theta oscillations could play a role in synaptic plasticity. It has been shown in both in vitro and in vivo recordings that long-term potentiation in the rat hippocampus can be induced by pairing of the inducing stimulus with the peak of the hippocampal theta rhythm (Huerta and Lisman, 1993; Hölscher et al., 1997). We propose that the right temporal theta activity reflects neuronal dynamics that are optimal for synaptic plasticity, which facilitates memory encoding.
Gamma activity in the visual system in humans
Strong sustained gamma activity in the visual system has been identified in a large number of studies on cats (Eckhorn et al., 1988; Gray and Singer, 1989) and monkeys (Kreiter and Singer, 1996; Gail et al., 2000; Fries et al., 2001). This gamma activity has been related to feature binding and selective attention. Several MEG studies have likewise shown a relationship between the gamma band activity and visual perception (Adjamian et al., 2004; Kaiser et al., 2004; Hoogenboom et al., 2006). Interestingly, the gamma activity we identified in the memory task bears some of the same features as the gamma activity identified by Hoogenboom et al. (2006) in terms of frequency range and loci. Given these resemblances, it would be interesting to further investigate whether the physiological mechanisms accounting for gamma activity in animals underlie the occipital gamma activity in humans as well.
Does the gamma activity reflect modulations in attention?
Several studies have indicated that modulations in gamma band activity reflect attention (Gruber et al., 1999; Fries et al., 2001; Tallon-Baudry et al., 2005). This raises the possibility that the increase in gamma power that we observe reflects an increase in attention that facilitates memory encoding and retrieval. However, multiple studies have demonstrated that attention is reflected in relatively early onsets of electrophysiological responses (Anllo-Vento et al., 1998; Torriente et al., 1999). Speaking against an attention effect, we found no differences in the electrophysiological activity during the early part of the stimulus presentation (0–0.3 s) with respect to the conditions of the task. This is consistent with a previous ERF study on the same data, in which we did not find modulations in the early evoked responses (N1, P1) either (Takashima et al., 2006). This is also in line with ERP studies that showed the onset of memory-dependent effects emerging 0.3 s after stimulus onset (for review, see Friedman and Johnson, 2000). Additionally, there were no differences in prestimulus alpha activity. We conclude that general changes in attention, e.g., attributable to changes in arousal, are unlikely to account for the correlation between declarative memory operations and gamma band activity. Conversely, attention associated with specific features of the pictorial stimuli could be reflected in the gamma band modulations.
Relationship to working memory
It has been proposed that there are strong links between working memory and processes involved in declarative encoding and retrieval (Baddeley, 2000). Both theta and gamma activity has been reported during maintenance of working memory representations in monkeys (Pesaran et al., 2002; Lee et al., 2005) and humans (Gevins et al., 1997; Sarnthein et al., 1998; Raghavachari et al., 2001; Tallon-Baudry et al., 2001; Jensen and Tesche, 2002; Howard et al., 2003; Kaiser and Lutzenberger, 2005). Additionally, a physiologically realistic computational model that accounts for the functional interaction between these two rhythms during working memory maintenance was proposed by Lisman and Idiart (1995). Predictions from the model has been tested in several studies (for review, see Jensen, 2006). The model was later extended to account for the encoding of long-term memory representations. In particular, it was argued that synaptically dependent hippocampal encoding requires working memory maintenance (Jensen and Lisman, 2005). These ideas are consistent with a recent study by Mormann et al. (2005) who observed that declarative memory operations were modulating the theta and gamma band activity recorded from the MTL in epileptic patients. Recent neuroimaging studies support that working memory plays a role for declarative encoding (Davachi and Wagner, 2002; Ranganath et al., 2005). This leaves open the possibility that the theta and gamma activity observed during encoding and retrieval in our study is related to working memory operations required for declarative memory operations. From that perspective, the gamma activity in visual areas might reflect representations being reinforced by top-down activity from areas directly engaged in encoding and retrieval (Ranganath et al., 2004, their Fig. 6). This reinforcement might be related to working memory operations supporting successful memory performances.
Relationship to fMRI and PET studies
It is of interest to compare the modulations in oscillatory activity reported here with findings from fMRI and PET studies using related paradigms. Modulations in oscillatory activity reflect changes in local neuronal synchronization, which does not necessarily change metabolic demands. Thus, the measure of oscillatory synchrony is complementary to fMRI and PET measuring variations in blood flow resulting from changing metabolic demands. Furthermore, MEG is less sensitive to certain neuronal generators, e.g., deep sources in the MTL. These differences are likely to explain the lack of overlapping regions engaged when comparing the current study and the findings of Weis et al. (2004) using very similar stimuli. For instance, Weis et al. (2004) did not find activations in the occipital BA18/19 when comparing the memory conditions, but they did find modulations in prefrontal and hippocampal areas. However, it should be pointed out that other fMRI studies have reported activation in BA18/19 associated with subsequent memory (Ranganath et al., 2005) and the old/new effect (Wheeler et al., 2000; Vaidya et al., 2002; Kahn et al., 2004; Lundstrom et al., 2005). Additionally, these studies identified a number of regions that we did not observe in our MEG study. We conclude that, to get a fuller understanding of the networks and temporal dynamics involved in declarative memory processing, both blood flow measures and electrophysiological techniques are important because they provide truly complementary information.
Relationships to previous electrophysiological studies on brain oscillations
Our results are consistent with previous EEG studies that have demonstrated an increase in theta power for the subsequent memory effect (Klimesch et al., 1996) and old/new effect (Klimesch et al., 2000). These studies applied words, whereas we used pictorial stimuli. In addition, we show that the effects in the theta bands most likely are originating in the right temporal lobe. The effects in the theta band over right temporal cortex are consistent with findings from iEEG recordings (Sederberg et al., 2003). In addition to these results, we identified a gamma band increase in the occipital cortex. Understandably, the locations of electrodes in iEEG depend on the type of pathology or on the requirements of presurgical mapping, which often leave the occipital region uncovered. Moreover, it is difficult to differentiate pathology- or medication-related high-frequency activity from regular gamma oscillations, whereas in the present study, we measured unmedicated healthy subjects. Overall, our findings support the iEEG data suggesting a role for both theta and gamma oscillations in declarative encoding.
In conclusion, we demonstrated that successful declarative encoding and retrieval are associated with increases in occipital gamma and right hemisphere theta power in healthy unmedicated subjects. Thus, we identified new correlates of declarative memory encoding and retrieval that are complementary to the existing data from electrophysiological and brain imaging studies.
Footnotes
The study was supported by the framework of the Netherlands Organization for Scientific Research Innovative Research Incentive Schemes, Academy of Finland, Huygens program, and the Volkswagen-Stiftung. We thank Dr. Pascal Fries for valuable discussions on methods and interpretations.
References
- Adjamian P, Holliday IE, Barnes GR, Hillebrand A, Hadjipapas A, Singh KD (2004). Induced visual illusions and gamma oscillations in human primary visual cortex. Eur J Neurosci 20:587–592. [DOI] [PubMed] [Google Scholar]
- Anllo-Vento L, Luck SJ, Hillyard SA (1998). Spatio-temporal dynamics of attention to color: evidence from human electrophysiology. Hum Brain Mapp 6:216–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A (2000). The episodic buffer: a new component of working memory? Trends Cogn Sci 4:417–423. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JDE (1998). Making memories: brain activity that predicts how well visual experience will be remembered. Science 281:1185–1187. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD (2002). Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. J Neurophysiol 88:982–990. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL (2001). Dissociating memory retrieval processes using fMRI: evidence that priming does not support recognition memory. Neuron 31:1047–1059. [DOI] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ (1988). Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern 60:121–130. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernandez G (2001). Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci 4:1259–1264. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernandez G (2003). Rhinal-hippocampal theta coherence during declarative memory formation: interaction with gamma synchronization? Eur J Neurosci 17:1082–1088. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson R (2000). Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microsc Res Tech 51:6–28. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R (2001). Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291:1560–1563. [DOI] [PubMed] [Google Scholar]
- Gail A, Brinksmeyer HJ, Eckhorn R (2000). Contour decouples gamma activity across texture representation in monkey striate cortex. Cereb Cortex 10:840–850. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D (1997). High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex 7:374–385. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W (1989). Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA 86:1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hämäläinen M, Timmermann L, Schnitzler A, Salmelin R (2001). Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci USA 98:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber T, Müller MM, Keil A, Elbert T (1999). Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clin Neurophysiol 110:2074–2085. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV (1993). Magnetoencephalography: theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys 65:413–497. [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ (1999). Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci 19:3962–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher C, Anwyl R, Rowan MJ (1997). Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can be depotentiated by stimulation on the negative phase in area CA1 in vivo J Neurosci 17:6470–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom N, Schoffelen JM, Oostenveld R, Parkes LM, Fries P (2006). Localizing human visual gamma-band activity in frequency, time and space. NeuroImage 29:764–773. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ (2003). Gamma oscillations correlate with working memory load in humans. Cereb Cortex 13:1369–1374. [DOI] [PubMed] [Google Scholar]
- Huang M, Mosher JC (1997). A novel head model for the MEG forward problem: BEM accuracy with only spherical model complexity. Proceedings of Third International Conference on Functional Mapping of the Human Brain June Denmark: Copenhagen.
- Huerta PT, Lisman JE (1993). Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature 364:723–725. [DOI] [PubMed] [Google Scholar]
- Jensen O (2006). Maintenance of multiple working memory items by temporal segmentation. Neuroscience 139:237–249. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE (2005). Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci 28:67–72. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD (2002). Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci 15:1395–1399. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD (2004). Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci 24:4172–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W (2005). Cortical oscillatory activity and the dynamics of auditory memory processing. Rev Neurosci 16:239–254. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Buhler M, Lutzenberger W (2004). Magnetoencephalographic gamma-band responses to illusory triangles in humans. NeuroImage 23:551–560. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE (2000). Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci 20:6173–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T (1996). Theta band power in the human scalp EEG and the encoding of new information. NeuroReport 7:1235–1240. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Winkler T, Gruber W (2000). Theta oscillations and the ERP old/new effect: independent phenomena? Clin Neurophysiol 111:781–793. [DOI] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL (2000). Neural correlates of episodic retrieval success. NeuroImage 12:276–286. [DOI] [PubMed] [Google Scholar]
- Kreiter AK, Singer W (1996). Stimulus-dependent synchronization of neuronal responses in the visual cortex of the awake macaque monkey. J Neurosci 16:2381–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, Rainer G (2005). Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron 45:147–156. [DOI] [PubMed] [Google Scholar]
- Liljeström M, Kujala J, Jensen O, Salmelin R (2005). Neuromagnetic localization of rhythmic activity in the human brain: a comparison of three methods. NeuroImage 25:734–745. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MAP (1995). Storage of 7 ± 2 short-term memories in oscillatory subcycles. Science 267:1512–1515. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM (2005). The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. NeuroImage 27:824–834. [DOI] [PubMed] [Google Scholar]
- Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, Elger CE, Fernandez G (2005). Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus 15:890–900. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E (2005). The normal EEG of the waking adult. In: Electroencephalography: basic principles, clinical applications, and related fields (Niedermeyer E, Lopes da Silva F, eds) pp. 167–192. Philadelphia: Lippincott Williams and Wilkins.
- Otten LJ, Henson RNA, Rugg MD (2001). Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain 124:399–412. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M, Mayes AR (1987). Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr Clin Neurophysiol 67:360–371. [DOI] [PubMed] [Google Scholar]
- Percival DB, Walden AT (1993). In: Spectral analysis for physical applications: multitaper and conventional univariate techniques Cambridge, UK: Cambridge UP.
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA (2002). Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci 5:805–811. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Zalaudek K, Neuper C (1998). Event-related beta synchronization after wrist, finger and thumb movement. Electroencephalogr Clin Neurophysiol 109:154–160. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE (2001). Gating of human theta oscillations by a working memory task. J Neurosci 21:3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Dam C, D’Esposito M (2004). Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci 24:3917–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Brozinsky CJ (2005). Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. J Cogn Neurosci 17:994–1010. [DOI] [PubMed] [Google Scholar]
- Rugg MD (1990). Event-related brain potentials dissociate repetition effects of high-frequency and low-frequency words. Mem Cognit 18:367–379. [DOI] [PubMed] [Google Scholar]
- Rugg MD (1995). Event-related potential studies of human memory. In: The cognitive neurosciences (Gazzaniga MS, ed.) pp. 1341–1356. Cambridge, MA: MIT.
- Rugg MD, Henson RNA (2002). Episodic memory retrieval: an (event-related) functional neuroimaging perspective. In: The cognitive neuroscience of memory: encoding and retrieval (Parker AE, Wilding EL, Bussey T, eds) pp. 7–28. Hove, UK: Psychology.
- Salinas E, Sejnowski TJ (2001). Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 2:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanquist TF, Rohrbaugh JW, Syndulko K, Lindsley DB (1980). Electrocortical signs of levels of processing: perceptual analysis and recognition memory. Psychophysiology 17:568–576. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von Stein A (1998). Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA 95:7092–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR (2003). Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci 23:10809–10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W (1999). Neuronal synchrony: a versatile code for the definition of relations? Neuron 24:49–65. [DOI] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ (2002). Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci 22:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Jensen O, Oostenveld R, Maris E, van de Coevering M, Fernandez G (2006). Successful declarative memory formation is associated with ongoing activity during encoding in a distributed neocortical network related to working memory: an MEG study. Neuroscience 139:291–297. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O (1999). Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3:151–162. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Fischer C (2001). Oscillatory synchrony between human extrastriate areas during visual short-term memory maintenance. J Neurosci 21:RC177(1–5) (1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Henaff MA, Isnard J, Fischer C (2005). Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cereb Cortex 15:654–662. [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Rugg M, Fell J, Vogt H, Scholz M, Hinrichs H, Heinze HJ (2000). A magnetoencephalographic study of brain activity related to recognition memory in healthy young human subjects. Neurosci Lett 280:69–72. [DOI] [PubMed] [Google Scholar]
- Torriente I, Valdes-Sosa M, Ramirez D, Bobes MA (1999). Visual evoked potentials related to motion-onset are modulated by attention. Vision Res 39:4122–4139. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Zhao M, Desmond JE, Gabrieli JDE (2002). Evidence for cortical encoding specificity in episodic memory: memory-induced re-activation of picture processing areas. Neuropsychologia 40:2136–2143. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL (1998). Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 281:1188–1191. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL (2005). Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9:445–453. [DOI] [PubMed] [Google Scholar]
- Weis S, Klaver P, Reul J, Elger CE, Fernandez G (2004). Temporal and cerebellar brain regions that support both declarative memory formation and retrieval. Cereb Cortex 14:256–267. [DOI] [PubMed] [Google Scholar]
- Weiss S, Muller HM, Rappelsberger P (2000). Theta synchronization predicts efficient memory encoding of concrete and abstract nouns. NeuroReport 11:2357–2361. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL (2000). Memory’s echo: vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci USA 97:11125–11129. [DOI] [PMC free article] [PubMed] [Google Scholar]