Abstract
There is increasing evidence to suggest that the hippocampus and perirhinal cortex may mediate processes beyond long-term declarative memory. We assessed patients with Alzheimer's disease (AD) or semantic dementia (SD) on a visual oddity judgment task that did not place an explicit demand on long-term memory and is known to be sensitive to hippocampal and perirhinal cortex lesions. Importantly, within the medial temporal lobe, AD is associated with predominant hippocampal atrophy, whereas SD patients have greater perirhinal cortex damage. The AD group was selectively impaired in oddity judgment for scenes, whereas the SD patients demonstrated a deficit in face oddity judgment only. This compelling double dissociation supports the idea that the hippocampus and perirhinal cortex may be critical for the processing of scenes and objects, respectively, in the domain of perception or very short-term working memory.
Keywords: memory, perception, amnesia, Alzheimer's disease, semantic dementia, discrimination
Introduction
Medial temporal lobe (MTL) structures are thought to subserve long-term declarative memory processes exclusively, either as a single unitary system (Squire et al., 2004) or with each structure subserving a distinct process, such as recollection versus familiarity (Brown and Aggleton, 2001). Recent studies challenge this idea, however, suggesting that the MTL may be critical for processes beyond long-term memory, such as very short-term working memory (Ranganath and D'Esposito, 2005) or even higher-order perception, with the hippocampus and perirhinal cortex subserving spatial and object perception, respectively (Buckley et al., 2001; Gaffan, 2001; Bussey et al., 2002; Lee et al., 2005a,b,c). For example, in the context of oddity judgment tasks that did not place an explicit long-term declarative memory demand, patients with selective hippocampal lesions were impaired in discriminating spatial scenes, whereas cases with larger MTL lesions, including the perirhinal cortex, exhibited further difficulties discriminating objects, including faces (Lee et al., 2005b).
Although the aforementioned studies provide compelling support for the idea that the hippocampus and perirhinal cortex may mediate distinct processes beyond long-term memory, an additional and more powerful demonstration in support of this view would be a functional double dissociation associated with damage to these two structures. There is no published report of patients with selective bilateral perirhinal cortex damage and preserved hippocampi, but two patient populations that allow one to investigate a possible double dissociation are Alzheimer's disease (AD) and semantic dementia (SD). AD is characterized by a primary deficit in episodic memory with at least one additional impairment in a different cognitive domain (McKahnn et al., 1984). In contrast, SD patients show a progressive, cross-modal loss of semantic knowledge (Snowden et al., 1989; Hodges et al., 1992), with relative preservation of other abilities early on. Importantly, although there is widespread MTL damage in both SD and AD (Chan et al., 2001; Galton et al., 2001), volumetric studies have revealed greater atrophy to the perirhinal cortex and anterior entorhinal cortex compared with other MTL structures (i.e., the hippocampus) in SD, whereas AD patients have damage to the entire hippocampus but less so to the perirhinal cortex (Davies et al., 2004). Although other structural regions are implicated in these two diseases, this skewing of MTL pathology provides a unique opportunity to determine the role of the MTL in processes beyond long-term memory.
The present study, therefore, assessed spatial scene and face discrimination in AD and SD patients, using a paradigm that did not demand long-term declarative memory and is highly sensitive to hippocampal and perirhinal cortex lesions in amnesic patients (Lee et al., 2005b). On the basis of previous findings and the profiles of MTL atrophy in these two diseases, we predicted that the AD group would be impaired in the discrimination of scenes but not faces, whereas the SD group would show difficulties when face, but not scene, discrimination was assessed.
Materials and Methods
Subjects
Eight SD patients [mean age, 61.63 years; SD, 5.80; mean education, 13.38 years; SD, 3.66; mean Mini-Mental State Examination (MMSE) score, 20.71 of 30; SD, 6.52] and seven AD patients (mean age, 70.14 years; SD, 5.55; mean education, 13.29 years; SD, 4.27; mean MMSE score, 23.57 of 30; SD, 3.91) participated in this study. Both patient groups were matched in terms of MMSE and education (both t < 1; p > 0.3), although the AD group was significantly older (t(13) = 2.89; p = 0.013). Subsequently, two groups of healthy controls were assessed: an SD control group (mean age, 63.90 years; SD, 3.57; mean education, 11.11 years; SD, 1.17) and an AD control group (mean age, 69.00 years; SD, 4.24; mean education, 13.11 years; SD, 2.52). There were no significant differences in age or education between each patient group and its controls (all t < 1.8; p > 0.1). Thus, any task performance differences between the patients and their respective controls could not be attributed to age or education differences.
The patients presented through the Memory Clinic, Addenbrooke's Hospital, Cambridge, UK and have been assessed longitudinally on an extensive neuropsychological battery. The SD patients fulfilled the Lund-Manchester consensus criteria for frontotemporal lobar degeneration (Neary et al., 1998), whereas the AD cases met the criteria from the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (McKahnn et al., 1984).
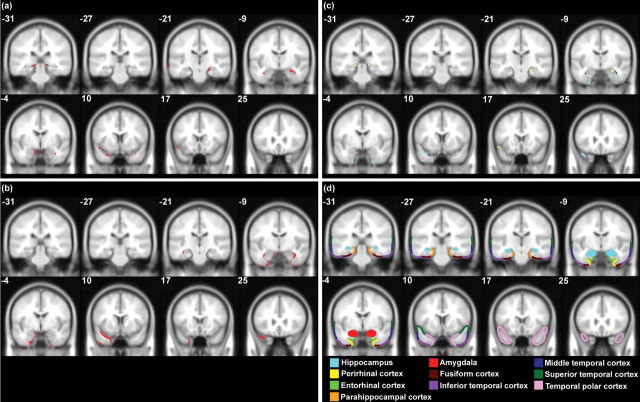
Regions of atrophy within the temporal lobe were delineated for those patients with structural magnetic resonance imaging (MRI) scans using MRIcro (Rorden and Brett, 2000) (see supplemental material, available at www.jneurosci.org). Within both groups, there was variability in the distribution of pathology, with differing degrees of atrophy to the hippocampus, perirhinal and enthorinal cortices, amygdala, temporal pole, fusiform gyrus, and superior, middle, and inferior temporal gyri. To determine, however, which brain regions were most likely to underlie the findings reported here, MRIcro was used to identify the areas that were commonly affected in each patient group. Figure 1, a and b, illustrates the regions that were atrophied in all AD or SD patients (i.e., the overlapping regions of damage for each group), whereas Figure 1c shows the damage that was specific to each group and those areas of atrophy that were shared by AD and SD patients. Consistent with Davies et al. (2004), the AD cases had overlapping atrophy throughout the hippocampus bilaterally, but not to the perirhinal cortex. In contrast, the SD patients had common damage to the left temporal pole, as well as the perirhinal cortex and anterior hippocampus bilaterally.
Figure 1.
Overlapping regions of atrophy within the temporal lobe (in red) are shown for the AD (a) and SD (b) patients with structural MRI scans, superimposed on a Montreal Neurological Institute average brain template. c shows the regions of atrophy that were specific to each patient group (AD, yellow; SD, light blue), as well as the damage that was shared by both groups (in dark purple). d illustrates different temporal lobe regions. The boundaries of these areas are approximate, because anatomical landmarks are unclear on the average brain template. The y-coordinate of each brain slice is shown.
The patients' cognitive abilities were quantified with various standardized neuropsychological tests and compared with published normative control data. Unfortunately, one SD patient could not be assessed within 6 months of experimental testing, although there were no indications that his cognitive profile differed to that of the other SD cases. For those patients whose cognitive abilities were assessed at the time of experimental testing, their performance (shown as mean percentage correct) reflected typical SD and AD deficits. Both patient groups were impaired on episodic memory tests, although the profiles of performance differed with the SD patients often showing better performance. For instance, on the Rey complex figure (RCF) delayed recall condition, the AD group were near floor (3.80%), whereas the SD patients demonstrated some residual recall memory (24.80%). Similarly, on the Recognition Memory Test (RMT) for scenes, the SD patients exhibited better recognition memory (74.76%) than the AD group (48.57%). Interestingly, however, this pattern was reversed on the RMT faces test, with the AD patients (71.14%) performing better than the SD group (65.71%). In terms of semantic memory, the AD group showed mild impairment compared with severe deficits in the SD patients on word–picture matching (AD, 98.43%; SD, 50.00%), picture naming (AD, 92.86%; SD, 26.56%), and the Pyramid and Palm Trees pictures test (AD, 94.78%; SD, 72.52%). Both patient groups performed within the normal range on tests of visuoperceptual skills, such as the Benton face test (AD, 87.30%; SD, 82.27%) and sub tests of the Visual Object Space Perception battery including letter identification (AD, 94.29%; SD, 94.00%), dot counting (AD, 95.71%; SD, 97.14%), and position discrimination (AD, 94.29%; SD, 97.86%). On the RCF copy condition, however, the AD group was impaired (80.36%), whereas the SD patients showed intact performance (94.44%). Finally, six SD and five AD patients were assessed on a computerized standard mental rotation task and all performed normally.
All participants gave informed consent, and this study was approved by the Cambridgeshire Health Authority Local Research Ethics Committee (UK).
Experimental tasks
Four computerized tasks [used by Lee et al. (2005b)] were administered on a 15 inch super video graphics array liquid crystal display touch screen (800 × 600 pixels). Instructions and practice trials were given before each task. These tests were based on an oddity paradigm (Buckley et al., 2001) in which the subjects had to select the odd-one-out from an array of stimuli as quickly but as accurately as possible, by touching the odd stimulus with the index finger of their dominant hand. Two tasks, “scene same views” and “face same views,” were designed to place a lower demand on spatial scene and object perception, respectively, and could be solved without using viewpoint-independent representations of the scenes/faces (e.g., a same views discrimination could be made by directly matching the four simultaneously presented images). We predicted, therefore, that the hippocampus and perirhinal cortex were less critical to these two tasks. In contrast, object and spatial scene perception were emphasized in the other two tasks, “face different views” and “scene different views,” because the participants were required to use complete three-dimensional representations of faces/scenes within each trial (e.g., be able to identify the same face/scene from different viewpoints).
In all of the tests, four grayscale images were presented on a gray background in a two-by-two array, three of the same stimulus and one of a different stimulus. On each trial, the location of each stimulus in this array was randomized. The four oddity tasks each consisted of 40 unique trials (the stimuli in each trial appeared only once) and were administered in a counterbalanced order across all participants. By piloting in healthy subjects, the two different views tasks were matched in terms of difficulty, as were the two same views tasks. The same views tasks were, however, easier than the different views tasks.
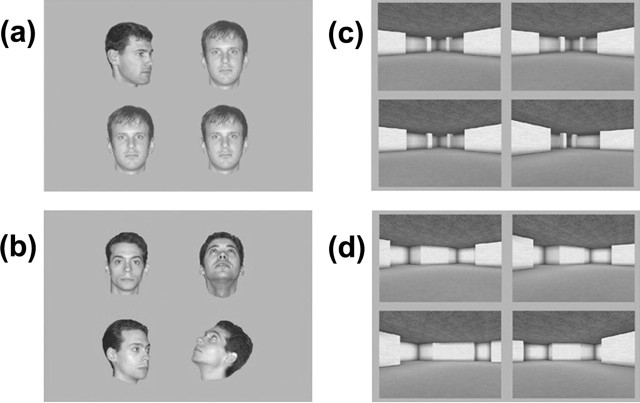
Face same views.
Four images of human faces were presented every trial (128 × 128 pixels) (Fig. 2a). Eighty unfamiliar male faces were used, each with six different views: face looking directly ahead, tilted back, looking 45° to the left, looking 45° to the right, tilted back and 45° to the left, and finally, tilted back and 45° to the right. In each trial, two views were presented, with three images of the same view of one face and one image of a different view of another face. Each face was presented only once and was paired with a second face matched for similar appearance (pairings were fixed across subjects).
Figure 2.
One trial is shown from the face same views (a), face different views (b), scene same views (c), and scene different views (d) tasks.
Face different views.
This was identical to the face same views task, except that on each trial, three different views of the same face were paired with another view of a different face (Fig. 2b).
Virtual scene same views.
Four images of virtual reality scenes were presented every trial (460 × 370 pixels) (Fig. 2c). Eighty scenes, created using a commercially available computer game (Deus Ex; Ion Storm, Austin, TX) and a freeware editor (Deus Ex SDK v1112f), were used and each had four different views. In each trial, two views were presented, with three images of the same view of one scene and one image of a different view of another scene. Each scene was presented only once and was paired with a scene of similar appearance, which only differed with respect to the dimensions or placement of one or more aspects of the scene such as a wall, window, or room cavity (pairings were fixed across subjects).
Virtual scene different views.
This was identical to the virtual scene same views task, except that on each trial, three different views of the same scene were paired with another view of a different scene (Fig. 2d).
Results
If MTL structures mediate all aspects of long-term declarative memory exclusively (Squire et al., 2004), then both patient groups should perform normally across all oddity task conditions, because this test stresses perception (Stark and Squire, 2000). Contrary to this view, statistical analyses revealed a double dissociation in the scores of the two groups: whereas AD performed poorly on scenes, but not faces, SD showed impaired face, but not scene, discrimination.
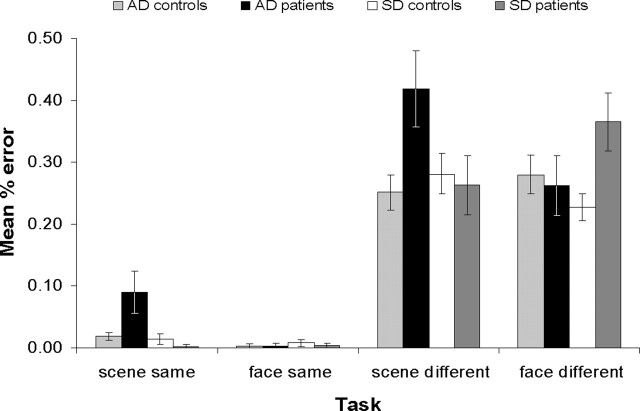
The performance accuracy data for all four participant groups on the four oddity tasks (shown as percentage error in Fig. 3) were subjected to a repeated-measures ANOVA. Two within-subject factors of stimuli (faces vs scenes) and viewpoint (same views vs different views) were incorporated. Because each patient group had its own age-matched control group, two between-subject factors, each with two levels, were included: (1) “health” with the levels patient (incorporating both patient groups) and control (incorporating both control groups) and (2) “disease type” with the levels SD (incorporating the SD patients and their controls) and AD (incorporating the AD group and their controls). This analysis showed that there was a significant interaction between health, disease type, stimuli, and viewpoint (F(1,30) = 8.87; p = 0.006), suggesting that the two patient groups, when compared with their matched controls, performed differently across the four different tasks. To investigate this further, the results from each individual task were analyzed separately in univariate ANOVAs with the factors health and disease type and a dependent variable of performance. The health-by-disease type interaction was significant in the face different views task (F(1,30) = 5.07; p = 0.032), and t tests to compare each patient group with their own controls revealed that this interaction was because of a significant impairment in the SD group (t(16) = 3.03; p = 0.004; one-tailed) but not the AD group (t(14) = 0.34; p = 0.370; one-tailed). There was also a significant health-by-disease type interaction in the scene different views task (F(1,30) = 5.53; p = 0.025), but in contrast, whereas the SD group demonstrated intact performance (t(16) = 0.35; p = 0.366; one-tailed), the AD patients were significantly impaired (t(14) = 2.84; p = 0.007; one-tailed). Unexpectedly, given the findings from our previous study in focal amnesic cases (Lee et al., 2005b), health-by-disease type was also significant for the scenes same views task (F(1,30) = 8.44; p = 0.007) and similar to the scenes different views task, this reflected a significant mild deficit in the AD group (t(14) = 2.51; p = 0.032; one-tailed) but not the SD group (t(16) = 1.32; p = 0.117; one-tailed). There was no significant health-by-disease type interaction in the face same views task (F(1,30) = 0.26; p = 0.615), indicating that both patient groups performed similarly to each other when compared with their respective controls. In this task, there was no significant effect of health (F(1,30) = 0.21; p = 0.653), and t tests between each patient group and their own controls confirmed that both patient groups performed within the normal range (both t < 1; p > 0.2; one-tailed).
Figure 3.
Mean ± SE percentage error for the four subject groups on the different oddity tasks.
Discussion
Using a paradigm without an overt long-term declarative memory component (there was no requirement to remember stimuli across trials nor were any items repeated), we have, to our knowledge, demonstrated the first double dissociation between spatial scene and face discrimination in MTL-damaged patients. The AD group had difficulties in oddity judgment for scenes (especially different views, but also to a lesser extent same views) but performed normally on same and different views face oddity tasks that were matched for difficulty with the scene tasks. In contrast, whereas the SD patients exhibited a significant deficit in oddity judgment for different views faces, they were unimpaired in both scene conditions, as well as the face same views task. Given the disproportionate damage to the perirhinal cortex compared with other MTL regions in SD and the significant hippocampal atrophy in the context of less perirhinal cortex atrophy in AD (Davies et al., 2004), as well as the striking concordance between these findings and those evident in patients with more selective MTL lesions (Lee et al., 2005b), these results strongly suggest that the human hippocampus and perirhinal cortex may be critical to processes beyond long-term declarative memory, with specialization for spatial scene and object processing, respectively.
It is possible that the observed discrimination deficits in the AD and SD patients are a result of pathology beyond the MTL. SD is associated with significant anterior and lateral temporal lobe damage (e.g., fusiform gyrus, area TE/TEO), whereas parietal lobe dysfunction is often a feature of AD. Although it is difficult to determine the precise contribution of such damage to the current findings, it is unlikely that these anatomical disturbances account entirely for the observed pattern of deficits. First, analyses of the available patient MRI scans revealed that the AD cases had consistent atrophy throughout the hippocampus, whereas the SD patients had anterior hippocampus, perirhinal cortex, and left temporal pole damage. Second, the AD and SD patients were each unimpaired in one of the two different views conditions, which were critically matched for difficulty. Subsequently, it is unlikely that other concomitant cognitive impairments (e.g., attentional and/or mental rotation difficulties in AD after parietal lobe damage, or general perceptual difficulties after lateral temporal lobe damage in SD) would produce this profile. Pathology beyond the MTL is, therefore, at most one of several contributing causes to the current findings, with cognitive difficulties after MTL damage being a significant other factor (as demonstrated by convergent findings in patients with static MTL damage) (Lee et al., 2005b).
The AD participants were impaired on same and different views scene oddity judgment, whereas amnesics with selective hippocampal damage only had difficulties on the different views scenes task (Lee et al., 2005b). The former pattern may reflect dysfunction within a network of regions closely linked to the hippocampus, thought to process aspects of space and episodic memory, including the parahippocampal cortex and posterior cingulate (Minoshima et al., 1997; Epstein et al., 1999; Chételat et al., 2003). Alternatively, the broader spatial discrimination deficit in the AD group may imply that the hippocampus is involved in scene perception in a manner beyond the representation of multiple viewpoints, with the same views task being a less demanding version of the different views condition (see later comment). Supporting this hypothesis, the range of scores on the scenes same views task was similar for the present AD group and the hippocampal amnesics from our previous study (0–20% error), although the latter were not impaired compared with controls.
It is perhaps surprising that, compared with the AD group, the SD patients exhibited intact oddity judgment for spatial scenes despite the presence of hippocampal atrophy in SD (Davies et al., 2004). Structural studies indicate that AD may be associated with greater atrophy to the posterior hippocampus in comparison to anterior regions, whereas the reverse profile is seen in SD, with disproportionate anterior compared with posterior hippocampal damage (Laakso et al., 2000; Chan et al., 2001). In rat and monkey brain, the anterior and posterior portions of the hippocampus receive differing afferents via the enthorinal cortex (Witter et al., 1989; Burwell and Amaral, 1998) and electrophysiological studies have observed a higher proportion of neurons signaling aspects of spatial memory in the posterior than anterior hippocampus (Jung et al., 1994; Colombo et al., 1998). Human neuroimaging studies also indicate that posterior hippocampus is important for spatial memory tasks (e.g., route recall and memory for the spatial arrangement of visually presented objects) (Burgess et al., 2001; Maguire et al., 2003; Pihlajamäki et al., 2004), whereas the anterior hippocampus and perirhinal cortex are involved in object memory (e.g., detecting changes in object identity in a visual array) (Pihlajamäki et al., 2004). It is conceivable, therefore, that regions within the hippocampus may be functionally distinct in aspects of space and object processing.
Because the current oddity tasks minimized the involvement of long-term declarative memory, our findings support the controversial view that MTL structures are not uniquely involved in long-term memory. One plausible account of MTL function is that the MTL may mediate working memory processes (Ranganath and D'Esposito, 2005) and that the patients reported here may suffer from short-term working memory problems that result in difficulties making comparisons across simultaneously presented items. A second explanation is that the hippocampus and perirhinal cortex may be critical for spatial scene and object perception, respectively (Buckley et al., 2001; Gaffan, 2001; Bussey et al., 2002). Both of these suggestions predict that lesions to the MTL structures can impair visual discrimination and that these difficulties may even contribute to the types of long-term memory impairment evident in our patients. To support this, the present SD group were disproportionately impaired on the RMT for faces compared with scenes, whereas the AD group, consistent with their difficulty on scene oddity judgment, showed a greater deficit on the RMT scenes task compared with the faces version.
The four oddity tasks were designed to place different demands on perception by varying the emphasis on viewpoint-independent representations. Thus, only the two different views, and not the same views, tasks required the subjects to perceive faces or virtual rooms from multiple viewpoints. One model of perirhinal cortex function suggests that this region may process conjunctions of object features (Bussey et al., 2002), and likewise, it is possible that the hippocampus may be critical for representing conjunctions of spatial information (Buckley et al., 2004). Individuals with perirhinal cortex or hippocampal damage may, therefore, struggle to discriminate stimuli that share a high degree of overlapping features, even if viewpoint-independent representations are not required. To support this, we demonstrated patient discrimination deficits with stimuli that do not require the processing of multiple viewpoints, but instead stress complex conjunctions of object and spatial features (Barense et al., 2005; Lee et al., 2005a).
The idea that different MTL structures may process conjunctions of scene and object features is not highly dissimilar to the theory that the hippocampus supports relational or associative memory (Eichenbaum et al., 1994; Eichenbaum and Cohen, 2002), of which the binding of perceptually distinct items (e.g., the objects within a spatial scene) is a critical component. Importantly, however, the latter is thought to be specific to long-term memory, in contrast to the perceptual theories discussed previously. Moreover, in the context of the current tasks, which lacked an explicit long-term declarative memory component, it is unclear how a deficit in long-term relational memory could explain the observed patient deficits.
The present findings appear at odds with the fact that patients with MTL damage often demonstrate intact performance on standard visual perceptual tasks (Lee et al., 2005c). For instance, the current AD and SD groups were unimpaired on the Benton face task, despite the latter showing difficulties with the different views faces condition. One possible explanation is that standard tasks do not demand sufficiently the perceptual or working memory processes that the hippocampus and perirhinal cortex may subserve. Thus, whereas tests such as the Benton face task can be solved using simple features, the present tests were designed to emphasize the processing of multiple, complex features and subsequently stress perception and very short-term working memory.
In summary, we have demonstrated a clear double dissociation on tests of scene and face oddity judgment in AD and SD patients. This double dissociation is the first of its kind in the context of a task that does not contain an explicit long-term memory component. When considered alongside other findings in cases with more selective involvement of MTL structures, the present results strongly support the idea that the human hippocampus and perirhinal cortex may mediate processes beyond long-term memory, with specialization for spatial scenes and objects respectively.
We thank all the participants for their time; H. Spiers for assistance with experimental design; V. Scahill for help with data collection; T. Bussey, E. Murray, and L. Saksida for feedback on these experiments; and the Alzheimer's Research Trust, United Kingdom and the Medical Research Council, United Kingdom for funding this research.
References
- Barense MD, Bussey TJ, Lee ACH, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS (2005). Functional specialization in the human medial temporal lobe. J Neurosci 25:10239–10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP (2001). Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2:51–61. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Booth MC, Rolls ET, Gaffan D (2001). Selective perceptual impairments after perirhinal cortex ablation. J Neurosci 21:9824–9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Charles DP, Browning PG, Gaffan D (2004). Learning and retrieval of concurrently presented spatial discrimination tasks: role of the fornix. Behav Neurosci 118:138–149. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O'Keefe J (2001). A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. NeuroImage 14:439–453. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG (1998). Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol 398:179–205. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA (2002). Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci 15:365–374. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, Rossor AM, Stevens JM, Cipolotti L, Rossor MN (2001). Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann Neurol 49:433–442. [PubMed] [Google Scholar]
- Chételat G, Desgranges B, de la Sayette V, Viader F, Berkouk K, Landeau B, Lalevée C, Le Doze F, Dupuy B, Hannequin D, Baron JC, Eustache F (2003). Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain 126:1955–1967. [DOI] [PubMed] [Google Scholar]
- Colombo M, Fernandez T, Nakamura K, Gross CG (1998). Functional differentiation along the anterior-posterior axis of the hippocampus in monkeys. J Neurophysiol 80:1002–1005. [DOI] [PubMed] [Google Scholar]
- Davies RR, Graham KS, Xuereb JH, Williams GB, Hodges JR (2004). The human perirhinal cortex and semantic memory. Eur J Neurosci 20:2441–2446. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ (2002). In: From conditioning to conscious recollection: memory systems of the brain Oxford: Oxford UP.
- Eichenbaum H, Otto T, Cohen NJ (1994). Two functional components of the hippocampal memory system. Behav Brain Sci 17:449–518. [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N (1999). The parahippocampal place area: recognition, navigation, or encoding? Neuron 23:115–125. [DOI] [PubMed] [Google Scholar]
- Gaffan D (2001). What is a memory system? Horel's critique revisited. Behav Brain Res 127:5–11. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, Sahakian BJ, Hodges JR (2001). Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology 57:216–225. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E (1992). Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain 115:1783–1806. [DOI] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL (1994). Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci 14:7347–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso MP, Frisoni GB, Könönen M, Mikkonen M, Beltramello A, Geroldi C, Bianchetti A, Trabucchi M, Soininen H, Aronen HJ (2000). Hippocampus and entorhinal cortex in frontotemporal dementia and Alzheimer's disease: a morphometric MRI study. Biol Psychiatry 47:1056–1063. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, Hodges JR, Graham KS (2005a). Perceptual deficits in amnesia: challenging the medial temporal lobe ‘mnemonic' view. Neuropsychologia 43:1–11. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, Gaffan D, Bussey TJ, Davies RR, Kapur N, Hodges JR, Graham KS (2005b). Specialisation in the medial temporal lobe for processing of objects and scenes. Hippocampus 15:782–797. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Barense MD, Graham KS (2005c). The contribution of the human medial temporal lobe to perception: bridging the gap between animal and human studies. Quart J Exp Psych 58B:300–325. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Spiers HJ, Good CD, Hartley T, Frackowiak RS, Burgess N (2003). Navigation expertise and the human hippocampus: a structural brain imaging analysis. Hippocampus 13:250–259. [DOI] [PubMed] [Google Scholar]
- McKahnn G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984). Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34:939–944. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE (1997). Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol 42:85–94. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S (1998). Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554. [DOI] [PubMed] [Google Scholar]
- Pihlajamäki M, Tanila H, Könönen M, Hänninen T, Hämäläinen A, Soininen H, Aronen HJ (2004). Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur J Neurosci 19:1939–1949. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M (2005). Directing the mind's eye: prefrontal, inferior and medial temporal mechanisms for visual working memory. Curr Opin Neurobiol 15:175–182. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M (2000). Stereotaxic display of brain lesions. Behav Neurol 12:191–200. [DOI] [PubMed] [Google Scholar]
- Snowden J, Goulding P, Neary D (1989). Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol 2:167–182. [Google Scholar]
- Squire LR, Stark CE, Clark RE (2004). The medial temporal lobe. Annu Rev Neurosci 27:279–306. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR (2000). Intact visual perceptual discrimination in humans in the absence of perirhinal cortex. Learn Mem 7:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Van Hoesen GW, Amaral DG (1989). Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci 9:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]