Abstract
Tumor necrosis factor-α (TNFα) is a proinflammatory cytokine involved in the development and maintenance of inflammatory and neuropathic pain conditions. TNFα can have long-lasting effects by regulating the expression of a variety of inflammatory mediators, including other cytokines and TNFα itself. However, the speed with which TNFα induces tactile and thermal hypersensitivity suggests that transcriptional regulation cannot fully account for its sensitizing effects, and some recent findings suggest that TNFα may act directly on primary afferent neurons to induce pain hypersensitivity. In the present study, we show that peripheral administration of TNFα induces thermal hypersensitivity in wild-type mice but not in transient receptor potential vanilloid receptor TRPV1–/– mice. In contrast, TNFα produced equivalent mechanical hypersensitivity in TRPV1–/– mice and wild-type littermates, suggesting a role for TRPV1 in TNFα-induced thermal, but not mechanical, hypersensitivity. Because tetrodotoxin (TTX)-resistant Na+ channels are a critical site of modulation underlying mechanical hypersensitivity in inflammatory and neuropathic pain conditions, we tested the effects of TNFα on these channels in isolated mouse dorsal root ganglion (DRG) neurons. We report that acute application of TNFα rapidly enhances TTX-resistant Na+ currents in isolated DRG neurons. This potentiation of TTX-resistant currents by TNFα is dramatically reduced in DRG neurons from TNF receptor 1 (TNFR1) knock-out mice and is blocked by the p38 mitogen-activated protein kinase inhibitor SB202190 [4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole]. Mechanical hypersensitivity induced by peripherally applied TNFα is also significantly reduced by SB202190. These results suggest that TNFα may induce acute peripheral mechanical sensitization by acting directly on TNFR1 in primary afferent neurons, resulting in p38-dependent modulation of TTX-resistant Na+ channels.
Keywords: pain, MAPK, TNF, nociceptor, DRG, Nav1.8, phosphorylation, sensitization
Introduction
Tumor necrosis factor-α (TNFα) is an important proinflammatory cytokine produced by a variety of cell types, including immune, neuronal, and glial cells (Tchelingerian et al., 1993; Wagner and Myers, 1996; Wagner et al., 1998; Schafers et al., 2002; Li et al., 2004). In response to injury or inflammation, TNFα serves as a trigger for activation of other cytokines and growth factors. In addition, TNFα plays a critical role in the development and maintenance of inflammatory and neuropathic pain (Woolf et al., 1997; Junger and Sorkin, 2000; Joseph and Levine, 2004; Sommer and Kress, 2004). For example, TNFα-neutralizing agents attenuate thermal hyperalgesia and mechanical allodynia in animal models of neuropathic pain (Lindenlaub et al., 2000; Sommer et al., 2001; Sweitzer et al., 2001), and intraplantar administration of TNFα produces hypersensitivity to thermal and mechanical stimuli in rats and mice (Cunha et al., 1992; Perkins and Kelly, 1994; Woolf et al., 1997; Wacnik et al., 2005). However, the mechanisms underlying the behavioral effects of TNFα are not fully understood.
Although TNFα can have long-lasting effects by regulating the expression of a variety of inflammatory mediators, the rapid onset of TNFα-induced pain hypersensitivity suggests that transcriptional regulation cannot fully account for the sensitizing effects of TNFα (Sommer and Kress, 2004). TNFα applied directly to dorsal root ganglion (DRG) neurons induces mechanical allodynia (Homma et al., 2002; Schafers et al., 2003c), and subcutaneous TNFα increases mechanical sensitivity of C-fibers (Junger and Sorkin, 2000). Furthermore, in vitro perfusion of TNFα to intact or injured DRG elicits neuronal discharges in A- and C-fibers (Liu et al., 2002; Zhang et al., 2002; Schafers et al., 2003b), and application of TNFα to isolated skin enhances heatevoked CGRP release from nociceptor terminals (Opree and Kress, 2000). Many of these effects occur in <5 min and are therefore too rapid to be mediated by changes in gene expression. However, the molecular mechanisms underlying these rapid sensitizing effects of TNFα have not been elucidated.
Peripheral sensitization can be induced by modulation of a variety of ion channels that mediate the transduction of thermal and mechanical stimuli or regulate excitability and action potential propagation (Bhave and Gereau, 2004). For example, the noxious heat transduction channel transient receptor potential vanilloid receptor 1 (TRPV1) can be sensitized by chronic TNFα treatment (Nicol et al., 1997), a possible mechanism for the development of heat hypersensitivity, but it is not obvious how TRPV1 modulation would mediate enhanced sensitivity to mechanical stimuli. Among other ion channels critical in the genesis of inflammatory and neuropathic pain are the TTX-resistant (TTX-R) sodium channels (Bhave and Gereau, 2004; Wood et al., 2004a,b). In the present study, we examined the modulation of TTX-R Na+ channels in mouse DRG neurons by TNFα. We show that acute application of TNFα to cultured mouse DRG neurons rapidly enhances TTX-R currents via a TNF receptor 1 (TNFR1)- and p38-dependent pathway. These studies provide the first evidence of rapid receptor-mediated modulation of nociceptor excitability by TNFα and may provide an explanation for the rapid sensitization to mechanical stimuli induced by TNFα.
Materials and Methods
Animals. Adult male mice, 6–8 weeks old, of the following strains, ICR (Taconic Farms, Germantown, NY), TRPV1–/–, TNFp55rKO (TNFR1–/–), TNFp75rKO (TNFR2–/–), and their appropriate wild-type (WT) control strain (C57BL/6J) were purchased from The Jackson Laboratory (Bar Harbor, ME). All studies were performed under the guidelines of the National Institutes of Health and The International Association for the study of Pain and were approved by the Animal Care and Use Committee of Washington University School of Medicine.
Behavioral analysis. Mice were allowed to acclimate for 1d before baseline testing. Mechanical sensitivity was assessed using von Frey hairs (North Coast Medical, San Jose, CA). Mice were placed on elevated wire mesh and allowed to acclimate to the testing environment for 2 h before testing. The plantar surface of the hindpaw was stimulated with a series of von Frey hairs. Each filament was applied five times, and threshold was determined as the lowest force that induced hindpaw withdrawal on at least three of five trials. Baseline values were defined as the mean of three measurements before injection. To test the effect of TNFα on the basal mechanical sensitivity, 1 ng of TNFα (in 10 μl) was injected into the hindpaw plantar surface, and the paw-withdrawal thresholds of the ipsilateral hindpaw were measured at 30, 45, 60, and 90 min after injection. For the inhibitor experiments, vehicle (0.12% DMSO, 10 μl) or SB202190 [4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1Himidazole] (12 μm in 10 μl) was injected in the paw 20 min before the injection of TNFα.
Thermal sensitivity was measured using radiant heat applied to the plantar surface of the hindpaw (IITC Life Sciences, Woodland Hills, CA). Paw-withdrawal latency was measured. The heat stimulus was terminated with a withdrawal response or cutoff at 20 s to avoid tissue damage. Before injections, three withdrawal latencies were recorded and averaged as the baseline for each animal. In TRPV1–/– mice, the average baseline withdrawal latency was 14.31 ± 2.17 s. This was significantly different from wild-type littermates in which the baseline withdrawal latency was 6.81 ± 0.97 s (p < 0.01, ANOVA). TNFα (1 ng in 10 μl) was injected intradermally in the planter surface of the hindpaw, and withdrawal latencies were measured at 30, 45, 60, and 90 min after injection.
Preparation of DRG neuronal cultures. DRG neuronal cultures were prepared using procedures similar to those described in our previous publications (Hu et al., 2002; Yang and Gereau, 2004). DRGs were removed and collected in cold (4°C) PBS without Ca2+ or Mg2+ (Mediatech, Herndon, VA). Ganglia were incubated in 15 U/ml papain in HBSS (Mediatech) for 18 min at 37°C. After this initial enzyme treatment, the ganglia were rinsed three times in HBSS and then incubated for 18 min with 1.5 mg/ml collagenase (Sigma, St. Louis, MO) in HBSS at 37°C. After washing three times with HBSS, ganglia were gently triturated with a flame-polished Pasteur pipette. The tissue fragments were centrifuged at 1000 rpm for 5 min, and the pellet was resuspended in neurobasal culture media (Invitrogen, Grand island, NY) with 5% FBS, 1% B27, 100 U/ml penicillin/streptomycin, and 2 mm glutamax. Cells were plated onto poly-d-lysine-coated 12 mm glass coverslips and maintained at 37°C in a 95% air–5% CO2 incubator overnight. Electrophysiological recordings were performed within 24 h of cell isolation.
Electrophysiological recording. Standard whole-cell patch-clamp recordings from cultured DRG neurons were performed at room temperature (∼22°C) using an Axopatch 200B amplifier. The pipettes were prepared on a Flaming-Brown horizontal puller (P-87; Sutter Instruments, Novato, CA) and fire polished. The resistance of the pipettes was 1–3 MΩ. In whole-cell voltage-clamp mode, the pipette capacitance current was cancelled using the amplifier circuitry, and series resistance was compensated by 70–80%. Currents were acquired using Clampex 8.2 software, filtered at 5 kHz, and digitized at a sampling rate of 20 kHz via a Digidata 1322 series interface (Axon Instruments, Union City, CA). To determine the voltage dependence of activation, channels were fully inactivated by holding at –100 mV for 500 ms and then stepped to various test potentials from –60 to 25 mV in +5 mV increments. The peak current amplitudes observed at the different test potentials were converted to macroscopic conductance using the equation G = I/(V – Vm), where V is the calculated reversal potential. To determine the voltage dependence of inactivation, a two-pulse protocol was used. Conditioning prepulses ranging from –100 to +20 mV were applied at 5 s intervals in +5 mV increments for 1000 ms, followed by test voltage 0 mV for 100 ms. The values for voltage of half-maximal activation or inactivation (V1/2) and slope factor k were estimated by fitting the activation and steady-state inactivation curves with the Boltzmann equation G = Gmax/(1 + exp[(V1/2 – Vm)/k]), where V1/2 is the potential at which activation is half-maximal, Vm is the membrane potential, and k is the slope factor.
For voltage-clamp experiments, the pipette solution contained the following (in mm): 120 KCl, 20 HEPES, 2.25 CaCl2, 5 EGTA, 5 ATP, and 0.4 GTP. The pH of the solutions was adjusted to 7.2 with KOH. To measure A-type transient potassium currents (IA), cells were bathed in Tyrode's solution containing the following (in mm): 140 NaCl, 4 KCl, 2 MgCl2, 2 CaCl2, 5 glucose, and 5 HEPES, pH 7.4 (with NaOH). To record Na+ currents, the solution was switched to an external solution that suppressed K+ and Ca2+ currents. This solution contained the following (in mm): 40 NaCl, 90 tetraethylammonium (TEA)-Cl, 10 4-aminopyridine (4-AP), 10 HEPES, 2 BaCl2, 0.1 CaCl2, 0.4 CdCl2, and 10 glucose. The pH was adjusted to 7.4 with HEPES.
All patch-clamp analysis of Na+ currents performed in this study was restricted to small-diameter DRG neurons with TTX-resistant sodium currents. These cells were identified in our studies by the presence of an IA-like current of >200 pA evoked during repolarization to –50 mV after a hyperpolarization to –110 mV. We found that 100% of IA expressing neurons expressed TTX-resistant sodium currents.
Immunohistochemistry. Cultured DRG neurons were washed with Ca2+-free PBS and treated with drugs diluted in the external solution used in Na+ current recording experiments. Cells were fixed with 4% paraformaldehyde in 0.1 m phosphate buffer for 30 min and blocked with 3% goat serum in 0.2% Triton X-100/PBS for 1 h at room temperature. The cells were then incubated with anti-phospho-p38 (p-p38) primary antibody (rabbit, 1:400; Cell Signaling Technology, Beverly, MA) over two nights at 4°C, followed by biotinylated secondary antibody (goat anti-rabbit, 1:100, Vector Laboratories, Burlingame, CA) for 1.5 h at room temperature and then extrAvidin peroxidase (1:100; Sigma) for 1 h at room temperature. DAB (Vector Laboratories) was used for detection. The level of p38 phosphorylation in the cultures was quantified by both counting p-p38-immunoreactive neurons and by measuring the intensity of staining using a computerized image analysis system (Simple PCI; Compix, Lake Oswego, OR). For each condition, 100 cells were selected at random, and the staining intensity (in arbitrary units, ranging from 0 to 255) was measured from each cell while subtracting background. For a given experiment, identical exposure times and illumination intensities were used for all treatment conditions, and the experimenter was blind to treatment during the random selection and measurement of staining intensities.
Drug application. Recombinant murine TNFα was obtained from R & D Systems (Minneapolis, MN) and prepared as concentrated stock solutions at 50 μg/ml in 0.1% BSA. 4-AP was purchased from Sigma. SB202190 and its inactive analog SB202474 [4-ethyl-2(p-methoxyphenyl)-5-(4′-pyridyl)-1 H-imidazole] were purchased from Calbiochem (La Jolla, CA) and dissolved in DMSO (final concentration, 0.12%).
Statistical analysis. All data are expressed as mean ± SEM. Treatment effects were statistically analyzed by one-way ANOVA, followed by post hoc analysis using the Bonferroni's correction, Tukey's test, or Dunnett's test for multiple comparisons as indicated using Statistica Software (Statsoft, Tulsa, OK). Student's t test was used when comparisons were restricted to two experimental groups. Error probabilities of p < 0.05 were considered statistically significant.
Results
TNFα-induced thermal and mechanical hypersensitivity: the role of TRPV1
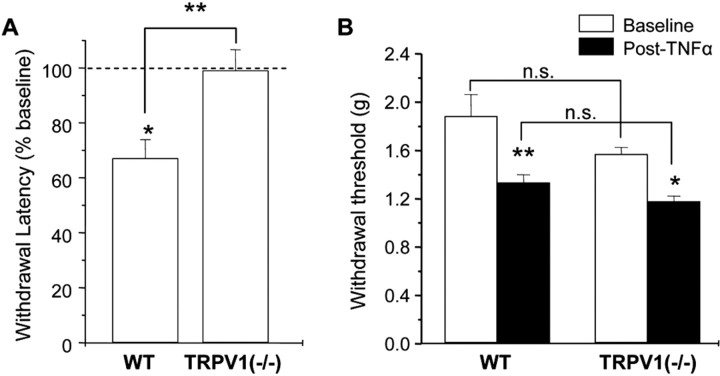
As mentioned above, application of TNF can sensitize the noxious heat transduction channel, TRPV1(Nicol et al., 1997). It is clear that this modulation of TRPV1 could underlie the induction of heat hypersensitivity by TNFα, but it is less obvious how TRPV1 modulation would mediate the enhanced sensitivity to mechanical stimuli induced by TNFα. To test the hypothesis that TRPV1 is required only for TNFα-induced thermal hypersensitivity, we examined the induction of thermal and mechanical hypersensitivity by intraplantar injection of TNFα in TRPV1–/– mice and wild-type littermates. Consistent with previous reports, we found that intraplantar injection of TNFα induced both thermal and mechanical hypersensitivity in wild-type mice (Fig. 1). However, thermal hypersensitivity was not observed in TRPV1–/– mice, whereas mechanical hypersensitivity in TRPV1–/– mice was not significantly different from that observed in WT littermates. These data support the hypothesis that TNFα-induced thermal hyperalgesia requires the expression of TRPV1, whereas TNFα-induced mechanical hypersensitivity occurs via a TRPV1-independent mechanism.
Figure 1.
Loss of TNFα-induced thermal hyperalgesia, but not mechanical hypersensitivity, in TRPV1–/– mice. Thermal withdrawal latencies (expressed as percentage of preinjection baseline values)(A)and mechanical withdrawal thresholds (ingrams)(B) before (baseline) and after intraplantar injection of TNFα (1 ng/10 μl) in TRPV1–/– mice and WT littermates. The experimenter was blind to the genotype of the animals. Three measurements were taken from each animal over a period of 30 –90 min after TNFα injection, and the average was taken as the postinjection withdrawal threshold. Asterisks above the bars indicate the results of comparisons for each condition to the preinjection baseline (paired t test for A; ANOVA for B). Asterisks above the brackets indicate results of comparisons between the genotypes (unpaired t test in A; ANOVA with post hoc Tukey's test for B). *p < 0.05; **p < 0.01. n = 6–9 animals per condition. n.s., Not significant.
Characterization of TTX-resistant Na+ currents in mouse DRG neurons
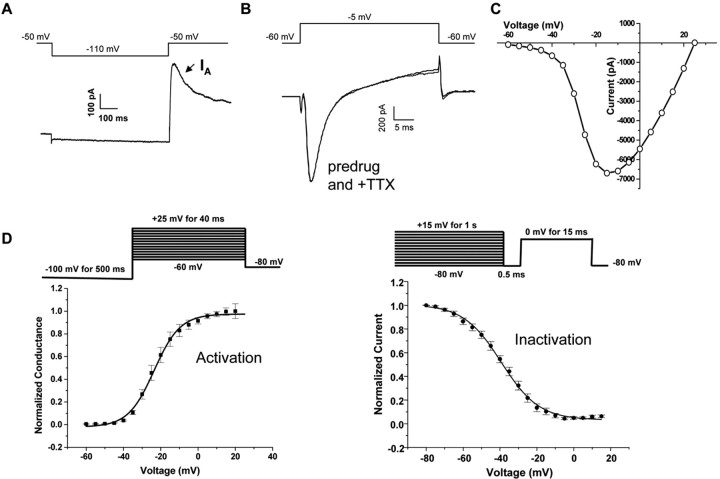
Previous studies suggest that TTX-R Na+ currents and TNF receptors are colocalized with TRPV1 and other nociceptive markers in a subpopulation of DRG neurons (Cardenas et al., 1995, 1997; Li et al., 2004). To minimize variability in our recordings, we chose to record from an electrophysiologically identified subclass of DRG neurons that is likely to include neurons expressing both TTX-R Na+ channels and TNF receptors. To do this, we used electrophysiological signatures to classify small-diameter DRG neurons, as described in Materials and Methods. Small-diameter DRG neurons with an IA of >200 pA were selected for study. IA was measured using the protocol as shown in Figure 2A. These were small-diameter DRG neurons, with an average diameter of 25 ± 2.5 μm. The average peak current of IA in these cells was 435 ± 26 pA. Following this classification, Na+ currents were measured using an external solution containing TEA and 4-AP to block K+ currents and CdCl2 to block Ca2+ currents. Figure 2B shows an example of original Na+ current records obtained from an IA-expressing DRG neuron. To define whether currents were TTX resistant, we perfused the cells with 250 nm TTX for 3 min. There was no significant reduction in current amplitude in the presence of TTX. This is in agreement with previous studies showing that rat DRG neurons that express a prominent IA also express only TTX-resistant Na+ currents (Cardenas et al., 1999, 2001). The peak current value at multiple potentials was recorded to generate I–V curves (Fig. 2C), and the voltage dependence of activation and steady-state inactivation were measured using protocols as shown in Figure 2D. The activation curve had an average V1/2 of –23.57 mV and a slope factor of 6.95 (n = 15), whereas the V1/2 for inactivation averaged –39.21 mV with a slope factor of 10.16 (n = 12).
Figure 2.
Characteristics of TTX-resistant sodium currents in mouse DRG neurons. A, Representative current response showing a prominent A-type K+ current used to identify TTX-R expressing DRG neurons. IA was evoked using the voltage protocol shown above the current trace. B, Representative inward Na+ current recorded before and after application of TTX (250 nm for 3 min). Note that traces of predrug and with TTX are superimposed. TTX had no effect on Na+ currents in IA-positive DRG neurons. C, Representative current–voltage relationship for peak TTX-R currents. D, Steady-state inactivation (n = 12) and activation (n = 15) curves for TTX-R currents obtained using the voltage protocols shown above the graphs.
TNFα enhances TTX-R Na+ currents
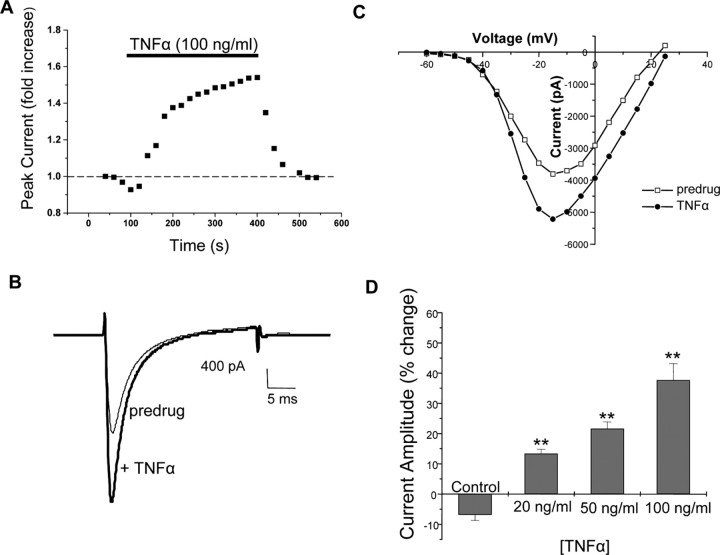
Previous studies have reported that TNFα can evoke action potentials and increase discharge rates when applied locally to nociceptive neurons (Sorkin et al., 1997; Junger and Sorkin, 2000; Sorkin and Doom, 2000; Liu et al., 2002). The mechanisms underlying this alteration in firing are not known, and we hypothesized that this effect of TNFα might be mediated in part by modulation of TTX-R Na+ channels. As illustrated from the representative recording in Figure 3A–C, bath application of mouse recombinant TNFα (100 ng/ml) rapidly enhanced peak TTX-R Na+ currents. The enhancement of TTX-R currents by TNFα was typically observed within ∼1 min of the onset of TNFα application and reached its peak effect within 3–5 min. This effect was concentration dependent, with significant potentiation of TTX-R currents seen at 20, 50, and 100 ng/ml TNFα, as shown in Figure 3D. The largest increase in currents was observed at 100 ng/ml TNFα, in which we observed a 37.7% increase in peak currents compared with the pre-TNFα current amplitude.
Figure 3.
TNFα enhances TTX-R Na+ currents. A, Plot of peak Na+ current amplitude versus time in a representative cell showing the effect of application of TNFα (recombinant murine TNFα, 100ng/ml). Peak current was measured every 20s. The duration of TNFα perfusion is indicated by the bar above the trace. Representative traces (B) and I–V plots (C) showing inward Na+ currents recorded before and during application of TNFα (100ng/ml).D, Dose–response relationship for TNFα-induced enhancement of TTX-R currents. Values represent the mean±SEM percentage change in amplitude of peak current induced by vehicle (BSA) or various doses of TNFα (n=7). The decreased current in the control represents time-dependent rundown of TTX-R currents over the duration of the recording. *p ≤ 0.05; **p ≤ 0.01 compared with the control condition, ANOVA with post hoc Dunnett's test.
We also determined the effects of TNFα on biophysical properties of TTX-R currents, including conductance, the voltage dependence, and slope factor in both activation and steady-state inactivation from each individual cell. The results of this analysis are shown in Table 1. Application of TNFα resulted in a significant increase in total TTX-R conductance from 121.23 ± 21.43 to 172.20 ± 25.30 pS (n = 8). However, TNFα had no effect on the gating properties as assessed by comparing V1/2 and k in the activation or steady-state inactivation curves.
Table 1.
Effects of TNFα (100 ng/ml) and SB202190 (10 μ m) on properties of TTX-R Na+ currents in mouse DRG neurons
|
|
V1/2 |
Slope factor |
Gmax |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Pre-TNFα |
Post-TNFα |
Pre-SB |
Post-SB |
Pre-TNFα |
Post-TNFα |
Pre-SB |
Post-SB |
Pre-TNFα |
Post-TNFα |
Pre-SB |
Post-SB |
|||||||||
| Activation | -24.19 ± 3.61 | -22.94 ± 6.2 | -18.86 ± 2.8 | -17.64 ± 3.3 | 4.01 ± 1.01 | 5.42 ± 1.03 | 6.76 ± 0.47 | 5.42 ± 0.52 | 121.59 ± 12.99 | 172.21 ± 25.3* | 118.92 ± 11.77 | 88.11 ± 11.34* | |||||||||
| Inactivation |
-34.13 ± 4.17 |
-43.88 ± 6.24 |
-40.64 ± 6.24 |
-39.41 ± 2.15 |
11.52 ± 1.67 |
10.65 ± 0.99 |
7.36 ± 0.76 |
8.04 ± 3.59 |
|
|
|
|
|||||||||
p < 0.05 indicates significant differences by paired t test. SB, SB202190.
p38 mitogen-activated protein kinase modulates TTX-R Na+ currents and mediates sensitization of TTX-R by TNFα
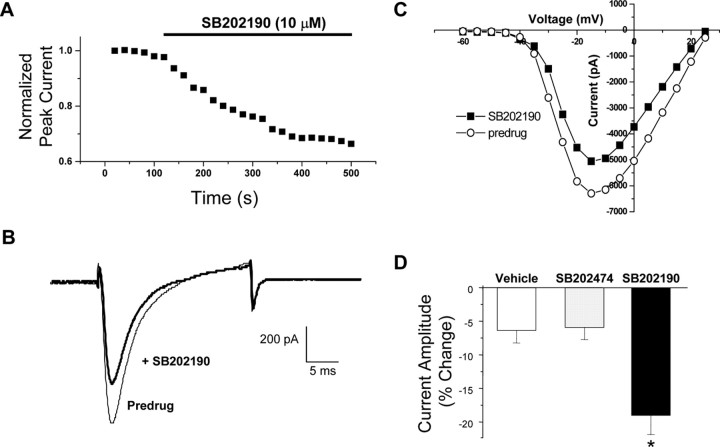
It has been reported that TNF receptors can signal through activation of the p38 mitogen-activated protein kinase (MAPK) cascade in DRG neurons as well as many other cell types (Pollock et al., 2002; Wu, 2004). Furthermore, p38 inhibitors can block TNF-induced pain sensitization in vivo (Schafers et al., 2003b). These data suggest the possibility that TNFα might modulate TTX-R Na+ currents via activation of p38 MAPK. We therefore investigated the role of p38 MAPK in the TNFα-induced enhancement in TTX-R Na+ currents in cultured mouse DRG neurons. First, we exposed DRG neurons to the p38 inhibitor SB202190 alone for at least 3 min. We found that SB202190 induced a significant decrease in basal TTX-R Na+ currents in these cells (Fig. 4). The average peak current was reduced by 19.03 ± 2.84% (n = 19) in the presence of SB202190 (10 μm), whereas in vehicle (0.1% DMSO)-treated cells under the same recording conditions, currents were reduced by an average of only 6.38 ± 1.84% (n = 13). The decrease under the control conditions represents the typical time-dependent rundown of TTX-R currents observed over a 5 min recording period under our conditions (Figs. 3D, 4 D) (see Fig. 7C). SB202190 had no effect on V1/2 or the slope factor but significantly decreased maximal conductance (Table 1). The Gmax was 118.92 ± 11.77 pS under control conditions and was 88.11 ± 11.34 pS (n = 8) after SB202190 application. In contrast to the effects of the p38 inhibitor SB202190, TTX-R currents were not significantly reduced relative to control in the presence of SB202474 (10 μm), an inactive structural analog of SB202190 that does not inhibit p38 (Lee et al., 1994) (Fig. 4 D).
Figure 4.
p38 MAPK modulates TTX-R Na+ currents. A, Representative time course showing inhibition of basal TTX-R Na+ currents after bath application of the p38 MAPK inhibitor SB202190 (10 μm). Current traces (B) and I–V curves (C) showing the inhibition of TTX-R currents under control conditions (vehicle of 0.1% DMSO) and after SB202190 treatment. D, Grouped data showing the mean percentage change in peak current amplitude over time induced by SB202190 (n = 19) or its inactive control analog SB202474 (10 μm)(n = 8) compared with vehicle (DMSO; n = 13). *p < 0.05 versus the vehicle control, ANOVA.
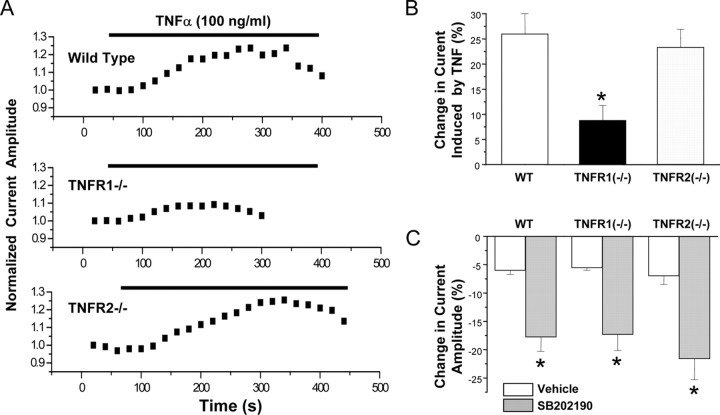
Figure 7.
TNFR1 mediates the TNFα-induced modulation of TTX-R Na+ currents. A, Representative time courses of the peak TTX-R current showing the effect of bath application of TNFα in DRG neurons prepared from WT, TNFR1–/–, or TNFR2–/– mice. B, Mean ± SEM percentage change of TTX-R peak currents induced by TNFα in TNFR1–/– and TNFR2–/– DRG cells compared with wild type. C, Mean ± SEM percentage change of TTX-R peak currents induced by the p38 inhibitor SB202190 (10 μm) in TNFR1–/– and TNFR2–/– DRG cells compared with wild type.
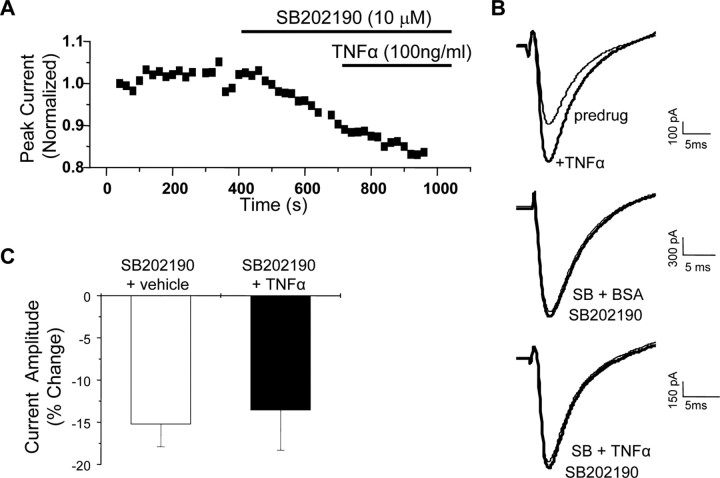
The effects of SB202190 were precisely the opposite of what was observed with TNFα application, suggesting that there might be basal p38 activation in these cells and further that p38 might mediate the potentiation of TTX-R currents induced by TNFα. To test this hypothesis, we next investigated whether the enhancement of TTX-R currents by TNF could be blocked by inhibition of p38. Cells were treated with SB202190 (10 μm) before and during application of TNFα (100 ng/ml). Under these conditions, TNF had no significant effect on TTX-R currents compared with vehicle-treated cells (Fig. 5). Pretreatment with SB202190 decreased peak currents and abolished the TNF-induced enhancement of TTX-R.
Figure 5.
p38 MAPK is required for TNFα modulation of TTX-R Na+ currents. A, Representative time course showing the change in peak current amplitude in response to application of TNFα in the presence of the p38 inhibitor SB202190. TNFα failed to produce enhancement when the cell was pretreated with SB202190 (10 μm) for 3 min. B, Representative current traces (truncated for clarity) showing the effects of TNFα in the absence (top) and presence (bottom) of SB202190. BSA is the vehicle used for TNFα, and BSA had no effect on TTX-R currents under control conditions (data not shown) or in the presence of SB202190 (middle). C, Grouped data showing the mean ± SEM percentage change in peak current after application of SB202190 plus BSA and SB202190 plus TNFα. The two groups are not significantly different.
It should be noted that, in some of our recordings, there was a residual unblocked outward current (Figs. 2B, 4B). It is conceivable that, in cells in which there is significant unblocked outward current, some of the effects of TNFα or the p38 inhibitor on the amplitude of the inward current may be impacted by effects on this outward current. However, we did not see significant effects on this outward current, and we have observed modulation of TTX-R sodium currents by TNFα and the p38 inhibitor in cells with no unblocked outward current. Thus, modulation of the unblocked outward current is not the primary mechanism for this modulation.
TNF activates p38 MAPK in mouse DRG neurons in vitro
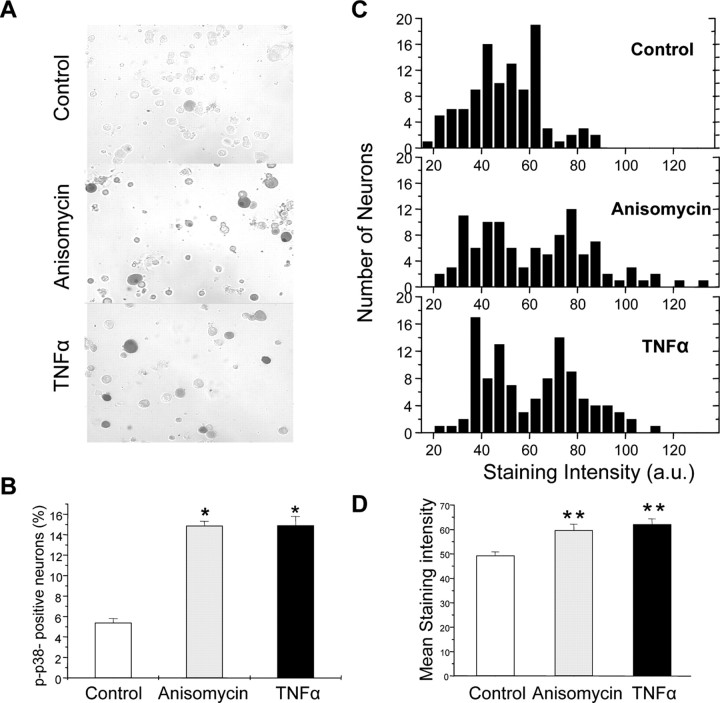
The electrophysiological data described above strongly suggest that p38 MAPK mediates the modulation of TTX-R Na+ currents by TNFα and suggest that TNFα activates p38 in mouse DRG neurons. To test this more directly, we used immunohistochemistry to determine whether TNF caused increases in active (phosphorylated) p38 in mouse DRG neurons under conditions similar to those used in our electrophysiological recordings. Cultured DRG neurons were treated with TNFα (100 ng/ml) or anisomycin (200 nm, as a positive control) for 20 min. The cells were then fixed and processed for immunohistochemistry using a phospho-specific p38 antibody. As illustrated in Figure 6A, there were few neurons labeled for p-p38 in control DRG cultures, consistent with previous reports (Pollock et al., 2002; Schafers et al., 2003b). Application of TNFα clearly induced p38 activation, both in terms of staining intensity and in the number of positive cells, to an extent similar to that observed in response to the positive control (anisomycin). TNFα increased the percentage of p-p38-immunoreactive neurons to 14.9% compared with 5.4% in control cultures; a similar enhancement was observed with anisomycin. The staining intensity (in arbitrary units ranging from 0 to 255) of p-p38 immunoreactivity was also increased to 62.04 ± 2.35 in TNFα treatment from 49.31 ± 1.51 in control conditions, again similar to what was observed with anisomycin.
Figure 6.
TNFα activates p38 MAPK in isolated mouse DRG neurons. Phospho-p38 immunohistochemical analysis of cultured DRG neurons exposed to TNFα (100 ng/ml) and anisomycin (200 nm, as a positive control) for 20 min. A, Representative images showing increased phospho-p38 immunoreactivity visualized by DAB in response to both anisomycin and TNFα relative to parallel control cultures (original magnification, 200×). B, Average data from three separate experiments showing the increase in the percentage of phospho-p38-positive cells in response to anisomycin and TNFα.*p < 0.05 compared with control. One hundred randomly selected neurons were measured from each condition. C, Quantification of staining intensity of 100 individual neurons in each condition showing an increase in more densely labeled neurons after TNFα and anisomycin treatment. Staining intensity is represented as arbitrary units (a.u.) ranging from 0 to 255 based on the grayscale intensity level for each pixel. D, Grouped data showing the mean ± SEM staining intensity of DRG neurons under the three conditions. *p < 0.01compared with control.
The role of TNFR1 in the modulation of TTX-R currents by TNFα
Recent evidence has indicated that TNFR1 (p55) and TNFR2 (p75) are expressed in rat DRG neurons (Shubayev and Myers, 2001; Pollock et al., 2002), although the presence of TNFR2 in these cells is controversial (Li et al., 2004; Inglis et al., 2005). To test whether the TNFα-induced enhancement of TTX-R currents occurs via activation of TNFR1, TNFR2, or both receptors, we compared the effects of TNFα on TTX-R Na+ currents in DRG neurons prepared from wild type with those prepared from TNFR1 or TNFR2 knock-out mice. The TNFR1–/– and TNFR2–/– mice were generated in the C57BL/6J strain (Pfeffer et al., 1993; Erickson et al., 1994), so C57BL/6J mice were used as wild-type controls for this experiment. We detected no major differences in DRG neurons in the C57BL/6J strain compared with the ICR mice used in the experiments above, including that there was no significant difference in the magnitude of potentiation of TTX-R by TNFα (potentiation in ICR mice by TNF was 37.68 ± 5.49%, and, in C57BL/6 mice, the potentiation averaged 26.14 ± 4.0%; p > 0.1, ANOVA). As shown in Figure 7, the TNFα (100 ng/ml)-induced enhancement of TTX-R currents was significantly reduced in TNFR1–/– mice relative to WT controls, with a 66% decrease in the enhancement of TTX-R currents in TNFR1–/– mice. In contrast, the enhancement of TTX-R currents by TNFα was not significantly reduced in the TNFR2–/– mice relative to WT controls. This suggests that TNFα sensitization of TTX-R Na+ channels is dependent on TNFR1 but not TNFR2.
The p38 inhibitor SB202190 induced a similar inhibition of basal TTX-R Na+ currents in TNFR1–/–, TNFR2–/–, and wild-type mice. This suggests that the basal p38 activity in mouse DRG neurons is not attributable to basal activity of TNFR1 or TNFR2. The inhibition by SB202190 was similar in neurons prepared from wild-type C57BL/6J and ICR mice.
p38 activity is required for mechanical hypersensitivity induced by TNFα
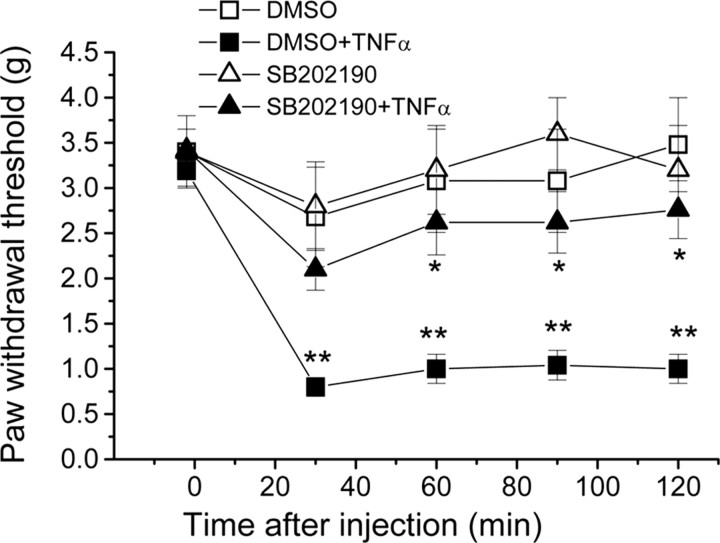
Thus far, our results suggest that TNFα promotes thermal hyperalgesia (but not mechanical hypersensitivity) via a TRPV1-dependent mechanism. Furthermore, our biochemical and electrophysiological studies show that TNFα can sensitize TTX-R sodium channels via activation of p38. If this sensitization of TTX-R sodium channels underlies mechanical hypersensitivity induced by TNFα, then this hypersensitivity should be reduced by inhibition of the p38 pathway. To test this hypothesis, we performed behavioral studies in which male ICR mice were pretreated with the p38 inhibitor SB202190 (12 μm in 10 μl) or vehicle (0.12% DMSO) 20 min before intraplantar injection of TNFα (1 ng in 10 μl). Intraplantar injection of TNFα induced robust mechanical hypersensitivity in the vehicle-pretreated mice. This hypersensitivity was significantly reduced when mice were pretreated with the p38 inhibitor SB202190, whereas treatment with vehicle or SB202190 alone did not produce any significant analgesia (Fig. 8). These data suggest that mechanical hypersensitivity induced by TNFα is dependent on the p38 pathway.
Figure 8.
p38 activity is required for mechanical hypersensitivity induced by TNFα. ICR mice were pretreated with the p38 inhibitor SB202190 (12 μm in 10 μl) or vehicle (0.1% DMSO) alone or 20 min before intraplantar injection of TNFα (1 ng in 10 μl). The graph shows the mean ± SEM mechanical withdrawal thresholds over time before and after injection of TNFα. The experimenter was blind to the identity of the preinjection drug. *p < 0.05 compared with DMSO plus TNFα-treated mice. n = 6 for each condition. **p < 0.001, ANOVA compared with DMSO-treated mice.
The present results show that TNFα induces mechanical and thermal hypersensitivity in WT mice but only mechanical hypersensitivity in TRPV1–/– mice (Fig. 1). This complete deficit in TNFα-induced thermal hyperalgesia in TRPV1–/– mice is curious given that TNFα still induces a robust sensitization of TTX-R Na+ currents in DRG neurons prepared from TRPV1–/– mice (TNFα-induced potentiation of TTX-R is 26.12 ± 4.0 (n = 7) in WT mice compared with 25.43 ± 3.33 (n = 7) in TRPV1–/– mice; data not shown). Together, these results collectively suggest that thermal hyperalgesia induced by peripherally applied TNFα is mediated via TRPV1 and not TTX-R sodium channels, whereas mechanical hypersensitivity induced by TNFα does not require TRPV1 and may involve modulation of TTX-R sodium channels.
Discussion
Peripheral administration of TNFα can induce rapid sensitization to both thermal and mechanical stimuli. Nicol et al. (1997) showed previously that TNFα can sensitize the noxious heat-sensing protein TRPV1. Whether this effect is mediated by transcriptional or posttranslational mechanisms is not known, although it requires the increased production of prostaglandin E2. TRPV1 sensitization was induced by prolonged pretreatment with TNFα (>4 h) (Nicol et al., 1997). If TNFα can also rapidly sensitize TRPV1, then this could explain the ability of TNFα to cause rapid sensitization to thermal stimuli. This hypothesis is supported by our findings that TNFα induces thermal hyperalgesia in wild-type mice but not in their TRPV1–/– littermates. TRPV1 modulation is not involved in peripheral mechanical sensitization induced by TNFα, however, because we found no reduction in tactile hypersensitivity in TRPV1–/– mice compared with WT littermates.
TNFα has been shown to regulate a variety of ion channels in the nervous system. For example, TNFα decreases outward K+ currents in retinal ganglion neurons (Diem et al., 2001) and increases L-type Ca2+ currents in hippocampal (Furukawa and Mattson, 1998) and superior cervical ganglion (Soliven and Albert, 1992) neurons. Modulation of similar channels in DRG neurons could mediate TNFα-induced mechanical hypersensitivity. Our results show that TNFα can acutely sensitize TTX-R sodium currents in nociceptors. In theory, this mechanism could account for acute sensitization to both thermal and mechanical stimuli induced by TNFα. However, our behavioral results suggest that modulation of TRPV1 is of greater importance than modulation of TTX-R in establishing thermal sensitization after exposure to TNFα. Although p38 sensitizes TTX-R and p38 inhibitors prevent mechanical sensitization induced by peripherally applied TNFα, this is only suggestive that modulation of TTX-R by p38 underlies this sensitization. In future studies, it will be important to address this question more directly by testing whether TNFα can induce mechanical hypersensitivity in mice lacking TTX-R channel subunits.
TNFα exerts its effects on cells via activation of two receptor subtypes: TNFR1 and TNFR2 (Wu, 2004). Previous studies have shown that TNFα, TNFR1, and TNFR2 are expressed in adult rat DRG neurons (Shubayev and Myers, 2001; Pollock et al., 2002; Schafers et al., 2003a), and both TNFα and its receptors are upregulated in DRG neurons after chronic constriction injury (Shubayev and Myers, 2001; Schafers et al., 2003a). TNFR1 appears to be particularly important in the pain-sensitizing actions of TNFα, because mechanical hypersensitivity induced by exogenous TNFα or by inflammation is reduced in TNFR1–/– mice (Cunha et al., 2005) and antisense knockdown of TNFR1 reduces hyperalgesic priming after inflammation in the rat (Parada et al., 2003). Furthermore, neutralizing antibodies against TNFR1, but not those against TNFR2, reduce thermal and mechanical hypersensitivity after nerve injury (Sommer et al., 1998). Our studies are consistent with these findings in that we demonstrate a critical role for TNFR1, but not TNFR2, in the sensitization of TTX-R by TNFα.
TNF receptors activate multiple signaling pathways, including ceramide signaling and activation of several MAPK pathways (Joseph and Levine, 2004; Wu, 2004). It is now recognized that the p38 MAPK pathway is an important regulator of inflammatory and neuropathic pain (Ji et al., 2002; Kim et al., 2002; Jin et al., 2003; Milligan et al., 2003; Svensson et al., 2003a,b; Inoue et al., 2004; Ji, 2004; Ji and Strichartz, 2004; Obata et al., 2004a,b; Sweitzer et al., 2004a,b; Mizushima et al., 2005; Svensson et al., 2005a,b; Tsuda et al., 2005). Peripheral inflammation results in p38 activation in nociceptive DRG neurons, and p38 participates in the maintenance of inflammatory heat hyperalgesia by increasing TRPV1 expression (Ji et al., 2002). TNFα activates p38 MAPK in cultured DRG neurons (Pollock et al., 2002), and treatment with the TNFα antagonist Etanercept attenuates mechanical hypersensitivity before but not after spinal nerve ligation, an effect that is mimicked by the p38 inhibitor SB203580 (Schafers et al., 2003b). Although these data provide substantial evidence that p38 activation is downstream of TNFα and participates in pain hypersensitivity after inflammation and nerve injury, we do not know the downstream targets of p38 that mediate this sensitization. We show here that p38 activity modulates TTX-R Na+ channels in mouse DRG neurons. Furthermore, we show that activation of TNFR1 induces p38 activity in mouse DRG neurons and enhances TTX-R currents in a p38-dependent manner.
We found that p38 inhibitors have effects on basal TTX-R Na+ currents in isolated DRG neurons, suggesting that there is basal p38 activity in our preparation. This is perhaps not surprising given that an isolated sensory neuron could be looked at as a rather severe axotomy model, and previous studies have shown that nerve injury leads to enhanced p38 activity in DRG somata (Kim et al., 2002; Jin et al., 2003). Indeed, our results indicate that there is a low level of active p38 in unstimulated DRG neurons (Fig. 6). It is interesting to note that previous studies have shown enhanced effects of TNFα when applied to injured and adjacent uninjured neurons in the DRG from nerve-injured rats (Schafers et al., 2003c). It is possible that the effects we are studying in our isolated DRG neurons reflects more the sensitized state observed in nerve-injured animals than it does the naive state. Nevertheless, we do find that peripherally administered TNFα sensitizes naive mice to both thermal and mechanical stimuli by a p38-dependent mechanism. Whether the enhanced response to TNFα in nerve-injured animals represents an amplification of the same mechanism seen in naive animals or a different mechanism that is induced by injury remains to be determined.
The studies reported here show that TNFα can directly sensitize nociceptors via a TNFR1- and p38-dependent mechanism. This effect is rapid, beginning ∼1 min after the application of TNFα, suggesting a direct action of TNFR1-mediated signaling pathways on TTX-R. Our finding that the potentiation of TTX-R by TNFα was significantly reduced in TNFR1–/– mice relative to wild types suggests a prominent role of TNFR1 in mediating this sensitization. The small potentiation of TTX-R by TNFα in TNFR1–/– DRG neurons suggests a possible role for a TNFR1-independent mechanism in mediating these effects in the TNFR1–/– mice. Although we observed no difference in potentiation of TTX-R in TNFR2–/– mice compared with wild type, it is possible that, in the context of the TNFR1 knock-out, TNFR2 might play a greater role. Nevertheless, our results show that activation of TNFR1 is the major mechanism by which TNFα modulates TTX-R.
Our findings provide support for the hypothesis that the pain-sensitizing actions of TNFα are mediated through activation of p38 and suggest that p38 may enhance TTX-R by phosphorylating TTX-R sodium channels or an associated protein. Previously, p38 has been reported to directly modulate other voltage-gated sodium channels. For example, Wittmack et al. (2005) showed that Nav1.6 is modulated by p38 activation, and this effect could be eliminated by mutating a p38 phosphorylation site (Wittmack et al., 2005). In this case, p38 activation resulted in a decrease in current density, whereas our results show that p38 activation leads to an increase in TTX-R currents in DRG neurons. Future studies should endeavor to identify p38 phosphorylation sites in sodium channel subunits to test whether the modulation of TTX-R is mediated by direct phosphorylation of the channel. For example, the published mouse Nav1.8 sequence contains 15 consensus sites for phosphorylation by MAPKs, including p38 (Souslova et al., 1997; Sharrocks et al., 2000). Twelve of these consensus sites are located on predicted intracellular domains, making them possible targets for p38-mediated phosphorylation and modulation of Nav1.8.
Although our results may help explain some of the more rapid pain-sensitizing effects of TNFα, it is clear that the overall role of TNFα and related cytokines in mediating various types of chronic pain is much more complex. Many effects of TNFα are mediated by synergistic interactions with other cytokines. For example, only the combination of TNFα, interleukin-1β, and interleukin-8 is capable of inducing nociceptive writhing responses after intraperitoneal injection in mice, whereas TNFα alone does not (Ribeiro et al., 2000). Here, we have shown induction of mechanical and thermal hypersensitivity by injection of TNFα alone and effects of TNFα on isolated DRG neurons in vitro, but whether these effects require the concomitant activity of other cytokines is not known. In addition, TNFα can have TNFR1- and TNFR2-independent effects on cells; in vitro, TNFα can have intrinsic ion channel-forming activity, and this action may underlie some physiological effects of TNFα (Kagan et al., 1992).
TNFα inhibitors are effective for the treatment of inflammatory pain conditions, including rheumatoid arthritis and inflammatory bowel diseases (Hanauer, 2004; Toussirot and Wendling, 2004; Wendling and Toussirot, 2004; Goldblatt and Isenberg, 2005). The agents currently on the market act by sequestering endogenous TNFα. Our findings together with previous reports showing amelioration of pain in a variety of models by reducing TNFR1 activation suggest that agents that specifically target TNFR1 or downstream signaling molecules, including p38, could also be effective in specifically targeting pain hypersensitivity induced by TNFα.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS42595 (R.W.G.). We thank the members of the Gereau laboratory for critical comments on this manuscript and C. S. Qiu for assistance with mouse colony maintenance and genotyping.
Correspondence should be addressed to Dr. Robert W. Gereau IV, Washington University Pain Center, Department of Anesthesiology, 660 South Euclid Avenue, St. Louis, MO 63110. E-mail: gereaur@wustl.edu.
DOI:10.1523/JNEUROSCI.3858-05.2006
Copyright © 2006 Society for Neuroscience 0270-6474/06/260246-10$15.00/0
References
- Bhave G, Gereau RW (2004) Posttranslational mechanisms of peripheral sensitization. J Neurobiol 61: 88–106. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Scroggs RS (1995) Variation in serotonergic inhibition of calcium channel currents in four types of rat sensory neurons differentiated by membrane properties. J Neurophysiol 74: 1870–1879. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Cooper BY, Scroggs R (1997) 5-HT4 receptors couple positively to tetrodotoxin-insensitive sodium channels in a subpopulation of capsaicin-sensitive rat sensory neurons. J Neurosci 17: 7181–7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas CG, Mar LP, Vysokanov AV, Arnold PB, Cardenas LM, Surmeier DJ, Scroggs RS (1999) Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. J Physiol (Lond) 518: 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas LM, Cardenas CG, Scroggs RS (2001) 5HT increases excitability of nociceptor-like rat dorsal root ganglion neurons via cAMP-coupled TTX-resistant Na+ channels. J Neurophysiol 86: 241–248. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH (1992) The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol 107: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Verri Jr WA, Silva JS, Poole S, Cunha FQ, Ferreira SH (2005) A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA 102: 1755–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem R, Meyer R, Weishaupt JH, Bahr M (2001) Reduction of potassium currents and phosphatidylinositol 3-kinase-dependent AKT phosphorylation by tumor necrosis factor-α rescues axotomized retinal ganglion cells from retrograde cell death in vivo J Neurosci 21: 2058–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson SL, de Sauvage FJ, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan KCF, Schreiber RD, Goeddel DV, Moore MW (1994) Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature 372: 560–563. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Mattson MP (1998) The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem 70: 1876–1886. [DOI] [PubMed] [Google Scholar]
- Goldblatt F, Isenberg DA (2005) New therapies for rheumatoid arthritis. Clin Exp Immunol 140: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer SB (2004) Efficacy and safety of tumor necrosis factor antagonists in Crohn's disease: overview of randomized clinical studies. Rev Gastroenterol Disord 4 [Suppl 3]: S18–S24. [PubMed] [Google Scholar]
- Homma Y, Brull SJ, Zhang JM (2002) A comparison of chronic pain behavior following local application of tumor necrosis factor alpha to the normal and mechanically compressed lumbar ganglia in the rat. Pain 95: 239–246. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Bhave G, Gereau RW (2002) Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci 22: 7444–7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis JJ, Nissim A, Lees DM, Hunt SP, Chernajovsky Y, Kidd BL (2005) The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthritis Res Ther 7: R807–R816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Tsuda M, Koizumi S (2004) Chronic pain and microglia: the role of ATP. Novartis Found Symp 261: 55–64; discussion 64–67, 149–154. [PubMed] [Google Scholar]
- Ji RR (2004) Mitogen-activated protein kinases as potential targets for pain killers. Curr Opin Investig Drugs 5: 71–75. [PubMed] [Google Scholar]
- Ji RR, Strichartz G (2004) Cell signaling and the genesis of neuropathic pain. Sci STKE 2004: reE14. [DOI] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ (2002) p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36: 57–68. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR (2003) p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci 23: 4017–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD (2004) Caspase signalling in neuropathic and inflammatory pain in the rat. Eur J Neurosci 20: 2896–2902. [DOI] [PubMed] [Google Scholar]
- Junger H, Sorkin LS (2000) Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain 85: 145–151. [DOI] [PubMed] [Google Scholar]
- Kagan BL, Baldwin RL, Munoz D, Wisnieski BJ (1992) Formation of ionpermeable channels by tumor necrosis factor-alpha. Science 255: 1427–1430. [DOI] [PubMed] [Google Scholar]
- Kim SY, Bae JC, Kim JY, Lee HL, Lee KM, Kim DS, Cho HJ (2002) Activation of p38 MAP kinase in the rat dorsal root ganglia and spinal cord following peripheral inflammation and nerve injury. NeuroReport 13: 2483–2486. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Keys JR, Landvatter SW, Strickler JE, McLaughlin MM, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR (1994) A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372: 739–746. [DOI] [PubMed] [Google Scholar]
- Li Y, Ji A, Weihe E, Schafer MK (2004) Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor α (TNFα) and TNF receptors in rat dorsal root ganglion. J Neurosci 24: 9623–9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenlaub T, Teuteberg P, Hartung T, Sommer C (2000) Effects of neutralizing antibodies to TNF-alpha on pain-related behavior and nerve regeneration in mice with chronic constriction injury. Brain Res 866: 15–22. [DOI] [PubMed] [Google Scholar]
- Liu B, Li H, Brull SJ, Zhang JM (2002) Increased sensitivity of sensory neurons to tumor necrosis factor alpha in rats with chronic compression of the lumbar ganglia. J Neurophysiol 88: 1393–1399. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR (2003) Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci 23: 1026–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima T, Obata K, Yamanaka H, Dai Y, Fukuoka T, Tokunaga A, Mashimo T, Noguchi K (2005) Activation of p38 MAPK in primary afferent neurons by noxious stimulation and its involvement in the development of thermal hyperalgesia. Pain 113: 51–60. [DOI] [PubMed] [Google Scholar]
- Nicol GD, Lopshire JC, Pafford CM (1997) Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J Neurosci 17: 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Noguchi K (2004a) Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur J Neurosci 20: 2881–2895. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K (2004b) Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci 24: 10211–10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opree A, Kress M (2000) Involvement of the proinflammatory cytokines tumor necrosis factor-α, IL-1β, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci 20: 6289–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Joseph EK, Levine JD (2003) Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci 17: 1847–1852. [DOI] [PubMed] [Google Scholar]
- Perkins MN, Kelly D (1994) Interleukin-1 beta induced-desArg9bradykinin-mediated thermal hyperalgesia in the rat. Neuropharmacology 33: 657–660. [DOI] [PubMed] [Google Scholar]
- Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW (1993) Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73: 457–467. [DOI] [PubMed] [Google Scholar]
- Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeele P, MacEwan DJ, Scott RH (2002) TNF-alpha receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurones. Neuropharmacology 42: 93–106. [DOI] [PubMed] [Google Scholar]
- Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato ABP, Poole S, Ferreira SH, Cunha FQ (2000) Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol 387: 111–118. [DOI] [PubMed] [Google Scholar]
- Schafers M, Geis C, Brors D, Yaksh TL, Sommer C (2002) Anterograde transport of tumor necrosis factor-alpha in the intact and injured rat sciatic nerve. J Neurosci 22: 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Sorkin LS, Geis C, Shubayev VI (2003a) Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci Lett 347: 179–182. [DOI] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS (2003b) Tumor necrosis factor-α induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci 23: 2517–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS (2003c) Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-α after spinal nerve ligation. J Neurosci 23: 3028–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks AD, Yang SH, Galanis A (2000) Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem Sci 25: 448–453. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR (2001) Axonal transport of TNF-alpha in painful neuropathy: distribution of ligand tracer and TNF receptors. J Neuroimmunol 114: 48–56. [DOI] [PubMed] [Google Scholar]
- Soliven B, Albert J (1992) Tumor necrosis factor modulates Ca2+ currents in cultured sympathetic neurons. J Neurosci 12: 2665–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, Kress M (2004) Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 361: 184–187. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schmidt C, George A (1998) Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol 151: 138–142. [DOI] [PubMed] [Google Scholar]
- Sommer C, Lindenlaub T, Teuteberg P, Schafers M, Hartung T, Toyka KV (2001) Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res 913: 86–89. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Doom CM (2000) Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J Peripher Nerv Syst 5: 96–100. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Xiao WH, Wagner R, Myers RR (1997) Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience 81: 255–262. [DOI] [PubMed] [Google Scholar]
- Souslova VA, Fox M, Wood JN, Akopian AN (1997) Cloning and characterization of a mouse sensory neuron tetrodotoxin-resistant voltage-gated sodium channel gene, Scn10a. Genomics 41: 201–209. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Hua XY, Protter AA, Powell HC, Yaksh TL (2003a) Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE(2) release and thermal hyperalgesia. NeuroReport 14: 1153–1157. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Fresh-water JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL (2003b) Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem 86: 1534–1544. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Schafers M, Jones TL, Powell H, Sorkin LS (2005a) Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci Lett 379: 209–213. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua XY, Yaksh TL (2005b) Spinal p38beta isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J Neurochem 92: 1508–1520. [DOI] [PubMed] [Google Scholar]
- Sweitzer S, Martin D, DeLeo JA (2001) Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience 103: 529–539. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Medicherla S, Almirez R, Dugar S, Chakravarty S, Shumilla JA, Yeomans DC, Protter AA (2004a) Antinociceptive action of a p38alpha MAPK inhibitor, SD-282, in a diabetic neuropathy model. Pain 109: 409–419. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Peters MC, Ma JY, Kerr I, Mangadu R, Chakravarty S, Dugar S, Medicherla S, Protter AA, Yeomans DC (2004b) Peripheral and central p38 MAPK mediates capsaicin-induced hyperalgesia. Pain 111: 278–285. [DOI] [PubMed] [Google Scholar]
- Tchelingerian JL, Quinonero J, Booss J, Jacque C (1993) Localization of TNF alpha and IL-1 alpha immunoreactivities in striatal neurons after surgical injury to the hippocampus. Neuron 10: 213–224. [DOI] [PubMed] [Google Scholar]
- Toussirot E, Wendling D (2004) The use of TNF-alpha blocking agents in rheumatoid arthritis: an overview. Expert Opin Pharmacother 5: 581–594. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW (2005) Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci 28: 101–107. [DOI] [PubMed] [Google Scholar]
- Wacnik PW, Eikmeier LJ, Simone DA, Wilcox GL, Beitz AJ (2005) Nociceptive characteristics of tumor necrosis factor-alpha in naive and tumorbearing mice. Neuroscience 132: 479–491. [DOI] [PubMed] [Google Scholar]
- Wagner R, Myers RR (1996) Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. NeuroReport 7: 2897–2901. [DOI] [PubMed] [Google Scholar]
- Wagner R, Janjigian M, Myers RR (1998) Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-alpha expression. Pain 74: 35–42. [DOI] [PubMed] [Google Scholar]
- Wendling D, Toussirot E (2004) Anti-TNF-alpha therapy in ankylosing spondylitis. Expert Opin Pharmacother 5: 1497–1507. [DOI] [PubMed] [Google Scholar]
- Wittmack EK, Rush AM, Hudmon A, Waxman SG, Dib-Hajj SD (2005) Voltage-gated sodium channel Nav1.6 is modulated by p38 mitogen-activated protein kinase. J Neurosci 25: 6621–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Boorman JP, Okuse K, Baker MD (2004a) Voltage-gated sodium channels and pain pathways. J Neurobiol 61: 55–71. [DOI] [PubMed] [Google Scholar]
- Wood JN, Abrahamsen B, Baker MD, Boorman JD, Donier E, Drew LJ, Nassar MA, Okuse K, Seereeram A, Stirling CL, Zhao J (2004b) Ion channel activities implicated in pathological pain. Novartis Found Symp 261: 32–40, discussion 40–54. [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S (1997) Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol 121: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H (2004) Assembly of post-receptor signaling complexes for the tumor necrosis factor receptor superfamily. Adv Protein Chem 68: 225–279. [DOI] [PubMed] [Google Scholar]
- Yang D, Gereau RW (2004) Group II metabotropic glutamate receptors inhibit cAMP-dependent protein kinase-mediated enhancement of tetrodotoxin-resistant sodium currents in mouse dorsal root ganglion neurons. Neurosci Lett 357: 159–162. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Li H, Liu B, Brull SJ (2002) Acute topical application of tumor necrosis factor alpha evokes protein kinase A-dependent responses in rat sensory neurons. J Neurophysiol 88: 1387–1392. [DOI] [PubMed] [Google Scholar]