Abstract
Recent evidence suggests that dopamine plays an important role in arousal, but the location of the dopaminergic neurons that may regulate arousal remains unclear. It is sometimes assumed that the dopaminergic neurons in the ventral tegmental area that project to the prefrontal cortex and striatum may regulate the state of arousal; however, the firing of these dopaminergic neurons does not correlate with overall levels of behavioral wakefulness. We identified wake-active dopaminergic neurons by combining immunohistochemical staining for Fos and tyrosine hydroxylase (TH) in awake and sleeping rats. Approximately 50% of the TH-immunoreactive (TH-ir) cells in the ventral periaqueductal gray matter (vPAG) expressed Fos protein during natural wakefulness or wakefulness induced by environmental stimulation, but none expressed Fos during sleep. Fos immunoreactivity was not seen in the substantia nigra TH-immunoreactive cells in either condition. Injections of 6-hydroxydopamine into the vPAG, which killed 55–65% of wake-active TH-ir cells but did not injure nearby serotoninergic cells, increased total daily sleep by ∼20%. By combining retrograde and anterograde tracing, we showed that these wake-active dopaminergic cells have extensive reciprocal connections with the sleep–wake regulatory system. The vPAG dopaminergic cells may provide the long-sought ascending dopaminergic waking influence. In addition, their close relationship with the dorsal raphe nucleus will require reassessment of previous studies of the role of the dorsal raphe nucleus in sleep, because many of those experiments may have been confounded by the then-unrecognized presence of intermingled wake-active dopaminergic neurons.
Keywords: dorsal raphe nucleus, dopamine, arousal, psychostimulants, neurotoxin lesions, neuronal tracing
Introduction
Arousal and waking behaviors are associated with increased forebrain dopamine (DA) secretion and can be enhanced by drugs that block DA reuptake or directly stimulate DA release (for instance, cocaine or amphetamine); in contrast, drugs that inhibit norepinephrine reuptake have little effect on wakefulness (Nishino et al., 1998; Kanbayashi et al., 2000). Wisor et al. (2001) found that mice with deletion of the DA transporter gene have 20% more wakefulness than control mice and do not respond to psychostimulant drugs such as amphetamine or modafinil, indicating that the DA transporter is involved in the control of wakefulness and is required for modafinil- and amphetamine-induced arousal. Patients with Parkinson's disease who have extensive loss of dopaminergic cells in the substantia nigra (SN; A9), and less extensive loss in the A10 dopaminergic group in the ventral tegmental area (VTA), often have increased sleepiness, which is made worse by D2 receptor (autoreceptor) agonists (Larsen and Tandberg, 2001; Arnulf et al., 2002). Despite this evidence, the specific population of dopaminergic neurons responsible for arousal is not known. VTA neurons that project to the prefrontal cortex and striatum (Steinfels et al., 1983; Freeman et al., 2001) are often assumed to play this role; however, the firing of VTA neurons or SN neurons that project to the striatum does not correlate with overall levels of behavioral wakefulness in rats (Miller et al., 1983). Furthermore, neurotoxic lesions of the VTA do not decrease behavioral wakefulness (Lai et al., 1999).
We therefore set out to identify the dopaminergic cells that may mediate regulation of wakefulness. We used expression of c-Fos protein as an indicator of neuronal activity to identify dopaminergic neurons that are active during wakefulness but not during sleep. We then used the neurotoxin 6-hydroxydopamine (6-OHDA) to eliminate specifically the identified wake-active dopaminergic neurons and examined changes in sleep–wake behavior. Finally, we determined the afferent and efferent projections of the wake-active dopaminergic cells by combining anterograde and retrograde tracer methods with tyrosine hydroxylase (TH) and Fos immunostaining.
Materials and Methods
Animals
Pathogen-free adult male Sprague Dawley rats (275–300 g; Harlan, Indianapolis, IN) were individually housed and had free access to food and water. All animals were housed under controlled conditions (12 h light/dark cycle, starting at 7:00 A.M.; 100 lux) in an isolated ventilated chamber maintained at 20–22°C. All protocols were approved by the Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center and Harvard Medical School.
Physiology
EEG/EMG lead implantation and sleep analysis. After animals were anesthetized with chloral hydrate (350 mg/kg), the skulls were exposed. Four EEG screw electrodes were implanted into the skull, in the frontal (two) and parietal bones (two) of each side, and two flexible EMG wire electrodes were placed into the neck muscles. The free ends of the leads were soldered into a socket that was attached to the skull with dental cement, and the incision was then closed by wound clips. One week after surgery, the sockets were connected via flexible recording cables and a commutator to a Grass polygraph and computer, and signals were digitized using an Apple Macintosh computer running the ICELUS sleep recording system (M. Opp, University of Michigan, Ann Arbor, MI). The EEG/EMG was recorded at the end of third week after surgery, for 24 h.
Wake–sleep states were manually scored in 12 s epochs on the digitized EEG/EMG. Wakefulness was identified by the presence of a desynchronized EEG and phasic EMG activity. Nonrapid eye movement (NREM) sleep consisted of a high amplitude slow wave EEG together with a low EMG tone relative to wake. Rapid eye movement (REM) sleep was identified by the presence of regular theta EEG activity coupled with low EMG tone relative to NREM sleep. The amount of time spent in wake, NREM sleep, and REM sleep was determined for each hour.
Forced wakefulness. During periods of forced wakefulness, rats were closely monitored by online EEG/EMG to be certain that they were awake. When EEG slowing was noted, the investigators removed the cover of the cage, which was sufficient to wake up the animals, and repeated this procedure as often as necessary to maintain wakefulness until the perfusion time. During the period of forced wakefulness, the rats were never touched, to minimize stress.
Injections of tracers or toxins and histology
Tracer or toxin injections. For tracer experiments, under chloral hydrate anesthesia (7% in saline; 350 mg/kg), a fine glass pipette containing 1.0% cholera toxin subunit B (CTB; List Biologic, Campbell, CA), 12.5% biotinylated dextran (BD), or a mixture of 1% CTB and 12.5% BD was lowered to the precalculated targets based on the rat atlas of Paxinos and Watson (1998), and 9 nl of a solution containing the tracers was injected by an air pressure system. Phaseolus vulgaris leukoagglutinin (PHA-L; 2.5%) was injected by iontophoresis with a current of 5 μA for 15 min (7 s on and 7 s off). After two additional minutes, the pipette was slowly withdrawn and the incision was closed with wound clips. Animals survived for 7 d. The coordinates for tracer injections were as follows: medial prefrontal cortex, anteroposterior (AP), 2.20 mm, medial–lateral (ML), 0.4 mm, dorsoventral (DV), 4.6 mm; midline thalamus, AP, –2.8 mm, ML, 0 mm, DV, 4.4 mm; intralaminar thalamus, AP, –2.8 mm, ML, 0.8 mm, DV, 5.6 mm; the ventrolateral preoptic nucleus (VLPO), AP, –0.4 mm, ML, 0.9 mm, DV, 8.5 mm; substantia innominata (SI), AP, –0.8 mm, ML, 2.6 mm, DV, 7.2 mm; perifornical region, AP, –3.6 mm, ML, 1.4 mm, DV, 8.4 mm; ventral periaqueductal gray matter (vPAG), AP, –7.3 mm, ML, 0.2 mm, DV, 5.4 mm; laterodorsal tegmental nucleus (LDT), AP, –8.8 mm, ML, 1.0 mm, DV, 5.6 mm; locus ceruleus (LC), AP, –9.8 mm, ML, 1.5 mm, DV, 5.6 mm.
For vPAG lesions, two injections were made by air pressure containing 100 nl of 6.0% 6-OHDA in saline, 20 nl of 10% ibotenic acid, or 50 nl of 25% 5,7-dihydroxytryptamine (5,7-DHT) into the rostral (AP, –6.3 mm; ML, 0.0 mm; DV, 5.4 mm) and caudal (AP, 7.3 mm; ML, 0.0 mm; DV, 5.6 mm) levels of the ventral PAG region. The lesion was later verified by TH and 5-HT immunostaining and by Nissl staining.
Perfusion. Animals were deeply anesthetized with chloral hydrate (500 mg/kg) and then perfused with 50 ml saline followed by 500 ml of 10% formalin through the heart. The brains were removed, postfixed for 4 h in 10% formalin, and then equilibrated in 20% sucrose in PBS overnight.
Immunohistochemistry. The brains were sectioned on a freezing microtome at 40 μm into four series. Sections were washed in 0.1 m PBS, pH 7.4 (two changes), and then incubated in the primary antiserum for 1 d at room temperature. For c-Fos, we used a rabbit polyclonal antiserum (AB5; 1:150,000; Oncogene Sciences, Cambridge, MA) against residues 4–17 from human c-Fos. This antiserum has been used extensively and stains only the nuclei of neurons based on recent activity patterns (Gaus et al., 2002; Lu et al., 2002). For TH, we used a mouse monoclonal antibody (22941; 1:20,000; DiaSorin, Stillwater, MN) raised against rat pheochromocytoma TH. This antibody stains a pattern of neuronal morphology and distribution identical to previous reports (Takada et al., 1990; Sauer and Oertel, 1994). For orexin/hypocretin, we used a rabbit antiserum (T4074; 1:50,000; Phoenix Pharmaceuticals, Belmont, CA) against Pyr-Pro-Leu-Pro-Asp-Cys-Cys-Arg-Gln-Lys-Thr-Cys-Ser-Cys-Arg-Leu-Tyr-Glu-Leu-Leu-His-Gly-Ala-Gly-Asn-His-Ala-Ala-Gly-Ile-Leu-Thr-Leu-NH2. Staining with this antibody is blocked by preadsorption with the immunizing peptide (Chou et al., 2003). For ChAT, we used a polyclonal goat antiserum (AB144; 1:2000; Chemicon, Temecula, CA) against human placental enzyme. For serotonin, we used a goat antiserum against 5-hydroxytryptamine-glutaraldehyde-poly-lysine (AB125; 1:10,000; Chemicon). This antiserum stains a pattern of cellular morphology and distribution identical to previous reports (Datiche et al., 1995). For aromatic amino acid decarboxylase (AADC), we used a rabbit antiserum against recombinant bovine AADC expressed in Escherichia coli (AB1569; 1:1,000; Chemicon). This serum identifies a single band of ∼50 kDa molecular weight on Western blot, and stains a pattern of cellular morphology and distribution consistent with the combination of TH and 5-HT in the midbrain. We also used goat anti-CTB (1:100,000; #703; List Biological) and goat anti-PHA-L (1:10,000; #AS-2300; Vector Laboratories, Burlingame, CA). Neither antiserum stained anything in brains with no tracer injection. Sections were then washed in PBS and incubated in biotinylated secondary antiserum (against appropriate species IgG; 1:1,000 in PBS) for 1 h, washed in PBS, and incubated in avidin-biotin-horseradish peroxidase conjugate (Vector Laboratories) for 1 h. Sections were then washed again and incubated in a 0.06% solution of 3,3-diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO) plus 0.02% H2O2. The sections were stained brown with DAB only or black by adding 0.05% cobalt chloride and 0.01% nickel ammonium sulfate to the DAB solutions.
For double staining of Fos and TH, we immunostained the nucleus black for Fos and cytoplasm brown for TH. For combined fluorescence labeling of CTB and TH, sections were labeled with cyanine 3 (CY3; red)-conjugated antiserum against goat IgG (CTB), and streptavidin Alexa Fluor 488 (green) to stain biotinylated second antibody against mouse IgG (TH). For fluorescence labels of TH and AADC, sections were labeled with CY3 (red)-conjugated antiserum against mouse (TH) and streptavidin Alexa Fluor 488 (green) for the secondary antibody for AADC.
The antibodies against Fos, TH, orexin, ChAT, PHA-L, and serotonin all have been used widely and their specificity has been established in rat brain in previous control studies from this laboratory (Scammell et al., 2000; Chou et al., 2002, 2003). Immunohistochemical control studies for AADC and TH dual staining were performed in which either or both antibodies were omitted, revealing in lack of the appropriate staining.
Cell counting. We counted TH versus TH plus Fos-labeled cells, or TH versus TH plus CTB-labeled cells in TH-immunoreactive (TH-ir) fields in five sections spacing 160 μm apart through the vPAG from AP 6.50 mm to 7.50 mm posterior to bregma (Paxinos and Watson, 1998). To determine TH-ir cell loss in the lesion region, we counted all the TH-ir cells in the vPAG in five sections at the same level. For double labeling of CTB with orexin in the lateral hypothalamic area (LHA), with ChAT in the LDT and SI, or with TH in the LC, we counted labeled cells for three sections through the center of each group. In the medial prefrontal cortex at levels 1.0 to 1.6 mm in AP (Paxinos and Watson, 1986), we counted all the retrogradely labeled cells in three sections. In the VLPO, we used a predetermined counting box as described in a previous study on three sections through the center of the nucleus (Lu et al., 2002). In each experiment, we counted only neurons in which there was a visible nucleus, and we also measured the sizes of the nuclei of neurons that were counted. The cell counts were then corrected by using Abercrombie's equation, N = n(T/T + D), where N is the calculated number of cells, n is the measured number of profiles, T is the thickness of the tissue section, and D is the diameter of the cell nucleus (Abercrombie, 1946), to control for potential double counting of nuclei that would extend into two or more sections. We did not use stereological estimation of cell numbers because the immunohistochemical methods we use do not penetrate the full section thickness and, hence, do not allow acquisition of a systematic random sample in which each element has an equal chance of being sampled.
Statistical methods. Data were analyzed by ANOVA followed by post hoc Student's t test to determine statistical differences in cell counts and sleep behavior. Pearson correlation coefficients were calculated for comparison of loss of dopamine cells with changes in sleep time.
Results
Dopaminergic neurons in the ventral PAG express Fos during wakefulness
We perfused five rats at 9:00 P.M. during the early part of the wake period (lights off at 7:00 P.M.) and five rats at 10:00 A.M. (lights on at 7:00 A.M.) during the height of the sleep period, and brain sections were immunohistochemically double labeled with TH (brown-colored cytoplasm) and Fos (black-colored nuclei). The five rats perfused at 9:00 P.M. spent 82.4 ± 7.2% of total time in wakefulness during the hour before death. The five rats killed at 10:00 A.M. spent >75% of the hour before perfusion asleep (NREM = 68.5 ± 4.3%; REM = 8.6 ± 2.3%).
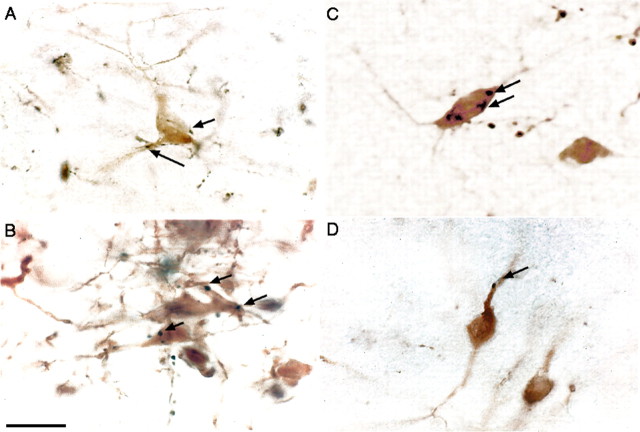
Wakeful rats showed marked Fos expression in TH-ir dopaminergic cells in the vPAG and extending into the adjacent dorsal raphe nucleus (DRN) (Fig. 1). The double-labeled cells were evenly distributed in the vPAG (Fig. 1). Cell counting indicated that 50.3 ± 6.2% of the TH-ir cells in the vPAG were Fos positive (Fig. 1A). In contrast, Fos was not seen in TH-ir cells in the vPAG in the sleeping control rats (Fig. 1C). No Fos-TH double-labeled cells were seen in the VTA or the SN in either awake or sleep conditions.
Figure 1.

Dopaminergic but not serotoninergic neurons in the vPAG contain Fos protein during wakefulness but not sleep. Immunohistochemical dual labeling of TH (brown) and Fos (black) is shown in animals that are spontaneously awake (A), kept awake by 2 h of environmental stimulation (B), and are spontaneously asleep (C). Fos-positive TH-ir neurons were seen only during wakefulness (arrows). In contrast, no double-labeled neurons were seen in immunohistochemical dual-labeling of serotonin (5-HT; brown) and Fos (black) in a spontaneously awake rat (D). Aq, Cerebral aqueduct; F wake, forced wakefulness. Scale bar: (in C) A, B, 200 μm; C, D, 100 μm. Arrows point to double-labeled neurons; arrowheads mark single-labeled neurons. An asterisk (*) marks the reference location in the inset box.
In spontaneously waking rats, we also found strong Fos expression in the tuberomammillary nucleus histaminergic cells, the perifornical orexin-ir (also called hypocretin-ir) cells, and the SI cholinergic neurons (but not other basal forebrain cholinergic neurons). However, we did not see Fos in the DRN serotoninergic cells (Fig. 1D), LC noradrenergic cells, or LDT/pedunculopontine tegmental (PPT) cholinergic cells. Some TH-ir cells in the medial preoptic area were Fos positive in both wake and sleep conditions. The remaining dopaminergic cell groups in the hypothalamus did not show Fos expression in either condition.
To determine whether TH and 5HT are synthesized in the same cells in the vPAG, we double labeled for TH and 5-HT and found that the TH-ir and 5-HT-ir neurons were separate populations (Fig. 2A,B). To ascertain that the TH-ir cells in the vPAG can synthesize dopamine, we double labeled sections through the PAG with antisera against AADC (the enzyme that converts l-dihydroxyphenylalanine, the product of TH, to dopamine; antiserum from goat) (Fig. 3, red) and TH (antiserum from mouse) (Fig. 3, green) in five rats. Nearly all of the TH-ir cells in these experiments were labeled with AADC (Fig. 3, cells containing both green and yellow; the small arrows indicate two such cells and the large arrow indicates a rare cell that is only TH positive). Because AADC also catalyzes the second step in serotonin synthesis, those AADC-ir cells that were not immunoreactive for TH (Fig. 3, red cells, one is indicated by the arrowhead) are likely to be serotoninergic.
Figure 2.
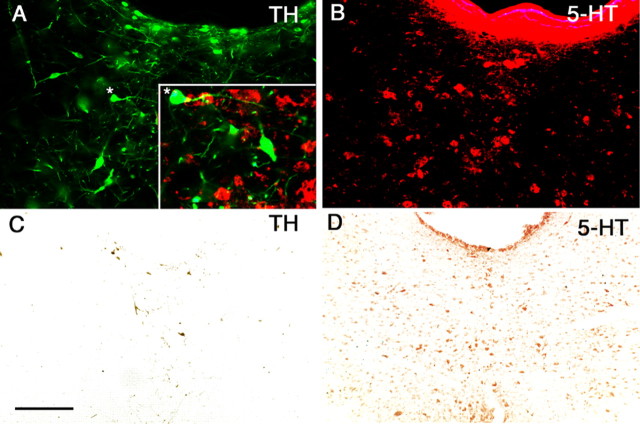
Dopamine (TH-immunoreactive) but not serotonin (5-HT-immunoreactive) neurons in the vPAG are depleted by 6-hydroxydopamine. Dual staining of the same tissue section for TH-ir (A) and 5-HT-ir (B) neurons in the DRN indicates that they are entirely separate populations (inset in A shows higher magnification in merged image). An asterisk (*) marks the identical location in A and in the inset. After 6-hydroxydopamine lesions, few TH-ir cells remained (C), but serotoninergic neurons were not affected (D). Scale bar: (in C) A, B, 100μm; C, D, 250 μm.
Figure 3.
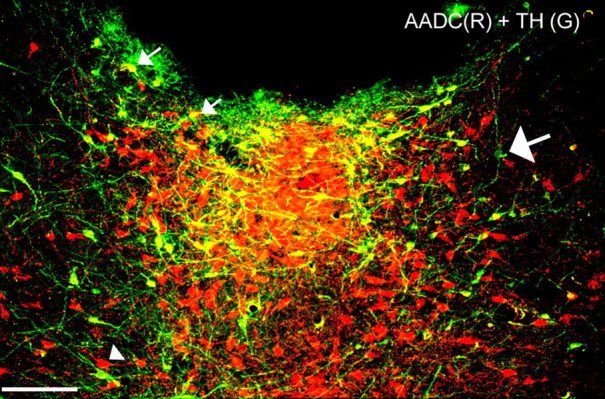
Colocalization of AADC (red) and TH (green) in the vPAG. Nearly all of the TH-ir neurons contained AADC (visualized as yellow color over green neurons; small white arrows); a rare TH-ir cell that did not contain AADC is indicated by the large white arrow. The AADC-ir neurons that were not TH-ir (arrowhead; red cells) are presumably serotoninergic. Scale bar, 100 μm.
vPAG TH-ir neurons express Fos after forced wakefulness
To control for time of day, and to determine how much wakefulness was necessary to promote Fos expression in the vPAG, we kept rats (n = 3 in each group) awake by environmental stimulation for periods of 30, 60, or 120 min, beginning at 10:00 A.M. (when they are usually at the height of their sleep period) and then examined their brains for Fos and TH immunoreactivity.
We found that vPAG TH-ir cells began expressing Fos after 30 min of forced wakefulness (5.3 ± 3.5% of TH-ir cells) and reached maximal expression at 120 min (41.4 ± 7.2% of TH-ir cells) (Fig. 1B). We saw a few double-labeled (Fos and TH) cells in the medial VTA but none in the SN in these rats. In agreement with previous results during natural or forced wakefulness (Scammell et al., 2000; Ko et al., 2003), wakefulness induced Fos expression in the ventrolateral tuberomammillary nucleus and perifornical orexin-ir cells in the lateral hypothalamus with similar kinetics. We did not see Fos immunoreactivity in the 5-HT-ir cells in the DRN or in the cholinergic cells in the LDT/PPT during forced wakefulness.
Compared with spontaneous wakefulness, forced wakefulness produced many additional Fos-ir cells in the CNS as a whole (particularly the cerebral cortex), but especially among nondopaminergic cells in the vPAG and the VTA and SN, and among basal forebrain (SI and the nucleus of the diagonal band) cholinergic cells (Greco et al., 2000) and noradrenergic cells in the LC.
The effects of dopaminergic cell loss in the PAG on sleep–wake cycles
We next tested the effect of lesions of the vPAG with the catecholamine-selective neurotoxin 6-OHDA on sleep and wakefulness. The typical lesion killed ∼55–65% (mean 63%) of the TH-ir cells in the vPAG (the intact vPAG contains ∼60 TH-ir cells per section) without damaging the VTA or the SN (Fig. 2C). Although the injected volume of 6-hydroxydopamine was quite large (two injections, 100 nl per injection), the TH-ir cell loss was never >70%. In the region of the lesion, we found no loss of 5-HT-ir cells, indicating that 6-OHDA did not affect serotoninergic cells (Fig. 2D).
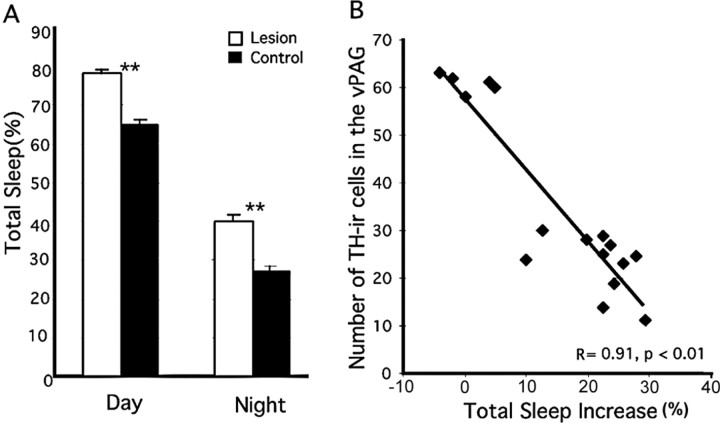
Behaviorally, the 6-OHDA-lesioned animals (n = 11) appeared to be normal. The rats with vPAG 6-OHDA lesions did not show the motor hyperactivity seen in animals with VTA lesions (Lai et al., 1999). They also showed a robust daily rhythm of total sleep 3 weeks after the surgery. However, these animals showed a statistically significant increase in NREM, REM, and total sleep (or decrease in wakefulness) by ∼20% over the sham-lesioned and saline controls during both the light and the dark cycles (p < 0.01) (Fig. 4A, Table 1). There was a strong correlation between the number of TH-ir cells lost and the percentage increase of total sleep over 24 h (r = 0.91; p < 0.01) (Fig. 4B).
Figure 4.
Loss of dopaminergic neurons in the vPAG causes an increase in sleep time. A, The loss of TH-ir neurons in the vPAG after 6-hydroxydopamine lesions (white bars) caused increased sleep compared with control animals (black bars) during both the light and the dark cycle. **p < 0.01. B, The loss of TH-ir neurons in the vPAG correlated closely with the amount of sleep increase. Data are represented as mean ± SEM.
Table 1.
Amounts of sleep during light (L) and dark (D) cycles after vPAG lesions by 6-OHDA, 5,7-DHT, and ibotenic acid, compared with sham-lesioned control animals
|
|
L-NREM sleep (%) |
L-REM sleep (%) |
D-NREM sleep (%) |
D-REM sleep (%) |
|---|---|---|---|---|
| Control | 58.0 ± 5.3 | 7.2 ± 1.6 | 21.0 ± 3.5 | 5.2 ± 1.9 |
| 6-OHDA | 67.8 ± 7.8* ↑ | 9.5 ± 2.1* ↑ | 31.0 ± 4.2* ↑ | 6.2 ± 1.9 |
| 5,7-DHT | 56.3 ± 1.3 | 7.2 ± 1.0 | 28.0 ± .2.1 | 4.7 ± 0.8 |
| Ibotenic acid |
68.5 ± 8.7* ↑ |
16.0 ± 4.5** ↑ |
34.3 ± 3.0* ↑ |
4 ± 1.4 |
<0.05, **<0.01 by t test; ANOVA: L-NREM, p = 4.32 × 106;L-REM, p = 0.0017; D-REM, p = 0.015; D-REM, p = 0.18. Arrows indicate direction of significant difference.
Injection of ibotenic acid into the vPAG (n = 5) produced more TH-ir cell loss (up to 85%) and some 5-HT-ir cell loss (up to 30% of total 5-HT cells) in the rostral DRN. These animals showed an even greater increase (p > 0.05) in total sleep than in the 6-OHDA-lesioned rats (Table 1). The major difference was that REM sleep was mostly increased only during the day, and was similar to control animals at night.
Injection of 5,7-dihydroxytryptamine into the vPAG caused extensive loss (∼80%) of the serotoninergic cells but not TH-ir cells, and did not have significant effects on NREM, REM, or total sleep (n = 5) compared with the control animals (p > 0.05) (Table 1).
Efferent and afferent projections of wake-active dopaminergic neurons
Anterograde tracing of vPAG projections
To examine the projections of the vPAG, we injected the anterograde tracer BD (6 nl; 12%) into the region that contains TH-ir cells in five rats. As controls, in three additional rats, injections were placed into the region just ventral to the PAG and in three other animals, injections were placed more laterally.
Because the projections of the PAG and the DRN have been reported previously (Moore et al., 1978; Krout and Loewy, 2000), we will just summarize here our observations on vPAG projections. After injections into the vPAG, we consistently found ascending fibers coursing through the central gray matter and taking a paramedian course through the ventral part of the midbrain tegmentum. A paramedian pathway traveled along the medial edge of the medial forebrain bundle to reach the lateral hypothalamus and basal forebrain targets. A central gray pathway passed into the periventricular gray matter of the third ventricle. Descending axons traveled to the LDT and the LC through the periaqueductal gray matter.
There were prominent projections to a number of sites related to forebrain arousal and sleep regulation. The major targets included the prefrontal cortex (especially the infralimbic cortex) (Fig. 5A), the horizontal limb of the diagonal band nucleus (HDB) and the SI (Fig. 6C), the VLPO (Fig. 7), the perifornical region that contains orexin cells (Fig. 5C), the midline thalamus (Fig. 5B), and the intralaminar thalamus. Double staining showed that the anterogradely labeled boutons had close appositions with ChAT-ir neurons in the SI and the HDB (Fig. 6C), thionin-stained neurons in the VLPO, and orexin-ir cells in the lateral hypothalamus (Fig. 5C). In addition, in the brainstem, vPAG axons were found in apposition to ChAT-ir neurons in the LDT (Fig. 6B) but not in the PPT, and noradrenergic cells in the LC (Fig. 5D). The densities of anterogradely labeled fibers were heaviest in the perifornical orexin group and the cholinergic LDT, moderate in the SI cholinergic group, the VLPO, and the midline thalamus, and sparse in the medial prefrontal cortex, the intralaminar thalamus, and the LC. The vPAG also projected to the DRN and median raphe nucleus, as has been reported previously (Kalen et al., 1988). It is not possible at the light-microscopic level to determine which of these appositions represent synaptic connections. However, we conclude from this pattern of projection that the dopaminergic cells of the vPAG target multiple areas associated with wake–sleep regulation.
Figure 5.
Anterograde tracer BD (black color) injected into the vPAG labels axons projecting to the medial prefrontal cortex (MPFC; A), the intralaminar thalamus (Th; B), the orexin-ir (brown) neurons in the perifornical field (PeriF; C), and the LC (D). Arrows in the inset in C point to appositions onto orexin-ir cell bodies. Scale bar: (in D) A–D, 250 μm; inset, 100 μm.
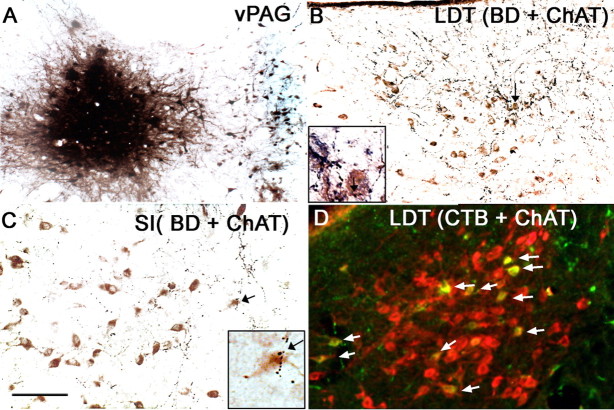
Figure 6.
Connections between the dopaminergic cells in the vPAG and cholinergic cells in the basal forebrain and the laterodorsal tegmental nucleus. After BD was injected into the vPAG (A; injection site), anterogradely labeled terminals apposed the ChAT-ir cells (brown) in the laterodorsal tegmental nucleus (B) and the substantia innominata (C; arrow points to a cell with multiple appositions, seen in the inset at higher magnification). CTB (green) injected into the vPAG was retrogradely transported to ChAT-ir cells (red) in the laterodorsal tegmental nucleus (D). Arrows point to doubled-labeled cells (yellow). Scale bar: (in C) A, 200 μm; B–D, 100 μm.
Figure 7.
Reciprocal connections between the wake-active dopaminergic cells in the vPAG and the sleep-active neurons in the VLPO. After CTB and BD together were injected into the vPAG, some CTB-labeled (brown) cells in the VLPO were also Fos-ir (black) in a sleeping rat (A; box is enlarged in B where the arrows indicate CTB–Fos double-labeled cells and the arrowheads show CTB single-labeled neurons). After an injection of both BD and CTB into the vPAG (C) in a sleeping rat, anterogradely labeled (black) terminals were apposed to double-labeled cells containing both CTB (brown cytoplasm) and Fos (black nucleus) in the VLPO (arrows). After an injection of anterograde tracer PHA-L into the VLPO (D), labeled terminals (black) formed appositions with the TH-ir cells in the vPAG (brown) (arrows indicate appositions). These results, together with Figure 8, D and D′, indicate a reciprocal connection of the dopaminergic cells in the vPAG and sleep-active neurons in the VLPO. Scale bar: (in C) A, 200 μm; B, 100 μm; C,50 μm; D,25 μm.
We also found smaller projections from the vPAG to the lateral septum, the piriform cortex, the lateral habenular nucleus, the zona incerta (including the A11 dopaminergic cell group), the bed nucleus of stria terminalis, the hippocampus, and the central amygdaloid nucleus. The contribution of dopaminergic inputs from the PAG to these projections has been identified previously (Pohle et al., 1984; Stratford and Wirtshafter, 1990; Li et al., 1993; Datiche et al., 1995; Hasue et al., 2002).
Retrograde tracing of vPAG projections
To verify that key projections from the vPAG indeed originate from the dopaminergic cells, we injected the retrograde tracer CTB (6 nl) into the medial prefrontal cortex, the VLPO, the midline and intralaminar thalamus, the SI, the perifornical region of the lateral hypothalamus, the LDT, and the LC.
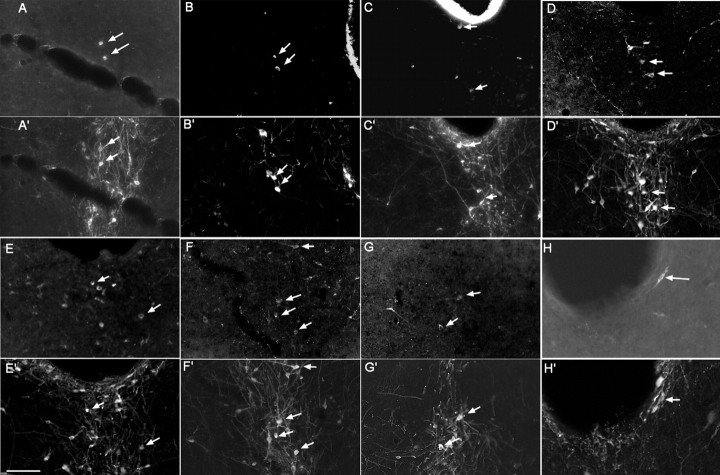
Injections into each of the targets produced retrogradely labeled neurons in the vPAG (Table 2, Fig. 8). After injections into the medial prefrontal cortex, the LC and the LDT, the majority (> 50%) of the retrogradely labeled cells in the vPAG were TH-ir. In the case of the SI, the VLPO, the midline and intralaminar thalamus, and the perifornical hypothalamus, the proportion of retrogradely labeled TH-ir neurons was lower, in the 30–45% range, but still substantial. The major targets of the vPAG dopaminergic neurons appeared to be the LHA and the LDT, because 10 and 25% of all vPAG TH-ir neurons were retrogradely labeled after injections into these sites. However, the SI (8%), LC (6%), and midline/paraventricular thalamus (5% each) also are substantial targets of the vPAG dopaminergic population.
Table 2.
Number of CTB–TH double-labeled neurons (per section) and as a percentage of total CTB-ir cells in the vPAG after injection in targets of the vPAG
|
CTB-injected regions |
CTB—TH-ir cells in PAG |
% of CTB-ir cells in PAG |
|---|---|---|
| Medial frontal cortex | 3.3 | 55.0 |
| VLPO | 3.6 | 33.5 |
| SI | 4.5 | 24.7 |
| Midline thalamus | 3.2 | 35.5 |
| Intralaminar thalamus | 5.5 | 47.6 |
| Perifornical hypothalamus | 5.8 | 45.1 |
| LDT | 17.0 | 54.9 |
| LC |
3.6 |
60.0 |
Figure 8.
Retrograde labeling of vPAG dopaminergic neurons after injections of CTB into axonal targets. Double labeling of retrograde tracer CTB (first panel in each pair) and TH immunoreactivity (second panel in each pair) in cells in the vPAG after CTB injection in the medial prefrontal cortex (A, A′), the midline paraventricular thalamus (B, B′), the intralaminar thalamus (C, C′), the VLPO (D, D′), the perifornical area (E, E′), the substantia innominata (F, F′), the LDT (G, G′), and the locus ceruleus (H, H′). Arrows point to double-labeled cells. Scale bar: (in E′) A–H′, 100 μm.
Retrograde tracing of vPAG afferents
To identify the afferents to the wake-active dopaminergic neurons, we injected CTB into the vPAG region. In five animals with injections confined to the vPAG, we found retrogradely labeled neurons in the VLPO (Fig. 7A,B) and the LC (Fig. 9C), and among the SI cholinergic cells, the lateral hypothalamic orexin neurons (Fig. 9A), the LDT cholinergic neurons (Fig. 6D), and the pyramidal cells in the medial prefrontal cortex (Fig. 9B). In the VLPO and medial prefrontal cortex, an average of ∼4.2 ± 0.7 and 39.4 ± 6.7 retrogradely labeled neurons per section were seen, respectively. In the LDT, there were ∼14.5 ± 1.1 double-labeled (CTB–ChAT) neurons per section; these represented ∼25% of ChAT-ir neurons. We did not see CTB and ChAT double-labeled cells in the SI, indicating that it is the noncholinergic cells in the SI that project to the vPAG. In the perifornical region, an average of 12.8 ± 2.3 double-labeled (CTB–orexin) cells were seen per section, which constituted ∼10% of orexinergic neurons and ∼4% of retrogradely labeled neurons in the lateral hypothalamic area. In the LC, an average of two neurons per section were retrogradely labeled, but this was <1% of the LC population.
Figure 9.
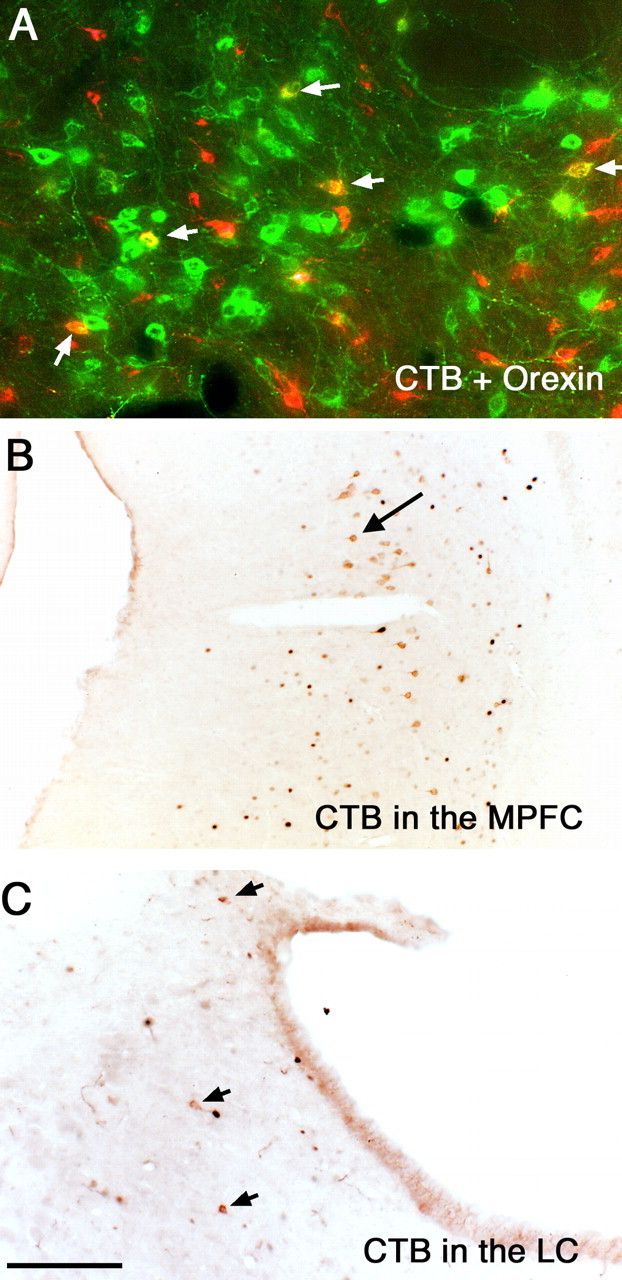
Sources of afferents to the vPAG. CTB injected into the vPAG retrogradely labels neurons (red) in the orexin-ir field (green) in the lateral hypothalamus. Arrows point to double-labeled neurons (yellow). After a similar injection, retrogradely labeled neurons (brown) are seen in the medial prefrontal cortex (B) and in the LC (C). Black fos staining is seen in both B and C in this sleeping animal. MPFC, Medial prefrontal cortex. Scale bar: (in C) A, 100 μm; B, C, 250 μm.
To further ascertain whether the projection to the vPAG from the VLPO originated from sleep-active neurons, at 10:00 A.M., we perfused three rats that had received CTB injections into the vPAG. Each rat had >70% total sleep time during the 2 h before perfusion, and about one-half of the CTB labeled cells in the VLPO in sleeping rats were also Fos positive (Fig. 7A–C). By comparison, very few Fos-positive cells are found in the VLPO during wakefulness (Sherin et al., 1996; Gaus et al., 2002).
Anterograde tracing of vPAG afferents
To confirm that afferents to the vPAG actually target dopaminergic neurons, we injected anterograde tracers into several of the regions identified above as vPAG afferents. PHA-L injections into the VLPO (n = 3) gave rise to many terminal boutons that apposed the TH-labeled cell bodies and dendrites in the vPAG (Fig. 7D). BD injections that filled the LDT (n = 2) (Fig. 10D), the LC (n = 3) (Fig. 10B), and the medial prefrontal cortex (n = 2) (Fig. 10C) also showed afferent labeled terminals apposing TH-ir cells in the vPAG. Because perifornical orexin-containing cells are the only sources of orexin in the brain, we double immunostained orexin (black) and TH (brown), and found that many orexin terminal boutons apposed TH-labeled cells in the vPAG region (Fig. 10A). However, the BD labeled fibers from the SI region appeared not to appose the TH-ir cells in the vPAG.
Figure 10.
Appositions of labeled afferents (black) onto the vPAG dopaminergic neurons (brown). Afferents are shown from orexin-ir neurons (A), the locus ceruleus (B), the medial prefrontal cortex (C), and the LDT (D). Arrows indicate terminal appositions with TH-ir cell bodies and dendrites. Scale bar: (in B) A–D,25 μm.
Discussion
We identified a population of dopaminergic neurons in the vPAG whose Fos immunoreactivity is closely related to previous wakefulness. These wake-active dopaminergic neurons project to major components of the wake–sleep regulatory system, including the midline and intralaminar thalamus, the medial prefrontal cortex, the basal forebrain cholinergic neurons, the VLPO, the lateral hypothalamic orexin/hypocretin cells, the pontine LDT cholinergic cells, and the LC. This same dopaminergic cell group receives afferents from most of the same sites, including the medial prefrontal cortex, the VLPO, the orexin cells, the LDT cholinergic cells, and the LC noradrenergic cells. 6-OHDA lesions that produce >60% loss of the wake-active dopaminergic cells (without affecting DRN serotoninergic cells, the VTA, or the SN) cause a significant and persistent 20% increase in total (NREM plus REM) sleep. Moreover we found that the percent loss of TH-ir cells in the vPAG significantly correlates with increases in total sleep time. We conclude that the identified wake-active dopaminergic cells may play a key role in promoting wakefulness. In addition, the presence of this dopaminergic cell group just adjacent to the DRN may have confounded results in many previous studies of the role of the DRN in sleep and wakefulness.
Technical consideration
One concern is whether 6-OHDA kills nondopaminergic neurons such as 5-HT cells. We used 6-OHDA at dosages that selectively damage catecholaminergic neurons (Sauer and Oertel, 1994) and produced little if any damage to the 5-HT cells in the vPAG. Ibotenic acid, which kills cell bodies but not fibers of passage, caused slightly greater increases in total sleep time, as well as more extensive loss of both dopaminergic and serotoninergic neurons in the vPAG. However, 5,7-DHT, which destroyed most of the dorsal raphe nucleus adjacent to the vPAG, had little or no effect on sleep, suggesting that the sleep increase is indeed attributable to the loss of dopaminergic cell bodies in the vPAG.
The doses of 6-OHDA that we used in this study only eliminated ∼70% of the TH-ir cells in the vPAG. It is possible that some of these dopaminergic cells may have low levels of the dopamine transporter (DAT), which is required to take up 6-OHDA into the dopaminergic cells. The 20% increase in total sleep after vPAG lesions is, thus, likely to underestimate the effects of the vPAG dopaminergic group on sleep. However, to put this finding into perspective, this change is much larger than changes in sleep that are seen after the lesions of the LC (Jones et al., 1977), the LDT (Webster and Jones, 1988), the DRN (Mouret and Coindet, 1980), the orexinergic cells (Hara et al., 2001), the basal forebrain cholinergic cells (Wenk et al., 1994), or the histaminergic tuberomammillary nucleus (Gerashchenko et al., 2004). Only large lesions of the lateral hypothalamic area (combining loss of orexinergic and other unidentified neurons) have been found to cause such substantial changes in wakefulness (Gerashchenko et al., 2001, 2003). Hence, it is likely that the vPAG dopaminergic neurons make a major contribution to maintaining wakefulness.
The role of the dopaminergic vPAG in the regulation of wakefulness
Dopaminergic neurons in the vPAG have been identified previously in rats (Hokfelt et al., 1984; Arsenault et al., 1988) and in humans (Saper and Petito, 1982). Because they share many efferent projections with the VTA (A10) dopaminergic neurons, such as to the ventral striatum and the prefrontal cortex (Stratford and Wirtshafter, 1990), they have been considered an extension of the A10 group. However, several unique anatomical and physiological traits of vPAG dopaminergic cells suggest that they constitute a distinct population. Compared with the VTA, vPAG dopaminergic cells have very little input to the ventral striatum but substantial input to the central nucleus of amygdala and the extended amygdala (Hasue and Shammah-Lagnado, 2002), whereas the VTA and SN do not project to the VLPO, which is a major target of the vPAG (Chou et al., 2002). Neurotoxic lesions of the VTA that spare the vPAG region cause a decrease rather than an increase in sleep in rats (Lai et al., 1999) and produce motor hyperactivity (Galey et al., 1977; Koob et al., 1981). However, 6-OHDA lesions that include both the VTA and the vPAG regions were found to produce a 20% increase in total sleep (Sakata et al., 2002), which is similar to our lesions of the vPAG alone. Finally, although dopaminergic cells in the VTA are responsive to behavioral stimuli (Horvitz et al., 1997; Lavin et al., 2005), they do not have a consistently wake-active firing pattern. Many neurons in the vPAG region are wake-active, but in the past it has been assumed that these cells were serotoninergic (Trulson and Jacobs, 1979). Because only the dopaminergic neurons in the vPAG have a distinctively wake-active pattern of Fos expression, this concept will clearly require re-evaluation.
The vPAG dopaminergic projections to the prefrontal cortex (Yoshida et al., 1989) and the midline and intralaminar thalamus (Takada et al., 1990; Freeman et al., 2001) have been identified previously. The dopamine transporter (Freeman et al., 2001) and D1 and D2 dopamine receptors (Huang et al., 1992; Lewis and O'Donnell, 2000) are present in both the thalamus and the cerebral cortex, suggesting that dopamine can directly influence thalamocortical activity. Similar dopaminergic projections have been reported in primates (Gaspar et al., 1992; Williams and Goldman-Rakic, 1998; Freeman et al., 2001; Sanchez-Gonzalez et al., 2005).
Our study also confirms the vPAG dopaminergic projections to the basal forebrain cholinergic cells (Eberhart et al., 1985; Semba et al., 1988), the noradrenergic LC (Ornstein et al., 1987), and the DRN (Kalen et al., 1988), as well as demonstrating these inputs to the lateral hypothalamic orexin cells, the LDT cholinergic cells, and to the VLPO. These projections provide a number of potential sites at which DA may influence sleep–wake regulation. DA has been reported to inhibit VLPO cells by activating α2 adrenoreceptors (Cornil et al., 2002; Gallopin et al., 2004), and stimulate cholinergic cells in the basal forebrain by D1/D5 receptors in vitro (Arrigoni et al., 2003).
Wake-active dopaminergic cells are under the control of the sleep-arousal system
Sherin et al. (1998) and Lu et al. (2002) demonstrated that the VLPO heavily innervates the lateral PAG and the vPAG/dorsal raphe nucleus; we now add dopaminergic neurons in the vPAG to this list of substantial VLPO targets. Because VLPO neurons are sleep-active and contain the inhibitory neurotransmitters GABA and galanin (Sherin et al., 1998; Lu et al., 2002), they would be expected to inhibit the wake-active dopaminergic neurons, promoting sleep. The mutually inhibitory circuitry of the VLPO and vPAG dopaminergic neurons should be added as a new component to the flip-flop switch for sleep–wake control (Saper et al., 2001, 2005).
Projections of the lateral hypothalamic orexinergic neurons, the DRN serotoninergic neurons (Kalen et al., 1988), the LDT cholinergic neurons, and the LC noradrenergic neurons to the dopaminergic cells in the vPAG suggest that these putative arousal systems may also work, in part, by influencing the activity of the dopaminergic arousal system. All of these neurotransmitters excite VTA dopaminergic neurons (Gronier and Rasmussen, 1998; Forster and Blaha, 2000; Lejeune and Millan, 2000; Uramura et al., 2001) and, hence, are probably also excitatory in the vPAG.
Can dopaminergic cells in the vPAG account for the arousal effects of drugs that block dopamine reuptake?
Many drugs that promote wakefulness, such as amphetamine, cocaine, and modafinil, block dopamine reuptake by the DAT, and mice in which the DAT gene is deleted do not respond to these arousing drugs (Wisor et al., 2001). However, the substrate of this dopaminergic influence on wakefulness has not been understood. Our results suggest that the vPAG dopaminergic system may provide a basis for this influence.
Possible substrates for the vPAG dopaminergic influence on wakefulness include excitation of the prefrontal cortex (Luoh et al., 1994), the lateral hypothalamus (particularly the orexin neurons), and the basal forebrain (particularly the cholinergic neurons), which are thought to promote wakefulness, as well as inhibition of the VLPO, which would prevent sleep. Of course, contributions from other A8–A10 dopaminergic fields (e.g., to the prefrontal cortex and the reticular nucleus of the thalamus) (Freeman et al., 2001), may also play a role in the response to stimulants, even if they are less important for maintaining baseline arousal.
Is loss of wake-active dopaminergic cells responsible for the sleep disorders seen in Parkinson's disease?
Patients with Parkinson's disease suffer loss of pigmented dopamine cells in the DRN/vPAG (Jellinger, 1999), and the loss of these neurons may play a role in the excessive daytime sleepiness seen in Parkinson patients (Matheson and Saper, 2003). However, periodic limb movements and REM behavior disorder seen during sleep in some Parkinson patients appear to be attributable to damage to descending dopaminergic projections that may regulate REM sleep motor phenomena, particularly atonia. The role of the vPAG in this system remains to be determined.
In summary, our observations indicate the presence of a powerful vPAG dopaminergic arousal system. The connections of these neurons, their wake-active activity pattern, and the substantial increases in sleep after they are damaged, all support this hypothesis. The vPAG dopamine neurons may play an important role both in sleepiness in Parkinson's disease and in the response to wake-promoting drugs.
Footnotes
This work was supported by Grants MHS051609, MH55772, AG09975, and HL60292. We thank Quan Hue Ha and Minh Ha for technical expertise.
Correspondence should be addressed to Dr. Jun Lu, Department of Neurology, Beth Israel Deaconess Medical Center, 77 Avenue Louis Pasteur, #819, Boston, MA 02215. E-mail: jlu@bidmc.harvard.edu.
DOI:10.1523/JNEUROSCI.2244-05.2006
Copyright © 2006 Society for Neuroscience 0270-6474/06/260193-10$15.00/0
References
- Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94: 239–247. [DOI] [PubMed] [Google Scholar]
- Arnulf I, Konofal E, Merino-Andreu M, Houeto JL, Mesnage V, Welter ML, Lacomblez L, Golmard JL, Derenne JP, Agid Y (2002) Parkinson's disease and sleepiness: an integral part of PD. Neurology 58: 1019–1024. [DOI] [PubMed] [Google Scholar]
- Arrigoni E, Lu J, Chamberlin NL, Saper CB (2003) Dopamine induces excitation of the basal forebrain cholinergic neurons. APSS Abstr 1032.A.
- Arsenault MY, Parent A, Seguela P, Descarries L (1988) Distribution and morphological characteristics of dopamine-immunoreactive neurons in the midbrain of the squirrel monkey (Saimiri sciureus). J Comp Neurol 267: 489–506. [DOI] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB (2002) Afferents to the ventrolateral preoptic nucleus. J Neurosci 22: 977–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J (2003) Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci 23: 10691–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V (2002) Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci 22: 9320–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datiche F, Luppi PH, Cattarelli M (1995) Serotonergic and nonserotonergic projections from the raphe nuclei to the piriform cortex in the rat: a cholera toxin B subunit (CTb) and 5-HT immunohistochemical study. Brain Res 671: 27–37. [DOI] [PubMed] [Google Scholar]
- Eberhart JA, Morrell JI, Krieger MS, Pfaff DW (1985) An autoradiographic study of projections ascending from the midbrain central gray, and from the region lateral to it, in the rat. J Comp Neurol 241: 285–310. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD (2000) Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci 12: 3596–3604. [DOI] [PubMed] [Google Scholar]
- Freeman A, Ciliax B, Bakay R, Daley J, Miller RD, Keating G, Levey A, Rye D (2001) Nigrostriatal collaterals to thalamus degenerate in parkinsonian animal models. Ann Neurol 50: 321–329. [DOI] [PubMed] [Google Scholar]
- Galey D, Simon H, Le Moal M (1977) Behavioral effects of lesions in the A10 dopaminergic area of the rat. Brain Res 124: 83–97. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Luppi PH, Rambert FA, Frydman A, Fort P (2004) Effect of the wake-promoting agent modafinil on sleep-promoting neurons from the ventrolateral preoptic nucleus: an in vitro pharmacologic study. Sleep 27: 19–25. [PubMed] [Google Scholar]
- Gaspar P, Stepniewska I, Kaas JH (1992) Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J Comp Neurol 325: 1–21. [DOI] [PubMed] [Google Scholar]
- Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB (2002) Ventrolateral preoptic nucleus contains sleep-active, galanin-immunoreactive neurons in multiple mammalian species. Neuroscience 115: 285–294. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ (2001) Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci 21: 7273–7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Blanco-Centurion C, Greco MA, Shiromani PJ (2003) Effects of lateral hypothalamic lesion with the neurotoxin hypocretin-2-saporin on sleep in Long–Evans rats. Neuroscience 116: 223–235. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Chou TC, Blanco-Centurion CA, Saper CB, Shiromani PJ (2004) Effects of lesions of the histaminergic tuberomammillary nucleus on spontaneous sleep in rats. Sleep 27: 1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco MA, Lu J, Wagner D, Shiromani PJ (2000) c-Fos expression in the cholinergic basal forebrain after enforced wakefulness and recovery sleep. NeuroReport 11: 437–440. [DOI] [PubMed] [Google Scholar]
- Gronier B, Rasmussen K (1998) Activation of midbrain presumed dopaminergic neurones by muscarinic cholinergic receptors: an in vivo electrophysiological study in the rat. Br J Pharmacol 124: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T (2001) Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30: 345–354. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ (2002) Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol 454: 15–33. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Martensson R, Bjorklund A, Kleinau S, Goldstein M (1984) Distribution of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Handbook of chemical neuroanatomy (Bjorklund A, Hokfelt T, eds), pp 277–379. Amsterdam: Elsevier Science.
- Horvitz JC, Stewart T, Jacobs BL (1997) Burst activity of ventral tegmental dopamine neurons is elicited by sensory stimuli in the awake cat. Brain Res 759: 251–258. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhou D, Chase K, Gusella JF, Aronin N, DiFiglia M (1992) Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc Natl Acad Sci USA 89: 11988–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA (1999) Post mortem studies in Parkinson's disease: is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl 56: 1–29. [DOI] [PubMed] [Google Scholar]
- Jones BE, Harper ST, Halaris AE (1977) Effects of locus coeruleus lesions upon cerebral monoamine content, sleep-wakefulness states and the response to amphetamine in the cat. Brain Res 124: 473–496. [DOI] [PubMed] [Google Scholar]
- Kalen P, Skagerberg G, Lindvall O (1988) Projections from the ventral tegmental area and mesencephalic raphe to the dorsal raphe nucleus in the rat. Evidence for a minor dopaminergic component. Exp Brain Res 73: 69–77. [DOI] [PubMed] [Google Scholar]
- Kanbayashi T, Honda K, Kodama T, Mignot E, Nishino S (2000) Implication of dopaminergic mechanisms in the wake-promoting effects of amphetamine: a study of d- and l-derivatives in canine narcolepsy. Neuroscience 99: 651–659. [DOI] [PubMed] [Google Scholar]
- Ko EM, Estabrooke IV, McCarthy M, Scammell TE (2003) Wake-related activity of tuberomammillary neurons in rats. Brain Res 992: 220–226. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M (1981) Hyperactivity and hypoactivity produced by lesions to the mesolimbic dopamine system. Behav Brain Res 3: 341–359. [DOI] [PubMed] [Google Scholar]
- Krout KE, Loewy AD (2000) Periaqueductal gray matter projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol 424: 111–141. [DOI] [PubMed] [Google Scholar]
- Lai YY, Shalita T, Hajnik T, Wu JP, Kuo JS, Chia LG, Siegel JM (1999) Neurotoxic N-methyl-d-aspartate lesion of the ventral midbrain and mesopontine junction alters sleep-wake organization. Neuroscience 90: 469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JP, Tandberg E (2001) Sleep disorders in patients with Parkinson's disease: epidemiology and management. CNS Drugs 15: 267–275. [DOI] [PubMed] [Google Scholar]
- Lavin A, Nogueira L, Lapisch CC, Wightman RM, Phillips PE, Seamans JK (2005) Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci 25: 5013–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune F, Millan MJ (2000) Pindolol excites dopaminergic and adrenergic neurons, and inhibits serotonergic neurons, by activation of 5-HT1A receptors. Eur J Neurosci 12: 3265–3275. [DOI] [PubMed] [Google Scholar]
- Lewis BL, O'Donnell P (2000) Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential “up” states in pyramidal neurons via D1 dopamine receptors. Cereb Cortex 10: 1168–1175. [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Shinonaga Y, Mizuno N (1993) The sites of origin of dopaminergic afferent fibers to the lateral habenular nucleus in the rat. J Comp Neurol 333: 118–133. [DOI] [PubMed] [Google Scholar]
- Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, Saper CB (2002) Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci 22: 4568–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoh HF, Kuo TB, Chan SH, Pan WH (1994) Power spectral analysis of electroencephalographic desynchronization induced by cocaine in rats: correlation with microdialysis evaluation of dopaminergic neurotransmission at the medial prefrontal cortex. Synapse 16: 29–35. [DOI] [PubMed] [Google Scholar]
- Matheson JK, Saper CB (2003) REM sleep behavior disorder: a dopaminergic deficiency disorder? Neurology 61: 1328–1329. [DOI] [PubMed] [Google Scholar]
- Miller JD, Farber J, Gatz P, Roffwarg H, German DC (1983) Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res 273: 133–141. [DOI] [PubMed] [Google Scholar]
- Moore RY, Halaris AE, Jones BE (1978) Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol 180: 417–438. [DOI] [PubMed] [Google Scholar]
- Mouret J, Coindet J (1980) Polygraphic evidence against a critical role of the raphe nuclei in sleep in the rat. Brain Res 186: 273–287. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mao J, Sampathkumaran R, Shelton J (1998) Increased dopaminergic transmission mediates the wake-promoting effects of CNS stimulants. Sleep Res Online 1: 49–61. [PubMed] [Google Scholar]
- Ornstein K, Milon H, McRae-Degueurce A, Alvarez C, Berger B, Wurzner HP (1987) Biochemical and radioautographic evidence for dopaminergic afferents of the locus coeruleus originating in the ventral tegmental area. J Neural Transm 70: 183–191. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. San Diego: Academic.
- Pohle W, Ott T, Muller-Welde P (1984) Identification of neurons of origin providing the dopaminergic innervation of the hippocampus. J Hirnforsch 25: 1–10. [PubMed] [Google Scholar]
- Sakata M, Sei H, Toida K, Fujihara H, Urushihara R, Morita Y (2002) Mesolimbic dopaminergic system is involved in diurnal blood pressure regulation. Brain Res 928: 194–201. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C (2005) The primate thalamus is a key target for brain dopamine. J Neurosci 25: 6076–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Petito CK (1982) Correspondence of melanin-pigmented neurons in human brain with A1–A14 catecholamine cell groups. Brain 105: 87–101. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE (2001) The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 24: 726–731. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J (2005) Hypothalamic regulation of sleep and circadian rhythms. Nature 437: 1257–1263. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH (1994) Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 59: 401–415. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB (2000) Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci 20: 8620–8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Reiner PB, McGeer EG, Fibiger HC (1988) Brainstem afferents to the magnocellular basal forebrain studied by axonal transport, immunohistochemistry, and electrophysiology in the rat. J Comp Neurol 267: 433–453. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB (1996) Activation of ventrolateral preoptic neurons during sleep. Science 271: 216–219. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB (1998) Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci 18: 4705–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfels GF, Heym J, Strecker RE, Jacobs B (1983) Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res 258: 217–228. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D (1990) Ascending dopaminergic projections from the dorsal raphe nucleus in the rat. Brain Res 511: 173–176. [DOI] [PubMed] [Google Scholar]
- Takada M, Campbell KJ, Moriizumi T, Hattori T (1990) On the origin of the dopaminergic innervation of the paraventricular thalamic nucleus. Neurosci Lett 115: 33–36. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL (1979) Raphe unit activity in freely moving cats: Correlation with level of behavioral arousal. Brain Res 163: 135–150. [DOI] [PubMed] [Google Scholar]
- Uramura K, Funahashi H, Muroya S, Shioda S, Takigawa M, Yada T (2001) Orexin-a activates phospholipase C- and protein kinase C-mediated Ca2+ signaling in dopamine neurons of the ventral tegmental area. NeuroReport 12: 1885–1889. [DOI] [PubMed] [Google Scholar]
- Webster HH, Jones BE (1988) Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res 458: 285–302. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Stoehr JD, Quintana G, Mobley S, Wiley RG (1994) Behavioral, biochemical, histological, and electrophysiological effects of 192 IgG-saporin injections into the basal forebrain of rats. J Neurosci 14: 5986–5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS (1998) Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex 8: 321–345. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM (2001) Dopaminergic role in stimulant-induced wakefulness. J Neurosci 21: 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Shirouzu M, Tanaka M, Semba K, Fibiger HC (1989) Dopaminergic neurons in the nucleus raphe dorsalis innervate the prefrontal cortex in the rat: a combined retrograde tracing and immunohistochemical study using anti-dopamine serum. Brain Res 496: 373–376. [DOI] [PubMed] [Google Scholar]