Abstract
Cannabinoids exert their psychomotor actions through the CB1 cannabinoid receptor in the brain. Genetic deletion of CB1 in mice causes various symptoms, including changes in locomotor activity, increased ring catalepsy, supraspinal hypoalgesia, and impaired memory extinction. Although the cerebellar cortex contains the highest level of CB1, severe cerebellum-related functional deficits have not been reported in CB1 knock-out mice. To clarify the roles of CB1 in cerebellar function, we subjected CB1 knock-out mice to a delay version of classical eyeblink conditioning. This paradigm is a test for cerebellum-dependent discrete motor learning, in which conditioned stimulus (CS) (352 ms tone) and unconditioned stimulus (US) (100 ms periorbital electrical shock) are coterminated. We found that delay eyeblink conditioning performance was severely impaired in CB1 knock-out mice. In contrast, they exhibited normal performance in a trace version of eyeblink conditioning with 500 ms stimulus-free interval intervened between the CS offset and the US onset. This paradigm is a test for hippocampus-dependent associative learning. Sensitivity of CB1 knock-out mice to CS or US was normal, suggesting that impaired delay eyeblink conditioning is attributable to defects in association of responses to CS and US. We also found that intraperitoneal injection of the CB1 antagonist SR141716A [N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazole carboxamide] to wild-type mice caused severe impairment in acquisition but not extinction of delay eyeblink conditioning. SR141716A treatment had no effect on trace eyeblink conditioning with a 500 or 750 ms trace interval. These results indicate that endogenous cannabinoid signaling through CB1 is essential for cerebellum-dependent discrete motor learning, especially for its acquisition.
Keywords: CB1 cannabinoid receptor, endocannabinoid, delay eyeblink conditioning, trace eyeblink conditioning, motor learning, associative learning, knock-out mice, cerebellum, Purkinje cell
Introduction
Inhalation of marijuana causes various psychomotor symptoms, including hypoalgesia, modulation of locomotor activity and control, and impairment of cognition and memory (Ameri, 1999). Receptors for Δ9-tetrahydrocannabinol, the psychoactive component of marijuana, belong to the seven-transmembrane-domain G-protein-coupled receptors and consist of two types, namely the CB1 and CB2 cannabinoid receptors (Matsuda et al., 1990; Munro et al., 1993; Howlett et al., 2002). Psychomotor actions of marijuana are mostly mediated through the interaction of Δ9-tetrahydrocannabinol with the CB1 cannabinoid receptor (Elphick and Egertova, 2001; Freund et al., 2003; Piomelli, 2003).
CB1 is abundant in the axons and axon terminals of subsets of central neurons (Tsou et al., 1998; Egertova and Elphick, 2000; Freund et al., 2003). It has been established that endogenous ligands for the cannabinoid receptors (endocannabinoids) are released from postsynaptic neurons, act retrogradely onto presynaptic CB1, and cause short-term or long-term suppression of transmitter release (Maejima et al., 2001a; Alger, 2002; Kreitzer and Regehr, 2002; Wilson and Nicoll, 2002; Kano et al., 2003). Endocannabinoids also control the excitability of neocortical GABAergic interneurons in an autocrine manner (Bacci et al., 2004).
At the behavioral level, CB1 knock-out mice have been reported to display various symptoms (Ledent et al., 1999; Steiner et al., 1999; Zimmer et al., 1999; Marsicano et al., 2002), including changes in locomotor activity (Steiner et al., 1999; Zimmer et al., 1999), increased ring catalepsy (Zimmer et al., 1999), supraspinal hypoalgesia (Zimmer et al., 1999), impaired extinction of aversive memory (Marsicano et al., 2002) and spatial memory (Varvel and Lichtman, 2002), and reduction of some aspects of anxiety (Degroot and Nomikos, 2004). The diversity of the symptoms reflects the wide distribution of CB1 in the brain (Tsou et al., 1998; Egertova and Elphick, 2000; Freund et al., 2003). However, severe cerebellum-related functional deficits have not been reported in CB1 knock-out mice, although the cerebellar cortex contains the highest level of CB1 in the brain (Tsou et al., 1998; Egertova and Elphick, 2000; Freund et al., 2003). Multiple forms of endocannabinoid-mediated retrograde modulation are found in both excitatory (Kreitzer and Regehr, 2001a; Maejima et al., 2001b, 2005; Brown et al., 2003; Brenowitz and Regehr, 2005) and inhibitory (Kreitzer and Regehr, 2001b; Diana et al., 2002; Yoshida et al., 2002) synapses in the cerebellar cortex. This apparent discrepancy between the behavioral and morphological/electrophysiological data led us to examine delay paradigm of classical eyeblink conditioning, a form of cerebellum-dependent discrete motor learning (McCormick and Thompson, 1984; Thompson et al., 1997), in CB1 knock-out mice. Delay eyeblink conditioning is impaired in several mouse models with lesions in cerebellar circuitry, spontaneous degeneration of Purkinje cells (PCs) (Chen et al., 1996, 1999), or gene deletions that result in defects in cerebellar synaptic function (Aiba et al., 1994; Shibuki et al., 1996; Kishimoto et al., 2001b,c, 2002; Miyata et al., 2001). Our present results indicate that CB1-mediated endocannabinoid signaling is specifically involved in discrete motor learning in the cerebellum.
Materials and Methods
Animals.
Breeding pairs of CB1 knock-out mice that have been backcrossed to the C57BL/6J strain were kindly provided by A. Zimmer (Molecular Neurobiology, University of Bonn, Bonn, Germany) (Zimmer et al., 1999). The CB1 knock-out mice (CB1−/−) and their littermate controls (CB1+/+) were obtained by intercrossing heterozygous (CB1+/−) breeding pairs. After behavioral analyses, genotypes of the mice were determined using tail biopsies and PCR amplification. For pharmacological experiments, we obtained C57BL/6J mice from SLC (Hamamatsu, Japan). All subjects were individually housed in a room and maintained under a 12 h light/dark cycle (lights off at 10:00 P.M.) with food and water available ad libitum. All behavioral tests were performed by operators who were blind to the genotype of the mice. During the course of the present study, the care of the animals conformed to the guidelines established by the Animal Investigation Committee of Osaka University, Kanazawa University, and the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Eyeblink classical conditioning.
Mice underwent surgery at the age of 8–18 postnatal weeks, and eyeblink conditioning experiments started at the age of 11–19 weeks (for experiments with CB1 knock-out mice) or 9–16 weeks (for pharmacological experiments). Surgery was made according to the procedure described previously (Kishimoto et al., 2001a,b, 2002). Mice were anesthetized with ketamine (80 mg/kg, i.p.; Sankyo, Tokyo, Japan) and xylazine (20 mg/kg, i.p.; Bayer, Tokyo, Japan). Four Teflon-coated stainless-steel wires (100 μm in diameter; A-M Systems, Carlsborg, WA) were implanted subcutaneously under the left eyelid. Two of the wires were used to record electromyogram (EMG) in the orbicuralis oculi muscle, which is responsible for eyeblink, and the remaining two to deliver electrical shocks.
At least 3 d were allotted for recovery and 1 d for acclimation to the conditioning chamber (∼50 min, with no stimulus) after the surgery. Tone with 352 ms duration (1 kHz, 80 dB) was used as conditioned stimulus (CS), and electrical shock with 100 ms duration (100 Hz square pulses) was as unconditioned stimulus (US). In the delay paradigm, the US overlapped the CS in time such that the two stimuli terminate simultaneously. The delay conditioning experiment consisted of a 7 d acquisition phase and a 4 d extinction phase (Kishimoto et al., 2001b,c). In the trace paradigm, the US started 500 or 750 ms after termination of the CS. The trace conditioning experiment consisted of a 10 d acquisition phase and a 4 d extinction phase (Kishimoto et al., 2001a, 2002, 2006). In pseudoconditioning, CS and US were randomly presented with an interstimulus interval ranging from 0 to 20 s. The US intensity was carefully determined as the minimal current amplitude required for eliciting an eyeblink response and constant amplitude of unconditioned response (UR). The US intensity was adjusted daily for each animal. A daily training consisted of 100 trials grouped in 10 blocks. The acquisition sessions consisted of 10 CS-only (every 10th trial) and 90 CS–US paired trials. The extinction sessions consisted of 100 CS-only trials. The intertrial interval was randomized between 20 and 40 s, with a mean of 30 s.
Spontaneous eyeblink frequency was measured during 100 “no stimulus” trials in the first session for acclimation before the conditioning experiment began. Startle response to a 352 ms tone was measured during the first 100 trials of the first session of delay eyeblink conditioning. UR amplitude was defined as the EMG amplitude at 50 ms after the US onset.
All experiments were performed during the light phase of the light/dark cycle in a container (10 cm in diameter) placed in a sound- and light-attenuating chamber. Data were analyzed as described previously (Kishimoto et al., 2001a,b, 2002).
Pharmacological blockade of CB1 receptors.
Male C56BL/6J mice (SLC) were used in all pharmacological experiments. For pharmacological blockade of CB1, the CB1 antagonist SR141716A [N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazole carboxamide] (3 mg/kg, i.p.) was dissolved in solution (3 ml of 2.5% dimethylsulfoxide and 10% Cremophor in saline) and administered to mice 20 min before the daily training. This dose of SR141716A were close to ED50 values shown for other functions (Rinaldi-Carmona et al., 1995; Compton et al., 1996) and similar to those used in previous studies on learning in mice (Marsicano et al., 2002; Suzuki et al., 2004). SR141716A was a generous gift from Sanofi (Montpellier, France).
Rotarod and fixed-bar tests.
The rotarod test and fixed-bar test were performed as described previously (Kadotani et al., 1996). The rotarod (Muromachi Kikai, Kyoto, Japan) consisted of a gritted metal roller (3 cm in diameter). Mice at the age of 10–18 postnatal weeks (for experiments with CB1 knock-out mice) or 8–15 postnatal weeks (for pharmacological experiments) were placed on the roller rotated at 25 rpm, and the time it remained on the rotating roller was measured. A maximum of 60 s was allowed per mouse. In the fixed-bar test, the time the animal remained on the wooden bar (5 mm in width and 40 cm above the ground) was measured. A maximum of 60 s was allowed per mouse.
Statistical analysis.
All data are presented as means ± SEM. Data were statistically analyzed by a two-tailed Student’s t test using the Microsoft (Seattle, WA) Excel program or by a two-way or repeated-measures ANOVA using the SPSS 6.1 program (SPSS, Chicago, IL). A post hoc comparison was made with the Scheffé’s test. The difference was considered significant when p was <0.05.
Results
Eyeblink conditioning experiments can be categorized into two distinct types: delay and trace paradigms (Thompson and Kim, 1996). In the delay paradigm, there is a temporal overlap of US with preceding CS, whereas in the trace paradigm, a stimulus-free interval intervenes between the CS offset and the US onset. It has been shown that delay eyeblink conditioning is dependent on the cerebellum (McCormick and Thompson, 1984; Thompson et al., 1997), whereas trace eyeblink conditioning with a long trace interval requires the hippocampus and cerebellum in rabbits and rats (Moyer et al., 1990; Weiss et al., 1999; Kishimoto et al., 2006). In the present study, we applied these two paradigms to wild-type and CB1 knock-out mice.
Impaired acquisition of delay eyeblink conditioning in CB1 knock-out mice
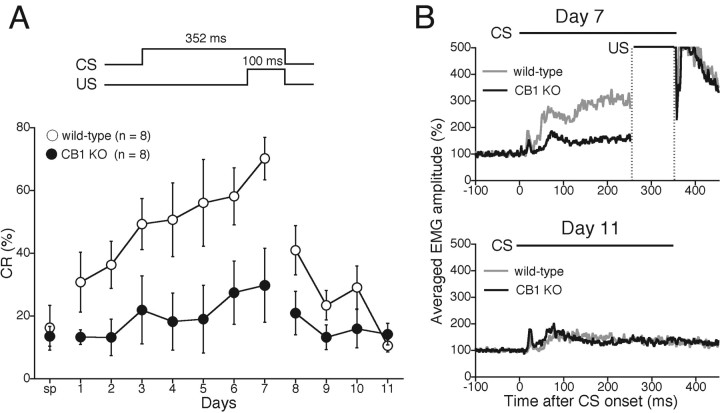
We began by investigating delay eyeblink conditioning in wild-type and CB1 knock-out mice. A 100 ms periorbital shock (100 Hz square pulses) was used as US, and a 352 ms tone (1.0 kHz, 80 dB) was used as CS. In the delay paradigm, the tone CS preceded the US and coterminated with the US, which yielded an interstimulus interval of 252 ms (Fig. 1A) (Kishimoto et al., 2001b,c, 2002). The acquisition session continued for 7 consecutive days, followed by the extinction session for 4 d. Figure 1A shows averaged percentage of successful trials of the conditioned response (CR%) in wild-type and CB1 knock-out mice during the 7 d acquisition and 4 d extinction sessions. On a day before the training, spontaneous eyeblink frequency (Fig. 1A, sp) was measured in the conditioning chamber without CS or US. The CR% for wild-type mice progressively increased to >70% during the 7 d acquisition session. In contrast, the CR% for CB1 knock-out mice failed to increase during the 7 d acquisition session. Thus, CR% for CB1 knock-out mice was significantly lower than that for wild-type mice (genotype, F(1,14) = 12.07, p = 0.0037; session and genotype interaction, F(6,98) = 0.498, p = 0.808). After the acquisition phase, extinction training with CS-only trials was performed. In this extinction phase, a significant interaction effect was revealed (F(3,56) = 3.648, p = 0.020). These results clearly indicate that acquisition of delay eyeblink conditioning is severely impaired in CB1 knock-out mice.
Figure 1.
Severe impairment in cerebellum-dependent delay eyeblink conditioning in CB1 knock-out mice. A, Development of CR during delay eyeblink conditioning in wild-type (open circles; n = 8) and CB1 knock-out (KO) (filled circles; n = 8) mice. In the acquisition phase from day 1 to day 7, daily session consists of 10 blocks of trials. Each block consists of nine CS–US paired trials and one CS-only trial. In the following 4 d extinction phase, 100 CS-only presentations were given daily. Temporal relationship between CS and US is depicted at the top. sp, Spontaneous eyeblink response. B, The averaged EMG amplitudes of eyeblink response for wild-type (gray trace) and CB1 knock-out (black trace) mice on day 7 (top) and day 11 (bottom). The top indicates significantly lower EMG response to tone CS during acquisition phase in CB1 knock-out mice. Experiments were performed with CB1 knock-out mice and their wild-type littermates at 11–19 weeks of age.
During eyeblink conditioning, animals learn the adaptive timing of CR expression. To illustrate the temporal pattern of CR, EMGs were averaged over the valid trials for each day and each mouse (Kishimoto et al., 2002). The averaged EMG data for each mouse were then accumulated for each genotypic group, and the resultant accumulated EMG was expressed by normalizing the level during 100 ms before CS onset (Fig. 1B). The accumulated EMG amplitude on day 7 for CB1 knock-out mice was significantly lower than that for the wild-type mice because of the lower CR%. Nevertheless, no difference was observed in the temporal pattern of EMG between the two groups on day 7 (Fig. 1B). The accumulated EMGs of CS-only trials on day 11 demonstrate that there was also no difference between the two groups after extinction learning.
Normal sensitivity to US and CS and normal performance of eyeblink in CB1 knock-out mice
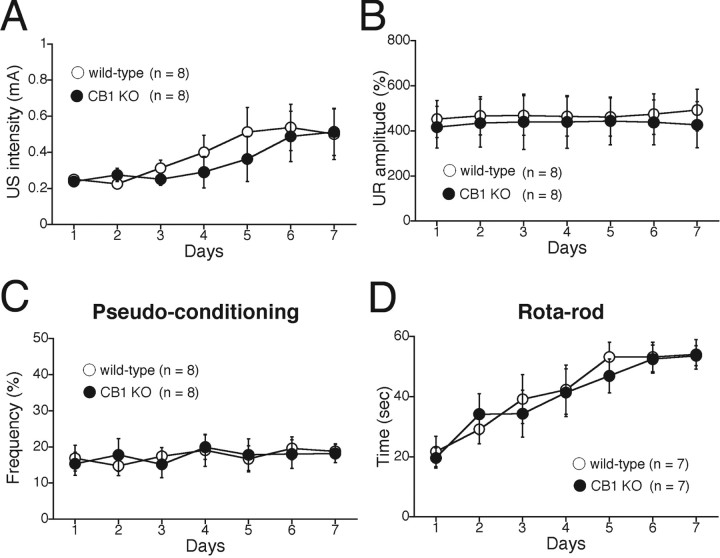
We examined whether the sensitivity to CS and US and the performance of eyeblink reflex differed between wild-type and CB1 knock-out mice (Kishimoto et al., 2002, 2006). First, we confirmed that there was no significant difference in spontaneous eyeblink frequency between wild-type and CB1 knock-out mice (Fig. 1A, sp). Second, we found that there was no significant difference in the frequency of startle response to the tone CS between the two genotypic groups (wild-type mice, 1.62 ± 0.62%, n = 8; CB1 knock-out mice, 0.92 ± 0.39%, n = 8; p > 0.05). Third, we measured the minimal intensity of US required for eliciting an eyeblink response during the 7 d acquisition phase (Fig. 2A). No significant difference was detected between the two groups (genotype, F(1,14) = 0.497, p = 0.492; session and genotype interaction, F(6,98) = 0.418, p = 0.865). Fourth, to check the performance of eyeblink reflex itself, we analyzed the amplitude of UR during the 7 d acquisition session (Fig. 2B). No significant difference was detected between wild-type and CB1 knock-out mice (genotype, F(1,14) = 0.222, p = 0.645; session and genotype interaction, F(6,98) = 0.345, p = 0.911). Next, to check nonassociative cheek movement, we tested pseudoconditioning with pseudorandomized presentations of CS and US (Fig. 2C). We found no significant difference between the two genotypic groups (genotype, F(1,14) = 2.69, p = 0.123; session and genotype interaction, F(6,98) = 0.38, p = 0.89). These results clearly indicate that the sensitivity to CS and US and the performance of eyeblink reflex are intact in CB1 knock-out mice.
Figure 2.
Normal sensitivity to stimuli and normal motor coordination in CB1 knock-out mice. A, The minimum US intensity (milliamperes) required for eliciting a UR in delay eyeblink conditioning. No difference was observed between wild-type (n = 8) and CB1 knock-out (KO) (n = 8) mice. B, Averaged UR amplitudes for wild-type (n = 8) and CB1 knock-out (n = 8) mice during delay eyeblink conditioning. In both genotypic groups, the UR amplitudes were nearly constant throughout the 7 d acquisition phase. No difference was observed between wild-type and CB1 knock-out mice. C, The eyeblink frequency during the pseudoconditioning did not increase in either wild-type mice (n = 8) or CB1 knock-out mice (n = 8). The CS and US were pseudorandomly presented with an interstimulus interval ranging from 0 to 20 s. D, Rotarod test. The stay time (seconds) of the mice on a rotating rod (25 rpm) is plotted versus the training day. There was no significant difference between wild-type (n = 7) and CB1 knock-out (n = 7) mice. Experiments were performed with CB1 knock-out mice and their wild-type littermates at 10–18 weeks of age.
Normal motor coordination in CB1 knock-out mice
We examined motor coordination of mature mice at the age of 10–18 postnatal weeks by using the fixed-bar test and the rotarod test (Fig. 2D). There was no significant difference in the retention time on the fixed bar between wild-type (50.29 ± 7.18 s; n = 6) and CB1 knock-out (52.01 ± 4.23 s; n = 6) mice (p > 0.05). There was also no significant difference in the retention time on the rotating rod between wild-type and CB1 knock-out mice during 7 consecutive days of examination (Fig. 2D) (genotype, F(1, 2) = 0.146, p = 0.709; session and genotype interaction, F(6,84) = 0.20, p = 0.976, for the rotarod test).
Normal acquisition and extinction of trace eyeblink conditioning in CB1 knock-out mice
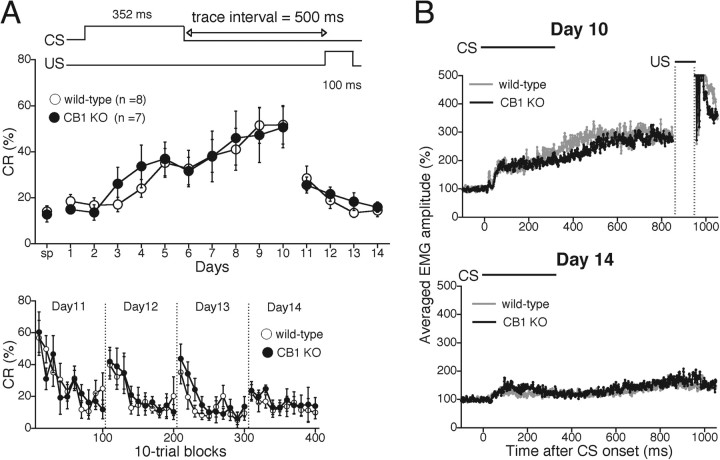
Next, we examined whether a trace version of eyeblink conditioning, a form of hippocampus-dependent associative learning (Moyer et al., 1990; Weiss et al., 1999; Kishimoto et al., 2006), was also impaired in CB1 knock-out mice. In the trace paradigm, a 500 ms stimulus-free interval intervened between the offset of 352 ms tone CS and the onset of 100 ms US (Fig. 3A, inset) (Kishimoto et al., 2001a,c, 2002). The acquisition session continued for 10 consecutive days, followed by the extinction session for 4 d. Figure 3A (top) shows the daily averaged percentage of successful CR trials in wild-type and CB1 knock-out mice during the 14 d of testing. The CR% for both wild-type and CB1 knock-out mice increased progressively to >50% during the 10 d acquisition session. Thus, in contrast to the delay conditioning paradigm, acquisition of CR for the trace paradigm is intact in CB1 knock-out mice (genotype, F(1,13) = 0.0783, p = 0.784; session and genotype interaction, F(6,91) = 0.253, p = 0.985). Furthermore, during the 4 d extinction session, the extinction effect was normal in CB1 knock-out mice compared with wild-type mice (genotype, F(1,13) = 0.267, p = 0.614; session and genotype interaction, F(3,52) = 0.540, p = 0.658). To examine in more detail the extinction of acquired CR, we calculated the frequency of the CR% in every 10 trial block of the daily extinction session (Fig. 3A, bottom). Both wild-type and CB1 knock-out mice exhibited a monotonic decrease in CR within 1 d. There was no difference between the two groups in terms of the intraday behavior in any of the 4 d during extinction phase, indicating that intraday-level extinction is normal in CB1 knock-out mice.
Figure 3.
Intact acquisition and extinction of hippocampus-dependent trace eyeblink conditioning in CB1 knock-out mice. A, Top, CR% in wild-type (open circles; n = 8) and CB1 knock-out (KO) (filled circles; n = 7) mice were analyzed daily during the trace conditioning paradigm. The trace conditioning experiment consisted of an acquisition phase (10 d) and an extinction phase (4 d). In both acquisition and extinction phases, no difference was observed between wild-type and CB1 knock-out mice. The temporal relationship between CS and US is depicted at the top. sp, Spontaneous eyeblink response. A, Bottom, CR% with every 10 trial block of the daily extinction session (day 11–14). CB1 knock-out mice exhibited normal extinction. B, The averaged EMG amplitude of eyeblink response for wild-type (gray trace) and CB1 knock-out (black trace) mice on day 10 (top) and day 14 (bottom), indicating no significant difference in EMG amplitude and the temporal pattern during both acquisition and extinction phases. Experiments were performed with CB1 knock-out mice and their wild-type littermates at 11–19 weeks of age.
Figure 3B shows the averaged EMG amplitudes of wild-type and CB1 knock-out mice on day 10 and day 14. The amplitude and temporal structure for CB1 knock-out mice was similar to those for wild-type mice both at the peak of trace eyeblink conditioning (day 10) and after extinction learning (day 14). These results clearly indicate that both acquisition and extinction of trace eyeblink conditioning are normal in CB1 knock-out mice.
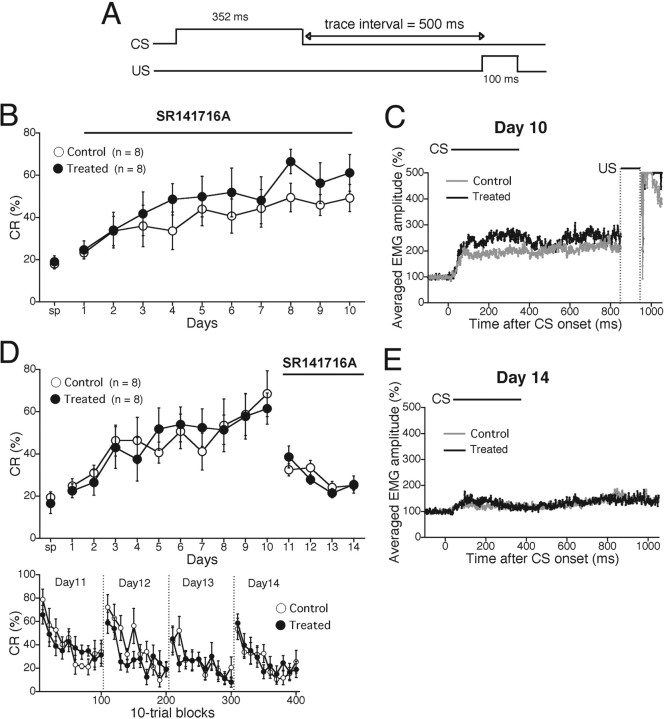
Impaired acquisition but normal extinction of delay eyeblink conditioning in mice treated with the CB1 antagonist SR141716A
The results from CB1 knock-out mice strongly suggest the importance of CB1 receptor in delay eyeblink conditioning. However, a possibility remains that CB1 deficiency may cause developmental alteration in the cerebellum, which may influence delay eyeblink conditioning. Furthermore, if the mice once exhibit impairment of memory acquisition, their performance of memory extinction cannot be evaluated. Therefore, we examined whether acute pharmacological blockade of CB1 caused similar impairment of delay eyeblink conditioning (Fig. 4A) as in CB1 knock-out mice.
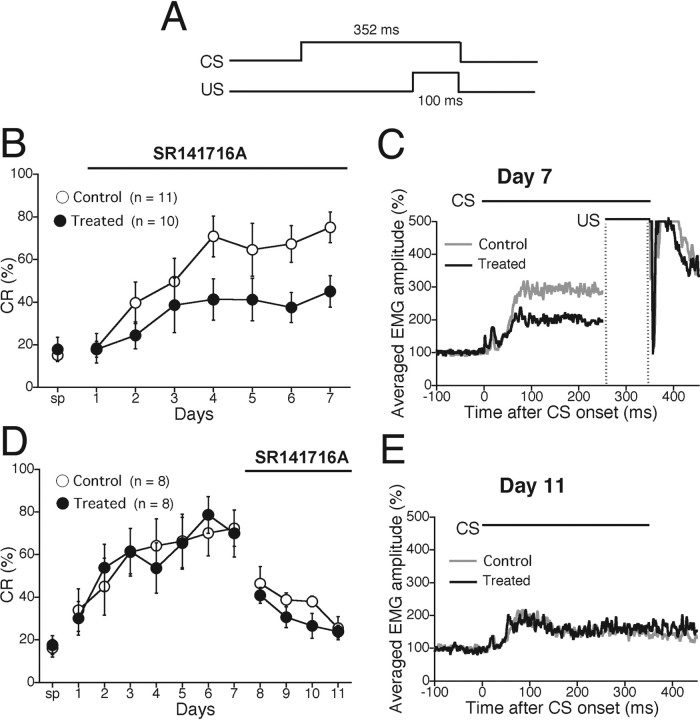
Figure 4.
The CB1 antagonist SR141716A impaired acquisition but not extinction of delay eyeblink conditioning in mice. A, Temporal relationship between CS and US for the delay eyeblink conditioning. B, C, The effect of CB1 antagonist on the acquisition of delay conditioning was tested. B, CR% during the delay conditioning were analyzed daily in wild-type (C57BL/6J) mice injected with vehicle (Control; open circles; n = 11) and those with the CB1 antagonist SR141716A (Treated; filled circles; n = 10). SR141716A or vehicle was administered 20 min before daily training during acquisition phase (bar). SR141716A-injected mice exhibited significantly lower CR% during 7 d acquisition sessions. sp, Spontaneous eyeblink response. C, The averaged EMG amplitudes of eyeblink response for the control (gray trace) and treated (black trace) mice on day 7, indicating significantly lower EMG response to tone CS in SR141716A-treated mice. D, E, The effect of CB1 antagonist on the extinction of delay conditioning was tested. D, During acquisition phase (day 1–7), both control (open circles; n = 8) and treated (filled circles; n = 8) groups underwent daily injection of vehicle 20 min before daily training. From day 8 (the 1st session of extinction phase), SR141716A was administered to the treated mouse group. There was no difference in CR% during extinction phase between the two groups of mice. E, The averaged EMG amplitude of eyeblink response for control (gray trace) and SR141716A-treated (black trace) mice on day 11, indicating no significant difference in EMG amplitude during extinction phases. Experiments were performed with male C57BL6/J mice at 9–16 weeks of age.
After we monitored spontaneous eyeblink frequency (Fig. 4B, sp), we made an intraperitoneal injection of the CB1 antagonist SR141716A or vehicle to mice 20 min before daily training during acquisition phase. CR% for vehicle-injected mice progressively increased to ∼70% during the 7 d acquisition session. In contrast, CR% for SR141716A-treated mice increased to ∼40% during the 7 d acquisition session. Thus, CR% for SR141716A-treated mice was significantly lower than that for vehicle-injected mice (treatment, F(1,19) = 8.329, p = 0.0094; session and treatment interaction, F(13,133) = 1.118, p = 0.356). The accumulated EMG amplitude on day 7 for SR141716A-treated mice was significantly lower than that for vehicle-injected mice because of the lower CR% (Fig. 4C). Nevertheless, no significant difference was noted in the temporal pattern of EMG between the two groups (Fig. 4C).
We then examined whether acute pharmacological blockade of CB1 affected extinction of delay eyeblink conditioning. After the 7 d acquisition session, mice underwent extinction training with an intraperitoneal administration of SR141716A or vehicle 20 min before daily extinction training (Fig. 4D). In marked contrast to impaired acquisition (Fig. 4B), extinction occurred normally in SR141716A-treated mice (Fig. 4D) (treatment, F(1,14) = 3.649, p = 0.0768; session and treatment interaction, F(7,56) = 0.443, p = 0.724). The accumulated EMG amplitude on day 11 for SR141716A-treated mice was similar to that for vehicle-injected mice (Fig. 4E). These results indicate that acute pharmacological blockade of CB1 impairs acquisition but has no effect on extinction of delay eyeblink conditioning in mice.
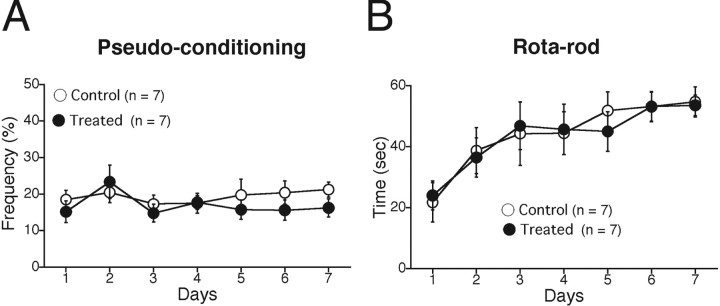
SR141716A treatment caused no sensory deficit or motor discoordination
We examined whether SR141716A treatment caused any change in sensory function or motor coordination in C57BL/6J mice (Kishimoto et al., 2002, 2006). We found that there was no significant difference in the frequency of startle response to the tone CS between the SR141716A-treated and vehicle-injected mice (vehicle-injected mice, 2.09 ± 0.46%, n = 12; SR141716A-treated mice, 2.31 ± 0.53%, n = 11; p > 0.05). We tested pseudoconditioning with pseudorandomized presentations of CS and US (Fig. 5A) and found no significant difference between the two groups (treatment, F(1,12) = 2.217, p = 0.162; session and treatment interaction, F(6,84) = 0.596, p = 0.733). Furthermore, we found that SR141716A treatment did not affect motor coordination, as assessed by the fixed-bar test (vehicle-injected mice, 45.29 ± 6.96 s, n = 7; SR141716A-treated mice, 47.0 ± 5.47 s, n = 7; p > 0.05) and the rotarod test (treatment, F(1,12) = 0.027, p = 0.873; session and treatment interaction, F(6,84) = 0.124, p = 0.993) (Fig. 5B). These results clearly indicate that acute pharmacological blockade of CB1 receptors by SR141716A treatment caused no detectable sensory deficit or motor discoordination in mice.
Figure 5.
SR141716A treatment caused no sensory deficit or motor discoordination. A, The eyeblink frequency during the pseudoconditioning did not increase in either control mice (n = 7) or treated mice (n = 7). The CS and US were pseudorandomly presented with an interstimulus interval ranging from 0 to 20 s. B, Rotarod test. The stay time (seconds) of the mice on a rotating rod (25 rpm) is plotted versus the training day. There was no significant difference between control (open circles; n = 7) and treated (filled circles; n = 7) mice. Experiments were performed with male C57BL6/J mice at 8–15 weeks of age.
Normal acquisition and extinction of trace eyeblink conditioning in mice treated with SR141716A
We then examined whether acute pharmacological blockade of CB1 receptors had any effect on trace eyeblink conditioning. We administered SR141716A or vehicle intraperitoneally to mice 20 min before daily acquisition training of trace eyeblink conditioning with a 500 ms stimulus-free interval (Fig. 6A). As shown in Figure 6B, CR% for SR141716A-treated mice increased progressively to the same level as that for vehicle-injected mice during the 10 d acquisition session. The accumulated EMGs for the two groups of mice were similar in their amplitudes and temporal patterns (Fig. 6C). There was no significant difference in the acquisition of the 500 ms trace eyeblink conditioning between the vehicle-treated and SR141716A-treated mice (treatment, F(1,14) = 2.044, p = 0.175; session and treatment interaction, F(19,140) = 0.498, p = 0.873).
Figure 6.
SR141716A affected neither acquisition nor extinction of 500 ms interval trace eyeblink conditioning. A, Temporal relationship between CS and US for trace conditioning paradigm with a trace interval of 500 ms. B, C, The effect of CB1 antagonist on the acquisition of trace conditioning (trace interval of 500 ms) was tested. B, CR% during the trace conditioning were analyzed daily in wild-type (C57BL/6J) mice injected with vehicle (Control; open circles; n = 8) and those with SR141716A (Treated; filled circles; n = 8). SR141716A or vehicle was administered 20 min before the daily training during acquisition phase (bar). There was no statistically significant difference in CR% between control and SR141716A-injected mice during acquisition phases. sp, Spontaneous eyeblink response. C, The averaged EMG amplitudes of eyeblink response for control (gray trace) and SR141716A-treated (black trace) mice on day 10, showing nearly the same amplitude of EMG response between control and treated mice. D, E, The effect of CB1 antagonist on the extinction of trace conditioning (trace interval of 500 ms) was investigated. D, During acquisition phase (day 1–10), both control (open circles; n = 8) and treated (filled circles; n = 8) groups underwent daily injection of vehicle 20 min before daily training. From day 11 (the 1st session of extinction phase), SR141716A was administered to the treated group. There was no difference in CR% during extinction phase between the two mouse groups. E, The averaged EMG amplitude of eyeblink response for control (gray trace) and SR141716A-treated (black trace) mice on day 11, indicating no significant difference in EMG amplitude during extinction phases. Experiments were performed with male C57BL6/J mice at 9–16 weeks of age.
We then examined whether acute pharmacological blockade of CB1 affected the extinction of trace eyeblink conditioning with a 500 ms stimulus-free interval. After the 10 d acquisition session, mice underwent extinction training with intraperitoneal administration of SR141716A or vehicle 20 min before the daily session. As shown in Figure 6D (top), extinction occurred normally in SR141716A-treated mice (treatment, F(1,14) = 0.020, p = 0.889; session and treatment interaction, F(7,56) = 2.021, p = 0.127). The accumulated EMG amplitude on day 14 for SR141716A-treated mice was similar to that for vehicle-injected mice (Fig. 6E). Furthermore, detailed examination of the daily extinction session shows that there was no difference between the two groups in terms of the intraday behavior in any of the 4 d during the extinction phase (Fig. 6D, bottom). These results indicate that acute pharmacological blockade of CB1 has no detectable effect on acquisition and extinction of the 500 ms trace eyeblink conditioning.
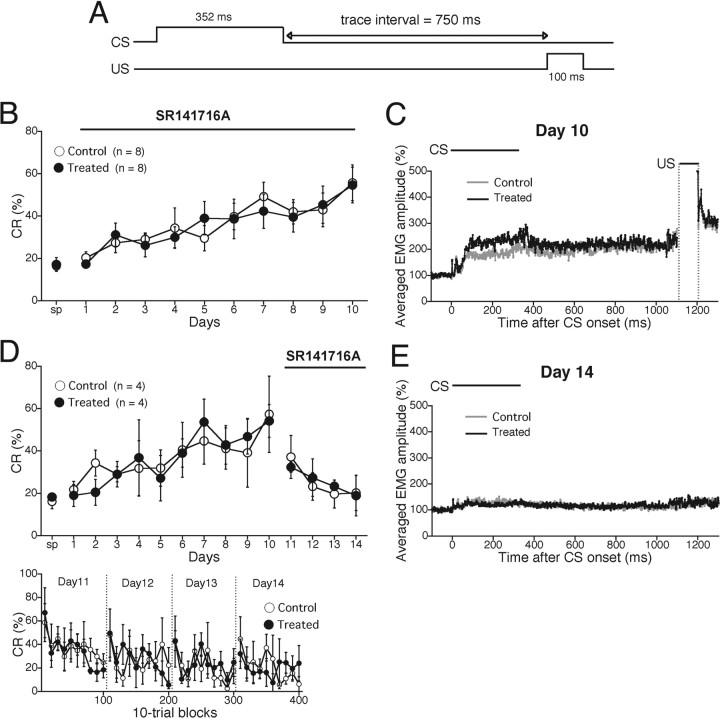
It is possible, however, that the contribution of CB1 in trace eyeblink conditioning could be detected with a more prolonged trace interval. We therefore tested whether SR141716A treatment affected trace eyeblink conditioning with a 750 ms stimulus-free interval (Fig. 7A). During the acquisition phase, we administered SR141716A or vehicle intraperitoneally to mice 20 min before daily training. As shown in Figure 7B, CR% for both groups increased progressively to ∼50% during the 10 d acquisition session (treatment, F(1,14) = 0.037, p = 0.848; session and treatment interaction, F(19,140) = 0.289, p = 0.977). The accumulated EMGs for the two groups of mice were also similar in their amplitudes and temporal patterns (Fig. 7C).
Figure 7.
SR141716A affected neither acquisition nor extinction of 750 ms interval trace eyeblink conditioning. A, Temporal relationship between CS and US for trace conditioning paradigm with a trace interval of 750 ms. B, C, The effect of CB1 antagonist on the acquisition of trace conditioning (trace interval of 750 ms) was tested. Data are illustrated similarly to Figure 6, B and C. B, There was no statistically significant difference in CR% between control and SR141716A-injected mice during the acquisition phase. C, The averaged EMG amplitudes of eyeblink response on day 10 for control (gray trace) and SR141716A-treated (black trace) mice showed similar amplitude and patterns. D, E, The effect of CB1 antagonist on the extinction of trace conditioning (trace interval of 750 ms) was investigated. Data are illustrated similarly to Figure 6, D and E. D, There was no difference in CR% during extinction phase for the two mouse groups. E, The averaged EMG amplitude of eyeblink response for control (gray trace) and SR141716A-treated (black trace) mice on day 11 showed similar amplitude and patterns. Experiments were performed with male C57BL6/J mice at 9–10 weeks of age.
Then, we examined whether SR141716A treatment affected extinction of the 750 ms trace eyeblink conditioning. After the 10 d acquisition session, mice underwent extinction training with intraperitoneal administration of SR141716A or vehicle 20 min before daily training. As shown in Figure 7D (top), the same degree of extinction of the CR was observed in both vehicle-injected mice and SR141716A-treated mice (treatment, F(1,6) = 0.010, p = 0.923; session and treatment interaction, F(7,32) = 0.149, p = 0.929). Furthermore, there was no difference between the two groups in terms of the intraday behavior in any of the 4 d during extinction phase (Fig. 7D, bottom). The accumulated EMG amplitude on day 14 for SR141716A-treated mice was similar to that for vehicle-injected mice (Fig. 7E). These results provide unequivocal evidence that acute pharmacological blockade of CB1 has no effect on both acquisition and extinction of trace eyeblink conditioning.
Discussion
The present study provides clear evidence that delay eyeblink conditioning is severely impaired, whereas trace eyeblink conditioning is intact, in CB1 knock-out mice. The sensitivity to CS and US and performance of eyeblink were normal. Intraperitoneal injection of the CB1 antagonist SR141716A into wild-type mice impaired acquisition but not extinction of delay eyeblink conditioning. SR141716A had no effect on the acquisition and extinction of trace eyeblink conditioning. These results indicate that CB1-mediated endocannabinoid signaling is essential for cerebellum-dependent discrete motor learning, especially for its acquisition.
Endocannabinoid-mediated retrograde suppression of synaptic transmission
Endocannabinoids act as retrograde messengers at various regions of the brain (Kreitzer and Regehr, 2001a; Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001). In the cerebellum, endocannabinoids are released from PCs, act retrogradely onto CB1 receptors at excitatory terminals from parallel fibers (PFs) and climbing fibers (CFs), and induce transient suppression of excitatory transmission (Kreitzer and Regehr, 2001b; Maejima et al., 2001b, 2005; Brown et al., 2003; Brenowitz and Regehr, 2005). Endocannabinoids also activate CB1 receptors at inhibitory terminals from basket/stellate cells and induce transient suppression of inhibitory transmission (Kreitzer and Regehr, 2001b; Diana et al., 2002; Yoshida et al., 2002). Endocannabinoid release is triggered by either strong depolarization of PCs that results in an elevation of the intracellular Ca2+ concentration to a micromolar range (Brenowitz and Regehr, 2003; Maejima et al., 2005) or activation of metabotropic glutamate receptor subtype 1 (mGluR1) in PCs (Maejima et al., 2001b). Furthermore, subthreshold weak activation of mGluR1 can effectively trigger endocannabinoid release when combined with weak depolarization of PCs that causes a submicromolar elevation of the intracellular Ca2+ concentration (Maejima et al., 2005). This phenomenon is attributable to the Ca2+-dependent enhancement of the activity of phospholipase Cβ4 (Maejima et al., 2005), a key enzyme for endocannabinoid biosynthesis that is activated downstream of mGluR1. Endocannabinoid-mediated associative short-term depression of PF synapse has been reported to occur after conjunctive activation of PF and CF inputs (Brenowitz and Regehr, 2005). This short-term plasticity requires mGluR1 activation and Ca2+ elevation in PC dendrites (Brenowitz and Regehr, 2005) and therefore is likely to involve the Ca2+-dependent enhancement of phospholipase Cβ4 activity. Because CB1 is enriched in PFs as well as the inhibitory axon terminals (Kawamura et al., 2006; Yoshida et al., 2006), endocannabinoid-mediated short-term plasticity must be absent in CB1 knock-out mice.
What would be the cellular mechanisms underlying motor learning that depends on endocannabinoid signaling in the cerebellum? Because the aforementioned endocannabinoid-mediated suppression can persist only for several tens of seconds (Kreitzer and Regehr, 2001b; Diana et al., 2002; Yoshida et al., 2002; Brown et al., 2003; Brenowitz and Regehr, 2005; Maejima et al., 2005), this short-term plasticity in itself cannot encode a memory trace for delay eyeblink conditioning. However, it is possible that the endocannabinoid-mediated short-term plasticity influences the induction of other forms of long-term synaptic plasticity that underlie delay eyeblink conditioning. Alternatively, endocannabinoid signaling itself may contribute to the process of such long-term synaptic plasticity.
Cellular mechanism underlying deficits in discrete motor learning in CB1 knock-out mice
Many previous studies strongly suggest that long-term depression (LTD) of PF to PC synaptic transmission underlies discrete motor learning in the cerebellum (Ito, 1989). Mice that have a deficiency in LTD display impairment of delay eyeblink conditioning. These mouse models include mutant mice deficient in mGluR1 (Aiba et al., 1994; Kishimoto et al., 2002), phospholipase Cβ4 (Kishimoto et al., 2001c; Miyata et al., 2001), and glutamate receptor δ2 subunit (Kishimoto et al., 2001b). We have shown that both LTD and delay eyeblink conditioning are restored in the mGluR1-rescue mice (Kishimoto et al., 2002) in which mGluR1α have been introduced into the PCs of the mGluR1 knock-out mice under the control of a PC-specific promoter (Ichise et al., 2000). Importantly, trace eyeblink conditioning is impaired in the mGluR1-rescue mice, presumably because mGluR1 in the hippocampus or other brain regions is crucial for trace eyeblink conditioning (Kishimoto et al., 2002). These results are consistent with the notion that cerebellar LTD is a cellular substrate of delay eyeblink conditioning (Thompson and Kim, 1996). Recently, Safo and Regehr (2005) have found that cerebellar LTD requires endocannabinoid production and CB1 activation in mice. They also reported deficient LTD in CB1 knock-out mice (Safo and Regehr, 2005). It is therefore highly likely that LTD deficiency is a cause of impaired delay eyeblink conditioning in CB1 knock-out mice.
In contrast to severe impairment of delay eyeblink conditioning, trace eyeblink conditioning was normal in CB1 knock-out mice. In rabbits and rats, trace eyeblink conditioning with a long trace interval requires the hippocampus and cerebellum (Moyer et al., 1990; Weiss et al., 1999). Our previous results indicate that trace eyeblink conditioning is impaired in mice that have deficits in hippocampal long-term potentiation (LTP) but have intact cerebellar structure and LTD (Kishimoto et al., 2001a, 2002). Conversely, trace eyeblink conditioning is normal, whereas delay eyeblink conditioning is impaired, in mice that have a deficit in cerebellar LTD (Kishimoto et al., 2001b,c). These results collectively indicate that trace eyeblink conditioning requires intact hippocampus, hippocampal LTP, and intact cerebellum but does not require cerebellar LTD.
CB1-mediated endocannabinoid signaling is essential for acquisition but not extinction of delay eyeblink conditioning
We showed that pharmacological blockade of CB1 caused severe impairment of acquisition but had no effect on extinction of delay eyeblink conditioning. This result indicates that CB1-mediated endocannabinoid signaling is specifically involved in acquisition phase of delay eyeblink conditioning. Given that cerebellar LTD is blocked by SR141716A and is deficient in CB1 knock-out mice (Safo and Regehr, 2005), LTD is expected to be blocked in mice injected with SR141716A. Therefore, these results collectively suggest that cerebellar LTD may be a cellular basis for acquisition but not for extinction of delay eyeblink conditioning.
Neither acquisition nor extinction of trace eyeblink conditioning requires CB1 activation
Previous studies suggest that CB1-mediated endocannabinoid signaling suppresses hippocampal LTP. LTP induced by moderate stimuli is facilitated in slices from CB1 knock-out mice (Bohme et al., 2000) and in slices from wild-type animals treated with CB1 antagonist (Slanina et al., 2005), whereas LTP elicited by robust stimuli is independent of CB1 activation (Chevaleyre and Castillo, 2004; Slanina et al., 2005). Notably, several behavioral studies have demonstrated that genetic deletion or pharmacological blockade of CB1 impairs the extinction of fear conditioning (Marsicano et al., 2002; Suzuki et al., 2004; Chhatwal et al., 2005) or spatial learning (Varvel and Lichtman, 2002). These results suggest that CB1-mediated endocannabinoid signaling facilitates extinction of hippocampus-dependent memories. Tonic suppression of hippocampal LTP by endocannabinoid signaling might underlie the facilitation of memory extinction. In contrast, CB1 knock-out mice exhibit normal memory acquisition and extinction in an appetitively motivated operant conditioning task in which food-deprived animals receive a food reward on nose poking into an illuminated hole (Holter et al., 2005). In the present study, we found that genetic deletion or pharmacological blockade of CB1 affected neither acquisition nor extinction of trace eyeblink conditioning. Thus, the endocannabinoid signaling through CB1 appears to facilitate extinction in several, but not all, hippocampus-dependent learning paradigms.
Normal motor coordination despite impairment in discrete motor learning in CB1 knock-out mice
Mature CB1 knock-out mice at 10–18 weeks of age exhibit normal motor coordination in our measurements on the fixed-bar test and rotarod test, whereas a recent study has demonstrated impaired performance of mature CB1 knock-out mice on the rotarod test (Bilkei-Gorzo et al., 2005). We assume that this apparent discrepancy is attributable to the different methods for rotarod test. We used a fixed speed of rotation (25 rpm), but Bilkei-Gorzo et al. (2005) adopted an accelerating rod from 4 to 20 rpm at an acceleration rate of 1 rpm/s. Importantly, mice deficient in mGluR1 (Aiba et al., 1994; Kishimoto et al., 2002), phospholipase Cβ4 (Kano et al., 1998; Kishimoto et al., 2001c), or glutamate receptor δ2 subunit (Kishimoto et al., 2001b) have clear deficits on the rotarod test with a fixed-rotation velocity. These mice also showed clear impairment of delay eyeblink conditioning. Therefore, the CB1 knock-out mouse is an exceptional case in which severe impairment of delay eyeblink conditioning and apparently normal motor coordination are observed in the same mouse strain. These phenotypes resemble those of mutant mice deficient in glial fibrillary acidic protein (Shibuki et al., 1996). Conversely, mice deficient in protein kinase Cγ is impaired in motor coordination but exhibits normal or rather enhanced performance in delay eyeblink conditioning (Chen et al., 1995). These results suggest that distinct cellular mechanisms underlie discrete motor learning and motor coordination. The establishment of CF mono-innervation of each PC is suggested to be required for normal motor coordination (Chen et al., 1995). Our preliminary experiments indicate that CF innervation of PCs is normal in CB1 knock-out mice (T. Maejima, H. Nakayama, K. Hashimoto, and M. Kano, unpublished data). Thus, the cerebellar phenotypes of CB1 knock-out mice are consistent with the aforementioned notion (Chen et al., 1995).
Footnotes
This work was supported by Grants-in Aid for Scientific Research 15-8589 (Y.K.), 17023021, and 17100004 (M.K.), and Special Coordination Funds for Promoting Science and Technology (M.K.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Y.K. was a recipient of the Research Fellowships for Young Scientists from the Japan Society for the Promotion of Science. We thank A. Zimmer for providing the original breeding pairs of CB1 knock-out mice and T. Tabata and T. Ohno-Shosaku for valuable comments on this manuscript.
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Valverde O, Otto M, Michel K, Sastre M, Zimmer A. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc Natl Acad Sci USA. 2005;102:15670–15675. doi: 10.1073/pnas.0504640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohme GA, Laville M, Ledent C, Parmentier M, Imperato A. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience. 2000;95:5–7. doi: 10.1016/s0306-4522(99)00483-2. [DOI] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci. 2003;23:6373–6384. doi: 10.1523/JNEUROSCI.23-15-06373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–431. doi: 10.1016/j.neuron.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6:1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim JJ, Hashimoto K, Thompson RF, Tonegawa S. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKCγ mutant mice. Cell. 1995;83:1233–1242. doi: 10.1016/0092-8674(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Chen L, Bao S, Thompson RF. Bilateral lesions of the interpositus nucleus completely prevent eyeblink conditioning in Purkinje cell-degeneration mutant mice. Behav Neurosci. 1999;113:204–210. doi: 10.1037//0735-7044.113.1.204. [DOI] [PubMed] [Google Scholar]
- Chen L, Bao S, Lockard JM, Kim JJ, Thompson RF. Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. J Neurosci. 1996;16:2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- Degroot A, Nomikos GG. Genetic deletion and pharmacological blockade of CB1 receptors modulates anxiety in the shock-probe burying test. Eur J Neurosci. 2004;20:1059–1064. doi: 10.1111/j.1460-9568.2004.03556.x. [DOI] [PubMed] [Google Scholar]
- Diana MA, Levenes C, Mackie K, Marty A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci. 2002;22:200–208. doi: 10.1523/JNEUROSCI.22-01-00200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Elphick MR, Egertova M. The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2001;356:381–408. doi: 10.1098/rstb.2000.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Holter SM, Kallnik M, Wurst W, Marsicano G, Lutz B, Wotjak CT. Cannabinoid CB1 receptor is dispensable for memory extinction in an appetitively-motivated learning task. Eur J Pharmacol. 2005;510:69–74. doi: 10.1016/j.ejphar.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- Kadotani H, Hirano T, Masugi M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J Neurosci. 1996;16:7859–7867. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Maejima T, Yoshida T, Hashimoto K. Retrograde modulation of synaptic transmission mediated by endogenous cannabinoids. Curr Neuropharmacol. 2003;2:49–57. [Google Scholar]
- Kano M, Hashimoto K, Watanabe M, Kurihara H, Offermanns S, Jiangs H, Wu Y, Jun K, Shin H-S, Inoue Y, Simon MI, Wu D. PLCβ4 is specifically involved in climbing fiber synapse elimination in the developing cerebellum. Proc Natl Acad Sci USA. 1998;95:15724–15729. doi: 10.1073/pnas.95.26.15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic site in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Mori H, Mishina M, Kirino Y. Long-trace interval eyeblink conditioning is impaired in mutant mice lacking the NMDA receptor subunit ε1. Eur J Neurosci. 2001a;13:1221–1227. doi: 10.1046/j.0953-816x.2001.01486.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Nakazawa K, Tonegawa S, Kirino Y, Kano M. Hippocampal CA3 NMDA receptors are crucial for adaptive timing of trace eyeblink conditioned response. J Neurosci. 2006;26:1562–1570. doi: 10.1523/JNEUROSCI.4142-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Suzuki M, Mori H, Mishina M, Kirino Y. Classical eyeblink conditioning in glutamate receptor subunit δ2 mutant mice is impaired in the delay paradigm but not in the trace paradigm. Eur J Neurosci. 2001b;13:1249–1253. doi: 10.1046/j.0953-816x.2001.01488.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Fujimichi R, Araishi K, Kano M, Aiba A, Kirino Y. mGluR1 in cerebellar Purkinje cells is required for normal association of temporally contiguous stimuli in classical conditioning. Eur J Neurosci. 2002;16:2416–2424. doi: 10.1046/j.1460-9568.2002.02407.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Hirono M, Sugiyama T, Kawahara S, Nakao K, Kishio M, Katsuki M, Yoshioka T, Kirino Y. Impaired delay but normal trace eyeblink conditioning in PLCβ4 mutant mice. NeuroReport. 2001c;12:2919–2922. doi: 10.1097/00001756-200109170-00033. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001a;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001b;21 doi: 10.1523/JNEUROSCI.21-20-j0005.2001. RC174(1–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde signaling by endocannabinoids. Curr Opin Neurobiol. 2002;12:324–330. doi: 10.1016/s0959-4388(02)00328-8. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert J-F, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Maejima T, Ohno-Shosaku T, Kano M. Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci Res. 2001a;40:205–210. doi: 10.1016/s0168-0102(01)00241-3. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001b;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase C β4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- Miyata M, Kim HT, Hashimoto K, Lee TK, Cho SY, Jiang H, Wu Y, Jun K, Wu D, Kano M, Shin H-S. Deficient long-term synaptic depression in the rostral cerebellum correlated with impaired motor learning in phospholipase C β4 mutant mice. Eur J Neurosci. 2001;13:1945–1954. doi: 10.1046/j.0953-816x.2001.01570.x. [DOI] [PubMed] [Google Scholar]
- Moyer JRJ, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Alonso R, Shire D, Congy C, Soubrie P, Breliere JC, Le Fur G. Biochemical and pharmacological characterization of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Shibuki K, Gomi H, Chen L, Bao S, Kim JJ, Wakatsuki H, Fujisaki T, Fujimoto K, Katoh A, Ikeda T, Chen C, Thompson RF, Itohara S. Deficient cerebellar long-term depression, impaired eyeblink conditioning, and normal motor coordination in GFAP mutant mice. Neuron. 1996;16:587–599. doi: 10.1016/s0896-6273(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Slanina KA, Roberto M, Schweitzer P. Endocannabinoids restrict hippocampal long-term potentiation via CB1. Neuropharmacology. 2005;49:660–668. doi: 10.1016/j.neuropharm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Steiner H, Bonner TI, Zimmer AM, Kitai S, Zimmer A. Altered gene expression in striatal projection neurons in CB1 cannabinoid receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5786–5790. doi: 10.1073/pnas.96.10.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Kim JJ. Memory systems in the brain and localization of a memory. Proc Natl Acad Sci USA. 1996;93:13438–13444. doi: 10.1073/pnas.93.24.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Bao S, Chen L, Cipriano BD, Grethe JS, Kim JJ, Thompson JK, Tracy JA, Weninger MS, Krupa DJ. Associative learning. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav Brain Res. 1999;99:123–132. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J Neurosci. 2002;22:1690–1697. doi: 10.1523/JNEUROSCI.22-05-01690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-α around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]