Abstract
The accessory α2δ subunits of voltage-gated calcium channels are highly glycosylated transmembrane proteins that interact with calcium channel α1 subunits to enhance calcium currents. We compared the membrane localization and processing of native cerebellar α2δ-2 subunits with α2δ-2 stably expressed in tsA-201 cells. We identified that α2δ-2 is completely concentrated in cholesterol-rich microdomains (lipid rafts) in cerebellum, in which it substantially colocalizes with the calcium channel α1 subunit CaV2.1, although CaV2.1 is also present in the Triton X-100-soluble fraction. In tsA-201 cells, unlike cerebellum, α2δ-2 is not completely proteolytically processed into α2-2 and δ-2. However, this processing is more complete in the lipid raft fraction of tsA-201 cells, in which α2δ-2 also colocalizes with CaV2.1. Cholesterol depletion of intact cells disrupted their lipid rafts and enhanced CaV2.1/α2δ-2/β4 currents. Furthermore, α2δ-2 coimmunoprecipitates with lipid raft-associated proteins of the stomatin family. The apparent affinity of α2δ-2 for its ligand gabapentin is increased markedly in the cholesterol-rich microdomain fractions, in both cerebellum and the stable α2δ-2 cell line. In contrast, α2δ-2 containing a point mutation (R282A) has a much lower affinity for gabapentin, and this is not enhanced in the lipid raft fraction. This R282A mutant α2δ-2 shows reduced functionality in terms of enhancement of CaV2.1/β4 calcium currents, suggesting that the integrity of the gabapentin binding site may be important for normal functioning of α2δ-2. Together, these results indicate that both α2δ-2 and CaV2.1 are normally associated with cholesterol-rich microdomains, and this influences their functionality.
Keywords: calcium, channel, α2δ, gabapentin, lipid raft, cerebellum, cholesterol
Introduction
Voltage-gated Ca2+ (CaV) channels are composed of a pore-forming α1 subunit, associated, at least in the case of the CaV1 and 2 subfamilies, with an intracellular β subunit responsible for trafficking (Walker and De Waard, 1998) and a transmembrane α2δ subunit whose function is less well defined (Canti et al., 2003). The α1 subunit determines the main biophysical properties of the channel and is modulated by the other subunits (Walker and De Waard, 1998). Mammalian genes encoding 10 α1, four β, and four α2δ subunits have been identified (Catterall, 2000; Ertel et al., 2000; Burgess et al., 2001).
The topology of the α2δ protein appears to generalize for all four α2δ subunits. They are all predicted to be type 1 transmembrane proteins, because all have a hydrophobic region in the C terminus (CT) that is likely to be a transmembrane domain. All have predicted N-terminal signal sequences, indicating that the N terminus is extracellular (for review, see Canti et al., 2003). From the early studies of α2δ-1 purified from skeletal and cardiac muscle, it was identified that the α2 subunit is disulfide bonded to a transmembrane δ subunit, and both subunits are the product of a single gene, encoding the α2δ protein, which is posttranslationally cleaved into α2 and δ (Ellis et al., 1988; De Jongh et al., 1990; Gurnett et al., 1996). The α2δ-2 subunit is also clearly reduced in molecular mass by disulfide-bond reduction (Gong et al., 2001), and, when expressed heterologously, the signal sequence is cleaved and the α2 moiety is entirely extracellular (Brodbeck et al., 2002). However, there is poor sequence conservation between α2δ-1 and α2δ-2 around the identified site of cleavage between α2 and δ in α2δ-1 (Jay et al., 1991).
All α2δ subunits enhance calcium currents through the high-voltage-activated (HVA) CaV1 and CaV2 channels (Gurnett et al., 1996; Klugbauer et al., 1999; Barclay et al., 2001). However, their mechanism of action remains unclear. Our recent work has shed light on this and suggests that the metal ion-dependent adhesion site (MIDAS) in the Von Willebrand factor-A (VWA) domain within α2δ-2 is of key importance in trafficking CaVα1 subunits (Canti et al., 2005). This VWA domain is present in all α2δ subunits and has a perfect MIDAS motif in α2δ-1 and α2δ-2 (Whittaker and Hynes, 2002). Both α2δ-1 and α2δ-2 bind the antiepileptic drugs gabapentin and pregabalin, which are also used in the treatment of neuropathic pain (Brown et al., 1998; Marais et al., 2001; Canti et al., 2003).
In this study, we identified that both native and heterologously expressed α2δ-2 subunits are concentrated together with CaV2.1 in Triton X-100-insoluble cholesterol-rich microdomain fractions (commonly known as lipid rafts), in which the affinity for binding [3H]gabapentin is strongly enhanced and in which they interact with proteins of the stomatin family.
Materials and Methods
Molecular biology.
Mouse α2δ-2 [GenBank accession number AF247139; common brain splice variant, lacking exon 23 and 6 bp of exon 38 (Barclay and Rees, 2000)] was engineered with an internal hemagglutinin (HA) tag between amino acids 652 (L) and 653 (Q), using standard molecular biology techniques to form α2δ-2(HA). The R282A mutation in mouse α2δ-2 was made by standard techniques. All constructs were sequenced before use. Other cDNAs used were rat CaV2.1 (GenBank accession number M64373), E1686R (Hans et al., 1999), and rat β4 (LO2315), cloned into the pMT2 vector for expression in mammalian cells.
Antibodies.
Ca channel antibodies (Abs) used were as follows: anti-mouse α2-2(16–29), α2-2(102–117) (Brodbeck et al., 2002), δ-2(1080–1094), and δ-2 CT (1133–1147) for α2δ-2 (all affinity-purified rabbit anti-peptide Abs) and anti-β1b serum (Moss et al., 2002). Other primary antibodies and their sources were as follows: anti-flotillin-1 (BD Biosciences, Cowley, Oxford, UK), anti-prohibitin (Abcam, Cambridge, UK), anti-stomatin-like protein-2 (SLP-2) (BD Biosciences), anti-Akt (Cell Signaling Technology, Danvers, MA), and anti-CaV2.1 (Alomone Labs, Jerusalem, Israel).
Heterologous expression of cDNAs.
Cos-7 or tsA-201 cells were transfected with either α2δ cDNA alone or with all calcium channel subunit cDNAs, using the ratios for α1, β, α2δ, and green fluorescent protein (GFP) of 3:2:2:0.4. For electrophysiological recording, the cDNA for GFP (mut3 GFP) was included in the transfection to identify transfected cells from which recordings were made.
Creation of a tsA-201 stable cell line containing α2δ-2 with internal HA tag.
The internally HA-tagged α2δ-2 cDNA was cloned in-frame into the mammalian expression vector pcDNA3.1/Zeo(+) (Invitrogen, Carlsbad, CA) using KpnI and NotI, linearized with PvuI, and transfected into cultured tsA-201 cells using Fugene 6 (Roche, Mannheim, Germany). The vector conferred Zeocin (Invitrogen) resistance to cells that stably incorporated the plasmid. Clonal foci were isolated using trypsin-impregnated 5-mm-diameter sterile paper discs (Sigma, Poole, UK). Expression of the full-length α2δ-2 protein was confirmed by Western blotting and functionality of the protein by electrophysiology.
Cholesterol depletion of tsA-201 cells.
A 50 mm stock of methyl-β-cyclodextrin (Sigma) was prepared in serum-free medium, sterile filtered, and kept at room temperature for no longer than 10 h. For electrophysiological experiments, cells were replated onto collagen-coated dishes, before addition of methyl-β-cyclodextrin. Cells were treated with methyl-β-cyclodextrin (5 mm) in serum-free medium for 1 h at 37°C (Christian et al., 1997), immediately before use in electrophysiological or biochemical experiments. Control cells were incubated in serum-free medium for the same length of time.
Cell membrane preparation.
Membrane fractions from mouse cerebellum or cultured cells were prepared using the following general method. Mouse cerebella were homogenized in a buffer containing the following (in mm): 20 HEPES, pH 7.4, 50 NaCl, 300 sucrose, 2 EDTA, incomplete protease inhibitor cocktail (Roche), and 1 orthophenanthroline. Cultured cell pellets were resuspended in 25 vol of ice-cold 10 mm HEPES, pH 7.4, containing complete protease inhibitor cocktail (Roche). Cells were lysed by 10 passages through a 23 gauge needle, followed by three 10-s rounds of sonication. Cell debris was removed by centrifugation (1000 × g, for 15 min at 4°C), and the resultant supernatants were recentrifuged (100,000 × g, for 60 min at 4°C) to pellet membranes. Protein concentrations of the membrane preparations were determined by BCA assay (Perbio, Tatenhall, Cheshire, UK). Membranes were used immediately or stored in aliquots at −80°C.
Preparation of Triton X-100-insoluble membrane fraction.
All steps were performed on ice. Confluent cells from four 175 cm2 flasks, or pelleted whole homogenate derived from two mouse cerebella, were taken up in 1.5 ml of MES-buffered saline (MBS) (25 mm MES, pH 6.5, 150 mm NaCl, and complete protease inhibitor cocktail) containing 1% (v/v) Triton X-100 (Perbio), resuspended by five passages through a 23 gauge needle and three 7-s rounds of sonication, and then left on ice for 1 h. An equal volume of 90% (w/v) sucrose in MBS was then added. The 3 ml sample was transferred to a 13 ml ultracentrifuge tube and overlaid with 10 ml of discontinuous sucrose gradient, consisting of 35% (w/v) sucrose in MBS (5 ml) and 5% (w/v) sucrose in MBS (5 ml). The sucrose gradients were centrifuged at 140,000 × g for 18 h at 4°C in a Beckman Instruments (Fullerton, CA) SW40 rotor; 1 ml fractions were subsequently harvested from the top to the bottom of the tube. When necessary, protein fractions from the gradient were washed free of sucrose by dilution into 25 vol of MBS and centrifugation (100,000 × g, for 60 min at 4°C) to pellet the cholesterol-enriched microdomain material.
Immunoprecipitation.
α2δ-2(HA) was immunoprecipitated from stably transfected tsA-201 cells as follows. Triton X-100-insoluble membranes prepared from these cells were resuspended in immunoprecipitation (IP) solubilization buffer (1% Igepal, 50 mm Tris, 75 mm NaCl, and protease inhibitor cocktail, pH 7.4) for 1 h at 4°C. Insoluble material was removed by centrifugation at 12,000 × g at 4°C for 15 min. The clarified material was incubated for 2 h at 4°C with ∼2 μg of the appropriate Ab. Protein G-agarose (30 μl of 50% slurry; Sigma) was added and incubated for 1 h at 4°C. Beads were then washed three times in low detergent buffer (0.15% Igepal, 50 mm Tris, 75 mm NaCl, and protease inhibitor cocktail, pH 7.4), and bound protein was removed from the beads by the addition of SDS sample buffer containing 100 mm DTT, with heating at 70°C for 10 min. For protein sequencing, samples were separated on Nu-Page 4–12% Bis-Tris gels (Invitrogen) and stained with Coomassie blue (Simply Blue safe stain; Invitrogen). Bands of interest were excised from the gel, and protein identification was performed at Imperial College London Proteomics Facility by tryptic mass fingerprinting, confirmed by quadrupole time of flight (Q-ToF) mass spectrometry. The probability of correct identification from peptide mass fingerprinting is based on the molecular weight search (MOWSE) score using the Matrix Science Mascot search engine, which is −10*Log(P), where P is the probability that the observed match is a random event. Individual peptide ion scores >52 indicate identity or extensive homology (p < 0.05).
Immunoblotting.
Immunoblot analysis was performed essentially as described previously (Page et al., 2004). SDS-PAGE-resolved samples were transferred to polyvinylidene difluoride membranes and probed with relevant primary Abs and the appropriate horseradish peroxidase-conjugated secondary Abs, followed by enhanced chemiluminescence detection. Ca channel and prohibitin primary Abs were used at 1–5 μg/μl, and anti-flotillin-1 and anti-SLP-2 were used at 1:500 and 1:250, respectively.
Electrophysiology.
Calcium channel expression in tsA-201 cells was investigated by whole-cell patch-clamp recording. The internal (pipette) and external solutions and recording techniques were similar to those described previously (Campbell et al., 1995). The patch pipette solution contained the following (in mm): 140 Cs-aspartate, 5 EGTA, 2 MgCl2, 0.1 CaCl2, 2 K2ATP, and 10 HEPES, pH 7.2 (310 mOsm with sucrose). The external solution for recording Ba2+ currents contained the following (in mm): 150 tetraethylammonium Br, 3 KCl, 1.0 NaHCO3, 1.0 MgCl2, 10 HEPES, 4 glucose, and 10 BaCl2, pH 7.4 (320 mOsm with sucrose). Pipettes of resistance 2–4 MΩ were used. An Axopatch 1D amplifier (Molecular Devices, Palo Alto, CA) was used, and data were filtered at 1–2 kHz and digitized at 5–10 kHz. Analysis was performed using pClamp7 (Molecular Devices) and Origin 7 (Microcal, Northampton, MA). Current records are shown after leak and residual capacitance current subtraction (P/4 protocol). For determination of the voltage for 50% current activation (V50,act), the current density–voltage (I–V) relationships were fitted between −30 and +50 mV with a modified Boltzmann equation as follows: I = Gmax × (V − Vrev)/(1 + exp(−(V − V50,act)/k)), where I is the current density (in picoamperes per picofarad), Gmax is the maximum conductance (in nanosiemans per picofarad), Vrev is the reversal potential, and k is a slope factor. Steady-state inactivation data were fitted with a single Boltzmann equation of the following form: I/Imax = ((A1 − A2)/[1 + exp((V − V50,inact)/k)] + A2, where Imax is the maximal current, V50,inact is the half-maximal voltage for current inactivation, and A1 and A2 represent the total and non-inactivating current, respectively.
Calcium current recording from isolated Purkinje cells was performed as described previously (Barclay et al., 2001), except for the following points: cerebellar slices (300 μm) were prepared from postnatal days 7–9 mice using a vibrating tissue slicer and kept in 95%O2/5% CO2-saturated Krebs' solution for 30 min at 36°C before being cooled to room temperature. Cells were isolated immediately before use by digestion of slices with papain (20 U/ml) for 5–15 min, washed and triturated in Ringer's solution, and plated onto poly-l-lysine-coated coverslips.
Gabapentin binding assay.
Binding of [3H]gabapentin was performed as described previously (Canti et al., 2005), in a final volume of 250 μl at room temperature for 2 h. Membranes (50 μg/tube) or cholesterol-rich microdomains (3 μg/tube) were incubated with various concentrations of [3H]gabapentin (ARC, St. Louis, MO) in 10 mm HEPES/KOH, pH 7.4, and then rapidly filtered through GF/B filters, presoaked with 0.3% polyethyleneimine. Filters were washed three times with 3 ml of ice-cold 50 mm Tris/HCl, pH 7.4, and the amount of bound [3H]gabapentin was determined by scintillation counting. Concentrations of [3H]gabapentin >50 nm were achieved by adding nonradioactive gabapentin and correcting the specific binding by the dilution factor, as described previously (Marais et al., 2001). Nonspecific binding was determined in the presence of 1000-fold excess of nonradioactive gabapentin (or pregabalin, with identical results). Replicate (three to five) independent experiments were performed, each in triplicate, and data were analyzed by fitting an equation for a rectangular hyperbola to the specific binding data from each experiment, to obtain the dissociation constant (KD) and maximum number of binding sites (Bmax).
Data are presented as mean ± SEM, and statistical significance of differences between data were determined using Student's unpaired, two-tailed t test.
Results
Processing of native α2δ-2 in cerebellar tissue
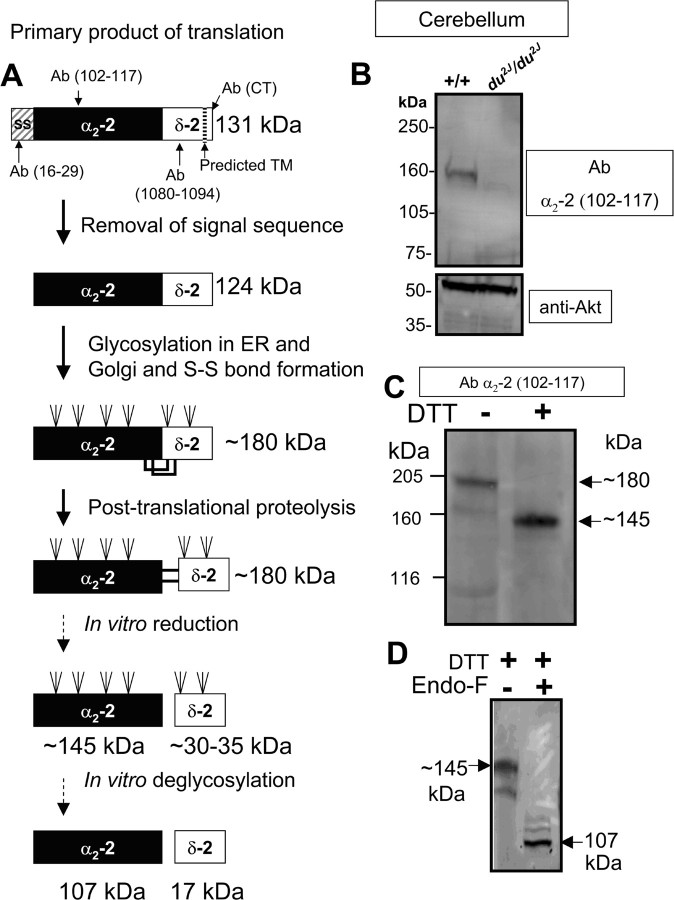
In parallel with the posttranslational processing that has been determined for native muscle α2δ-1 (Jay et al., 1991), the theoretical processing of α2δ-2 is shown in Figure 1A. The mature form of α2δ-2 is thought to be proteolytically cleaved into α2-2 and δ-2, which remain disulfide bonded, until subjected to disulfide-bond reduction in vitro (Hobom et al., 2000; Brodbeck et al., 2002). The cerebellum is rich in native α2δ-2, and we confirmed that the protein from cerebellum that is recognized by the α2-2(102–117) Ab used in these studies is indeed derived from α2δ-2, because it is absent from du2J/du2J cerebellum (Fig. 1B). This mouse strain has a 2 bp mutation in the Cacna2d2 gene (calcium channel, voltage-dependent, alpha2delta subunit-2), which is predicted to result in a complete loss of α2δ-2 protein (Barclay et al., 2001).
Figure 1.
Processing of α2δ-2 from cerebellum. A, Schematic showing the proposed products of posttranslational and in vitro modifications of the α2δ-2 subunit. SS, Signal sequence; S-S, disulfide bond; TM, transmembrane domain. B, Presence of α2-2(102–117)-immunoreactive band in wild-type but absence from du2J/du2J cerebellar membranes, separated under reducing conditions, in the presence of DTT (100 mm). Akt immunoreactivity was used as a loading control. C, Western blot of cerebellar membranes separated under nonreducing conditions (−) or reducing conditions (+100 mm DTT) and probed with Ab α2-2(102–117). Immunolabeled bands corresponding to mature full-length α2δ-2 (∼180 kDa) and glycosylated α2-2 (∼145 kDa) are arrowed. D, Western blot of cerebellar membranes prepared under reducing conditions, treated without (−) or with (+) endo-F and then probed with Ab α2-2(102–117). Bands corresponding to glycosylated α2-2 and deglycosylated α2-2 (107 kDa) are arrowed.
Additional examination of the native species shows it to have the expected molecular mass of ∼180 kDa before in vitro reduction with DTT and 145 kDa for the reduced form (Fig. 1A,C). Subsequent in vitro deglycosylation with endoglycosidase F (endo-F) resulted in the expected reduction in mass for the reduced α2-2(102–117)-immunoreactive species from ∼145 kDa before deglycosylation to ∼107 kDa after deglycosylation (Fig. 1D). This is in accordance with the predicted processing shown in the schematic diagram in Figure 1A. The specificity of the α2-2(102–117) Ab was further confirmed by showing that it recognized heterologously expressed α2δ-2 but not α2δ-1 or α2δ-3 transfected into tsA-201 cells (data not shown).
Native α2δ-2 from cerebellum is colocalized in cholesterol-rich microdomains with CaV2.1
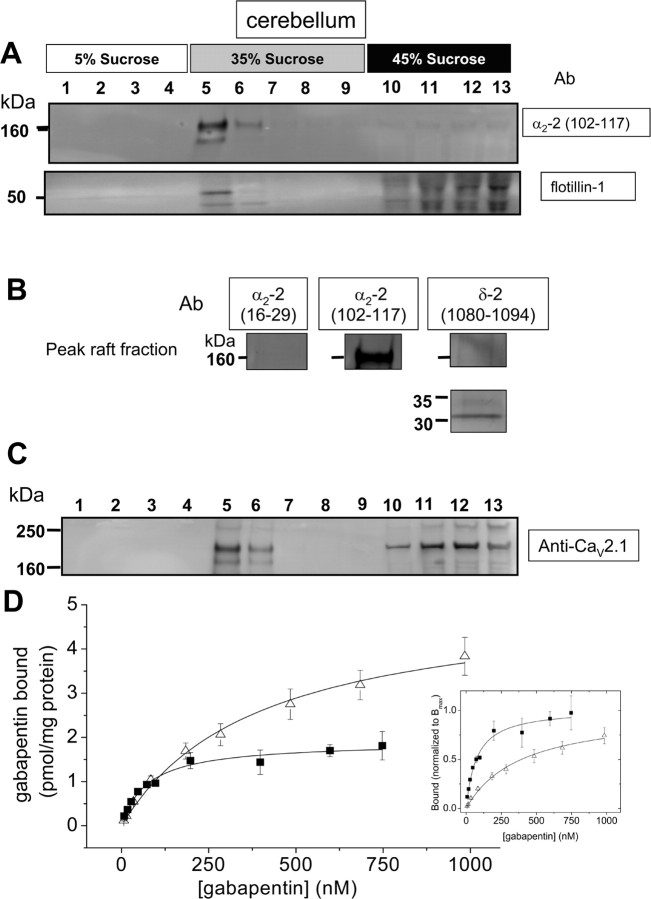
The punctate cell-surface distribution of heterologously expressed α2δ-2 that we observed previously after heterologous expression of α2δ-2 (Canti et al., 2005) suggested to us that it might be concentrated in cholesterol-rich microdomains. Cell lysates were prepared from mouse cerebellum and separated on a sucrose gradient. These showed native α2δ-2 to be entirely concentrated in cholesterol-rich lipid raft microdomains (Fig. 2A, fractions 5, 6), in which it colocalizes with the lipid raft marker flotillin-1 (Fig. 2A). Within the lipid raft fraction, α2δ-2 is entirely in its mature disulfide-bond linked form, as shown by the fact that, when subjected to SDS-PAGE under reducing conditions, it is separated into α2-2 (∼145 kDa) (Fig. 2A,B) and δ-2 (∼32 kDa) (Fig. 2B) and, as expected, has a cleaved signal sequence (Fig. 2B). No α2δ-2 was present in the Triton X-100-soluble fraction (Fig. 2A, fractions 10–13). We also examined the distribution of native CaV2.1 in the cerebellar sucrose gradient fractions and found that it substantially colocalized with α2δ-2 in the cholesterol-rich microdomain fractions, although some was also present in the Triton X-100-soluble fractions (fractions 10–13), which contain a large percentage of the total protein (Fig. 2C) (for quantification, see Fig. 6A). Two main immunoreactive bands were observed for CaV2.1, of mass ∼250 and 190 kDa, with the lower band being the predominant species. Multiple mass species of CaV2.1 have been attributed previously to the existence of different C-terminal splice variants and to proteolytic cleavage of the C terminus (Sakurai et al., 1995). Furthermore, there is evidence that these species may have differing locations (Sakurai et al., 1996).
Figure 2.
Presence of α2δ-2 and CaV2.1 in cholesterol-rich microdomains from mouse cerebellum. A, Western blot analysis of the distribution of proteins from a typical discontinuous sucrose gradient derived from whole cerebellar homogenate. Fractions under reducing conditions were probed with Ab α2-2(102–117) (top) and anti-flotillin-1 (bottom). B, The flotillin-1-positive peak fraction from the sucrose gradient shown in A was further characterized using Abs α2-2(16–29) (left), α2-2(102–117) (middle), and δ-2(1080–1094) (right). C, Western blot analysis of the distribution of CaV2.1 from the same sucrose gradient as in A. Fractions under reducing conditions were probed with anti CaV2.1 Ab. D, Measurement of the affinity of [3H]gabapentin binding sites in a membrane fraction (Δ; fitted to a hyperbola with KD 392 nm, mean of n = 3) and cholesterol-rich microdomains (■; fitted to a hyperbola with KD 74.6 nm, mean of n = 3) derived from mouse cerebellum. Data in the inset graph have been normalized to each mean Bmax to illustrate the difference in KD values.
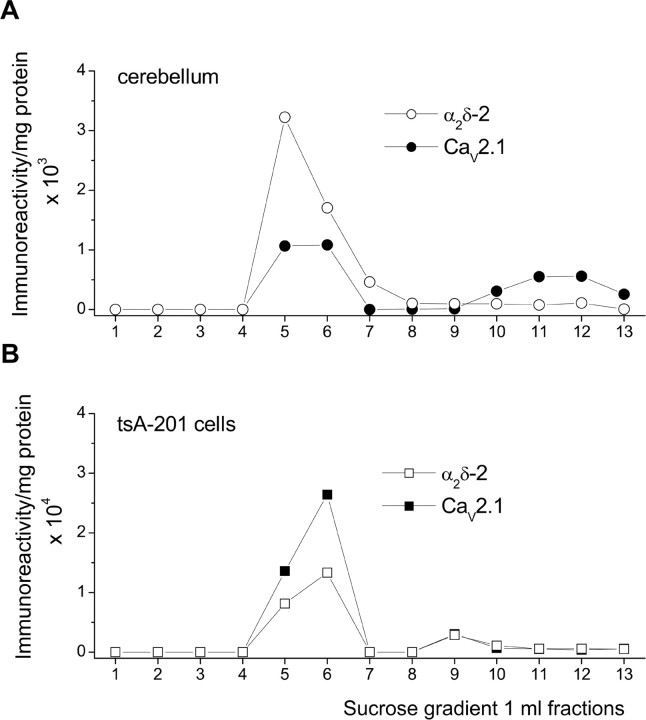
Figure 6.
Quantification of the localization of α2δ-2 and CaV2.1 in cholesterol-rich microdomains. The presence of α2δ-2 (open symbols) and CaV2.1 (filled symbols) in sucrose gradient fractions from cerebellum (A, circles) and tsA-201 cells (B, squares). The band intensities were quantified using ImageQuant5.2 (Molecular Dynamics, Sunnyvale, CA) and expressed as arbitrary density units per milligram of protein in each fraction.
Gabapentin binding to cerebellar α2δ-2 is enhanced in cholesterol-rich microdomains
Gabapentin is a ligand for α2δ-2 as well as α2δ-1 (Canti et al., 2003, 2005; Klugbauer et al., 2003). We therefore examined the [3H]gabapentin binding affinity in cholesterol-rich microdomains compared with membrane preparations from cerebellum. The affinity for [3H]gabapentin was increased 4.8-fold in the lipid raft fraction, with the KD being reduced from 385 ± 56 nm in membranes (n = 3) to 79.7 ± 19.8 nm (n = 3) in lipid rafts (Fig. 2D). The Bmax was 5.23 ± 0.73 pmol/mg protein in cerebellar membranes and 1.67 ± 0.45 pmol/mg protein for cerebellar rafts.
Gabapentin has no acute effect on Purkinje cell calcium currents
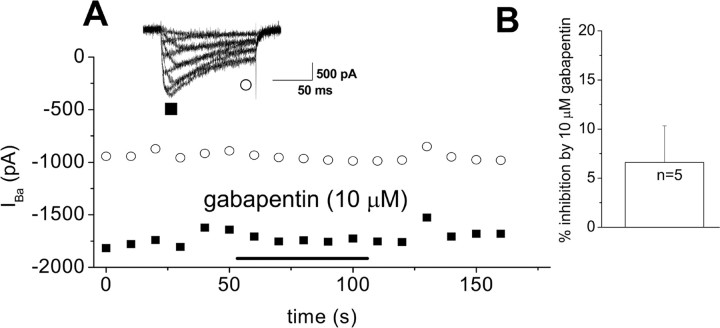
It is assumed in much of the literature that gabapentin has its effect via binding to α2δ subunits and consequently inhibiting calcium currents (for review, see Sutton and Snutch, 2001) and reducing transmitter release (van Hooft et al., 2002). However, the effect of gabapentin on calcium currents is controversial because, although it has been found to produce a small inhibition of native calcium currents in sensory neurons (Sutton et al., 2002), it does not produce acute inhibition of calcium currents in heterologous expression systems (Vega-Hernandez and Felix, 2002; Canti et al., 2003). Because α2δ-2 is very strongly expressed in cerebellar Purkinje cells (Barclay et al., 2001) and has also been found to be associated with many other GABAergic neurons (Cole et al., 2005), we therefore examined the effect of gabapentin on Purkinje cell calcium currents. An inhibition of calcium currents in GABAergic neurons and consequent inhibition of GABA release would not be compatible with the well established antiepileptic action of gabapentin. We found no significant effect of 1–10 μm gabapentin on Purkinje cell calcium currents from wild-type mice (Fig. 3A,B).
Figure 3.
Lack of effect of gabapentin on calcium channel currents recorded from cerebellar Purkinje cells. A, Example of the lack of effect of gabapentin (10 μm) on the time course of calcium channel currents recorded from a holding potential of −90 mV to a test potential of +20 mV, every 10 s from an isolated cerebellar Purkinje cell, measured at peak (■) and end (○) of pulse. The inset shows current traces from a current–voltage relationship performed before the time course, with voltage pulses of −50 to +30 mV in 10 mV steps. Gabapentin application is indicated by the horizontal bar. B, Inhibition (mean ± SEM) by gabapentin (10 μm) (white bar).
Colocalization of heterologously expressed CaVα1 subunits with α2δ-2 in cholesterol-rich microdomains
We wanted to examine whether heterologously expressed α2δ-2 was also localized in lipid rafts in a similar manner to native α2δ-2. For these experiments, we used either transient transfection of wild-type α2δ-2 or a cell line stably expressing either wild-type α2δ-2 or α2δ-2 with an internal HA tag [α2δ-2(HA)].
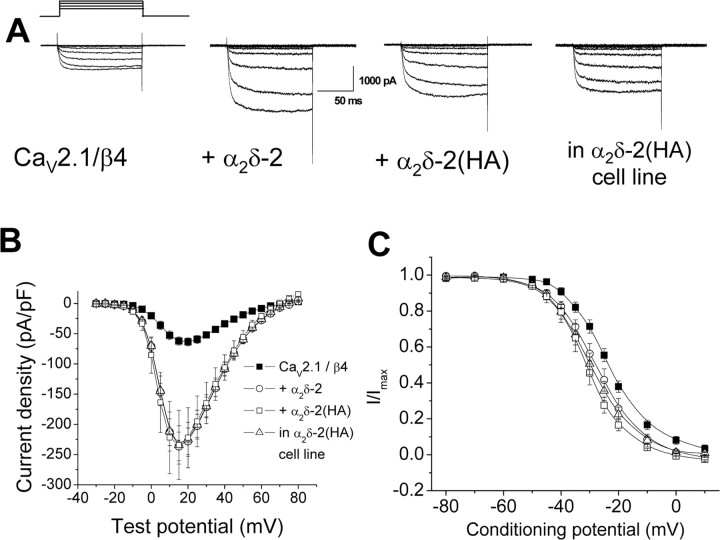
It was first important to determine whether α2δ-2 with an internal HA tag was fully functional. Coexpression of α2δ-2 enhanced calcium currents through all HVA calcium channels in all expression systems we tested (Canti et al., 2005). In the example shown in Figure 4, A and B, α2δ-2 increased currents through CaV2.1/β4 channels in tsA-201 cells by 3.8 fold (n = 16). This is a physiologically relevant subunit combination, likely to be the main functional heteromeric calcium channel in cerebellar Purkinje cells. Transient transfection of the α2δ-2(HA) construct enhanced CaV2.1/β4 currents to the same extent as wild-type α2δ-2, and these currents were very similar to those obtained after transient transfection of CaV2.1/β4 into the stable α2δ-2(HA) cell line (Fig. 4B). Furthermore, both α2δ-2 and α2δ-2(HA) produced a similar small hyperpolarization of the steady-state inactivation compared with CaV2.1/β4 alone (Fig. 4C).
Figure 4.
Comparison of the effect of expression of α2δ-2 and α2δ-2(HA) on currents through Cav2.1/β4 channels in tsA-201 cells. Cav2.1/β4 were expressed alone or with α2δ-2 or α2δ-2 with internal HA tag [α2δ-2(HA)] in tsA-201 cells, and currents were recorded using Ba2+ as charge carrier. A, Representative current traces elicited between −30 and +15 mV in 5 mV steps from a holding potential of −90 mV for Cav2.1/β4 (left), Cav2.1/β4/α2δ-2 (middle left), Cav2.1/β4/α2δ-2(HA) (middle right), or Cav2.1/β4 in stable α2δ-2(HA) cell line (right). B, Current density–voltage relationships (mean ± SEM) for the four experimental conditions. ■, Cav2.1/β4 (n = 13); ○, with α2δ-2 (n = 16); □, with α2δ-2(HA) (n = 7); Δ, Cav2.1/β4 in α2δ-2(HA) cell line (n = 21). C, Steady-state inactivation (mean ± SEM) for the four experimental conditions: Cav2.1/β4 (■; n = 7; V50 = −23.7 ± 1.2 mV); with α2δ-2 (○; n = 2; V50 = −27.3 mV); with α2δ-2(HA) (□; n = 3; V50 = −30.6 ± 1.9 mV; p < 0.01 compared with no α2δ-2), and Cav2.1/β4 in α2δ-2(HA) cell line (Δ; n = 8; V50 = −29.5 ± 1.9 mV; p < 0.03 compared with no α2δ-2).
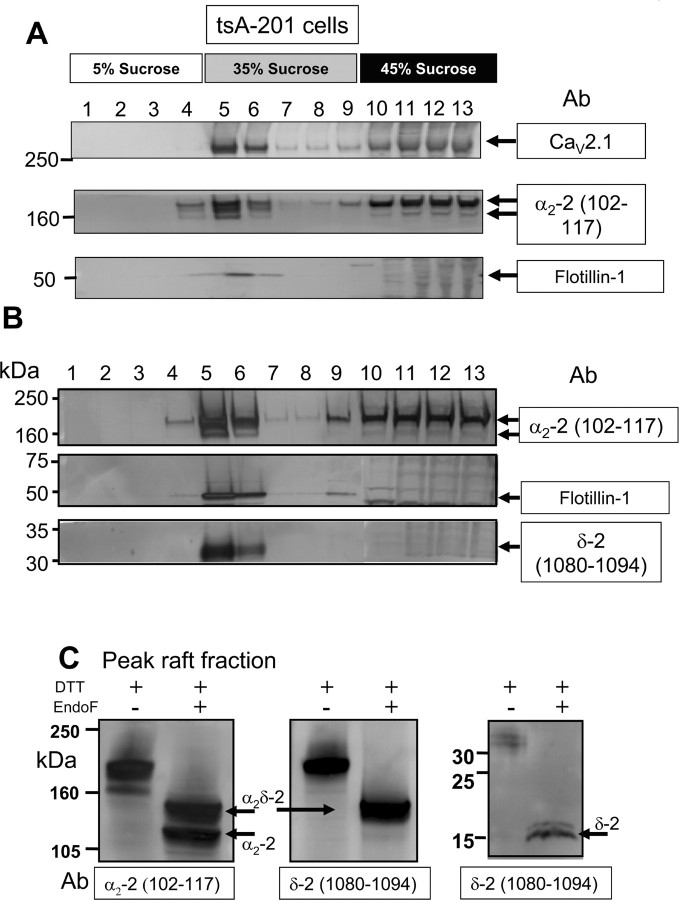
The processing of stably expressed α2δ-2(HA) was examined in either the additional presence of transiently transfected CaV2.1 and β4 (Fig. 5A) or their absence (Fig. 5B). Cells were lysed, and the Triton X-100-soluble and -insoluble fractions were separated on a discontinuous sucrose gradient. Fractions from the sucrose gradient were analyzed by SDS-PAGE under reducing conditions in the presence of DTT. As was the case for cerebellar α2δ-2, the heterologously expressed α2δ-2 was clearly present in the flotillin-1-positive lipid raft fractions (Fig. 5A,B, fractions 5, 6), although it was also present in the Triton X-100 soluble fractions (fractions 10–13). This was true in both the presence (Fig. 5A) and absence (Fig. 5B) of coexpressed CaV2.1.
Figure 5.
Colocalization of α2δ-2 and CaV2.1 in cholesterol-rich microdomains from α2δ-2(HA) cell line. A, Stably transfected tsA-201 cells containing α2δ-2(HA) were transiently transfected with Cav2.1 and β4, and Western blot analysis was performed of the distribution of proteins found in fractions from a typical discontinuous sucrose gradient. Fractions (separated on SDS-PAGE under disulfide-bond-reducing conditions) were probed for CaV2.1 (top) and α2δ-2 with Ab α2-2(102–117) (middle). The bottom shows the location of the cholesterol-rich microdomain marker protein flotillin-1 (arrowed). B, Western blot analysis was performed as in A, except that the α2δ-2(HA) cell line was not additionally transfected with other subunits. Fractions (separated under disulfide-bond-reducing conditions) were probed for α2δ-2-derived products with Abs α2-2(102–117) and δ-2(1080–1094) (top and bottom). The middle shows the location of the cholesterol-rich microdomain marker protein flotillin-1 (arrowed). For Ab α2-2(102–117), the top and bottom arrows indicate bands corresponding in size to the nonreducible full-length α2δ-2 (∼180 kDa) and free α2-2 (145 kDa) proteins, respectively. For Ab δ-2(1080–1094), the arrow indicates free δ-2 peptide (32 kDa). C, Aliquots of the peak lipid raft fraction from the sucrose gradient in B were treated in vitro under reducing conditions without (−) or with (+) endo-F, and the products were analyzed on Western blots with Abs α2-2(102–117) (left) and δ-2(1080–1094) (middle and right). The positions of bands corresponding in size to nonreducible deglycosylated full-length α2δ-2 and deglycosylated free α2-2 are indicated with arrows (left and middle). A band corresponding to deglycosylated δ-2 peptide is arrowed in the right.
Whereas a single α2-2(102–117)-immunoreactive band of ∼180 kDa was predominant in the Triton X-100-soluble fractions (fractions 10–13), two bands of ∼180 and 145 kDa were present in the lipid raft fractions 5 and 6 (Fig. 5A,B), which are likely to represent intact noncleaved α2δ-2 and fully mature α2-2 derived from disulfide-bonded α2δ-2, respectively. In agreement with this, a δ-2(1080–1094)-immunoreactive band at ∼32 kDa was only observed in the lipid raft fractions (Fig. 5B). Similar results were obtained using transient transfection of wild-type α2δ-2, indicating that this partial processing of heterologously expressed α2δ-2 is not a result of introduction of the HA tag (data not shown).
Additional investigation of the peak lipid raft fraction was undertaken to confirm the nature of the two protein bands that were immunoreactive against α2-2(102–117), by subjecting this fraction to in vitro deglycosylation before separation under reducing conditions on SDS-PAGE (Fig. 5C). Deglycosylation tended to increase the immunoreactivity with the anti-peptide Abs, probably by increasing the exposure of the peptide epitopes. This analysis showed that the 180 and 145 kDa bands were reduced in mass to ∼125 and 107 kDa after in vitro deglycosylation (Fig. 5C, left panel), consistent with their identification as full-length α2δ-2 and free α2-2. Additional confirmation of this conclusion is that only the upper band is immunoreactive against the δ-2(1080–1094) Ab (Fig. 5C, middle panel). Free δ-2 is also present (Fig. 5C, right panel).
We also observed that a substantial proportion of coexpressed CaV2.1 was present in the Triton X-100-insoluble lipid raft fractions in tsA-201 cells (Fig. 5A). In this study, the long C-terminal isoform of CaV2.1 was expressed, and the mass of ∼250 kDa is in agreement with its predicted mass. To compare the localization in lipid rafts of CaV2.1 and α2δ-2 in cerebellum and tsA-201 cells, we quantified the α2-2(102–117) and CaV2.1 immunoreactivity in the sucrose gradient fractions per milligram of total protein (Fig. 6). This shows that α2δ-2 is strongly concentrated in the lipid raft fraction in both cerebellum (Fig. 6A) and tsA-201 cells (Fig. 6B), whereas CaV2.1 is more strongly concentrated in lipid raft fractions in tsA-201 cells than in cerebellum. This suggests that, in cerebellum, some CaV2.1 is either associated with an α2δ that is not concentrated in lipid rafts or that not all native CaV2.1 is associated with α2δ. In this regard, we observed that α2δ-1 is also associated with lipid rafts (data not shown). It is therefore possible that some native CaV2.1 is not associated with these α2δ subunits.
Effect of cholesterol depletion of tsA-201 cells
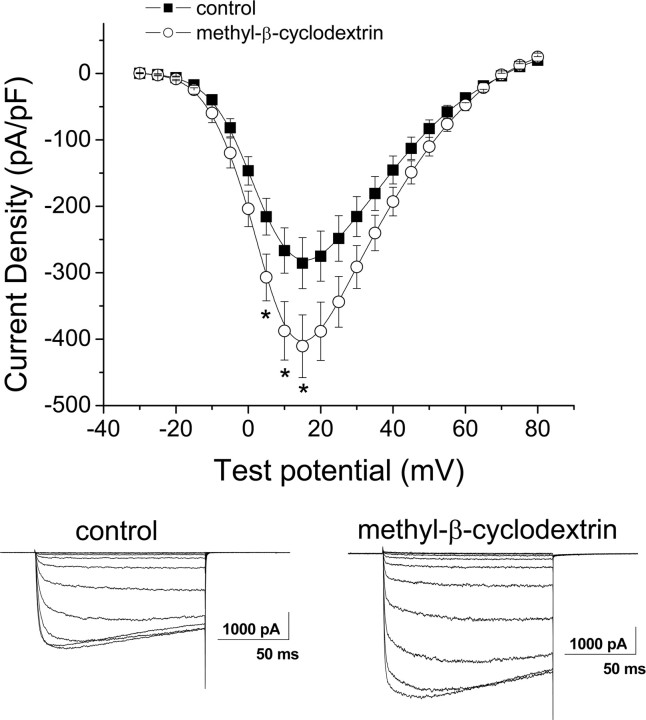
The α2δ-2(HA) tsA-201 cell line was incubated with methyl-β-cyclodextrin (5 mm) for 1 h to deplete membrane cholesterol (Christian et al., 1997), and calcium channel currents were recorded in cells that had also been transiently transfected with CaV2.1 and β4 (Fig. 7). Lipid rafts became disrupted by the methyl-β-cyclodextrin treatment, as shown previously (Fagan et al., 2000). We confirmed there was a reduced concentration of flotillin-1 in the Triton X-100-insoluble fraction (data not shown). Calcium channel currents were significantly elevated after cholesterol depletion (Fig. 7). The increase at +15 mV was 44.1 ± 16.5% (n = 17; p < 0.05). There was no effect on the V50,act, which was +2.8 ± 1.2 mV after treatment with cyclodextrin compared with +3.7 ± 0.8 mV for controls performed in parallel (n = 17 for both).
Figure 7.
Effect of cholesterol depletion with methyl-β-cyclodextrin on calcium channel currents. Cav2.1/β4 were expressed in the stable α2δ-2(HA) cell line and current density–voltage relationships (mean ± SEM; top) were determined in parallel, either after treatment with methyl-β-cyclodextrin (5 mm) for 1 h at 37°C (○; n = 17) or after identical treatment without methyl-β-cyclodextrin (■; n = 17). *p < 0.05, Student's unpaired t test. Bottom, Example traces under the two conditions, from a holding potential of −90 mV to test potentials of between −30 and +15 mV at 5 mV intervals.
Coimmunoprecipitation of α2δ-2 with other lipid raft constituent proteins
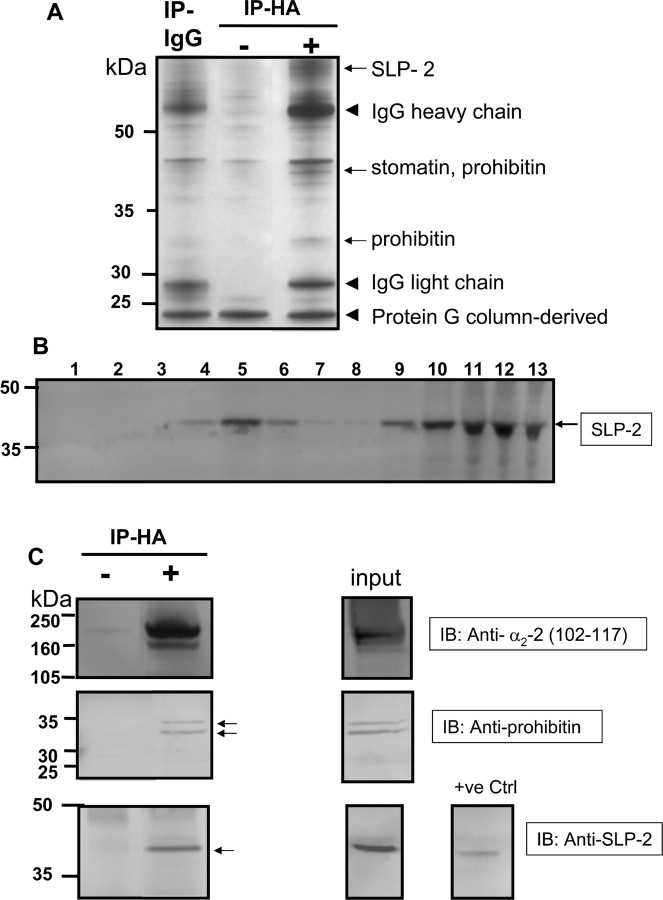
We examined whether α2δ-2 is associated with other protein constituents of lipid rafts and found that IP of α2δ-2(HA) from the lipid raft fraction, by means of its HA tag, consistently resulted in coimmunoprecipitation with three related proteins of the SPFH family (stomatin/prohibitin/flotillin/HflK), which have been identified to be lipid raft constituent proteins (for review, see Langhorst et al., 2005). They were identified by peptide mass fingerprinting and Q-ToF mass spectrometry. The proteins identified were SLP-2 (Owczarek et al., 2001), which was present in the excised ∼60 kDa band, presumably complexed to another protein or as a dimer, because its monomer protein mass is 39 kDa. Three peptides were identified for this protein giving an MOWSE score of 85 (p < 0.05). Stomatin (for review, see Rivera-Milla et al., 2006) was identified to be present in the excised ∼40 kDa band. Four peptides were identified for stomatin, giving an MOWSE score of 108 (p < 0.05). Prohibitin was also identified in the excised 40 kDa band, with an MOWSE score of 285 (p < 0.05), and, in addition, the same protein was identified in the excised ∼32 kDa band, which is similar to its protein mass of 30 kDa (Fig. 8A). We found that SLP-2 is strongly, although not exclusively, expressed in the peak Triton X-100-insoluble lipid rafts from tsA-201 cells (Fig. 8B), as is prohibitin (data not shown). Immunoprecipitation of α2δ-2, followed by immunoblotting confirmed the presence of coimmunoprecipitating SLP-2 and prohibitin (Fig. 8C). No commercial Ab was available for stomatin, and thus its interaction with α2δ-2 has not yet been verified by immunoblotting.
Figure 8.
Coimmunoprecipitation of α2δ-2(HA) with stomatin, SLP-2, and prohibitin. A, Silver stain showing proteins coimmunoprecipitating with α2δ-2(HA) from the α2δ-2(HA) cell line using an anti-HA Ab (right lane) and the α2-2(102–117) Ab (data not shown) for immunoprecipitation. Bands indicated with arrows that were absent from the lanes showing proteins immunoprecipitated with control IgG (left lane) or no Ab (middle lane) were excised and identified as described in Materials and Methods. The nonspecific IgG heavy (55 kDa) and light (28 kDa) chains and a protein G-column-derived band (15 kDa) are also indicated with arrowheads. B, Western blot analysis was performed of the distribution of SLP-2 (arrowed) in fractions from a typical discontinuous sucrose gradient. C, Anti-HA Ab was used to immunoprecipitate α2δ-2(HA) from lipid rafts (top). The input is shown on the right. Prohibitin was coimmunoprecipitated from lipid rafts with the anti-HA Ab (middle lane; two bands arrowed) and was absent from the control IP (left lane). SLP-2 was also coimmunoprecipitated from lipid rafts with α2δ-2(HA), using either the δ-2 (CT) Ab (data not shown) or the anti-HA Ab (bottom). SLP-2 was absent from the control IP (left) and present in the input and in the positive control supplied with the SLP-2 Ab (human epidermoid carcinoma cell line lysate).
Gabapentin binding to heterologously expressed α2δ-2 is enhanced in cholesterol-rich microdomains
For comparison with cerebellum, we examined the concentration of [3H]gabapentin binding sites and their affinity for [3H]gabapentin in cholesterol-rich microdomains compared with membrane preparations, both derived from the α2δ-2(HA) cell line (Fig. 9A).
Figure 9.
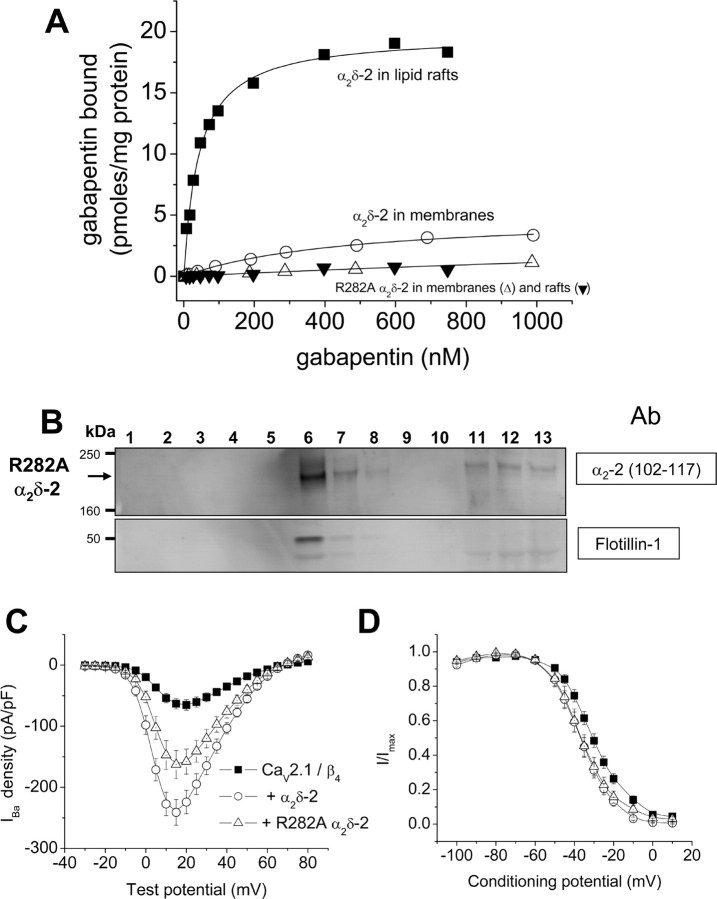
Gabapentin binding to α2δ-2(HA) cell line is enhanced in cholesterol-enriched microdomains and prevented by R282A mutation. A, Examples of experiments to determine the concentration of [3H]gabapentin binding sites and affinity for [3H]gabapentin of a membrane fraction (○; KD of 468 nm; representative example from n = 3) and cholesterol-rich microdomains (■; KD of 42.6 nm; representative example from n = 4) derived from the α2δ-2(HA) cell line. Also included on this graph are examples of the lack of binding of [3H]gabapentin to membranes (Δ; representative example from n = 5) or lipid rafts (▾; representative example from n = 2) prepared from Cos-7 cells transiently expressing the R282A α2δ-2 mutant. B, Western blot analysis of the distribution of proteins found in fractions from a typical discontinuous sucrose gradient, derived from transient expression of R282A α2δ-2. Fractions 1–13 from the sucrose gradient (run under disulfide-bond-reducing conditions) were probed for α2δ-2-derived products with Ab α2-2(102–117) (top). The bottom shows the location of the cholesterol-rich microdomain marker protein flotillin-1. C, Comparison of the effect of expression of α2δ-2 and R282A α2δ-2 on currents through Cav2.1/β4 channels in tsA-201 cells, using Ba2+ as charge carrier. Current density–voltage relationships (mean ± SEM) are shown for the three experimental conditions: ■, Cav2.1/β4 (n = 14); ○, with α2δ-2 (n = 14); and Δ, with R282A α2δ-2 (n = 18). D, Comparison of the effect of expression of α2δ-2 and R282A α2δ-2 on the steady-state inactivation of currents through Cav2.1/β4 channels in tsA-201 cells. Conditioning prepulses of 5 s to the potentials shown were given before a constant test pulse to +10 mV. The normalized data (mean ± SEM) are shown for the three experimental conditions: ■, Cav2.1/β4 (n = 11); ○, with α2δ-2 (n = 11); and Δ, with R282A α2δ-2 (n = 9). The V50 values are −30.5 ± 0.4, −36.2 ± 0.5, and −36.5 ± 0.4 mV, and the k values are 8.4 ± 0.4, 8.0 ± 0.4, and 8.1 ± 0.4 mV, respectively.
Initial studies showed that the presence of the HA tag had no effect on the gabapentin binding affinity. For wild-type α2δ-2, stably expressed in tsA-201 cells, the apparent KD for [3H]gabapentin in membranes was 450.6 ± 36.9 nm (n = 4), very similar to the apparent KD for gabapentin binding to membranes prepared from the α2δ-2(HA) cell line, which was 474.4 ± 32.9 nm (n = 3). Just as we observed in the cerebellum, the affinity for [3H]gabapentin was enhanced in the lipid raft fraction compared with the membrane fraction. The affinity of α2δ-2(HA) for [3H]gabapentin was approximately ninefold greater in the cholesterol-rich microdomain fraction compared with the membrane fraction, the apparent KD decreasing to 53.3 ± 8.4 nm (n = 4). Unlike in the cerebellum, the Bmax also increased from 5.1 ± 0.4 pmol/mg protein (n = 3) in the membrane fraction to 71.1 ± 29.8 pmol/mg protein (n = 4) in the lipid raft fraction, indicating a 14-fold purification of [3H]gabapentin binding sites in this fraction. No specific [3H]gabapentin binding was observed in the absence of transfected α2δ-2 (data not shown). One explanation for the increase in Bmax is that it may relate to the lower relative concentration of total protein in the lipid raft fraction of the tsA-201 cell line.
The effect of a mutation R282A in α2δ-2 that prevents [3H]gabapentin binding
The binding of [3H]gabapentin was almost completely abrogated by a mutation R282A in α2δ-2, which is situated just before the VWA domain. The affinity for [3H]gabapentin was reduced very substantially by this mutation to an unmeasurably high KD of >2 μm (n = 5) (Fig. 9A). Although the R282A mutant α2δ-2 did partition into cholesterol-rich microdomains (Fig. 9B), its affinity for gabapentin was not enhanced in this fraction (Fig. 9A). An equivalent R217A mutation in α2δ-1 has been described previously to prevent [3H]gabapentin binding to α2δ-1 (Wang et al., 1999), and a knock-in mouse carrying this mutation has been reported to be nonresponsive to gabapentin (Taylor, 2004). We confirmed that the R282A mutant α2δ-2 was still functional, although it enhanced CaV2.1/β4 currents to a significantly smaller extent than wild-type α2δ-2 in tsA-201 cells. The peak IBa at +15 mV was enhanced only 2.6-fold by R282A α2δ-2 compared with 3.8-fold for wild-type α2δ-2 (p < 0.05) (Fig. 9C). However, both the wild-type and R282A α2δ-2 hyperpolarized the steady-state inactivation to similar extents, indicating that, despite a reduction in the peak currents, the R282A mutant α2δ-2 is still able to influence the properties of the expressed channels (Fig. 9D). For α2δ-1, hyperpolarization of the steady-state inactivation was found to be a property of the δ subunit (Felix et al., 1997).
Discussion
Previous in vitro studies have shown that α2δ-1, α2δ-2, and α2δ-3 subunits all increase the maximum conductance of a number of expressed calcium channel α1/β subunit combinations at the whole-cell level in several different expression systems (Mori et al., 1991; Gurnett et al., 1996; Walker and De Waard, 1998; Klugbauer et al., 1999; Hobom et al., 2000; Barclay et al., 2001). However, this may be dependent to some extent on the specific combination of α1 and β subunits expressed (Klugbauer et al., 1999). Furthermore, the effects of α2δ subunits on the kinetics and voltage dependence of inactivation, while present, are relatively minor (Walker and De Waard, 1998; Klugbauer et al., 1999; Canti et al., 2003). We previously investigated the effect of α2δ-2 on currents resulting from several combinations of CaV α1 and β subunits and have shown that, whereas there was an approximately threefold increase in maximum whole-cell conductance (Barclay et al., 2001; Canti et al., 2005), α2δ-2 had no influence on single-channel conductance (Barclay et al., 2001; Brodbeck et al., 2002). This implies that α2δ-2 probably has its main effect on the number of channel complexes in the plasma membrane, either by enhancing trafficking to the plasma membrane or reducing turnover of channels. In agreement with this proposed mechanism, it has been found previously that α2δ-1 increased the amount of CaV1.2 protein expressed in Xenopus oocytes (Shistik et al., 1995). We have shown recently that the MIDAS motif in the VWA domain within α2δ-2 is of key importance in trafficking CaVα1 subunits (Canti et al., 2005).
Localization of α2δ-2 in lipid microdomains
In the present study, we have shown that α2δ-2 from cerebellum partitions completely into the Triton X-100-insoluble lipid raft fraction. Flotillin-1 was used as a marker of the lipid raft fraction, because it shows ubiquitous tissue expression (for review, see Morrow and Parton, 2005; Rivera-Milla et al., 2006), whereas caveolins, which are markers for the caveolar lipid rafts, have been reported to be absent or poorly expressed in brain (Scherer et al., 1996).
We also found α2δ-2 to be strongly concentrated in the lipid raft fractions isolated from an α2δ-2(HA)-expressing cell line, in which the mature species that is proteolytically processed into α2-2 and δ-2 is only present in these cholesterol-rich microdomains. In addition, CaV2.1 was concentrated with α2δ-2 in lipid rafts when expressed in tsA-201 cells and to a lesser extent in cerebellum. Evidence that association with cholesterol-rich microdomains is of functional importance comes from our finding that depletion of cholesterol from intact cells significantly enhanced CaV2.1/α2δ-2/β4 calcium channel currents. In general agreement with this, it has been shown that native N-type channel activity is reduced by enhancing membrane cholesterol (Toselli et al., 2005). Of relevance to the localization of calcium channel complexes in specific membrane microdomains, CaV2.1 has also been found to localize in synaptosomal lipid rafts with certain SNARE (soluble N-ethylmaleimide-sensitive factor attached protein receptor) complex proteins (Taverna et al., 2004), whereas a similar localization was not identified for L-type channels.
Additional evidence that α2δ-2 interacts with other lipid raft constituent proteins comes from the proteomic identification of several proteins of the SPFH family: SLP-2, stomatin, and prohibitin, which coimmunoprecipitate with α2δ-2. These proteins are submembrane scaffold proteins that are known constituents of lipid rafts in both the plasma membrane and endocytic vesicles (Foster et al., 2003; Langhorst et al., 2005; Morrow and Parton, 2005; Rivera-Milla et al., 2006). Furthermore, a stomatin homolog in Caenorhabditis elegans [MEC-2 (mechanotransducing channel subunit-2)] was identified to be involved in mechanosensation (Chalfie and Au, 1989). However, whereas MEC-2 enhanced C. elegans degenerin/epithelial Na+ channel (DEG/ENac) currents (Goodman et al., 2002), stomatin inhibited the related mammalian ASIC3 acid-sensing ion channels (Price et al., 2004). The functional importance of the interaction between α2δ-2 and proteins of the SPFH family, and whether it is direct, remains to be determined. However, it is of interest that a role for the related lipid raft protein flotillin in endocytosis has recently been defined (Glebov et al., 2006).
Affinity of gabapentin binding to α2δ-2 is enhanced in lipid rafts
Gabapentin is a ligand for α2δ-2 as well as α2δ-1, and we monitored [3H]gabapentin binding to membranes and lipid raft fractions from both the cerebellum and the α2δ-2 cell line. In the cerebellum, we found that the apparent KD decreased 4.8-fold from 385 nm in membranes to 80 nm in lipid rafts. In the tsA-201 cell line, we found that the apparent KD decreased ninefold from 474 to 53 nm. A reduction in KD of up to 10-fold has been observed previously after purification or dialysis of native α2δ-1. This was attributed to the loss of an unknown endogenous ligand (Dissanayake et al., 1997; Brown et al., 1998). Given that we find most α2δ-2 to be present in lipid rafts, the increased affinity for gabapentin when α2δ-2 is isolated in the lipid raft fraction may therefore be a result of the disruption of interaction of α2δ-2 with a soluble endogenous ligand or one that has been solubilized by Triton X-100, with which gabapentin normally competes for binding.
Effect of a mutation in α2δ-2 that abrogates gabapentin binding
We then made a mutation R282 in α2δ-2, because an equivalent mutation in α2δ-1 was found to prevent gabapentin binding to α2δ-1 (Wang et al., 1999). In agreement with this, the R282A mutant α2δ-2 showed a marked reduction in affinity for gabapentin, and the affinity of the R282A mutant α2δ-2 for [3H]gabapentin was not enhanced in the cholesterol-rich microdomain fraction. It is therefore likely that, if gabapentin binds at the same site as an endogenous ligand, to an exofacial binding site on α2δ-2, and if the apparent affinity for gabapentin is enhanced in the cholesterol-rich microdomain fraction because of loss of interaction with this endogenous ligand, then R282A α2δ-2 does not bind the endogenous ligand. It was therefore of interest to determine whether binding of this unknown endogenous ligand is relevant to the functionality of α2δ-2. Comparison of the effect of wild-type α2δ-2 and R282A α2δ-2 on calcium channel currents showed this mutation to significantly reduce the functionality of α2δ-2 by ∼33%. This finding suggests that, if there is an endogenous ligand associated with α2δ-2, as predicted for α2δ-1, that normally binds in the same site as gabapentin (Dissanayake et al., 1997; Brown et al., 1998), this might be essential for its full functionality or for the stability of the protein.
Conclusion
The identification here of α2δ-2 as a lipid raft protein opened the question as to whether this subunit associates with particular proteins in these cholesterol-rich microdomains and also whether it has other signaling roles. We identified that the properties of α2δ-2 are altered in the lipid raft fraction, as evidenced by the increased affinity for [3H]gabapentin. We identified CaV2.1 to be strongly concentrated with α2δ-2 in lipid rafts from both cerebellum and after heterologous expression and have shown that cholesterol depletion enhances CaV2.1/α2δ-2/β4 currents. We also identified that lipid raft proteins of the SPFH family coimmunoprecipitate with α2δ-2. These proteins are submembrane scaffold proteins that interact with several types of signaling complex (Langhorst et al., 2005). In the future, we will examine whether they play a role in anchoring the calcium channel proteins in these microdomains or in regulating their endocytosis.
Footnotes
This work was supported by Medical Research Council, Biotechnology and Biological Sciences Research Council, and The Wellcome Trust. We thank K. Chaggar for technical assistance.
References
- Barclay J, Rees M. Genomic organization of the mouse and human α2δ2 voltage-dependent calcium channel subunit genes. Mamm Genome. 2000;11:1142–1144. doi: 10.1007/s003350010211. [DOI] [PubMed] [Google Scholar]
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, PerezReyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck J, Davies A, Courtney J-M, Meir A, Balaguero N, Canti C, Moss FJ, Page KM, Pratt WS, Hunt SP, Barclay J, Rees M, Dolphin AC. The ducky mutation in Cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated α2δ-2 protein with abnormal function. J Biol Chem. 2002;277:7684–7693. doi: 10.1074/jbc.M109404200. [DOI] [PubMed] [Google Scholar]
- Brown JP, Dissanayake VU, Briggs AR, Milic MR, Gee NS. Isolation of the [3H]gabapentin-binding protein/alpha 2 delta Ca2+ channel subunit from porcine brain: development of a radioligand binding assay for alpha 2 delta subunits using [3H]leucine. Anal Biochem. 1998;255:236–243. doi: 10.1006/abio.1997.2447. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Gefrides LA, Foreman PJ, Noebels JL. A cluster of three novel Ca2+ channel gamma subunit genes on chromosome 19q13.43: evolution and expression profile of the gamma subunit gene family. Genomics. 2001;71:339–350. doi: 10.1006/geno.2000.6440. [DOI] [PubMed] [Google Scholar]
- Campbell V, Berrow NS, Fitzgerald EM, Brickley K, Dolphin AC. Inhibition of the interaction of G protein Go with calcium channels by the calcium channel β-subunit in rat neurones. J Physiol (Lond) 1995;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canti C, Davies A, Dolphin AC. Calcium channel α2δ subunits: structure, functions and target site for drugs. Curr Neuropharmacol. 2003;1:209–217. [Google Scholar]
- Canti C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, Richards MW, Hendrich J, Douglas L, Page KM, Davies A, Dolphin AC. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of α2δ subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci USA. 2005;102:11230–11235. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Au M. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science. 1989;243:1027–1033. doi: 10.1126/science.2646709. [DOI] [PubMed] [Google Scholar]
- Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- Cole RL, Lechner SM, Williams ME, Prodanovich P, Bleicher L, Varney MA, Gu G. Differential distribution of voltage-gated calcium channel α2δ (alpha2delta) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. J Comp Neurol. 2005;491:246–269. doi: 10.1002/cne.20693. [DOI] [PubMed] [Google Scholar]
- De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels. α2 and δ are encoded by the same gene. J Biol Chem. 1990;265:14738–14741. [PubMed] [Google Scholar]
- Dissanayake VUK, Gee NS, Brown JP, Woodruff GN. Spermine modulation of specific [3H]-gabapentin binding to the detergent-solubilized porcine cerebral cortex α2δ calcium channel subunit. Br J Pharmacol. 1997;120:833–840. doi: 10.1038/sj.bjp.0700988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, Schwartz A, Harpold MM. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Fagan KA, Smith KE, Cooper DM. Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J Biol Chem. 2000;275:26530–26537. doi: 10.1074/jbc.M001369200. [DOI] [PubMed] [Google Scholar]
- Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel α2δ subunit. J Neurosci. 1997;17:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- Gong HC, Hang J, Kohler W, Li L, Su TZ. Tissue-specific expression and gabapentin-binding properties of calcium channel α2δ subunit subtypes. J Membr Biol. 2001;184:35–43. doi: 10.1007/s00232-001-0072-7. [DOI] [PubMed] [Google Scholar]
- Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- Hans M, Urrutia A, Deal C, Brust PF, Stauderman K, Ellis SB, Harpold MM, Johnson EC, Williams ME. Structural elements in domain IV that influence biophysical and pharmacological properties of human α1A-containing high-voltage-activated calcium channels. Biophys J. 1999;76:1384–1400. doi: 10.1016/S0006-3495(99)77300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobom M, Dai S, Marais E, Lacinova L, Hofmann F, Klugbauer N. Neuronal distribution and functional characterization of the calcium channel α2δ-2 subunit. Eur J Neurosci. 2000;12:1217–1226. doi: 10.1046/j.1460-9568.2000.01009.x. [DOI] [PubMed] [Google Scholar]
- Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP. Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. J Biol Chem. 1991;266:3287–3293. [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel α2-δ subunit. J Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N, Marais E, Hofmann F. Calcium channel alpha2delta subunits: differential expression, function, and drug binding. J Bioenerg Biomembr. 2003;35:639–647. doi: 10.1023/b:jobb.0000008028.41056.58. [DOI] [PubMed] [Google Scholar]
- Langhorst MF, Reuter A, Stuermer CA. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell Mol Life Sci. 2005;62:2228–2240. doi: 10.1007/s00018-005-5166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais E, Klugbauer N, Hofmann F. Calcium channel alpha(2)delta subunits: structure and gabapentin binding. Mol Pharmacol. 2001;59:1243–1248. doi: 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- Mori Y, Friedrich T, Kim M-S, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, Mikoshiba K, Imoto K, Tanabe T, Numa S. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Morrow IC, Parton RG. Flotillins and the PHB domain protein family: rafts, worms and anaesthetics. Traffic. 2005;6:725–740. doi: 10.1111/j.1600-0854.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- Moss FJ, Viard P, Davies A, Bertaso F, Page KM, Graham A, Canti C, Plumpton M, Plumpton C, Clare JJ, Dolphin AC. The novel product of a five-exon stargazin-related gene abolishes CaV2.2 calcium channel expression. EMBO J. 2002;21:1514–1523. doi: 10.1093/emboj/21.7.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owczarek CM, Treutlein HR, Portbury KJ, Gulluyan LM, Kola I, Hertzog PJ. A novel member of the STOMATIN/EPB72/mec-2 family, stomatin-like 2 (STOML2), is ubiquitously expressed and localizes to HSA chromosome 9p13.1. Cytogenet Cell Genet. 2001;92:196–203. doi: 10.1159/000056902. [DOI] [PubMed] [Google Scholar]
- Page KM, Heblich F, Davies A, Butcher AJ, Leroy J, Bertaso F, Pratt WS, Dolphin AC. Dominant-negative calcium channel suppression by truncated constructs involves a kinase implicated in the unfolded protein response. J Neurosci. 2004;24:5400–5409. doi: 10.1523/JNEUROSCI.0553-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, Thompson RJ, Eshcol JO, Wemmie JA, Benson CJ. Stomatin modulates gating of acid-sensing ion channels. J Biol Chem. 2004;279:53886–53891. doi: 10.1074/jbc.M407708200. [DOI] [PubMed] [Google Scholar]
- Rivera-Milla E, Stuermer CA, Malaga-Trillo E. Ancient origin of reggie (flotillin), reggie-like, and other lipid-raft proteins: convergent evolution of the SPFH domain. Cell Mol Life Sci. 2006;63:343–357. doi: 10.1007/s00018-005-5434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Hell JW, Woppmann A, Miljanich GP, Catterall WA. Immunochemical identification and differential phosphorylation of alternatively spliced forms of the α1A subunit of brain calcium channels. J Biol Chem. 1995;270:21234–21242. doi: 10.1074/jbc.270.36.21234. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Westenbroek RE, Rettig J, Hell J, Catterall WA. Biochemical properties and subcellular distribution of the BI and rbA isoforms of α1A subunits of brain calcium channels. J Cell Biol. 1996;134:511–528. doi: 10.1083/jcb.134.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA. 1996;93:131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shistik E, Ivanina T, Puri T, Hosey M, Dascal N. Ca2+ current enhancement by α2/δ and β subunits in Xenopus oocytes: contribution of changes in channel gating and α1 protein level. J Physiol (Lond) 1995;489:55–62. doi: 10.1113/jphysiol.1995.sp021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton KG, Snutch TP. Gabapentin: a novel analgesic targeting voltage-gated calcium channels. Drug Dev Res. 2001;54:167–172. [Google Scholar]
- Sutton KG, Martin DJ, Pinnock RD, Lee K, Scott RH. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol. 2002;135:257–265. doi: 10.1038/sj.bjp.0704439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna E, Saba E, Rowe J, Francolini M, Clementi F, Rosa P. Role of lipid microdomains in P/Q-type calcium channel (Cav2.1) clustering and function in presynaptic membranes. J Biol Chem. 2004;279:5127–5134. doi: 10.1074/jbc.M308798200. [DOI] [PubMed] [Google Scholar]
- Taylor CP. The biology and pharmacology of calcium channel alpha2-delta proteins Pfizer Satellite Symposium to the 2003 Society for Neuroscience Meeting. Sheraton New Orleans Hotel, New Orleans, LA November 10, 2003. CNS Drug Rev. 2004;10:183–188. doi: 10.1111/j.1527-3458.2004.tb00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toselli M, Biella G, Taglietti V, Cazzaniga E, Parenti M. Caveolin-1 expression and membrane cholesterol content modulate N-type calcium channel activity in NG108–15 cells. Biophys J. 2005;89:2443–2457. doi: 10.1529/biophysj.105.065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca2+ influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol. 2002;449:221–228. doi: 10.1016/s0014-2999(02)02044-7. [DOI] [PubMed] [Google Scholar]
- Vega-Hernandez A, Felix R. Down-regulation of N-type voltage-activated Ca2+ channels by gabapentin. Cell Mol Neurobiol. 2002;22:185–190. doi: 10.1023/A:1019865822069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D, De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels. Trends Neurosci. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- Wang MH, Offord J, Oxender DL, Su TZ. Structural requirement of the calcium-channel subunit α2δ for gabapentin binding. Biochem J. 1999;342:313–320. [PMC free article] [PubMed] [Google Scholar]
- Whittaker CA, Hynes RO. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell. 2002;13:3369–3387. doi: 10.1091/mbc.E02-05-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]