Abstract
Conditioned taste aversion (CTA) is a form of aversive memory in which an association is made between a consumed substance and a subsequent malaise. CTA is a critical mechanism for the successful survival, and hence evolution, of most animal species. The role of excitatory neurotransmitters in the neurochemical mechanisms of CTA is well recognized; however, less is known about the involvement of inhibitory receptor systems. In particular, the potential functions of metabotropic GABAB receptors in CTA have not yet been fully explored. GABAB receptors are metabotropic GABA receptors that are comprised of two subunits, GABAB(1) and GABAB(2), which form heterodimers. The Gabbr1 gene is transcribed into two predominant isoforms, GABAB(1a) and GABAB(1b), which differ in sequence primarily by the inclusion of a pair of sushi domains (also known as short consensus repeats) in the GABAB(1a) N terminus. The behavioral function of mammalian GABAB(1) receptor isoforms is currently unknown. Here, using a point mutation strategy in mice, we demonstrate that these two GABAB(1) receptor isoforms are differentially involved in critical components of CTA. In contrast to GABAB(1b)−/− and wild-type mice, GABAB(1a)−/− mice failed to acquire CTA. In contrast, GABAB(1b)−/− mice robustly acquired CTA but failed to show any extinction of this aversion. The data demonstrate that GABAB receptors are involved in both the acquisition and extinction of CTA; however, receptors containing the GABAB(1a) or the GABAB(1b) isoform differentially contribute to the mechanisms used to learn and remember the salience of aversive stimuli.
Keywords: conditioned taste aversion, GABAB, anxiety, inhibitory, learning, extinction
Introduction
Conditioned taste aversion (CTA) is an associative learning phenomenon whereby the characteristics of a consumed substance are paired with the memory of a subsequent malaise (Bermudez-Rattoni, 2004). CTAs are long-lasting and specific memories that can be induced with a single pairing of the conditioned stimulus (CS) (the consumed substance) and the unconditioned stimulus (the malaise). As such, CTA is a critical mechanism for the successful survival, and hence evolution, of most animal species (Bures, 1998). Moreover, because CTA declines reliably with repeated nonreinforced exposure to the CS, the use of CTA in the laboratory allows the investigation of the processes involved in both the acquisition and extinction of aversive memories (Bahar et al., 2003; Bermudez-Rattoni, 2004).
The importance of excitatory neurotransmitters in the acquisition and extinction of CTA is well known (Berman and Dudai, 2001; Bermudez-Rattoni, 2004). In contrast, there is a paucity of studies investigating the role of inhibitory neurotransmitters in CTA. Some studies have demonstrated that ionotropic GABAA-modulating drugs such as benzodiazepines alter the acquisition and certain aspects of extinction of CTA (Roache and Zabik, 1986; Delamater and Treit, 1988; Yasoshima and Yamamoto, 2005), whereas the role of GABAB receptors is largely uninvestigated.
GABAB receptors are comprised of two subunits, GABAB(1) and GABAB(2), which form heterodimers. The Gabbr1 gene is predominantly transcribed into two differentially expressed isoforms, GABAB(1a) and GABAB(1b), which differ in sequence primarily by the inclusion of a pair of evolutionary conserved sushi repeats (also known as short consensus repeats) in the GABAB(1a) N terminus (Bettler et al., 2004; Cryan and Kaupmann, 2005). The function of the sushi repeats, and hence of the different receptor isoforms, has been a mystery until recently. Vigot et al. (2006) demonstrated that the sushi repeats define the morphological localization of GABAB receptors: the GABAB(1a) isoform was mainly a presynaptic heteroreceptor at glutamatergic terminals, whereas the GABAB(1b) isoform was predominantly located postsynaptically. Given the importance of GABAergic mechanisms in emotional learning (Akirav, 2006; Davis et al., 2006), we used GABAB(1a)−/− and GABAB(1b)−/− mice to address the hypothesis that GABAB receptor isoforms could play distinctive roles in the acquisition and extinction of aversive memories.
Materials and Methods
Establishment of a conditioned taste aversion protocol in BALB/c mice.
Because there are marked strain differences in emotional behavior in mice (Cryan and Holmes, 2005), it was important first to establish an appropriate CTA protocol in the background strain of our genetically modified mice. A two-bottle choice CTA protocol was validated using singly housed male mice ]BALB/cByJIco (Charles River Laboratories, L‘ Abresle Cedex, France); ∼12 weeks of age; n = 30[. Mice were trained to drink water from a 15 ml plastic drinking tube in two 30 min sessions (morning and afternoon) per day for 5 d. Mice were then presented with a saccharin solution (0.5% in tap water) in their drinking tube. Thirty minutes after the end of the 30 min saccharin-drinking period, they were injected (i.p., 10 ml/kg) with either vehicle (saline, unconditioned mice) or the malaise-inducing agent lithium chloride (LiCl; Sigma-Aldrich Chemie, Steinheim, Germany) at a dose of either 3 or 6 mEq/kg (0.3 or 0.6 m LiCl) (conditioned mice). Over the subsequent 7 d, mice were presented with both water and the saccharin solution in the morning drinking sessions. Drinking tubes containing the saccharin solution were always presented in the same spatial order relative to the water tube (e.g., always on the right). Afternoon drinking sessions remained water-only throughout the experiment.
Conditioned taste aversion in wild-type, GABAB(1a)−/−, and GABAB(1b)−/− mice.
The generation of GABAB(1a)−/− and GABAB(1b)−/− has been described previously (Vigot et al., 2006). Briefly, a knock-in point mutation strategy was adopted, whereby GABAB(1a) and GABAB(1b) initiation codons were converted to stop codons by targeted insertion of a floxed neo cassette. Gene targeting constructs and embryonic stem cells were of BALB/c origin. Embryonic stem cells were injected into C57BL/6 blastocysts and chimeras crossed with BALB/c mice to generate heterozygotic founding mice. The neo cassette was excised by crossing to BALB/c mice expressing Cre recombinase and breeding to homozygosity. Consequently, all mutant and wild-type mice were maintained on a pure inbred BALB/c genetic background. GABAB(1a)−/− and GABAB(1b)−/− mice used for the evaluation of CTA were derived from subsequent homozygous breeding (F5–6) of siblings originating from the founding heterozygotic mice. Homozygous wild-type controls for the GABAB(1) isoform mutant mice were derived from mating together wild-type siblings generated from GABAB(1a)+/− and GABAB(1b)+/− heterozygous breedings (F5–F6). The breeding strategy was applied in accordance with the recommendations proposed by The Jackson Laboratory (Bar Harbor, ME) to obviate genetic drift and the formation of substrains (http://jaxmice.jax.org/geneticquality/guidelines.html).
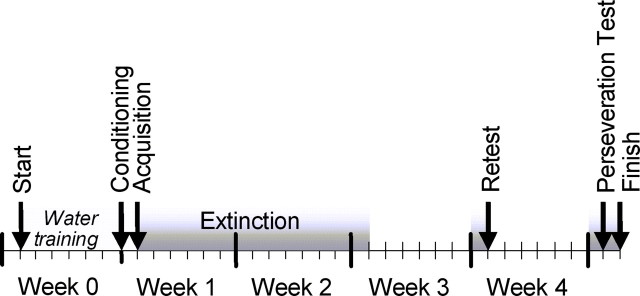
A similar protocol to that validated in house (see above) was used to evaluate CTA in singly housed male wild-type (n = 19; 29.3 ± 0.6 weeks of age), GABAB(1a)−/− (n = 15; 26.8 ± 0.6 weeks of age), and GABAB(1b)−/− (n = 18; 26.4 ± 0.6 weeks of age) mice (Fig. 1). Mice from each genotype were allocated to either an unconditioned (saline injection after saccharin presentation) or a conditioned (6 mEq/kg LiCl after saccharin presentation) treatment. The dose of lithium was selected based on the validation experiment. Furthermore, mice were subjectively scored in a blind manner for the presence or absence of malaise behavior after LiCl or saline injections (Hayley et al., 1999; Anisman et al., 2001). Malaise was defined as prolonged periods of nonsleeping immobility, piloerection, contraction of the flanks, prostrate elongated body posture, and/or excessive defecation or diarrhea. Mice displaying malaise behavior were given a score of 1. Animals not showing malaise behavior were given a score of 0. Sleeping animals were not scored. For 2 weeks after conditioning, mice experienced a once-daily preference test in which they were presented with both saccharin and water for 30 min. In the afternoons, they were given only water for 30 min. They were then returned to an ad libitum water regimen for an additional week. Thereafter, animals were water deprived overnight and again presented with the choice of saccharin or water in the morning. This allowed us to assess whether the aversion was altered over 1 week in the absence of saccharin exposure. Animals were then returned to an ad libitum water regimen for an additional week. Thereafter, animals were water deprived overnight and again presented with the choice of saccharin or water in the morning, with the difference that the spatial order of tube presentation was reversed, which allowed us to determine whether or not perseverative behavior was contributing to the choice of drinking fluid. The following day, the saccharin or water option was presented again, but in the usual order.
Figure 1.
Schematic of a CTA protocol used in GABAB(1) isoform mutant and wild-type mice.
Calculations and statistical analyses.
All drinking tubes were weighed before and after presentation to the mice to obtain the weight of fluid consumed. An aversion index (AI) for the saccharin solution was calculated as follows: AI (%) = ]water intake (g)/]saccharin intake (g) + water intake (g)[[ × 100. Data were analyzed with one-way, two-way, or two-way repeated-measure ANOVA, followed by Fisher’s LSD post hoc comparisons, where appropriate.
Results
Conditioned taste aversion in BALB/c mice
BALB/c mice acquired a robust aversion to both 3 and 6 mEq/kg doses of LiCl compared with the unconditioned (saline-treated) animals (Fig. 2A) (LiCl dose; F(2,29) = 9.87; p < 0.001). Mice treated with LiCl at 3 mEq/kg had extinguished the aversion by 5 d, whereas the mice treated with LiCl at 6 mEq/kg took 7 d to extinguish (Fig. 3A) (p > 0.05), respectively. Therefore, this protocol was deemed as appropriate for detecting alterations in CTA acquisition or extinction in genetically modified mice bred on a BALB/c genetic background.
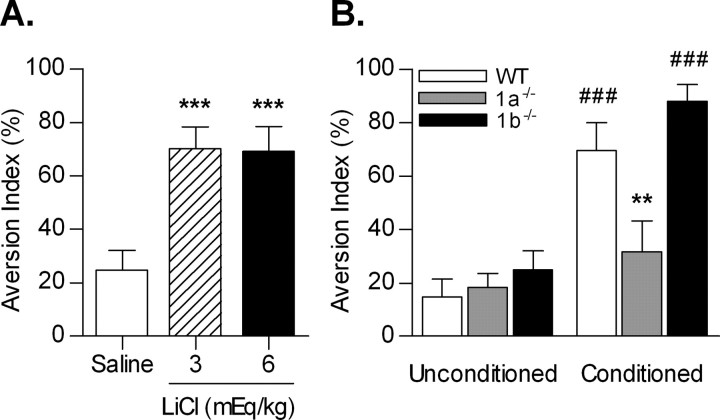
Figure 2.
Acquisition of CTA. A, BALB/c mice acquire CTA to a saccharin solution when paired with malaise induced by LiCl at 3 and 6 mEq/kg (***p < 0.001 vs saline). B, The GABAB(1a) receptor isoform is essential for acquisition of a CTA. WT, Wild type; 1a−/−, GABAB(1a)−/−; 1b−/−, GABAB(1b)−/−. **p < 0.01 versus conditioned wild type; ###p < 0.001 versus unconditioned within genotype.
Figure 3.
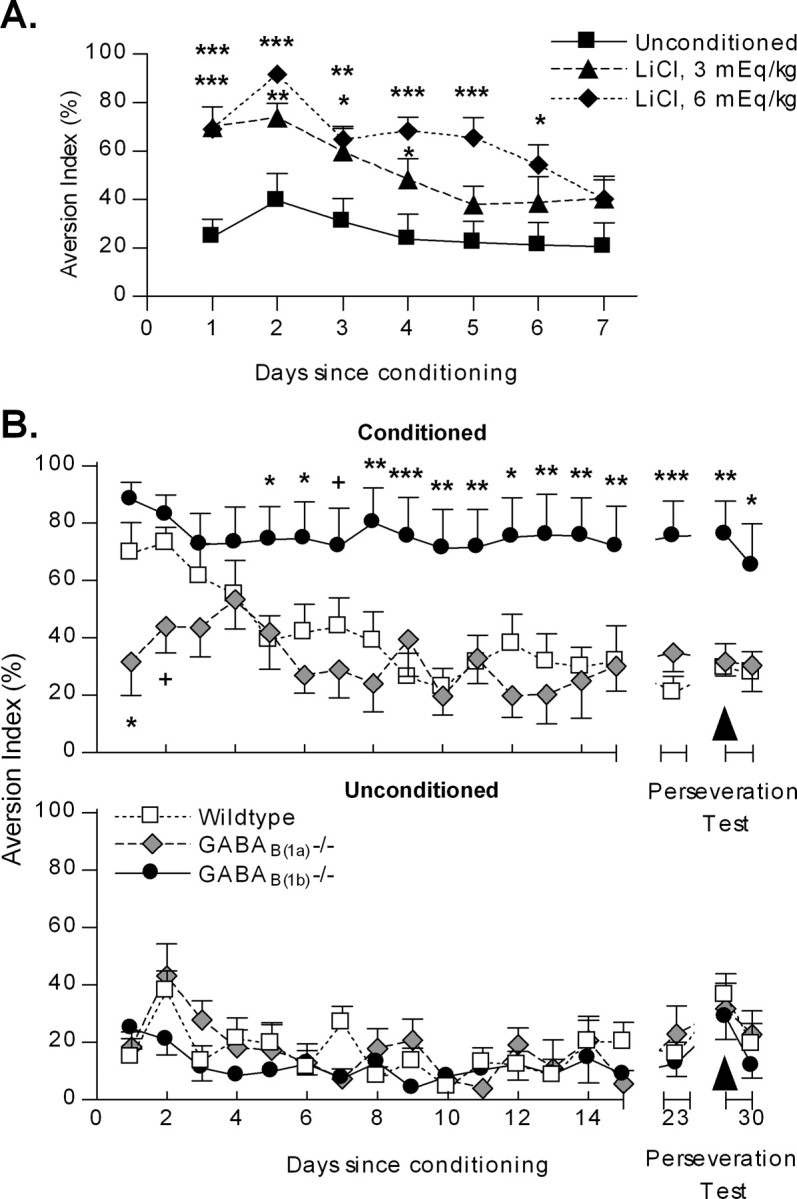
Extinction of CTA. A, Time to extinguish a CTA in BALB/c mice was determined by dose of the malaise-inducing agent LiCl (***p < 0.001, **p < 0.01, *p < 0.05 vs saline, within day). B, Conditioned, Deletion of the GABAB(1b) receptor isoform profoundly impairs extinction of CTA (***p < 0.001, **p < 0.01, *p < 0.05, +p < 0.10 vs wild type). Unconditioned, GABAB(1) isoforms do not influence the development of preference for a saccharin solution in unconditioned mice.
Conditioned taste aversion in GABAB(1a)−/−, GABAB(1b)−/−, and wild-type mice
Mice of all three genotypes readily consumed the saccharin solution on the day of conditioning (mean ± SEM saccharin solution intake: wild type, 1.91 ± 0.08 ml; GABAB(1a)−/−, 2.12 ± 0.13 ml; GABAB(1b)−/−, 1.95 ± 0.09 ml; genotype, F(2,51) = 11.11, p = 0.34).
GABAB(1a)−/− mice that received LiCl after saccharin exposure (conditioned) failed to acquire an aversion to the saccharin solution, relative to conditioned wild-type and GABAB(1b)−/− mice (p < 0.01), and showed a preference for the saccharin solution to a level not different from that of unconditioned GABAB(1a)−/−, GABAB(1b)−/−, or wild-type mice. In comparison, both conditioned wild-type and GABAB(1b)−/− mice developed similar, robust levels of aversion to the saccharin solution, relative to unconditioned controls (p < 0.001) (Fig. 2B) (LiCl, F(1,51) = 39.77, p < 0.001; genotype, F(2,51) = 6.57, p < 0.01; interaction, F(2,51) = 4.60, p < 0.05). The failure of the conditioned GABAB(1a)−/− mice to acquire an aversion to the saccharin solution was not attributable to an insensitivity to LiCl-induced malaise, as indicated by the demonstration of malaise behavior in 100% of the GABAB(1a)−/− mice 1 h after LiCl injections (Table 1).
Table 1.
LiCl (6 mEq/kg, i.p.) induced malaise to an equivalent degree in wild-type, GABAB(1a)−/−, and GABAB(1b)−/− mice
| Time (h) | Wild type |
GABAB(1a)−/− |
GABAB(1b)−/− |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline (n = 9) |
LiCl (n = 10) |
Saline (n = 7) |
LiCl (n = 8) |
Saline (n = 9) |
LiCl (n = 9) |
|||||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Sick | ||||||||||||||||||
| % | 0 | 0 | 0 | 90 | 90 | 67 | 0 | 0 | 0 | 100 | 83 | 57 | 11 | 0 | 0 | 89 | 78 | 100 |
| n | 9 | 9 | 6 | 8 | 6 | 4 | 1 | 8 | 7 | 7 | ||||||||
| Sleep | ||||||||||||||||||
| % | 11 | 87 | 44 | 0 | 0 | 10 | 0 | 29 | 71 | 0 | 25 | 13 | 0 | 44 | 38 | 0 | 0 | 22 |
| n | 1 | 8 | 4 | 1 | 2 | 5 | 2 | 1 | 4 | 3 | 2 | |||||||
Only mice that were awake were scored for the presence or absence of malaise (% sick).
In striking contrast, although GABAB(1b)−/− mice readily acquired the aversion, they failed to show any reduction of this aversion over the following 30 d of the experiment, relative to both unconditioned GABAB(1b)−/− mice and conditioned wild-type and GABAB(1a)−/− mice (Fig. 3B) (conditioning within GABAB(1b)−/−: LiCl, F(1,323) = 27.39, p < 0.001; time, F(17,323) = 2.71, p < 0.001; interaction, F(17,323) = 0.72, p = 0.78; genotype within conditioned treatment: genotype, F(2,480) = 7.32, p < 0.01; time, F(17,480) = 5.06, p < 0.001; interaction, F(34,480) = 1.90, p < 0.01).
Post hoc comparisons revealed significant differences between the conditioned GABAB(1b)−/− and conditioned wild-type mice from day 5 onward (Fig. 3B). This was because of extinction in the conditioned wild-type mice, which reached a low level of aversion not different from that of unconditioned wild-type mice by day 5 after conditioning (Fig. 3B) (conditioning within wild type: LiCl, F(1,326) = 8.22, p = 0.01; day, F(17,326) = 5.2, p < 0.001; interaction, F(17,326) = 2.85, p < 0.001). The reversal of drinking tube presentation on day 29 demonstrated that the GABAB(1b)−/− mice were not simply drinking from the same tube position each day but were actively avoiding the saccharin solution.
In the unconditioned mice, there was no effect of genotype on the AI, although the overall AI decreased over the duration of the experiment until the perseveration test on day 29, when it was transiently elevated (Fig. 3B) (genotype within unconditioned, F(2,434) = 1.49, p = 0.25; day, F(17,434) = 5.04, p < 0.001; interaction, F(34,434) = 1.08, p = 0.36).
Discussion
Our data demonstrate a critical role for GABAB receptors in CTA. Specifically, the two GABAB(1) subunit isoforms are differentially involved in the acquisition and extinction of CTA. Acquisition of CTA requires the GABAB(1a) isoform, whereas extinction requires the GABAB(1b) receptor isoform.
It has recently been shown that the presence of specific sushi domains directs GABAB(1a) isoforms to a presynaptic localization and that this localization is critical for cognitive performance as assessed using an object-recognition task (Vigot et al., 2006). Furthermore, GABAB(1a)−/− mice have impaired hippocampal long-term potentiation and lack presynaptic GABAB-ergic inhibition of glutamatergic excitability (Vigot et al., 2006). Given that glutamate signaling is essential for the acquisition of CTA (Yasoshima et al., 2000; Bermudez-Rattoni, 2004; Akirav, 2006), it therefore seems plausible that GABAB(1a) isoform modulation of presynaptic glutamate release may underlie the mechanisms of CTA acquisition. The brain regions involved in such modulation are unknown presently, because the GABAB(1a) receptor isoform is widely expressed throughout the brain (Benke et al., 1999; Bischoff et al., 1999; Fritschy et al., 1999). However, lesion or inactivation of the pontine parabrachial nucleus, amygdala, or insular cortex disrupts the acquisition of CTA (Bermudez-Rattoni and Yamamoto, 1998; Bermudez-Rattoni, 2004), which points to GABAB(1a) receptors in these structures as being crucial for CTA acquisition.
Given the differential localization of GABAB(1) isoforms (Perez-Garci et al., 2006; Vigot et al., 2006), the very dissimilar phenotype of GABAB(1b)−/− compared with GABAB(1a)−/− mice was not entirely unexpected. Indeed, unlike GABAB(1a)−/− mice, GABAB(1b)−/− mice readily acquired CTA but failed to extinguish the aversion despite repeated unreinforced exposures to the CS. Similarly to the acquisition of associative learning, its extinction is believed to be a learning process that results from the formation of new memories, as opposed to simple forgetting (Myers and Davis, 2002; Davis et al., 2006). It has been suggested that the study of CTA may have direct implications for the study of anxiety disorders associated with altered emotional learning (Bahar et al., 2003; Bermudez-Rattoni, 2004; Guitton and Dudai, 2004; Cryan and Holmes, 2005). Therefore, the understanding of the molecular mechanisms that underlie the extinction of established aversive memories would be a considerable breakthrough in the treatment and management of anxiety disorders (Ressler et al., 2004; Barad, 2005; Davis et al., 2006).
Until recently, no unique pharmacological or functional properties could be assigned to GABAB(1a) or GABAB(1b) (Perez-Garci et al., 2006; Vigot et al., 2006). However, it has been proposed that auxiliary proteins exist that modify receptor activity, pharmacology, and localization (Marshall et al., 1999). Our data clearly show differential functions of GABAB(1) receptor isoforms in the acquisition (GABAB(1a)) and extinction (GABAB(1b)) of CTA. Thus, future studies must focus on uncovering potential novel protein interacting sites at either receptor isoform to enable pharmaceutical intervention. Together, our data demonstrate that isoforms of the GABAB(1) receptor, which differ only in the presence or absence of a pair of sushi repeats at their N-terminal ectodomain, play differential yet critical roles in the evolutionary conserved mechanisms used to learn and remember the salience of aversive stimuli.
Footnotes
This work was supported by National Institutes of Mental Health/National Institute on Drug Abuse Grant U01 MH69062 (L.H.J., P.H.K., K.K., and J.F.C.) and by Swiss Science Foundation Grant 3100-067100.01 (B.B.). We thank Prof. Daniel Hoyer for critical reading of this manuscript.
References
- Akirav I. NMDA partial agonist reverses blocking of extinction of aversive memory by GABA(A) agonist in the amygdala. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301050. in press. [DOI] [PubMed] [Google Scholar]
- Anisman H, Hayley S, Kelly O, Borowski T, Merali Z. Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: mouse strain-dependent outcomes. Behav Neurosci. 2001;115:443–454. [PubMed] [Google Scholar]
- Bahar A, Samuel A, Hazvi S, Dudai Y. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. Eur J Neurosci. 2003;17:1527–1530. doi: 10.1046/j.1460-9568.2003.02551.x. [DOI] [PubMed] [Google Scholar]
- Barad M. Fear extinction in rodents: basic insight to clinical promise. Curr Opin Neurobiol. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Benke D, Honer M, Michel C, Bettler B, Mohler H. Gamma aminobutyric acid type B receptor splice variant proteins GBR1a and GBR1b are both associated with GBR2 in situ and display differential regional and subcellular distribution. J Biol Chem. 1999;274:27323–27330. doi: 10.1074/jbc.274.38.27323. [DOI] [PubMed] [Google Scholar]
- Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Yamamoto T. Neuroanatomy of CTA: lesion studies. In: Bures J, Bermudez-Rattoni F, Yamamoto T, editors. Conditioned taste aversion: memory of a special kind. New York: Oxford UP; 1998. pp. 28–46. [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bischoff S, Leonhard S, Reymann N, Schuler V, Shigemoto R, Kaupmann K, Bettler B. Spatial distribution of GABA(B)R1 receptor mRNA and binding sites in the rat brain. J Comp Neurol. 1999;412:1–16. [PubMed] [Google Scholar]
- Bures J. Ethological, physiological psychology, and neurobiology of CTA. In: Bures J, Bermudez-Rattoni F, Yamamoto T, editors. Conditioned taste aversion: memory of a special kind. New York: Oxford UP; 1998. pp. 1–13. [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K. Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM, Chhatwal J, Ressler KJ. Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx. 2006;3:82–96. doi: 10.1016/j.nurx.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR, Treit D. Chlordiazepoxide attenuates shock-based and enhances LiCl-based fluid aversions. Learn Motiv. 1988;19:221–238. [Google Scholar]
- Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Guitton MJ, Dudai Y. Anxiety-like state associates with taste to produce conditioned taste aversion. Biol Psychiatry. 2004;56:901–904. doi: 10.1016/j.biopsych.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Hayley S, Brebner K, Lacosta S, Merali Z, Anisman H. Sensitization to the effects of tumor necrosis factor-α: neuroendocrine, central monoamine, and behavioral variations. J Neurosci. 1999;19:5654–5665. doi: 10.1523/JNEUROSCI.19-13-05654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall FH, Jones KA, Kaupmann K, Bettler B. GABA(B) receptors—the first 7TM heterodimers. Trends Pharmacol Sci. 1999;20:396–399. doi: 10.1016/s0165-6147(99)01383-8. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Perez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABA(B1b) isoform mediates long-lasting inhibition of dendritic Ca(2+) spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Roache JD, Zabik JE. Effects of benzodiazepines on taste aversions in a two-bottle choice paradigm. Pharmacol Biochem Behav. 1986;25:431–437. doi: 10.1016/0091-3057(86)90020-1. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Bräuner-Osborne H, Turecek R, Shigemoto R, Zhang Y-P, Luján R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Müller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoshima Y, Yamamoto T. Effects of midazolam on the expression of conditioned taste aversion in rats. Brain Res. 2005;1043:115–123. doi: 10.1016/j.brainres.2005.02.070. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Morimoto T, Yamamoto T. Different disruptive effects on the acquisition and expression of conditioned taste aversion by blockades of amygdalar ionotropic and metabotropic glutamatergic receptor subtypes in rats. Brain Res. 2000;869:15–24. doi: 10.1016/s0006-8993(00)02397-0. [DOI] [PubMed] [Google Scholar]