Abstract
Schwann cell phenotype is classified as either myelinating or nonmyelinating. Additional phenotypic specialization is suggested, however, by the preferential reinnervation of muscle pathways by motoneurons. To explore potential differences in growth factor expression between sensory and motor nerve, grafts of cutaneous nerve or ventral root were denervated, reinnervated with cutaneous axons, or reinnervated with motor axons. Competitive reverse transcription-PCR was performed on normal cutaneous nerve and ventral root and on graft preparations 5, 15, and 30 d after surgery. mRNA for nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor, hepatocyte growth factor, and insulin-like growth factor-1 was expressed vigorously by denervated and reinnervated cutaneous nerve but minimally by ventral root. In contrast, mRNA for pleiotrophin (PTN) and glial cell line-derived neurotrophic factor was upregulated to a greater degree in ventral root. ELISA confirmed that NGF and BDNF protein were significantly more abundant in denervated cutaneous nerve than in denervated ventral root, but that PTN protein was more abundant in denervated ventral root. The motor phenotype was not immutable and could be modified toward the sensory phenotype by prolonged reinnervation of ventral root by cutaneous axons. Retrograde labeling to quantify regenerating neurons demonstrated that cutaneous nerve preferentially supported cutaneous axon regeneration, whereas ventral root preferentially supported motor axon regeneration. Schwann cells thus express distinct sensory and motor phenotypes that are associated with the support of regeneration in a phenotype-specific manner. These findings suggest that current techniques of bridging gaps in motor and mixed nerve with cutaneous graft could be improved by matching axon and Schwann cell properties.
Keywords: peripheral nerve, deafferentation, retrograde labeling, Schwann cell, RT-PCR, reinnervation, pleiotrophin, BDNF, NGF, GDNF
Introduction
Schwann cells are routinely described as expressing one of two possible phenotypes, myelinating or nonmyelinating (Mirsky and Jessen, 1999). We now present evidence that Schwann cells also express distinct sensory and motor phenotypes and that these phenotypes are associated with modality-specific promotion of axon regeneration.
The idea that sensory and motor pathways might differ was first suggested by studies of muscle spindle reinnervation (Ramon y Cajal, 1928) and crossed repair of cutaneous and muscle nerve (Langley and Anderson, 1904), both of which revealed motor axons escaping from cutaneous pathways to reinnervate appropriate targets. Ramon y Cajal (1928) attributed this behavior to “a neurotropic influence which has an individual and specific character” for each type of end organ. When forcefully misdirected, however, motor axons extended over long distances within cutaneous nerve and remained stable in this environment (Kilvington, 1941), suggesting that sensory and motor pathways were equivalent.
A functional difference between sensory and motor nerve was not unmasked until experiments were designed to provide regenerating motor axons with equal access to both environments. Under these conditions, motor axons preferentially reinnervated muscle pathways, even when these pathways had been blocked off distally to prevent axons from reaching muscle (Brushart, 1993). These motoneurons were also found to support significantly more myelinated collaterals in cutaneous nerve than in muscle nerve, indicating a modality-specific difference in the ability of pathways to stimulate and/or maintain collateral sprouts (Redett et al., 2005). Cutaneous and muscle nerve thus differ in ways that can be detected by regenerating motor axons and that can significantly modify their behavior.
The search for differences between sensory and motor nerve revealed a strong association between expression of the L2 carbohydrate epitope and the preferential reinnervation of muscle nerve by motoneurons (Martini et al., 1994), yet a direct functional link could not be established by antibody blocking experiments (Mears et al., 2003). Examination of growth factor expression by denervated dorsal and ventral root was more fruitful. In preliminary experiments, semiquantitative reverse transcription (RT)-PCR demonstrated that nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and glial cell line-derived neurotrophic factor (GDNF) were all upregulated in dorsal root, whereas only GDNF was upregulated in ventral root (Höke et al., 2001).
The current experiments were designed to search more broadly for differential expression of growth factors that could characterize sensory or motor nerve. Expression of these factors was determined by competitive RT-PCR on grafts of cutaneous nerve, a pure population of sensory axons, or ventral root, a pure population of motor axons. Dorsal root was not used because of the possibility that Schwann cells might in some way reflect the limited regenerative capacity of dorsal root axons (Moyer and Kimmel, 1948). Grafts were either denervated, or denervated and reinnervated by pure populations of cutaneous or motor axons (see Fig. 1). Cutaneous nerve and ventral root were found to differ strikingly in their expression profiles. Additional experiments in which reinnervating neurons were retrogradely labeled demonstrated that cutaneous nerve preferentially supported cutaneous axons, whereas ventral root preferentially supported motor axons. The unique expression patterns of cutaneous nerve and ventral root were thus found to correlate with their ability to support modality-specific regeneration.
Figure 1.
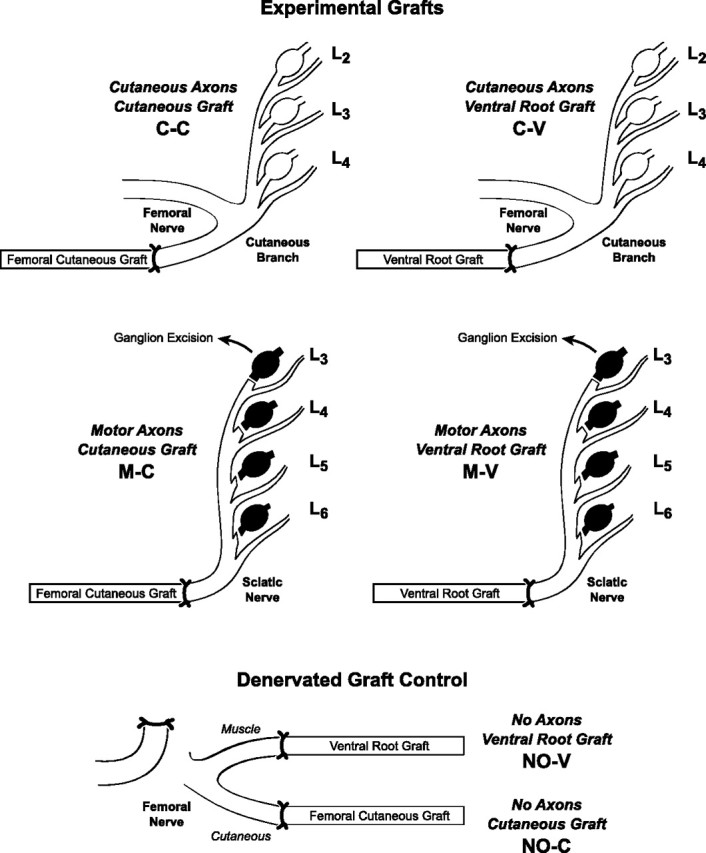
Surgical preparations analyzed in these experiments. The cutaneous branch of the femoral nerve was used as a source of cutaneous axons and the sciatic nerve, deafferented by removal of dorsal root ganglia L3, L4, L5, and L6, as a pure source of motor axons. Grafts were obtained from the femoral cutaneous nerve or the L4 or L5 ventral roots. Experimental groups are identified as to axon source (C, cutaneous axons; M, motor axons) and as to graft type (C, cutaneous nerve graft; V, ventral root graft); the label C-V thus identifies preparations in which cutaneous axons grow into ventral root grafts. Denervated graft controls were provided by suturing cutaneous nerve and ventral root grafts to the femoral cutaneous and muscle branches and then interrupting the donor femoral nerve proximally during the same procedure to allow normal graft revascularization while preventing axon ingrowth. Because no axons are present in these controls, the letters NO identify the axon source (e.g., NO-V describes preparations in which ventral root grafts are revascularized without the possibility of axon ingrowth).
Materials and Methods
Axon counts.
Axon counts were performed on ventral roots to select grafts that were structurally similar to the femoral cutaneous nerve. L4 (n = 3) and L5 (n = 3) ventral roots were harvested from 250 g Lewis rats, fixed in 5% glutaraldehyde, osmicated, and embedded in Epon–Araldite. Sections were cut at 1 μm and counterstained with methylene blue. Myelinated axons were counted on composite reconstructions made from overlapping photographs taken at 500×. Myelinated axon counts in the femoral cutaneous branch were obtained from a previous study (Brushart, 1988).
Surgical preparations.
Female Lewis rats were anesthetized by intramuscular injection of ketamine (87 mg/kg) and xylazine (13 mg/kg). All procedures were performed under sterile conditions and were approved by the Johns Hopkins Animal Care and Use Committee. Four experimental configurations were generated to reinnervate cutaneous nerve graft with cutaneous [cutaneous axons, cutaneous nerve graft (C-C)] or motor axons [motor axons, cutaneous nerve graft (M-C)] and to similarly reinnervate grafts of ventral root with cutaneous [cutaneous axons, ventral root graft (C-V)] or motor axons [motor axons, ventral root graft (M-V)] (Fig. 1). A pure source of sensory axons was obtained by transecting the femoral cutaneous nerve of 250 g rats distal to its separation from the motor branches to pectineus and quadriceps muscles. At this level, it provided an excellent size match for both cutaneous and ventral root grafts. A pure population of motor axons was obtained in 100 g rats by unilateral excision of the L3, L4, L5, and L6 dorsal root ganglia (DRGs), followed by sciatic nerve transection. Younger rats were used for these preparations to ensure that the sciatic nerve would be the same diameter as the grafts and because young animals better withstood the stress of DRG excision.
Grafts of both cutaneous nerve and ventral root were harvested from 250 g female Lewis rats and were thus identical throughout all experiments. The femoral cutaneous nerve was transected proximally just below the femoral bifurcation, where it is unifascicular, and again 2 cm distally. Two-centimeter lengths of L4 and L5 ventral root were harvested bilaterally from donor rats that were then killed. Grafts were maintained in sterile chilled saline until implantation and then sutured to the donor femoral cutaneous or sciatic nerve with two 11-0 sutures placed under 20–40× magnification.
Denervated graft controls were designed to permit normal graft revascularization and Wallerian degeneration but without axon ingrowth (Fig. 1). Cutaneous nerve grafts were sewn to the femoral cutaneous nerve [no axons, cutaneous nerve graft (NO-C)], and ventral root grafts were sewn to the femoral muscle branch [no axons, ventral root graft (NO-V)]. The femoral nerve was then divided proximally to prevent axon regeneration. This configuration was chosen entirely on the basis of a good donor-graft size match to permit normal graft revascularization; the sensory/motor identity of the proximal stump was irrelevant, because no axons were present.
Additional animals were prepared to determine the response of Schwann cell phenotype to prolonged inappropriate reinnervation (Fig. 2, phenotype plasticity experiment). Ventral root grafts were harvested from female Lewis rats and sutured to the femoral cutaneous nerve of male Lewis rats. Cutaneous axons were allowed to reinnervate the grafts for 1 month, after which the grafts were transected 2 mm distal to the original host/graft juncture in an attempt to exclude migrating Schwann cells. The remaining ventral root graft was allowed to degenerate for 1 week and was then subjected to RT-PCR analysis to detect growth factor gene expression as described below. Potential migration of host Schwann cells into the harvested portion of the graft was evaluated using PCR for a Y-chromosome-specific gene, also described below.
Figure 2.
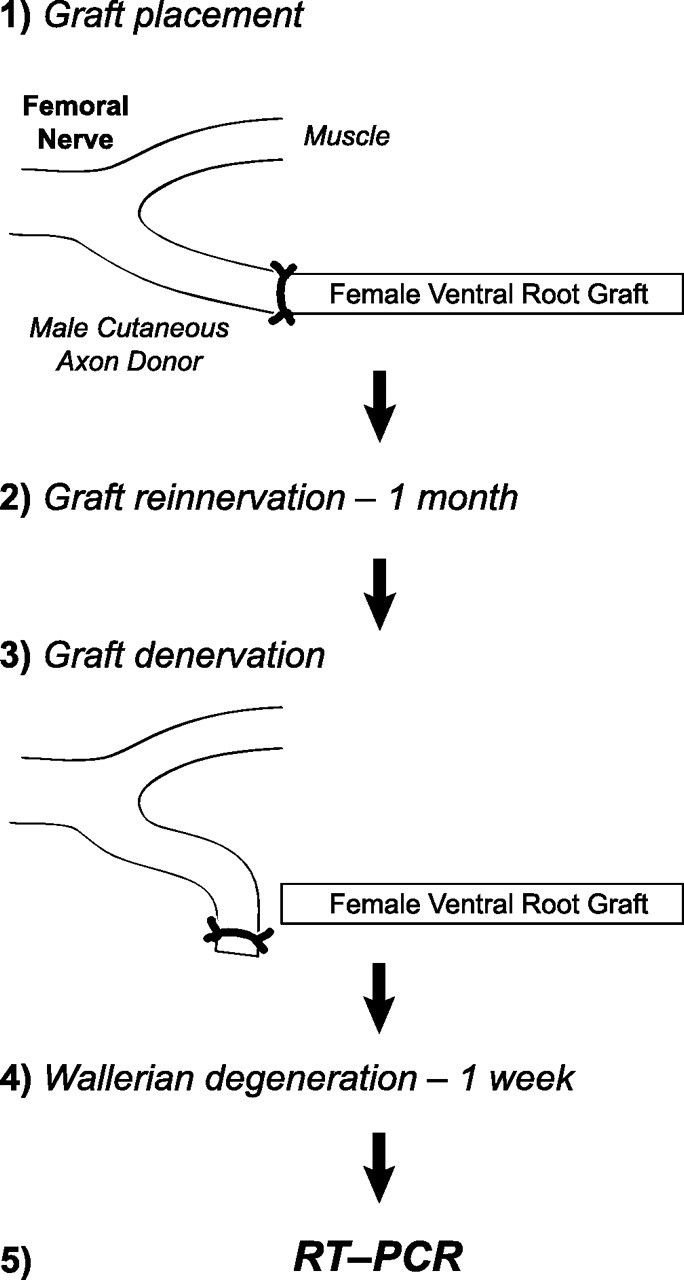
Phenotype plasticity experiment. Ventral root grafts harvested from female Lewis rats were sewn to the femoral cutaneous branch of male Lewis rats. After 1 month of inappropriate reinnervation by cutaneous axons, the grafts were denervated for 1 week and their expression profiles were determined by RT-PCR. A portion of the proximal graft was excised at the time of denervation to exclude tissue that could have been invaded by host Schwann cells. PCR for a Y-chromosome-specific gene was performed on genomic DNA isolated from the grafts to ensure that the remaining Schwann cells were of graft origin.
PCR experiments.
Grafts from the two denervation control (NO-C and NO-V) and four experimental preparations (C-C, C-V, M-C, and M-V) were examined at 5, 15, and 30 d after surgery, yielding a total of 18 groups of nerves. An additional group of ventral root grafts from the phenotype plasticity experiment was examined after reinnervation by sensory axons for 1 month and denervation for 1 week. Twelve grafts were set up for each group, requiring a total of 228 surgical preparations for PCR analysis. Additional normal femoral cutaneous nerves and L4 and L5 ventral roots were used for normalization of the mRNA levels (i.e., day 0).
Total RNA was extracted from the nerves using Trizol (Invitrogen, Carlsbad, CA). The cDNA was synthesized using 2 μg of total RNA in the presence of Ready-to-Go You-Prime First-Strand Beads (Amersham Biosciences, Arlington Heights, IL) and random primers (Invitrogen). Measurements of mRNA levels were performed by real-time RT-PCR using two-color DNA Engine Opticon System (MJ Research, Watertown, MA), and the relative amount of gene of interest was normalized to the mRNA amount of an internal control (glyceraldehyde-3-phosphate dehydrogenase) in the same PCR reaction. To avoid the possibility of amplifying contaminating DNA, all of the primers for real-time RT-PCR were designed with an intron sequence inside the cDNA to be amplified. Reactions were performed with appropriate negative control samples (template-free control samples). A uniform amplification of the products was rechecked by analyzing the melting curves of the amplified products (dissociation graphs). The melting temperature (Tm) was 57–60°C. The probe Tm was at least 10°C higher than primer Tm, and gel electrophoresis was performed to confirm the correct size of the amplification and the absence of nonspecific bands. Protein products were sequenced, and the sequences were validated at the time each probe was constructed. The RT-PCR experiments were conducted by a technician who was blinded to the nature of groups. The real-time PCR parameters and the primer and probe sequences are presented in supplemental Table 1 (available at www.jneurosci.org as supplemental material). The data were analyzed using ANOVA with post hoc correction for multiple comparisons (the critical a level was set at p = 0.005).
Tracing experiments.
Reinnervation was assessed in groups C-C, C-V, M-C, and M-V after 2 weeks of regeneration, because trophic factor expression usually peaked at that time. The graft was crushed with a narrow microforceps 1 cm from the host/graft juncture. A micropipette was then introduced through the epineurium and perineurium distal to the crush and advanced intraneurally to the crush zone. Approximately 0.5 μl of a 5% solution of Fluoro-Ruby (Invitrogen) was injected with a Picospritzer (Parker Hannefin, Fairfield, NJ) to restore the flattened crush zone to its normal rounded contour (Brushart et al., 2002). Forty-eight hours later, the animals were deeply anesthetized before being perfused through the left ventricle. A warm saline flush (150 ml) was followed by 500 ml of 4% paraformaldehyde in 0.1 m Sorensen's phosphate buffer. Postfixation in the same solution overnight followed by immersion in 20% sucrose, also in 0.1 m Sorensen's phosphate buffer, prepared the tissue for sectioning. Sections (40 μm) of spinal cord or L2, L3, and L4 DRGs were cut on a freezing microtome, serially mounted on glass slides, dried, and overlaid with coverslips using dimeric paraxylene (Aldrich, Milwaukee, WI) to minimize extraneous fluorescence.
Spinal cord and DRG sections were viewed with fluorescent light (555 nm excitation, 580 nm emission) by an observer unaware of the graft type used. All nuclei within labeled neurons were counted, and the presence of split nuclei was corrected for as described by Abercrombie (1946). Counts were performed on 11 rats in which motor axons reinnervated ventral root (M-V) and on eight rats from each of the other three groups (M-C, C-C, and C-V) and were compared by unpaired t test analysis.
Determination of graft Schwann cell origin.
To evaluate a potential confounding factor in the phenotype plasticity experiment, migration of host Schwann cells into the graft, we placed ventral root grafts from female rats into male hosts. At the end of the study, we performed PCR for a Y-chromosome-specific gene in genomic DNA isolated from the grafts. We isolated genomic DNA using the Trizol method as explained above and performed PCR for a Y-chromosome-specific gene (An et al., 1997). Forward (gca caa gtt ggc tca aca ga) and reverse (acc ctt cga tga ggc tga ta) primers were designed based on GenBank accession number NM_012772. Positive controls were ventral roots from male rats, and negative controls were female ventral roots that had not been grafted.
Protein isolation and ELISA.
Protein measurements of NGF, GDNF, and BDNF were made using kits (catalog numbers G7630, 7620, and 7611) from Promega (Madison, WI) according to the instructions of the manufacturer. Measurement of pleiotrophin (PTN) was done by developing an ELISA assay based on standard protocols. Briefly, the 96-well ELISA microplates were coated with a goat anti-PTN antibody (catalog number P3743; Sigma, St. Louis, MO) at 1:200 dilution overnight at 4°C and then blocked at room temperature for 1 h using blocking buffer. Samples (10 μg of total protein per well) and standards (starting at 500 pg/ml) were loaded onto the wells, incubated at room temperature on an orbital shaker for 6 h, washed five times, and incubated with second anti-PTN antibody at 1:1000 dilution (rabbit polyclonal; catalog number AB14025; Abcam, Cambridge, MA) overnight at 4°C. This was followed by five washes and incubation with anti-rabbit IgG HRP (catalog number W4011; Promega) at 1:2000 dilution at room temperature for 2 h. After final washes, chromogen was added, optical density was read using SPECTRAmax 340PC (Molecular Devices, Palo Alto, CA), and concentrations were calculated based on the standards curve.
Results
Axon counts
A crucial element of the experimental model is the provision of cutaneous nerve and ventral root grafts that are approximately equal in size, so that the phenotype of the graft Schwann cells will be the only significant variable. We chose the L4 and L5 ventral roots and the femoral cutaneous nerve because they were the best match possible in the rat. The L4 ventral root contained a mean ± SD of 1578 ± 81 myelinated axons (n = 3), and the L5 ventral root contained a mean ± SD of 1462 ± 19 myelinated axons (n = 3). Previous analysis revealed a mean ± SD of 1672 ± 46 myelinated axons in the femoral cutaneous nerve (n = 4) (Brushart, 1988).
Neuron labeling
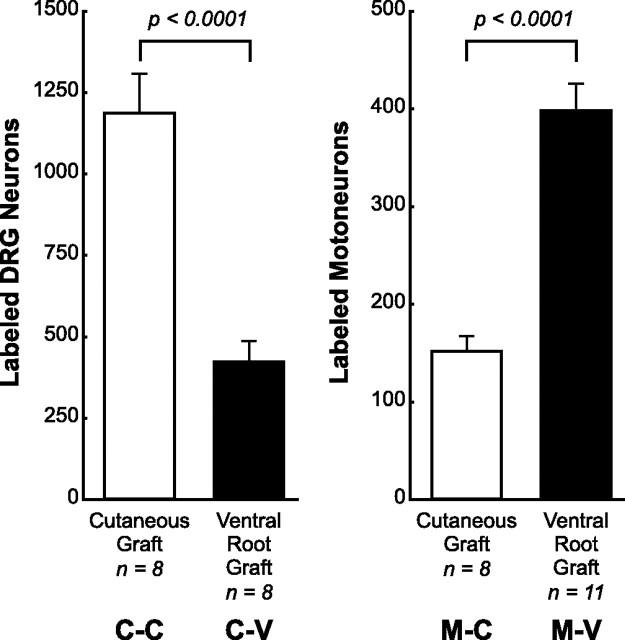
Regeneration in the four experimental groups was assessed by retrograde labeling to ensure that significant axon ingrowth had occurred and to compare the performance of the four combinations of axon and pathway (Fig. 3). After 2 weeks of regeneration, a mean ± SE of 1188 ± 120 DRG neurons projected axons 1 cm down the graft when it was cutaneous nerve (C-C), but a mean ± SE of only 423 ± 61 extended this far when it was ventral root (C-V) (p < 0.0001). Conversely, a mean ± SE of 398 ± 28 motoneurons projected their axons 1 cm down the graft when it was ventral root (M-V), but a mean ± SE of only 151 ± 16 reached this far when it was cutaneous nerve (M-C) (p < 0.0001). Cutaneous nerve thus selectively promotes the regeneration of sensory axons, whereas ventral root selectively promotes the regeneration of motor axons. One consequence of this differential support is that more Schwann cells will be contacted by axons when regeneration is modality specific than when it is heterologous. This could, in turn, translate into more growth factor expression in the groups in which axon and pathway are matched (groups C-C and M-V). As we will show, growth factor expression in cutaneous nerve and ventral root often differed dramatically, minimizing the impact of differential axon growth on differences among the experimental groups.
Figure 3.
Results of retrograde labeling in the four experimental groups, identified as to axon source and graft type as in Figure 1. Label was applied to the center of the graft after reinnervation had progressed for 2 weeks. Labeled neurons were then counted in the spinal cord or L2, L3, and L4 DRGs as appropriate. Sensory neuron regeneration was preferentially supported by cutaneous nerve grafts, whereas motor axon regeneration was preferentially supported by ventral root grafts.
Differences in growth factor expression at baseline
We used competitive RT-PCR to examine the baseline expression levels of 11 growth factors in intact ventral root and femoral cutaneous nerve. This information will serve as the basis for comparing subsequent upregulation of these factors after denervation or reinnervation of ventral root and cutaneous nerve grafts. Factors were characterized as more prominent in ventral root, more prominent in cutaneous nerve, or expressed equally in both (Fig. 4). Expression of vascular endothelial growth factor-1 (VEGF-1), insulin-like growth factor-1 (IGF-1), and PTN was much higher in intact ventral root than in intact cutaneous nerve. In contrast, baseline expression of BDNF, neurotrophin-3 (NT-3), hepatocyte growth factor (HGF), and GDNF was higher in cutaneous nerve than in ventral root. NGF, the prototypical sensory growth factor, was expressed similarly in both nerve types, as were IGF-2, fibroblast growth factor-2 (FGF-2), and ciliary neurotrophic factor (CNTF).
Figure 4.
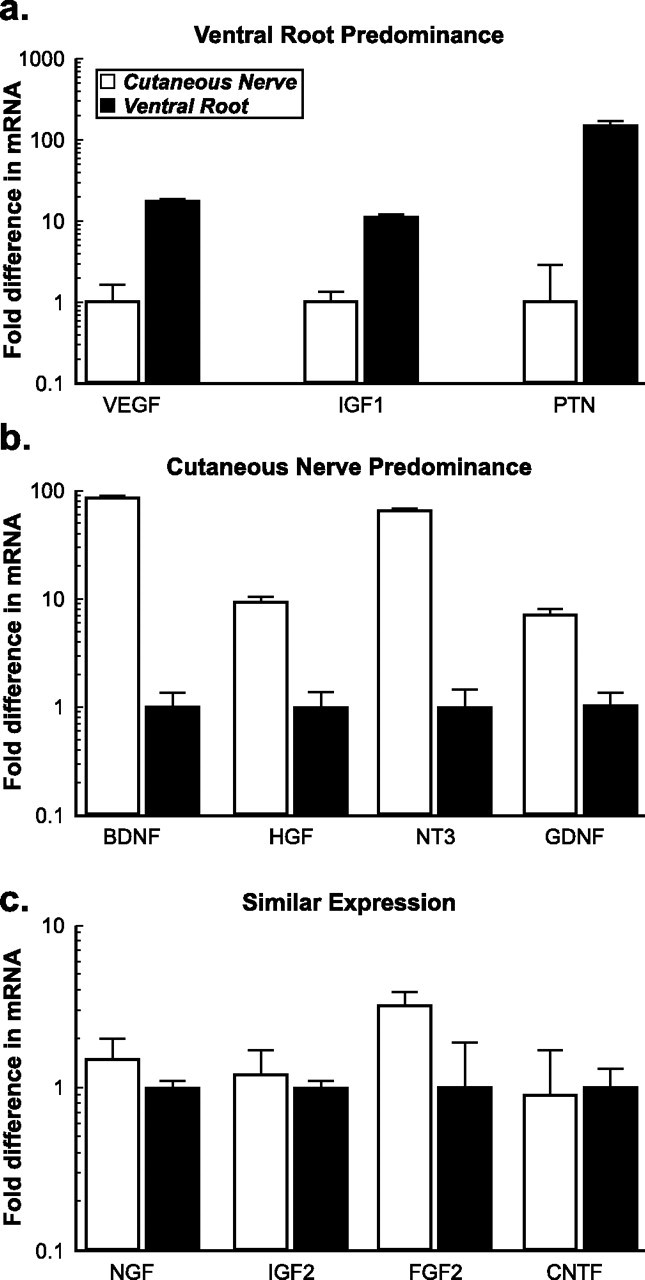
Comparison of baseline growth factor expression in intact ventral root and cutaneous nerve. Each factor is characterized as to whether relative mRNA levels were higher in ventral root (a), higher in cutaneous nerve (b), or similar in both types of nerve (c). For each comparison, the lower expression level is normalized as 1 arbitrary unit of expression.
Differences in growth factor expression after denervation
Current evidence suggests that denervated motor and sensory Schwann cells resume a more primitive, nonmyelinating phenotype and express similar growth factors (Mirsky and Jessen, 1996). As we have shown (see Introduction), however, studies of regeneration specificity indicate that degenerating cutaneous and motor nerves differ in ways that influence motor axon behavior. To search for potential sensory/motor differences, we used competitive RT-PCR to examine the expression of 11 growth factors 5, 15, and 30 d after denervation of the femoral cutaneous nerve (group NO-C) and the L4 and L5 ventral roots (group NO-V). Substantial differences emerged (Figs. 5–7). Five of the growth factors were significantly upregulated in denervated cutaneous nerve but minimally if at all in denervated ventral root. These growth factors included not only the traditional sensory neurotrophins NGF and BDNF but also IGF-1, HGF, and VEGF. That these five growth factors were minimally upregulated by denervated ventral root after even 15 or 30 d of denervation suggests that the motor phenotype survives denervation and Schwann cell division. When viewed in light of baseline expression levels (Fig. 4), the predominance of BDNF and HGF in sensory nerve is accentuated further and that of NGF is unchanged; the relative levels of VEGF and IGF-1 are lowered.
Figure 5.
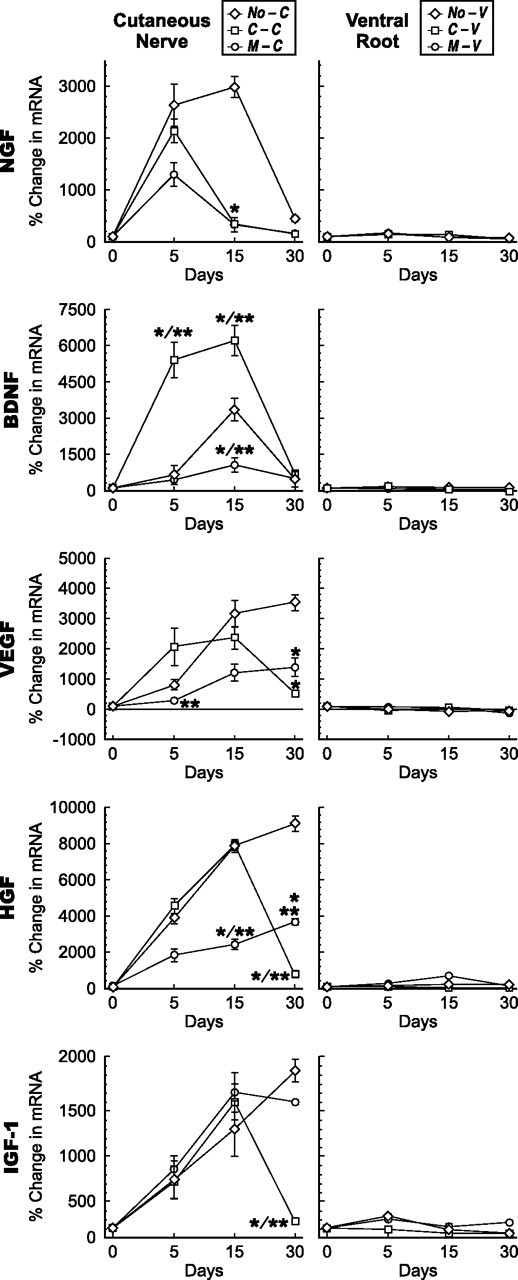
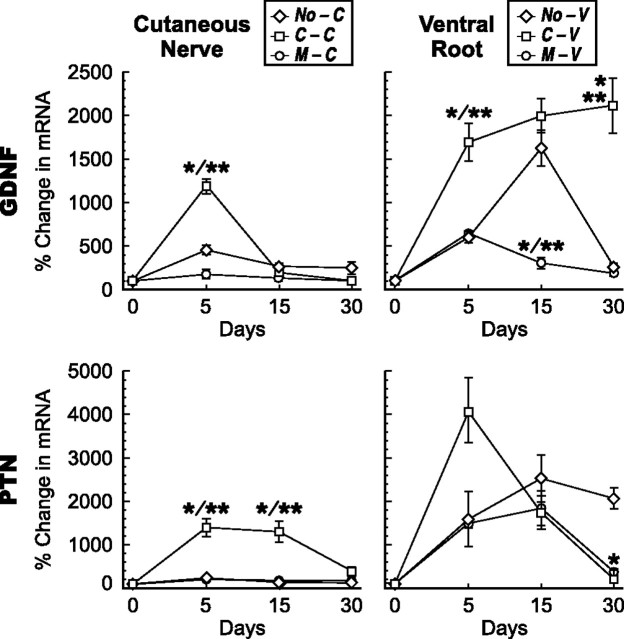
Growth factors expressed predominately in cutaneous nerve. Changes in mRNA levels in cutaneous nerve grafts (left column) and ventral root grafts (right column) after denervation, and reinnervation by motor or sensory axons at 5, 15, and 30 d. The abbreviations established in Figure 1 are used to identify the groups. *p < 0.005 compared with denervation alone; **p < 0.005 motor versus sensory reinnervation.
Figure 6.
Growth factors expressed predominately in ventral root. Changes in mRNA levels in cutaneous nerve grafts (left column) and ventral root grafts (right column) after denervation, and reinnervation by motor or sensory axons at 5, 15, and 30 d. The abbreviations established in Figure 1 are used to identify the groups. *p < 0.005 compared with denervation alone; **p < 0.005 motor versus sensory reinnervation.
Figure 7.
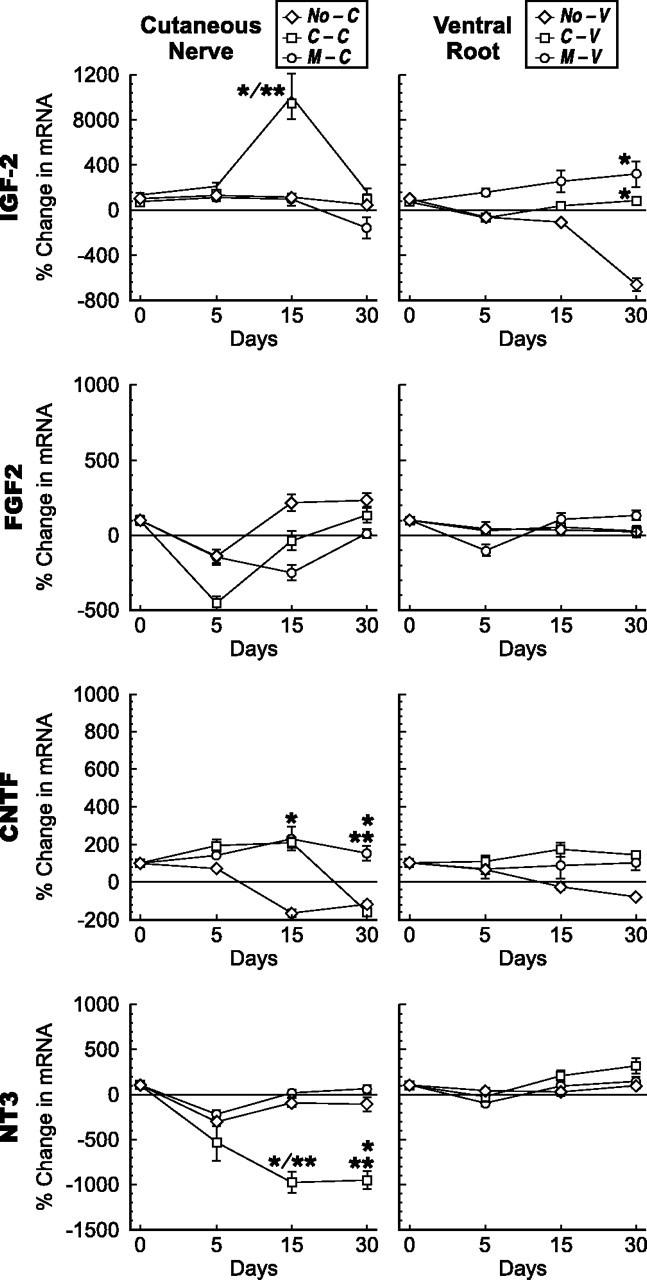
Growth factors that lack modality specificity. Changes in mRNA levels in cutaneous nerve graft (left column) and ventral root graft (right column) after denervation, and reinnervation by motor or sensory axons at 5, 15, and 30 d. The abbreviations established in Figure 1 are used to identify the groups. *p < 0.005 compared with denervation alone; **p < 0.005 motor versus sensory reinnervation.
In contrast to these five growth factors, GDNF and PTN were similarly upregulated by denervated ventral root and cutaneous nerve, suggesting a possible role in both motor and sensory axon regeneration (Fig. 6). This upregulation must also be considered in light of baseline expression (Fig. 4). Normal PTN expression is 100-fold greater in ventral root, whereas GDNF expression is 10-fold higher in cutaneous nerve. The levels of PTN expression achieved after denervation are thus dramatically higher in ventral root, whereas final GDNF expression is similar in both pathways. Other factors that were clearly not modality specific included IGF-2, FGF-2, CNTF, and NT-3 (Fig. 7).
Differences in growth factor expression during reinnervation
Cutaneous and motor pathways demonstrated several different patterns of response to reinnervation (Figs. 5–7). Minimal upregulation in ventral root after denervation (NGF, BDNF, VEGF, HGF, and IGF-1) accurately predicted a similar response after reinnervation by either cutaneous or motor axons. Schwann cells of ventral root are thus not induced to produce these factors during the period of study, no matter what their axonal partners. These same factors also responded variably to reinnervation of cutaneous nerve. Upregulation of NGF in denervated cutaneous nerve peaked at 15 d and returned toward baseline by 30 d. When the nerve was reinnervated by either motor or sensory axons, however, downregulation of NGF occurred by day 15 and thus potentially in response to axonal contact (Fig. 5). A second pattern was displayed by HGF. Denervated cutaneous nerve maintained high levels of HGF. Reinnervation by cutaneous axons caused a sharp downregulation from these levels between 15 and 30 d, whereas reinnervation by motor axons resulted in a more gradual, continued rise in expression. The response to cutaneous axons thus mirrored that of NGF, although more slowly, whereas contact with motor axons caused a dampening of expression. A third pattern was seen with IGF-1, in which reinnervation by cutaneous axons mirrored the HGF pattern, but motor reinnervation had no effect.
GDNF and PTN were upregulated by both cutaneous nerve and ventral root but more vigorously by the latter (Fig. 6). In cutaneous nerve, GDNF and PTN responded only to cutaneous axons, with transient upregulation of GDNF at day 5 and a low but more sustained upregulation of PTN. Reinnervation of ventral root by cutaneous axons caused prolonged expression of GDNF but only a brief burst of PTN expression, whereas reinnervation by motor axons had little effect on GDNF but occasioned a more sustained expression of PTN. When these patterns are viewed in light of baseline expression (Fig. 4), PTN clearly emerges as the most specific motor factor.
The expression patterns of four other growth factors (IGF-2, FGF-2, NT-3, and CNTF) were also investigated (Fig. 7). The changes that occurred in these growth factors were less prominent than those displayed by the previous seven.
Growth factor protein levels
ELISA for NGF, GDNF, PTN, and BDNF was performed on fresh cutaneous nerve and ventral root grafts (groups NO-C and NO-V) and on grafts that had been allowed to undergo Wallerian degeneration for 2 weeks (Fig. 8). NGF protein was present at a significantly higher concentration in degenerated cutaneous nerve (14.6 pg/ml) than in normal cutaneous nerve (5.8 pg/ml) or in either fresh or degenerated ventral root (5.7 and 5.2 pg/ml, respectively). GDNF levels did not differ significantly among preparations (fresh cutaneous graft, 17.6 pg/ml; degenerated cutaneous graft, 22.5 pg/ml; fresh ventral root, 22.1 pg/ml; degenerated ventral root, 20.1 pg/ml), confirming the view, based on both baseline expression and percentage upregulation of GDNF, that protein levels in cutaneous nerve and ventral root would be similar. PTN levels were elevated dramatically in denervated ventral root (95.3 pg/ml) compared with the other preparations (fresh cutaneous nerve, 20.2 pg/ml; degenerated cutaneous nerve, 14.7 pg/ml; fresh ventral root, 24.0 pg/ml), confirming the observed pattern of mRNA expression. Similarly, BDNF was most abundant in denervated cutaneous nerve (187.8 pg/ml) as opposed to fresh cutaneous nerve (94.1 pg/ml) and both ventral root preparations (fresh ventral root, 109.2 pg/ml; denervated ventral root, 125.5 pg/ml).
Figure 8.
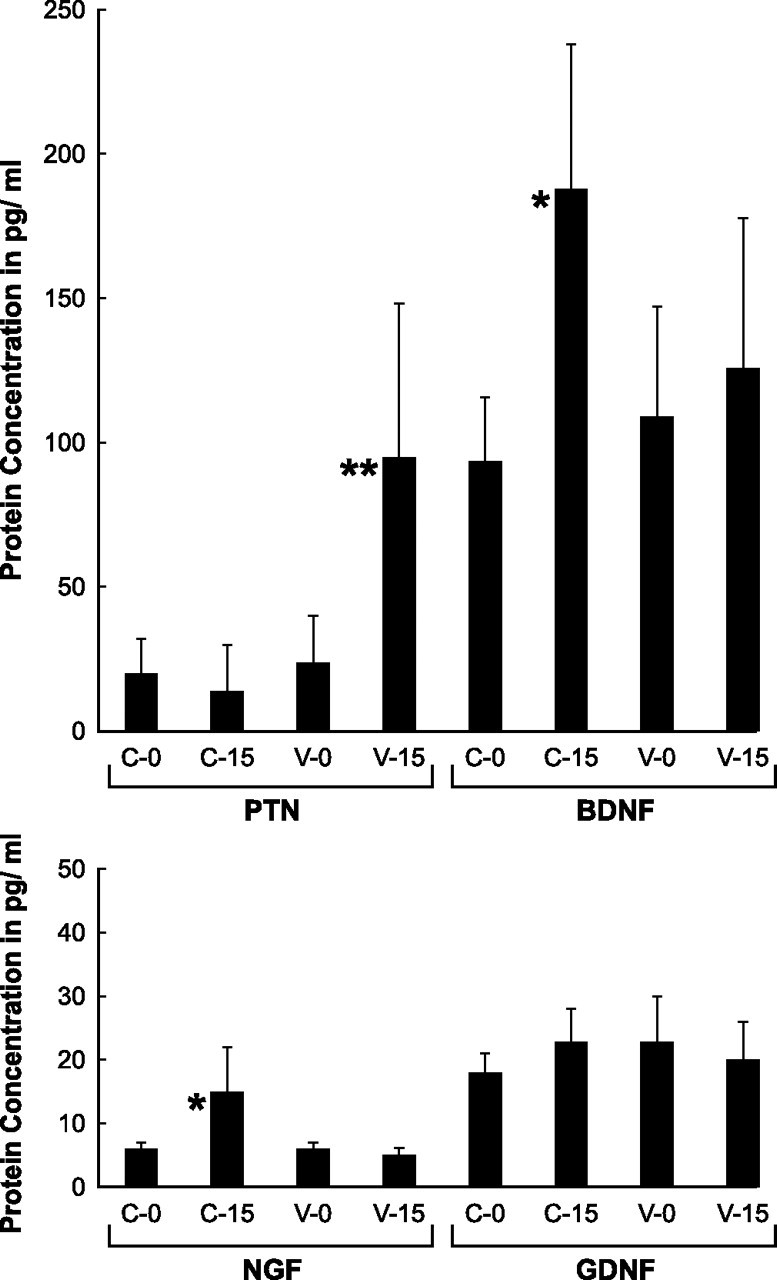
ELISA for NGF, GDNF, PTN, and BDNF was performed on protein extracted from normal cutaneous nerve (C-0), normal ventral root (V-0), denervated cutaneous nerve (C-15), and denervated ventral root (V-15). *p < 0.05; **p < 0.005. Protein levels confirm selective upregulation of NGF and BDNF in denervated cutaneous nerve and PTN in denervated ventral root. Error bars represent 1 SD from the mean.
Phenotypic plasticity
Schwann cells clearly maintain their cutaneous or motor phenotype despite the cellular divisions that accompany Wallerian degeneration. We next set out to determine the extent to which these phenotypic differences survive in the face of a more severe challenge, prolonged reinnervation by inappropriate axons. We surgically reinnervated a ventral root graft with cutaneous axons for 1 month and then transected the sensory axons proximally and let the grafts degenerate for 1 week (Fig. 2). Growth factor expression by the initially motor Schwann cells was then examined to see whether they would retain their initial motor pattern or assume a more sensory profile. To evaluate a potential confounding factor, migration of sensory Schwann cells into the graft, we placed ventral root grafts from female rats into male hosts. At the end of the study, we performed PCR for a Y-chromosome-specific gene in genomic DNA isolated from the grafts. Compared with normal male ventral roots, the reinnervated grafts expressed the Y-chromosome-specific sequence at only 2–4% of normal levels, indicating that migrating host Schwann cells had little impact on the outcome.
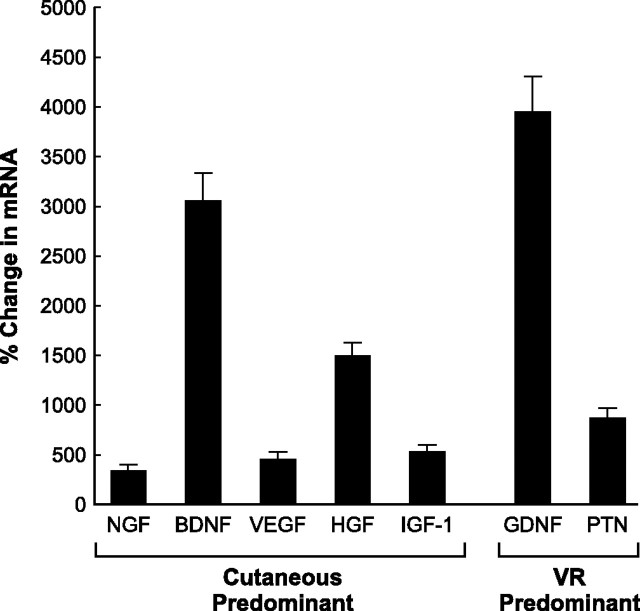
As shown in Figure 9, the majority of factors were expressed to a degree intermediate between the expression levels in normal cutaneous and motor nerve. There were, however, two glaring exceptions: BDNF was elevated to three times the level seen in cutaneous nerve and GDNF to eight times the level in either cutaneous nerve or ventral root. These findings suggest that motor Schwann cells, when forcibly reinnervated by cutaneous axons, modify their phenotype to be more like that of cutaneous Schwann cells but still retain evidence of their original motor identity.
Figure 9.
Results of phenotypic plasticity experiment (Fig. 2). Changes in mRNA levels of ventral root grafts that were transected after 30 d of forced reinnervation by sensory axons and allowed to degenerate for 7 d. Growth factors are identified as cutaneous predominant or ventral root (VR) predominant based on their upregulation after denervation of normal nerve (see Figs. 5–7). All changes were significantly different from baseline (p < 0.005). After prolonged sensory reinnervation, motor Schwann cells continue to produce PTN but also generate excessive levels of mRNA for BDNF and GDNF. Motor Schwann cells reinnervated by sensory axons thus retain a partial memory of their previous phenotype.
Discussion
The experimental model
The key components of this model are donor nerves of equal diameter from which only cutaneous or motor axons will regenerate and unifascicular recipient grafts that contain only cutaneous or motor axons in similar numbers. The femoral cutaneous nerve was chosen for both cutaneous donor and graft because it is the largest purely cutaneous nerve in the rat and because it is unifascicular proximally where it is transected and sutured. Comparison of unifascicular grafts avoids the unwanted variable of extraneural regeneration in the space between fascicles. The sciatic nerve of 100 g rats was used as motor axon donor; it is the same diameter as the femoral cutaneous nerve in 250 g rats, and deafferentation is well tolerated in young animals. Extensive controls have demonstrated the effectiveness of the deafferentation procedure (Redett et al., 2005). The femoral cutaneous nerve and the L4 and L5 ventral roots were well suited for recipient grafts because they matched the size of the donor nerves, contained similar numbers of myelinated axons, and could each be harvested in 2 cm lengths.
Schwann cell phenotype
Differentiated Schwann cells are traditionally thought to assume one of two possible phenotypes, myelinating or nonmyelinating (Jessen and Mirsky, 2002). Axonal neuregulin-1 type III is now known to control which of these phenotypes is expressed (Taveggia et al., 2005). Myelinating Schwann cells associate with axons in a 1:1 relationship and support saltatory conduction, whereas nonmyelinating Schwann cells often associate with several axons that conduct as cables. The phenotypes are further distinguished by characteristic patterns of protein expression. This distinction is not fixed, however, because denervation of myelinated Schwann cells restores a more primitive phenotype. To date, the assumption has been that this phenotype is similar for all Schwann cells and that they will produce the same growth factors regardless of whether they had previously associated with motor or sensory axons (Mirsky and Jessen, 1996). We now demonstrate that Schwann cells of cutaneous nerve and ventral root exhibit differing growth factor profiles at baseline and respond differently to denervation and reinnervation by cutaneous or motor axons.
The possibility of distinct sensory and motor phenotypes was first suggested by the discovery that Schwann cells of peripheral cutaneous and motor nerve differ in their expression of the L2/HNK-1 carbohydrate epitope. This epitope is expressed during regeneration only when previous motor Schwann cells are recontacted by motor axons (Martini et al., 1994). Schwann cells are thus capable of maintaining a record of their previous association with sensory or motor axons that survives their division during Wallerian degeneration. Our experiments extend these findings by revealing that cutaneous and motor Schwann cells also differ in their patterns of trophic factor expression after both denervation and reinnervation and that these differences also survive despite the Schwann cell division that accompanies Wallerian degeneration. This phenotypic variability has remained concealed because the entire rat sciatic nerve, which contains the full spectrum of sensory and motor axons, has been the standard model for investigation of trophic factor expression (Funakoshi et al., 1993; Henderson et al., 1994).
The relative abundance of factors that characterize cutaneous nerve is consistent with the heterogeneity of sensory neurons in the DRG. Many neuronal subgroups have been identified and characterized by overlapping yet distinct trophic requirements (McMahon et al., 1994). Motoneurons, in contrast, are more homogeneous and thus less likely to have widely differing trophic requirements. It is possible that a portion of the unique sensory profile is contributed by nonmyelinating Schwann cells. These cells do respond to denervation by upregulating growth factors (Friedman et al., 1996), but the extent of this upregulation has not been determined with quantitative PCR techniques. It is also likely that the division into sensory and motor phenotypes understates the true diversity of Schwann cell expression profiles, as suggested by the diverse neurotrophin sensitivities of their parent neurons.
The sensory/motor Schwann cell phenotype, in addition to surviving the cell divisions that accompany Wallerian degeneration, is resistant to prolonged contact with axons of the opposite phenotype. When reinnervated by cutaneous axons for 1 month and then denervated for 1 week (Figs. 2, 8), ventral root Schwann cells upregulate five of the seven specific factors to a level intermediate between denervated ventral root and denervated cutaneous nerve. A strong record of their initial association with motor axons thus persists despite the challenge of heterologous reinnervation.
Growth factor expression
Although many elements must participate for peripheral nerve regeneration to be successful, growth factor production by denervated Schwann cells in the distal nerve segment is among the most crucial. During regeneration, the distance to target tissue is often so long that axons are deprived of target-derived growth factors for months or even years. In response to denervation, Schwann cells of the nerve segment distal to injury secrete a variety of growth factors and act as “presumed targets” until regenerating axons reach their target muscle or sensory end organs (Taniuchi et al., 1988; Höke et al., 2000, 2002; Fenrich and Gordon, 2004). Elimination of endogenous production of these factors may interfere significantly with regeneration (Zhang et al., 2000).
The RT-PCR data obtained in these experiments substantiate our hypothesis that Schwann cells of cutaneous nerve and ventral root respond differently to denervation and reinnervation. Five factors, NGF, BDNF, VEGF, HGF, and IGF-1, were upregulated vigorously by cutaneous nerve but minimally by ventral root. Within this group, the response of cutaneous nerve to denervation and reinnervation by appropriate or inappropriate axons also varied significantly. Beginning with the neurotrophins, rapid upregulation of NGF in cutaneous nerve followed by downregulation on axon contact is consistent both with previous findings (Heumann et al., 1987a,b) and with the hypothesis that pathway-derived NGF stimulates axon elongation (Taniuchi et al., 1988). Upregulation of BDNF, in contrast, has been found to increase more slowly (Meyer et al., 1992; Funakoshi et al., 1993). Our data confirm this observation and reveal that reinnervation of cutaneous nerve by cutaneous axons stimulates additional significant upregulation of BDNF, whereas reinnervation by motor axons does not. This disparity is surprising given the prominent role of BDNF in the support of motoneurons (for review, see Boyd and Gordon, 2003).
Expression of other predominately cutaneous growth factors is less well characterized. IGF-1 concentrations have been shown to rise rapidly and peak 2 weeks after nerve repair (Hansson et al., 1986). We confirm this pattern, extending it to find rapid downregulation when cutaneous nerve is reinnervated by cutaneous axons, but not when it is reinnervated by motor axons. HGF, undetectable by Northern blot (Hashimoto et al., 2001), follows a pattern almost identical to that of IGF-1. VEGF protein was found to increase only by 2.5 times after nerve crush (Pola et al., 2004), but, in the current experiments, VEGF mRNA was upregulated 30-fold after nerve transection and to a lesser degree after reinnervation with cutaneous or motor axons.
Two factors, GDNF and PTN, were upregulated substantially by both cutaneous nerve and ventral root. As discussed previously, the 10-fold higher baseline expression of GDNF in cutaneous nerve tends to equalize the final expression of GDNF in cutaneous nerve and ventral root, as confirmed by relatively equal GDNF protein concentrations in the two graft types. GDNF supports both motoneurons and a population of DRG neurons (Henderson et al., 1994; Trupp et al., 1995). Interestingly, GDNF was expressed strongly when ventral root Schwann cells were reinnervated by cutaneous axons and to an even greater extent when these constructs were denervated after 1 month (Figs. 2, 9). The 100-fold higher baseline expression of PTN in fresh ventral root, coupled with substantially higher protein levels in degenerated ventral root, suggests that PTN is predominately a motor factor. PTN, a heparin-binding growth factor that is expressed by Schwann cells after peripheral nerve injury (Blondet et al., 2005), has in fact been identified as a potent growth factor for motoneurons (Höke, 2005).
Modality-specific regeneration
Matching pure populations of cutaneous and motor axons with homogenous groups of cutaneous or motor Schwann cells provides a clear test of axon–Schwann cell interactions. Regeneration is quantified by identifying the number of neurons that have projected to mid-graft. Axon counts, used as an outcome measure in previous graft comparisons, have variously demonstrated that cutaneous nerve is superior to (Bernardo Corte et al., 1984), equal to (Ghalib et al., 2001), or inferior to (Nichols et al., 2004) muscle nerve as a graft for motor axons. Moreover, motor axons maintain more collaterals in cutaneous nerve than in muscle nerve (Redett et al., 2005), confounding the use of axon counts as an outcome measure. Under the current experimental conditions, cutaneous Schwann cells preferentially support cutaneous axon regeneration, and ventral roots preferentially support motor axon regeneration. For the past century, cutaneous nerve has been used almost exclusively for clinical nerve reconstruction. The present findings thus help to explain why the results of grafting motor nerves consistently lag behind those of end-to-end nerve repair (Brushart, 1998) and suggest strategies to improve outcome by manipulating Schwann cell phenotype.
Ultimately, only a thorough analysis of protein expression and receptor upregulation in as many types of axon and Schwann cell as possible will fully outline the selectivity of axon–Schwann cell partnering. This information will help integrate the data we provided on baseline and postinjury expression of growth factors, as well as determine which axon populations are equipped to respond to which growth factors. In addition to revealing the presence of more than one Schwann cell phenotype, our experiments suggest that cutaneous and motor axons apply different strategies to regeneration. Cutaneous pathways supply a broad menu of growth factors, whereas motor pathways forgo the expression of factors such as BDNF and IGF-1 that are potentially supportive to motoneurons. Only when we understand the full spectrum of axon–pathway interactions in the context of neuronal regeneration strategies will we be equipped to promote regeneration in a truly specific manner.
Footnotes
This work was supported by National Institutes of Health Grant RO1 NS034484 and the Johns Hopkins Department of Surgery. We thank Kate Weaver for preparation of artwork and Dr. Manuela Aspalter for surgical assistance.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- An J, Beauchemin N, Albanese J, Abney TO, Sullivan AK. Use of a rat cDNA probe specific for the Y chromosome to detect male-derived cells. J Androl. 1997;18:289–293. [PubMed] [Google Scholar]
- Bernardo Corte MJ, Suarez Nieto C, Ablanedo Ablanedo P. Motor and sensory facial nerve grafts. An experimental comparative study. Arch Otolaryngol. 1984;110:378–383. doi: 10.1001/archotol.1984.00800320032007. [DOI] [PubMed] [Google Scholar]
- Blondet B, Carpentier G, Lafdil F, Courty J. Pleiotrophin cellular localization in nerve regeneration after peripheral nerve injury. J Histochem Cytochem. 2005;53:971–977. doi: 10.1369/jhc.4A6574.2005. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993;13:2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Nerve repair and grafting. In: Green D, Hotchkiss R, Pederson W, editors. Operative hand surgery. New York: Churchill Livingston; 1998. pp. 1381–1403. [Google Scholar]
- Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrich K, Gordon T. Canadian Association of Neuroscience review: axonal regeneration in the peripheral and central nervous systems: current issues and advances. Can J Neurol Sci. 2004;31:142–156. doi: 10.1017/s0317167100053798. [DOI] [PubMed] [Google Scholar]
- Friedman HC, Jelsma TN, Bray GM, Aguayo AJ. A distinct pattern of trophic factor expression in myelin-deficient nerves of Trembler mice: implications for trophic support by Schwann cells. J Neurosci. 1996;16:5344–5350. doi: 10.1523/JNEUROSCI.16-17-05344.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalib N, Houst'ava L, Haninec P, Dubovy P. Morphometric analysis of early regeneration of motor axons through motor and cutaneous nerve grafts. Ann Anat. 2001;183:363–368. doi: 10.1016/S0940-9602(01)80183-7. [DOI] [PubMed] [Google Scholar]
- Hansson HA, Dahlin LB, Danielsen N, Fryklund L, Nachemson AK, Polleryd P, Rozell B, Skottner A, Stemme S, Lundborg G. Evidence indicating trophic importance of IGF-I in regenerating peripheral nerves. Acta Physiol Scand. 1986;126:609–614. doi: 10.1111/j.1748-1716.1986.tb07862.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Yamanaka H, Fukuoka T, Dai Y, Obata K, Mashimo T, Noguchi K. Expression of HGF and cMet in the peripheral nervous system of adult rats following sciatic nerve injury. NeuroReport. 2001;12:1403–1407. doi: 10.1097/00001756-200105250-00022. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [Erratum (1995) 267:777] [DOI] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987a;104:1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci USA. 1987b;84:8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höke A. Neuroprotection in the peripheral nervous system. Ann Neurol. 2005;58:S2. [Google Scholar]
- Höke A, Cheng C, Zochodne DW. Expression of glial cell line-derived neurotrophic factor family of growth factors in peripheral nerve injury in rats. NeuroReport. 2000;11:1651–1654. doi: 10.1097/00001756-200006050-00011. [DOI] [PubMed] [Google Scholar]
- Höke A, Rosenberg B, Hoffman P, Griffin JW. Differential expression of neurotrophin mRNA in denervated Schwann cells of motor and sensory nerve fibers. J Peripher Nerv Syst. 2001;6:130–193. [Google Scholar]
- Höke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Signals that determine Schwann cell identity. J Anat. 2002;200:367–376. doi: 10.1046/j.1469-7580.2002.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilvington B. Some experiments on nerve regeneration. Aust NZJ Surg. 1941;10:266–272. [Google Scholar]
- Langley JN, Anderson HK. The union of different kinds of nerve fibers. J Physiol (Lond) 1904;31:365–391. doi: 10.1113/jphysiol.1904.sp001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schachner M, Brushart TM. The L2/HNK-1 carbohydrate is preferentially expressed by previously motor axon-associated Schwann cells in reinnervated peripheral nerves. J Neurosci. 1994;14:7180–7191. doi: 10.1523/JNEUROSCI.14-11-07180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Mears S, Schachner M, Brushart TM. Antibodies to myelin-associated glycoprotein accelerate preferential motor reinnervation. J Peripher Nerv Syst. 2003;8:91–99. doi: 10.1046/j.1529-8027.2003.03012.x. [DOI] [PubMed] [Google Scholar]
- Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119:45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R, Jessen KR. Schwann cell development, differentiation and myelination. Curr Opin Neurobiol. 1996;6:89–96. doi: 10.1016/s0959-4388(96)80013-4. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Jessen KR. The neurobiology of Schwann cells. Brain Pathol. 1999;9:293–311. doi: 10.1111/j.1750-3639.1999.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer EK, Kimmel DL. The repair of severed motor and sensory spinal roots by the arterial sleeve method of anastomosis. J Comp Neurol. 1948;88:285–317. doi: 10.1002/cne.900880205. [DOI] [PubMed] [Google Scholar]
- Nichols CM, Brenner MJ, Fox IK, Tung TH, Hunter DA, Rickman SR, Mackinnon SE. Effects of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp Neurol. 2004;190:347–355. doi: 10.1016/j.expneurol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Pola R, Aprahamian TR, Bosch-Marce M, Curry C, Gaetani E, Flex A, Smith RC, Isner JM, Losordo DW. Age-dependent VEGF expression and intraneural neovascularization during regeneration of peripheral nerves. Neurobiol Aging. 2004;25:1361–1368. doi: 10.1016/j.neurobiolaging.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. New York: Hafner; 1928. Degeneration and regeneration of the nervous system. [Google Scholar]
- Redett R, Jari R, Crawford T, Chen YG, Rohde C, Brushart TM. Peripheral pathways regulate motoneuron collateral dynamics. J Neurosci. 2005;25:9406–9412. doi: 10.1523/JNEUROSCI.3105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, Schweitzer JB, Johnson EM., Jr Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000;12:4171–4180. [PubMed] [Google Scholar]