Abstract
Recent neuroimaging studies have successfully identified encoding mechanisms that support different forms of subsequent episodic recognition memory. In our everyday lives, however, much of our episodic memory retrieval is accomplished by means of free recall, i.e., retrieval without an external recognition cue. In this study, we used functional magnetic resonance imaging to investigate the encoding mechanisms that support later free recall and their relationship to those that support different forms of later recognition memory. First, in agreement with previous work, we found that activation in the left inferior frontal gyrus and hippocampus correlated with later associative/relational recognition. In these regions, activation was further enhanced for items later freely recalled, pointing to shared underlying relational encoding mechanisms whose magnitude of activation differentiates later successful free recall from successful associative recognition. Critically, we also found evidence for free recall-specific encoding mechanisms that did not, in our paradigm, support later associative recognition compared with item recognition. These free recall-specific effects were observed in left mid/dorsolateral prefrontal (DLPFC) and bilateral posterior parietal cortices (PPC). We speculate that the higher-level working memory operations associated with DLPFC and attention to internal mnemonic representations perhaps mediated via PPC may serve to embed an item into a rich associative network during encoding that facilitates later access to the item. Finally, activation in the perirhinal cortex correlated with successful associative binding regardless of the form of later memory, i.e., recognition or free recall, providing novel evidence for the role of the perirhinal cortex in episodic intra-item encoding.
Keywords: episodic encoding, free recall, associative recognition, prefrontal cortex, posterior parietal cortex, perirhinal cortex
Introduction
Using the subsequent memory (SM) paradigm (Sanquist et al., 1980), neuroimaging studies have helped to elucidate the brain mechanisms supporting successful episodic encoding (Paller and Wagner, 2002; Henson, 2005). Motivated by dual-process theories of recognition memory (Yonelinas, 2002), recent studies have distinguished encoding mechanisms supporting associative recognition (AR) (accompanied by various kinds of episodic detail) from those supporting item recognition (IR) (no episodic details available). These studies have consistently revealed that encoding activation in the hippocampus and left inferior frontal gyrus (LIFG) supports later AR relative to IR (Davachi et al., 2003; Jackson and Schacter, 2004; Ranganath et al., 2004), which is in line with neuropsychological findings suggesting a disproportionate deficit in AR compared with IR after damage to the hippocampus (Yonelinas et al., 2002; Turriziani et al., 2004) (but see Manns et al., 2003; Wixted and Squire, 2004) and prefrontal cortex (PFC) (Janowsky et al., 1989b).
However, despite the evidence that recognition and free recall may be mediated by distinct neurocognitive mechanisms, SM studies have focused almost exclusively on recognition memory. For instance, free recall has been shown to be differentially impaired in amnesic patients compared with IR (Hirst et al., 1986) (but see Haist et al., 1992), and the extant cognitive literature suggests that free recall benefits from encoding or retrieval processes that encourage inter-item or organizational processing (Tulving, 1962; Bahrick, 1970; Anderson and Bower, 1972; Sternberg and Tulving, 1977; Hunt and Einstein, 1981; Kahana, 1996), whereas recognition benefits from encoding processes that make individual items more distinctive, regardless of their relationships to other items in the study list or other contextual variables (Tulving, 1968; Kintsch, 1970; Tversky, 1973; Mandler, 1980). However, these distinctions stem from comparisons between free recall and IR and do not take into account how processes supporting AR may differ from those supporting free recall. Thus, it is possible that the same encoding operations that differentially enhance AR compared with IR (e.g., relational encoding) are similarly engaged or perhaps enhanced for later free recall. The present study is designed to elucidate the encoding mechanisms that support later free recall and their relationship to those that support later AR.
To date, only two neuroimaging SM studies have used free recall as an assessment of subsequent memory (Alkire et al., 1998; Strange et al., 2002), and they have shown that engagement of the hippocampus, perirhinal cortex, and prefrontal cortex is correlated with later free recall performance. However, because neither study assessed IR or AR, the critical question of what encoding operations may be specific to later free recall remains open.
Together, extant data and theory offer different scenarios of how encoding mechanisms that support free recall and AR might diverge or overlap. Free recall may be supported by similar or enhanced engagement of the same mechanisms that support AR (e.g., relational processing, potentially mediated by the hippocampus and LIFG). Conversely, there may exist encoding mechanisms that selectively support our ability to freely recall episodic information and that do not differentially support AR.
Materials and Methods
Subjects.
Ten female and eight male right-handed native English speakers participated in the study. All subjects had normal or corrected-to-normal vision. Informed consent was obtained in a manner approved by the institutional review board at New York University, and subjects were paid for their participation. Two female subjects had to be excluded from additional analyses because of scanner malfunction and early termination of the experiment, respectively. Mean ± SD age of the remaining 16 subjects was 21.3 ± 2.5.
Item material.
Four hundred sixty English nouns were obtained from the Medical Research Council Psycholinguistics database (http://www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm). Words were three to eight letters long, with a Kucera–Francis written frequency of 10–100. Only words with concreteness and imageability ratings ranging from 400 to 700 (of 700) were included. For counterbalancing purposes, the whole item pool was divided into five groups of 92 words, and these groups were equated for concreteness and imageablility (both F(4,455) < 0.41; p > 0.80). A total of 276 words (three groups) were used as study items, and the remainder (two groups) served as lures for the recognition tests. The assignment of each group to study and test list, respectively, was counterbalanced across subjects. Each study word was assigned one of four colors (red, yellow, green, and blue) for the experimental procedure (see below), and every word was paired with every color at study across subjects. Stimuli were created and displayed using Matlab (MathWorks, Sherborn, MA) in combination with Psychophysics Toolbox extensions (Brainard, 1997).
Behavioral procedures.
The experiment consisted of three blocks, each containing an encoding, a distracter, and a test phase. The entire experiment was conducted inside the scanner, although brain-imaging data were only acquired during the encoding phases. Each encoding phase consisted of 92 trials. For a given trial (4 s), subjects were presented with a noun (printed in black uppercase letters) that was superimposed on a color square (Fig. 1A). The task was to create a mental image of the referent of the noun in the given color and to decide whether this word/color combination was plausible, i.e., whether it was possible for this word/color combination to exist in real life/nature or not. Subjects were given 3 s to conjure up a vivid mental image of the word/color combination. After 3 s, the frame of the color square changed from black to white, prompting subjects to indicate their plausibility judgment within the remaining second (“plausible” or “implausible”). Responses were given with a button box positioned under the subject’s left hand. Importantly, subjects were also instructed to press a separate button in case they were unable to create a mental image of the given word/color combination. These trials were excluded from all subsequent analyses. Encoding trials were intermixed with baseline trials involving an active, sensorimotor task. Arrows that randomly pointed to the left or to the right for 1 s were repeatedly presented for the length of a baseline trial, and subjects had to press the middle finger key if the arrow pointed to the left and the index finger key if it pointed to the right. An active baseline condition was chosen following previous suggestions that subjects are more likely to engage in uncontrolled cognitive processes during passive baseline conditions (e.g., looking at a fixation cross), which in turn might attenuate the sensitivity to detect task-related brain activation, particularly in medial temporal lobe (MTL) structures (Stark and Squire, 2001).
Figure 1.
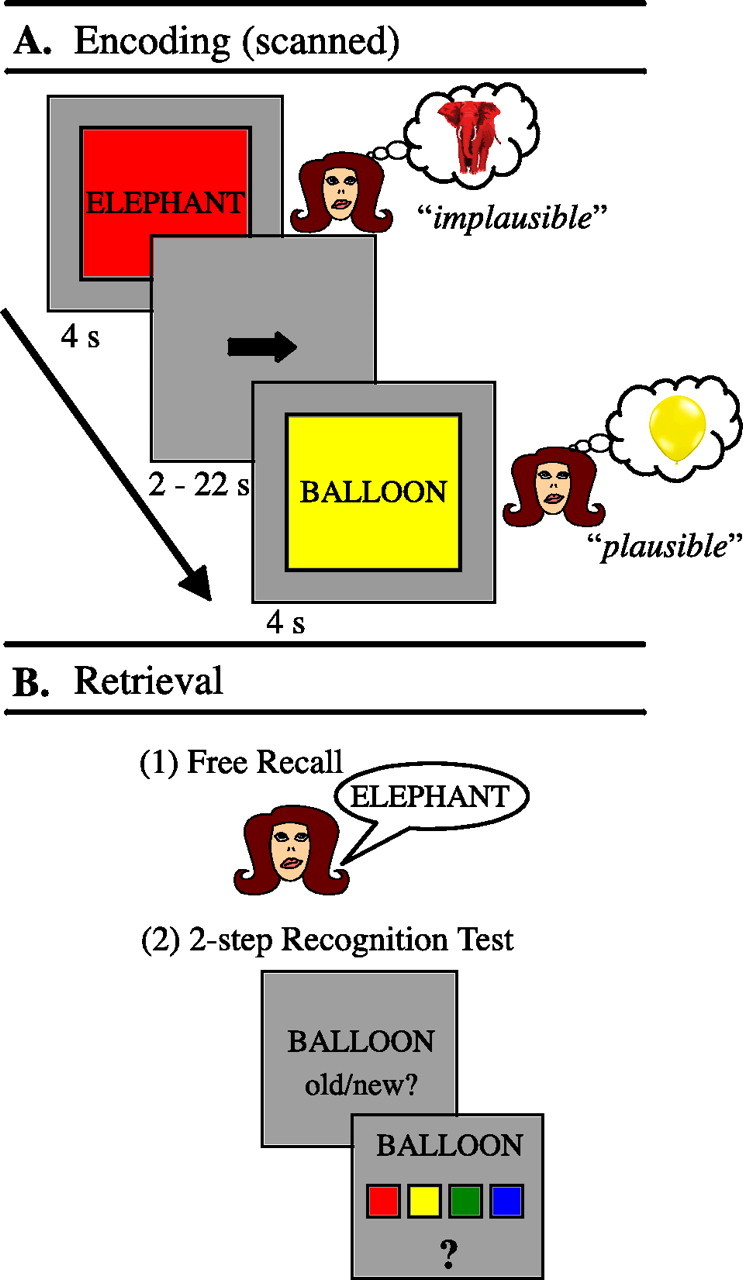
Experimental design. A, Encoding. Two example trials from the scanned encoding task. Subjects were instructed to vividly imagine the referent of the noun in the color presented and to decide whether this combination was plausible or not. B, Three-step subsequent memory test, consisting of a free recall phase (1), followed by assessment of both item and associative recognition (2).
The order of word/color trials and baseline trials was determined by using a sequencing program designed to maximize the efficiency of the event-related design (Dale, 1999). Conditions were jittered using variable duration (2–22 s) baseline trials. Each encoding phase was immediately followed by a 1 min distracter phase in which subjects had to solve four mathematical equations with a multiple-choice answering format. This distracter task, because it requires working memory (WM) maintenance as well as manipulation processes, was used to prevent active rehearsal of the most recently presented items before free recall performance (Shiffrin, 1970). Immediately after this distracter phase, subjects were given 5 min to freely recall verbally as many words from the previous encoding block as possible (Fig. 1B). Responses were recorded digitally and transcribed by the experimenter. Finally, a two-step recognition test was administered immediately after the free recall block. Subjects were presented with the 92 previously seen study words as well as 61 new words. For the first step, subjects were presented with a word in the center of the screen and were prompted to indicate via button press whether they thought it was “old or ”new,“ i.e., whether it had been presented in the previous encoding block or not. If they responded old, the four colors and a question mark appeared on the screen as a second step and subjects were prompted to indicate the color with which the word was associated at encoding or to press the question mark key if they did not remember the color (Fig. 1B). The additional question mark was used to circumvent forced guesses about the correct color. Critically, these memory tests allowed separation of encoding trials into four different conditions with regard to subsequent memory: (1) items later freely recalled (F trials), (2) items later recognized with the correct color (AR trials), (3) items later recognized without the correct color (IR trials, both question mark and incorrect color responses), and (4) items later forgotten (misses). Items that were both freely recalled and recognized thereafter were counted as freely recalled in both functional magnetic resonance imaging (fMRI) and behavioral analyses. However, associative recognition for those items was also assessed to determine whether the color was incorporated into the representation of the freely recalled items.
fMRI procedures.
Scanning was performed on a 3T Siemens Allegra MRI system using a whole-head coil. Functional data were acquired using a gradient-echo echo-planar pulse sequence (repetition time, 2 s; echo time, 30 ms; 35 slices oriented perpendicular to the hippocampal axis; 3 × 3 × 3 mm voxel size; 0.6 mm interslice gap; 368 volume acquisitions per run). High-resolution T1-weighted (magnetization-prepared rapid-acquisition gradient echo) images were collected for anatomical visualization. A vacuum pillow minimized head motion. Visual stimuli were projected onto a screen that was viewed through a mirror, and responses were collected with a magnet-compatible button box. Data were analyzed using SPM2 (Wellcome Department of Cognitive Neurology, University College London, London, UK). During preprocessing, images were corrected for differences in slice acquisition timing, followed by motion correction across all runs. Structural and functional data were spatially normalized to an echo planar imaging template, and voxels were spatially smoothed with a 6 mm full-width half-maximum isotropic Gaussian kernel.
General statistical analyses.
Data analysis was performed using the general linear model implemented in SPM2. Encoding trials were sorted according to the subsequent memory conditions described above and modeled using a canonical hemodynamic response function and its temporal derivative. The three runs were concatenated and modeled as one continuous run to improve parameter estimability. Accordingly, mean signal and drift per run were separately modeled as confounds. Effects were estimated by using a subject-specific fixed-effects model. To reveal brain regions involved in our encoding task regardless of subsequent memory performance, all later memory conditions were collapsed and contrasted with sensorimotor baseline trials. Subject-specific contrast images for task-related activation were entered into a second-level random-effects analysis (one-sample t test). Regions consisting of at least five contiguous voxels that exceeded an uncorrected threshold of p < 0.001 were considered reliable. Second-level analyses across the three memory conditions (IR, AR, and F; see below) were conducted in the context of a within-subjects one-way ANOVA (corrected for nonsphericity), with subject-specific parameter estimates for the memory conditions (β estimates) included as dependent measures. For comparisons between two memory conditions, subsidiary pairwise contrasts were computed via paired-samples t tests (two-tailed unless otherwise noted) on subject-specific β estimates for each condition, averaged across all significant voxels within a cluster. Although all statistical analyses were conducted on subject-specific β estimates, we additionally present the deconvolved blood oxygenation level-dependent (BOLD) time courses for each condition to provide a complementary illustration of the data. Time course data were extracted using the MarsBaR toolbox (Brett et al., 2002).
Increasing encoding activation across subsequent memory conditions.
As outlined in Introduction, one hypothesized scenario is that free recall may be supported by the same mechanisms as associative recognition, but those mechanisms may be more engaged during encoding of items that are later freely recalled, reflecting, perhaps, additional inter-item associative processing. Thus, in this case, one would expect greater activation during AR trials compared with IR trials and, furthermore, an additional enhancement during F trials. A well suited statistical procedure to reveal regions in which activation increases linearly from IR to AR to F trials is a parametric analysis (Buchel et al., 1998). Specifically, the parametric analysis can be designed to detect regions in which the condition β estimates derived from the general linear model show a specific pattern. For the purpose of the current question, we modeled a regressor reflecting the parametric modulation of all encoding trials in terms of a linear trend (first polynomial order) across subsequent memory conditions (IR = 1, AR = 2, F = 3). This analysis was conducted on a subject-specific fixed-effects level, yielding estimates for the slope of the parametric regressor. Subject-specific estimates of the parametric effect were then entered into a second-level random-effects group analysis (one-sample t test), and regions showing a positive slope and consisting of at least five contiguous voxels that exceeded an uncorrected threshold of p < 0.001 were considered reliable. To account for a generally lower signal-to-noise ratio in MTL regions, the threshold was adjusted to p < 0.005 to assess MTL effects (Ojemann et al., 1997; Schacter and Wagner, 1999; Davachi and Wagner, 2002; Strange et al., 2002; Dobbins et al., 2003; Weis et al., 2004; O’Kane et al., 2005). Finally, because a parametric analysis may identify regions in which the underlying linear trend may be driven by a large difference in the outlier conditions (F > IR), we statistically confirmed pairwise differences between all three memory conditions (AR > IR, F > IR, and F > AR) using planned one-tailed t tests on the subject-specific β estimates for the three memory conditions (IR, AR, and F trials). The results of this subsidiary analysis are reported in Table 1.
Table 1.
Regions resulting from subsequent memory analyses
| Region | Approximate BA | x, y, z | t | No. of voxels | Contrast analysis | |
|---|---|---|---|---|---|---|
| Increasing activation across subsequent memory conditions (parametric analysis) | ||||||
| Posterior inferior frontal gyrus | L | 44/45 | −51, 18, 18 | 4.13 | 7 | IR < AR < F |
| Hippocampus | R | 21, −21, −21 | 4.56 | 18 | IR < AR < F | |
| Middle temporal gyrus | L | 21 | −66, −45, −9 | 6.52 | 62 | IR < AR < F |
| Frontal operculum | L | 47 | −33, 21, −3 | 4.48 | 5 | IR < AR < F |
| Fusiform gyrus | R | 37 | 42, −39, −27 | 6.41 | 5 | IR < AR < F |
| Anterior inferior frontal gyrus | L | 45 | −51, 30, 9 | 4.44 | 5 | IR < AR = F |
| Hippocampus | L | −24, −15, −18 | 3.41 | 7 | IR ∼ AR < F | |
| Posterior cingulate/retrosplenial cortex | L | 30 | −3, −51, 18 | 4.14 | 5 | IR ∼ AR < F |
| Inferior parietal lobule/intraparietal sulcus | L | 40/7 | −39, −54, 57 | 4.86 | 15 | IR = AR < F |
| Free recall-specific network (conjunction/masking analysis) | ||||||
| Prefrontal cortex | ||||||
| Inferior/middle frontal gyrus | L | 45/46/9 | −45, 21, 21 | 3.77 | 22 | |
| Middle frontal gyrus | L | 45/46/9 | −51, 21, 30 | 3.73 | 10 | |
| L | 8 | −39, 15, 57 | 3.33 | 23 | ||
| L | 8 | −30, 30, 48 | 3.03 | 8 | ||
| Superior frontal gyrus | L | 9 | −18, 54, 33 | 3.15 | 6 | |
| Frontal operculum | L | 47 | −30, 21, −3 | 3.69 | 5 | |
| Superior frontal gyrus | R | 9 | 3, 51, 42 | 3.16 | 7 | |
| Posterior parietal cortex | ||||||
| Inferior parietal lobule/intraparietal sulcus | L | 40/7 | −39, −54, 57 | 4.4 | 75 | |
| Supramarginal gyrus | L | 40 | −57, −54, 30 | 3.16 | 6 | |
| Intraparietal sulcus | L | 19 | −36, −69, 42 | 4.14 | 29 | |
| Inferior parietal lobule | R | 40/7 | 54, −60, 39 | 2.75 | 5 | |
| Intraparietal sulcus | R | 40 | 39, −57, 42 | 3.12 | 6 | |
| Posterior cingulate/retrosplenial cortex | L/R | 30 | −3, −51, 15 | 3.76 | 50 | |
| Lateral/ventral temporal cortex | ||||||
| Middle temporal gyrus | L | 21/37 | −60, −54, −3 | 2.89 | 6 | |
| L | 21 | −66, −48, −9 | 4.37 | 44 | ||
| Fusiform gyrus | L | 20/37 | −48, −33, −18 | 2.98 | 5 | |
| R | 36/37 | 42, −39, −30 | 3.27 | 5 | ||
| Subcortical regions | ||||||
| Thalamus (anterior nucleus) | L | −3, 0, 6 | 4.86 | 35 | ||
| Caudate | L | −15, 0, 18 | 3.86 | 6 | ||
| Successful word/color binding | ||||||
| Perirhinal cortex | L | 35/36 | −30, −6, −36 | 4.66 | 10 |
Complete list of regions resulting from three separate analyses, corresponding Brodmann’s areas, Montreal Neurological Institute coordinates of the peak voxel, t values, and cluster sizes in number (No.) of voxels. For the parametric analysis, results from pairwise contrast analyses across the three memory conditions are shown. Bold indicates regions in which the pairwise contrasts did not show a stepwise increase across memory conditions; ∼ indicates trending toward significance at p = 0.085. L, Left hemisphere; R, right hemisphere.
Selective encoding activation for subsequent free recall.
The parametric analysis was designed to reveal encoding mechanisms important for both later associative recognition and later free recall. To reveal regions that may selectively bolster later free recall and not associative recognition, we used the following two-step analysis on the three memory conditions (IR, AR, and F trials). First, within the ANOVA model described above, a conjunction analysis (Nichols et al., 2005) was performed to reveal regions that showed greater activation for subsequent free recall compared with both subsequent item recognition (F > IR) and associative recognition (F > AR). Both individual contrasts were thresholded at p < 0.01, so that the conjunction of the two free recall effects (in the sense of the logical AND operator) was assessed at the conservative criterion of at least five contiguous voxels exceeding the uncorrected conjoint threshold of p < 0.0001. Next, because the resulting regions from this conjunction may also display enhanced activation for AR trials compared with IR trials (i.e., F > AR > IR), which we want to avoid, we excluded (via an exclusive masking procedure) all regions that showed a significant effect of AR > IR at a liberal threshold of p < 0.1. It is worth noting that the more liberal the threshold for the exclusive mask (AR > IR), the more likely the resulting regions are to show a free recall-specific pattern of activation.
Results
Behavioral results
Encoding task
At encoding, an equal number of plausible and implausible judgments for word/color combinations were made: 48.03 ± 13.17% (mean ± SD) of encoding trials were judged plausible, and 46.54 ± 13.06% were judged implausible (t(15) = 0.23; p = 0.82). The remaining trials were those for which subjects either indicated that they could not come up with an image or no response was made within the allotted response time of 4 s.
Subsequent memory
Performance on the subsequent memory tests (free recall and two-step recognition) revealed that, on average, 15.15 ± 3.24% (mean ± SD) of the study words were freely recalled (F), 52.88 ± 8.78% were correctly recognized with the correct color (AR), 15.63 ± 6.25% were recognized without the correct color (IR), and 10.71 ± 6.52% of the study words were forgotten. Of the IR trials, 64.17% were question mark responses, and 35.83% were incorrect color responses. Of the words that were freely recalled, 73.42% ± 10.18% were remembered with the correct color during the subsequent recognition test. This indicates that encoding for free recall primarily included successful binding of the color but not necessarily in all cases (further addressed below). The false alarm rate for new items during recognition was 8.14 ± 8.95%, and, on average, 8.88 ± 9.61 words during free recall were intrusion errors.
Overall memory performance, collapsing across all successful memory outcomes, varied as a function of plausibility judgments during encoding: word/color combinations that were judged plausible were less likely to be forgotten than trials judged to be implausible (t(15) = 5.08; p < 0.001). Furthermore, trials that were judged plausible were more likely to be later freely recalled (t(15) = 2.47; p < 0.05) and recognized with the correct color (t(15) = 7.01; p < 0.001) than trials judged implausible. Importantly, the results from the fMRI subsequent memory analyses presented below did not qualitatively differ when plausible and implausible trials were considered separately. Because this factor is beyond the scope of the present manuscript, all data presented herein are collapsed across plausibility judgments.
fMRI results
Compared with the sensorimotor baseline condition, performance of the encoding task required engagement of numerous cognitive processes, including associative object/color imagery, semantic retrieval, and evaluative processes. Correspondingly, the comparison of the encoding task with our baseline condition resulted in significant activation of a large number of brain regions (Fig. 2) (full list of coordinates available on request).
Figure 2.
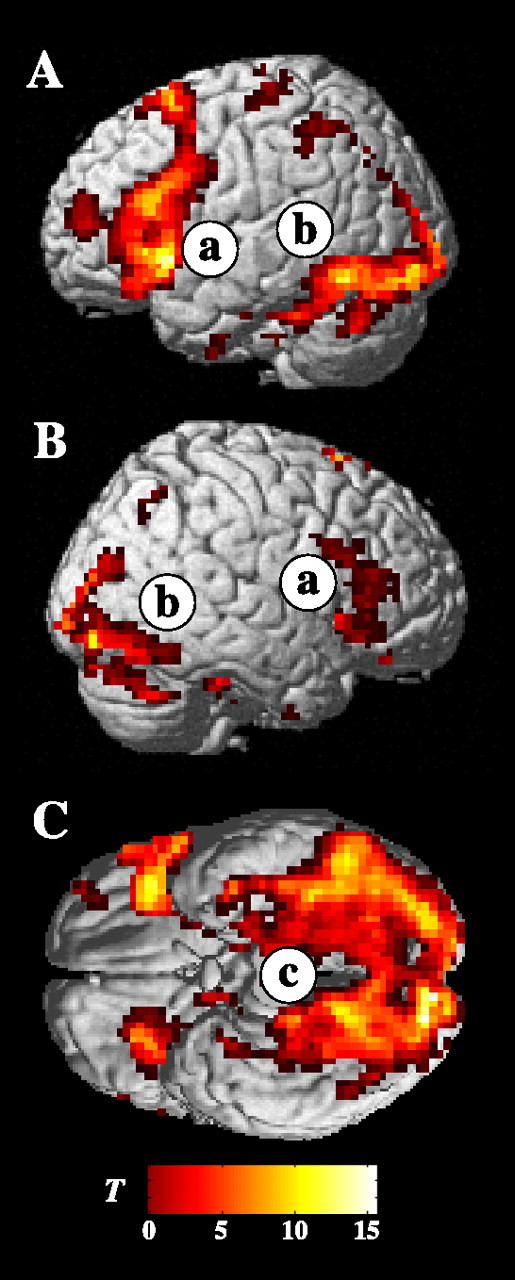
Task effects. Statistical parametric maps (T-maps) of activation during encoding task performance (collapsed across all subsequent memory outcomes) compared with the sensorimotor baseline condition, rendered on a canonical brain template. A, Left lateral view. B, Right lateral view. C, Ventral view (cerebellum removed for illustration purposes). Robust activation was found in the prefrontal cortex (a), in ventrolateral temporal lobe regions (b), and in the medial temporal lobes (c).
Of note, robust activation during encoding trials compared with baseline was revealed in LIFG, a region observed in numerous neuroimaging studies involving orientation to and retrieval of semantic information and the recruitment of generalized control mechanisms (Kapur et al., 1994; Demb et al., 1995; Vandenberghe et al., 1996; Fiez, 1997; Thompson-Schill et al., 1997; Wagner et al., 1998; Kirchhoff et al., 2000; Gold and Buckner, 2002; Badre et al., 2005; Gold et al., 2005; Wig et al., 2005). In addition, significant activation was revealed in ventral and lateral temporal lobe regions that have consistently been correlated with object processing (Ungerleider and Haxby, 1994; Chao et al., 1999; Ishai et al., 1999, 2000b), color processing (Martin et al., 1995; Howard et al., 1998; Chao and Martin, 1999), and mental imagery of concrete items (D’Esposito et al., 1997; Ishai et al., 2000a; Mellet et al., 2000). Significant activation during encoding task performance was also found in the MTL, including the hippocampus and perirhinal cortex.
Linearly increasing activation across subsequent memory conditions
All clusters emerging from our parametric analysis are presented in Table 1. Subsidiary pairwise contrasts confirmed a linear stepwise increase of activation from IR to AR to F trials in the LIFG [approximately Brodmann’s areas (BA) 44/45] (Fig. 3C), the left frontal operculum (approximately BA 47), the left middle temporal gyrus (MTG) (approximately BA 21), and the right fusiform gyrus (approximately BA 37) (all t values, t(15) > 1.81; p < 0.05). Importantly, these contrasts also revealed regions whose pattern of activation did not show statistically significant (or even trending) effects between all conditions. For example, in a second cluster in LIFG (approximately BA 45) (Fig. 3D), localized more anterior and ventral than the LIFG cluster just mentioned, contrasts between the memory conditions revealed that activation during both AR trials and F trials was enhanced compared with IR trials (both t values, t(15) > 4.13; p < 0.001), but AR and F trials did not differ statistically from each other (t(15) = 1.11; p = 0.14).
Figure 3.
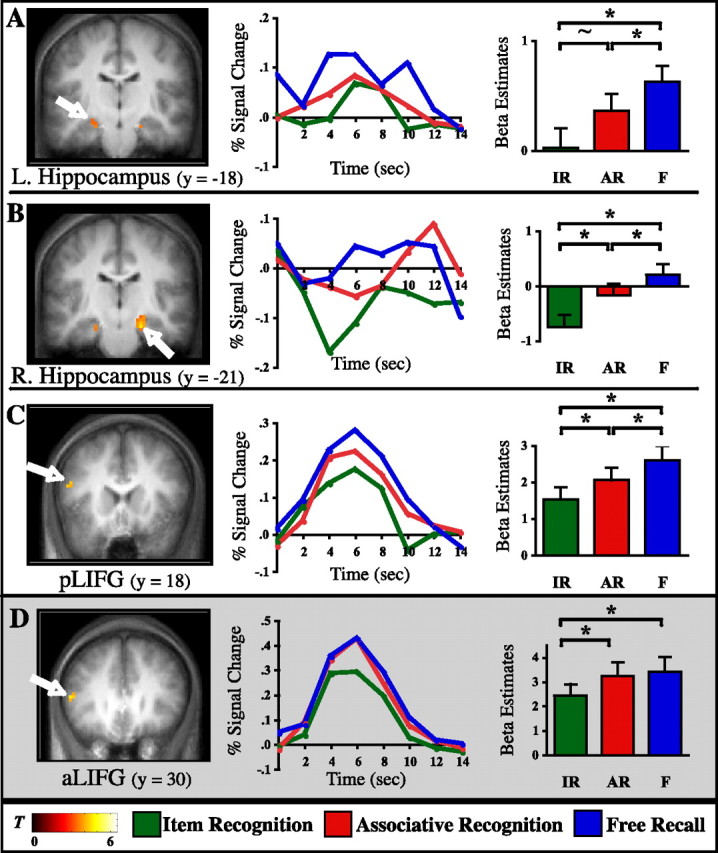
Linearly increasing activation across subsequent memory conditions. Left column, Statistical parametric maps (T-maps) revealing regions that show linearly increasing encoding activation across subsequent IR, AR, and F, superimposed on the coronal slices of the mean anatomical image across subjects. A, Left hippocampus (L. Hippocampus). B, Right hippocampus (R. Hippocampus). C, Posterior left inferior frontal gyrus. Highlighted in gray (D) is the anterior left inferior frontal gyrus, in which the statistical pattern is distinguished from A–C because IR < AR = F. Middle column, Deconvolved BOLD time course data are shown for the peak voxel (A, −24, −15, −18; B, 21, −21, −21; C, −51, 18, 18; D, −51, 30, 9). Percentage signal change is graphed for each memory condition across 14 s (7 time points) after trial onset. Right column, Results from pairwise contrasts across memory conditions. Bar graphs reflect the mean of the β parameter estimates across subjects for each condition, averaged across all voxels in a given cluster. Error bars represent the SEM. * indicates statistically significant at p < 0.05; ∼ indicates trending toward significance at p = 0.085.
Thus, distinct activation patterns were seen along the anteroposterior extent of the inferior frontal gyrus, with anterior left inferior frontal gyrus (aLIFG) showing the pattern IR < AR = F and posterior left inferior frontal gyrus (pLIFG) showing IR < AR < F. This is interesting because there has been some controversy in the recent literature about the precise contributions of aLIFG and pLIFG to semantic processing and more general control mechanisms (Thompson-Schill et al., 1997, 1999; Poldrack et al., 1999; Wagner et al., 2001a,b; Gold and Buckner, 2002; Badre et al., 2005; Gold et al., 2005). We will return to this debate and how it might relate to the pattern seen in our current study in Discussion.
Finally, one cluster in posterior parietal cortex (PPC) that emerged in the parametric analysis was, however, found to show selective enhancement of activation for later free recall [inferior parietal lobule/intraparietal sulcus, F > AR and F > IR (both t values, t(15) > 4.41; p < 0.001), AR = IR (NS, t(15) = 1.25; p > 0.1)], and we will elaborate on these effects in the next section.
Within the MTL, the parametric analysis revealed clusters in both the left and right hippocampal formation (referring to the hippocampus proper, the dentate gyrus, and the subiculum) (Fig. 3A,B). In the right hippocampal region, the contrast analysis confirmed the linear increase across the three memory conditions statistically (all t values, t(15) > 2.16; p < 0.05). In the left hippocampal region, the contrasts of F > AR and F > IR were both significant (both t values, t(15) > 1.92; p < 0.05), and the contrast of AR > IR trended toward significance (t(15) = 1.44; p = 0.085). We believe the lack of a significance between IR and AR in this left hippocampal region should be interpreted with caution given that overall statistical power is reduced in the MTL by a relatively low signal-to-noise ratio in this region and that a corresponding region in the opposite hemisphere shows a very similar pattern that reached statistical significance.
To summarize the results of the parametric analysis, we found that regions in the LIFG and the right hippocampus, in addition to lateral temporal and fusiform cortices, showed a reliable stepwise increase of activation during encoding from IR to AR to F trials. Not only do these results replicate previous findings showing that enhanced encoding activation in both LIFG and hippocampus correlated with subsequent relational/associative recognition compared with simple item recognition (Henson et al., 1999; Davachi et al., 2003; Sperling et al., 2003; Jackson and Schacter, 2004; Kirwan and Stark, 2004; Ranganath et al., 2004; Eldridge et al., 2005), but it extends these findings by suggesting that an enhancement of the processes supported by these regions may further contribute to subsequent episodic free recall.
Free recall-specific network
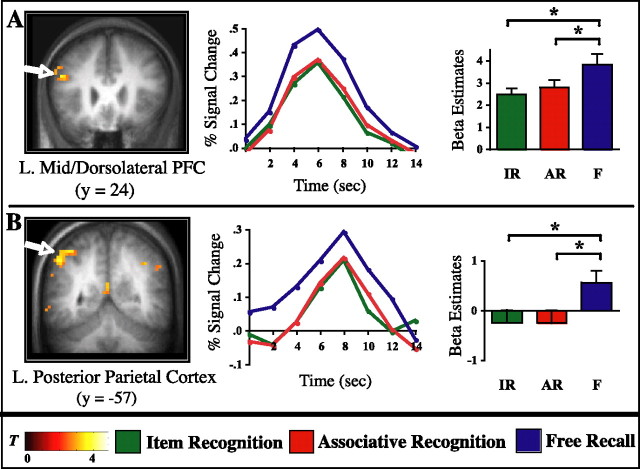
To reveal encoding mechanisms that selectively support later free recall and that, critically, do not differentiate between later AR and later IR, a conjunction analysis and exclusive masking procedure were performed (see Materials and Methods). As expected, pairwise contrasts on subject-specific β estimates confirmed the statistical pattern F > IR and F > AR (all t values, t(15) > 2.25; p < 0.05) and AR = IR (all t values, t(15) < 1.51; p > 0.1) in all clusters emerging from this analysis. The full list of regions resulting from this analysis is provided in Table 1. Most notably, regions found to selectively support later free recall were located in a cluster in the left mid/dorsolateral prefrontal cortex (DLPFC) (including middle and superior frontal gyri, approximately BA 45/46/9 and BA 8) (Fig. 4A) and in bilateral PPC (including inferior parietal lobule, intraparietal sulcus, and retrosplenial cortex, approximately BA 40/7, BA 19, and BA 30) (Fig. 4B).
Figure 4.
Free recall-specific effects. Encoding activation is significantly greater in these regions for subsequent F (blue) compared with both subsequent IR (green) and subsequent AR (red), without differing between IR and AR. Left column, Statistical parametric maps (T-maps) are superimposed on the coronal slices of the mean anatomical image across subjects. A, Left mid/dorsolateral prefrontal cortex. B, Left inferior parietal lobule/intraparietal sulcus. Middle column, Time course data are shown for the peak voxel (A, −45, 21, 21; B, −39, −54, 57). Percentage signal change is graphed for each memory condition across 14 s (7 time points) after trial onset. Right column, Results from pairwise contrasts across memory conditions. Bar graphs reflect the mean of the β parameter estimates across subjects for each condition, averaged across all voxels in a given cluster. Error bars represent the SEM. *p < 0.05. Note that bar graphs are mainly shown here for confirmatory and illustrative purposes, because the analysis was specifically designed to reveal regions that show this statistical pattern of activation. L., Left.
DLPFC regions have not typically emerged in previous subsequent memory studies of recognition memory (but see Blumenfeld and Ranganath, 2006) but have consistently been highlighted in neuroimaging studies of WM and have been hypothesized to support executive cognitive processes (Petrides et al., 1993; D’Esposito et al., 1995) (for review, see Smith and Jonides, 1999; Wager and Smith, 2003). Lateral PPC (including inferior parietal lobule/supramarginal gyrus) has been consistently shown to be involved in WM tasks requiring phonological rehearsal and/or storage (Awh et al., 1996) (for review, see Becker et al., 1999; Chein et al., 2003) as well as in visuospatial and more domain-general attentional processing (Corbetta et al., 2000; Ravizza et al., 2004) (for review, see Corbetta and Schulman, 2002; Pessoa and Ungerleider, 2004). Likewise, parietal subsequent memory effects have been reported during phonological encoding tasks (Davachi et al., 2001; Otten et al., 2002), but these effects were observed in superior parietal cortex, located more dorsally than the PPC regions identified in the present study. In contrast to the sparse reporting of PPC subsequent memory effects, PPC activation has consistently been revealed in studies of episodic memory retrieval (Konishi et al., 2000; McDermott et al., 2000; Donaldson et al., 2001; Velanova et al., 2003; Wheeler and Buckner, 2003, 2004; Kahn et al., 2004; Shannon and Buckner, 2004; Henson et al., 2005) (for review, see Wagner et al., 2005), perhaps indicating that some forms of episodic retrieval mechanisms are important during encoding as well. Additional discussion of these different accounts of DLPFC and PPC and how they might relate to episodic encoding that selectively supports later free recall will be addressed in Discussion.
Successful word/color binding
Finally, given that the behavioral data revealed that only ∼75% of the freely recalled items were also recognized with the correct color, this suggested to us that the mechanisms supporting later free recall operate, at least in part, independently from those that are involved in associative word/color binding. Because both the parametric analysis and the conjunction/masking analysis put special emphasis on encoding mechanisms that support later free recall, we conducted a final analysis to reveal brain regions involved in successful word/color binding independent of whether the word was freely recalled or recognized. First, F trials were split into those for which the correct color was subsequently remembered (F_AR trials) and those for which the correct color was subsequently not remembered (F_IR trials). Next, to reveal regions that showed greater activity for successful word/color binding than for unsuccessful word/color binding regardless of recognition or recall performance, we contrasted AR and F_AR trials to both IR and F_IR trials (contrast weights: AR, +1; F_AR, +1; IR, −1; F_IR, −1 within the ANOVA). Finally, to ensure that the results were not driven by effects within one form of memory (recognition or free recall), statistical comparisons of the β estimates using paired t tests were conducted within both subsequent memory levels (AR vs IR and F_AR vs F_IR).
As shown in Figure 5, the only region that emerged from the contrast analysis was the left perirhinal cortex (approximately BA 35/36). The comparison of successful versus unsuccessful word/color binding was significant within both forms of memory (AR > IR, t(15) = 4.23, p < 0.001; F_AR > F_IR, t(15) = 2.34, p < 0.05). Furthermore, activation during AR trials did not differ from F_AR trials (t(15) = 0.50; p > 0.62), and IR trials did not differ from F_IR trials (t(15) = 1.16; p > 0.26), suggesting that perirhinal activation was greater during successful than during unsuccessful word/color binding regardless of whether the item was freely recalled or recognized. Finally, to test whether this effect in perirhinal cortex might be driven by plausible or implausible trials, we separated both AR trials and IR trials according to the plausibility judgment and conducted a repeated-measures ANOVA including the factors memory (AR, IR) and judgment (plausible, implausible), with the β estimates for each condition as dependent variables. Critically, the results revealed a significant main of memory (F(1,15) = 14.25; p = 0.002) and no significant main effect of judgment (F(1,15) = 0.34; p > 0.85) or a significant interaction between memory × judgment (F(1,15) = 0.96; p > 0.34). In short, encoding activation in left perirhinal cortex was highly selective for successful associative binding of intra-item information, i.e., binding of the color to the word or object representation, regardless of whether the word/color combination was judged plausible or implausible during encoding or whether the item was later freely recalled or recognized. [We use “intra-item associative binding” here to denote successful episodic binding of a concrete item feature (color) with the item (word/object representation) itself, as opposed to binding of more contextual/abstract information such as the experimental task in which the item was encountered (Davachi et al., 2003).] Importantly, this pattern was distinct from that seen in the neighboring bilateral hippocampal regions that were revealed in the parametric analysis, in which activation did not differ between F_IR and F_AR trials (both t values, t(15) < 0.26; p > 0.8). Thus, the hippocampus shows a pattern consistent with involvement in relational binding processes that are important for both AR and free recall, whereas perirhinal cortex exhibits a pattern selective to the binding of the item with a feature of that item, regardless of whether this knowledge was revealed after free recall performance or within the recognition test.
Figure 5.
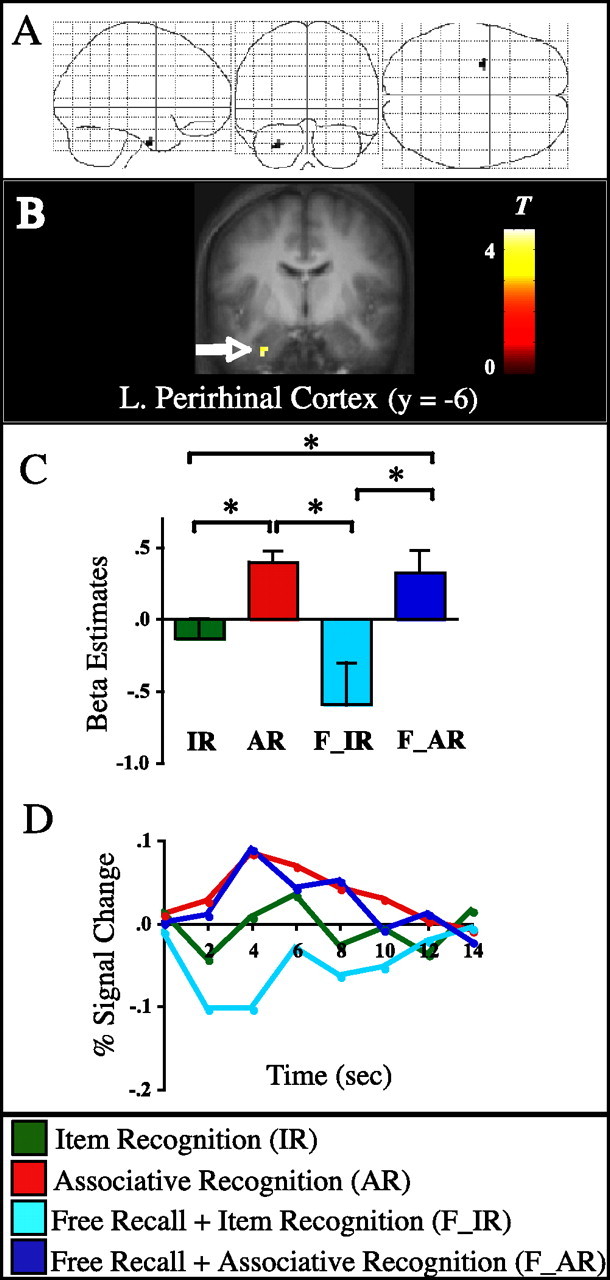
Successful word/color binding in left perirhinal cortex. A, SPM2 “glass brain” view of regions selectively involved in successful word/color binding regardless of form of memory, thresholded at five contiguous voxels exceeding an uncorrected p < 0.001. B, Statistical parametric map (T-map) superimposed on the coronal slice of the mean anatomical image across subjects. C, Results from pairwise contrasts across all memory conditions. Bar graphs show the mean of the β parameter estimates across subjects for each condition, averaged across all voxels from the perirhinal cortex cluster. Error bars represent the SEM. *p < 0.05. D, Time course data for the peak voxel (−30, −6, −36). Percentage signal change is graphed for each memory condition across 14 s (7 time points) after trial onset.
Discussion
These results are, to our knowledge, the first investigating the encoding processes correlated with later episodic free recall in direct comparison with those supporting later IR and AR memory. Importantly, the data provide evidence for free recall-specific encoding processes supported by left mid/dorsolateral prefrontal and bilateral posterior parietal cortices. Furthermore, we found that encoding activation in a number of regions, including LIFG and bilateral hippocampus, correlated with later AR, and these regions showed either equivalent or enhanced engagement during encoding for items later freely recalled. Finally, we report that activation in only one brain region, the perirhinal cortex, correlated strictly with later successful AR for the color of the presented item, regardless of whether the item was freely recalled or recognized.
Shared encoding mechanisms
Activation in pLIFG, bilateral hippocampus (Fig. 3A–C), left MTG, and right fusiform gyrus was graded with respect to later memory performance (IR < AR < F), suggesting that the processes supported by these regions contribute to both AR and free recall. Importantly, the data show that the magnitude of activation correlates with the probability of later free recall over recognition.
Notably, as opposed to pLIFG, aLIFG showed equivalent activation for F and AR trials, both exhibiting significantly greater activation than IR trials (Fig. 3D). A recent study by Badre et al. (2005) addressed the putative functional dissociation between aLIFG and pLIFG (the latter labeled “mid-ventrolateral” prefrontal cortex in their manuscript) and concluded that aLIFG activation varied with “semantic retrieval effort,” whereas pLIFG activation was related to a generalized “semantic control mechanism.” In their study, activation in left MTG correlated with the amount of internally generated or externally provided associated semantic representations, leading the authors to suggest that MTG may support the storage of semantic knowledge. Although not directly manipulated in our study, these putative processes might suggest that both AR and F trials engendered the same initial effort of semantic retrieval, as evidenced by equivalent aLIFG engagement, with a reduction of this effort resulting in the relatively impoverished episodic memory outcome of item recognition (IR trials). Furthermore, it is conceivable that, despite similar top-down retrieval efforts mediated via aLIFG, engagement of left MTG is enhanced during F trials by the enhanced availability of, perhaps pre-experimentally acquired, associations for certain items. Finally, activation in pLIFG may reflect additional control processes (e.g., elaboration) during F trials operating on the enhanced semantic information actually available during those trials.
Within MTL, a linear increase across memory conditions was seen in bilateral hippocampal regions. Previous work has revealed activation in the hippocampus during relational processing compared with item processing (Davachi and Wagner, 2002), and, importantly, hippocampal encoding activation has been shown to correlate with later relational recognition compared with item recognition (Davachi et al., 2003; Sperling et al., 2003; Jackson and Schacter, 2004; Kirwan and Stark, 2004; Ranganath et al., 2004; Eldridge et al., 2005). Because none of these previous SM studies have used the recovery of intra-item information as criterial for relational recognition, the present results extend the role of the hippocampus in episodic relational encoding to include the encoding of relations within an item or item feature information (word/color binding). Moreover, activation in bilateral hippocampus was greater for F trials compared with AR trials, suggesting that additional relational encoding, perhaps reflecting the incorporation of additional contextual or extra-item representations made available by left MTG and pLIFG mechanisms, contributes to later free recall ability. These graded effects nicely align with neuropsychological findings suggesting that hippocampal damage impairs both AR and free recall, with more pronounced deficits for free recall (Holdstock et al., 2002, 2005).
Free recall-specific network
Unlike the regions discussed above, encoding activation in left DLPFC and bilateral lateral and medial PPC correlated selectively with later free recall (Fig. 4). The lateral prefrontal and parietal cortices have consistently been implicated in attentional and WM functions (for review, see Goldman-Rakic, 1996; Pessoa and Ungerleider, 2004). Within PFC, it has been proposed that ventrolateral PFC supports maintenance of items, whereas DLPFC supports additional cognitive manipulations of the maintained information (Petrides, 1995; D’Esposito et al., 1999; Owen et al., 1999). It is likely that these higher-level WM operations underlie organizational encoding processes previously identified to benefit later free recall performance (Tulving, 1968). Importantly, our data suggest that this organizational processing, in conjunction with PPC mechanisms, is differentially important for later free recall and does not necessarily contribute to AR. In support of this is evidence that patients with damage to DLPFC are impaired at free recall performance (Gershberg and Shimamura, 1995) (but see Janowsky et al., 1989a) but not impaired in making “remember” or “know” recognition judgments relative to controls (Wheeler and Stuss, 2003). However, a recent fMRI study by Blumenfeld and Ranganath (2006) showed that DLPFC activation during the delay period of a WM encoding task correlated with later recognition memory, but this was the case only using an encoding task that required relational inter-item processing. Future work will address the possibility that DLPFC processes may also support later AR in a paradigm in which AR is conditionalized on the recovery of more contextual relational information presumably supported by inter-item processing.
Although the PPC regions selectively involved during F trials in the present study have not been reported in previous SM studies, they have consistently been found in studies of episodic memory retrieval (for review, see Wagner et al., 2005). One intriguing speculation put forth by Wagner et al. (2005) is that activation seen in PPC during retrieval might reflect attention to internal mnemonic representations. It is possible that attention to mnemonic representations also arises during item encoding, and the availability of mnemonic associations for an item might increase the probability that internally generated cues during later free recall performance successfully lead to the recovery of that item. In other words, free recall may benefit from the embedding of a study item in a rich associative network that may include episodic mnemonic representations because this may provide effective routes to the target item during retrieval. The common finding that free recall performance is enhanced for high-frequency compared with low-frequency words (Hall, 1954) is consistent with this interpretation, because high-frequency words likely activate more mnemonic representations. Interestingly, a recent fMRI study by Kuo et al. (2003) found greater activation in the left supramarginal gyrus (close to regions showing F-selective effects in the present study) for high-frequency compared with low-frequency words. Along the same vein, because the advantage for high-frequency words does not hold for recognition memory (Gorman, 1961), our data also suggest that these putative PPC processes may not be as important for later AR.
Associative encoding in perirhinal cortex
A novel, and somewhat surprising, finding in this study was that encoding activation in left perirhinal cortex correlated with later AR (Fig. 5). Previously, SM investigations have shown that activation in perirhinal cortex during episodic encoding of verbal stimuli correlates with later IR and not recovery of episodic details such as a cognitive source or a paired associate (Davachi et al., 2003; Kirwan and Stark, 2004; Ranganath et al., 2004). One interesting possibility for this apparent discrepancy is that the criterial information on which AR was conditionalized in our study is quite distinct from that used in other studies, namely the recovery of a feature of the item itself instead of the recovery of an extra-item detail of the episode. Importantly, numerous findings from animal lesion and electrophysiological work (for review, see Murray and Richmond, 2001) as well as a recent fMRI study in humans (Taylor et al., 2006) suggest that the perirhinal cortex is critical for high-level object processing. Monkeys with perirhinal lesions are impaired at tasks that require decisions based on overlapping item features that are ambiguous but not at performance of the same tasks when featural overlap is minimal (Bussey et al., 2003; Buckley and Gaffan, 2006). Thus, enhanced perirhinal activation for AR trials in our study compared with others may be a consequence of AR being conditionalized on the binding of an item with a concrete item feature. Importantly, this novel finding provides evidence in the human for a role of the perirhinal cortex in supporting some forms of associative mnemonic processing (namely, intra-item). An important question for future research is how intra-item binding contributes to different subjective phenomenologies of recognition (e.g., familiarity vs recollection) (Yonelinas et al., 1999). Importantly, as described in the Results, activation in the perirhinal cortex, unlike in the neighboring hippocampus, did not show an additional enhancement for F trials, suggesting that intra-item binding may not be critical for later free recall. Rather, our results suggest that the free accessibility of episodic information without the aid of an external cue (Tulving and Pearlstone, 1966) may be supported by encoding processes that might involve attention to and binding of both semantic and episodic associations, mediated by interactions between DLPFC and PPC.
Footnotes
This work was supported by the Seaver Foundation. We thank Shannon Tubridy for assistance with data acquisition, Liz Phelps and Kevin Ochsner for insightful comments on a previous version of this manuscript, and Robert Welsh for technical support.
References
- Alkire MT, Haier RJ, Fallon JH, Cahill L. Hippocampal, but not amygdala, activity at encoding correlates with long-term, free recall of nonemotional information. Proc Natl Acad Sci USA. 1998;95:14506–14510. doi: 10.1073/pnas.95.24.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, Bower GH. Recognition and retrieval processes in free recall. Psychol Rev. 1972;79:97–123. [Google Scholar]
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: evidence from positron emission tomography. Psychol Sci. 1996;7:25–31. [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Bahrick HP. Two-phase model for prompted recall. Psychol Rev. 1970;77:215–222. [Google Scholar]
- Becker JT, MacAndrew DK, Fiez JA. A comment on the functional localization of the phonological storage subsystem of working memory. Brain Cogn. 1999;41:27–38. doi: 10.1006/brcg.1999.1094. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox; The Eighth International Conference on Functional Mapping of the Human Brain, Sendai, Japan, June; 2002. [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. NeuroImage. 1998;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortical contributions to object perception. Trends Cogn Sci. 2006;10:100–107. doi: 10.1016/j.tics.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Impairments in visual discrimination after perirhinal cortex lesions: testing “declarative” vs. “perceptual-mnemonic” views of perirhinal cortex function. Eur J Neurosci. 2003;17:649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Cortical regions associated with perceiving, naming, and knowing about colors. J Cogn Neurosci. 1999;11:25–35. doi: 10.1162/089892999563229. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Chein JM, Ravizza SM, Fiez JA. Using neuroimaging to evaluate models of working memory and their implications for language processing. J Neurolinguistics. 2003;16:315–339. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Davachi L, Maril A, Wagner AD. When keeping in mind supports later bringing to mind: neural markers of phonological rehearsal predict subsequent remembering. J Cogn Neurosci. 2001;13:1059–1070. doi: 10.1162/089892901753294356. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Aguirre GK, Stallcup M, Alsop DC, Tippet LJ, Farah MJ. A functional MRI study of mental image generation. Neuropsychologia. 1997;35:725–730. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL. Dissociating memory retrieval processes using fMRI: evidence that priming does not support recognition memory. Neuron. 2001;31:1047–1059. doi: 10.1016/s0896-6273(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci. 2005;25:3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- Gershberg FB, Shimamura AP. Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia. 1995;33:1305–1333. doi: 10.1016/0028-3932(95)00103-a. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Kirchhoff BA, Buckner RL. Common and dissociable activation patterns associated with controlled semantic and phonological processing: evidence from FMRI adaptation. Cereb Cortex. 2005;15:1438–1450. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Gorman AM. Recognition memory for nouns as a function of abstractness and frequency. J Exp Psychol. 1961;61:23–29. doi: 10.1037/h0040561. [DOI] [PubMed] [Google Scholar]
- Haist F, Shimamura AP, Squire LR. On the relationship between recall and recognition memory. J Exp Psychol Learn Mem Cogn. 1992;18:691–702. doi: 10.1037//0278-7393.18.4.691. [DOI] [PubMed] [Google Scholar]
- Hall JF. Learning as a function of word-frequency. Am J Psychol. 1954;67:138–140. [PubMed] [Google Scholar]
- Henson R. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q J Exp Psychol B. 2005;58:340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Hornberger M, Rugg MD. Further dissociating the processes involved in recognition memory: an FMRI study. J Cogn Neurosci. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- Hirst W, Johnson MK, Kim JK, Phelps EA, Risse G, Volpe BT. Recognition and recall in amnesics. J Exp Psychol Learn Mem Cogn. 1986;12:445–451. [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O’Reilly RC, Norman KA. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12:341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15:203–215. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- Howard RJ, ffytche DH, Barnes J, McKeefry D, Ha Y, Woodruff PW, Bullmore ET, Simmons A, Williams SC, David AS, Brammer M. The functional anatomy of imagining and perceiving colour. NeuroReport. 1998;9:1019–1023. doi: 10.1097/00001756-199804200-00012. [DOI] [PubMed] [Google Scholar]
- Hunt RR, Einstein GO. Relational and item-specific information in memory. J Verbal Learn Verbal Behav. 1981;20:497–514. [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci USA. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV. Distributed neural systems for the generation of visual images. Neuron. 2000a;28:979–990. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Haxby JV. The representation of objects in the human occipital and temporal cortex. J Cogn Neurosci. 2000b;12(Suppl 2):35–51. doi: 10.1162/089892900564055. [DOI] [PubMed] [Google Scholar]
- Jackson O, III, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. NeuroImage. 2004;21:456–462. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Kritchevsky M, Squire LR. Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav Neurosci. 1989a;103:548–560. doi: 10.1037//0735-7044.103.3.548. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989b;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Kahana MJ. Associative retrieval processes in free recall. Mem Cognit. 1996;24:103–109. doi: 10.3758/bf03197276. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: levels of processing effect. Proc Natl Acad Sci USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintsch W. Models for free recall and recognition. In: Norman DA, editor. Models of human memory. New York: Academic; 1970. pp. 331–373. [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for novelty encoding and subsequent memory. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. NeuroImage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee CY, Wu YT, Chou CC, Ho LT, Hung DL, Tzeng OJ, Hsieh JC. Frequency effects of Chinese character processing in the brain: an event-related fMRI study. NeuroImage. 2003;18:720–730. doi: 10.1016/s1053-8119(03)00015-6. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: the judgment of previous occurrence. Psychological Rev. 1980;87:252–271. [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HL., III Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: an event-related fMRI study. J Cogn Neurosci. 2000;12:965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- Mellet E, Tzourio-Mazoyer N, Bricogne S, Mazoyer B, Kosslyn SM, Denis M. Functional anatomy of high-resolution visual mental imagery. J Cogn Neurosci. 2000;12:98–109. doi: 10.1162/08989290051137620. [DOI] [PubMed] [Google Scholar]
- Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory, and associations. Curr Opin Neurobiol. 2001;11:188–193. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. NeuroImage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- O’Kane G, Insler RZ, Wagner AD. Conceptual and perceptual novelty effects in human medial temporal cortex. Hippocampus. 2005;15:326–332. doi: 10.1002/hipo.20053. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. State-related and item-related neural correlates of successful memory encoding. Nat Neurosci. 2002;5:1339–1344. doi: 10.1038/nn967. [DOI] [PubMed] [Google Scholar]
- Owen AM, Herrod NJ, Menon DK, Clark JC, Downey SP, Carpenter TA, Minhas PS, Turkheimer FE, Williams EJ, Robbins TW, Sahakian BJ, Petrides M, Pickard JD. Redefining the functional organization of working memory processes within human lateral prefrontal cortex. Eur J Neurosci. 1999;11:567–574. doi: 10.1046/j.1460-9568.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Top-down mechanisms for working memory and attentional processes. In: Gazzaniga MS, editor. The new cognitive neurosciences. Ed 3. Cambridge, MA: MIT; 2004. pp. 919–930. [Google Scholar]
- Petrides M. Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann NY Acad Sci. 1995;769:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci USA. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. NeuroImage. 2004;22:562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Sanquist TF, Rohrbaugh JW, Syndulko K, Lindsley DB. Electrophysiological signs of levels of processing: perceptual analysis and recognition memory. Psychophysiology. 1980;17:568–576. doi: 10.1111/j.1469-8986.1980.tb02299.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffrin RM. Memory search. In: Norman DA, editor. Models of human memory. New York: Academic; 1970. pp. 375–447. [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. NeuroImage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg RJ, Tulving E. The measurement of subjective organization in free recall. Psychol Bull. 1977;84:539–556. [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal and parahippocampal roles during verbal encoding. J Neurosci. 2002;15:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KI, Moss HE, Stamatakis EA, Tyler LK. Binding crossmodal object features in perirhinal cortex. Proc Natl Acad Sci USA. 2006;103:8239–8244. doi: 10.1073/pnas.0509704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Tulving E. Subjective organization in free recall of “unrelated” words. Psychol Rev. 1962;69:344–354. doi: 10.1037/h0043150. [DOI] [PubMed] [Google Scholar]
- Tulving E. Theoretical issues in free recall. In: Dixon TR, Horton DL, editors. Verbal behavior and general behavior theory. Englewood Cliffs, NJ: Prentice Hall; 1968. pp. 2–36. [Google Scholar]
- Tulving E, Pearlstone Z. Availability versus accessibility of information in memory for words. J Verbal Learn Verbal Behav. 1966;5:381–391. [Google Scholar]
- Turriziani P, Fadda L, Caltagirone C, Carlesimo GA. Recognition memory for single items and for associations in amnesic patients. Neuropsychologia. 2004;42:426–433. doi: 10.1016/j.neuropsychologia.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Tversky B. Encoding processes in recognition and recall. Cognit Psychol. 1973;5:275–287. [Google Scholar]
- Ungerleider LG, Haxby JV. “What” and “where” in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;23:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. NeuroImage. 2001a;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001b;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Weis S, Klaver P, Reul J, Elger CE, Fernandez G. Neural correlates of successful declarative memory formation and retrieval: the anatomical overlap. Cortex. 2004;40:200–202. doi: 10.1016/s0010-9452(08)70950-x. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT. Remembering and knowing in patients with frontal lobe injuries. Cortex. 2003;39:827–846. doi: 10.1016/s0010-9452(08)70866-9. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. NeuroImage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nat Neurosci. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. Recall and recognition are equally impaired in patients with selective hippocampal damage. Cogn Affect Behav Neurosci. 2004;4:58–66. doi: 10.3758/cabn.4.1.58. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollecion and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NE, Dobbins IG, Soltani M. Recognition memory for faces: when familiarity supports associative recognition judgments. Psychon Bull Rev. 1999;6:654–661. doi: 10.3758/bf03212975. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]