Abstract
Many of the same regions of the human brain are activated during conscious attention to signals of fear and in the absence of awareness for these signals. The neural mechanisms that dissociate level of awareness from activation in these regions remain unknown. Using functional magnetic resonance imaging with connectivity analysis in healthy human subjects, we demonstrate that level of awareness for signals of fear depends on mode of functional connectivity in amygdala pathways rather than discrete patterns of activation in these pathways. Awareness for fear relied on negative connectivity within both cortical and subcortical pathways to the amygdala, suggesting that reentrant feedback may be necessary to afford such awareness. In contrast, responses to fear in the absence of awareness were supported by positive connections in a direct subcortical pathway to the amygdala, consistent with the view that excitatory feedforward connections along this pathway may be sufficient for automatic responses to “unseen” fear.
Keywords: functional brain imaging, connectivity, amygdala, medial prefrontal cortex, amygdala, emotion
Introduction
Human emotions are more easily influenced when we are not consciously aware of the influence (Zajonc, 1980). Fearful expressions are biologically salient signals of potential danger (Ekman, 1982), with the capacity to influence our physiology in the absence of awareness (Hatfield et al., 1992; Zald, 2003), but the neural mechanisms that dissociate conscious from nonconscious perception of these signals remain unknown.
Neuroimaging studies have considered whether distinct neural systems determine responses to consciously “seen” versus “unseen” signals of fear. The amygdala is implicated in fear processing regardless of awareness (Hatfield et al., 1992; Zald, 2003) but may be recruited by parallel visual inputs. In response to masked fearful faces, the amygdala is activated together with the superior colliculus and pulvinar (Morris et al., 1998; Whalen et al., 1998; Liddell et al., 2005), components of a direct subcortical pathway that bypasses the visual (striate) cortex (Linke et al., 1999). Consciously seen fear, conversely, elicits extensive visual cortex activation (Morris et al., 1999; Hariri et al., 2003; Das et al., 2005), consistent with an indirect striate–lateral geniculate nucleus (LGN) pathway to the amygdala.
Other findings indicate that a simple subcortical–cortical division is not sufficient to dissociate levels of awareness. Early visual association areas of the ventral processing stream are engaged by both unseen (Liddell et al., 2005; Williams et al., 2006c) and seen (Hariri et al., 2003; Das et al., 2005) fear stimuli. These areas have direct white matter links to the amygdala (Catani et al., 2003). Regardless of level of awareness, fear signals also elicit medial prefrontal cortex (MPFC) activation, although nonconscious perception may be distinguished by more rostral ventral activity and conscious fear by more prolonged activity in a comparatively dorsal portion (Williams et al., 2006c).
Lesion studies highlight the lack of a neat dissociation between level of awareness and neural systems. Blind-sight patients cannot consciously report seeing fearful faces but nonetheless discriminate them in forced-choice tasks (de Gelder et al., 1999). Conversely, parietal lesion patients show residual activity in striate and association cortices but remain unable to report the presence of extinguished fearful faces (Vuilleumier et al., 2002). This evidence indicates that striate–LGN input to the amygdala is not sufficient to afford awareness for fear and that lack of such input (Morris et al., 1999, 2001) is not sufficient to account for loss of awareness.
Connectivity between activated brain regions may be necessary for determining awareness (Crick, 1984; Tononi and Edelman, 1998; Lamme and Roelfsema, 2000). In the visual system, fast-latency responses are thought to occur via an initial sweep of activation, supported by feedforward connections along a direct superior colliculus–pulvinar pathway and that is too rapid for feedback reentrants to exert an effect. In contrast, reentrant and lateral feedback occurs at slower timescales, supported by the indirect cortical pathway (Lamme and Roelfsema, 2000). Here, we drew on this model to test the hypothesis that distinct modes of functional connectivity within cortical and subcortical amygdala pathways distinguish consciously seen from unseen signals of fear.
Materials and Methods
Participants.
Fifteen predominantly right-handed healthy subjects (eight females, seven males; mean ± SD age of 35.80 ± 9.04 years; range of 21–48 years) participated in the study [in collaboration with the Brain Resource International Database (Gordon, 2003; Gordon et al., 2005)]. We have shown previously that neural responses to fear are relatively stable over this age range (Williams et al., 2006b). All subjects were within the normal range of tested intelligence (mean of 105) based on the Spot-the-Word estimate of intelligence quotient (Baddeley et al., 1993). Exclusion criteria included Axis-I psychiatric diagnosis, brain injury [via radiological assessment of structural magnetic resonance imaging (MRI) scans], history of loss of consciousness (>10 min), history of other neurological disorder or genetic disorder, and substance abuse.
All participants provided written informed consent to participate, in accordance with Medical Health and Research Council guidelines.
Threshold setting for behavioral task.
The parameters for presenting face stimuli under unseen (nonconscious) and seen (conscious) conditions were based on signal detection findings from an initial psychophysics study (Williams et al., 2004). In this study, a series of equal numbers of fear, neutral, and blank screen stimuli were presented at durations below and above 20 ms, each followed immediately by a neutral face mask. Stimulus series were presented in counterbalanced order, and, for each duration, subjects were asked to respond (via button press) if they could (1) detect the presence of a face versus a blank screen stimulus and (2) discriminate the valence (fear vs neutral) of the face stimuli. At durations of <20 ms, the ability to detect the presence of face versus blank screen stimuli did not differ from chance level. At the duration of 170 ms and above, stimulus detection and emotion discrimination were well above chance level (Williams et al., 2004). These psychophysics data provided the thresholds for nonconscious and conscious perception of fearful faces, respectively.
Behavioral task.
Fear and neutral face stimuli (four female and four male individuals) were selected from a standardized series of grayscale images (Gur et al., 2002), matched for luminosity and size. Facial signals of fear have been found to elicit more robust amygdala activation than visual scenes (Hariri et al., 2002). Face stimuli were presented under both conscious and nonconscious perception conditions, based on the thresholds determined in the above psychophysics study (Williams et al., 2004). In the conscious perception condition, face stimuli were presented for 500 ms, well above the threshold for conscious discrimination. In the nonconscious perception condition, stimulus duration was 16.7 ms [less than the 20 ms threshold established previously (Williams et al., 2004)], followed immediately by a neutral face mask (150 ms). The presentation of the mask after a very short delay (<20 ms) meant that it would not impact the initial feedforward sweep but would interrupt reentrant feedback from higher areas (Lamme and Roelfsema, 2000).
Experimental software ensured that stimulus onset was synchronized with projector refresh cycles. Each condition comprised 240 stimuli (120 fear and 120 neutral), presented in a pseudorandom sequence of 30 mini-blocks (containing eight fear or eight neutral stimuli each). In the nonconscious condition, a stimulus was defined as a stimulus–mask pairing. The interstimulus interval (ISI) was 767 ms in the conscious perception condition and 1100 ms in the nonconscious condition to ensure that the total stimulation period (stimulus plus ISI) was equivalent across conditions (1267 ms). For each condition, the ISI was jittered by ±500 ms to ensure that stimulus onset did not coincide with a constant slice position during image acquisition.
Instructions were presented in synchronized visual and audio (through headphones) format. Subjects were asked to actively attend to the face stimuli, in preparation for a postscanning briefing about these stimuli. For the nonconscious perception condition, they were instructed to focus on the first face in particular.
Skin conductance response acquisition and analysis.
Given that nonconscious as well as conscious fear stimuli, including conditioned fear, have been found to elicit skin conductance responses (SCRs) (Ohman and Soares, 1998; Williams et al., 2004, 2006c), we recorded SCRs in this study as an independent index that physiological responses were produced in the absence of awareness. SCRs were recorded simultaneously with functional MRI data via a customized system (Williams et al., 2001), using a pair of silver–silver chloride electrodes with 0.05 m sodium chloride gel placed on the distal phalanges of digits II and III of the left hand. The electrode pairs were supplied by a constant voltage, and the current change representing conductance was recorded using the direct current amplifier.
The presence of a phasic SCR to each stimulus event was defined by an unambiguous increase (>0.05 μS) with respect to each pretarget baseline and occurring 1–3 s after the event (Lim et al., 1997). In the nonconscious condition, an event was defined by the target face in each target–mask pair. Customized software was based on a sigmoid-exponent mathematical model that allows each SCR to be linked to the individual eliciting stimulus and potentially overlapping SCRs in short interstimulus interval paradigms to be disentangled (Lim et al., 1997). In both conscious and nonconscious conditions, there was an average of 16 SCR events. SCR amplitude was analyzed using within-subjects, repeated-measures ANOVA, with condition (conscious vs nonconscious) and stimulus (fear vs neutral) as within-subject factors. Paired t tests were used to explore the a priori contrasts of interest.
Functional MRI acquisition.
Imaging was performed on a 1.5 T Siemens Vision Plus scanner using an echo planar protocol. A total of 90 functional T2*-weighted volumes (three per stimulus block) were acquired: 15 noncontiguous slices parallel to the intercommissural (anterior commissure–posterior commissure) line, with 6.6 mm thickness; repetition time, 3.3 s; echo time, 40 ms; flip angle, 90°; field of view, 24 × 24 cm2; and matrix size, 128 × 128. Three initial “dummy” volumes were acquired to ensure blood oxygen level-dependent (BOLD) saturation. Analysis of signal-to-noise ratio has demonstrated previously that this functional imaging protocol is able to elicit robust signal change in the amygdala (Williams et al., 2006a) and other subcortical regions (Liddell et al., 2005).
Image processing and analysis.
Preprocessing and statistical analysis was implemented within the statistical parametric mapping software package SPM2 (http://www.fil.ion.ucl.ac.uk/spm/software). For each subject, functional images were first corrected for susceptibility-by-movement artifacts and then realigned to the first volume of the time series. Realigned images were spatially normalized into the standard stereotactic space of the Montreal Neurologic Institute and smoothed using a Gaussian kernel (full-width half-maximum of 8 mm) to minimize anatomical differences. The experimental sequences (conscious/nonconscious fear vs neutral) were modeled using a canonical hemodynamic response function with temporal derivative, and a high-pass filter was applied to remove low-frequency fluctuations in the BOLD signal.
Statistical analysis followed three stages: (1) single-condition statistical parametric maps were generated for the contrast of fear versus neutral for the conscious and nonconscious perception conditions separately; (2) a conjunction analysis was performed to determine the regions activated in common for both conscious and nonconscious conditions, and (3) functional connectivity analysis was undertaken to identify the relationships between regions of activation in response to fear (vs neutral) for the conscious and nonconscious conditions considered separately.
In each stage of analysis, we used a priori, hypothesis-driven search regions of interest (ROIs). To define the search ROIs, we used standardized masks based on neuroanatomical divisions and defined by the automated anatomical labeling protocol (Tzourio-Mazoyer et al., 2002). To test our hypotheses, we focused on the bilateral amygdala, brainstem, thalamus, striate visual cortex [Brodmann’s area 17 (BA17)], extrastriate visual regions (fusiform and inferior occipital gyri, encompassing BA18 and BA37), and medial prefrontal regions, including the anterior cingulate cortex (ACC) (encompassing BA24, BA25, and BA32, and medial orbital to superior frontal structures extending to BA9/10). The utility of this search ROI approach has been demonstrated in our previous study of functional connectivity (Das et al., 2005).
Within-condition activation analyses.
Brain activation for each search ROI was first examined for the contrast of fear relative to neutral face stimuli, within both conscious and nonconscious perception conditions separately. Given the a priori nature of the hypotheses, we used an α level of p < 0.05 with (small-volume correction) and a spatial extent of at least 5 voxels per cluster, consistent with previous studies of fear processing (Das et al., 2005). These individual contrast images were then used in the second-level random effects model to determine regions of significant activation in response to fear, within conscious and nonconscious conditions separately.
Conjunction analyses.
We used conjunction analysis to determine the search ROIs that were significantly activated during both conscious and nonconscious perception of fear versus the neutral baseline. These two experimental conditions were entered into the conjunction analysis.
Functional MRI connectivity analysis.
Psychophysiological interaction (PPI) analysis is used to assess how activity in brain regions of interest may covary together in relation to a source region, in response to the experimental condition (Friston et al., 1997). This method of assessing condition-dependent covariation has been used as an index of “functional connectivity” between these regions (White and Alkire, 2003). We examined functional covariation specific to fear (vs the neutral baseline) within both conscious and nonconscious conditions using the specifically developed function in SPM2. Within each condition, we examined functional connectivity between the amygdala as the source region of interest and each search ROI shown to be significant in the within-condition activation analyses. Connectivity analyses were undertaken separately for the left and right amygdala.
PPI analysis relied on two first-order effect terms, representing the time series of activity in the amygdala source region and the condition (conscious and nonconscious fear vs neutral). Time series were based on the first eigenvariate, extracted from a sphere of 6 mm radius centered on the most significantly active voxel revealed in the ROI contrasts. The interaction between the amygdala time series and condition represented the second-order effect. Before creating this interaction term, the BOLD signal was deconvolved with the hemodynamic response function to represent the interaction at the neuronal rather than hemodynamic level.
This interaction term was fitted at each voxel in the other search ROIs using the contrast [1 0 0]. The resulting individual contrast images were then taken to the second level to perform a random effect analysis. Given the use of a stringent random effects model with a priori defined regions, we used a statistical threshold of p < 0.01 (uncorrected), with an extent threshold of at least 5 voxels per cluster. This procedure produces a regression coefficient for the interaction term as a measure of PPI. A significant coefficient indicates significant functional connectivity between the amygdala source region and the ROI in response to conscious and nonconscious fear (relative to neutral), and the direction of this coefficient indicates the direction (positive or negative) of the covariation. However, it is noted that, because this is a correlational technique, the causal direction of covariation cannot be determined.
In this study, we also used physiophysiological interaction analysis (a variation of the psychophysiological interaction analysis outlined above) to examine how the interaction between components of the cortical and subcortical visual pathways covaries with activation in the other regions of interest. This analysis focuses on the “physiological” interaction between two brain regions rather than the interaction between one brain region and the “psychological” impact of the condition manipulation. Thus, in the analysis steps outlined above, the interaction between the time series for the amygdala source region and the condition was replaced by the interaction between two time series. For the conscious condition, we examined the effect of the interaction between the striate visual cortex and thalamic LGN and, for the nonconscious condition, the effect of the interaction between the thalamic pulvinar and brainstem superior colliculus. Time series (using the first eigenvariate) were extracted from the most significantly active voxel in these regions (Table 1).
Table 1.
Regions of interest activated in response to conscious and nonconscious fearful (vs neutral) faces
| Region | BA | MNI coordinates |
Cluster size (voxels) | p value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Conscious fear > neutral | ||||||
| Amygdala | −26 | 2 | −16 | 37 | 0.015 | |
| 22 | −6 | −12 | 3 | 0.07* | ||
| Thalamus | 2 | −12 | 4 | 502 | 0.001 | |
| Thalamic LGN | −22 | −24 | −2 | 62 | 0.002 | |
| 62 | ||||||
| Superior colliculus/central gray | 0 | −38 | −8 | 21 | 0.025 | |
| Hypothalamus | −2 | −8 | −2 | 13 | 0.002 | |
| Prefrontal cortices | ||||||
| Dorsal MPFC | BA8 | 8 | 40 | 42 | 809 | <0.0001 |
| Dorsal ACC | BA24/33 | −4 | 6 | 28 | 177 | <0.0001 |
| Ventral ACC | 8 | 6 | 28 | |||
| BA32 | −14 | 46 | −4 | 10 | 0.025 | |
| Striate visual cortex | ||||||
| Lingual gyrus (V1) | BA17 | −10 | −94 | −14 | 16 | 0.004 |
| 14 | −92 | −14 | 5 | 0.031 | ||
| Extrastriate visual cortices | ||||||
| Fusiform gyrus | BA37 | −42 | −40 | −24 | 85 | 0.001 |
| BA20 | 42 | −32 | −24 | 16 | 0.027 | |
| Inferior occipital gyrus | BA18 | 30 | −92 | −8 | 62 | 0.002 |
| Nonconscious fear > neutral | ||||||
| Amygdala | −16 | 2 | −16 | 11 | 0.008 | |
| 18 | 2 | −16 | 8 | 0.010 | ||
| Thalamic pulvinar | −8 | −32 | 4 | 12 | 0.023 | |
| Brainstem | ||||||
| Superior colliculus | −4 | −32 | 0 | 43 | 0.019 | |
| Hypothalamus | −2 | −6 | −2 | 9 | 0.007 | |
| 4 | −8 | −2 | 25 | 0.010 | ||
| Prefrontal cortices | 0.019 | |||||
| Ventral rostral MPFC | BA11/25 | 8 | 58 | −12 | 12 | |
| Extrastriate visual cortices | ||||||
| Fusiform gyrus | BA37 | |||||
| Inferior occipital gyrus | BA18 | 42 | −86 | −14 | 90 | 0.006 |
| Conjunction of conscious and nonconscious fear > neutral | ||||||
| Amygdala | 20 | 0 | −12 | 6 | 0.046 | |
| −20 | 2 | −16 | 8 | 0.048 | ||
| Thalamus | 12 | −8 | 0 | 229 | 0.04 | |
| Thalamic LGN | −18 | −22 | −2 | 0.03 | ||
| 18 | −22 | −4 | 0.09* | |||
| Brainstem | 0 | −34 | 0 | 40 | 0.035 | |
| Superior colliculus | −2 | −6 | −2 | 115 | 0.007 | |
| Hypothalamus | 4 | −8 | −2 | 0.040 | ||
| Prefrontal cortices | BA8/24 | −10 | 30 | 46 | 19 | 0.07* |
| Dorsal MPFC/ACC | 8 | 28 | 48 | 23 | 0.09* | |
| Extrastriate visual cortices | ||||||
| Fusiform gyrus | BA18 | 42 | −86 | −14 | 76 | 0.006 |
| Inferior occipital gyrus | 34 | −96 | −8 | 0.017 | ||
Regions are defined in terms of Brodmann’s area, standardized coordinates [in Montreal Neurological Institute (MNI) format], size of activated cluster, and level of significance at p < 0.05 (small-volume corrected). The asterisks indicate trend level. V1, Striate visual cortex.
Results
Behavioral data
In postscan briefings, subjects confirmed they were unable to detect the presence of face stimuli in the nonconscious condition with above chance accuracy. In contrast, consciously presented expressions were recognized with well above chance accuracy (fear, 83%; neutral, 78%), indicating that any differential effects in brain activity were unlikely to be attributable to visual processing or discrimination difficulties.
SCR data
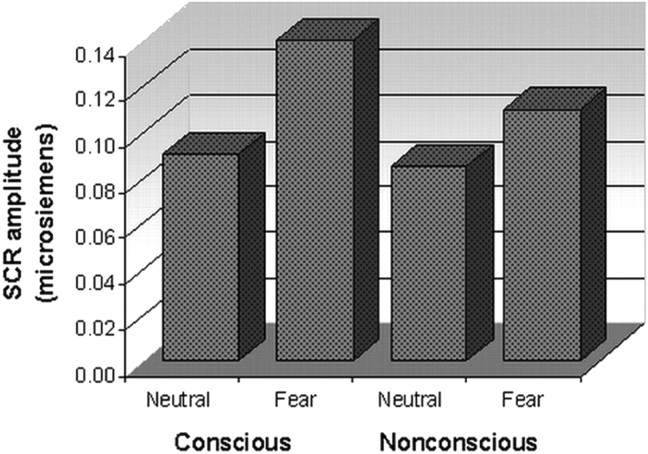
Fearful faces elicited significantly enhanced SCRs (relative to neutral) in both conditions (Fig. 1), although this enhancement was most pronounced in response to conscious fear, reflected in the trend level interaction between condition and stimulus (F(28) = 2.74; p = 0.07). Contrasts confirmed that SCRs to conscious fear were significantly greater than those to neutral (t(14) = 4.28; p = 0.001), whereas there was a trend toward a difference for nonconscious fear relative to neutral (t(14) = 1.89; p = 0.08). These SCR data provide independent evidence that physiological responses occur in the absence of awareness.
Figure 1.
Skin conductance responses elicited by fear relative to neutral under conscious and nonconscious perception conditions. The amplitude of SCRs is shown in microsiemens. Fear elicited enhanced SCRs relative to neutral under both awareness conditions.
Within-condition activation analyses
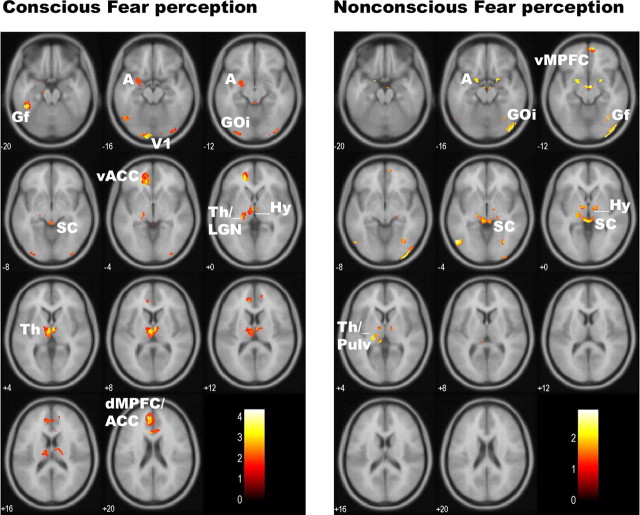
Consciously viewed fear elicited significant activation in components of a cortical visual pathway to the amygdala: left amygdala (and right amygdala at trend level), bilateral thalamus (including a region consistent with the LGN), and striate cortex (Table 1, Fig. 2). Significant, albeit less pronounced, activation was also revealed in a brainstem region corresponding to the superior colliculus, suggesting parallel input from the subcortical visual pathway (Table 1, Fig. 2). Additional cortical activation was observed in the extrastriate visual cortices [both inferior occipital gyrus (GOi) and fusiform gyrus (Gf)] and in both dorsal and ventral portions of the MPFC, including ACC (Table 1, Fig. 2).
Figure 2.
Statistical parameter maps for the regions of interest activated in response to conscious fear (left) (vs neutral) and nonconscious fear (right) (vs neutral). For conscious fear, significant (p < 0.05, small-volume corrected) activations were observed in the amygdala, thalamus (including LGN), anterior cingulate (dorsal and ventral portions), and visual cortices, both striate and extrastriate (including fusiform and inferior occipital gyri). Activations were also observed in the superior colliculus and hypothalamus regions of the brainstem. For nonconscious fear, significant (p < 0.05, small-volume corrected) activity was observed in the bilateral amygdala and brainstem regions consistent with the superior colliculus and hypothalamus. Activity was also observed in the thalamic pulvinar (rather than LGN), in the ventral (but not dorsal) anterior cingulate, and in the extrastriate (right inferior occipital gyrus) but not striate visual cortex. Coordinates for these regions of significant activation are presented in Table 1. A, Amygdala; V1, striate visual cortex; SC, superior colliculus of the brainstem; Pulv, pulvinar of the thalamus; Th, thalamus; Hy, hypothalamus; dMPFC, dorsal MPFC; vMPFC, ventral MPFC.
Nonconscious fear (relative to neutral) perception elicited activity in corresponding subcortical regions, including the bilateral amygdala, pulvinar region of the thalamus, and brainstem region consistent with the superior colliculus (Table 1, Fig. 2). Although cortical activity was not prominent in response to nonconscious fear, it was nonetheless observed in extrastriate regions, GOi and Gf, and ventral rostral MPFC (Table 1, Fig. 2).
Conjunction analysis
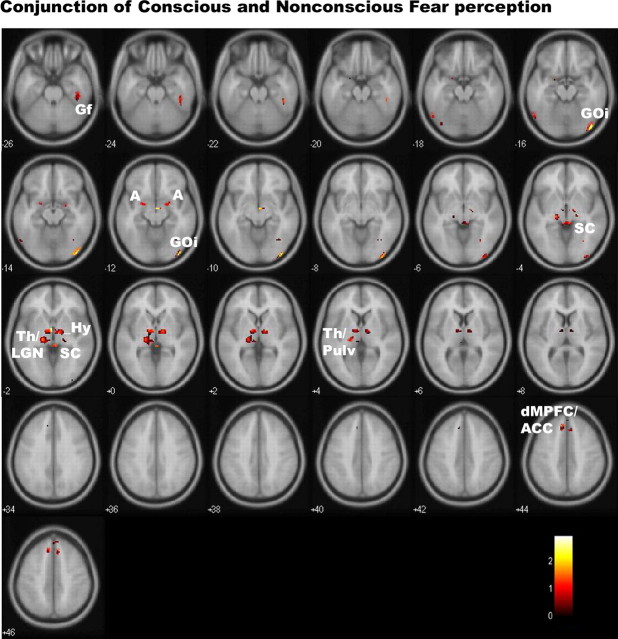
Using conjunction analysis, we determined which regions of activation were common to both conscious and nonconscious fear (relative to neutral). In common, conscious and nonconscious fear elicited activity in the amygdala, thalamus (encompassing regions consistent with LGN and pulvinar), brainstem, and extrastriate GOi and Gf regions, albeit primarily at marginal levels of significance (Table 1, Fig. 3). Small regions of common, trend level activation were also observed in a dorsal portion of the MPFC and ACC (Table 1, Fig. 3), although activation in these regions reached significance only in response to conscious fear for within-condition analyses. There were no corresponding clusters of common activity in the ventral prefrontal cortices. Common activation was also not apparent in the striate visual cortex.
Figure 3.
Statistical parameter maps for the conjunction of activation in regions of interest to both conscious and nonconscious fear (vs neutral). In conjunction, these conditions showed significant (p < 0.05, small-volume corrected) activations in the amygdala, thalamus (encompassing regions consistent with LGN and pulvinar), brainstem, and extrastriate GOi and Gf regions. Small regions of common, trend-level activation were also observed in a dorsal portion of the MPFC and ACC. There were no corresponding clusters of common activity in the ventral prefrontal or striate visual cortices. A, Amygdala; dMPFC, dorsal MPFC; Hy, hypothalamus; Pulv, pulvinar of the thalamus; SC, superior colliculus of the brainstem; Th, thalamus.
Functional MRI connectivity analysis
Using PPI analysis, we examined functional connectivity between the amygdala and components of the visual pathways, as well as the medial prefrontal and visual association cortices. For conscious fear, the interaction between striate visual cortex and thalamic LGN covaried negatively with both left and right amygdala, suggesting negative neuromodulation within the indirect cortical pathway (Table 2, Fig. 4). Activation in the bilateral amygdala also covaried negatively with both the striate cortex and thalamus, which was specific to the LGN for the right amygdala (Table 2, Fig. 4). Similarly, in the parallel subcortical pathway, the amygdala showed negative connectivity with the midbrain region of the brainstem.
Table 2.
Functional connectivity between the amygdala and both cortical and subcortical regions of interest during conscious fear (vs neutral) perception
| Region | MNI coordinates |
Cluster size (voxels) | z score | p value | Direction of connectivity | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Connectivity with left amygdala | |||||||
| Thalamus | −16 | −16 | 4 | 156 | 3.17 | 0.001 | Negative |
| 12 | −10 | 4 | 108 | 2.74 | 0.003 | ||
| Brainstem | |||||||
| Midbrain | −4 | −18 | −2 | 6 | 2.67 | 0.004 | Negative |
| 12 | −10 | −2 | 8 | 2.41 | 0.008 | ||
| Rostral dorsal MPFC (BA8/9/10) | −6 | 62 | 34 | 9 | 2.68 | 0.004 | Negative |
| 12 | 58 | 40 | 12 | 2.56 | 0.005 | ||
| Striate cortex (V1) | 14 | −78 | 8 | 37 | 3.20 | 0.001 | Negative |
| Extrastriate visual cortex: | |||||||
| Fusiform gyrus | −38 | −78 | −14 | 54 | 3.90 | 0.000 | Negative |
| Inferior occipital gyrus | −40 | −86 | −12 | 37 | 3.53 | 0.000 | |
| 42 | −68 | −10 | 42 | 2.95 | 0.002 | ||
| Dorsal ACC (BA24/33) | 6 | 8 | 24 | 16 | 3.16 | 0.001 | Positive |
| Connectivity with right amygdala | |||||||
| Thalamic LGN | 22 | −28 | 10 | 123 | 3.16 | 0.001 | Negative |
| Brainstem | Negative | ||||||
| Midbrain | −8 | −16 | −4 | 55 | 3.34 | 0.000 | |
| Rostral dorsal MPFC (BA9/10) | −6 | 60 | 24 | 10 | 2.50 | 0.005 | Negative |
| Ventral ACC (BA25/32) | −8 | 20 | −2 | 8 | 2.75 | 0.003 | |
| 4 | 18 | −2 | 5 | 2.55 | 0.005 | ||
| Striate cortex (V1) | −14 | −84 | 4 | 5 | 2.76 | 0.003 | Negative |
| Extrastriate visual cortex | |||||||
| Fusiform gyrus | 20 | −56 | −12 | 5 | 2.65 | 0.004 | Positive |
| Dorsal ACC (BA24/33) | 10 | 10 | 28 | 4 | 2.94 | 0.002 | Positive |
| Connectivity with striate Cortex (V1)–LGN interaction | |||||||
| Right amygdala | 30 | 2 | −26 | 56 | 2.18 | 0.015 | Negative |
| Left amygdala | −26 | −4 | −22 | 43 | 2.29 | 0.011 | |
Regions showing functional connectivity with the left and right amygdala are summarized first, followed by the interaction of activity in the striate cortex (V1) and LGN and its functional connectivity with the amygdala. Regions are defined by standardized coordinates [Montreal Neurological Institute (MNI) format] and size of activated cluster, with extent (z score), significance (p value), and direction of connectivity.
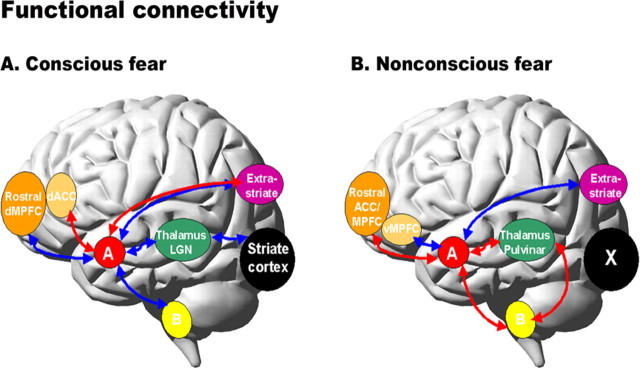
Figure 4.
Psychophysiological interaction analyses showing the functional connectivity between the amygdala and the regions of interest during conscious fear (A) and nonconscious fear (B) relative to neutral. Red arrows represent positive functional connectivity between the amygdala and regions of interest, and blue arrows represent negative connectivity. Conscious fear (A) was distinguished by negative functional connectivity in a cortical (striate–thalamic LGN) amygdala pathway, with links to both left and right amygdala. Negative connectivity was also observed between the bilateral amygdala and other cortical regions (extrastriate and dorsal medial prefrontal), as well as the subcortical brainstem region. Conscious fear elicited positive functional connectivity between the bilateral amygdala and dorsal ACC. The right amygdala in particular also covaried positively with the ventral ACC, extrastriate, and periaqueductal gray regions of the brainstem (data not shown). Nonconscious fear (B), in contrast, elicited positive functional connectivity in a subcortical (brainstem–thalamic pulvinar) amygdala pathway, localized to the right amygdala. Positive relationships were also observed between the bilateral amygdala and rostral regions of the medial prefrontal and ACC. For nonconscious fear, the right amygdala showed negative relationships with additional cortical regions: the extrastriate and ventral medial prefrontal cortices (data not shown). A, Amygdala; B, brainstem; dACC, dorsal ACC; dMPFC, dorsal MPFC; vMPFC, ventral MPFC.
During conscious fear, connectivity between the amygdala and visual association and medial prefrontal cortices varied with both laterality and dorsoventral gradient. Whereas the left amygdala showed negative functional connectivity with extrastriate visual regions, the right amygdala covaried positively with the right extrastriate fusiform gyrus (Table 2, Fig. 4). We observed negative connections between the right amygdala and both dorsal MPFC and ventral ACC but positive connections between bilateral amygdala and dorsal ACC (Table 2, Fig. 4).
In contrast to conscious fear, PPI analysis revealed a predominance of positive functional connections with the amygdala for nonconscious fear. Within the subcortical visual pathway, the interaction of activation in brainstem superior colliculus and thalamic pulvinar showed positive functional connectivity with the right amygdala (Table 3, Fig. 4). The bilateral amygdala also covaried positively with the thalamic pulvinar, and the right amygdala showed additional positive covariation with the midbrain region (Table 3, Fig. 4). Conversely, there was an absence of functional connectivity between the striate cortex and amygdala, confirming the preferential reliance on positive connections within the subcortical pathway.
Table 3.
Functional connectivity between the amygdala and both cortical and subcortical regions of interest during nonconscious fear (vs neutral) perception
| Region | MNI coordinates |
Cluster size (voxels) | z score | p value | Direction of connectivity | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Connectivity with left amygdala | |||||||
| Brainstem | Negative | ||||||
| Hypothalamus | −4 | −4 | −10 | 5 | 3.83 | 0.000 | |
| Thalamic pulvinar | −20 | −26 | −2 | 10 | 3.03 | 0.001 | Positive |
| Brainstem | Positive | ||||||
| Midbrain | 14 | −14 | −8 | 9 | 2.75 | 0.003 | |
| Rostral MPFC (BA9/10) | −12 | 58 | 20 | 6 | 2.59 | 0.005 | Positive |
| Rostral ACC (BA24/32) | 16 | 40 | 6 | 11 | 2.79 | 0.003 | Connectivity with right amygdala |
| Extrastriate visual cortex: GOi | 44 | −86 | −10 | 62 | 3.27 | 0.001 | Negative |
| −50 | −76 | −10 | 42 | 2.90 | 0.002 | ||
| Ventral MPFC (BA11) | 4 | 36 | −14 | 31 | 3.67 | 0.000 | Negative |
| Thalamic pulvinar | −22 | −24 | −2 | 8 | 2.86 | 0.002 | Positive |
| 10 | −26 | 2 | 3 | 2.51 | 0.006 | ||
| Brainstem | 12 | −24 | −30 | 8 | 2.40 | 0.008 | Positive |
| Connectivity with colliculo-pulvinar interaction | |||||||
| Right amygdala | 24 | 2 | −18 | 17 | 2.21 | 0.014 | Positive |
| Rostral dorsal ACC (BA24/32) | −4 | 30 | 24 | 125 | 2.48 | 0.001 | Positive |
Regions showing functional connectivity with the left and right amygdala are summarized first, followed by results for the interaction of activity in the superior colliculus and thalamic pulvinar and its functional connectivity with the amygdala. Regions are defined by standardized coordinates [Montreal Neurological Institute (MNI) format] and size of activated cluster (in number of active voxels), with extent (z score), significance (p value), and direction of connectivity.
Nonconscious fear also elicited distinctive profiles of functional connectivity between the amygdala and visual and prefrontal association cortices. Activation in the bilateral amygdala covaried positively with the rostral portion of the ACC, extending to MPFC (as opposed covariation with the dorsal portion for conscious fear) (Table 3, Fig. 4). Conversely, there was negative functional connectivity between the right amygdala and ventral MPFC for nonconscious fear, akin to that for the conscious condition (Table 3, Fig. 4). Additionally, we observed negative functional connectivity between the right amygdala and bilateral extrastriate inferior occipital gyrus for nonconscious fear, an inverse relationship to that observed for conscious fear (Table 3, Fig. 4).
Discussion
These findings demonstrate for the first time that the mode of functional connectivity in cortical and subcortical amygdala pathways, and not the pattern of activation in these pathways, provides a striking dissociation of the level of awareness for biologically significant signals of fear.
In common, conscious and nonconscious perception of fear elicited activation in the amygdala, brainstem, thalamus, and extrastriate visual cortices, along with a small region of the MPFC and ACC. Clusters of activation that distinguished level of awareness for fear were observed in the striate and prefrontal cortices. The striate visual cortex was engaged only by consciously perceived fear signals, consistent with a cortical pathway to the amygdala. In the absence of awareness, the amygdala may receive only crude visual input via a parallel ventral stream involving the brainstem superior colliculus, pulvinar, and extrastriate regions. Conscious perception of fear was also distinguished by pronounced dorsal MPFC/ACC activity, extending ventrally for the ACC, whereas MPFC activation localized to the ventral portion for unseen fear. These results extend previous neuroimaging and lesion evidence that facial signals of fear elicit amygdala activity even in the absence of awareness (Morris et al., 1998; Whalen et al., 1998; Liddell et al., 2005; Williams et al., 2006c). They also accord with the view that visual input to the amygdala relies on the preferential engagement of parallel ventral and dorsal pathways, according to level of awareness (Morris et al., 1999, 2001; Williams et al., 2006c). However, the overlap in regions of activation indicates it is unlikely that level of awareness for fear is determined solely by recruitment of localized amygdala networks.
Psychophysiological and physiophysiological interaction analyses indicated that it is the mode of functional connectivity in amygdala pathways that is crucial to determining level of awareness. Conscious attention to fear elicited negative functional connectivity within visual pathways to the amygdala. In contrast, we observed only positive connectivity within the subcortical pathway to the amygdala in the absence of awareness. Although BOLD activity changes over the slower timescale of neuromodulator action, rather than the millisecond timescale of neural coupling, evidence concerning the relationship between neural firing and subsequent hemodynamic changes is accumulating (Attwell and Iadecola, 2002; Logothetis, 2003). In this context, opposing functional relationships between significant regions of BOLD activity may reflect a different balance of excitatory and inhibitory neuromodulatory action on synaptic activity. Although it is also not possible to infer causal direction from the connectivity analysis, this proposal also accords with the distinct modes of neural cooperation described in models of visual consciousness (Lamme and Roelfsema, 2000). That is, negative functional connectivity may reflect the reliance of conscious fear perception on reentrant feedback, whereas positive connectivity may reflect the excitatory feedforward connections that support processing of fear signals in the absence of awareness.
Reentrant feedback to posterior visual areas is thought to be necessary in affording conscious awareness for visual stimuli (Lamme and Roelfsema, 2000). Feedback involving visual areas may require a greater balance of inhibitory action in cortical neuromodulators such as GABA. Indeed, the mutual inhibition of GABAergic interneurons is implicated as a fundamental mechanism in the neural synchrony that binds information for awareness (Bush and Sejnowski, 1996; Lee et al., 2003). Numerical modeling demonstrates that neural synchrony requires an optimal ratio of 4:1 inhibitory to excitatory connections (Bush and Sejnowski, 1996), consistent with the predominance of negative connections in cortical pathways to the amygdala.
Complementary animal evidence demonstrates the presence of inhibitory GABAergic circuitry in the amygdala (Szinyei et al., 2000). Across the sustained timescale of conscious attention to fear, inhibition may serve to downregulate parallel sources of visual input to the amygdala, allowing a shift toward stimulus elaboration in the amygdala and higher-order association areas. This proposal accords with models of visual consciousness, in which selective stimulus processing is facilitated by the broad inhibition of surrounding sensory networks (Dehaene et al., 2003). Terms such as “searchlight” and “surround inhibition” have been used to describe this phenomenon (Crick, 1984). Biophysical and large-scale network models also highlight the importance of inhibitory modulation for se lective attention to salient information (Rennie et al., 2002; Dehaene et al., 2003).
Masking of fear faces may disrupt reentrant feedback from higher areas, such that nonconscious fear perception proceeds with rapid feedforward connections between the amygdala and components of the subcortical pathway. Such connections may be reflected in the positive relationships within the brainstem–amygdala pathway revealed by PPI analysis. The excitatory action of rapid-acting neuromodulators, such as glutamate, may facilitate feedforward connections along direct amygdala pathways, allowing automatic responses to low-level fear stimulation to proceed despite an absence of awareness. Whereas feedforward connections may also be sufficient to activate visual and medial prefrontal association areas, the blocking of reentrant feedback by the mask may prevent information supported by activation of these areas from reaching conscious awareness (Lamme and Roelfsema, 2000).
Despite the preponderance of negative connectivity for conscious fear and positive connectivity for nonconscious fear, covariation between the amygdala and association areas was more complex under both conditions. The lateralized profile of positive and negative connectivity between visual association areas and the amygdala during conscious fear processing accords with evidence from activation studies (Das et al., 2005). Modulation of amygdala activity during the maintenance of conscious visual attention may involve a dynamical shift in the balance of excitation and feedback, according to laterality. In contrast, the reversal of the right amygdala–visual association connection during nonconscious fear perception might reflect the absence of feedback to the striate cortex in this condition and thus a lack of visual elaboration in the higher-order areas to which it projects.
For medial prefrontal areas, awareness was distinguished by the topography as well as direction of connections. The amygdala showed a positive connection with the dorsal ACC for conscious fear but with the rostral portion for nonconscious fear. This three-way dissociation provides compelling support within the one study for previous evidence using consciously seen negative facial expressions (Kim et al., 2003, 2004) and unseen fearful eye whites (Whalen et al., 2004), that the direction of amygdala relationship varies according to dorsal versus rostral ventral medial prefrontal regions and level of awareness. The findings also contradict the view that the ACC has a specific role in the conscious modulation of emotional states (Damasio, 1996). Rather, a feedforward sweep of positive connections may be the means by which the ACC is recruited as part of the networks for automatic alerting to potential danger. The ventral medial prefrontal cortex may have a more general role in emotion regulation, regardless of awareness, consistent with the negative connections with the amygdala under both awareness conditions (Damasio, 1996). Overall, the amygdala showed far fewer connections with cortical association areas when conscious awareness was prevented, adding weight to the view that the degree of neural connectivity within the association cortices may be critical to the emergence of conscious awareness (Vuilleumier et al., 2002).
Our findings support a model of fear processing that goes beyond a focus on the neuroanatomical specialization of amygdala pathways and highlights the importance of the mode of functional connectivity in determining the level of awareness with which innate signals of potential danger are perceived (Williams, 2006). The presence of positive connectivity in the absence of awareness may provide a mechanism to explain the contagious nature of fear (Hatfield et al., 1992), in which a cascade of automatic physiological responses to fear to spread rapidly from one individual to the next, without time for inhibitory feedback. On a macro-scale, a similar cycle of covert contagion may underlie the global escalation in fear about traumatic events and the corresponding rise in anxiety and posttrauma reactions (Hoven et al., 2005).
Footnotes
This work was supported in part by Australian Research Council Grants DP0452237 and DP0345481 (L.M.W., E.G.). L.M.W. was supported by a Pfizer Senior Research fellowship and is also an affiliated scientist of Neuroscience Institute of Schizophrenia and Allied Disorders (NISAD). P.D. was supported by NISAD, and A.H.K. was supported by a National Health and Medical Research Council Peter Doherty fellowship. We thank the Brain Resource International Database (under the auspices of the BRAINnet scientific division of Brain Resource Company) for data acquisition support.
References
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Emslie H, Nimmo-Smith I. The Spot-the-Word test: a robust estimate of verbal intelligence based on lexical decision. Br J Clin Psychol. 1993;32:55–65. doi: 10.1111/j.2044-8260.1993.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Bush P, Sejnowski T. Inhibition synchronizes sparsely connected cortical neurons within and between columns in realistic network models. J Comput Neurosci. 1996;3:91–110. doi: 10.1007/BF00160806. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, Gordon E, Williams LM. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. NeuroImage. 2005;26:141–148. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Vroomen J, Pourtois G, Weiskrantz L. Non-conscious recognition of affect in the absence of striate cortex. NeuroReport. 1999;10:3759–3763. doi: 10.1097/00001756-199912160-00007. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Sergent C, Changeux JP. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc Natl Acad Sci USA. 2003;100:8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. Emotion in the human face. Cambridge, UK: Cambridge UP; 1982. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gordon E. Integrative neuroscience. Neuropsychopharmacology. 2003;28:S2–S8. doi: 10.1038/sj.npp.1300136. [DOI] [PubMed] [Google Scholar]
- Gordon E, Cooper N, Rennie C, Hermens D, Williams LM. Integrative neuroscience: the role of a standardised database. Clin EEG Neurosci. 2005;36:64–75. doi: 10.1177/155005940503600205. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hatfield E, Cacioppo JT, Rapson RL. Primitive emotional contagion. Emot Social Behav. 1992;14:151–177. [Google Scholar]
- Hoven CW, Duarte CS, Lucas CP, Wu P, Mandell DJ, Goodwin RD, Cohen M, Balaban V, Woodruff BA, Bin F, Musa GJ, Mei L, Cantor PA, Aber JL, Cohen P, Susser E. Psychopathology among New York city public school children 6 months after September 11. Arch Gen Psychiatry. 2005;62:545–552. doi: 10.1001/archpsyc.62.5.545. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. NeuroReport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM. A direct brainstem-amygdala-cortical “alarm” system for subliminal signals of fear. NeuroImage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Lim CL, Rennie C, Barry RJ, Bahramali H, Lazzaro I, Manor B, Gordon E. Decomposing skin conductance into tonic and phasic components. Int J Psychophysiol. 1997;25:97–109. doi: 10.1016/s0167-8760(96)00713-1. [DOI] [PubMed] [Google Scholar]
- Linke R, De Lima AD, Schwegler H, Pape HC. Direct synaptic connections of axons from superior colliculus with identified thalamo-amygdaloid projection neurons in the rat: possible substrates of a subcortical visual pathway to the amygdala. J Comp Neurol. 1999;403:158–170. [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, de Gelder B, Weiskrantz L, Dolan RJ. Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain. 2001;124:1241–1252. doi: 10.1093/brain/124.6.1241. [DOI] [PubMed] [Google Scholar]
- Ohman A, Soares JJ. Emotional conditioning to masked stimuli: expectancies for aversive outcomes following nonrecognized fear-relevant stimuli. J Exp Psychol Gen. 1998;127:69–82. doi: 10.1037//0096-3445.127.1.69. [DOI] [PubMed] [Google Scholar]
- Rennie CJ, Robinson PA, Wright JJ. Unified neurophysical model of EEG spectra and evoked potentials. Biol Cybern. 2002;86:457–471. doi: 10.1007/s00422-002-0310-9. [DOI] [PubMed] [Google Scholar]
- Szinyei C, Heinbockel T, Montagne J, Pape HC. Putative cortical and thalamic inputs elicit convergent excitation in a population of GABAergic interneurons of the lateral amygdala. J Neurosci. 2000;20:8909–8915. doi: 10.1523/JNEUROSCI.20-23-08909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Edelman GM. Consciousness and complexity. Science. 1998;282:1846–1851. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Clarke K, Husain M, Driver J, Dolan RJ. Neural response to emotional faces with and without awareness: event-related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia. 2002;40:2156–2166. doi: 10.1016/s0028-3932(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, Johnstone T. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. NeuroImage. 2003;19:402–411. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Williams LM. An integrative neuroscience model of significance processing. J Integr Neurosci. 2006;5:1–47. doi: 10.1142/s0219635206001082. [DOI] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, Bahramali H, Olivieri G, David AS, Peduto A, Gordon E. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. NeuroImage. 2001;14:1070–1079. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Rathjen J, Brown KJ, Gray J, Phillips M, Young A, Gordon E. Mapping the time course of nonconscious and conscious perception of fear: an integration of central and peripheral measures. Hum Brain Mapp. 2004;21:64–74. doi: 10.1002/hbm.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto AS, Gordon E, Bryant RA. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. NeuroImage. 2006a;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer P, Liddell BJ, Kemp AH, Olivieri G, Peduto AS, Gordon E. The mellow years?: neural basis of improving emotional stability over age. J Neurosci. 2006b;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, Gordon E. Amygdala-prefrontal dissociation of subliminal and supraliminal fear. Hum Brain Mapp. 2006c;27:652–661. doi: 10.1002/hbm.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc R. Feeling and thinking: preferences need no inferences. Am Psychol. 1980;35:151–175. [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]