Abstract
Corticotropin-releasing factor (CRF), a 41 amino acid peptide, mediates endocrine, autonomic, and behavioral responses to stress. Whereas the CRF1 receptor appears to contribute to anxiety associated with stress, the role of the CRF2 receptor remains unclear and may depend on drug dose, brain location, or testing environment. Results involving treatments with selective CRF2 receptor agonists or antagonists and the behavior of CRF2 receptor knock-out mice suggest both anxiogenic and anxiolytic effects of CRF2 receptor activation. The present study tested the hypothesis that the effect of CRF2 receptor activation on anxiety depends on the stress level of the animal. The selective CRF2 receptor agonist urocortin 2 was infused into the lateral septum of mice under low- or high-stress (30 min of immobilization) testing conditions, and then behavior in the light–dark box, open-field, and novel-object tests was assessed. In the low-stress environment, 240 pmol of septal urocortin 2 increased anxiety, but lower doses (0.48, 4.8, and 48 pmol) did not have consistent effects. However, in the high-stress condition, 48 pmol of septal urocortin 2 significantly increased anxiety compared with control in wild-type but not CRF2 receptor knock-out mice in the light–dark box. Septal administration of the relatively selective CRF2 antagonist astressin-2B, but not the CRF1- selective antagonist antalarmin, blocked the anxiogenic effects of urocortin 2. Urocortin 2 infusion into the medial septum or lateral ventricle did not affect anxiety measures. These results indicate that the effect of septal CRF2 receptor activation on anxiety is dependent on stress level.
Keywords: CRF receptor 1, CRF receptor 2, urocortin 2, lateral septum, anxiety, stress
Introduction
Corticotropin-releasing factor (CRF), a 41 amino acid peptide, mediates the endocrine, autonomic, and behavioral responses to stress (Vale et al., 1981), initiating the release of glucocorticoids from the hypothalamic–pituitary–adrenal axis. CRF plays a critical role in the “fight or flight” response to stress, increasing blood sugar and heart rate, inhibiting immune and digestive function (Dunn and Berridge, 1990), and increasing anxiety (Takahashi et al., 1989). CRF binds to G-protein-coupled receptors, CRF1 and CRF2, expressed in discrete brain regions. Whereas the CRF1 receptor is expressed in many rodent brain areas (Van Pett et al., 2000), the CRF2 receptor has a restricted distribution, with strong expression in the ventromedial hypothalamus, dorsal raphe, and lateral septum.
Considerable evidence indicates that CRF1 receptor activation mediates the anxiogenic effects of CRF (Smith et al., 1998; Zorrilla et al., 2002), but the role of the CRF2 receptor in anxiety remains controversial. CRF2 receptor knock-out mice exhibited greater anxiety-like behavior in the elevated plus maze and the light–dark box compared with wild-type mice in two of three mouse lines tested (Bale et al., 2000; Coste et al., 2000; Kishimoto et al., 2000), suggesting that activation of CRF2 receptors reduces anxiety. Selective CRF2 receptor agonists (urocortin 2 and 3) also decreased anxiety-like behavior in the elevated plus maze in rats (Valdez et al., 2002, 2003) and in the open field and light–dark box in C57BL/6 mice (Venihaki et al., 2004). In contrast, other studies report anxiogenic effects of CRF2 receptor agonists in the elevated plus maze and the acoustic startle test in mice (Pelleymounter et al., 2002, 2004; Risbrough et al., 2003, 2004). Furthermore, antagonism of CRF2 receptors in the lateral septum also decreases anxiety (Radulovic et al., 1999; Bakshi et al., 2002).
It is relevant to note that studies reporting anxiolytic effects of CRF2 receptor agonists (Valdez et al., 2002, 2003; Venihaki et al., 2004) tested animals under relatively low-stress conditions, in which the animals were handled by the experimenter and habituated to the testing environment. In contrast, reports of anxiogenic effects of CRF2 receptor activation are from subjects tested in high-stress environments [e.g., during mild restraint in the acoustic startle chamber while exposed to loud noises (Risbrough et al., 2003, 2004), shock-induced freezing (Ho et al., 2001; Bakshi et al., 2002), or immobilization stress (Radulovic et al., 1999)].
We propose that the effect of CRF2 receptor activation on anxiety may depend on the level of stress in the testing environment, a variable that may alter the degree of receptor activation in specific brain regions or affect interaction between the CRF1 and CRF2 receptors. In the current study, we examined the effects of the selective CRF2 receptor agonist urocortin 2 in the mouse lateral septum on anxiety-related behaviors in the light–dark box, open-field, and novel-object tests under low-stress (no immobilization) or high-stress (30 min of immobilization) testing conditions.
Materials and Methods
Subjects.
Male ICR mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). CRF2 receptor wild-type and knock-out mice on a mixed C57BL/6 × 129 background were generated as described previously (Bale et al., 2000) and bred mating heterozygous mice. Animals were group housed under a 12 h light/dark cycle. All experiments were conducted during the dark cycle, and all procedures were approved by the Institutional Animal Care and Use Committee of The Salk Institute and The Scripps Research Institute. All experimental protocols and animal facilities were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care and the National Institutes of Health guidelines.
Drugs.
Urocortin 2 and astressin-2B were synthesized under the direction of Dr. Jean Rivier (Clayton Foundation Laboratories for Peptide Biology, The Salk Institute). Urocortin 2 was dissolved in sterile physiological saline containing 0.1 m bovine serum albumin, pH 7.4. Astressin-2B was initially dissolved in a small amount of acetic acid and then diluted with sterile physiological saline containing 0.1 m bovine serum albumin to a final pH of 6.5. Antalarmin was purchased from Sigma (St. Louis, MO) and dissolved in a solution of 10% Cremaphor (Sigma), 5% ethanol, and 85% sterile saline with a final pH of 6.0. Peptides were prepared immediately before use.
It is relevant to note that urocortin 1 and 3, but not urocortin 2, are known to project to the rodent lateral septum. Urocortin 1 exhibits a high affinity for both the CRF1 and CRF2 receptor and was thus not the optimal choice for a study designed to examine the effect of selective CRF2 receptor activation. Although both urocortin 2 and 3 exhibit low affinity for the CRF1 receptor (Ki of >100 nm), urocortin 1 and 2 have similar affinity for the mouse CRF2 receptor (Ki of 0.8 and 0.9 nm, respectively), a significantly higher affinity compared with urocortin 3 (14.2 nm) (Chen et al., 2004). Urocortin 2 was thus selected as an appropriate choice for infusion into the lateral septum.
Surgery, drug administration, and histology.
Mice under isofluorane anesthesia were prepared with 26 gauge bilateral cannulas aimed at the intermediate lateral septum (+0.30 mm anterior to bregma, 0.5 mm lateral from the midline, −3.2 ventral from skull surface; flat skull) or the central nucleus of the amygdala (−1.4 mm posterior to bregma, 2.4 mm lateral to the midline, −4.6 mm ventral from skull surface; flat skull) or a single cannula targeting the medial septum (+0.30 anterior to bregma, 0.5 mm lateral from the midline, −3.5 mm ventral from skull surface) or the lateral ventricle (−0.5 mm posterior to bregma, 1.0 mm lateral from the midline, −2.5 mm ventral from skull surface; flat skull) (Franklin and Paxinos, 1997). Cannulas were attached to the skull using dental acrylic. Peptides were infused through 33 gauge injector cannula (Plastics One, Roanoke, VA) attached to 10 μl Hamilton microsyringes mounted on an automated pump (Harvard Apparatus, Holliston, MA). Animals were allowed 1 week to recover from the surgery before drug administration. Peptides were infused over a period of 120 s (volume of 0.25 μl per side for the bilateral lateral septal cannula, 0.5 μl in the medial septum and central amygdala, and 5 μl in the ventricle to allow for diffusion throughout the brain), and the injector cannula remained in the brain for 60 s after drug administration. After the completion of each experiment, methylene blue dye was infused into each cannula using the drug delivery volume, the brains were removed and frozen, and sections were cut on the cryostat to assess cannula placement. Mice with lateral ventricle placement were examined for dye in both lateral and the third and fourth ventricles; animals without dye in distal ventricles were removed from analysis. Mice with lateral or medial septal placement were examined for cannula placement within the appropriate regions (Fig. 1). Animals that did not have correct cannula placement (9% for lateral septum, 5% for medial septum, 8% for lateral ventricle, and 10% for central amygdala) were excluded from the analyses.
Figure 1.
Histological verification of the cannula injector placements. Cannula placement was verified after each experiment with the infusion of methylene blue dye as shown in the lateral (b) and medial (c) septum. Scale bars, 500 μm. a is an illustration of the septum showing successful cannula placements in the intermediate lateral septum (filled circles) and placements excluded from data analyses (open circles). d illustrates the central amygdala region targeted for antalarmin infusion, with an example section shown in e. LS, Lateral septum; MS, medial septum; LV, lateral ventricle; cc, corpus callosum; aca, anterior commissure, IC internal capsule; OP, optic tract; CEA, central nucleus of the amygdala; BLA, basolateral amygdala.
Behavioral testing.
Mice were handled for 5 min/d for 7 d before drug administration and behavioral testing. On the test day, each animal was taken from the housing room and habituated to a dark testing room with a 65 dB white-noise background for 1 h before drug administration. After drug infusion, mice were returned to their cage or restrained for 30 min by taping their limbs to a Plexiglas surface (Radulovic et al., 1999). Preliminary work in our laboratory indicated that 30 min of immobilization was sufficient to increase anxiety-like behavior in the behavioral tests used in the present study (data not shown) and was thus chosen as the high-stress condition. After this 30 min period, each mouse was tested in the light–dark box, open-field, and novel-object tests. Mice were placed into the light–dark box for 10 min, then immediately removed from the light–dark box, and placed into the open field for 10 min. At the end of that period, a novel object was placed into the center of the open field, and behavior was measured for a third 10 min period. This procedure and testing order was used for all experiments. Behavior in these tests was filmed, with the experimenter absent from the room during the test.
The light–dark box apparatus consisted of a small dark chamber 27 × 15 × 27 cm high connected by 10 × 10 cm opening to a larger white chamber 27 × 29 × 27 cm high. The light intensity in the light compartment was 1000 lux compared with 10–50 lux in the dark compartment. The mouse was placed in the dark compartment facing away from the opening to the light side, and the latency to first enter the light compartment, number of transitions from the dark to the light compartment, and total time spent in the light compartment were measured for 10 min. Entry into either side of the light–dark box was defined as the placement of all four paws into that side. Immediately after the light–dark test, mice were placed into a open-field chamber divided into nine regions: four corner regions, four side regions, and a center region. The corner regions were ∼7 × 7 cm, the side regions were 7 × 14 cm, and the center region was 14 × 14 cm. Locomotion was assessed as the total number of crossings between regions of the open field during the test. The time spent in the center square and percentage entries into the center region were assessed as measures of anxiety. After 10 min in the open field, a novel object (white plastic cup) 3 cm in diameter and 5 cm high was placed into the middle of the center square and secured to the floor with tape affixed to the inside of the cup. During the novel-object test, locomotion was assessed as the total number of crossings between regions. The time spent in the center square with the novel object and percentage entries into the center region were quantified as measures of anxiety. The method in the current study follows the procedures used by Dulawa et al. (1999) to assess anxiety-like behavior in dopamine D4 receptor knock-out mice.
Experiment procedures.
All experiments were conducted using a between-subjects design for drug administration and immobilization stress. Each mouse received either vehicle or one dose of urocortin 2. In experiments that examined the effect of stress on behavior and/or tested the effect of CRF receptor antagonists, each drug dose and stress condition was tested in separate groups of mice.
Experiment 1: urocortin 2 infused into the lateral septum.
ICR mice were prepared with a bilateral cannula in the lateral septum and were infused with vehicle or 0.48, 4.8, 48, or 240 pmol (2 ng, 20 ng, 200 ng, and 1 μg) of urocortin 2 per mouse (n = 9–10 per group).
Experiment 2: urocortin 2 infused into the medial septum.
ICR mice were prepared with a cannula in the medial septum and infused with vehicle or 120 or 240 pmol of urocortin 2 per mouse (n = 6–7 per group).
Experiment 3: urocortin 2 infused in the lateral ventricle.
ICR mice were prepared with a cannula in the lateral ventricle and infused with vehicle or 24, 120, 240, or 1200 pmol (5 μg) of urocortin 2 per mouse (n = 7–8 per group).
Experiment 4: septal urocortin 2 combined with stress.
ICR mice were prepared with a bilateral cannula in the lateral septum and infused with vehicle or 48 pmol of urocortin 2 per mouse combined with no stress or 30 min of immobilization stress (n = 8–9 per group).
Experiment 5: septal urocortin 2 combined with stress in CRF2 receptor wild-type mice (C57BL/6 × 129 mixed background).
Mice were prepared with a bilateral cannula in the lateral septum and infused with vehicle or 0.48, 4.8, or 48 pmol of urocortin 2 per mouse combined with no stress or 30 min of immobilization stress (n = 6–7 per group).
Experiment 6: septal urocortin 2 combined with stress in CRF2 receptor knock-out and wild-type mice.
CRF2 receptor wild-type and knock-out mice were prepared with a bilateral cannula in the lateral septum and infused with vehicle or 48 pmol of urocortin 2 per mouse in combination with no stress or 30 min of immobilization stress (n = 8–10 per group).
Experiment 7: septal urocortin 2 with CRF2 receptor antagonist.
CRF2 receptor wild-type mice prepared with a bilateral cannula in the lateral septum were infused with vehicle or 24, 96, or 192 pmol (100, 400, and 800 ng) of the CRF2 receptor antagonist astressin-2B. Ten minutes later, they were infused with vehicle or 48 pmol of urocortin 2 in combination with no stress or 30 min of immobilization stress (n = 7–16 per group).
Experiment 8: septal urocortin 2 with CRF1 receptor antagonist.
ICR mice prepared with a bilateral cannula in the lateral septum were treated with the CRF1 receptor antagonist antalarmin before the septal infusion of urocortin 2. This experiment was conducted to determine whether the effect of septal urocortin 2 was mediated by CRF1 receptors in the lateral septum. The mice were infused with vehicle or 264 pmol (100 ng) or 792 pmol (300 ng) of antalarmin, followed 10 min later by an infusion of vehicle or 48 pmol of urocortin 2. After the second septal infusion, all mice were restrained for 30 min before testing in the light–dark box, open-field, and novel-object tests (n = 7–9 per group). These doses of antalarmin were selected based on the results of a previous study (Robison et al., 2004) reporting a decrease in stress-related behavior in mice treated with an equivalent dose of this drug.
Experiment 9: CRF1 receptor antagonist infused into the amygdala.
To determine whether the doses of antalarmin infused into the lateral septum were sufficient to antagonize CRF1 receptor-mediated behavior, ICR mice were prepared with a bilateral cannula targeting the central nucleus of the amygdala. Mice were infused with vehicle or 264 pmol (100 ng) or 792 pmol (300 ng) of antalarmin into the amygdala 10 min before 30 min of immobilization stress and were then tested in the light–dark box, open-field, and novel-object tests (n = 7–9 per group).
Data analyses.
SPSS (Chicago, IL) software was used to perform ANOVAs of peptide dose, stress level, and genotype on measures of anxiety and locomotion in the light–dark box, open-field, and novel-object tests. The effect of urocortin 2 infusion into the lateral septum, medial septum, and lateral ventricle described in experiments 1–3 was assessed with one-way between-subjects ANOVA. In experiments 4 and 5, a two-way ANOVA (stress level × peptide dose) was used to assess the interaction between urocortin 2 and immobilization stress. Three-way ANOVAs were used to assess the data in experiment 6 (stress × peptide × genotype) and experiment 7 (antagonist × agonist × stress), and two-way ANOVA assessed the effect of the CRF1 receptor antagonist antalarmin in experiments 8 and 9. Dunnett's t test and Tukey's test were used for appropriate post hoc comparisons. The correlation between locomotion (total squares entered) and “anxiety” (percentage time in the center square) in the open-field and novel-object tests was assessed with Pearson's correlation coefficient. The level of significance for all statistical tests was set to 0.05.
Results
Experiment 1: effects of urocortin 2 in the lateral septum
Septal administration of the CRF2 agonist urocortin 2 increased anxiety-related behavior in the light–dark box and novel-object tests, with a trend toward an anxiogenic-like effect in the open-field test (Fig. 2). Statistical analyses revealed that the highest dose of septal urocortin 2 (240 pmol) significantly decreased the time spent in the light side of the light–dark box (F(4,46) = 3.058; p < 0.05) (Fig. 2b) and the percentage entries (F(4,46) = 4.223; p < 0.01) and percentage time (F(4,46) = 7.288; p < 0.001) (Fig. 2f) spent in the center region with the novel object, with a trend toward fewer percentage entries (p = 0.057) and less time (p = 0.065) (Fig. 2d) in the center of the open field compared with wild-type mice. Lower doses of the peptide had no effect in the light–dark box, except for a decrease in transitions observed with the 48 pmol dose (F(4,46) = 3.041; p < 0.05). None of the lower doses had any effect in the open field. In the novel-object test, the 0.48 and 48 pmol doses had no effect on anxiety-like behavior, whereas 4.8 pmol of urocortin 2 decreased time spent in the center (p < 0.05). Urocortin 2 had no effect on locomotion in the open-field and the novel-object tests at any dose tested.
Figure 2.
Septal urocortin 2 increased anxiety. Effect of bilateral septal infusion of urocortin 2 (vehicle or 0.48, 4.4, 48, or 240 pmol) into ICR mice on the following: a, latency to enter the light side of light–dark box (seconds); b, total time spent in the light side of light–dark box (seconds); c, locomotion in open field (total square entries); d, percentage of time in the center of the open field; e, locomotion in the open field with a novel object (total square entries); and f, percentage of time spent in center with novel object. Transitions in the light–dark box and percentage of center entries in the open-field and novel-object tests not shown. The high dose of 240 pmol increased anxiety-like behavior in the light–dark box and novel-object tests compared with vehicle, with a trend toward increased anxiety-like behavior in the open-field test, but did not alter locomotion. Lower doses of 0.48 and 48 pmol did not significantly alter anxiety. Urocortin 2 at 4.8 pmol increased anxiety-like behavior in the novel-object test but had no effect in the light–dark box or open-field tests. Data are presented as mean ± SEM. *p < 0.05; **p < 0.01.
Experiment 2: effects of urocortin 2 in the medial septum
Infusion of the peptide into the medial septum did not have a significant effect on any measure of behavior in the light–dark box, open-field, or novel-object tests (data not shown).
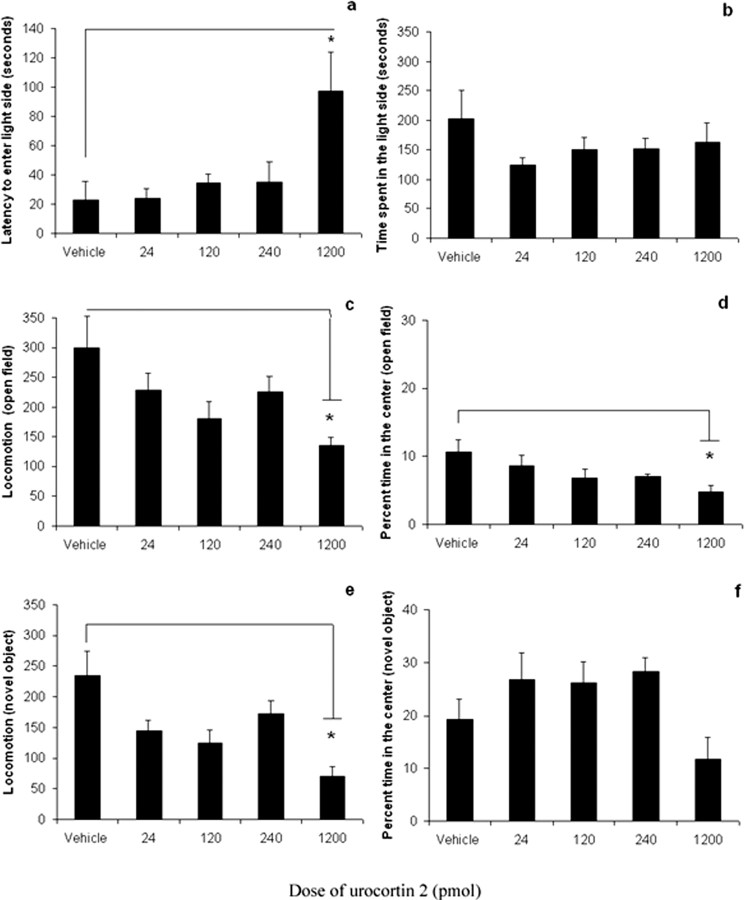
Experiment 3: effects of urocortin 2 in the lateral ventricle
Urocortin 2 infused into the lateral ventricle decreased locomotion at the highest dose tested (1200 pmol) (Fig. 3c,e) but did not affect locomotor or anxiety-related behavior at lower doses. Data analyses showed that 1200 pmol of the peptide increased latency to enter the light side of the light–dark box (F(4,32) = 4.260; p < 0.05) (Fig. 3a) and decreased the percentage time spent in the center of the open field (F(4,32) = 2.914; p < 0.05) (Fig. 3d), suggesting an increase in anxiety-related behavior; however, this dose also significantly decreased locomotion in both the open-field (F(4,32) = 3.623; p < 0.05) and novel-object (F(4, 32) = 3.324; p < 0.05) tests. Because the 1200 pmol dose decreased overall locomotion, the effects on the light–dark box and open field cannot be unambiguously interpreted as an anxiogenic effect.
Figure 3.
Intracerebroventricular urocortin 2 decreased motor activity at high doses. Effect of urocortin 2 infusion into the lateral ventricle of ICR mice (vehicle or 24, 120, 240, or 1200 pmol) on the following: a, latency to enter the light side of the light–dark box (seconds); b, total time spent in the light side of the light–dark box (seconds); c, locomotion in the open field (total square entries); d, percentage time in the center of the open field; e, locomotion in the open field with a novel object (total square entries); and f, percentage time spent in the center with a novel object. Transitions in the light–dark box and percentage center entries in open-field and novel-object tests are not shown. The highest dose of intracerebroventricular urocortin 2 (1200 pmol) significantly reduced locomotion in the open-field and novel-object tests compared with vehicle, but the peptide had no effect on anxiety or locomotion at lower doses. Data are presented as mean ± SEM. *p < 0.05.
Infusion of urocortin 2 into the medial septum or the lateral ventricle did not have significant effects on anxiety measures, consistent with the hypothesis that the anxiogenic effects of the peptide were mediated by CRF2 receptors in the lateral septum. High doses of a CRF2 receptor agonist delivered into the ventricle would be expected to occupy some CRF2 receptors in the septum; however, infusion of the peptide into the ventricle may also activate CRF2 receptors in other brain areas that may oppose the septal effects on anxiety. Urocortin 2 infused into the lateral septum did not alter locomotion in the open-field or novel-object tests in the low- or high-stress conditions, demonstrating that the effects on anxiety measures were not confounded by changes in motor activity.
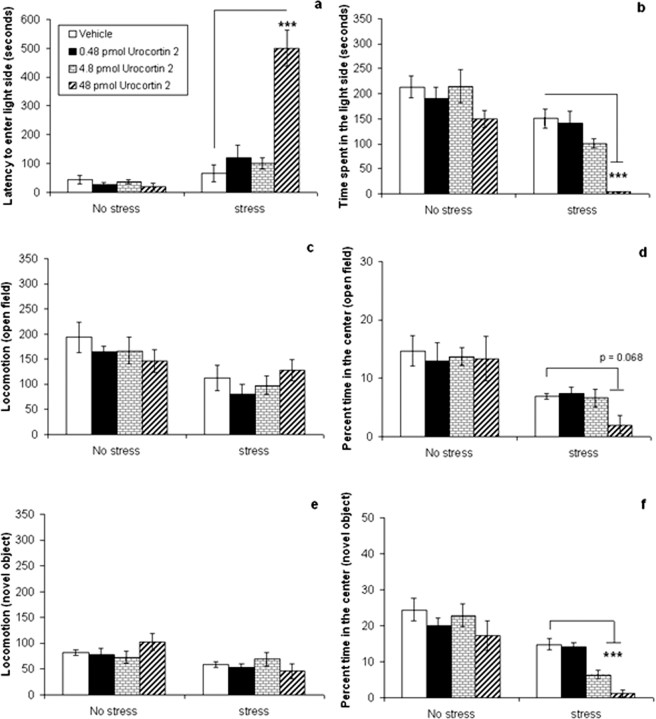
Experiment 4: effects of septal urocortin 2 combined with stress
In this experiment, ICR mice were infused with 48 pmol of septal urocortin 2 combined with 30 min of immobilization stress or no stress. The purpose of this experiment was to determine whether stimulation of the septal CRF2 receptor would have a different effect on anxiety-related behavior when tested during low- versus high-stress conditions. In experiment 1, septal administration of 48 pmol of urocortin 2 did not have a significant effect on anxiety. This dose was thus chosen in the current and subsequent experiments to determine whether stress would interact with a dose of the CRF2 agonist that was inactive in the no-stress condition. The results indicated that this dose of urocortin 2 had no effect on anxiety-related behavior in the no-stress condition but significantly increased anxiety in the stress condition compared with vehicle-treated mice (Fig. 4). Analyses of the data (two-way ANOVA, followed by Tukey's post hoc test) revealed significant interactions between urocortin 2 and stress on time spent in the light-side of the light–dark box (F(1,29) = 7.901; p < 0.01), the percentage center entries (F(1,29) = 4.498; p < 0.05), and percentage time in the center (F(1,29) = 4.338; p < 0.05) of the open field, and the percentage center entries (F(1,29) = 4.603; p < 0.05) and percentage time spent in the center (F(1,29) = 4.881; p < 0.05) with the novel object. Post hoc testing showed that urocortin 2 significantly increased anxiety-related behavior compared with vehicle only in stressed mice, decreasing the time spent in the light side of the light–dark box (p < 0.01) (Fig. 4b), the percentage center entries (p < 0.01), and percentage time in the center (p < 0.01) (Fig. 4d) of the open field, and the percentage center entries (p < 0.01) and percentage time in the center (p < 0.001) (Fig. 4f) with the novel object.
Figure 4.
Effect of septal urocortin 2 on anxiety is dependent on stress. Effect of septal urocortin 2 (48 pmol) in ICR mice treated with no stress or 30 min of immobilization stress on the following: a, latency to enter the light side of the light–dark box (seconds); b, total time spent in the light side of the light–dark box (seconds); c, locomotion in the open field (total square entries); d, percentage time in the center of the open field; e, locomotion in the open field with a novel object (total square entries; and f, percentage time spent in the center with a novel object. Transitions in the light–dark box and percentage center entries in open-field and novel-object tests are not shown. This dose of urocortin 2 had no effect on anxiety-like behavior in the low-stress condition but significantly increased anxiety-like behavior compared with vehicle in the light–dark box, open-field, and novel-object tests when combined with 30 min of immobilization stress. There was no difference in locomotion between vehicle- and peptide-treated mice in the stress condition. Data are presented as mean ± SEM. **p < 0.01;***p < 0.001.
Immobilization stress increased anxiety-related measures in the light–dark box, open-field, and novel-object tests (increased latency to enter the light side of light–dark box, F(1,29) = 10.479; p < 0.01) (Fig. 4a), decreased time in the light side of the light–dark box (F(1,29) = 25.02; p < 0.001) (Fig. 4b) and decreased percentage center entries (F(1,29) = 13.023; p < 0.01) and percentage time (F(1,29) = 29.551; p < 0.001) (Fig. 4d) in the center of the open field, and decreased percentage center entries (F(1,29) = 16.005; p < 0.001) and percentage time (F(1,29) = 22.48; p < 0.001) (Fig. 4f) in the center with the novel object, but also decreased overall locomotion in the open field (F(1,29) = 10.352; p < 0.01) (Fig. 4c) and with the novel object (F(1,29) = 4.849; p < 0.05) (Fig. 4e). However, urocortin 2 had no significant effect on locomotor activity.
There was a nonsignificant trend toward a decrease in locomotion in urocortin 2-treated mice in the stress condition compared with the vehicle-treated mice in the novel-object test. The correlation between total locomotion (squares entered) and anxiety (percentage time in the center square) was assessed for both the open-field and novel-object tests. The analyses indicated that there was no significant correlation between locomotion and anxiety-like behavior in the no-stress (r = 0.203; NS) or stress (r = 0.210; NS) conditions in the open-field test or the no-stress (r = 0.016; NS) or stress (r = 0.333; NS) conditions in the novel-object test. These data suggest that the effects of the peptide on anxiety-related behavior were not confounded by alterations in locomotor activity.
Experiment 5: effects of multiple doses of septal urocortin 2 in CRF2 receptor wild-type mice
The results from this experiment show a dose-dependent effect of urocortin 2 in the lateral septum, with the highest dose (48 pmol) increasing anxiety-related behavior only in the stress condition, whereas lower doses had little effect, regardless of stress (Fig. 5). There was a significant interaction between urocortin 2 and stress on the latency to enter the light side of the light–dark box (F(1,44) = 30.909; p < 0.001), time spent in the light side (F(1,44) = 23.871; p < 0.001), and percentage time in the center with a novel object (F(3,44) = 3.518; p < 0.05). Post hoc testing showed that 48 pmol of urocortin 2 significantly increased anxiety-related behavior compared with vehicle in stressed mice, increased the latency to enter the light side of the light–dark box (p < 0.001) (Fig. 5a), decreased time spent in the light side of the light–dark box (p < 0.001) (Fig. 5b), and decreased the percentage time in the center with the novel object (p < 0.001) (Fig. 5f), with a trend toward decreased percentage time in the center of the open field (p < 0.068) (Fig. 5d). There were no significant effects of lower peptide doses, with the exception that 4.8 pmol of urocortin 2 also significantly decreased percentage time in the center with the novel object when compared with vehicle in the stress condition.
Figure 5.
Septal urocortin 2 increased anxiety when combined with stress in CRF2 receptor wild-type mice on a mixed C57BL/6 × 129 background. Effect of septal urocortin 2 (vehicle or 0.48, 4.8, or 48 pmol) in CRF2 receptor wild-type mice treated with no stress or 30 min of immobilization stress on the following: a, latency to enter the light side of the light–dark box (seconds); b, total time spent in the light side of the light–dark box (seconds); c, locomotion in the open field (total square entries); d, percentage of time in the center of the open field; e, locomotion in the open field with a novel object (total square entries); and f, percentage of time spent in the center with a novel object. Transitions in the light–dark box and percentage of center entries in the open-field and novel-object tests are not shown. Urocortin 2 did not affect anxiety-like behavior in the no-stress condition at any dose tested. In the stress condition, 48 pmol of urocortin 2 increased anxiety-like behavior in the light–dark box and novel-object tests; the 4.8 pmol dose increased anxiety in the novel-object test in the stress condition but not the no-stress condition. Data are presented as mean ± SEM. ***p < 0.001.
As in the previous experiment, immobilization stress increased anxiety-related measures in the light–dark box, open-field, and novel-object tests but also decreased overall locomotion in the open field (F(1,44) = 4.775; p < 0.05) (Fig. 5c) and with the novel object (F(1,44) = 8.214; p < 0.01) (Fig. 5e). However, treatment with urocortin 2 did not affect locomotor activity in the stress or no-stress conditions, supporting the contention that the effects of the peptide are mediated by changes in anxiety-related behavior.
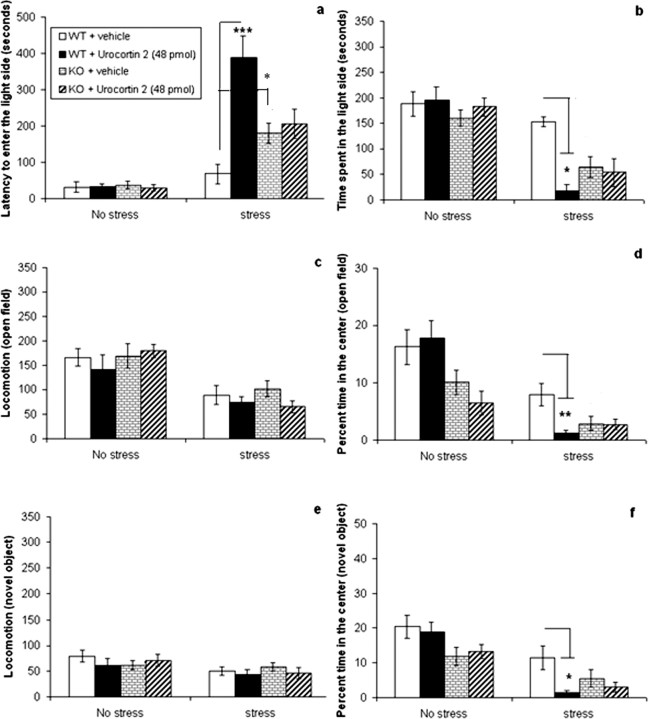
Experiment 6: effects of septal urocortin 2 combined with stress in CRF2 receptor knock-out and wild-type mice
The data show that treatment with urocortin 2 increased anxiety-related behavior in wild-type but not knock-out mice in the stress condition and had no effect on either genotype in the no-stress condition. Statistical analysis (three-way ANOVA, followed by the Tukey's post hoc test) revealed a three-way interaction between stress, genotype, and peptide treatment on the latency to enter the light side of the light–dark box (F(1,71) = 11.749; p < 0.01) and the percentage time spent in the center of the open field (F(1,71) = 4.014; p < 0.05). In wild-type mice in the stress condition, urocortin 2 significantly increased the latency to enter the light side of the light–dark box (p < 0.001) (Fig. 6a) and decreased time spent in the center of the open field (p < 0.01) (Fig. 6d) compared with vehicle, whereas the peptide had no effect in knock-out mice. A two-way ANOVA in wild-type mice (conducted according to our a priori hypothesis that urocortin 2 will affect behavior in this group of mice) showed a significant interaction between stress and peptide on the time spent in the light side of the light–dark box (F(1,33) = 4.491; p < 0.05) and percentage time spent in the center with the novel object (F(1,33) = 5.119; p < 0.05). Post hoc tests revealed that urocortin 2 decreased time spent in the light side of the light–dark box (p < 0.05) (Fig. 6b) and decreased percentage time in the center with the novel object (p < 0.05) (Fig. 6f) only in the stress condition when compared with vehicle. There were no effects of septal urocortin 2 on anxiety in CRF2 receptor knock-out mice in the light–dark box, with both vehicle- and drug-treated mice spending 50–60 s in the light side in the stress condition. However, the stressed knock-out mice spent very little time in the center of both the open-field and novel-object tests (<6% of the time) regardless of drug treatment (Fig. 6d,f), so it is difficult to determine whether urocortin 2 had any effect in these behavioral assays. It is possible that the anxiety response reached a floor effect under these conditions, given the increased anxiety observed in the knock-out mice combined with immobilization stress.
Figure 6.
No effect of septal urocortin 2 in CRF2 receptor knock-out mice. Effect of septal urocortin 2 (48 pmol) in CRF2 receptor wild-type (WT) and knock-out (KO) mice treated with no stress or 30 min of immobilization stress on the following: a, latency to enter the light side of the light–dark box (seconds); b, total time spent in the light side of the light–dark box (seconds); c, locomotion in the open field (total square entries); d, percentage of time in the center of the open field; e, locomotion in the open field with a novel object (total square entries); and f, percentage of time spent in the center with a novel object. Transitions in the light–dark box and percentage of center entries in the open-field and novel-object tests are not shown. In wild-type mice, urocortin 2 had no effect on anxiety-related behavior in the no-stress condition but increased anxiety-related behavior in the light–dark box, open-field, and novel-object tests when combined with immobilization stress. Urocortin 2 had no effect in CRF2 receptor knock-out mice in the light–dark box (a, b). CRF2 receptor knock-out mice showed increased anxiety compared with wild-type, spending less time in the center of the open-field (d) and novel-object (f) tests, regardless of drug treatment. Knock-out mice treated with vehicle also exhibited greater latency to enter the light side of the light–dark box compared with vehicle-treated wild-type mice (a). Data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
CRF2 receptor knock-out mice exhibited more anxiety-related behavior compared with wild-type mice, spending significantly less time in the center of both the open field (F(1,71) = 13.517; p < 0.001) (Fig. 6d) and the novel-object (F(1,71) = 4.778; p < 0.05) (Fig. 6f) tests but did not demonstrate any differences in overall locomotion. Immobilization stress increased anxiety-related measures in the light–dark box, open-field, and novel-object tests and decreased locomotion in both the open-field (F(1,33) = 36.662; p < 0.001) and the novel-object (F(1,33) = 6.319; p < 0.05) tests as demonstrated previously, but there was no effect of urocortin 2 on locomotion in the stress or no-stress conditions.
The increased anxiety-related behavior observed in CRF2 receptor knock-out mice compared with wild-type mice is in agreement with previous reports (Bale et al., 2000; Kishimoto et al., 2000; Bale and Vale, 2004). This result is in apparent contradiction to the increased anxiety reported with the administration of urocortin 2, a CRF2 receptor agonist. It is possible that the anxiogenic phenotype observed in the knock-out mice may be attributable to other factors besides the absence of the CRF2 receptor; Bale et al. (2000) reported increased CRF expression in the amygdala of these mice, which may contribute to greater anxiety, perhaps through stimulation of the CRF1 receptor.
Experiment 7: effects of septal urocortin 2 combined with a CRF2 receptor antagonist
Septal administration of the CRF2 receptor antagonist astressin-2B did not affect anxiety-related behavior in the no-stress condition but did reduce anxiety-related behavior in mice receiving urocortin 2 or vehicle in the stress condition (Fig. 7). There was a significant three-way interaction between stress, urocortin 2, and astressin-2B on the latency to enter the light side of the light–dark box (F(3,148) = 7.216; p < 0.001) and the time spent in the light side of the light–dark box (F(3,148) = 2.835; p < 0.05) and a significant interaction between stress and astressin-2B on the percentage time in the center of the open field (F(3,148) = 13.342; p < 0.001) and with the novel object (F(3,148) = 12.512; p < 0.001).
Figure 7.
CRF2 antagonist astressin-2B blocked the effect of urocortin 2 and stress on anxiety. Effect of septal CRF2 receptor antagonist astressin-2B (24, 96, or 192 pmol) administered 10 min before urocortin 2 (48 pmol) in CRF2 receptor wild-type mice treated with no stress or 30 min of immobilization stress on the following: a, latency to enter the light side of the light–dark box (seconds); b, total time spent in the light side of the light–dark box (seconds); c, locomotion in the open field (total square entries); d, percentage of time in the center of the open field; e, locomotion in the open field with a novel object (total square entries); and f, percentage of time spent in the center with a novel object. Transitions in the light–dark box and percentage of center entries in the open-field and novel-object tests are not shown. Astressin-2B decreased anxiety-like behavior in the light–dark box, open-field, and novel-object tests in the stress condition but had little effect in the no-stress condition. In the light–dark box, 24 and 96 pmol of astressin-2B decreased the anxiogenic effect of urocortin 2 in the stress condition but had no effect in vehicle-treated mice, whereas the 192 pmol dose reduced anxiety in all stressed mice, decreasing the latency to enter the light side of the light–dark box (a) and increasing time spent in the light side (b). In the open field, 24 pmol of astressin-2B decreased the anxiogenic effect of urocortin 2 but had no effect in vehicle-treated mice in the stress condition (d). In the novel-object test, 24 and 96 pmol of the antagonist inhibited the anxiogenic effect of urocortin 2 but had no significant effect in vehicle-treated mice (f), whereas 192 pmol decreased anxiety in both groups. Data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
Post hoc tests showed that the highest dose of astressin-2B (192 pmol) decreased anxiety-related behavior in the light–dark box in vehicle-treated mice in the stress condition, decreasing the latency to enter the light side (p < 0.001) (Fig. 7a) and increasing time spent in the light (p < 0.001) (Fig. 7b), but lower doses of the antagonist had no effect on anxiety in these mice. In contrast, 24 and 96 pmol of astressin-2B decreased anxiety in urocortin 2-treated mice in the stress condition, significantly decreasing latency to enter the light side (24 pmol, p < 0.01; 96 pmol, p < 0.001) (Fig. 7a) and increasing time spent in the light (24 pmol, p < 0.01; 96 pmol, p < 0.001) (Fig. 7b) compared with control.
In the open-field test, one-way ANOVA for vehicle-treated mice in the stress condition (conducted according to our a priori hypothesis that astressin-2B will have an effect in this group of mice) showed that the CRF2 receptor antagonist significantly increased time in the center square (F(3,37) = 13.109; p < 0.001). Astressin-2B also reduced anxiety-related behavior (increased time in the center) in urocortin 2-treated mice in the stress condition (F(3,37) = 40.467; p < 0.001) (Fig. 7d). However, post hoc tests showed that 24 pmol of astressin-2B reduced anxiety-related behavior in mice treated with urocortin 2 (p < 0.05) but had no significant effect in vehicle-treated mice (Fig. 7d); the higher doses of astressin-2B decreased anxiety in both groups. In the novel-object test, one-way ANOVA showed that astressin-2B decreased anxiety-related behavior in stressed mice treated with either vehicle (F(3,37) = 5.211; p < 0.01) or urocortin 2 (F(3,37) = 30.638; p < 0.001) (Fig. 7f). Post hoc tests showed that 24 and 96 pmol of urocortin 2 significantly decreased anxiety-related behavior in mice treated with urocortin 2 (24 pmol, p < 0.01; 96 pmol, p < 0.001), but these doses had no significant effect on vehicle-treated mice (Fig. 7f). Only the highest dose of astressin-2B (192 pmol) decreased anxiety-related behavior in both groups (vehicle, p < 0.01; urocortin 2, p < 0.001).
Immobilization stress increased anxiety-related measures in the light–dark box, open-field, and novel-object tests and decreased locomotion in the open-field (F(3,148) = 6.010; p < 0.05) (Fig. 7c) and the novel-object (F(3,148) = 11.239; p < 0.001) (Fig. 7e) tests, but neither urocortin 2 nor astressin-2B had a significant effect on locomotion.
In summary, septal administration of lower doses of the CRF2 receptor antagonist astressin-2B (24 and 96 pmol) decreased the anxiogenic effect of urocortin 2 combined with stress in the light–dark box and novel-object tests but had no effect on anxiety-related behavior in vehicle-treated mice. In the open-field test, 24 pmol of urocortin 2 had no effect on vehicle-treated mice in the stress condition but increased the time spent in the center for urocortin 2-treated mice, whereas the higher doses of the antagonist decreased anxiety-related in both groups. The highest tested dose of urocortin 2 (192 pmol) decreased anxiety-related behavior in the stress condition for both vehicle- and drug-treated mice in all three behavioral assays. These results suggest the possibility that the lowest dose of septal astressin-2B blocked the anxiogenic effect of septal urocortin 2, but the higher doses may also reverse the additive anxiogenic effect of the immobilization stress.
Experiment 8: effects of septal urocortin 2 combined with a CRF1 receptor antagonist
Septal administration of the CRF1 receptor antagonist antalarmin did not affect anxiety-related behavior in immobilization-stressed mice treated with septal urocortin 2 (Fig. 8). In this experiment, urocortin 2 significantly increased the latency to enter the light side of the light–dark box (F(1,42) = 52.369; p < 0.001) (Fig. 8a), decreased the time spent in the light side of the light–dark box (F(1,42) = 19.609; p < 0.001) (Fig. 8b), decreased percentage center entries (F(1,42) = 11.083; p < 0.01) and percentage time (F(1,42) = 39.893; p < 0.001) (Fig. 8d) in the center of the open field, and decreased percentage entries (F(1,60) = 11.885; p < 0.01) and percentage time (F(1,42) = 22.105; p < 0.001) (Fig. 8f) in the center with the novel object when compared with vehicle. There were no significant differences between mice treated with septal antalarmin or vehicle on any behavioral measure tested.
Figure 8.
Septal administration of the CRF1 antagonist antalarmin does not block the effect of urocortin 2. Effect of septal administration of the CRF1 receptor antagonist antalarmin (vehicle, 264 pmol/100 ng, of 792 pmol/300 ng) on the anxiogenic effect of septal urocortin 2 (48 pmol) on the following: a, latency to enter the light side of the light–dark box (seconds); b, total time spent in the light side of the light–dark box (seconds); c, locomotion in the open field (total square entries); d, percentage of time in the center of the open field; e, locomotion in the open field with a novel object (total square entries); and f, percentage of time spent in the center with a novel object. Transitions in the light–dark box and percentage of center entries in the open-field and novel-object tests are not shown. Urocortin 2 significantly increased anxiety-like behavior in the light–dark box (a, b), open-field (d), and novel-object (f) tests in immobilization-stressed mice pretreated with septal antalarmin or vehicle. Antalarmin did not block the anxiogenic effect of urocortin 2.
Experiment 9: CRF1 receptor antagonist infused into the amygdala
Administration of antalarmin into the central nucleus of the amygdala decreased anxiety-related behavior in the light–dark box, decreasing the latency to enter the light side (F(2,42) = 5.461; p < 0.01) (Fig. 9a) and increasing time spent in the light (F(2,42) = 4.942; p < 0.05) (Fig. 9b). Antalarmin also decreased anxiety in the open field, significantly increasing time spent in the center (F(2,42) = 5.274; p < 0.01) (Fig. 9d). There was a significant interaction between stress and antalarmin on the latency to enter the light side of the light–dark box (F(2,42) = 8.055; p < 0.05), time spent in the light side (F(2,42) = 10.326; p < 0.001), and time spent in the center of the open field (F(2,42) = 4.568; p < 0.05). Post hoc tests showed that antalarmin had no significant effect on anxiety in the no-stress condition for any test. In the stress condition, the highest dose of antalarmin (792 pmol) significantly decreased the latency to enter the light side of the light–dark box (p < 0.01), increased time spent in the light side of the light–dark box (p < 0.001), and increased time spent in the center of the open field (p < 0.001), with a trend toward increased time in the center with the novel object (p = 0.052) (Fig. 9f). The lower dose of antalarmin (264 pmol) did not significantly affect anxiety-related behavior, and neither dose had a significant effect on locomotion.
Figure 9.
Administration of the CRF1 antagonist antalarmin into the central amygdala reduced the anxiogenic effect of immobilization stress. Effect of administration of the CRF1 receptor antagonist antalarmin into central amygdala (vehicle or 264 or 792 pmol) on the anxiogenic effect of 30 min of immobilization stress on the following: a, latency to enter the light side of the light–dark box (seconds); b, total time spent in the light side of the light–dark box (seconds); c, locomotion in the open field (total square entries); d, percentage time in the center of open field; e, locomotion in the open field with a novel object (total square entries); and f, percentage time spent in the center with a novel object. Transitions in the light–dark box and percentage center entries in the open-field and novel-object tests are not shown. Antalarmin at 792 pmol reduced the anxiogenic effect of immobilization stress in the light–dark box (a, b) and open field (d), with a trend toward decreased anxiety in the novel-object test (f).The lower dose of 264 pmol did not have a significant effect. Data are presented as mean ± SEM. **p < 0.01; ***p < 0.001.
The data from these experiments (8 and 9) indicate that infusion of antalarmin into the lateral septum did not block the anxiogenic effect of septal urocortin 2 in the high-stress condition, but the higher dose of antalarmin (792 pmol) significantly decreased anxiety-related behavior induced by immobilization stress when infused into the amygdala, indicating that this dose was sufficient to antagonize anxiety putatively mediated by the CRF1 receptor. These data support the conclusion that the effects of urocortin 2 in the current study are mediated through the septal CRF2 receptor.
Discussion
The present study demonstrated that the effect of septal urocortin 2 on anxiety is influenced by the stress level of the mouse. Under low-stress conditions, only the high dose of 240 pmol of septal urocortin 2 consistently increased anxiety-related behavior in both the light–dark box and novel-object tests, with a strong trend toward anxiety-related behavior in the open-field test. Forty-eight picomoles of septal urocortin 2 had no significant effect on anxiety-related behavior under low-stress conditions; however, when combined with 30 min of immobilization stress, this dose significantly increased anxiety-related behavior in the light–dark box, open-field, and novel-object tests compared with the effects of immobilization stress alone. Septal urocortin 2 did not affect locomotion and had no measurable effect in CRF2 receptor knock-out mice, and the anxiogenic effects of the agonist were blocked by septal administration of the CRF2 receptor antagonist astressin-2B but not the CRF1 receptor antagonist antalarmin.
There is some indication that the mice showed increased sensitivity to the anxiogenic effects of septal urocortin 2 during the novel-object test. The results showed that a low dose of urocortin 2 (4.8 pmol) increased anxiety in the novel-object test by itself (experiment 1) and also in combination with immobilization stress (experiment 5), but this dose had no effect on anxiety-like behavior in the light–dark box or open-field tests. In all experiments, mice were tested first in the light–dark box, second in the open field, and last in the novel-object test. Exposure to a novel environment by itself has been shown to induce a stress response in mice (Hebda-Bauer et al., 2004). It is possible that the mice were more sensitive to the anxiogenic effects of urocortin 2 after 30 min of stress induced by successive exposure to three novel situations, resulting in increased anxiety in the novel-object test. Another possibility is that urocortin 2 may have time-dependent effects on behavior. Previous studies have reported delayed effects of CRF2 receptor agonists on anxiety (Valdez et al., 2002) and feeding (Zorrilla et al., 2004), and it is conceivable that the effect of septal urocortin 2 on anxiety-like behavior may differ 30–60 min after administration. Finally, whereas the light–dark box, open-field, and novel-object tests all measure approach-avoidance behavior, the novel-object test elicits the greatest approach or exploration behavior (Dulawa et al., 1999) and may also be the most sensitive measure of the behavioral effects of urocortin 2 during stress.
The current literature suggests that stress may play a role in determining the function of the CRF2 receptor in anxiety-related behaviors. Valdez et al. (2002, 2003) reported that intraventricular treatment with urocortin 2 or 3 decreased anxiety in rats previously handled for 1 week and habituated to the testing environment for 2 h before peptide delivery and behavioral testing. Venihaki et al. (2004) reported anxiolytic effects of intraventricular urocortin 3 in C57BL/6 mice habituated to human handling and cannula manipulation. In contrast, Risbrough et al. (2003, 2004) reported that urocortin 2 increased acoustic startle in mice, interpreted as an increase in anxiety-related defensive behavior. This latter effect is measured during a stressful procedure in which animals are restricted in movement inside a small chamber and subjected to loud noises.
Some data suggest that stress level could influence the function of CRF2 receptors located specifically in the lateral septum. Ho et al. (2001) reported that septal administration of CRF2 receptor antisense oligonucleotides decreased shock-induced freezing in rats. Bakshi et al. (2002) also reported that antagonism of septal CRF2 receptors decreased freezing behavior in response to shock. Both studies reported an anxiolytic effect of blocking septal CRF2 receptors in animals tested in high-stress conditions. However, Radulovic et al. (1999) reported that septal administration of the CRF2 receptor antagonist antisauvagine-30 had no effect on anxiety under baseline (low-stress) conditions but decreased anxiety-like behavior induced by administration of CRF into the lateral septum. In the current study, the selective CRF2 receptor antagonist astressin-2B had no effect during low stress but decreased anxiety-related behavior induced by immobilization stress.
The rodent lateral septum innervates brain areas that play an important role in regulating affect and anxiety, including the amygdala, hypothalamus, bed nucleus of the stria terminalis, and the dorsal raphe (Sheehan et al., 2004). Many lateral septal neurons extend collaterals to other neurons in the septum as they descend to target various brain regions. Electrical stimulation of the fimbria activates an excitatory hippocampal glutamatergic projection to the lateral septum, inducing an initial depolarization of septal neurons, followed by a hyperpolarization attributed to intraseptal GABAergic release (DeFrance et al., 1975; Stevens et al., 1987; Gallagher and Hasuo, 1989). Thus, neurons within the septum can inhibit other septal neurons via the release of GABA from recurrent axon collaterals. Eberly et al. (1983) reported that in vitro application of CRF on slices of rat lateral septum inhibited spontaneous firing in approximately half of the neurons tested. There is also evidence that CRF2 receptor activation may exert different effects in the septum depending on the stress level of the animal. Liu et al. (2005) reported that urocortin 1 depressed the activity of septal neurons by activating CRF2 receptors in the mediolateral nucleus of the lateral septum (intermediate lateral septum) but had the opposite effect after the stress of cocaine withdrawal, facilitating excitatory glutamatergic postsynaptic currents. Pernar et al. (2004) demonstrated that low doses of urocortin 2 inhibit serotonergic neurons in the rat dorsal raphe, but higher doses excite these neurons, hypothesizing that such a reversal of the effect of urocortin 2 may be mediated by activation of CRF2 receptors on GABAergic interneurons.
It is possible that CRF2 receptors are located on lateral septum neurons that inhibit each other via GABAergic collaterals and/or send inhibitory GABAergic projections toward brain regions that mediate anxiogenic behavior, such as the amygdala. Low CRF2 receptor activation may have little overall effect on septal output, but high activation (induced by immobilization stress combined with urocortin 2 administration) may increase the activity of inhibitory intraseptal GABAergic neurons, decreasing overall GABA release from the septum to other brain areas, and thus disinhibiting the amygdala and increasing anxiety. In contrast, the complete absence of any CRF2 receptor activity on septal neurons that directly inhibit the amygdala (in the CRF2 receptor knock-out mice) may also increase anxiety. In other words, both the hyperactivation and the complete removal of the septal CRF2 receptor could increase anxiety compared with a low activation state.
Immobilization stress in mice has been demonstrated to increase urocortin 1 mRNA in the Edinger–Westphal nucleus (Weninger et al., 2000) and urocortin 3 mRNA in the perifornical hypothalamic region (Venihaki et al., 2004). Previous studies have demonstrated a robust urocortin 1 projection from the Edinger–Westphal nucleus to the lateral septum in mouse (Bachtell et al., 2003) and a dense urocortin 3 projection to the rat lateral septum (Li et al., 2002). It is possible that immobilization stress in mouse increases the release of one or both of these CRF2 receptor agonists in the lateral septum, contributing to the anxiogenic effect of urocortin 2 administration.
The present set of studies demonstrated that the effect of septal CRF2 receptor activation on anxiety-related behavior appears dependent on stress level. In the low-stress condition, a high dose of 240 pmol of urocortin 2 was required to increase anxiety-related behavior, but lower doses did not have a consistent effect. However, in combination with immobilization stress, 48 pmol of septal urocortin 2 increased anxiety-related behavior in the behavioral paradigms used. The current findings do not resolve the controversy over the reported anxiolytic effects of CRF2 receptor activation but suggest that environmental factors influence the effect of receptor activation on behavior. Our data indicate that septal CRF2 receptor activation is more likely to increase anxiety-related behavior in a high-stress environment but has less effect on anxiety-related behavior under lower stress conditions.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant R01 MH62527, NIH Consortium Grant U01 MH69062, NIH Program Project Grant DK 26741, the Kelberg Foundation, the Chapman Charitable Trust, the Oxley Foundation, and the Philips Foundation. We thank Mike Arends for outstanding editorial assistance and Dr. Mark A. Geyer for valuable intellectual contributions and assistance. This is publication 17219-NP of The Scripps Research Institute.
References
- Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, Ryabinin AE. The Edinger–Westphal–lateral septum urocortin pathway and its relationship to alcohol consumption. J Neurosci. 2003;23:2477–2487. doi: 10.1523/JNEUROSCI.23-06-02477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–527. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KE. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behavior and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Chen A, Perrin M, Brar B, Li C, Jamieson P, Digruccio M, Lewis K, Vale W. Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol. 2004;19:441–458. doi: 10.1210/me.2004-0300. [DOI] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- DeFrance J, Yoshihara H, McCrea RA, Kitai ST. Pharmacology of inhibition in the lateral septal region. Exp Neurol. 1975;48:502–523. doi: 10.1016/0014-4886(75)90009-6. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J Neurosci. 1999;19:9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Eberly LB, Dudley CA, Moss RL. Iontophoretic mapping of corticotropin releasing factor (CRF) sensitive neurons in the rat forebrain. Peptides. 1983;4:837–841. doi: 10.1016/0196-9781(83)90077-3. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. San Diego: Academic; 1997. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Gallagher JP, Hasuo H. Bicuculline and phaclofen sensitive components of N-methyl-d-aspartate-induced hyperpolarizations in rat dorsolateral septal nucleus neurons. J Physiol (Lond) 1989;418:367–377. doi: 10.1113/jphysiol.1989.sp017846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebda-Bauer EK, Watson SJ, Akil H. CREBαδ- deficient mice show inhibition and low activity in novel environments without changes in stress reactivity. Eur J Neurosci. 2004;20:503–513. doi: 10.1111/j.1460-9568.2004.03487.x. [DOI] [PubMed] [Google Scholar]
- Ho SP, Takahashi LK, Livanov V, Spencer K, Lesher T, Maciag C, Smith MA, Rohrbach KW, Hartig PR, Arneric SP. Attenuation of fear conditioning by antisense inhibition of brain corticotropin releasing factor-2 receptor. Mol Brain Res. 2001;89:29–40. doi: 10.1016/s0169-328x(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of Crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Orozco-Cabal L, Grigoradis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Chronic cocaine administration switches corticotropin-releasing factor2 receptor mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci. 2005;25:577–583. doi: 10.1523/JNEUROSCI.4196-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor(2) receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Ther. 2002;302:145–152. doi: 10.1124/jpet.302.1.145. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides. 2004;25:659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology (Berl) 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison CL, Meyerhoff JL, Saviolakis GA, Chen WK, Rice KC, Lumley LA. A CRH1 antagonist into the amygdala of mice prevents defeat-induced defensive behavior. Ann NY Acad Sci. 2004;1032:324–328. doi: 10.1196/annals.1314.052. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale WW, Lee K. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Stevens DR, Gallagher JP, Shinnick-Gallagher P. In vitro studies of the role of γ-aminobutyric acid in inhibition in the lateral septum of the rat. Synapse. 1987;1:184–190. doi: 10.1002/syn.890010206. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE. Corticotropin-releasing factor modulates defensive withdrawal and exploratory behavior in rats. Behav Neurosci. 1989;103:648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Inoue K, Koob GF, Rivier J, Vale W, Zorrilla EP. Human urocortin II: mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002;943:142–150. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003;980:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, River J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale WW, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Venihaki M, Sakihara S, Subramanian S, Dikkes P, Weninger SC, Liapakis G, Graf T, Majzoub JA. Urocortin III, a brain neuropeptide of the corticotropin releasing hormone family: modulation by stress and attenuation of some anxiety-like behaviors. J Neuroendocrinol. 2004;16:411–422. doi: 10.1111/j.1365-2826.2004.01170.x. [DOI] [PubMed] [Google Scholar]
- Weninger SC, Peters LL, Majzoub JA. Urocortin expression in the Edinger-Westphal nucleus is up-regulated by stress and corticotrophin-releasing hormone deficiency. Endocrinology. 2000;141:256–263. doi: 10.1210/endo.141.1.7277. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Reinhardt LE, Valdez GR, Inoue K, Rivier JE, Vale WW, Koob GF. Human urocortin 2, a corticotropin-releasing factor (CRF)2 agonist and ovine CRF, a CRF1 agonist differentially alter feeding and motor activity. J Pharmacol Exp Ther. 2004;310:1027–1034. doi: 10.1124/jpet.104.068676. [DOI] [PubMed] [Google Scholar]