Abstract
Endogenous adenosine acting at the adenosine A2A receptor (A2AR) can modify brain injury in a variety of neurological disorder models. However, both A2AR activation and inactivation have been shown to be neuroprotective in different situations, raising the intriguing possibility that A2ARs in distinct cellular elements may have different and even opposing effects. In this study, we developed three novel transgenic models to dissect out cell-type-specific actions of A2ARs on striatal damage by the mitochondrial toxin 3-nitropropionic acid (3-NP). Whereas global inactivation of A2ARs exacerbated 3-NP-induced neurological deficit behaviors and striatal damage, selective inactivation of A2ARs in forebrain neurons (using the Cre/loxP strategy) did not affect neurological deficit or striatal damage after the acute systemic treatment of 3-NP and intrastriatal injection of malonate. However, selective inactivation of A2ARs in bone marrow-derived cells (BMDCs) by transplanting bone marrow cells from global A2AR knock-out (KO) mice into wild-type C57BL/6 mice produced a similar phenotype of global A2AR KO mice, i.e., exacerbation of 3-NP-induced striatal damage. Thus, cell-type-selective inactivation of A2ARs reveals that A2ARs in BMDCs but not in forebrain neurons are an important contributor to striatal damage induced by mitochondrial dysfunction.
Keywords: adenosine A2A receptor, 3-nitropropionic acid, forebrain, bone marrow-derived cells, striatum, Huntington's disease
Introduction
Adenosine levels rise markedly in response to hypoxic, traumatic, and inflammatory insults in the brain (Pedata et al., 2001). Recently, we learned that, despite the well documented neuroprotective properties of adenosine (Dunwiddie and Masino, 2001), under some circumstances, adenosine [probably acting at the A2A receptor (A2AR)] may contribute to neuronal damage and death (de Mendonca et al., 2000). The potential neuroprotection by A2AR antagonists was first reported in a global ischemia model (Gao and Phillis, 1994) and further substantiated in other cerebral ischemia and excitotoxicity models (Jones et al., 1998a,b; Monopoli et al., 1998; Popoli et al., 2002). Consistently, brain injury induced by transient focal ischemia is significantly attenuated in A2AR knock-out (KO) mice (Chen et al., 1999). Furthermore, recent pharmacological, genetic, and epidemiological studies suggest that blockade of A2ARs may attenuate dopaminergic neurotoxicity (Ross et al., 2000; Ascherio et al., 2001; Chen et al., 2001) and striatal damage induced by the mitochondrial toxin 3-nitropropionic acid (3-NP) (Blum et al., 2003b; Fink et al., 2004). Thus, A2AR inactivation offers neuroprotection against various brain insults in diverse brain regions.
However, A2AR agonists have also been shown to offer neuroprotection under some conditions. A2AR agonist CGS21680 [2-p-(2-carboxyethyl)phenethylamino-5-N-ethylcarboxamidoadenosine] attenuates kainate-induced hippocampal lesions and cerebral hemorrhage injury (Jones et al., 1998b; Mayne et al., 2001). Similarly, A2AR agonist ATL-146e (4-{3-[6-amino-9-(5-ethylcarbamoyl-3,4-dihydroxy-tetrahydro-furan-2-yl)-9H-purin-2-yl]-prop-2-ynyl}-cyclohexanecarboxylic acid methyl ester) has been shown to attenuate traumatic and ischemic injury in the spinal cord (Cassada et al., 2001). Interestingly, a recent report showed that CGS21680 attenuated neuropathological changes in R6/2 mice (a genetic model of Huntington's disease) (Chou et al., 2005). An even larger body of evidence supports the view that A2AR activation functions as a braking mechanism to prevent excessive tissue damage after ischemic, hypoxic, and inflammatory insults in the liver, kidney, and other peripheral organs (Ohta and Sitkovsky, 2001; Day et al., 2003; Sitkovsky et al., 2004).
The fact that both A2AR inactivation and, in some cases, A2AR activation have been shown to provide neuroprotection implies that adenosine acting at A2ARs can either be detrimental or protective to neurons after brain insults. A2ARs are expressed at high levels in striatal neurons and at low to moderate levels in a variety of cellular elements, including glial cells, endothelium, neutrophils, and platelets (Svenningsson et al., 1999; Hettinger et al., 2001). A2ARs on neurons and bone marrow-derived cells (BMDCs) may produce opposite effects in brain injury. Thus, it is critical to distinguish the contribution of A2ARs in distinct cells in modulating striatal damage. In this study, we developed three novel transgenic mouse models to evaluate the importance of A2ARs in forebrain neurons versus BMDCs in modulation of 3-NP-induced striatal damage. Using an acute treatment paradigm, we found that global inactivation of A2ARs exacerbated 3-NP-induced striatal damage. Importantly, chimeric mice with selective inactivation of A2ARs in BMDCs recaptured the phenotype of global A2AR KO mice, whereas selective inactivation of A2ARs in forebrain neurons did not. These results provide compelling evidence that A2ARs in BMDCs are critical to 3-NP-induced striatal damage, whereas A2ARs in forebrain neurons are not.
Materials and Methods
Generation and genotyping of global and forebrain A2A receptor KO mice.
The generation and genotyping by PCR analysis of global A2AR KO (gKO) mice were described previously (Chen et al., 1999; Yu et al., 2004). Congenic gKO mice on a C57BL/6 background were made by backcrossing gKO on mixed (129-Steel × C57BL/6) genetic background to C57BL/6 mice for 13–15 generations. Heterozygous cross-breeding was used to generate gKO and global wild-type (gWT) mice. The gKO and gWT mice with matched age and sex (male, 110–130 d old) were used for this study.
The generation of the forebrain-specific A2AR conditional KO mice using the Cre/loxP strategy has been described previously (Bastia et al., 2005). Genotyping of the forebrain A2AR KO (fKO) mice was performed by PCR analysis using a three primer set as described previously (Bastia et al., 2005). Forebrain A2AR KO and forebrain WT (fWT) mice have been backcrossed to C57BL/6 mice for six generations to achieve a near congenic line. Because our initial analysis showed that A2AR immunoreactivity disappeared at postnatal day 60 and A2AR antagonist-induced motor effect had completely disappeared at postnatal day 90, respectively (E. K. Rapp and J.-F. Chen, unpublished data), the matched fKO mice and their WT littermates (male, 120–132 d old) were used in this study. In a pilot study, we noted that there were no difference among Cre(+)A2ARflox(−/−), Cre(−)A2ARflox(+/+), and Cre(−)A2ARflox(−/−) to 3-NP induced neurotoxicity, and Cre(−)A2ARflox(+/+) was used as a control for fKO mice [Cre(+)A2ARflox(+/+)].
Creation of chimeric mice with selective inactivation of the A2AR in bone marrow-derived cells by transplantation.
Chimeric mice were produced using bone marrow transplantation (BMT) as described previously (Yu et al., 2004). Briefly, male C57BL/6 mice from Charles River Laboratories (Wilmington, MA) (6–9 weeks old) were irradiated with a total dose of 12.5 Grays from a 137Cs source. After irradiation, the mice received transplantation of bone marrow cells (2–3 million per 0.2–0.3 ml) via tail vein injection. Bone marrow cells were isolated from female gKO mice and their WT littermates (8–10 weeks old; weight, 24–26 g) by flushing the tibia and femur with RPMI 1640 medium under sterile conditions. During each irradiation, two control mice did not receive BMT, and they died within 10 d. Seven weeks after irradiation and transplantation, we assessed the efficiency of the reconstitution of the BMDC by transplantation in chimeric mice by two independent methods. First, we took advantage of sex-mismatched BMT in the study (from female donor to male recipient or from male donor to female recipient). Seven weeks after BMT, peripheral blood mononuclear cells (PBMCs) were collected using Histopaque 1083 (Sigma, St. Louis, MO). After isolation of DNA, genotyping of sex chromosome-linked genes (Jarid1d and Jarid1c) was performed as described previously (Mroz et al., 1999; Yu et al., 2004). Standard PCR conditions were used, and the annealing temperature was set at 55°C. A single primer pair (forward, 5′-TGAAGCTTTTGGCTTTGAG-3′; and reverse, 5′-CCGCTGCCAAATTCTTTGG-3′) amplifies both the X-linked (Jarid1c) and the Y-linked (Jarid1d) genes but yields different sized products, which were separated on 2% agarose gels (see Fig. 3B). Second, we used a substrain of C57BL/6 mice that are positive for CD45.1 (B6.SJL-Ptprca Pep3b/BoyJ) as the BMT donor source. The CD45.1 epitope is absent in cells of the recipient mice. Seven weeks after the transplantation, PBMCs were isolated from the chimeric mice. After washing and incubating with PE (phycoerythrin)-conjugated anti-mouse CD45.1 monoclonal antibody (BD PharMingen, San Diego, CA), subsequent flow cytometry data acquisition and analysis were performed using the flow cytometer FACSCalibur (BD PharMingen). Eight weeks after the transplantation, chimeric mice were subjected to 3-NP injection.
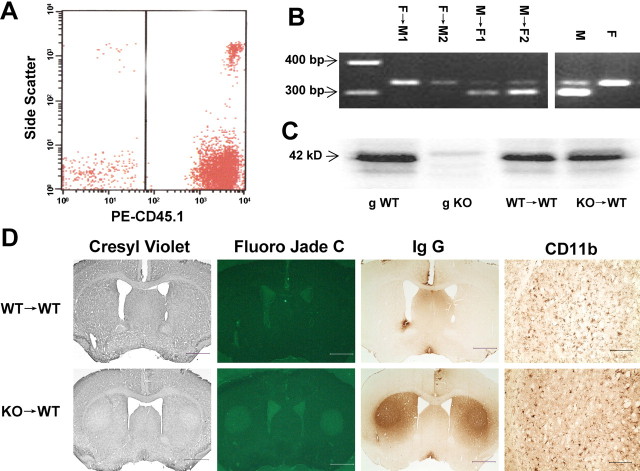
Figure 3.
A2ARs in bone marrow-derived cells contribute critically to exacerbation of 3-NP-induced striatal damage. A, Reconstitution of BMDCs in recipient mice after BMT. Irradiated mice in CD45 gene background received BMDCs from CD45.1-positive animals. Seven weeks after transplantation, PBMCs were isolated and examined by fluorescence-activated cell sorting for CD45.1-positive cells. The percentage of CD45.1-positive cells in PBMCs is 89.1 ± 3.1% (n = 8), indicating efficient reconstitution of BMDCs in chimeric mice. B, PCR amplification of the X- and Y-linked genes Jarid1c and Jarid1d yields two bands at 300 and 330 bp for male (M) and one band at 330 bp for female (F). Seven weeks after BMT, PCR bands of PBMCs changed from the recipient pattern to the donor pattern: lanes 1, 2, two male recipients (female donor, F→M); lanes 3, 4, two female recipients (male donor, M→F). C, Western blot analysis shows that A2AR immunoreactivity in striatum was not different in WT→WT mice and KO→WT mice. D, Representative sections of cresyl violet and Fluoro-Jade C staining and IgG and CD11b immunohistochemistry in WT→WT and KO→WT mice after the 3-NP treatment. Scale bars: columns 1–3,1 mm; column 4, 0.2 mm.
3-NP intoxication and assessment of neurological deficit behaviors.
3-NP (Sigma) was dissolved in saline (5 or 8 mg/ml), and the pH was adjusted to 7.4 with 1N NaOH. The solution was filtered (0.22 μm; Millipore, Bedford, MA) and kept at −80°C until use. 3-NP was intraperitoneally injected twice daily for 2 d at 12 h intervals (8:00 A.M. and 8:00 P.M.) at a dose of 60 mg/kg on the first day and 80 mg/kg on the second day (i.e., a 60–60-80–80 dose regimen).
We adopted a behavioral score system that combined the previously published methods (Gabrielson et al., 2001; Fernagut et al., 2002) to provide optimal assessment of neurological behavioral deficits after 3-NP treatment. After intoxication of 3-NP, mice were monitored for neurological deficit signs referred to as stages I, II, and III as follows. In stage I, mice were hypoactive, with reduced grooming activity and interaction with other mice but retained a normal posture and gait. In stage II, mice had marked reduction of general activity, less exploratory behavior, decreased body weight, and intermittent or intermediate clasping of hindlimbs after the mouse was grasped by the midtail. Finally, the mice in stage III were characterized by no exploratory behavior, dramatic weight loss, hindlimbs fully drawn up to the abdomen after the mouse was grasped by the midtail, or marked hindlimb dystonea with wide interlimb space, tremors, and even recumbency (moribund state).
Intrastriatal injection of malonate.
Malonate was dissolved in PBS, adjusted to pH 7.4, and filtered (0.22 μm; Millipore). One microliter of PBS containing 2 μmol of malonate was injected over 10 min into the left striatum at coordinates of 0.9 mm anterior, 2 mm lateral, and 3.5 mm deep to the bregma, and the needle was left in place for 5 min before being slowly withdrawn. An equal volume of PBS was administrated to the right striatum as a control.
Histological assessment of striatal damage.
Twenty-four hours after the last 3-NP injection or 7 d after malonate intrastriatal injection, mice were anesthetized with tribromoethanol (Avertin, 125 mg/kg) and perfused transcardially with saline, followed by ice-cold 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4. Brains were immediately removed and postfixed overnight in the same fixative and then cryoprotected in 30% sucrose in 0.1 m phosphate buffer, pH 7.4. Sequential coronal sections (30 μm) were made on a freezing microtome, starting from the anterior aspect of the corpus callosum (bregma 1.40 mm) throughout the entire striatum (bregma −1.30 mm), according to the mouse brain atlas (Hof et al., 2000). For histological assessment, every sixth section (∼210 μm interval) was processed for cresyl violet staining or Fluoro-Jade C staining to assess cell loss and neuronal degeneration. Cresyl violet staining was performed with standard protocols. For Fluoro-Jade C staining, the method described by Schmued et al. (2005) was used (Fluoro-Jade C; Histo-Chem, Jefferson, AR)
Stereological analysis of the lesion volumes was performed by digitally acquiring cresyl violet-stained sections through the striatum at 4× objective using a computerized image analysis system (Spot Insight; Diagnostic Instruments, Sterling Heights, MI). The lesion area was characterized by extensive neuronal loss (in the case of 3-NP injection) and extensive gliosis (in the case of malonate injection), as determined on cresyl violet-stained sections. Lesion volumes were calculated by summing the cross-sectional areas of the lesion in each section and multiplying this value by the distance between sections. Fluoro-Jade C-stained sections were only assessed in the core of the striatal lesion.
Immunohistochemistry.
For detecting microglial and astrocyte activation and neurotrophil infiltration in striatum after 3-NP lesioning, we used specific monoclonal antibodies against CD11b (Mac-1α chain, a marker for microglia, 1:10; BD PharMingen), glial fibrillary acidic protein (GFAP) (a marker for astrocyte, 1:100; Sigma), and Ly-6G (recognizing myeloid differentiation antigen Gr-1, a marker for neutrophil, 1:10; BD PharMingen). Brain sections (30 μm) were incubated with the antibody overnight, followed by biotin-conjugated goat anti-rat IgG-B (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and avidin–biotin complex (Vectastain ABC Elite kit; Vector Laboratories, Burlingame, CA). For CD11b and Ly-6G staining, peroxidase activity was localized using DAB as a chromogenic substrate (Vector Laboratories). For astrocyte staining, the sections were incubated for 72 h at 4°C and visualized under a fluorescence microscope. For IgG staining, biotinylated goat anti-mouse IgG (1:200; Jackson ImmunoResearch, West Grove, PA) was used.
Western blot.
The analysis of A2AR immunoreactivity was performed in total membranes of the striatum as described previously (Rebola et al., 2005). Briefly, the proteins isolated from the striatum were separated by SDS-PAGE (10% with a 4% concentrating gel) under reducing conditions. After blocking with 5% milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T), pH 7.6, the membranes were incubated overnight at 4°C with a mouse anti-A2AR antibody (raised against the third intracellular loop of the receptor, 1:500; Millipore, Billerica, MA) and washed with TBS-T containing 0.5% milk. The membranes were then incubated with an alkaline phosphatase-conjugated anti-mouse secondary antibody (1:5000; GE Healthcare, Piscataway, NJ) in TBS-T containing 1% milk for 60 min at room temperature and followed up with washing in TBS-T with 0.5% milk and developed with Enhanced Chemi-Fluorescence system. The membranes were then reprobed and tested for tubulin immunoreactivity to confirm that equal amounts of protein were loaded to the gels.
Statistical analysis.
Statistical comparisons between two groups were performed by using an unpaired Student's t test. The comparison of survival rate and lesioned animal rate of two genotypes was analyzed using the χ2 test.
Results
Effects of global inactivation of the A2AR on 3-NP-induced striatal damage
Congenic gKO mice and their WT littermates (in C57BL/6 genetic background) were tested with various treatment paradigms, including the chronic treatment regimen [i.e., 50 mg/kg, at 12 h interval, for 5 d as reported in previous studies (Fink et al., 2004)] We found that the chronic treatment paradigm did not produce clear and reproducible striatal damage. After several pilot studies, we found that the four doses of 3-NP (60–60-80–80 paradigm) resulted in clear and reproducible striatal damage with the low mortality (<25%). Consequently, we used this treatment paradigm for the rest of our experiments. After the first two injections (60 mg/kg, i.p.), neither gKO mice nor their WT littermates showed any visible neurological deficit (behavior) symptoms. After the third injection of 3-NP (80 mg/kg), five of eight gKO mice displayed clear neurological deficit symptoms of decreased body weight, marked reduction of general activity, less exploratory behavior, and intermittent or intermediate clasping of hindlimbs after the mouse was grasped by the midtail. In contrast, seven of the eight gWT mice showed normal or slightly reduced general activity. After the fourth injection of 3-NP (80 mg/kg), most of the gKO mice (seven of eight) displayed severe (stage III) neurological deficit symptoms (i.e., no exploratory behavior, fully drawn up hindlimbs to the abdomen after being grasped by the midtail, or marked hindlimb dystonea with wide interlimb space, tremors, recumbence), whereas most of the gWT mice (five of eight) displayed only slight (stage I) neurological deficit symptoms (Table 1). However, the survival rate in gKO mice (six of eight) was similar to that of gWT mice (seven of eight) at the end of the experiment.
Table 1.
3-NP-induced behavioral and pathological effects in global A2AR KO and forebrain A2AR KO mice and A2AR KO→ WT chimeric mice
| Genotype | Neurodeficit score |
Survival rate | Lesioned mice | Lesion volume (mm3) | ||
|---|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | ||||
| Global WT | 5 | 1 | 1 | 7/8 | 1/7 | 1.52 ± 1.52 |
| Global A2AR KO | 1 | 0 | 5 | 6/8 | 5/6* | 3.28 ± 1.38 |
| Forebrain WT | 2 | 1 | 4 | 7/8 | 4/7 | 2.87 ± 1.72 |
| Forebrain A2AR KO | 4 | 0 | 4 | 8/8 | 3/8 | 2.33 ± 1.20 |
| WT→WT | 2 | 5 | 3 | 10/12 | 0/10 | 0.00 ± 0.00 |
| A2AR KO→WT | 0 | 4 | 6 | 10/12 | 6/10** | 0.79 ± 0.29*** |
Global A2AR KO, forebrain A2AR KO, and A2AR KO→ WT chimeric mice as well as their corresponding WT littermates were treated with 3-NP as described in Materials and Methods. Numerals represent the number of animals with varied neurological deficit signs at 24 h after last injection of 3-NP. Values are mean ± SE.
*p < 0.05 compared with global WT (χ2 test);
**p< 0.01 compared with WT→ WT (χ2 test);
***p< 0.01 compared with WT→ WT (Student's t test).
Consistent with behavioral results, histological evaluation showed that five of the six surviving gKO mice had bilateral striatal lesions, whereas only one of the seven surviving gWT mice had a striatal lesion (p = 0.017, χ2 test). Figure 1 shows representative brain sections from gKO and gWT mice after 3-NP treatment. Cresyl violet staining demonstrates bilateral symmetrical lesioned areas in the striatum of a gKO mouse. Fluoro-Jade C staining shows neuronal degeneration. 3-NP intoxication induced striatal lesions in which degenerating soma, neuropil, and terminals were all Fluoro-Jade C positive. The Fluoro-Jade C-positive neuronal degenerative areas were consistent with the pale areas showed by cresyl violet. Similar to the behavioral results, the lesion volume in gKO mice (3.28 ± 1.38 mm3) was apparently higher than that of gWT mice (1.52 ± 1.52 mm3), but it did not reach statistical significance because of the unusually large lesion volume in the only damaged gWT mouse (Table 1). Nevertheless, these behavioral and histological data together suggest that global inactivation of A2ARs exacerbates 3-NP induced-striatal damage.
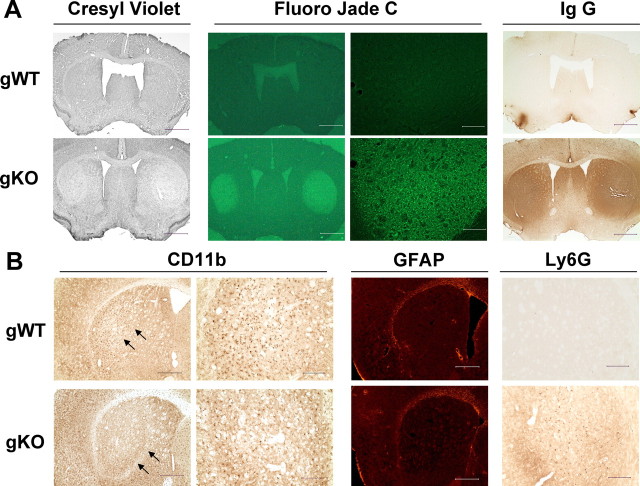
Figure 1.
Global inactivation of the A2AR exacerbates 3-NP-induced striatal damage. A, Representative sections from gKO and gWT mice show neuronal loss as detected by cresyl violet staining, neuronal degeneration as detected by Fluoro-Jade C staining, and impaired blood brain–barrier as detected by IgG immunohistochemical staining after the 3-NP treatment. Scale bars: column 3, 0.2 mm; columns 1, 2, and 4, 1 mm. B, Representative sections from gKO mice and gWT littermates show CD11b-positive microglia, GFAP-positive astrocytes, and Ly-6G-positive neutrophil immunohistochemical staining. Arrows in B indicate the border of the lesioned area of gKO mice and the corresponding area of gWT mice. Scale bars: columns 2 and 4, 0.2 mm; columns 1 and 3, 0.5 mm.
Interestingly, IgG immunoreactivity was detected in all lesioned striatum of five gKO mice (Fig. 1) and one gWT littermate after 3-NP treatment. In most of the brain sections, the areas of IgG staining were generally larger than the areas of neuronal loss shown by cresyl violet and Fluoro-Jade C staining. No IgG-positive staining was found in the unlesioned striatum of six gWT mice (Fig. 1) and one gKO mice. In some brain sections, the area of IgG staining was limited to within the lesion. Furthermore, two of the six lesioned animals exhibited histological evidence of intrastriatal hemorrhage. These results indicate an impaired blood–brain barrier (BBB) in the striatum after 3-NP treatment in both gKO and WT littermates.
Finally, we also examined the inflammatory status after 3-NP treatment by determining microglial cell activation (CD11b+), astrocyte (GFAP+) activation, and neutrophil infiltration (Ly-6G+) using these cell-type-specific markers. Activated microglia were detected within the lateral half of the striatum, the area vulnerable to damage by 3-NP, in five of the six unlesioned gWT mice (Fig. 1B) and one unlesioned gKO mouse (as evidenced by Fluoro-Jade C, cresyl violet, and IgG staining). The activated microglia showed intensified CD11b immunoreactivity, shortening and thickening of processes, and enlarged cell bodies, indicating microglial activation by 3-NP treatment. However, within the core of the lesioned area, the presence of activated microglia was significantly less, and the remaining microglia cells lost their processes (Fig. 1B). These results indicate that microglial activation proceeded (in unlesioned striatum) and was generally associated with 3-NP-induced striatal damage (in the lesioned striatum). Other than these effects associated with the striatal damage, there was no difference in microglial activation between gKO mice and their WT littermates.
Infiltrated neutropils (Ly-6G-positive cells) were only present in the core of lesioned area but not in the unlesioned striata (Fig. 1B), indicating a correlation between the degree of striatal lesion and neutrophil infiltration in these areas. GFAP-positive astrocytes were detected along the paraventricular zone and the corpus callosum but were sparse in the striatum and cortex in both lesioned and unlesioned mice (Fig. 1B).
Effects of selective inactivation of the A2ARs in forebrain neurons on 3-NP-induced striatal damage
In this set of experiments, we determined the contribution of A2ARs in forebrain neurons to 3-NP-induced striatal damage using the fKO mice in which A2ARs in forebrain neurons have been selectively inactivated. The generation and characterization of fKO mice has been described previously (Bastia et al., 2005). As an illustration of the selective inactivation of the A2AR in forebrain neurons, we determined the A2AR protein levels in the striatum and kidney by Western blot in fKO mice, gKO mice, and their corresponding WT littermates. Figure 2A shows that high levels of A2AR protein were detected in the striata of WT littermates at the expected molecular size 42 kDa, but this band was completely abolished in fKO mice. Furthermore, disappearance of the striatal A2AR band in fKO mice was comparable with that of gKO mice (Fig. 2A, compare lanes 3, 4 with lanes 1, 2), indicating that A2ARs were abolished to the background level of gKO mice. In contrast to A2ARs in the striatum, A2AR protein in peripheral organs (e.g., kidney) was not affected in fKO mice. Similar levels of A2AR proteins were detected in the kidneys of fKO mice and their WT littermates (Fig. 2A, compare lanes 7, 8 with lanes 5, 6). As expected, A2AR proteins in the kidney were completely abolished in gKO mice.
Figure 2.

A2ARs in forebrain neurons are not critical contributors to exacerbation of 3-NP-induced striatal damage. A, Western blot analysis shows that high levels of A2AR protein were detected in striatum of fWT mice, but this band was completely abolished in fKO mice. The disappearance of striatal A2AR band in fKO mice was comparable with the gKO mice. In contrast, A2AR protein in kidney was not affected in fKO mice. B, Representative sections from fKO and fWT mice show cresyl violet staining, Fluoro-Jade C staining, and IgG immunohistochemical staining after the 3-NP treatment. The lesioned mice and lesion volumes between these two groups were indistinguishable. Scale bars, 1 mm. C, Representative photographs show cresyl violet-, Fluoro Jade C-, and GFAP-stained sections of fKO and fWT mice 7 d after the intrastriatal injection of malonate. Scale bars, 0.5 mm.
After administration of 3-NP, both fKO mice and their WT littermates developed similar neurological deficit symptoms at various time points after the treatment. Neurological deficit scores were indistinguishable between fKO mice and their WT littermates (Table 1). Moreover, the survival rates of fKO (eight of eight) and fWT mice (seven of eight) were also comparable. Consistent with behavioral and survival results, 3-NP treatment also produced similar striatal damage in fKO mice and their WT littermates (Table 1). Three of eight fKO mice and four of seven surviving fWT littermates displayed macroscopic striatal damage (p = 0.45, χ2 test). Figure 2B shows representative brain sections of fKO mice and their WT littermates after 3-NP treatment. At a histological level, bilateral neuronal loss as detected by cresyl violet staining was comparable between fKO mice and their WT littermates. Furthermore, Fluoro-Jade C staining also revealed similar bilateral neuronal degeneration in fKO and their WT littermates. In addition, the extravasation of IgG was detectable in all lesioned striata and was indistinguishable between fKO mice and their WT littermates. The lesion volumes calculated from the cresyl violet-stained sections between these two groups (2.87 ± 1.72 mm3 for fWT and 2.33 ± 1.20 mm3 for fKO) were indistinguishable (p = 0.79; n = 7–8) (Table 1). Similar to the gKO mice, activated microglial cells were also noted in the lateral part of all three unlesioned fWT littermates and three of five unlesioned fKO mice. However, activated microglia significantly decreased within the lesioned areas in both fKO and their WT littermates (data not shown).
To further evaluate the contribution of A2ARs in forebrain neurons to brain injury by other mitochondrial toxins, we also determined the extent of striatal damage after intrastriatal injection of the reversible succinate dehydrogenase inhibitor malonate. Figure 2C shows representative sections of fKO and fWT mice 7 d after intrastriatal injection of malonate. GFAP staining showed a generalized increase throughout the lesioned striatum. However, the GFAP staining in the core lesion area was significantly reduced or completely abolished, indicating that malonate may also be toxic to astrocytes. Furthermore, Fluoro-Jade C-positive degenerating neurons were around the edge of lesioned area but not in the core lesioned area, indicating neuronal loss in the core lesion area after the malonate treatment. Consistent with the result of 3-NP-induced striatal damage, striatal damage was similar between the fKO and their WT littermates. There was no significant difference in the lesion volume induced by malonate in the fKO mice and their WT littermates (mean ± SE; 0.64 ± 0.19 mm3 for fWT, n = 12; 0.79 ± 0.27 mm3 for fKO, n = 13; p = 0.45, unpaired Student's t test).
Together, these behavioral and histological results demonstrate that selective inactivation of A2ARs in forebrain neurons does not affect striatal damage or neurological deficient behavior induced by 3-NP (intraperitoneal) or malonate (intrastriatal) injections.
Effects of selective inactivation of A2ARs in bone marrow-derived cells on 3-NP-induced striatal damage
The lack of effect of A2ARs in forebrain neurons on 3-NP-induced striatal damage and the demonstration of BBB impairment after 3-NP treatment prompted us to examine the contribution of A2ARs on peripheral inflammatory cells to 3-NP-induced striatal damage. In this set of experiments, we created chimeric mice with selective inactivation of A2ARs in BMDCs by transplanting BMDCs from gKO mice to wild-type C57BL/6 mice. The efficiency of the reconstitution of BMDCs was determined by flow cytometry analysis using CD45.1 as a donor mouse strain marker, i.e., recipient mice lacking CD45.1 received bone marrow cells from the CD45.1-positive substrain of C57BL/6 mice. Seven weeks after the transplantation, the percentage of CD45.1-positive cells was 89.1 ± 3.1% in PBMCs of chimeric mice (Fig. 3A). This result is entirely consistent with our previous report by A2AR immunohistochemistry in PBMC of chimeric mice that ∼90% of BMDCs have been successfully reconstituted by our transplantation protocol (Yu et al., 2004). Furthermore, PCR amplification of the sex chromosome-linked genes in BMDCs from chimeric mice confirmed successful reconstitution of BMDCs. PCR amplification of the X- and Y-linked genes, Jarid1c and Jarid1d, yields different sized products, giving two bands at 300 and 330 bp for male mice and one band at 330 bp for female mice. Seven weeks after bone marrow transplantation, PCR bands of PBMCs in chimeric mice had changed from the recipient pattern to the donor pattern (Fig. 3B). Finally, Western blot analysis shows that A2AR immunoreactivity in the striatum was not different in WT→WT mice and KO→WT mice, as expected (Fig. 3C). These results indicate that the A2ARs on BMDCs were successfully inactivated in KO→WT chimeric mice.
At 8 weeks after the transplantation, WT→WT mice and KO→WT mice received four doses of 3-NP. After the 3-NP treatment, both KO→WT and WT→WT mice developed similar neurological deficit symptoms (Table 1). Histological analysis revealed that whereas WT→WT mice did not display striatal lesions (0 of 10), six of ten KO→WT mice showed obvious bilateral striatal damage (vs WT→WT mice, p < 0.01, χ2 test) (Table 1). Figure 3D shows representative brain sections of WT→WT and KO→WT mice after 3-NP treatment. Bilateral neuronal loss in KO→WT mice was evident by cresyl violet or Fluoro-Jade C staining. Similarly, extravasation of IgG staining was detected in the striatum corresponding to the lesioned area. In contrast, there was essentially no striatal damage in the WT→WT group, as measured by cresyl violet, Fluoro-Jade C, and IgG staining. The lesion volume calculated from cresyl violet-stained sections was significantly higher in the KO→WT group than that of the WT→WT group (Table 1). These results suggest that A2ARs in BMDCs are the critical contributor to the exacerbation of 3-NP-induced striatal damage in global A2AR KO mice.
Similar to the gKO mice, activated microglial cells were also detected in the lateral portion of the unlesioned striatum in 6 of 10 from the WT→WT group (Fig. 3D) as well as three of four from the KO→WT group after the 3-NP treatment. Also, activated microglial cells were reduced in the core lesioned striatum in the KO→WT group (Fig. 3D). These results indicate that microglial activation precedes the 3-NP-induced striatal neuronal loss and that, other than their close association with striatal damage, there was no significant difference in microglial activation between the WT→WT and KO→WT groups.
Discussion
Using novel fKO mice and chimeric mice with selective inactivation of A2ARs in BMDCs, our study provides compelling evidence that A2AR activity in BMDCs (not in forebrain neurons) is a critical contributor to 3-NP-induced striatal damage. We demonstrated that, whereas global inactivation of A2ARs exacerbates 3-NP-induced striatal damage and neurological behavioral deficits, selective inactivation of A2ARs in forebrain neurons does not affect either of these readouts. Importantly, selective inactivation of A2ARs in BMDCs by transplanting BMDCs from gKO mice to WT mice exacerbates 3-NP-induced striatal damage. Thus, cell-type-specific genetic inactivation of A2ARs results in identification of A2ARs in BMDCs as a critical contributor to 3-NP-induced striatal damage. These results challenge a widely accepted notion that neuronal A2ARs in the forebrain play an important role in influencing brain injury outcomes and reinforce the important role of A2ARs in BMDCs in modulating brain injury. Dissection of distinct actions by A2ARs in BMDCs and forebrain neurons will facilitate the development of A2AR-based therapeutic strategies for neurological disorders such as Huntington's and Parkinson's diseases.
Different 3-NP intoxication outcomes in global A2AR KO mice may reflect complex actions of A2ARs in different cellular elements
Several groups, including ours, have provided results supporting the notion that A2AR antagonists reduce striatal damage induced by excitotoxicity and mitochondrial toxins (for review, see Cunha, 2005; Fredholm et al., 2005). However, several recent studies strongly suggest that A2AR modulation of brain injury is complex, depending on the nature of insults and the treatment paradigm (Blum et al., 2003b; Tebano et al., 2004). 3-NP-induced striatal damage can be exacerbated or attenuated in gKO mice, depending on the dose administered and the administration schedule (Blum et al., 2003b). Similarly, we observed that 3-NP-induced striatal damage was significantly enhanced in gKO mice (Fig. 1, Table 1), in contrast to our previous report of the attenuation in gKO mice (Fink et al., 2004). It should be noted that we used different 3-NP treatments (60–60-80–80 mg/kg at 12 h interval) compared with the regimen in the previous study (daily treatment with 50 mg/kg 3-NP at 12 h interval for 5 d). The change in treatment paradigm was attributable to the use of congenic A2AR KO mice in C57BL/6 background, which differ from the gKO mice in a mixed C57BL/6 × 129-Steel or pure 129-Steel background used in the previous study (Fink et al., 2004). However, several pilot studies using congenic A2AR KO mice with the same 3-NP paradigm used in the previous study (Fink et al., 2004) failed to produce significant striatal damage. Taking Blum's findings into consideration, we tested various 3-NP treatment paradigms and found that injection of 3-NP (the 60–60-80–80 mg/kg dose regimen at 12 h interval) resulted in clear striatal damage with <25% mortality. Using this protocol, we found that gKO mice were more susceptible to 3-NP than their WT littermates. The different effects of global A2AR inactivation in 3-NP-induced striatal damage with various treatment paradigms (the present and previous studies) may reflect complex or sometimes opposing effects of A2ARs in different cellular elements. For example, modulation of glutamate release by neuronal A2ARs and of neuroinflammation by A2ARs of the BMDCs may produce opposite outcomes of A2AR inactivation on striatal damage. Thus, it is critical to dissect out the cell-type-specific actions of A2ARs in the 3-NP intoxicated striatum before an adenosine-based therapeutic strategy can be considered.
A2ARs in forebrain neurons are not a critical contributor to the exacerbation of 3-NP-induced striatal damage
It has been widely accepted that A2ARs may influence the outcome of brain injury by modulating glutamate and aspartate release in various brain regions under different pathological conditions (Dunwiddie and Masino, 2001; Latini and Pedata, 2001). A2AR agonists enhance the glutamate release under normal and pathological conditions (O'Regan et al., 1992; Latini and Pedata, 2001; Marcoli et al., 2003). This facilitating effect by A2AR agonists has been primarily attributed to an effect on glutamatergic terminals (Nikbakht and Stone, 2001; Rosin et al., 2003). To our surprise, selective inactivation of A2ARs in forebrain neurons failed to affect striatal damage after the acute systemic 3-NP treatment paradigm. Moreover, direct injection of malonate into the striatum, thus avoiding potential systemic effects from intraperitoneal injection, produced similar striatal damage in fKO mice and their WT littermates. This result obtained using the acute 3-NP treatment paradigm argues that selective deletion of A2ARs in forebrain neurons (presumably also abolishing A2AR-mediated facilitation of glutamate release) does not affect the sensitivity of striatal neurons to mitochondrial toxins. The result is consistent with our recent finding that selective inactivation of A2ARs in the forebrain abolished CGS21680-mediated facilitation of glutamate release in synaptosomes but still failed to confer neuroprotection against ischemic brain injury (L. Yu and Chen, unpublished data). Thus, A2AR-mediated facilitation of glutamate release at presynaptic sites does not seem to play a critical role in 3-NP-induced brain injury.
This interpretation is, however, limited by the potentially opposing effects of A2ARs in presynaptic and postsynaptic neurons. Recent studies showed that blockade of postsynaptic A2ARs is detrimental to striatal neurons as demonstrated by amplified NMDA current, increased intracellular calcium efflux, and enhanced hippocampal excitoxicity after exposure to excitotoxins (Robledo et al., 1999; Popoli et al., 2002; Tebano et al., 2004). Thus, A2AR inactivation may not only exert beneficial effects by reducing glutamate levels at presynaptic sites but may also produce potentially detrimental effects by modulating NMDA receptor-mediated currents at postsynaptic sites (Blum et al., 2003a). Because fKO mice inactivate both presynaptic (corticostriatal neurons) and postsynaptic (intrinsic striatal neurons) striatal neurons, the lack of the effect of A2ARs in forebrain neurons may result from a delicate balance between presynaptic and postsynaptic actions. Additional experiments are needed to clarify the specific effect of A2ARs at presynaptic and postsynaptic sites.
A2ARs in bone marrow-derived cells contribute critically to the exacerbation of 3-NP-induced striatal damage
The failure of forebrain-specific inactivation of A2ARs to produce the exacerbation of 3-NP-induced striatal damage present in gKO mice suggests that A2ARs in cells other than forebrain neurons may play a critical role in modulating 3-NP-induced striatal damage. A study by Jones et al. (1998b) suggests that A2AR agonists might work at peripheral A2ARs because only peripheral injection (but not local intrahippocampal injection) of CGS21680 reduces kainate-induced hippocampal lesions. The possibility of a contribution by peripheral A2ARs has been strengthened by our finding that IgG staining correlates well with the striatal damage area in the 3-NP-treated striatum, indicating BBB impairment after 3-NP treatment. Together with our recent finding of the critical contribution of A2ARs in BMDCs to modulation of transient ischemic injury (Yu et al., 2004), these lines of evidence prompted us to evaluate the contribution of the A2AR in BMDCs to 3-NP-induced striatal damage using chimeric mice with selective inactivation of A2ARs in BMDCs. Using this novel model, we found that, after challenge with 3-NP, none of the WT→WT mice displayed any striatal lesions, whereas 60% of the surviving KO→WT mice showed obvious bilateral striatal damage. Although similar exacerbation was not noted at the neurological behavioral level in KO→WT mice, this discrepancy may be attributable to the overall smaller striatal damage in the KO→WT groups, which is likely attributable to an immunosuppressive effect, as suggested in a previous study showing protection against spinal cord injury by BMT (Kipnis et al., 2004). Nonetheless, this result suggests that A2ARs on BMDCs are critical to the modulation of 3-NP-induced striatal damage observed in gKO mice. The mechanism by which selective inactivation of A2ARs in BMDCs exacerbates 3-NP-induced striatal damage is not clear. However, A2AR-mediated vascular effects, including vasodilation (Phillis, 2004), inhibition of platelet aggregation (Sandoli et al., 1994), and suppression of super-oxygen species generation by neutrophils (Cronstein et al., 1990), may provide partial explanations for the exacerbation of 3-NP-induced striatal damage in the KO→WT mice. The finding of BBB dysfunction in 3-NP-intoxicated mice raises the possibility that A2ARs on BMDCs may affect 3-NP-induced striatal damage through their influence on the BBB. Additional experiments are required to clarify the exact role of the A2AR in BMDCs in this pathological process.
Although our studies using three different A2AR KO genotypes reveal an important role for A2ARs in BMDCs in modulation of 3-NP-induced striatal damage, A2ARs in glial cells may also contribute critically to the modulation of 3-NP-induced striatal damage. A contribution of A2ARs in glial cells would be consistent with the finding that selective inactivation of A2ARs on forebrain neurons did not affect 3-NP-induced striatal damage, whereas the global inactivation of A2ARs exacerbated the 3-NP-induced striatal damage. Our immunohistochemical analysis of glial activation and neutrophil infiltration indicate that microglial activation precedes striatal damage (as detected by Fluoro-Jade C staining and cresyl violet staining) but was significantly reduced in the core lesioned area after striatal damage. Other than these general observations, it is not clear how A2ARs influence microglial cells and neutrophils and consequently the striatal damage. Additional experiments involving different time points with glial cell-specific A2AR manipulation are required to clarify the exact role of the glial A2AR in the modulation of 3-NP-induced striatal damage.
Footnotes
This work was supported by National Institutes of Health Grants NS41083 and NS37403, the Bumpus Foundation, and the Jerry McDonald Research Fund in Huntington's Disease. We thank Dr. Robert Ferrante for his assistance with 3-nitropropionic acid injection paradigm, Dr. Dong-sheng Xu for his assistance in fluorescence-activated cell sorting analysis, and Erica Kirsten Rapp for her assistance with fKO mice.
References
- Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- Bastia E, Xu YH, Scibelli AC, Day YJ, Linden J, Chen JF, Schwarzschild MA. A crucial role for forebrain adenosine A(2A) receptors in amphetamine sensitization. Neuropsychopharmacology. 2005;30:891–900. doi: 10.1038/sj.npp.1300630. [DOI] [PubMed] [Google Scholar]
- Blum D, Hourez R, Galas MC, Popoli P, Schiffmann SN. Adenosine receptors and Huntington's disease: implications for pathogenesis and therapeutics. Lancet Neurol. 2003a;2:366–374. doi: 10.1016/s1474-4422(03)00411-3. [DOI] [PubMed] [Google Scholar]
- Blum D, Galas MC, Pintor A, Brouillet E, Ledent C, Muller CE, Bantubungi K, Galluzzo M, Gall D, Cuvelier L, Rolland AS, Popoli P, Schiffmann SN. A dual role of adenosine A2A receptors in 3-nitropropionic acid-induced striatal lesions: implications for the neuroprotective potential of A2A antagonists. J Neurosci. 2003b;23:5361–5369. doi: 10.1523/JNEUROSCI.23-12-05361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada DC, Gangemi JJ, Rieger JM, Linden J, Kaza AK, Long SM, Kron IL, Tribble CG, Kern JA. Systemic adenosine A2A agonist ameliorates ischemic reperfusion injury in the rabbit spinal cord. Ann Thorac Surg. 2001;72:1245–1250. doi: 10.1016/s0003-4975(01)03057-0. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr, Schwarzschild MA. Neuroprotection by caffeine and A2A adenosine receptor inactivation in a model of Parkinson's disease. J Neurosci. 2001;21(RC143):1–6. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SY, Lee YC, Chen HM, Chiang MC, Lai HL, Chang HH, Wu YC, Sun CN, Chien CL, Lin YS, Wang SC, Tung YY, Chang C, Chern Y. CGS21680 attenuates symptoms of Huntington's disease in a transgenic mouse model. J Neurochem. 2005;93:310–320. doi: 10.1111/j.1471-4159.2005.03029.x. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Puringergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mendonca A, Sebastiao AM, Ribeiro JA. Adenosine: does it have a neuroprotective role after all? Brain Res Brain Res Rev. 2000;33:258–274. doi: 10.1016/s0165-0173(00)00033-3. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Stefanova N, Biran M, Wenning GK, Canioni P, Bioulac B, Tison F. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57Bl/6 mice: behavioural and histopathological characterisation. Neuroscience. 2002;114:1005–1017. doi: 10.1016/s0306-4522(02)00205-1. [DOI] [PubMed] [Google Scholar]
- Fink JS, Kalda A, Ryu H, Stack EC, Schwarzschild MA, Chen JF, Ferrante RJ. Genetic and pharmacological inactivation of the adenosine A2A receptor attenuates 3-nitropropionic acid-induced striatal damage. J Neurochem. 2004;88:538–544. doi: 10.1046/j.1471-4159.2003.02145.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B, Chen J-F, Masino SA, Vaugeois J-M. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Gabrielson KL, Hogue BA, Bohr VA, Cardounel AJ, Nakajima W, Kofler J, Zweier JL, Rodriguez ER, Martin LJ, de Souza-Pinto NC, Bressler J. Mitochondrial toxin 3-nitropropionic acid induces cardiac and neurotoxicity differentially in mice. Am J Pathol. 2001;159:1507–1520. doi: 10.1016/S0002-9440(10)62536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Phillis JW. CGS 15943, an adenosine A2 receptor antagonist, reduces cerebral ischemic injury in the Mongolian gerbil. Life Sci. 1994;55:PL61–PL65. doi: 10.1016/0024-3205(94)00889-2. [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative cytoarchitectonic atlas of the C57BL/6 and 129/SV mouse brain. Amsterdam: Elsevier Science; 2000. [Google Scholar]
- Jones PA, Smith RA, Stone TW. Protection against hippocampal kainate excitotoxicity by intracerebral administration of an adenosine A2A receptor antagonist. Brain Res. 1998a;800:328–335. doi: 10.1016/s0006-8993(98)00540-x. [DOI] [PubMed] [Google Scholar]
- Jones PA, Smith RA, Stone TW. Protection against kainate-induced excitotoxicity by adenosine A2A receptor agonists and antagonists. Neuroscience. 1998b;85:229–237. doi: 10.1016/s0306-4522(97)00613-1. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Avidan H, Markovich Y, Mizrahi T, Hauben E, Prigozhina TB, Slavin S, Schwartz M. Low-dose gamma-irradiation promotes survival of injured neurons in the central nervous system via homeostasis-driven proliferation of T cells. Eur J Neurosci. 2004;19:1191–1198. doi: 10.1111/j.1460-9568.2004.03207.x. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Marcoli M, Raiteri L, Bonfanti A, Monopoli A, Ongini E, Raiteri M, Maura G. Sensitivity to selective adenosine A1 and A2A receptor antagonists of the release of glutamate induced by ischemia in rat cerebrocortical slices. Neuropharmacology. 2003;45:201–210. doi: 10.1016/s0028-3908(03)00156-4. [DOI] [PubMed] [Google Scholar]
- Mayne M, Fotheringham J, Yan HJ, Power C, Del Bigio MR, Peeling J, Geiger JD. Adenosine A2A receptor activation reduces proinflammatory events and decreases cell death following intracerebral hemorrhage. Ann Neurol. 2001;49:727–735. doi: 10.1002/ana.1010. [DOI] [PubMed] [Google Scholar]
- Monopoli A, Lozza G, Forlani A, Mattavelli A, Ongini E. Blockade of adenosine A2A receptors by SCH 58261 results in neuroprotective effects in cerebral ischaemia in rats. NeuroReport. 1998;9:3955–3959. doi: 10.1097/00001756-199812010-00034. [DOI] [PubMed] [Google Scholar]
- Mroz K, Carrel L, Hunt PA. Germ cell development in the XXY mouse: evidence that X chromosome reactivation is independent of sexual differentiation. Dev Biol. 1999;207:229–238. doi: 10.1006/dbio.1998.9160. [DOI] [PubMed] [Google Scholar]
- Nikbakht MR, Stone TW. Suppression of presynaptic responses to adenosine by activation of NMDA receptors. Eur J Pharmacol. 2001;427:13–25. doi: 10.1016/s0014-2999(01)01171-2. [DOI] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- O'Regan MH, Simpson RE, Perkins LM, Phillis JW. The selective A2 adenosine receptor agonist CGS 21680 enhances excitatory transmitter amino acid release from the ischemic rat cerebral cortex. Neurosci Lett. 1992;138:169–172. doi: 10.1016/0304-3940(92)90498-v. [DOI] [PubMed] [Google Scholar]
- Pedata F, Corsi C, Melani A, Bordoni F, Latini S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann NY Acad Sci. 2001;939:74–84. doi: 10.1111/j.1749-6632.2001.tb03614.x. [DOI] [PubMed] [Google Scholar]
- Phillis JW. Adenosine and adenine nucleotides as regulators of cerebral blood flow: roles of acidosis, cell swelling, and KATP channels. Crit Rev Neurobiol. 2004;16:237–270. doi: 10.1615/critrevneurobiol.v16.i4.20. [DOI] [PubMed] [Google Scholar]
- Popoli P, Pintor A, Domenici MR, Frank C, Tebano MT, Pezzola A, Scarchilli L, Quarta D, Reggio R, Malchiodi-Albedi F, Falchi M, Massotti M. Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J Neurosci. 2002;22:1967–1975. doi: 10.1523/JNEUROSCI.22-05-01967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Canas PM, Oliveira CR, Cunha RA. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience. 2005;132:893–903. doi: 10.1016/j.neuroscience.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Robledo P, Ursu G, Mahy N. Effects of adenosine and gamma-aminobutyric acid A receptor antagonists on N-methyl-d-aspartate induced neurotoxicity in the rat hippocampus. Hippocampus. 1999;9:527–533. doi: 10.1002/(SICI)1098-1063(1999)9:5<527::AID-HIPO6>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61:S12–S18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- Sandoli D, Chiu PJ, Chintala M, Dionisotti S, Ongini E. In vivo and ex vivo effects of adenosine A1 and A2 receptor agonists on platelet aggregation in the rabbit. Eur J Pharmacol. 1994;259:43–49. doi: 10.1016/0014-2999(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Tebano MT, Pintor A, Frank C, Domenici MR, Martire A, Pepponi R, Potenza RL, Grieco R, Popoli P. Adenosine A2A receptor blockade differentially influences excitotoxic mechanisms at pre- and postsynaptic sites in the rat striatum. J Neurosci Res. 2004;77:100–107. doi: 10.1002/jnr.20138. [DOI] [PubMed] [Google Scholar]
- Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10:1081–1087. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]