Abstract
The mechanisms underlying the development of painful and nonpainful neuropathy associated with diabetes mellitus are unclear. We have obtained microneurographic recordings from unmyelinated fibers in eight patients with diabetes mellitus, five with painful neuropathy, and three with neuropathy without pain. All eight patients had large-fiber neuropathy, and seven patients had pathological thermal thresholds in their feet, indicating the involvement of small-caliber nerve fibers. A total of 163 C-fibers were recorded at knee level from the common peroneal nerve in the patients (36–67 years old), and these were compared with 77 C-fibers from healthy controls (41–64 years old). The ratio of mechano-responsive to mechano-insensitive nociceptors was ∼2:1 in the healthy controls, whereas in the patients, it was 1:2. In patients, a fairly large percentage of characterized fibers (12.5% in nonpainful and 18.9% in painful neuropathy) resembled mechano-responsive nociceptors that had lost their mechanical and heat responsiveness. Such fibers were rarely encountered in age-matched controls (3.2%). Afferent fibers with spontaneous activity or mechanical sensitization were found in both patient groups. We conclude that small-fiber neuropathy in diabetes affects receptive properties of nociceptors that leads to an impairment of mechano-responsive nociceptors.
Keywords: neuropathy, diabetes, pain, C-fibers, microneurography, degeneration
Introduction
Diabetes mellitus is a common cause of neuropathic pain worldwide. However, not all patients who develop diabetic neuropathy (DN) experience pain (Calcutt, 2002). The prevalence of pain varies from 13 to 24% (O'Hare et al., 1994; Schmader, 2002). Among those with positive symptoms, the clinical findings often differ from those found in other neuropathic pain patients, such as for example postherpetic neuralgia and traumatic nerve lesions. Tactile allodynia is rare in the later stages, possibly because of loss of myelinated fibers, and many patients report a loss of tactile sensation coexisting with ongoing pain (Vinik et al., 2001; Otto et al., 2003).
The symptoms of pain seen in patients with DN have been assigned to probable changes in small afferent fibers (Vinik et al., 2001). However, so far, most clinical studies on painful and nonpainful diabetic neuropathy have not been able to prove any clear distinction in indirect tests of C-fiber function [quantitative sensory tests (QST)] and in neuropathological studies (nerve biopsies) between those patients who experience pain and those that do not (Llewelyn et al., 1991; Vrethem et al., 2002; Sommer, 2003; Kramer et al., 2004a).
The only method that directly measures receptive and axonal properties of C-fibers in humans is microneurography. In this study, recordings from patients with diabetic neuropathy with (DNP group) and without (DN group) pain were performed to assess axonal and receptive properties of C-fibers, which might contribute to pain in this disease. In healthy elderly people, there is a well known decline of large-fiber function, C-fiber function, and epidermal nerve fiber density (Mackenzie and Phillips, 1981; Hilz et al., 1999; Goransson et al., 2004). Because most of our knowledge on C-fibers from microneurography in the past was based on recordings from young subjects, we included a control group of healthy subjects (HC group) in the same age range as our diabetic neuropathy patients.
Materials and Methods
Patients.
Eight patients, three female and five male diagnosed with diabetes mellitus aged 36–67 years, participated in this study (see Table 2). Anamnestic details regarding duration of disease, sensory symptoms including pain symptoms, and maximal intensity of pain with the use of a visual analog scale were obtained. Patients with no clinical motor impairment and only mild neuropathy were chosen to avoid the risk of decreasing motor function further by the experiments.
Table 2.
Anamnestic and clinical data of the patients
| Pat 1 | Pat 2 | Pat 3 | Pat 4 | Pat 5 | Pat 6 | Pat 7 | Pat 8 | |
|---|---|---|---|---|---|---|---|---|
| Sex | M | M | M | F | M | M | F | F |
| Age (years) | 57 | 53 | 36 | 60 | 60 | 37 | 67 | 61 |
| Duration diabetes (years) | 33 | 2 | 25 | 1 | 3 | 30 | 9 | 20 |
| Duration | ||||||||
| Pain (years) | 3,5 | 3 | 9 | 1,5 | 3 | 0 | 0 | 0 |
| Treatment | Insulin | Oral | Insulin | Diet | Insulin | Insulin | Insulin | Insulin |
| Ongoing pain | Feet, pricking, aching | Feet, burning, pricking | Feet, legs, burning, pressing | Feet and toes, aching | Feet and legs aching, tightening | 0 | 0 | 0 |
| VASmax | 3,2 | 5,8 | 9,7 | 2,5 | 2,2 | |||
| Paroxysmal pain VASmax | 0 | 6,7 | 7,7 | 2,9 | 0 | 0 | 0 | 0 |
| Reported evoked pain VASmax | 0 | 6,7 | 8,2 | 3,2 | 2,2 | 0 | 0 | 0 |
| Tendon reflexes | N | N | Absent ankle, patellar, and biceps | Absent ankle | Absent ankle, reduced patellar | N | Absent ankle | Reduced ankle |
| Vibration | N | Reduced | N | N | Absent | Reduced | N | N |
| Light touch/pinprick | N | Reduced | Reduced | Reduced | N | N | N | N |
| Joint position | Reduced | N | N | N | Reduced | N | N | Reduced |
Pat, Patient; M, male; F, female; N, normal; VASmax, maximum intensity of pain on a visual analog scale.
All patients gave their written informed consent. The study was performed in the Department of Neurology, Rikshospitalet-Radiumhospitalet Medical Center (Oslo, Norway) and in the Department of Clinical Neurophysiology, University Hospital (Uppsala, Sweden), and experiments were performed according to the Helsinki Declaration with the approval of the local ethic committees connected to each hospital.
Healthy subjects.
Nine healthy subjects aged 41–64 years were used as controls for the microneurography experiments. These subjects had no known neurological diagnosis, symptoms, or diseases suspicious of causing neurological impairment. They did not use any medication known to influence the nervous system.
Part of the study in healthy subjects was done in the Department of Physiology and Experimental Pathophysiology, University of Erlangen/Nürnberg (Erlangen, Germany) and was approved by the local ethic committee of the University. All participants gave their written informed consent.
Neurological examination.
The patients underwent a neurological examination, including testing of muscular strength in the upper and lower extremities, tendon reflexes, and sensibility for painful stimuli with a needle, vibration sense, and joint-position sense of the first metatarso-phalangeal joint.
Hypersensitivity to gentle touch (allodynia) was assessed by lightly stroking all four extremities with a brush (Somedic, Hörby, Sweden) (LaMotte et al., 1991). The borders of hyperalgesia to punctate stimuli were determined by an 83.7 mN von Frey filament. Temporal summation was tested with a stiff von Frey monofilament tapping the same spot at a rate of 2–3 Hz for 20 s. The subjects were instructed to report if an intense painful, radiating sensation was felt early on. This test was applied to one of the thenar eminences and to the dorsal aspects of both feet.
Nerve conduction velocities, amplitudes, and distal latencies of the median, ulnar (motor and sensory), peroneal, tibial, and sural nerves were examined in all patients.
Quantitative sensory testing.
Threshold temperatures for sensations of warmth, cold, heat pain, and cold pain were determined by standardized computerized equipment using the method of limits (Thermotest; Somedic) as described in detail previously (Ørstavik et al., 2004). The results were compared with data from 38 healthy subjects (aged 22–66 years old) obtained in the laboratory in Oslo. The individual thresholds of the patients were considered pathological if they were greater than the 95 percentile (warmth, heat pain) or less than the fifth percentile (cold) of values found in healthy subjects. Cold pain detection <20°C were considered normal.
Microneurographic recording technique.
Methods of microneurography used in this study have been described in detail previously (Vallbo and Hagbarth, 1968; Torebjörk and Hallin, 1974; Schmidt et al., 1995). Microelectrodes were inserted at the level of the fibular head into cutaneous branches of the peroneal nerve. Single units were identified by action potentials that could be recorded after long latency following each electrical stimulus applied in their innervation territory in the foot. Additional responses evoked by nonelectrical activity were assigned to the fibers by applying the “marking” method (Torebjörk, 1974; Schmidt et al., 1995).
The latency of a C-fiber response to the first electrical stimulus after an initial rest period of at least 2 min was used to estimate average conduction velocity (CV). The distance between the stimulating needles in the skin and the recording needle in the nerve was assessed using a measuring tape, and 2 m/s was set as the upper limit of CV for C-fiber classification.
Activity-dependent slowing.
Activity-dependent slowing of conduction velocity is a well known characteristic of unmyelinated nerve fibers and can be used for differentiating functional subtypes of C-fibers (Serra et al., 1999; Weidner et al., 1999). It is much more pronounced for mechano-insensitive C-nociceptors than for mechano-responsive C-nociceptors (Weidner et al., 1999).
After a rest period of at least 2 min, an electrical protocol was performed for each stimulation site on the foot; 20 electrical stimuli were applied intracutaneously at 0.125 Hz, immediately followed by a second train of 20 pulses at 0.25 Hz and a third of 30 pulses at 0.5 Hz. Changes in latency were calculated relative to the initial latency (i.e., that immediately after the 120 s rest period). After this protocol, the stimulation frequency was set to 0.25 Hz for the rest of the experiments. At the end of some experiments, in which the recording conditions were still optimal, we performed a second stimulation protocol that consisted of a train of pulses at 2 Hz for 3 min (Serra et al., 1999), which were given after a rest period of 2 min without any stimulation.
Efferent and afferent responses.
Sympathetic fibers were identified by their marking response to sympathetic arousal stimuli, e.g., an unexpected loud noise, mental stress, or during deep inspiration (Hallin and Torebjörk, 1970; Hagbarth et al., 1972). This was performed after the stepwise electrical protocol, and the efficacy of these maneuvers was controlled by recording background activity of sympathetic burst discharges.
A set of von Frey nylon monofilaments (Stoelting, Chicago, IL) was used to test for mechanical responsiveness. Units unresponsive to 750 mN were regarded as mechano-insensitive. When possible, heat thresholds were assessed using a radiant heat lamp, the temperature of which was feedback controlled from a thermocouple gently pressed on the skin (Beck et al., 1974). The skin temperature was slowly increased by 0.25°C/s, from an initial temperature of 32°C to a maximum of 50°C. Heating could be stopped by the subjects at any time if the pain tolerance limit was reached. In this study, units unresponsive to heat when the heat lamp was switched off automatically or by the subject were regarded as heat negative. Transcutaneous electrical thresholds were obtained in a few fibers using a standardized electrode consisting of a Perspex holder with a round cotton disk, 5 mm in diameter, soaked with saline and giving additional pulses at the skin (pulse width of 500 μs).
Classification of C-fibers.
According to the receptive and reflex properties described above and their degree of activity-induced slowing, C-fibers were classified into three main classes: efferent sympathetic fibers (symp), afferent mechano-responsive fibers (CM) with a low degree of activity-dependent slowing and a clear and reproducible response to mechanical stimuli, and afferent mechano-insensitive fibers (CMi), with a high degree of activity-dependent slowing and no response to mechanical stimuli. In the present study, the CM and CMi class also include fibers responding to heat (CMH and CH, respectively). In this study, a large number of fibers were found that did not fall into any of these three categories. These fibers showed signs of either desensitization (Cxi/Cdes) or sensitization to mechanical stimuli or spontaneous activity (Cspont/Csens) (Table 1). The Cxi fibers had axonal properties resembling CM fibers but were negative to afferent and efferent stimuli, whereas Cdes had the same axonal properties but were responsive to heat or showed a minute response to mechanical stimuli. The Cspont/Csens represented afferent fibers with either mechanical sensitization or spontaneous activity.
Table 1.
Characterization of fibers
| CM | CM spont | C des | Cxi | CMi spont | CMi sens | CMi | symp | |
|---|---|---|---|---|---|---|---|---|
| Classification of C-fibers | ||||||||
| Sympathetic stimuli | − | − | − | − | − | − | − | + |
| Mechanical stimuli | + | + | +/− | − | − | + | − | − |
| Relative slowing | + | + | + | + | +++ | +++ | +++ | ++ |
| Spontaneous activity | − | + | − | − | + | − | − | + |
| Number of fibers | ||||||||
| Subject group | ||||||||
| HC | 27 | 1 | 0 | 2 | 1 | 2 | 12 | 17 |
| DN | 9 | 1 | 2 | 5 | 3 | 3 | 15 | 2 |
| DNP | 10 | 1 | 5 | 14 | 3 | 3 | 24 | 14 |
For the classification of C-fibers, normal C-fibers (CM, CMi, and symp) are mainly characterized by their response to efferent stimuli [Sympathetic stimuli: positive (+) or negative (−)], afferent mechanical stimuli [Mechanical stimuli: positive (+) or negative (−)], and relative degree of activity-dependent slowing to a stepwise low-frequency electrical stimulus protocol [Relative slowing: low degree <5% (+) and high degree >5% (+++) or borderline and symp positive (++)]. The number of fibers in the different subject groups are also shown. CMi fibers have a high degree of relative total slowing (+++ indicates >5%) and a high degree of slowing to 0.125 Hz, whereas these parameters are all less in CM fibers. Cxi fibers have a relative total slowing of <5% (+), no spontaneous activity, and no afferent or efferent response. One Cxi fiber showed borderline (5.05%) relative slowing, and the additional parameter of low degree of relative slowing to 0.125 Hz (<0.65%) was required for classification. Cdes represent fibers with other signs of desensitization. Abnormal afferent fibers with spontaneous activity (Cspont) or mechanical sensitization (Csens) were also characterized in the three subject groups.
Data analyses and statistics.
Microneurography signals were amplified, processed on-line, and stored to disk using custom-written Spike2 software and a micro1401 DAC (Cambridge Electronics Design, Cambridge, UK). Pearson's χ2 test and Fisher's exact test were used for categorical data, and Student's t test or one-way ANOVA was used for numerical data. The activity-dependent slowing of conduction velocity after electrical stimulation was analyzed by ANOVA with post hoc Dunnett's T3 and Scheffé's tests. Values are given as mean ± SEM. p values <0.05 were considered statistically significant. When comparing categorical data in subgroups of subjects, only p values <0.01 were considered statistically significant.
Results
Clinical testing
The clinical characteristics and findings from QST are presented in Tables 2 and 3. Patients 3 and 6 had type 1 diabetes mellitus, whereas the others had type 2 diabetes mellitus. All five patients with painful neuropathy had burning or pricking pain at least on the sole of their feet. One patient with pain (patient 2) had brush-evoked allodynia on his toes. None of these patients had punctate hyperalgesia or abnormal temporal summation, and none of these five patients described pain in their hands. Among the three patients without pain, patients 7 and 8 described numbness on the soles of their feet.
Table 3.
Thermal thresholds (in °C) from QST and C-fiber types obtained from each patient in microneurographic recordings
| Pat 1 | Pat 2 | Pat 3 | Pat 4 | Pat 5 | Pat 6 | Pat 7 | Pat 8 | |
|---|---|---|---|---|---|---|---|---|
| CDT | ||||||||
| Thenar | 31.6 | 29.5* | 30 | 29.7 | 30.7 | 30.7 | 29.3* | 30.2 |
| Thigh | 23* | 26.5* | 23.4* | - | 28.9 | 29 | 29.1 | 29.1 |
| Leg | 18.7* | 26.3* | 27.2* | 28.1 | 27.6 | 21.7* | 28.5 | 29 |
| Feet | 26.5 | 24.9* | 18.1* | 27.3 | 24.9* | 19.6* | 26.5 | 26.5 |
| WDT | ||||||||
| Thenar | 33.6 | 34.2 | 36* | 34.3* | 34 | 33.6 | 34.7* | 33.6 |
| Thigh | 42.3* | 35.6 | 35.9 | - | 36.1 | 35.6 | 42.9* | 35.6 |
| Leg | 48.8* | 45.9* | 44.4* | 37.3 | 41.2 | 36.6 | 43.8* | 39.5 |
| Feet | 44.9* | 43.9* | 42.6 | 39.4 | 44.2* | 48.4* | 44.4* | 45.8* |
| HPDT | ||||||||
| Thenar | 49.1* | 44.3 | 44.3 | 39.4 | 47.2 | 41 | 41.4 | 44.5 |
| Thigh | >50* | 45.5 | 48.9* | - | 44.5 | 43.7 | 49.6* | 45.5 |
| Leg | >50* | 50* | 47.5 | 43.7 | 48.2 | 47 | 49.2* | 48.1 |
| Feet | 49.5* | 49.9* | 47.3 | 46.9 | 48.6* | 50* | 49.7* | >50* |
| MNG days | two | two | two | two | one | two | one | two |
| Fiber types | ||||||||
| CM | 0 | 0 | 6 | 2 | 2 | 2 | 2 | 5 |
| CMi | 2 | 11 | 3 | 8 | 0 | 5 | 2 | 8 |
| Symp | 0 | 4 | 3 | 5 | 2 | 2 | 0 | 0 |
| Cxi + Cdes | 3 | 3 | 9 | 3 | 1 | 4 | 1 | 2 |
| Csp + Csens | 3 | 1 | 0 | 1 | 2 | 3 | 0 | 4 |
| Total | 8 | 19 | 21 | 19 | 7 | 16 | 5 | 19 |
Thresholds for cold detection (CDT), warmth detection (WDT), and heat pain detection (HPDT) obtained at different locations in each patient. An asterisk shows that the threshold was pathological compared to healthy controls. Classification of normal C-fibers obtained in microneurography as described in the methods. Abnormal units with either varying degrees of desensitization (Cxi + Cdes) or sensitized or spontaneously active afferent fibers (Csp + Csens) are shown in italics.
Patient 6 had neurophysiologic changes compatible with carpal tunnel syndrome in one arm. With the exception of patients 2 and 5, all other patients had pathological amplitudes or conduction velocities of motor or sensory nerves in the upper extremity. All patients had some degree of large-fiber neuropathy in their lower extremities as measured by conventional neurography. Patients 1 and 3 had decreased CV and amplitudes of both the peroneal and tibial nerves and no measurable responses from the sural nerve bilaterally. Patients 2, 4, 5, 6, and 8 all had pathological changes involving both motor and sensory nerves, patient 2 mainly decreased conduction velocities, and patient 4 mainly decreased amplitudes. Patients 5, 6, and 8 had mixed changes involving both amplitudes and conduction velocities. Patient 7 had only pathological changes in the sensory nerves, including both amplitude and conduction velocity in her left leg. All patients except patient 4 had pathologically elevated thermal thresholds in the lower extremities at least on the dorsal aspect of the foot (Table 3). Cold allodynia (as measured by cold pain thresholds) was not present in any of the patients (data not shown).
Microneurography
In this study, microneurographic recordings were obtained from cutaneous fascicles of the peroneal nerve at knee level in three different groups of subjects: five DNP patients, three DN patients, and nine HCs.
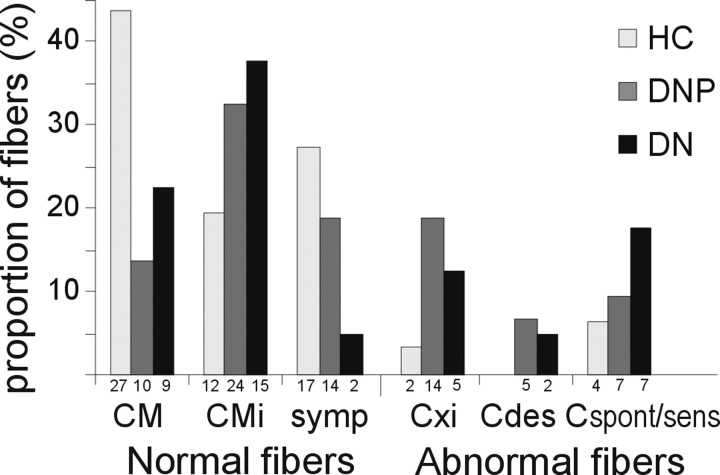
A total of 163 C-fibers were recorded in patients with diabetic neuropathy. The mean ± SEM CV of these units was 0.82 ± 0.02 m/s compared with a mean CV of 0.87 ± 0.02 m/s in the control subjects (n = 77) (p = 0.058, t test). Seventy-four fibers in the DNP patients, 40 in the DN patients, and 62 fibers from HC were tested for their degree of relative total slowing, their mechanical responsiveness, and response to sympathetic stimuli. The characterization of the fibers into three main classes and pathological subgroups is shown in Table 1, whereas the distribution of categorized fibers obtained from each patient is shown in Table 3. The relative distribution of fiber types identified in the three subject groups was significantly different (Fig. 1) (p < 0.001, Pearson's χ2 test).
Figure 1.
Percentages of characterized C-fibers in the three subject groups. The distribution of fibers characterized within the groups of HC, DNP, and DN was significantly different. This difference was most apparent regarding the CM, symp, and Cxi fibers. The absolute numbers of the different fiber types found within each group are given below the bars. CM and CMi fibers include fibers responding to heating (CMH and CH, respectively). Cxi are fibers with axonal properties resembling CM fibers but with no afferent or efferent response. Cdes are fibers with axonal properties resembling CM but with only an afferent response to heat or with a rudimentary response to mechanical stimulation. Cspont/sens represents afferent fibers with either mechanical sensitization or spontaneous activity.
Afferent fibers (CM, CMi) with normal characteristics
Mechano-responsive fibers
In the DNP patients, we found 10 CM fibers with CV 0.92 ± 0.04 m/s and a relative total slowing of 2.98 ± 0.3%, whereas in the DN patients, nine CM fibers were classified with a CV of 0.90 ± 0.06 m/s and a relative total slowing of 2.36 ± 0.3%. There were significantly fewer normal CM fibers in the patients compared with the controls (p < 0.001, Fisher's exact test), and the CV of the 19 CM fibers from the two patient groups was significantly lower than the CV of the 27 CM fibers in healthy controls (0.91 ± 0.03 vs 1.0 ± 0.02 m/s; p = 0.03, t test). All of these fibers responded to mechanical stimulation with thresholds ≤750 mN.
As seen in Figure 2, the relative total slowing of the CM fibers in the patients was not significantly different from healthy controls (2.69 ± 0.2 vs 2.69 ± 0.2%) (p = 0.99, t test). Fifteen of 19 CM fibers obtained from the patients were tested for heat responses and 13 were positive to heat (CMH) (total range, 38–45°C), whereas 5 of 27 CM fibers obtained from the healthy controls were tested for heat and four were positive (total range, 40–47.9°C).
Figure 2.
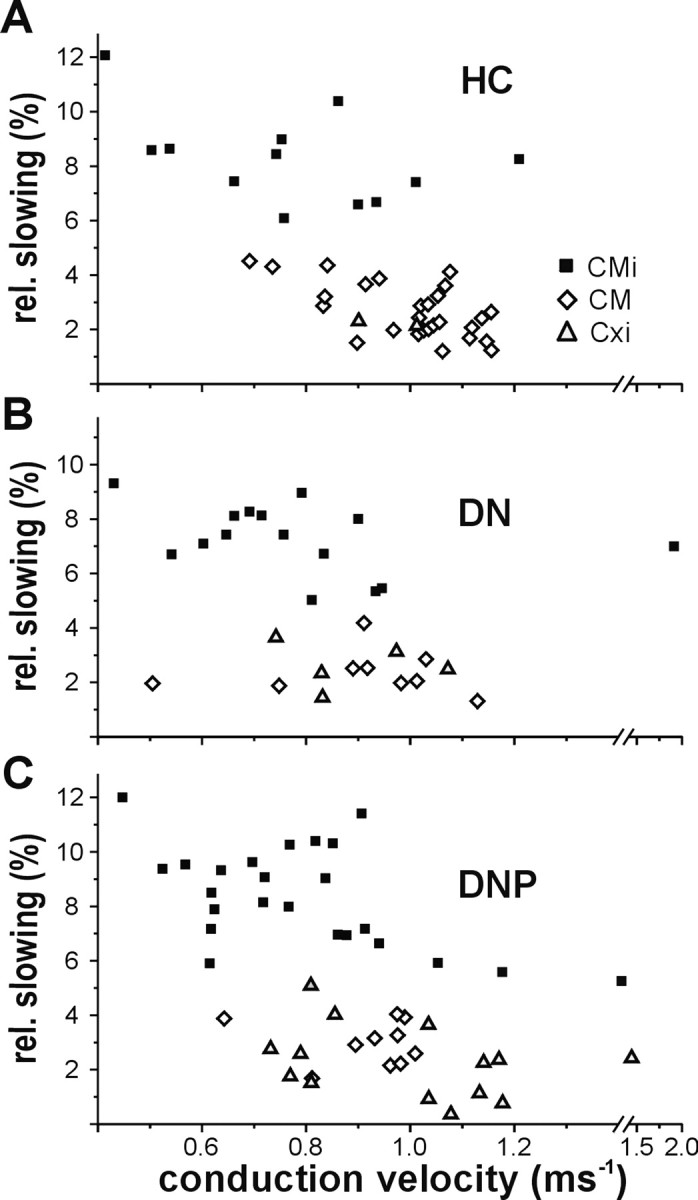
CV and total slowing of three fiber types in healthy controls and patients. CV and total slowing of CM, CMi, and abnormal fibers with axonal properties of CM but no response to afferent or efferent stimuli and no spontaneous activity (Cxi) identified as follows: A, healthy controls (CM, 27; CMi, 12; Cxi, 2); B, patients with nonpainful diabetic neuropathy (CM, 9; CMi, 15; Cxi, 5); and C, patients with painful diabetic neuropathy (CM, 10; CMi, 24; Cxi, 14). The CV and relative total slowing (to the increasing electrical stimulus frequency protocol) of the Cxi fibers were in the range of the CM fibers, although they did not respond to mechanical stimuli.
Mechano-insensitive fibers
In the DNP patients, 24 CMi fibers were classified, 15 in the DN patients and 12 in the HC group. There were more CMi fibers in the patients, but this difference did not reach statistical significance (p = 0.08, Fisher's exact test). The CV and relative total slowing of the CMi fibers did not differ significantly between the three groups and were, respectively, as follows: 0.79 ± 0.05 m/s, 8.34 ± 0.4% (DNP); 0.81 ± 0.09 m/s, 7.30 ± 0.3% (DN); and 0.77 ± 0.07 m/s, 8.30 ± 0.5% (HC). Thirty-two of 39 CMi fibers obtained in the two patient groups were tested for a heat response and 17 were positive to heat (total range, 40–49°C), whereas 4 of 12 CMi fibers obtained in the HC were tested for heat and two responded (thresholds, 40 and 48°C).
In the healthy subjects, 27 CM and 12 CMi fibers were found, whereas in the patients, only 19 CM but 39 CMi were classified (p < 0.001, χ2 test). Thus, the ratio of CM to CMi fibers being ∼2:1 in the control group was reversed to ∼1:2 in the patients.
Efferent fibers (symp)
Fourteen fibers in the symptomatic patients, two in the asymptomatic patients, and 17 in the healthy control subjects were characterized as symp. The CV and relative total slowing of the symp fibers were 0.83 ± 0.06 m/s and 3.91 ± 0.2% (DNP), 0.88 ± 0.01 m/s and 4.42 ± 0.4% (DN), and 0.83 ± 0.02 m/s and 3.45 ± 0.4% (HC) (no significant group effect, one-way ANOVA). However, as seen in Figure 1, fewer sympathetic fibers were found in the pain-free patients (DN), and the proportion of these fibers was significantly different in the three groups (p = 0.01, Fisher's exact test), with significantly fewer sympathetic fibers in the pain-free patients compared with healthy controls (p = 0.004, Fisher's exact test).
Mechano-insensitive fibers with moderate activity-dependent slowing: Cxi fibers
A large number of fibers in the patients could not be classified according to the standard criterion because of an apparent mismatch between degree of slowing and mechanical responsiveness. These units were not mechanically excitable (similar to mechano-insensitive fibers), and they showed only moderate activity-dependent slowing of conduction velocity in the low-frequency electrical stimulation protocol (in the range of mechano-responsive fibers). In the DNP group, we found 14 of these fibers (18.9% of characterized fibers) and five (12.5%) in the DN group. Two (3.5%) such fibers were observed in the HC group that were unresponsive to mechanical poking. There was a significant difference in the incidence of these fibers between the three groups (p = 0.01, Fisher's exact test), with a higher incidence in the patients (p = 0.007, Fisher's exact test).
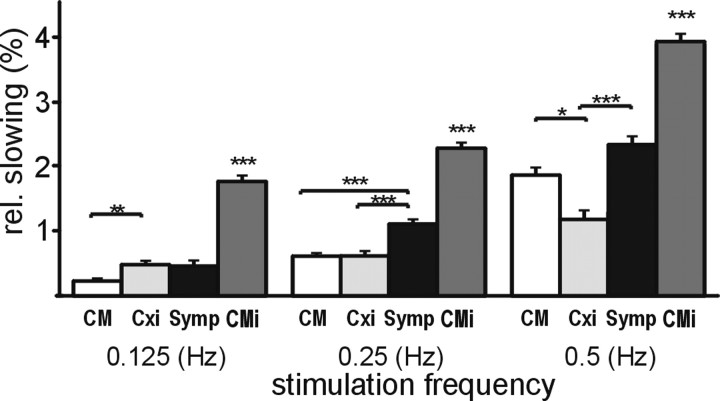
Conduction velocity of these fibers (pooled from all recordings) was 0.97 ± 0.04 m/s, similar to CM fibers (0.96 ± 0.02 m/s; p = 1, ANOVA, post hoc Dunnett's T3 test), but significantly faster than the CMi fibers (0.79 ± 0.04 m/s; p = 0.01, ANOVA, post hoc Dunnett's T3 test) and significantly faster than the symp fibers recorded in this study (0.83 ± 0.02 m/s; p = 0.04, ANOVA, post hoc Dunnett's T3 test). The relative total slowing of these fibers (2.34 ± 0.3%) was significantly less than that in the CMi (8.02 ± 0.2%; p < 0.001, ANOVA, post hoc Dunnett's T3 test) and symp fibers (3.73 ± 0.2%; p = 0.001, ANOVA, post hoc Dunnett's T3 test) but not significantly different from that found in the CM fibers (2.69 ± 0.1%; p = 0.8, ANOVA, post hoc Dunnett's T3 test). An afferent response to mechanical or heat stimulation was not observed in these fibers. Transcutaneous electrical thresholds were determined for eight of them and ranged from 15 to 60 mA (pulse width of 500 μs), with a mean threshold of 34.9 mA. The slowing pattern of these fibers (designated “Cxi” in the figures) for different stimulation frequencies showed significantly reduced slowing compared with mechano-insensitive fibers at all frequencies (Fig. 3) but a virtually identical slowing pattern as that seen for mechano-responsive fibers at a stimulation frequency of 0.25 Hz. Interestingly, the slowing pattern differed slightly between these atypical fibers and CM fibers, with a more pronounced slowing at a low-stimulus frequency of 0.125 Hz (0.48 ± 0.06 vs 0.23 ± 0.04%) and a less pronounced slowing at the higher stimulation frequency of 0.5 Hz (1.23 ± 0.2 vs 1.86 ± 0.1%) (Fig. 3).
Figure 3.
Mean relative slowing at increasing stimulus frequencies in CM, Cxi, symp, and CMi fibers. Fibers from both patient groups and healthy controls are pooled to look at conductive properties of Cxi (n = 21) fibers compared with CM (n = 45), symp (n = 23), and CMi (n = 50) fibers. The relative slowing to all three frequencies was significantly different in the four groups (p < 0.001, ANOVA). There was significantly more slowing to all frequencies in CMi fibers compared with CM, symp, and Cxi fibers (p < 0.001, post hoc Dunnett's T3 test for all 3 comparisons at all 3 frequencies). There was also significantly more pronounced slowing in the Cxi fibers compared with the CM fibers at 0.125 Hz (p = 0.008, post hoc Dunnett's T3 test) and less pronounced slowing of the Cxi fibers compared with the CM fibers at 0.5 Hz (p = 0.02, ANOVA, post hoc Dunnett's T3 test). The symp fibers slowed significantly more than the Cxi at both 0.25 and 0.5 Hz (p < 0.001, post hoc Dunnett's T3 test, at both paces), whereas the relative slowing did not differ significantly between Cxi fibers and symp fibers at 0.125 Hz. The CM fibers slowed significantly less than the symps at 0.250 Hz (p < 0.001, ANOVA, post hoc Dunnett's T3 test), whereas the relative slowing at 0.125 and 05.Hz did not differ significantly between these two fiber types. *p < 0.05 and ≥0.01; **p < 0.01 and ≥0.001; ***p < 0.001.
In both the DNP group (n = 5) and the DN group (n = 2), fibers with other signs of desensitization were identified. These fibers showed a slowing profile similar to CM fibers. Five of these responded to heat but had no mechanical response, whereas two fibers had no heat response and an unclear and not reproducible mechanical response. The CV and relative total slowing of these seven fibers were in the range of CM and Cxi fibers (0.91 ± 0.1 m/s and 2.66 ± 0.6%).
Response to 2 Hz, 3 min stimulation
Electrical stimulation at 2 Hz for 3 min (Serra et al., 1999) induced characteristic patterns of conduction velocity slowing in the different fiber classes. Maximum slowing to this stimulation frequency did not differ significantly between healthy subjects and patients with or without pain in CM, CMi, or symp fibers (Fig. 4). The Cxi fibers (DNP, n = 5; DN, n = 2) showed less slowing than normal afferent fibers (DNP, 7.9 ± 2.2%; DN, 10.6 ± 3.2%) compared with both CM (p = 0.04, ANOVA, post hoc Scheffé's test) and CMi (p = 0.003, ANOVA, post hoc Scheffé's test) fibers (Fig. 4). The slowing pattern of these atypical fibers also differed from the sympathetic fibers in which the initial slowing had a much steeper slope (half-maximum time of slowing, 22 ± 3.1 vs 55 ± 4.0 s; p < 0.01, ANOVA, post hoc Scheffé's test).
Figure 4.
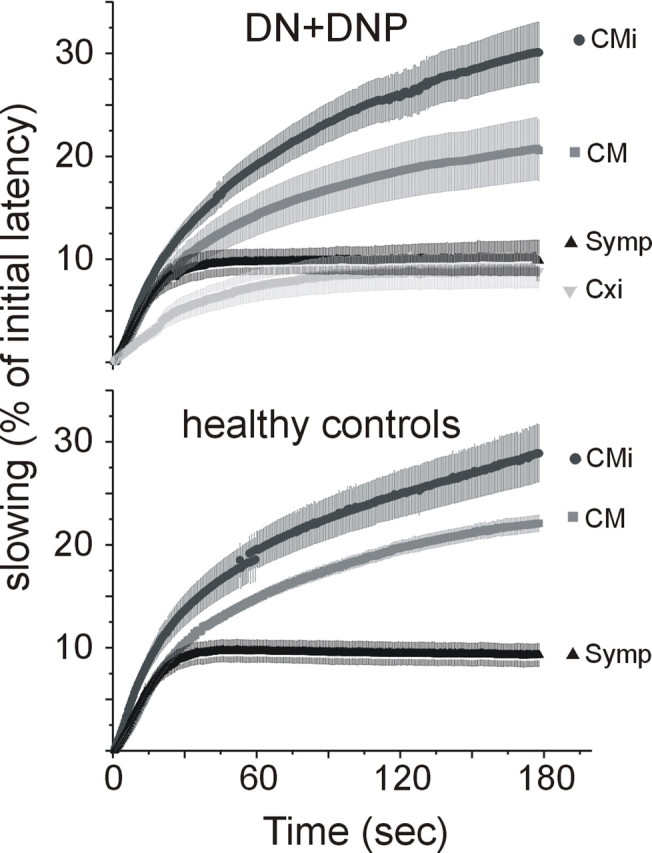
Slowing to 2 Hz, 3 min protocol. A 2 Hz, 3 min protocol was performed in some of the fibers recorded from patients (CM, 13; CMi, 14; symp, 4; Cxi, 8) and in some from healthy controls (CM, 26; CMi, 4; symp, 8; Cxi, 0). This additional protocol for testing the slowing at higher stimulus repetition rates can be used to distinguish between afferent and efferent C-fibers. In the patients, Cxi fibers slowed in a manner similar to symp fibers and significantly less than CM and CMi fibers (CM, p = 0.04; CMi, p = 0.003; ANOVA, post hoc Scheffé's test). However, the shape of the curve of the Cxi fibers (steepness of slope and no plateau or speeding after 1 min of stimulation) differed from the shape seen in sympathetic fibers with a significantly longer half-maximum time in Cxi compared with symp (p = 0.04, ANOVA post hoc Scheffé's test). In contrast to the relative total slowing parameter in the low repetition rate protocol, in this protocol, Cxi fibers showed less slowing than CM in healthy and diseased nerves.
In addition, normalization of conduction velocity after this protocol in CM fibers showed different patterns in the two patient groups (DN, n = 7; DNP, n = 6) and the healthy controls (HC, n = 17). The maximum slowing, which was reached after the 2 Hz, 3 min stimulation, gradually normalized when the stimulation frequency was reduced to 0.25 Hz. After 150 s with the lower-stimulation frequency, slowing of conduction velocity was still 28 ± 5.9% of the maximum in HC but 21.4 ± 4.8% in DN patients and only 9.9 ± 2.8% in DNP patients. This indicates that the patients had a tendency toward a faster recovery after a slowed state compared with the controls. This was most apparent in patients with pain (DNP), although it did not reach statistical significance (p = 0.07, ANOVA, post hoc Scheffé's test). Because of the low number of recovery profiles recorded from CMi fibers, we were not able to compare the results from the three subject groups for this fiber type.
Spontaneous activity and sensitized fibers
Bursts of unprovoked spontaneous activity were seen in afferent fibers in patients with pain (n = 4) but also in those without pain (n = 4) (Table 1). Six of these were CMi fibers, whereas two were CMs. Three units with axonal properties resembling CMi fibers but with some responsiveness to mechanical poking (threshold ≤750 mN) regarded as sensitized mechano-insensitive (CMi) fibers were also found in each patient group. Two spontaneously active fibers (one CM and one CMi) and two sensitized CMi fibers were also observed in the healthy controls (Table 1).
Discussion
None of the routinely used clinical methods test the conductive properties of small fibers, and microneurography studies in patients are few. Recordings from sympathetic fibers in patients with diabetic neuropathy have not shown changes in conduction velocity (Fagius and Wallin, 1980). In one microneurographic study on afferent C-fibers that included patients with diabetes but with little or no neuropathy, no specific changes of conductive properties in the C-fibers from the patients were found (Serra et al., 1999).
We describe for the first time a clear difference in the distribution of subtypes of C-fibers in patients with diabetic neuropathy compared with healthy controls. The ratio of normal mechano-responsive to mechano-insensitive nociceptors was ∼1:2 in patients with diabetic neuropathy compared with 2:1 in healthy controls. Microneurography experiments do not provide information on the absolute number of C-fibers in a nerve. However, histological studies suggest that diabetic neuropathy leads to a decrease of unmyelinated nerve endings in the skin (Pittenger et al., 2004; Polydefkis et al., 2004; Smith et al., 2001; Sumner et al., 2003), and psychophysical studies have revealed elevated thermal thresholds in patients with diabetic neuropathy (Kramer et al., 2004a). From these findings, we may conclude that our present results most probably reflect a shift in the relative proportion of fiber subtypes on the background of a general loss of C-fibers.
Previous studies have shown that CM and CMi fibers have different functions. Whereas the CM fibers are mainly responsible for the temporal and spatial resolution of thermal pain and for the heat pain threshold (Tillman et al., 1995b), CMi fibers contribute mainly to the axon reflex flare and thus to neurogenic inflammation (Schmelz et al., 2000a; Schmelz and Petersen, 2001) and are also crucial for phenomena of primary and secondary hyperalgesia (Schmelz et al., 2000b). If the CMi fibers are less affected than CM fibers, this may explain hyperalgesia observed in early stages of diabetic neuropathy on the background of elevated thresholds for painful stimuli. However, the efferent function of the CMi fibers, which has been found to be impaired in diabetic neuropathy (Kramer et al., 2004b), was not tested in this study.
The conduction velocity of the normal CM fibers found in the patients was significantly slower than in the healthy controls, whereas the conduction velocity of the CMi fibers was unchanged. Our results fit well with a morphological study from sural nerve biopsies in patients with diabetic neuropathy, which has shown smaller axonal diameters of unmyelinated fibers (Malik et al., 2001). Axonal atrophy with thinning of the axons and thereby a decrease in the length constant could explain the decreased CV of CM fibers in the patients in our study. Similar reductions in the CV of afferent fibers were observed in patients with erythromelalgia and have been attributed to an early stage of small-fiber neuropathy (Ørstavik et al., 2003).
Neuropathy: receptive changes
The relative lack of normal mechano-responsive fibers in the patients was accompanied by a large group of C-fibers with a degree of activity-dependent slowing of conduction velocity in the range of CM fibers, which were unresponsive to mechanical and heat stimulation of their nerve terminals (Cxi fibers). These fibers were also unresponsive to sympathetic stimulation and not spontaneously active. In a few additional fibers, heat responses were preserved (Cdes), indicating several stages of sensory degeneration.
The pattern of activity-dependent slowing of the abnormal Cxi fibers was similar to CM fibers, but the relative slowing was less pronounced at higher stimulation frequencies (Fig. 4). Sympathetic fibers show a characteristic response with pronounced initial slowing and later partial reversal to a 3 min, 2 Hz stimulation (Campero et al., 2004), which is the opposite pattern seen in the Cxi fibers. The Cxi fibers can therefore most probably be classified as afferent units with impaired transduction mechanisms, although we cannot rule out that they originate from mechano-insensitive units, sympathetic fibers, or even from Aδ fibers, which have acquired conduction velocities in the C-fiber range attributable to demyelination. However, the sensory and axonal characteristics of these fibers would best fit with a “dying back” of CM fibers, resulting in loss of normal receptor function and the reduced number of regular CM fibers observed in the patients. This interpretation is also in accordance with the reduced epidermal nerve fiber density found in diabetic neuropathy patients (Smith et al., 2001; Sumner et al., 2003; Pittenger et al., 2004). The constant growth of the keratinocytes in the epidermis with a regeneration cycle of 28 d (Kennedy et al., 2005) forces the most superficially located C-fibers to constantly extend their endings. Skin biopsies from diabetic patients have shown that sensory axons sometimes terminate abruptly at the epidermal border (Fernyhough and Schmidt, 2002; Kennedy et al., 2005). This indicates that superficial epidermal innervation is more difficult to sustain than axons deep in the skin and is reflected by a reduction of functional CM fibers in our material.
Another aspect of axonal degeneration is sensitization of neighboring uninjured units (Ali et al., 1999; Wu et al., 2001; Ringkamp and Meyer, 2005). A higher production of nerve growth factor by non-neuronal cells and impaired uptake by injured fibers would result in local NGF concentrations, high enough to sensitize the surviving C fibers (Griffin, 2005). Indeed, in patients, we found CMi fibers with spontaneous activity or sensitization to mechanical stimulation, which could be linked to the lack of NGF uptake by dying back mechano-responsive units. However, these probably “hypertrophed” units were more frequent in DN patients without pain than in those with pain. Overall, their number was small. One may hypothesize that these signs of sensitization are more prominent in the early development of a diabetic neuropathy than in the later stages studied here.
Thermal thresholds, including the heat pain detection threshold (HPDT), are often elevated in patients with diabetic neuropathy, in some cases causing serious slowly healing burns (Morbach et al., 2004). Because heat pain thresholds in normal hairy skin are determined by heat-responsive CM nociceptors (CMH) (Torebjörk et al., 1984; Tillman et al., 1995a,b), the finding of a relative loss of CM fibers could explain the elevation of HPDT, also seen in our patients. Supporting this hypothesis, the CM/CMi ratio in the three patients with only slightly elevated or normal heat pain detection thresholds (Table 3, patients 3–5) was 10:11, whereas in the other five patients with elevated HPDT, the CM/CMi ratio was 9:28. The heat pain responses with elevated thresholds in the patients may be mediated by preserved heat responses in CMi fibers (CH), which have higher heat activation thresholds compared with mechano- and heat-responsive (CMH) nociceptors (Weidner et al., 1999).
Is neuropathic pain attributable to spontaneous activity in C-fibers?
Ectopic activity in C-nociceptors is one of the favorite theories for spontaneous pain in neuropathic pain patients (Woolf and Mannion, 1999). Recently, results from microneurography studies on patients with limb pain (one with diabetes) found several categories of pathological behavior in C-fibers, including double spikes and spontaneous discharges in afferent fibers (Bostock et al., 2005; Ochoa et al., 2005). In the present study, we found spontaneously active afferent fibers in patients, but we did not find significantly more of these in patients with pain compared with those without pain. However, a possible explanation might be that, with advanced dying back, some afferent fibers with ongoing activity may have been unexcitable at the stimulation site but intact at sites closer to the spinal cord. Additional studies in patients with newly developed painful neuropathy are therefore needed to clarify the importance of spontaneous activity in C-nociceptors for the development of a painful diabetic neuropathy.
Conclusions
We found a clear difference in the distribution of C-fibers in patients with diabetic neuropathy compared with healthy controls, with a decrease in the number of mechano-responsive nociceptors and the presence of probable degenerated fibers without responsiveness to mechanical or heat stimuli, indicating an extensive dying back of mechano-responsive nociceptors. A change in the distribution of C-nociceptors in the skin has never been shown and might be important for understanding the mechanisms responsible for the development of small-fiber neuropathy, especially in patients with diabetes.
Footnotes
This work was supported by a grant from the Norwegian Research Council (K.Ø.), Swedish Medical Research Council Grant K2005-04X-15300-01A (R.S., M.H., H.E.T.), German Research Foundation Grant HA 831/14-1 (B.N.), and a grant from the International Association for the Study of Pain.
References
- Ali Z, Ringkamp M, Hartke TV, Chien HF, Flavahan NA, Campbell JN, Meyer RA. Uninjured C-fiber nociceptors develop spontaneous activity and alpha-adrenergic sensitivity following L6 spinal nerve ligation in monkey. J Neurophysiol. 1999;81:455–466. doi: 10.1152/jn.1999.81.2.455. [DOI] [PubMed] [Google Scholar]
- Beck PW, Handwerker HO, Zimmermann M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Res. 1974;67:373–386. doi: 10.1016/0006-8993(74)90488-0. [DOI] [PubMed] [Google Scholar]
- Bostock H, Campero M, Serra J, Ochoa JL. Temperature-dependent double spikes in C-nociceptors of neuropathic pain patients. Brain. 2005;128:2154–2163. doi: 10.1093/brain/awh552. [DOI] [PubMed] [Google Scholar]
- Calcutt NA. Potential mechanisms of neuropathic pain in diabetes. Int Rev Neurobiol. 2002;50:205–228. doi: 10.1016/s0074-7742(02)50078-7. [DOI] [PubMed] [Google Scholar]
- Campero M, Serra J, Bostock H, Ochoa JL. Partial reversal of conduction slowing during repetitive stimulation of single sympathetic efferents in human skin. Acta Physiol Scand. 2004;182:305–311. doi: 10.1111/j.1365-201X.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in patients with polyneuropathy. J Neurol Sci. 1980;47:449–461. doi: 10.1016/0022-510x(80)90099-4. [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Schmidt RE. Neurofilaments in diabetic neuropathy. Int Rev Neurobiol. 2002;50:115–144. doi: 10.1016/s0074-7742(02)50075-1. [DOI] [PubMed] [Google Scholar]
- Goransson LG, Mellgren SI, Lindal S, Omdal R. The effect of age and gender on epidermal nerve fiber density. Neurology. 2004;62:774–777. doi: 10.1212/01.wnl.0000113732.41127.8f. [DOI] [PubMed] [Google Scholar]
- Griffin JW. The pathophysiology of painful peripheral neuropathies. In: Koltzenburg M, Scadding JW, editors. Neuropathic pain: bench to bedside. London: The Royal Society of Medicine; 2005. pp. 1–6. [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjörk HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972;84:164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hallin RG, Torebjörk HE. Afferent and efferent C units recorded from human skin nerves in situ. A preliminary report. Acta Soc Med Ups. 1970;75:277–281. [PubMed] [Google Scholar]
- Hilz MJ, Stemper B, Axelrod FB, Kolodny EH, Neundorfer B. Quantitative thermal perception testing in adults. J Clin Neurophysiol. 1999;16:462–471. doi: 10.1097/00004691-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb 3G, Polydefkis M, McArthur J. Pathology and quantitation of cuatenous innervation. In: Dyck PJ, Thomas PK, editors. Peripheral neuropathy. Philadelphia: Elsevier; 2005. pp. 869–895. [Google Scholar]
- Kramer HH, Rolke R, Bickel A, Birklein F. Thermal thresholds predict painfulness of diabetic neuropathies. Diabetes Care. 2004a;27:2386–2391. doi: 10.2337/diacare.27.10.2386. [DOI] [PubMed] [Google Scholar]
- Kramer HH, Schmelz M, Birklein F, Bickel A. Electrically stimulated axon reflexes are diminished in diabetic small fiber neuropathies. Diabetes. 2004b;53:769–774. doi: 10.2337/diabetes.53.3.769. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- Llewelyn JG, Gilbey SG, Thomas PK, King RH, Muddle JR, Watkins PJ. Sural nerve morphometry in diabetic autonomic and painful sensory neuropathy. A clinicopathological study. Brain. 1991;114:867–892. doi: 10.1093/brain/114.2.867. [DOI] [PubMed] [Google Scholar]
- Mackenzie RA, Phillips LH. Changes in peripheral and central nerve conduction with aging. Clin Exp Neurol. 1981;18:109–116. [PubMed] [Google Scholar]
- Malik RA, Veves A, Walker D, Siddique I, Lye RH, Schady W, Boulton AJ. Sural nerve fibre pathology in diabetic patients with mild neuropathy: relationship to pain, quantitative sensory testing and peripheral nerve electrophysiology. Acta Neuropathol. 2001;101:367–374. doi: 10.1007/s004010000287. [DOI] [PubMed] [Google Scholar]
- Morbach S, Lutale JK, Viswanathan V, Mollenberg J, Ochs HR, Rajashekar S, Ramachandran A, Abbas ZG. Regional differences in risk factors and clinical presentation of diabetic foot lesions. Diabet Med. 2004;21:91–95. doi: 10.1046/j.1464-5491.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- Ochoa JL, Campero M, Serra J, Bostock H. Hyperexcitable polymodal and insensitive nociceptors in painful human neuropathy. Muscle Nerve. 2005;32:459–472. doi: 10.1002/mus.20367. [DOI] [PubMed] [Google Scholar]
- O'Hare JA, Abuaisha F, Geoghegan M. Prevalence and forms of neuropathic morbidity in 800 diabetics. Ir J Med Sci. 1994;163:132–135. doi: 10.1007/BF02965972. [DOI] [PubMed] [Google Scholar]
- Ørstavik K, Weidner C, Schmidt R, Schmelz M, Hilliges M, Jørum E, Handwerker H, Torebjörk E. Pathological C-fibres in patients with a chronic painful condition. Brain. 2003;126:567–578. doi: 10.1093/brain/awg060. [DOI] [PubMed] [Google Scholar]
- Ørstavik K, Mørk C, Kvernebo K, Jørum E. Pain in primary erythromelalgia: a neuropathic component? Pain. 2004;110:531–538. doi: 10.1016/j.pain.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Otto M, Bak S, Bach FW, Jensen TS, Sindrup SH. Pain phenomena and possible mechanisms in patients with painful polyneuropathy. Pain. 2003;101:187–192. doi: 10.1016/s0304-3959(02)00313-5. [DOI] [PubMed] [Google Scholar]
- Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care. 2004;27:1974–1979. doi: 10.2337/diacare.27.8.1974. [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Meyer RA. Injured versus uninjured afferents: who is to blame for neuropathic pain? Anesthesiology. 2005;103:221–223. doi: 10.1097/00000542-200508000-00002. [DOI] [PubMed] [Google Scholar]
- Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Petersen LJ. Neurogenic inflammation in human and rodent skin. News Physiol Sci. 2001;16:33–37. doi: 10.1152/physiologyonline.2001.16.1.33. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Michael K, Weidner C, Schmidt R, Torebjörk HE, Handwerker HO. Which nerve fibers mediate the axon reflex flare in human skin? NeuroReport. 2000a;11:645–648. doi: 10.1097/00001756-200002280-00041. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Handwerker HO, Torebjörk HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000b;123:560–571. doi: 10.1093/brain/123.3.560. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjörk HE, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J Physiol (Lond) 1999;515:799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology. 2001;57:1701–1704. doi: 10.1212/wnl.57.9.1701. [DOI] [PubMed] [Google Scholar]
- Sommer C. Painful neuropathies. Curr Opin Neurol. 2003;16:623–628. doi: 10.1097/01.wco.0000093106.34793.06. [DOI] [PubMed] [Google Scholar]
- Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–111. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- Tillman DB, Treede RD, Meyer RA, Campbell JN. Response of C fibre nociceptors in the anaesthetized monkey to heat stimuli: estimates of receptor depth and threshold. J Physiol (Lond) 1995a;485:753–765. doi: 10.1113/jphysiol.1995.sp020766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman DB, Treede RD, Meyer RA, Campbell JN. Response of C fibre nociceptors in the anaesthetized monkey to heat stimuli: correlation with pain threshold in humans. J Physiol (Lond) 1995b;485:767–774. doi: 10.1113/jphysiol.1995.sp020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torebjörk HE. Afferent C units responding to mechanical, thermal and chemical stimuli in human non-glabrous skin. Acta Physiol Scand. 1974;92:374–390. doi: 10.1111/j.1748-1716.1974.tb05755.x. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, Hallin RG. Identification of afferent C units in intact human skin nerves. Brain Res. 1974;67:387–403. doi: 10.1016/0006-8993(74)90489-2. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, LaMotte RH, Robinson CJ. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: simultaneous recordings in humans of sensory judgments of pain and evoked responses in nociceptors with C-fibers. J Neurophysiol. 1984;51:325–339. doi: 10.1152/jn.1984.51.2.325. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol. 1968;21:270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Vinik AI, Erbas T, Stansberry K, Pittenger GL. Small fiber neuropathy and neurovascular disturbances in diabetes mellitus. Exp Clin Endocrinol Diabetes. 2001;109:S451–S473. doi: 10.1055/s-2001-18602. [DOI] [PubMed] [Google Scholar]
- Vrethem M, Boivie J, Arnqvist H, Holmgren H, Lindstrom T. Painful polyneuropathy in patients with and without diabetes: clinical, neurophysiologic, and quantitative sensory characteristics. Clin J Pain. 2002;18:122–127. doi: 10.1097/00002508-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker H, Torebjörk E. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci. 1999;19:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21(RC140):1–5. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]