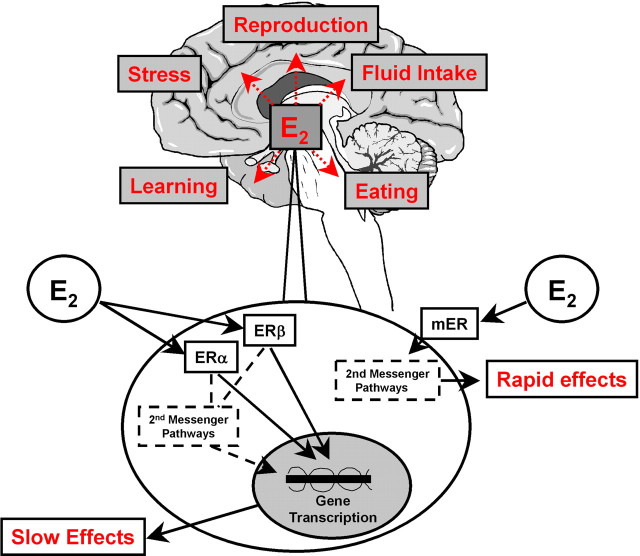
The actions of gonadal steroid hormones in the brain produce myriad physiological and behavioral effects. One of the most potent gonadal steroids is estradiol (E2), which, in addition to positive and negative feedback effects on gonadotropin-releasing hormone release, affects sexual and social behavior, food intake, locomotor activity, and many other brain functions. The site(s) and the estrogen receptor (ER) species involved in most of these effects remain unknown. The vast majority of investigations have focused on the classical nuclear ERs, which are now known to have at least two subtypes, ERα and ERβ. Both ERα and ERβ are present in the cytosol, membrane, or other cellular compartments and, after binding E2, initiate gene transcription by either nuclear translocation or activating second-messenger pathways (Fig. 1). (Lagrange et al. 1997) identified a different, membrane-associated ER (mER) on hypothalamic neural membranes. Binding of E2 to this mER results in increases in cAMP levels and rapid activation of phospholipase C/protein kinase C/protein kinase A (PLC/PKC/PKA) pathways that can immediately desensitize μ-opioid and GABAB receptors. In a recent issue of The Journal of Neuroscience, (Qiu et al., 2006) reported exciting additional investigations of this mER.
Figure 1.
Schematic of intracellular mechanisms underlying E2 effects on sexual and social behavior, energy homeostasis, and many other brain-mediated functions. Most of these functions occur as a result of gene transcription elicited by binding of E2 to the classic ERα and ERβ. The newly characterized mER (Qiu et al., 2006) that seems to mediate some of the effects of E2 on energy homeostasis opens new possibilities in the investigation of the role of E2 in normal physiology.
The new results are based mainly on studies of the newly developed compound STX, a novel ER antagonist that does not bind to ERα or ERβ. Remarkably, ∼75% of neurons in the hypothalamic arcuate nucleus, including all of the pro-opiomelanocortin (POMC)-expressing neurons, were found to be ∼40% less sensitive to the GABAB agonist baclofen after STX treatment [(Qiu et al., 2006), their Fig. 2 (http://www.jneurosci.org/cgi/content/full/26/21/5649/F2)]. The initial experiments in female guinea pigs were replicated in female ERα and ERβ knock-out (KO) mice and male ERαβ KO mice, indicating that these electrophysiological responses do not require classical ER, occur in other species, and occur in both sexes [(Qiu et al., 2006), their Fig. 3 (http://www.jneurosci.org/cgi/content/full/26/21/5649/F3)]. Additional pharmacological tests indicated that this response is mediated by a Gαq-coupled PLC/PKC/PKA pathway. These data open up exciting new frontiers in the molecular pharmacology of the arcuate nucleus and E2 in general. Finally, the finding that STX potently reduced the excess body weight gain of ovariectomized guinea pigs links the in vitro findings to in vivo, physiological functions [(Qiu et al., 2006), their Fig. 4a,b (http://www.jneurosci.org/cgi/content/full/26/21/5649/F4)]. The authors suggest that E2 is likely to inhibit feeding via mER-mediated maintenance of normal excitability of POMC, in particular, melanocortinergic, neurons in the arcuate nucleus, which are considered to be critical elements of the neural network controlling feeding.
Because estradiol appears to play a physiological role in the regulation of eating, energy homeostasis, and adiposity in both animals and women (Geary, 2004; Asarian and Geary, 2006), this functional conclusion deserves close scrutiny. Several issues require additional evaluation. First, of course, it will be important in future work to measure food intake directly, not just body weight. Although the effect of E2 on energy homeostasis is mediated mainly by changes in food intake, effects on physical activity and metabolic energy expenditure can also be important, especially in rodents. Second, because STX was given systemically in the energy balance tests, the site of action is uncertain. (Qiu et al., 2006) cite (Butera and Cjaza 1984) to support the idea that the anorexigenic effects of E2 originate in the arcuate/ventromedial hypothalamus; in fact, those authors found that paraventricular nucleus E2 administration was more effective than arcuate administration. The same is true in rats (for review, see Geary, 2004). Moreover, since that time, many investigators have shown that implantation of pure E2, as done by (Butera and Cjaza 1984), does not lead to a localized action of the hormone (for review, see Geary, 2004).
A third critical issue regards the involvement of ERα in body weight regulation: E2 treatment does not normalize food intake or body weight gain in ERα KO mice (Geary et al., 2001), indicating that ERα is necessary for this effect of E2. This, of course, does not eliminate the possibility that mER are also involved but does indicate that the relative contributions of the E2 receptors require additional work. A fourth and related point concerns the latency of the effect of E2 on feeding in ovariectomized animals, which is normally over 24 h. For example, the subcutaneous injection of a physiological dose of E2 (2 μg) in ovariectomized rats does not affect food intake for over 1 d (Asarian and Geary, 2002). This long latency seems inconsistent with the hypothesis that immediate mER signaling in the arcuate mediates the effect of E2 on feeding. It is possible, of course, that other, slower effects of mER activation do contribute.
Finally, the hypothesis that hypothalamic melanocortinergic neurons are involved in the feeding-inhibitory effect of E2 also deserves additional testing in light of several apparently inconsistent reports. For example, E2 treatment failed to affect the feeding response to intracerebroventricular injections of either the melanocortin 3/4 receptor agonist melanotan II or the melanocortin 3/4 receptor antagonists SHU9119 [acetyl-(Nle4,Asp5,D-2-Nal7,Lys10)-cyclo-α-MSH(4:10)amide] and agouti-related protein (for review, see Asarian and Geary, 2006). If E2 were to act, as hypothesized, by affecting the tone of hypothalamic melanocortinergic neurons, one would predict that E2 would indirectly affect the response of their postsynaptic neurons to melanocortin 3/4 agonists or antagonists. Furthermore, the feeding-inhibitory effect of leptin, which directly activates arcuate melanocortinergic neurons, is also not affected by E2 treatment in ovariectomized rats (for review, see Geary, 2004; Asarian and Geary, 2006).
Footnotes
Editor's Note: These short reviews of a recent paper in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to mimic the journal clubs that exist in your own departments or institutions. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
References
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera PC, Cjaza JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;19:41–48. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- Geary N. The estrogenic inhibition of eating. In: Stricker EM, Woods SC, editors. Handbook of behavioral neurobiology, Vol 14, Neurobiology of food and fluid intake. Ed 2. New York: Kluwer Academic/Plenum; 2004. pp. 307–345. [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of G-protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlon TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]