Abstract
Recent evidence suggests that neurons in the medullary raphe are critical to the activation of brown adipose tissue (BAT), the major source of nonshivering heat production in the rat. Yet it is unclear which medullary raphe cells participate in cold defense and how participating cells contribute to BAT activation. Therefore, we recorded extracellularly from raphe cells during three thermoregulatory challenges that evoked an increase in BAT temperature in anesthetized rats: central cold, ambient cold, or intracerebroventricular prostaglandin E2 (PGE2) injection. Physiologically identified serotonergic (p5HT) cell discharge increased in response to cold or PGE2 administration and was positively correlated with BAT temperature. However, none of the 147 physiologically identified non-serotonergic (non-p5HT) cells recorded responded to thermoregulatory challenges that evoked an increase in BAT temperature. To test for modulation of BAT activation by non-p5HT cells that are either excited (ON cells) or inhibited (OFF cells) by noxious cutaneous stimulation, noxious stimuli were applied during evoked BAT temperature increases. Noxious stimulation suppressed BAT activation, suggesting that cells inhibited by noxious stimulation facilitate spinal circuits controlling BAT. To test whether medullary OFF cells modulate BAT activity, the μ-opiate receptor agonist (d-Ala2, N-Me-Phe4, Gly-ol5)-enkephalin (DAMGO) was microinjected into the raphe magnus, a manipulation that selectively activates OFF cells. DAMGO microinjection blocked noxious stimulation-evoked suppression of PGE2-induced BAT temperature increases. Thus, both p5HT and non-p5HT OFF cells in the medullary raphe facilitate BAT activation in response to cold challenge or pyrogen.
Keywords: ventromedial medulla, autonomic modulation, nociceptive modulation, raphe pallidus, raphe magnus, thermoregulation
Introduction
Recent evidence from a number of different laboratories suggests that the medullary raphe is critical to heat production during cold defense and fever. Placing rats in a cold environment results in c-fos-labeled cells scattered throughout the raphe magnus (RM), raphe pallidus (RP), and the adjacent reticular nuclei (Morrison, 1999; Morrison et al., 1999; Martinez et al., 2001; Cano et al., 2003). Bicuculline microinjection into areas throughout the ventral medulla including the RP, RM, and the lateral reticular nuclei activates brown adipose tissue (BAT) (Morrison et al., 1999; Nason and Mason, 2004). Medullary raphe inactivation blocks BAT activation (Morrison, 2001, 2003; Nakamura et al., 2002). RM–RP neurons project to the intermediolateral cell column, where targeted cells include preganglionic sympathetic neurons, as well as to the superficial dorsal horn, where thermoreceptors terminate (Henry and Calaresu, 1974; Basbaum et al., 1978; Holstege and Kuypers, 1982; Bacon et al., 1990). Furthermore, RM–RP neurons project oligosynaptically to BAT tissue (Cano et al., 2003).
Although strongly supportive of a role for the medullary raphe in the control of BAT activation, available studies lack the temporal resolution critical for understanding the role of raphe in BAT activation. Therefore, we recorded from individual cells during physiological thermoregulatory challenges that elicited increases in BAT temperature. Here, we report that the discharge of physiologically identified serotonergic (p5HT) cells in the medullary raphe increases during evoked BAT temperature rises.
Because non-serotonergic cells target BAT-controlling sympathetic neurons in greater proportions than do serotonergic cells (Nakamura et al., 2004), it was surprising that none of the 147 cells physiologically identified as non-serotonergic (non-p5HT) were activated by thermoregulatory challenges. To test for an involvement of non-serotonergic cells in modulating BAT-controlling circuits, we took advantage of the fact that most non-serotonergic cells respond to noxious cutaneous stimulation (Leung and Mason, 1998). Non-serotonergic medullary raphe cells that respond to noxious stimulation have been well characterized physiologically and are critical to nociceptive modulation (Fields et al., 1991; Leung and Mason, 1998) but have been implicated in other physiological functions as well (Mason, 2005a). ON cells are excited by noxious cutaneous stimulation, inhibited by opioids, and facilitate nociceptive transmission, whereas OFF cells are inhibited by noxious cutaneous stimulation, excited by opioids, and suppress nociceptive transmission (Fields et al., 1991; Porreca et al., 2002). If ON cells facilitate BAT-controlling circuits, then noxious stimulation will enhance BAT activation, whereas if OFF cells do so, noxious stimulation will attenuate BAT activation. Here, we report that noxious stimulation interrupts increases in BAT temperature evoked by cold or prostaglandin E2 (PGE2) administration. To directly test for the involvement of OFF cells in modulating BAT-controlling circuits, (d-Ala2, N-Me-Phe4, Gly-ol5)-enkephalin (DAMGO), a μ-opioid receptor agonist that selectively excites OFF cells (Heinricher et al., 1994), was microinjected into the RM during a PGE2-evoked BAT temperature increase. We found that DAMGO microinjection attenuated the noxious stimulus-evoked inhibition of the BAT temperature rise, demonstrating that raphe OFF cells facilitate BAT activation.
Materials and Methods
Surgical preparation.
The methods for all experiments were approved by the University of Chicago Institutional Animal Care and Use Committee and conformed to the guidelines of the National Institutes of Health and the International Association for the Study of Pain.
The surgical preparation used is described in detail in the study by Nason and Mason (2004). Briefly, male Sprague Dawley rats (250–450 g; n = 129; Charles River, Portage, MI) were anesthetized with halothane, placed on a heated (36°C) water pad, and covered with a plastic blanket. A Y-tube was inserted into the trachea, and 2% halothane was administered during surgery. Needle electrodes (Grass Instruments, Warwick, RI) were inserted into multiple muscles of the left thigh and right forearm to record electromyograms (EMGs) from paw withdrawals. A thermistor (Thermometrics, Edison, NJ) with a time constant of 90 ms was inserted into the interscapular BAT pad. Core temperature, blood pressure, electrocardiogram, and expired [CO2] were monitored throughout the experiment. A craniotomy allowed for insertion of a tungsten recording electrode (0.01 inch; A-M Systems, Carlsborg, WA) or microinjection pipette. Peltier thermodes (2 × 2 cm) were placed on the ventrum of the left hindfoot and right forefoot, including the palm and all digits. Paws were affixed with Velcro straps. Animals were allowed to equilibrate for ≥1 h at 1% halothane after surgery.
Hypothalamic temperature changes.
A water-perfused hypothalamic heating and cooling probe constructed from steel tubing [outer diameter, 0.7 mm; Small Parts, Miami Lakes, FL) was placed in the preoptic/anterior hypothalamus (relative to bregma: −1.0 anteroposterior; 0.5 lateral; 8.0 dorsoventral). After a successful experiment, histology confirmed placement of the probe in the medial preoptic area (MPO) of the anterior hypothalamus. A thermocouple (Omega Engineering, Stamford, CT) was affixed to the outside of the probe tip, and a reference thermistor (Thermometrics) was epoxied to a hollow steel tube with outer diameter of 0.6 mm. The reference thermistor was placed 1–1.5 mm anterior and slightly dorsal to the probe. The reference temperature was linearly related to the thermode temperature with a slope <1.0 and a crossing point at 35.7°C. Therefore, reference temperatures were buffered toward 35.7°C, so that they were less than thermode temperatures elevated above 35.7°C and relatively greater than thermode temperatures cooled below 35.7°C.
After baseline cell recordings, hypothalamic temperature was changed to either 35°C or 39°C. The direction (hypothermia or hyperthermia) and length (5 or 15 min) of temperature changes were randomized.
Ambient temperature changes.
Ambient cold changes were achieved by blowing room air through crushed dry ice contained in a fenestrated plastic bottle. The air stream was ∼4°C when tested 2 cm from the end of the bottle and was judged cold, but not noxiously so, when directed at the experimenter’s inner wrist at a distance of 10–15 cm. Air was typically directed at the animal’s right hip where surface temperature was monitored. The stimulus temperature averaged 16.9 ± 1.3°C and was applied for an average duration of 684 ± 72 s, sufficient to elevate BAT temperature.
PGE2 administration.
PGE2 is a pyrogen released by venous endothelial tissue in response to cytokines as part of the immunological response to pathogens (Yamagata et al., 2001). PGE2 (4–8 ng, 50–100 nl; Sigma, St. Louis, MO) dissolved in sterile saline was inserted into the lateral ventricle. Subsequent injections of PGE2 were not administered until after BAT temperature returned to baseline levels or had remained constant for at least 60 min. The change in BAT temperature evoked by a second administration of PGE2 was the same as that evoked by the first application (p = 0.20).
Cell recording protocol during thermoregulatory challenges.
Neurons with spontaneous activity were isolated, and each extracellular unit that was successfully isolated was tested. Cell data were acquired at 10–40 kHz using Spike2 acquisition software (CED, Cambridge, UK) to digitize 20–36 points from the waveform. Individual waveforms were discriminated off-line using template-matching software. In several experiments, multiple cells were recorded simultaneously. After 500–900 s of baseline cellular activity, several trials of noxious thermal stimulation (3 s ramp from 32°C to a plateau of 56°C for 3 s) were applied at 5 min intervals. After baseline measurements, one of three thermoregulatory challenges was applied. Trials of noxious thermal stimuli then continued as before. After recording from the final cell, anodal current was passed (20 μA for 4 min), marking the site of the last recording. The locations of previously recorded cells were reconstructed by their relative distance from this burned location.
Protocol for noxious stimulation-evoked BAT inhibition experiments.
After application of a cold challenge or PGE2 administration, the experimenter waited until BAT temperature rose steadily for 45–150 s. The forepaw was then heated to 46.5°C. The forepaw temperature was increased by increments of 2°C until BAT temperature stopped increasing or until the forepaw reached a maximum temperature of 52.5°C. Typically, a forepaw temperature of 49–50°C was required to stop BAT temperature increases. The period of forepaw heating before the reversal of the BAT temperature slope was termed the Noxious I period. The period of forepaw heating after this inflection point was termed the Noxious II period. The Noxious I and II periods (see Fig. 5) had average durations of 49 ± 5 and 35 ± 3 s, and the average stimulus temperatures during these periods were 47.8 ± 0.4 and 49.6 ± 0.4°C, respectively.
Figure 5.
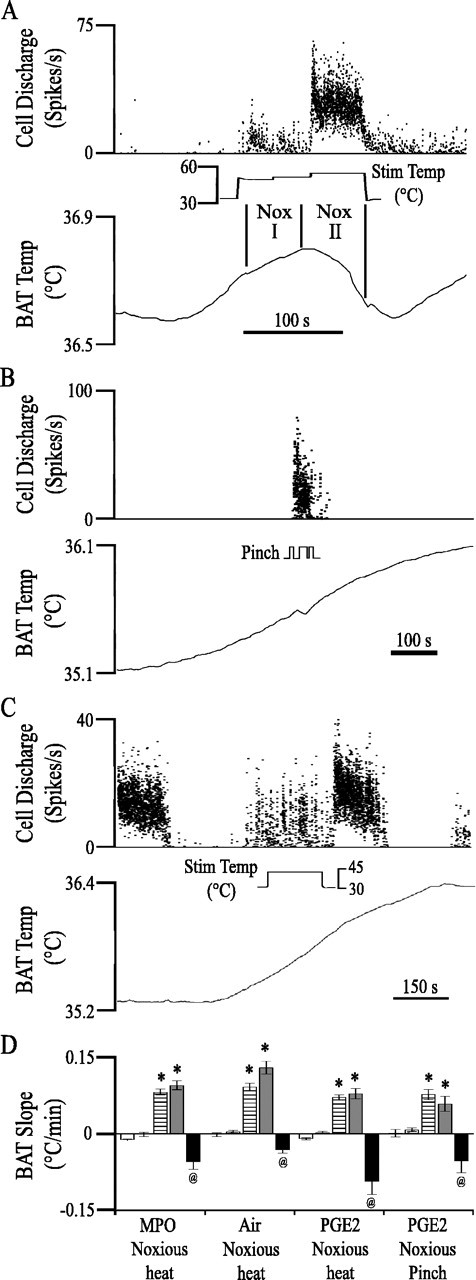
Noxious stimulation blocks BAT temperature rise. A, The BAT temperature increase elicited by cold air challenge (onset was 197 s before beginning of the traces) reversed direction and began to decrease in response to long-duration noxious thermal stimulation of the forepaw (inset). An RM ON cell was excited by noxious forepaw stimulation. The timing of the Noxious I (Nox I) and Noxious II (Nox II) periods are labeled in the inset. B, Noxious pinch reversed the BAT temperature slope and excited an RM ON cell. C, Warm stimulation (inset) of the forepaw had no effect on either BAT temperature slope or ON cell firing. D, BAT temperature slope during the four periods described above as well as during the “baseline” period (5 min before stimulation). In each of the four groupings, the bars represent sequentially baseline, post-challenge and pre-BAT, BAT plus 3 min, Noxious I, and Noxious II periods (as described in Materials and Methods). Statistically significant differences from baseline are marked by an asterisk. Differences from both baseline and BAT plus 3 min values are marked by the @ symbol. Error bars indicate SEM. Stim, Stimulation; Temp, temperature.
The above protocol was also performed with noxious cutaneous pinch of the forepaw or ear pinna substituting for the thermal stimulation of the forepaw. Skin on the dorsal aspect of the forepaw or ear pinna was pinched using serrated forceps. Pinches were applied over a 20–30 s period with 3–5 s of stimulation alternating with 3–5 s of no stimulation to prevent tissue damage, until BAT temperature decreased or stayed at a steady level. An effective noxious pinch of the ear pinna was ensured by using stimulation that elicited movements of the scalp. Noxious pinch of the forepaw always elicited forepaw withdrawal movements.
Spontaneous BAT temperature rises.
BAT temperature sometimes (n = 35) increased without an obvious precipitating stimulus. Such “spontaneous” rises occurred during experiments using all three paradigms: central cold (n = 13), ambient cold (n = 13), and PGE2 (n = 9). In four of nine PGE2 cases, a previous injection of PGE2 occurred, on average, 171 min before the spontaneous rise in BAT temperature.
Protocol for DAMGO modulation of noxious stimulation-evoked BAT inhibition.
BAT temperature increases were induced by PGE2 administration, and noxious stimulation inhibition experiments were performed as above to form a baseline response pattern for each animal (n = 25). After BAT temperature had returned to pre-injection levels, or had remained stable for at least 1 h, a second application of PGE2 was administered. Four minutes after BAT temperature began to rise, either 250 nl of sterile saline or 30 ng of the μ-opiate receptor agonist DAMGO (Mulitple Peptide Systems, San Diego, CA) dissolved in 250 nl of sterile saline was microinjected into the RM (Hurley et al., 2003). Fluorescent beads (0.1 μm; FluoSpheres; Molecular Probes, Eugene, OR) were coinjected to mark the microinjection location. Two minutes after DAMGO microinjection, noxious stimulation was applied.
Data collection.
Data were collected onto a Pentium IV personal computer using a Power 1401 A/D converter (CED) and analyzed using Spike2 4.0 (CED). Halothane concentration and expired [CO2] were monitored by a Datex Capnomac (Tewksbury, MA). EMG signals were collected at 1.2 kHz, whereas temperature (stimulus, core, and BAT) and [CO2] were collected at 100 Hz.
Animals were overdosed with halothane, perfused transcardially with PBS, followed by 10% formalin, and immersed in 30% sucrose in 0.1 m PBS until equilibration. Brains were sectioned (50 μ m) in the coronal plane, and marked injection sites were visualized and plotted on standard sections. The raphe nuclei were considered to include a region 400 μm wide centered on the midline. A small region between the pyramids with densely packed small cells was considered to be the RP. The RM was considered to be the raphe region stretching from the dorsal boundary of the RP to a point 1.5 mm dorsal to the ventral surface.
Cellular classification.
For all cells recorded, the mean (x), SD (SDISI), and coefficient of variation (CVISI) of the interspike interval were calculated from a 15 min record of background spontaneous activity. The function y(x, SDISI) = 146 − x + 0.98 SDISI was used to physiologically classify cells as serotonergic or non-serotonergic on the basis of the rate and variability of the background cellular discharge (Mason, 1997). Cells with a function value <0 were classified as p5HT, and cells with a function value >0 were classified as non-p5HT. The accuracy of this classification system is >90% (Mason, 2001).
Cells were also characterized by their responses to acute noxious thermal stimulation of the hindpaw using a quantitative method that was described in detail previously (Leung and Mason, 1998). Briefly, the variability in background discharge was first quantified as the SD of change across 10 s bins normalized to impulses per second (SD10 s). Then the response to noxious paw heat was calculated as the difference in spikes after the stimulus (2–12 s after stimulus onset) relative to the baseline period (10 s before stimulus onset). Heat-evoked decreases in discharge that were >2 ×SD10 s (the “threshold”) were considered inhibitory responses, and heat-evoked increases in discharge that were >2 ×SD10 s were considered excitatory responses. This method provides confidence at the p < 0.05 level that the response evoked by a stimulus on any single trial is unlikely to have occurred spontaneously. The percentage of inhibitory and excitatory responses to hindpaw heat stimulations were calculated for each cell. Cells inhibited by a majority of heat applications were considered OFF cells, whereas cells excited by a majority of heat applications were considered ON cells. Cells without a majority of either inhibitory or excitatory responses were considered NEUTRAL cells.
Thermoregulatory classification.
To characterize cellular reactions to thermoregulatory challenges, several criteria were added to those outlined above: (1) the response differed by more than the threshold value for at least seven of nine consecutive bins; (2) changed bins were consistent in valence; (3) changed bins occurred after or during the thermoregulatory stimulus and previous to or concurrent with the increase in BAT temperature; and (4) changes observed were consistent across multiple trials (each cell was tested with one to four thermoregulatory challenges).
Serotonergic cell correlations.
p5HT cell discharge was compared with arrays of BAT, hypothalamic, and/or ambient temperature when available and appropriate. To compare neuronal discharge with temperatures that were sampled at 50 samples/s, we converted the discharge data into an array containing 50 samples/s. This was achieved by rounding the time of each spike to the nearest 1/50th of a second, and constructing an array of ones and zeroes, with the ones marking when a spike occurred. Cross-correlations were then performed for each pair of arrays (discharge vs BAT, hypothalamic, or ambient temperature).
To determine the significance of each correlation, the interspike intervals present in the cell array were shuffled randomly 10 times, and the correlation between each random pattern and the temperature record was calculated. The average and SD of all random correlations at each point were computed. If the true correlation differed from the mean random correlation by more than 3 SDs, it was considered significantly different. All correlational analyses were performed using IgorPro (WaveMetrics, Lake Oswego, OR).
Drug effects were compared using two-way, repeated-measures ANOVAs and the Student–Newman–Keuls post hoc test. A p ≤ 0.05 was considered significant. Statistical analyses were completed using Excel 9.0 (Microsoft, Redmond, WA) and SigmaStat 2.0 (SPSS, Chicago, IL).
Results
Each of the thermoregulatory challenges, central cold (n = 53), ambient cold (n = 41), and PGE2 administration (n = 35), successfully evoked increases in BAT temperature. The average slope of BAT temperature increase was not different across the three challenges (range, 0.07–0.09°C/min).
p5HT cell discharge during BAT temperature rises
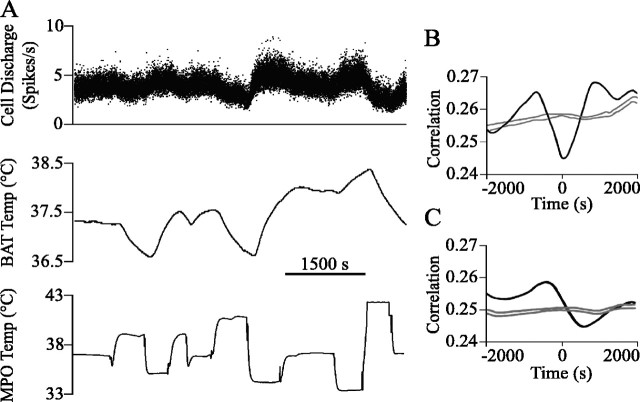
The discharge of seven p5HT raphe cells was recorded in response to central cold (n = 4), ambient cold (n = 1), or PGE2 administration (n = 2). Six of the seven p5HT cells discharged either in relation to BAT temperature (four of five; two experiments had no recorded BAT temperature) or hypothalamic or cold air temperature (four of four; three experiments had no recorded thermal challenge temperature), or both (n = 2; Fig. 1). Neither thermoregulatory challenge nor noxious stimulation significantly altered the firing pattern of the final p5HT cell (Fig. 2A, circle). Figure 1 shows a p5HT cell, the discharge rate of which increased when hypothalamic temperature was decreased and decreased when hypothalamic temperature was increased. The cross-correlation in Figure 1B shows that the discharge of this p5HT cell was inversely related to hypothalamic temperature, following hypothalamic temperature changes at a lag of tens of seconds. The discharge of this cell was directly related to BAT temperature with cell firing preceding BAT temperature changes by hundreds of seconds (Fig. 1C). p5HT cells with discharge correlated to BAT and/or challenge temperature were located in the RP (n = 3) or the ventral part of the RM (n = 3; Fig. 2A).
Figure 1.
The discharge of a p5HT cell (the cell marked by the arrow in Fig. 2A) was inversely correlated to hypothalamic temperature. A, The cell firing rate and BAT temperature (Temp) increased after hypothalamic temperature (MPO) was decreased and decreased after increases in hypothalamic temperature. B, The discharge of this cell was inversely correlated with hypothalamic temperature and lagged behind the hypothalamic temperature change by 44 s. The trough of this correlation was significantly different from the average correlation value for 10 randomized spike trains (see Materials and Methods). The gray lines in B and C show the mean correlation for 10 randomized spike trains ± 3 SDs. C, The discharge of this cell was positively correlated with BAT temperature, and changes in cell discharge preceded BAT temperature changes by 361 s. The peak of this correlation was significantly different from the average correlation value for 10 randomized spike trains.
Figure 2.
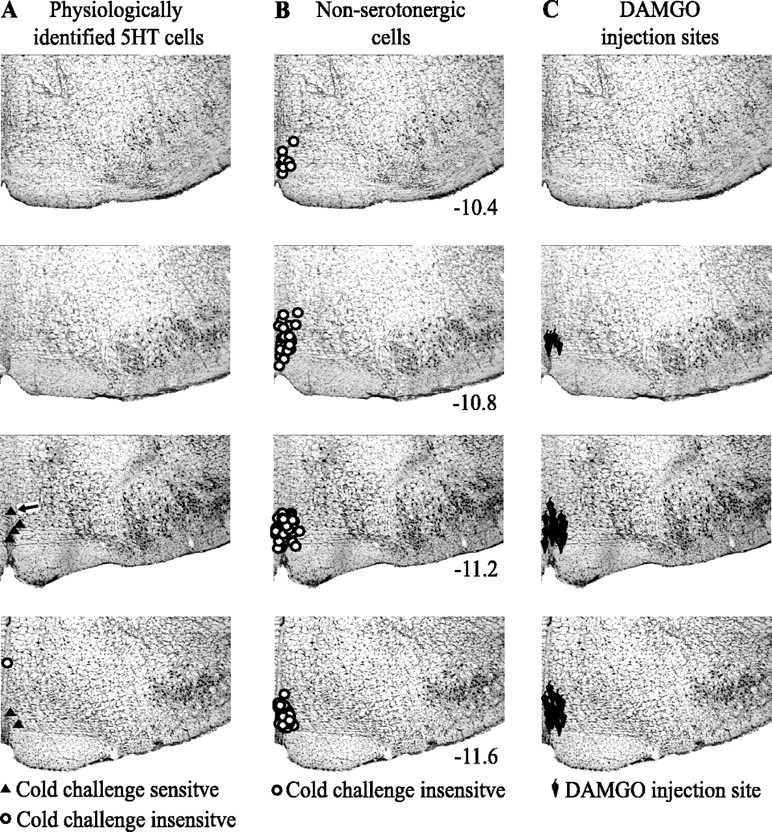
Locations of recorded cells and sites of microinjections. A, p5HT cells with discharge correlated to BAT or challenge temperature are represented by filled triangles. The single p5HT cell without correlated activity is represented by an open circle. B, Non-p5HT cells. C, Microinjection sites.
Non-p5HT cell discharge during BAT temperature rises
Non-p5HT raphe cells (n = 147) were recorded during central cold (n = 69), ambient cold (n = 36), or after PGE2 administration (n = 40). The number of challenges (n = 145) is less than the number of cells because two cells were recorded simultaneously in two instances. In addition, 39 of the 147 raphe cells were recorded during a spontaneous rise in BAT temperature. We did not observe any non-p5HT cells, in either the RP (n = 25) or the RM (n = 122), with consistent discharge alterations just preceding and/or during BAT temperature rises (Fig. 2B).
The discharge rate of OFF cells (n = 25) was not significantly changed by central cold (n = 9), ambient cold (n = 6), or PGE2 administration (n = 10) that evoked an increase in BAT temperature. Figure 3 shows OFF cell discharge that dramatically decreased in frequency in response to noxious paw heat (arrowheads below trace) but did not change in relation to either hypothalamic thermode or BAT temperature. Figure 4A shows mean discharge rates for all OFF cells in response to thermoregulatory challenges. The discharge rate of ON cells (n = 54) was also not significantly changed by central cold (n = 23), ambient cold (n = 13), or PGE2 administration (n = 18) that evoked an increase in BAT temperature (Fig. 4B). Finally, the discharge of NEUTRAL cells (n = 68) was not significantly changed by central cold (n = 26), ambient cold (n = 31), or PGE2 administration (n = 11) that evoked an increase in BAT temperature (Fig. 4C).
Figure 3.
A non-p5HT RM OFF cell that was inhibited by noxious stimulation (arrowheads under top trace) but not affected by a decrease in hypothalamic temperature (Temp) that evoked an increase in BAT temperature is shown.
Figure 4.
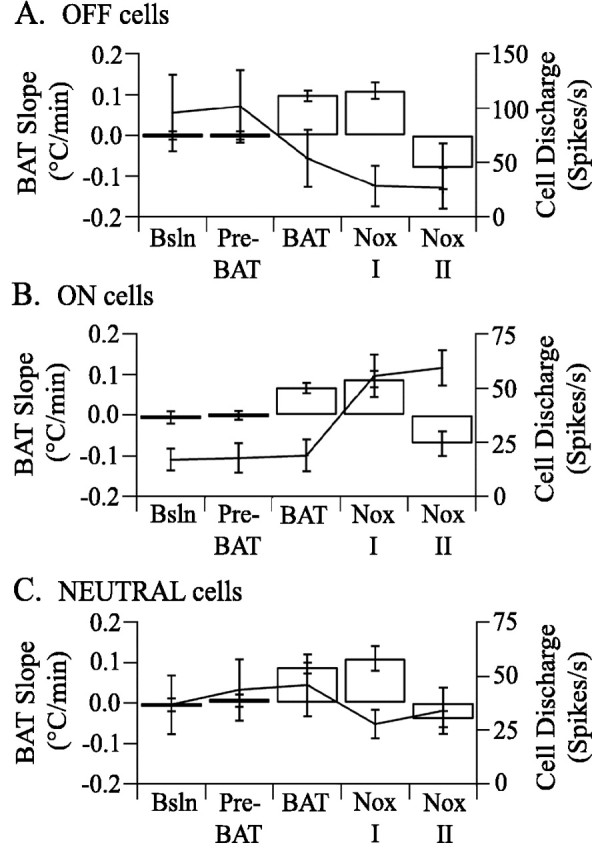
Experimental changes in BAT temperature do not correlate with changes in medullary raphe cellular discharge. Each panel shows BAT temperature slopes in open columns and non-p5HT raphe cell discharge as points joined by a line for five experimental periods (see Materials and Methods or Fig. 6 legend). A, OFF cell discharge was not significantly affected by thermoregulatory challenges but was inhibited by noxious stimulation. B, ON cell discharge was not significantly affected by thermoregulatory challenges but was excited by noxious stimulation. C, NEUTRAL cell discharge was not consistently affected by thermoregulatory challenges or by noxious stimulation. Error bars indicate SEM. Bsln, Baseline; Nox I and II, Noxious I and II.
Inhibition of BAT temperature increases by noxious stimulation
Given the accumulated evidence that non-serotonergic raphe cells are a critical relay for BAT activation (see Introduction), we were surprised that no non-p5HT cells were excited by cold challenge or pyrogen administration. Therefore, we hypothesized that non-serotonergic cells powerfully modulate BAT-controlling circuits rather than drive these circuits. To test this hypothesis, we examined the effect of sustained noxious stimulation, which elicits responses in most non-serotonergic cells (Leung and Mason, 1998), on BAT temperature increases.
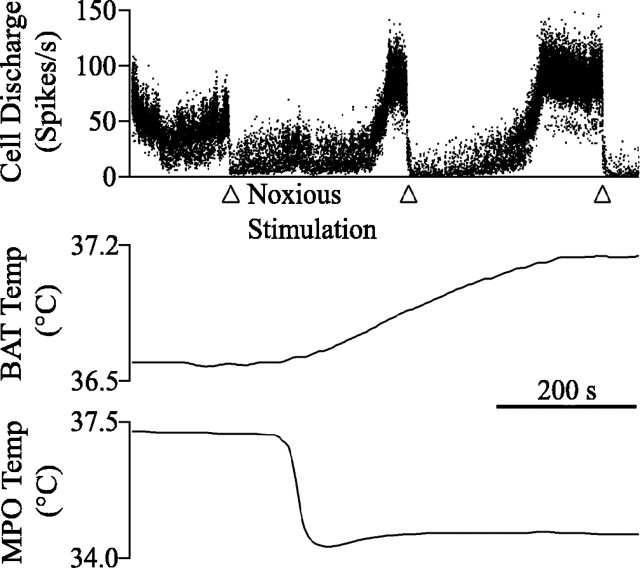
Noxious heating of the forepaw inhibited BAT temperature increases evoked by central cold, ambient cold, or central PGE2 administration. Before forepaw stimulation, BAT temperature increased at an average rate of 0.072–0.093°C/min and continued to increase at similar rates during the Noxious I period (Fig. 5). During the Noxious II period, the BAT temperature slope reversed direction and began to decrease, regardless of the stimulus used to elicit the initial BAT temperature rise (Fig. 5) (Kruskal-Wallis one-way ANOVA on ranks/Dunn’s method).
To test whether sustained noxious heat antagonized the increase in BAT temperature or simply evoked a large decrease that was added to the increase, the effect of noxious forepaw stimulation was tested on unstimulated BAT temperatures at rest (data not shown). In seven experiments, the forepaw was heated to an average temperature of 49.6 ± 0.4°C and was stimulated for an average total of 74 ± 8 s. These values were not different from the intensity (49.6 ± 0.4°C) and duration (84 ± 6 s) of noxious heat applications applied during BAT temperature increases. When BAT temperature was steady, forepaw stimulation had no effect on either BAT temperature or its rate of change.
To determine whether noxious forepaw heating antagonized BAT temperature increases because it provided a thermal or a noxious input, two experiments were done. First, in 14 experiments, we applied a noxious pinch to the ear (Fig. 5B). Noxious pinch significantly decreased the rate of BAT temperature rise from 0.115 ± 0.023°C/min to −0.016 ± 0.031°C/min (p < 0.001; n = 14). This demonstrates that a noxious mechanical stimulus blocked the BAT temperature rise. Second, in six experiments, an innocuous warm stimulus (40.0°C) was applied to the forepaw for an average of 174 ± 38 s, more than twice the average duration of the noxious thermal stimulus. Despite its long duration, warming had no effect on BAT temperature slope (Fig. 5C), nor did it change the discharge of five concurrently recorded raphe cells, evidence that a thermal but not noxious stimulus is not sufficient to block BAT temperature increases. Together, these experiments demonstrate that BAT temperature increases are blocked by noxious stimuli, regardless of thermal content.
Raphe neuronal discharge during noxious stimulation-evoked block of BAT temperature rises
Forepaw stimulation that blocked the BAT temperature rise inhibited 19 of 25 OFF cells (76%), excited 48 of 54 ON cells (88%), and did not change the discharge of any NEUTRAL cell (n = 52). Figure 4 shows the average population responses of each non-p5HT cell type to noxious forepaw stimulation. No p5HT cells were recorded during the suppression of BAT temperature increases by noxious stimulation.
DAMGO antagonism of noxious stimulation-evoked block of BAT temperature rises
To determine whether pain modulatory cells of the medullary raphe mediate noxious stimulus-evoked inhibition of BAT temperature increases, the effect of DAMGO microinjection into raphe on PGE2-evoked BAT temperature increases was tested. DAMGO is a μ-opiate receptor agonist that excites OFF cells and inhibits ON cells (Heinricher et al., 1994). As shown in Figure 2C, microinjection sites were concentrated in the RM. Four minutes after BAT temperature began to rise, 30 ng of DAMGO (in 250 nl of sterile saline) or saline vehicle was microinjected. BAT temperature increased at the same rate before and after DAMGO or saline microinjection, showing that in the absence of noxious stimulation, neither saline nor DAMGO microinjection into the RM influences a BAT temperature rise (p = 0.51) (Fig. 6).
Figure 6.
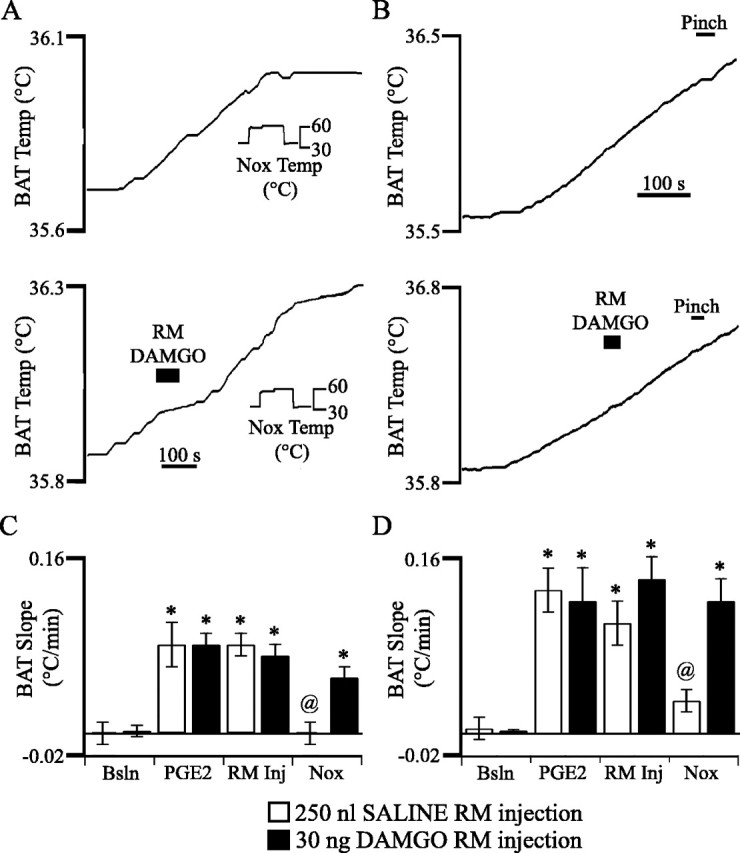
The μ-opiate receptor agonist DAMGO inhibits the decrease in BAT temperature evoked by noxious forepaw stimulation. A, The BAT temperature increase elicited by PGE2 administration (345 s before the start of the trace) reversed direction and began to decrease in response to long-duration noxious thermal stimulation of the forepaw. After a subsequent PGE2 administration (469 s before the start of the trace), BAT temperature began to rise again. DAMGO microinjection into the RM inhibited the decrease in BAT temperature evoked by noxious forepaw stimulation. B, The BAT temperature increase elicited by PGE2 administration (126 s before the start of the trace) reversed direction and began to decrease in response to noxious forepaw pinch. After a subsequent PGE2 administration (425 s before the start of the trace), BAT temperature began to rise again. DAMGO microinjection into the RM inhibited the decrease in BAT temperature evoked by noxious pinch. C, D, Average BAT temperature slope at five time points: 3 min before PGE2 administration (Bsln), after the onset of an increase in BAT temperature evoked by PGE2 administration and before microinjection of DAMGO (PGE2), after microinjection of either saline (□) or 30 ng of the μ-opiate receptor agonist DAMGO (▪), and after application of noxious thermal (C) or mechanical (D) stimulation (Nox). Statistically significant differences from baseline are marked by an asterisk. Differences from DAMGO are marked by the @ symbol. Error bars indicate SEM. Nox, Noxious; Temp, temperature; Bsln, baseline; Inj, injection.
Both noxious heating and pinch successfully inhibited the increase in BAT temperature (heat, n = 2; pinch, n = 5) after microinjection of saline into the RM (Fig. 6). In contrast, after DAMGO microinjection, both noxious heat (n = 16) (Fig. 6A, C) and noxious pinch (n = 8) (Fig. 6B, D) failed to reduce the rate of BAT temperature rise. Noxious stimulation evoked forepaw or pinnal withdrawals, even after DAMGO administration, evidence that the effects of DAMGO were not secondary to antinociception.
Discussion
p5HT cells and cold defense
Our findings that the discharge of most p5HT cells is directly correlated with BAT temperature and inversely so with stimulus temperature agree with previous findings that serotonergic medullary raphe cells are important in cold defense. Innocuous cold applied to the scrotum consistently excited cells with a slow and steady discharge that were presumably serotonergic (Dickenson, 1977). Exposure to ambient cold increases the discharge of presumed serotonergic cells in the medullary raphe (Martin-Cora et al., 2000) as well as levels of spinal serotonin metabolites (Passerin and Henley, 1994; Passerin et al., 1999). Serotonin facilitates preganglionic sympathetic neurons (Lewis and Coote, 1990; Pickering et al., 1994). Reducing serotonergic cell discharge increases the tail temperature of rats during ambient cold, thereby increasing heat loss (Berner et al., 1999), and inhibits the activation of BAT after intracerebroventricular injections of leptin (Morrison, 2004b). Importantly, our data provide the additional stringency of individual cell recordings concurrent with records of the appropriate physiological measure, BAT temperature. Thus, we have demonstrated a direct correlation between the discharge of medullary raphe p5HT cells and the activation of BAT, strong evidence that serotonergic cells facilitate BAT activation. In this light, it is important to note that bicuculline microinjection into raphe that elicits BAT activation also increases the discharge of local serotonergic cells (Levine and Jacobs, 1992).
Despite the evidence cited above that implicates serotonergic cells in cold defense, most medullary raphe cells that express c-fos after PGE2 administration fail to stain for serotonin immunoreactivity (Nakamura et al., 2002). Yet the proportion of serotonergic RP cells labeled with c-fos is not different after PGE2 than after saline [t test for proportions using numbers by Nakamura et al. (2002), their Table 1], evidence that serotonergic cells simply are a minority of the raphe population rather than that only non-serotonergic cells are activated during cold defense or fever.
Non-serotonergic cells and cold defense
Non-p5HT cells were not activated by thermoregulatory challenges that evoked an increase in BAT temperature. This result contrasts with previous findings that are themselves in disagreement: innocuous cold activated non-serotonergic cells that were either activated (Young and Dawson, 1987) or inhibited (Rathner et al., 2001) by noxious pinch. The different results obtained may be attributable to the different methods for cold application used in the present and in each of the previous studies. Alternatively, it is possible that the cellular responses to cold occurred but were unrelated to any thermoregulatory reaction. Because medullary raphe cells cycle between periods of activity and inactivity (Barbaro et al., 1986; Leung and Mason, 1999), it is easy to mistake a spontaneous change in discharge for a “response.”
Modulation versus drive
We propose that both serotonergic and non-serotonergic medullary raphe cells modulate rather than drive evoked BAT increases. In the present experiments, acute noxious stimulation had no effect on BAT temperature, evidence that under the conditions of light anesthesia and 25°C ambient temperature, OFF cells do not modulate resting BAT activity. Serotonergic raphe cells were excited by thermoregulatory challenges that elicited BAT temperature increases, thereby fulfilling one prerequisite of a premotor neuron to BAT-controlling sympathetic cells. Yet noxious stimulation that is effective in decreasing BAT temperature does not consistently decrease serotonergic cell discharge (Gao and Mason, 2000). Furthermore, the pattern of serotonergic cell firing in awake animals is consistent with a more global role in movement and arousal rather than a specific function in mediating BAT activation (Jacobs and Fornal, 1997).
None of the 147 non-p5HT cells recorded were activated by manipulations that elicited BAT temperature increases, evidence that continuous excitation of this raphe cell type is not needed to drive BAT activation. The possibility remains that a small number of non-serotonergic cells, none of which were recorded in the present study, are critical drivers of BAT activation. Yet the same OFF cells that were not excited before or during BAT temperature rises were inhibited by noxious stimulation that blocked increases in BAT temperature. Furthermore, DAMGO microinjection into the medullary raphe reversed this noxious stimulation-evoked block, showing that raphe OFF cells, the only cell type excited by DAMGO microinjection (Heinricher and Tortorici, 1994), facilitate BAT activation.
Morrison (2004a) proposed a heuristic model for cold defense that includes an obligatory pathway from the hypothalamus to medullary raphe neurons that in turn activate preganglionic neurons targeting the BAT. He bases this model on an anatomical connection between RM–RP and BAT-controlling spinal neurons, c-fos expression of raphe cells after cold challenge or pyrogen administration, and the block of BAT activation effected by raphe inactivation. Although a monosynaptic connection from raphe neurons, both serotonergic and not, to preganglionic neurons targeting the BAT exists, this does not imply that activity in the postsynaptic cell mirrors the activity of each presynaptic input. This disassociation is clearly illustrated by the example of lateral geniculate cells, the activity of which bares no relation to that of brainstem monoaminergic cells that provide a large proportion of their monosynaptic input (Sherman and Guillery, 1998). Instead, the discharge of a lateral geniculate cell is virtually identical to that of retinal ganglion cells that contribute only ∼5% of afferent input to a thalamic cell. Related to the present argument, the discharge of sympathetic efferents bares no resemblance to that of medullary raphe cells (Habler et al., 1994; Johnson and Gilbey, 1998).
The medullary raphe has no special relationship with BAT because RM–RP neurons project oligosynaptically to all sympathetic and parasympathetic tissues tested to date (Mason, 2005a, b). Whereas one could interpret this as evidence that raphe neurons are obligate relays for all autonomic outflow from thermogenesis to tear production to salivation and so on, it appears more likely that medullary raphe cells modulate rather than drive all autonomic outflow.
Satinoff (1978) proposed that the mammal is equipped with multiple hierarchical thermoneutral zone comparators. Under this model, the spinal cord is the most basic site of thermoregulatory integration but can only defend a relatively wide (several degrees) thermoneutral zone, and descending modulation from the brainstem and hypothalamus progressively narrows the thermoneutral range. Indeed, the spinal cord maintains body temperature, but within a large thermoneutral zone (Simon, 1974; Banet et al., 1978). Medullary raphe lesions result in alterations of the thermoneutral zone (Szelenyi and Hinckel, 1987). Raphe cells may modulate the excitability of preganglionic sympathetic neurons through connections to preganglionic cells, to pre-motoneurons in the intermediate gray, or to thermoreceptive cells in the superficial dorsal horn that are themselves presynaptic to preganglionic sympathetic neurons (Henry and Calaresu, 1974; Basbaum et al., 1978; Holstege and Kuypers, 1982; Bacon et al., 1990; Cabot, 1996). Because electrical stimulation in the raphe alters the responses of thermoreceptive neurons in the dorsal horn (Sato, 1993), RM–RP may modulate BAT activation via effects on thermoreceptive afferents to local preganglionic sympathetic neurons.
Disinhibition of RM–RP cells increases BAT nerve activity from almost nothing to continuous, near maximal activity (Morrison et al., 1999). Conversely, medullary raphe inactivation blocks the increase in BAT activity evoked by cold or pyrogen administration (Berner et al., 1999; Nakamura et al., 2002; Morrison, 2003, 2004b). These dramatic effects may result from a modulatory change, as is the case for the most robust examples of heat production and heat loss. After pyrogen administration, neutral temperatures are interpreted as cold. In the case of hot flashes, a small increase in core temperature that does not elicit any vasomotor reaction in asymptomatic women, results in a remarkable cutaneous vasodilation, followed by sweating and the sensation of being hot in symptomatic women (Freedman, 2005). Thus, a change in the interpretation of a steady, unchanging temperature can elicit large thermoeffector reactions. It is therefore not surprising that the activation or inactivation of a modulatory influence on BAT-controlling circuits can either generate BAT activation or block increases in BAT activity.
We speculate that ON cell function opposes that of OFF cells by negatively modulating cold defense and/or facilitating warm defense as it explains several findings. First, during naloxone-precipitated opioid withdrawal, when ON cells are active, tail temperature increases several degrees, leading to increased heat loss (Bederson et al., 1990; Kaplan and Fields, 1991). Second, raphe lesions lower the threshold for cold defense in awake guinea pigs (Szelenyi and Hinckel, 1987), possibly resulting from the loss of waking-associated discharge of ON cells (Leung and Mason, 1999) and consequent suppression of BAT-controlling neurons in the spinal cord. Finally, raphe lesions abolish the circadian temperature rhythm of pigeons while allowing appropriate thermoregulatory changes to ambient temperature alterations (Szelenyi and Hinckel, 1987).
Functional implications
Serotonergic cell discharge, which is tightly correlated with motor activity, serves to facilitate motor output and concomitant autonomic function (Jacobs and Fornal, 1997). Activation of serotonergic cells by cold may facilitate somatic motor activity and non-shivering thermogenesis, maximizing the influence of thermogenic machinery on core temperature. Indeed, in addition to activating BAT, bicuculline disinhibition of RM neurons frequently elicits shivering (Nason and Mason, 2004). Conversely, inhibition of raphe serotonergic cells decreases shivering in awake rats (Berner et al., 1999).
Why would antinociceptive OFF cells, specifically, be involved in cold defense? Any explanation that fits with the traditional role of the raphe in pain modulation is contrived. However, if one instead views the medullary raphe as part of a homeostatic modulatory system, then a parsimonious understanding of the participation of the raphe in thermoregulation emerges. The principle benefit of the facilitation of the OFF cells of BAT-controlling circuits is to ensure efficient cold defense during periods of inactivity such as chronic pain or sleep. OFF cells are active in animals with persistent pain that also leads to animals’ being immobile and therefore at risk of hypothermia (Montagne-Clavel and Oliveras, 1994). OFF cells are also active during sleep when animals are very still and could easily get too cold. Thus, an important and daily consequence of OFF cell activity is to facilitate cold defense when an animal is particularly vulnerable to hypothermia.
Footnotes
M. W. Nason’s present address: Center for Neurobiology and Behavior, Psychiatric Institute Annex 837, Columbia University, 1051 Riverside Drive, New York, NY 10032.
This work was supported by the Brain Research Foundation and National Institute of General Medical Sciences Grant T32 GM-07839 (M.W.N.). We thank Drs. Philip Lloyd, Robert McCrea, and Naoum Issa for helpful discussions.
References
- Bacon SJ, Zagon A, Smith AD (1990). Electron microscopic evidence of a monosynaptic pathway between cells in the caudal raphe nuclei and sympathetic preganglionic neurons in the rat spinal cord. Exp Brain Res 79:589–602. [DOI] [PubMed] [Google Scholar]
- Banet M, Hensel H, Liebermann H (1978). The central control of shivering and non-shivering thermogenesis in the rat. J Physiol (Lond) 283:569–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaro NM, Heinricher MM, Fields HL (1986). Putative pain modulating neurons in the rostral ventral medulla: reflex-related activity predicts effects of morphine. Brain Res 366:203–210. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Clanton CH, Fields HL (1978). Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol 178:209–224. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Fields HL, Barbaro NM (1990). Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res 7:185–203. [DOI] [PubMed] [Google Scholar]
- Berner NJ, Grahn DA, Heller HC (1999). 8-OH-DPAT-sensitive neurons in the nucleus raphe magnus modulate thermoregulatory output in rats. Brain Res 831:155–164. [DOI] [PubMed] [Google Scholar]
- Cabot JB (1996). Some principles of the spinal organization of the sympathetic preganglionic outflow. Prog Brain Res 107:29–42. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF (2003). Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460:303–326. [DOI] [PubMed] [Google Scholar]
- Dickenson AH (1977). Specific responses of rat raphe neurones to skin temperature. J Physiol (Lond) 273:227–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P (1991). Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci 14:219–245. [DOI] [PubMed] [Google Scholar]
- Freedman RR (2005). Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med 23:117–125. [DOI] [PubMed] [Google Scholar]
- Gao K, Mason P (2000). Serotonergic raphe magnus cells that respond to noxious tail heat are not ON or OFF cells. J Neurophysiol 84:1719–1725. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Krummel M, Peters OA (1994). Reflex patterns in postganglionic neurons supplying skin and skeletal muscle of the rat hindlimb. J Neurophysiol 72:2222–2236. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tortorici V (1994). Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience 63:533–546. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Tortorici V, Fields HL (1994). Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience 63:279–288. [DOI] [PubMed] [Google Scholar]
- Henry JL, Calaresu FR (1974). Excitatory and inhibitory inputs from medullary nuclei projecting to spinal cardioacceleratory neurons in the cat. Exp Brain Res 20:485–504. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HG (1982). The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res 57:145–175. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Banfor P, Hammond DL (2003). Spinal pharmacology of antinociception produced by microinjection of mu or delta opioid receptor agonists in the ventromedial medulla of the rat. Neuroscience 118:789–796. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA (1997). Serotonin and motor activity. Curr Opin Neurobiol 7:820–825. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Gilbey MP (1998). Focally recorded single sympathetic postganglionic neuronal activity supplying rat lateral tail vein. J Physiol (Lond) 508:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H, Fields HL (1991). Hyperalgesia during acute opioid abstinence: evidence for a nociceptive facilitating function of the rostral ventromedial medulla. J Neurosci 11:1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CG, Mason P (1998). A physiological survey of medullary raphe and magnocellular reticular neurons in the anesthetized rat. J Neurophysiol 80:1630–1646. [DOI] [PubMed] [Google Scholar]
- Leung CG, Mason P (1999). Physiological properties of medullary raphe neurons during sleep and waking. J Neurophysiol 81:584–595. [DOI] [PubMed] [Google Scholar]
- Levine ES, Jacobs BL (1992). Neurochemical afferents controlling the activity of serotonergic neurons in the dorsal raphe nucleus: microiontophoretic studies in the awake cat. J Neurosci 12:4037–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DI, Coote JH (1990). The influence of 5-hydroxytryptamine agonists and antagonists on identified sympathetic preganglionic neurones in the rat, in vivo. Br J Pharmacol 99:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL (2000). Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience 98:301–309. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Tache Y (2001). Central TRH receptor 1 antisense blocks cold-induced gastric emptying but not brain c-Fos induction. Peptides 22:81–90. [DOI] [PubMed] [Google Scholar]
- Mason P (1997). Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol 77:1087–1098. [DOI] [PubMed] [Google Scholar]
- Mason P (2001). Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci 25:737–777. [DOI] [PubMed] [Google Scholar]
- Mason P (2005a). Deconstructing endogenous pain modulation. J Neurophysiol 94:1659–1663. [DOI] [PubMed] [Google Scholar]
- Mason P (2005b). The ventromedial medulla: pain modulation and beyond. J Comp Neurol 493:2–8. [DOI] [PubMed] [Google Scholar]
- Montagne-Clavel J, Oliveras JL (1994). Are ventromedial medulla neuronal properties modified by chronic peripheral inflammation? A single-unit study in the awake, freely moving polyarthritic rat. Brain Res 657:92–104. [DOI] [PubMed] [Google Scholar]
- Morrison SF (1999). RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol 276:R962–R973. [DOI] [PubMed] [Google Scholar]
- Morrison SF (2001). Differential control of sympathetic outflow. Am J Physiol 281:R683–R698. [DOI] [PubMed] [Google Scholar]
- Morrison SF (2003). Raphe pallidus neurons mediate prostaglandin E2-evoked increases in brown adipose tissue thermogenesis. Neuroscience 121:17–24. [DOI] [PubMed] [Google Scholar]
- Morrison SF (2004a). Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci 19:67–74. [DOI] [PubMed] [Google Scholar]
- Morrison SF (2004b). Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am J Physiol 286:R832–R837. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Sved AF, Passerin AM (1999). GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol 276:R290–R297. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M (2002). The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci 22:4600–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel H-J, Gerstberger R, Kobayashi S, Kaneko T (2004). Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci 24:5370–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason MW Jr, Mason P (2004). Modulation of sympathetic and somatomotor function by the ventromedial medulla. J Neurophysiol 92:510–522. [DOI] [PubMed] [Google Scholar]
- Passerin AM, Henley WN (1994). Activation of spinal cord serotonergic neurons accompanies cold-induced sympathoexcitation. Can J Physiol Pharmacol 72:884–892. [DOI] [PubMed] [Google Scholar]
- Passerin AM, Bellush LL, Henley WN (1999). Activation of bulbospinal serotonergic neurons during cold exposure. Can J Physiol Pharmacol 77:250–258. [PubMed] [Google Scholar]
- Pickering AE, Spanswick D, Logan SD (1994). 5-Hydoxytryptamine evokes depolarizations and membrane potential oscillations in rat sympathetic preganglionic neurones. J Physiol (Lond) 480:109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF (2002). Chronic pain and medullary descending facilitation. Trends Neurosci 25:319–325. [DOI] [PubMed] [Google Scholar]
- Rathner JA, Owens NC, McAllen NC (2001). Cold-activated raphe-spinal neurons in rats. J Physiol (Lond) 535:841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satinoff E (1978). Neural organization and evolution of thermal regulation in mammals. Science 201:16–22. [DOI] [PubMed] [Google Scholar]
- Sato H (1993). Raphe-spinal and subcoeruleo-spinal modulation of temperature signal transmission in rats. J Therm Biol 18:211–221. [Google Scholar]
- Sherman SM, Guillery RW (1998). On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators.”. Proc Natl Acad Sci USA 95:7121–7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E (1974). Temperature regulation: the spinal cord as a site of extrahypothalamic thermoregulatory functions. Rev Physiol Biochem Pharmacol 71:1–76. [DOI] [PubMed] [Google Scholar]
- Szelenyi Z, Hinckel P (1987). Changes in cold- and heat-defence following electrolytic lesions of raphe nuclei in the guinea-pig. Eur J Physiol 409:175–181. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Matsumura K, Inoue W, Shiraki T, Suzuki K, Yasuda S, Sugiura H, Cao C, Watanabe Y, Kobayashi S (2001). Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J Neurosci 21:2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AA, Dawson NJ (1987). Static and dynamic response characteristics, receptive fields, and interaction with noxious input of midline medullary thermoresponsive neurons in the rat. J Neurophysiol 57:1925–1936. [DOI] [PubMed] [Google Scholar]