Abstract
Synaptic inhibition is determined by the properties of postsynaptic receptors, neurotransmitter release, and clearance, but little is known about how these factors shape sensation-evoked inhibition. The retina is an ideal system to investigate inhibition because it can be activated physiologically with light, and separate inhibitory pathways can be assayed by recording from rod bipolar cells that possess distinct glycine, GABAA, and GABAC receptors (R). We show that receptor properties differentially shape spontaneous IPSCs, whereas both transmitter release and receptor properties shape light-evoked (L) IPSCs. GABACR-mediated IPSCs decayed the slowest, whereas glycineR- and GABAAR-mediated IPSCs decayed more rapidly. Slow GABACRs determined the L-IPSC decay, whereas GABAARs and glycineRs, which mediated rapid onset responses, determined the start of the L-IPSC. Both fast and slow inhibitory inputs distinctly shaped the output of rod bipolar cells. The slow GABACRs truncated glutamate release, making the A17 amacrine cell L-EPSCs more transient, whereas the fast GABAAR and glycineRs reduced the initial phase of glutamate release, limiting the peak amplitude of the L-EPSC. Estimates of transmitter release time courses suggested that glycine release was more prolonged than GABA release. The time course of GABA release activating GABACRs was slower than that activating GABAARs, consistent with spillover activation of GABACRs. Thus, both postsynaptic receptor and transmitter release properties shape light-evoked inhibition in retina.
Keywords: inhibition, light, retina, GABAA receptor, GABAC receptor, glycine receptor, spillover, IPSC, glycine, GABA, patch-clamp
Introduction
Inhibitory signals are determined by the properties of neurotransmitter receptors, release, and clearance. Changes in GABAA and glycine receptor (R) subunit expression during development contribute to the speeding of inhibition (Takahashi et al., 1992; Dunning et al., 1999; Okada et al., 2000). In the cerebellum, α1-containing GABAA receptors mediate fast, synaptic inhibition, whereas α6-containing GABAA receptors mediate slow, tonic inhibition (Brickley et al., 1996; Hamann et al., 2002). Differences in GABA release contribute to hippocampal signaling, because either synchronous or asynchronous release from distinct interneurons produces either brief or prolonged inhibition (Hefft and Jonas, 2005). Additionally, at some synapses neurotransmitter diffuses or spills over from release sites and activates receptors at neighboring synapses. When spillover transmission is enhanced by decreasing transmitter clearance, the decay time of inhibitory synaptic currents are prolonged, suggesting that spillover between synapses shapes inhibition (Chiu et al., 2005; Keros and Hablitz, 2005). However, little is known about how these properties determine the time course of inhibition evoked by sensory stimuli.
To investigate the roles of receptor properties, neurotransmitter release, and clearance on sensory processing, we studied visually evoked inhibition in the retina, which can be activated physiologically with light. Furthermore, the circuitry and receptor expression patterns of the retina are well defined. Rod bipolar cells, which receive inhibition that is mediated by glycine, GABAA, and GABAC receptors (Eggers and Lukasiewicz, 2006), are ideal for studying distinct inhibitory inputs. We do not know whether the different temporal properties of these receptors (Amin and Weiss, 1994; Feigenspan and Bormann, 1994; Lukasiewicz and Shields, 1998; McCall et al., 2002; Frech and Backus, 2004) shape light-evoked (L) inhibition or whether the receptors are activated by distinct neurotransmitter release time courses. Using receptor-specific blockers and mice that lack GABAC receptors (McCall et al., 2002), we determined that GABA and glycine receptor properties, transmitter release, and spillover shaped L-IPSCs. These diverse presynaptic inhibitory signals differentially shaped the output of rod bipolar cells: prolonged, GABAC receptor-mediated inhibition limited the duration of A17 amacrine cell L-EPSCs, and briefer GABAA and glycine receptor-mediated inhibition affected the initial peak of the L-EPSCs.
Materials and Methods
Preparation of mouse retinal slices.
Animal protocols were approved by the Washington University School of Medicine Animal Studies Committee. In this study, we use wild-type (WT) mice (C57BL/6J strain; The Jackson Laboratory, Bar Harbor, ME) and GABAC ρ1 null mice that lacked functional GABAC receptors (crossed onto the C57BL/6J background) (McCall et al., 2002). The experimental techniques were similar to those described in our previous studies (Shields et al., 2000; McCall et al., 2002; Eggers and Lukasiewicz, 2006). Briefly, mice were dark-adapted overnight, and all dissection and recording procedures were performed under infrared illumination to preserve the light sensitivity of the preparations. Mice 28–90 d of age were killed using carbon dioxide, their eyes were enucleated, and the cornea, lens, and vitreous were removed. The eyecup was incubated for 20 min in dissection and storage solution (see electrode and bath solutions) with hyaluronidase (0.5 mg/ml; Sigma, St. Louis, MO) to facilitate vitreous removal. The hyaluronidase solution was replaced with cold, oxygenated storage solution, the retina was dissected out of the eyecup, and 250 μm slices were prepared from the isolated retina and maintained in oxygenated storage solution at room temperature (Werblin, 1978; McCall et al., 2002).
Whole-cell recordings.
Whole-cell patch recordings were made from bipolar cells and amacrine cells from retinal slices as described previously (McCall et al., 2002; Eggers and Lukasiewicz, 2006). IPSCs were recorded from retinal bipolar cells voltage clamped to 0 mV, the reversal potential for currents mediated by nonselective cation channels. EPSCs were recorded from amacrine cells voltage clamped to −60 mV, the reversal potential for IPSCs. All recordings were made at 32°C. Liquid junction potentials of 15 mV were corrected for at the beginning of each recording.
The recording procedures and microscope system have been described previously (Lukasiewicz and Roeder, 1995). Electrodes were pulled from borosilicate glass (1B150F-4; World Precision Instruments, Sarasota, FL) on a P97 Flaming/Brown puller (Sutter Instruments, Novato, CA) and had resistances of <5 MΩ. Patchit software (White Perch Software, Somerville, MA) was used to generate voltage command outputs, acquire data, and gate the drug perfusion valves. The data were digitized and stored with a personal computer using a Labmaster DMA data acquisition board (Scientific Solutions, Solon, OH). Responses were filtered at 1 kHz with the four-pole Bessel filter on the Axopatch 200B (Molecular Devices, Palo Alto, CA) and sampled at 2–5 kHz. The preparation was heated by temperature-controlled thin-stage and in-line heaters (Cell Microcontrols, Norfolk, VA).
Morphological identification of retinal cell types.
Bipolar cells and amacrine cells were identified by their characteristic morphology and cell stratification within the ON and OFF sublaminas of the retinal inner plexiform layer after labeling with either Lucifer yellow (0.05%) or Sulforhodamine B (0.005%), included in the recording electrode (Euler and Wässle, 1998; Shields et al., 2000; Eggers and Lukasiewicz, 2006).
Solutions and drugs.
The dissection and storage solution contained the following (in mm): 137 NaCl, 2.5 KCl, 1 MgCl2, 2.5 CaCl2, 28 glucose, and 10 HEPES, adjusted to pH 7.4 with NaOH and bubbled with oxygen. The extracellular recording solution contained the following (in mm): 125 NaCl, 2.5 KCl, 1 MgCl2, 1.25 NaH2PO4, 2 CaCl2, 20 glucose, and 26 NaHCO3, bubbled with carbogen (95% O2–5% CO2). The intracellular solution contained the following (in mm): 120 Cs gluconate, 1 CaCl2, 1 MgCl2, 10 Na-HEPES, 11 EGTA, 10 tetraethylammonium-Cl, adjusted to pH 7.2 with CsOH. To isolate receptor types, strychnine (500 nm) was used to block glycine receptors, bicuculline methobromide (50 μm; Research Biomedicals, Natick, MA) to block GABAA receptors and (1,2,5,6-tetrahydropyridine-4yl) methylphosphinic acid (TPMPA) (50 μm; Research Biomedicals) to block GABAC receptors. Kainate (10 μm) was used to elicit spontaneous GABAC receptor-mediated IPSCs. NO-711 (1-[2([(diphenylmethylene)imino]oxy)ethyl]-1,2,5,6-tetrahydro-3-pyridinecarboxylic acid hydrochloride) was used (5 μm) to block GAT-1 GABA transporters. Antagonists were applied to the slice by a gravity-driven superfusion system, as described previously (Lukasiewicz and Roeder, 1995). Unless otherwise indicated, all chemicals were obtained from Sigma.
Light stimulation.
To evoke L-IPSCs and L-EPSCs, full-field light stimuli were generated using a light-emitting diode (LED) (HLMP-3950, λpeak = 565 nm; Agilent, Palo Alto, CA) that was positioned near the microscope stage. The intensity of the unattenuated light was 1.85 × 105 photons/μm2/s. Light intensity was controlled by varying the current through the LED. Light stimuli were attenuated −2 log units, unless otherwise stated.
Data analysis.
Tack (White Perch Software) and Clampfit (Molecular Devices) software were used to average records and to measure the peak, time-to-peak, rise time, charge transfer (Q), decay time, and half-width of the light-evoked current responses for each cell or the simulated current responses. Unless otherwise stated, all analysis and traces displayed of light-evoked currents were from the average of two responses from the same cell. The decay time was measured by computing the D37, the time at which the current had declined to 37% of its peak amplitude. Student’s t tests (two-tailed, unequal variance) were used to compare these response parameters from wild-type and GABACR null neurons. Paired Student’s t tests were used to compare values between conditions for the same cell. Differences were considered significant when p ≤ 0.05. All data are reported as mean ± SEM.
Spontaneous IPSCs (sIPSCs) were analyzed using Mini Analysis (Synaptosoft, Decatur, GA). sIPSCs were selected so that the rise and decay phases did not contain any overlapping events. For each individual sIPSC, the amplitude was measured, and the τdecay was calculated by fitting an exponential function to the decay from the peak to baseline. The distributions of sIPSC amplitude and τdecay values were compared using the Kolmogorov–Smirnov test (K–S). To compute the average sIPSC, the events were aligned by the 50% rise time. GABAC receptor-mediated sIPSCs were aligned by hand, using the Clampfit program, because the events had too slow a rise time to be effectively aligned using the Mini Analysis program.
For intensity–response curves, L-IPSCs were normalized to the maximal response in control solution in WT mice, which was the maximum Q of the L-IPSCs. The normalized data were plotted versus the log10 of the stimulus luminance (L). The values were fitted using the sigmoid function: Y = a/(1 + e−(X − L50)/b), where a is the maximum response, b the Hill slope of the response, and L50 is the log10 of the light intensity at the half-maximum response. The luminance evoking a half-maximal response (L50) and the dynamic range for the curves, which is the difference in light intensity between 20 and 80% of the maximal response (L20–80), were calculated using the fitted curve.
For the transmitter release time course estimates, idealized L-IPSC and sIPSC curves were computed using the average rise time, peak, and D37 or τdecay of the L-IPSCs and sIPSCs mediated by isolated GABAC, GABAA, and glycine receptors. The rising phase was described by a line, using the peak/rise time as the slope. The decay phase was calculated as an exponential decay function from the peak, using either the D37 or τdecay value as the exponential τ. Release functions were calculated by convolution analysis (Diamond and Jahr, 1995) using the following relationship:
such that
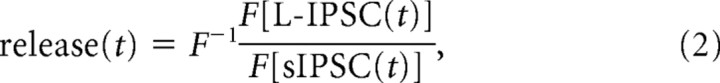 |
where F and F−1 represent the Fourier transform and inverse Fourier transform of the function, respectively. The release estimates and simulation were calculated using Igor Pro (WaveMetrics, Oswego, OR).
Results
Eliminating GABAC receptors shortened rod bipolar cell L-IPSC duration
Rod bipolar cell terminals receive light-evoked presynaptic inhibition mediated by distinct glycine, GABAA, and GABAC receptors (Eggers and Lukasiewicz, 2006). Although these receptors have distinct kinetic properties (Amin and Weiss, 1994; Shields et al., 2000; Frech and Backus, 2004), it is not known whether these different receptors shape light-evoked inhibition. If receptor properties are a major determinant of the light response time course, then eliminating slow GABAC receptors, either genetically or pharmacologically, should make L-IPSCs briefer. To determine whether the distinct inhibitory inputs have distinct temporal properties, we recorded L-IPSCs elicited by brief (30 ms) light stimuli.
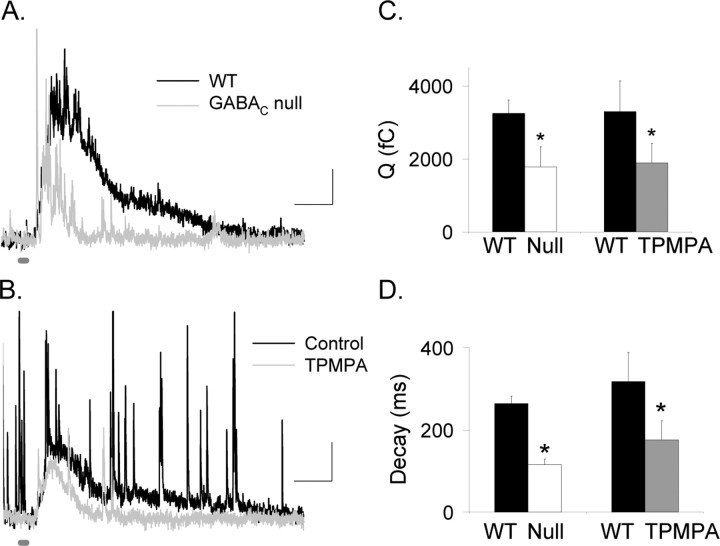
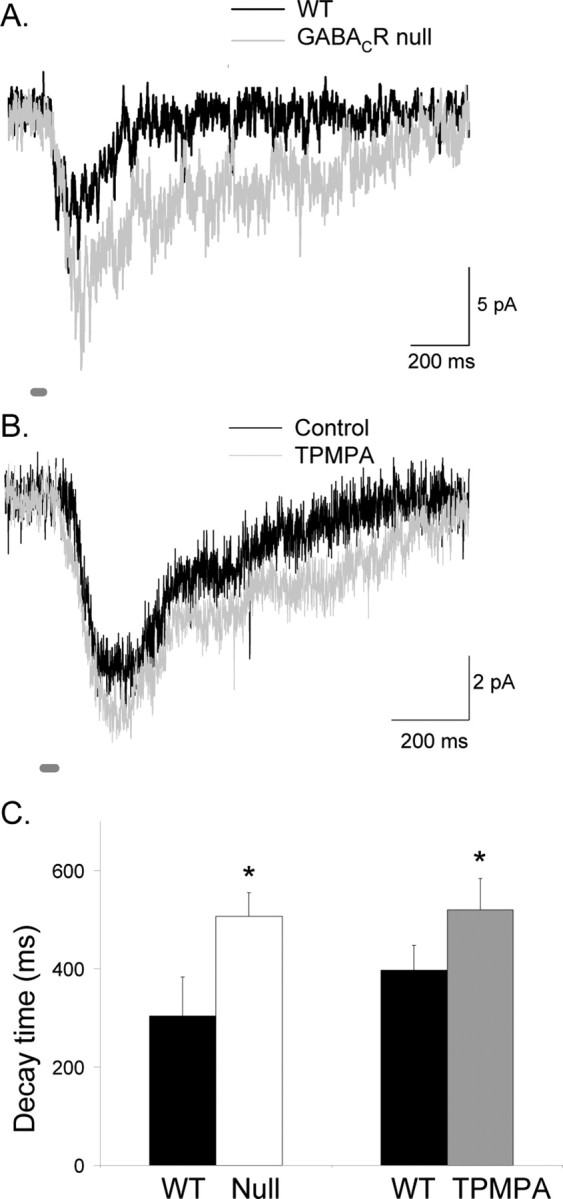
Figure 1A shows that the L-IPSCs recorded in mice that lacked GABAC receptors (GABACR null) had smaller charge transfers (p < 0.05) (Fig. 1C, Table 1) and briefer decay times (p < 0.001) (Fig. 1D, Table 1) compared with those recorded in WT mice. Similarly, when GABAC receptors in WT mice were blocked with TPMPA, the charge transfer (Q of 3290 ± 846 fC reduced to 1886 ± 538 fC; n = 5; p < 0.05) (Fig. 1B–D) and decay time were decreased (control D37, 318 ± 31 ms to TPMPA, 176 ± 46 ms; p < 0.05), demonstrating that the responses from GABACR null mice were not attributable to circuitry alterations caused by the elimination of GABAC receptors, in agreement with previous reports (McCall et al., 2002; Eggers and Lukasiewicz, 2006). In GABAC null mice, the briefer L-IPSCs presumably reflect the contributions of the more rapidly decaying responses of glycine and GABAA receptors.
Figure 1.
GABAC receptors prolong rod bipolar cell L-IPSCs. A, L-IPSCs evoked by brief light stimuli (30 ms; thick dark gray bar) were smaller and decayed faster in GABACR null mice (gray trace) than in WT mice (black trace). Calibration: 5 pA, 200 ms. B, Blocking GABAC receptors with TPMPA decreased and shortened L-IPSCs (gray trace) compared with control conditions (black trace). C, The Q in GABACR null mice was significantly smaller than in WT mice (*p < 0.05). The Q in rod bipolar cells after TPMPA application was significantly smaller than in control retinas (*p < 0.05). D, The L-IPSC decay (D37) in GABACR mice was significantly briefer than for WT mice (*p < 0.001). Similarly, the L-IPSC decay measured in the presence of TPMPA also was briefer than that measured in control conditions (*p < 0.05).
Table 1.
Parameters for L-IPSCs (30 ms light stimulus) from rod bipolar cells
| L-IPSC type | D37 (ms) | Q (fC) | Peak (pA) | Time-to-peak (ms) | n |
|---|---|---|---|---|---|
| GABAA | 134.1 ± 35.5 | 954 ± 371 | 7.1 ± 1.2 | 158.9 ± 12.5 | 12 |
| GABAC | 472.4 ± 82.7 | 3667 ± 522 | 10.4 ± 1.4 | 225.7 ± 17.9 | 14 |
| Glycine | 279.1 ± 28.9 | 1635 ± 421 | 10.6 ± 1.6 | 154.0 ± 17.5 | 13 |
| WT control | 275.3 ± 19.8 | 3215 ± 368 | 13.5 ± 1.3 | 168.2 ± 9.2 | 43 |
| GABACR null control | 119.6 ± 14.3 | 1775 ± 559 | 13.1 ± 2.2 | 156.7 ± 8.9 | 15 |
Glycine, GABAA, and GABAC receptor-mediated L-IPSCs have distinct kinetics
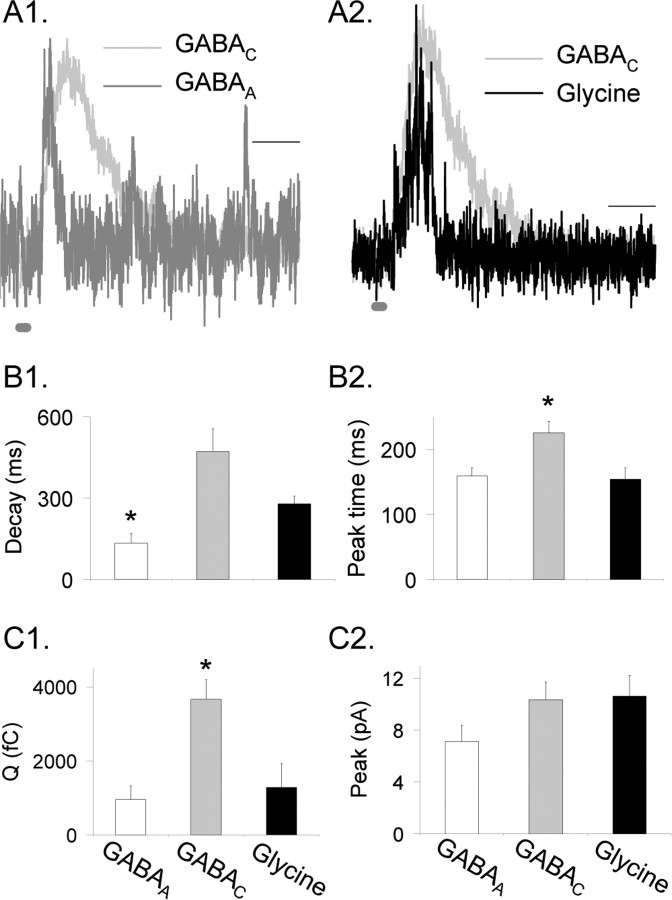
Our results suggest that GABAC receptors determine the duration of L-IPSCs, but it is not known how GABAA and glycine receptors shape L-IPSCs. To determine the contributions of individual receptor types to rod bipolar cell L-IPSCs, we recorded L-IPSCs mediated by pharmacologically isolated GABAC, GABAA, and glycine receptors, from WT mice and GABACR null mice, which lack GABAC receptors (see Materials and Methods), because no significant differences were observed between WT and GABACR null GABAA and glycine receptor-mediated L-IPSCs as described previously (Eggers and Lukasiewicz, 2006) or in this study (p > 0.4). Examples of normalized, pharmacologically isolated, glycine, GABAC, and GABAA receptor-mediated currents, in response to a brief light stimulus (30 ms), are shown in Figure 2. The GABAA (Fig. 2A1,B1, Table 1) and the glycine (Fig. 2A2,B1) receptor-mediated L-IPSCs decayed more rapidly than the GABAC receptor-mediated L-IPSCs. The decay of the GABAC receptor-mediated L-IPSCs was significantly longer than the decay of the GABAA receptor-mediated L-IPSCs (p < 0.01) (Fig. 2B1, Table 1) and, on average, longer than glycine receptor-mediated L-IPSCs (p < 0.08). Additionally, the glycine receptor-mediated L-IPSCs were significantly longer than the GABAA receptor-mediated L-IPSCs (p < 0.01).
Figure 2.
Glycine, GABAA, and GABAC receptors mediated L-IPSCs, with different properties. A1 and A2 show pharmacologically isolated and peak-scaled L-IPSCs evoked by a 30 ms light stimulus (dark gray bar at bottom of trace) mediated by GABAA, GABAC, and glycine receptors. GABAC receptors mediated L-IPSCs with a slower decay time than those mediated by GABAA or glycine receptors. Calibration, 200 ms. B1, The D37 was measured from L-IPSCs mediated by isolated glycine (black), GABAA (white), and GABAC (gray) receptors. GABAA L-IPSCs had a significantly briefer D37 than either glycine receptors or GABAC receptors; *p < 0.01. Glycine receptor-mediated L-IPSCs were on average briefer than GABAC receptor-mediated L-IPSCs (p < 0.08). B2, GABAC receptor-mediated L-IPSCs had a longer time-to-peak than either glycine or GABAA receptor-mediated L-IPSCs; *p < 0.01. There was no significant difference in time-to-peak between GABAA and glycine L-IPSCs (p = 0.8). C1, GABAC receptor-mediated L-IPSCs had significantly larger Q than either GABAA or glycine L-IPSCs (p < 0.01). There was no significant difference in Q between GABAA and glycine L-IPSCs (p = 0.3). C2, GABAA receptor-mediated L-IPSCs on average had a smaller peak value than GABAC and glycine L-IPSCs (p < 0.1).
The times to the peak of the pharmacologically isolated L-IPSCs (measured from stimulus onset) differed; GABAA and glycine receptor-mediated responses increased faster than GABAC receptor-mediated responses (Fig. 2B2). The time-to-peak of GABAA and glycine receptor-mediated L-IPSCs were similar to each other (p = 0.8) (Table 1) but significantly faster than the time-to-peak for the GABAC receptor-mediated L-IPSCs (p < 0.01) (Table 1). These findings suggest that the initial peak value of WT bipolar cell L-IPSCs was determined by GABAA and glycine receptor-mediated inputs because the GABAC receptor-mediated current increased too slowly to affect the initial part of the response. This idea was confirmed by comparing the L-IPSCs from rod bipolar cells in WT and GABACR null mice. There were no significant differences between their time-to-peak (p = 0.4) (Table 1) or peak amplitudes (p = 0.9) (Table 1), although, as expected, their decay times differed (Fig. 1, Table 1). There was also no significant difference between the time-to-peak values for WT L-IPSCs and the isolated glycine and GABAA receptor-mediated L-IPSCs (p = 0.5), whereas the isolated GABAC receptor-mediated L-IPSCs had a significantly slower time-to-peak than WT L-IPSCs (p < 0.05).
The time course of L-IPSCs is determined not only by the kinetic properties of individual receptor-mediated components but also by the magnitude of the current mediated by each receptor type. We therefore determined the charge transfer (Q) (Fig. 2C1, Table 1) and peak current amplitude (Fig. 2C2) of each receptor-mediated component, in response to brief light stimuli. GABAC receptors mediated the largest charge transfer, whereas glycine (p < 0.01) (Table 1) and GABAA (p < 0.01) (Table 1) mediated significantly smaller charge transfers.
The peak amplitude of the rapidly responding GABAA and glycine receptors is important in determining how inhibition affects the early component of glutamate release. We compared the peak amplitudes of L-IPSCs mediated by glycine, GABAA, and GABAC receptors. GABAA receptor-mediated L-IPSCs were the smallest (Table 1) and were smaller, but not significantly different, than the glycine (p < 0.1 vs GABAA) and the GABAC (p < 0.1 vs GABAA) receptor-mediated L-IPSCs. The peak values of glycine and GABAC receptor-mediated L-IPSCs were similar (p = 0.9). Because the GABAA or glycine receptor-mediated L-IPSCs rise faster than GABAC L-IPSCs and are similar to the time-to-peak values of the total WT L-IPSCs, our findings suggest that glycine and GABAA receptors determine the time-to-peak and GABAC receptors determine the decay time of WT L-IPSCs.
GABAC receptor-mediated inhibition limits the extent of rod bipolar cell glutamate release
If GABAC receptors prolong the duration of inhibition at rod bipolar cell terminals, then GABAC receptor-mediated inhibition should limit glutamate release from rod bipolar cells. To assess light-evoked glutamate release, we recorded L-EPSCs from morphologically identified A17 amacrine cells that receive excitatory input from rod bipolar cells. To determine whether GABAC receptor-mediated inhibition limits glutamate release from rod bipolar cells, we compared L-EPSCs recorded from GABAC null and WT mice in the absence or presence of TPMPA.
Figure 3A shows that L-EPSCs recorded from A17 amacrine cells in response to a brief light stimulus (30 ms) had a slower decay in mice that lacked GABAC receptors compared with WT cells. Consistent with the notion that GABAC receptor-mediated inhibition limits glutamate output from rod bipolar cells, we found that GABACR null L-EPSCs decayed more slowly (WT D37, 304.2 ± 78.7 ms, n = 9; GABACR null D37, 506.6 ± 48.3 ms, n = 7; p < 0.05) (Fig. 3C) and exhibited larger, but not significantly different, charge transfers than WT L-EPSCs (WT Q, −9960 ± 2548 fC; GABACR null Q, −15804 ± 6402 fC). We obtained similar results when L-EPSCs were recorded from WT A17 amacrine cells and GABAC receptors were blocked with TPMPA (Fig. 3B,C); TPMPA increased the D37 32.3 ± 6.4% (p < 0.01; n = 9) and the Q 23 ± 8.9% (p < 0.05). These results suggest that GABAC receptors limit the extent of glutamate release from rod bipolar cells.
Figure 3.
Presynaptic GABAC receptors make L-EPSCs from A17 amacrine cells more transient. A, Shown are L-EPSCs (30 ms light stimulus; dark gray bay) from A17 amacrine cells from WT and GABACR null mice. The absence of GABAC receptors in GABACR null mice causes the L-EPSC to have a longer decay and larger charge transfer. B, A similar effect was observed in WT mice when TPMPA was added to block GABAC receptors. C, The decay (D37) of A17 amacrine cells from GABACR null mice (*p < 0.05) and WT mice in TPMPA (*p < 0.01) was significantly longer than WT mice in control conditions.
Glycine and GABAA receptor-mediated inhibition decreased the initial phase of glutamate release from rod bipolar cells
We show above (Fig. 3) that the slowly responding GABAC receptors have a large influence on the time course of glutamate release from rod bipolar cells. Because GABAC receptors primarily affect the decay time and not the peak amplitude or peak time of L-IPSCs, we would expect that GABAC receptors would not have a significant effect on the initial peak of A17 amacrine cell L-EPSCs. We observed no significant change in peak (WT, −19.1 ± 3.1 pA; GABACR, −21.7 ± 7.0; p = 0.8) or time-to-peak (WT, 185.0 ± 30.6; GABACR null, 160.2 ± 19.4; p = 0.5) between L-EPSCs in WT and GABACR null mice, suggesting that the early phase of glutamate release from rod bipolar cells was determined by the more rapidly responding GABAA and glycine receptors.
To test this, we recorded L-EPSCs from A17 amacrine cells from GABACR null mice in response to a brief (30 ms) light stimulus to isolate the effects of GABAA and glycine receptors. We used GABACR null mice to assess the contribution of GABAA receptors to bipolar cell inhibition because blocking GABAA receptors with bicuculline produced network effects in WT mice, resulting in increased GABAC receptor-mediated inhibition (Eggers and Lukasiewicz, 2006). The contributions of glycine and GABAA receptors were determined after the addition of strychnine or bicuculline, respectively (Fig. 4A,B). Strychnine increased the peak L-EPSC amplitude 26.8 ± 7.7% (p < 0.05; n = 5) (Fig. 4C) but had no significant effect on the L-EPSC decay (D37 reduced 5.2 ± 8.9%; p = 0.4). Similarly, bicuculline increased the peak L-EPSC amplitude (14.0 ± 2.3%; p < 0.05) (Fig. 4C) but did not alter the L-EPSC decay (D37 reduced 14.4 ± 14.1%; p = 0.5; n = 5). Our results demonstrate that the rapidly responding glycine and GABAA receptors limited the initial component of glutamate release, whereas the slowly responding GABAC receptors limit prolonged glutamate release and had little effect on the initial component of release.
Figure 4.
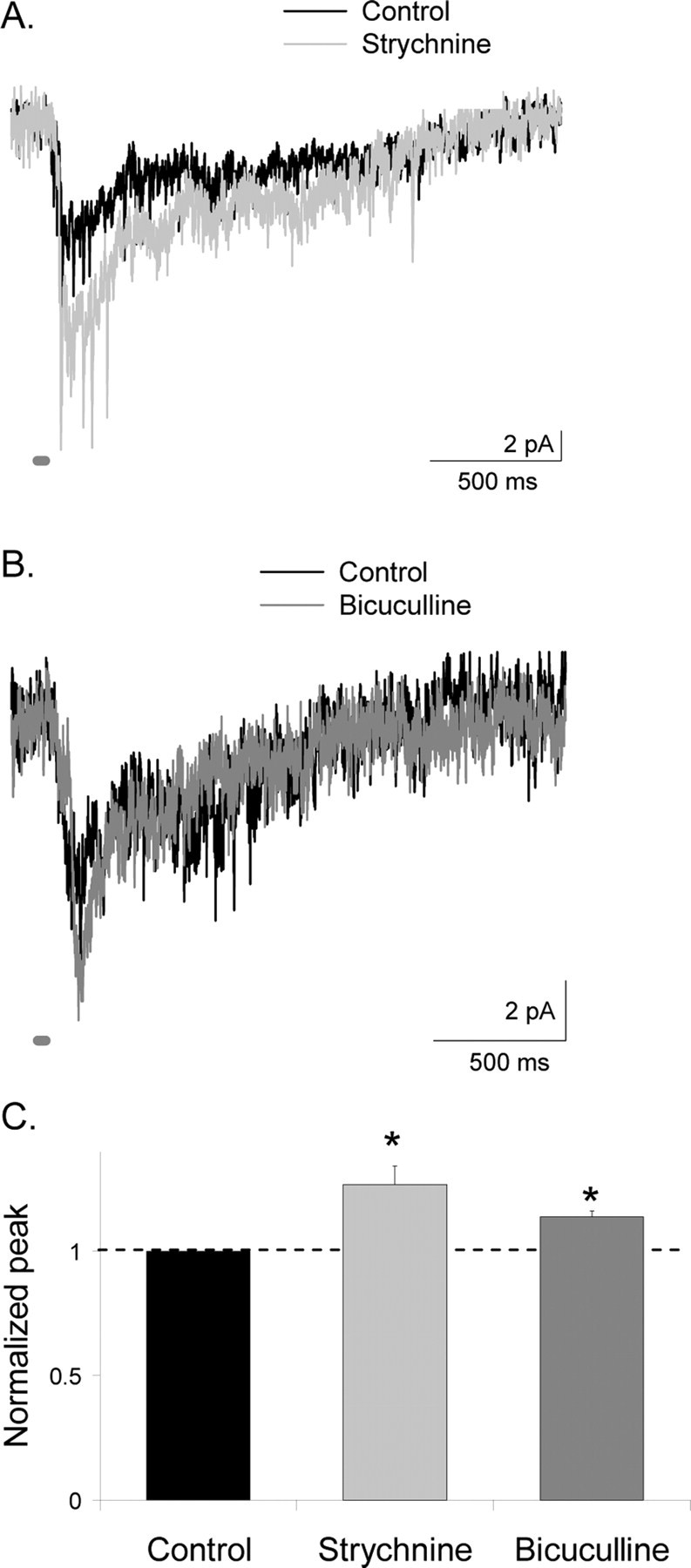
Presynaptic glycine and GABAA receptors decrease the peak response of L-EPSCs from A17 amacrine cells. A, The peak amplitude of L-EPSCs (30 ms light stimulus; dark gray bar) from A17 amacrine cells from GABACR null mice was increased by the addition of strychnine to block glycine receptors, but the decay time of the response was unaffected. B, Similarly, the peak amplitude of L-EPSCs from A17 amacrine cells from GABACR null mice was increased by addition of bicuculline to block GABAA receptors, but the response decay was unaffected. C, The amplitude of L-EPSCs was significantly larger with the addition of both strychnine (*p < 0.05) and bicuculline (*p < 0.05). Peak values in bicuculline and strychnine are normalized to control values, represented by the dotted line.
GABAA, glycine, and GABAC receptor-mediated sIPSCs have different properties
Our results suggest that the distinct inhibitory receptors mediate different temporal properties of light-evoked inhibition. However, L-IPSCs are also shaped by the time course of neurotransmitter release. Therefore, we determined the receptor properties that underlie the L-IPSCs by characterizing the temporal properties of pharmacologically isolated GABAA, glycine, and GABAC receptor-mediated sIPSCs, which are not affected by transmitter release properties. Figure 5 shows examples of temporally distinct glycine, GABAA, and GABAC receptor-mediated sIPSCs. Glycine receptor-mediated sIPSCs (Fig. 5A) had an average peak amplitude of 10.14 ± 0.35 pA (n = 193) and an average τdecay of 3.62 ± 0.1 ms, which are shown in the distribution of τdecay values and inset average in the right panel. GABAA receptor-mediated sIPSCs (Fig. 5B) had an average peak amplitude of 6.24 ± 0.08 pA (n = 972) and an average τdecay of 1.98 ± 0.1 ms, which are shown in the distribution of τdecay values and inset average in the right panel. The glycine receptor-mediated sIPSCs had larger amplitudes than the GABAA sIPSCs (K–S, glycine vs GABAA, p < 0.0001) (Fig. 5, insets).
Figure 5.
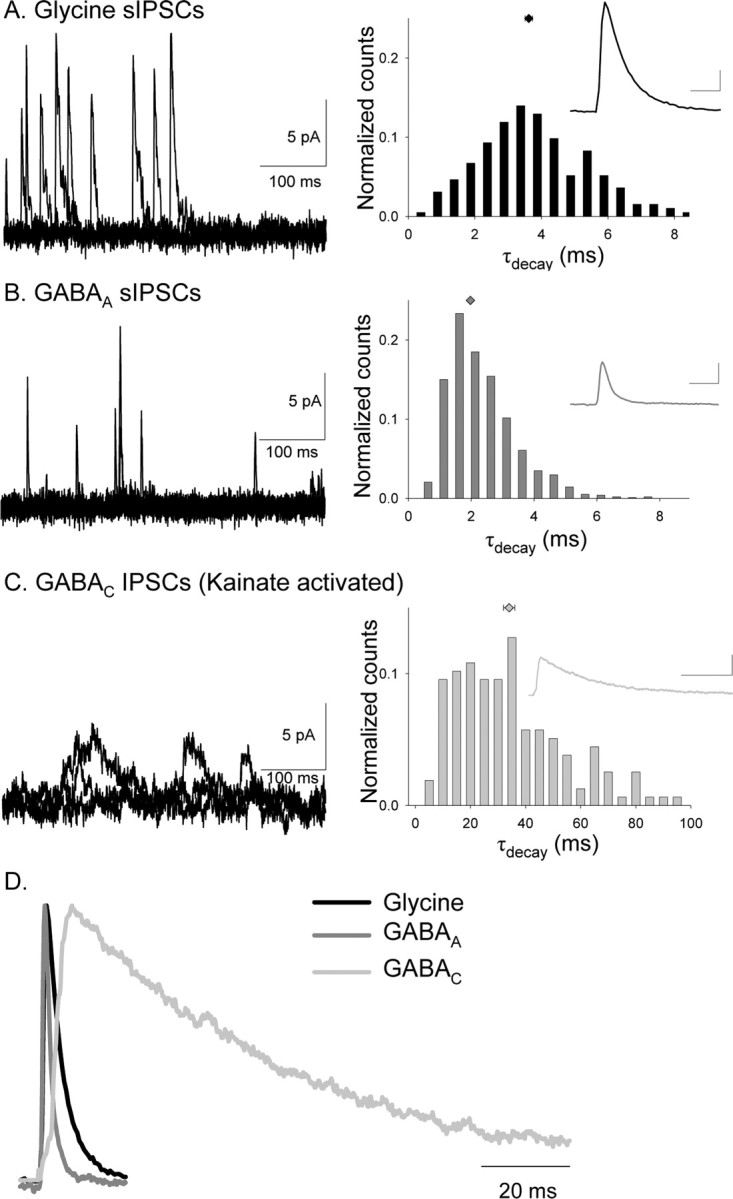
Glycine, GABAA, and GABAC receptors mediate sIPSCs with distinct kinetics in rod bipolar cells. A, Examples of isolated glycine receptor sIPSCs that were measured in the presence of bicuculline and TPMPA (left). The τdecay histogram distribution (normalized to the total number of events) for all glycine receptor-mediated sIPSCs recorded are shown in the right, and the inset shows the average glycine receptor-mediated sIPSC. Calibration:2 pA, 5 ms. B, Examples of isolated GABAA receptor sIPSCs that were measured in the presence of strychnine and TPMPA (left). The normalized τdecay histogram distribution for all GABAA receptor-mediated sIPSCs recorded are shown in the right, and the inset shows the average GABAA receptor-mediated sIPSC. Calibration: 2 pA, 5 ms. GABAA receptor-mediated sIPSCs had a significantly shorter τdecay than glycine receptor-mediated sIPSCs (K–S, p < 0.0001). C, Examples of isolated GABAC receptor sIPSCs, measured in the presence of kainate, strychnine, and bicuculline (left). The normalized τdecay histogram distribution for all GABAC receptor-mediated sIPSCs recorded is shown in the right, and the inset shows the average GABAC receptor-mediated sIPSC. Calibration: 2 pA, 50 ms. GABAC receptor-mediated sIPSCs had a significantly longer τdecay than glycine (K–S, p < 0.0001) and GABAA (K–S, p < 0.0001) receptor-mediated sIPSCs. D, Average normalized sIPSCs mediated by glycine, GABAA, and GABAC receptors. GABAC receptor-mediated sIPSCs have the longest decay time.
GABAC receptor-mediated sIPSCs were difficult to observe, as reported previously (Frech and Backus, 2004). However, in WT mice, we observed slow discrete events and an increase in tonic current after the addition of kainate (10 μm) to the bath solution, along with strychnine and bicuculline. TPMPA blocked both the individual events and the tonic current, indicating that they were mediated by GABAC receptors. GABAC receptor-mediated, kainate-activated sIPSCs (Fig. 5C) had an average peak amplitude of 5.7 ± 0.2 pA (n = 157). The GABAC receptor-mediated events were smaller in amplitude than the glycine receptor-mediated events (K–S, glycine vs GABAC, p < 0.0001) (Fig. 5, insets) but similar in amplitude to the GABAA sIPSCs. Because GABAC receptors have a smaller single-channel conductance than GABAA receptors, this suggests that more GABAC receptors mediate sIPSCs than GABAA receptors (Qian and Dowling, 1995). GABAC receptor-mediated sIPSCs had an average τdecay of 34.1 ± 2.0 ms, shown in the distribution of τdecay values and inset average in the right panel of Figure 5C.
Comparisons of the normalized sIPSCs mediated by three receptor types in Figure 5D and the distributions of decay times in Figure 5A–C shows that GABAC receptor-mediated sIPSCs had the slowest decay time, followed by the rapidly decaying glycine receptor-mediated sIPSCs (K–S vs GABAC, p < 0.0001) and the more rapidly decaying GABAA receptor-mediated sIPSCs (K–S vs GABAC and glycine, p < 0.0001). Figure 5D also shows the differences in the rising phase of the currents mediated by the three receptor types. GABAC receptor-mediated sIPSCs had significantly longer rise times (7.2 ± 0.5 ms; p < 0.001) than glycine (1.02 ± 0.03 ms) and GABAA (1.03 ± 0.03 ms). Our results suggest that these receptors mediate distinct temporal components of rod bipolar cell inhibition at near physiological temperatures, consistent with the observations of previous studies on evoked GABAA, GABAC, and glycine receptor-mediated IPSCs at room temperature (Cui et al., 2003; Frech and Backus, 2004).
Neurotransmitter release properties also determine L-IPSC time course
The similarities between the time courses of glycine, GABAA, and GABAC receptor-mediated sIPSCs and L-IPSCs suggest that receptor properties shaped the evoked responses. This idea is supported by our findings that eliminating GABAC receptors speeds the decays of L-IPSCs (Fig. 1), suggesting that slow GABAC receptors determine the decay of L-IPSCs. However, these observed differences may not be attributable to receptor properties alone because neurotransmitter release may differ for the inputs to specific receptor types. Although GABA and glycine release are most likely from distinct amacrine cells, it is not known whether GABAA and GABAC receptors, which are clustered at distinct synapses (Koulen et al., 1996), receive common or separate amacrine cell inputs.
L-IPSCs are made up of quantal events that reflect the activation of synaptic receptors by a vesicle of neurotransmitter. If these quantal events sum linearly, then the L-IPSC is the convolution of the sIPSC waveform and the neurotransmitter release time course (Cohen et al., 1981). Thus, deconvolution analysis can be used to estimate the neurotransmitter release underlying an evoked current (Van der Kloot, 1988; Diamond and Jahr, 1995). If receptors at synapses are saturated or desensitized during evoked release, or spillover occurs between synapses, then the estimated release may be an underestimate (saturation and desensitization) or overestimate (spillover) of the actual neurotransmitter released (Sargent et al., 2005). However, this “apparent release function” will reflect the neurotransmitter release necessary to produce the evoked response recorded and may also provide information about the factors that influence these currents (e.g., receptor saturation and spillover).
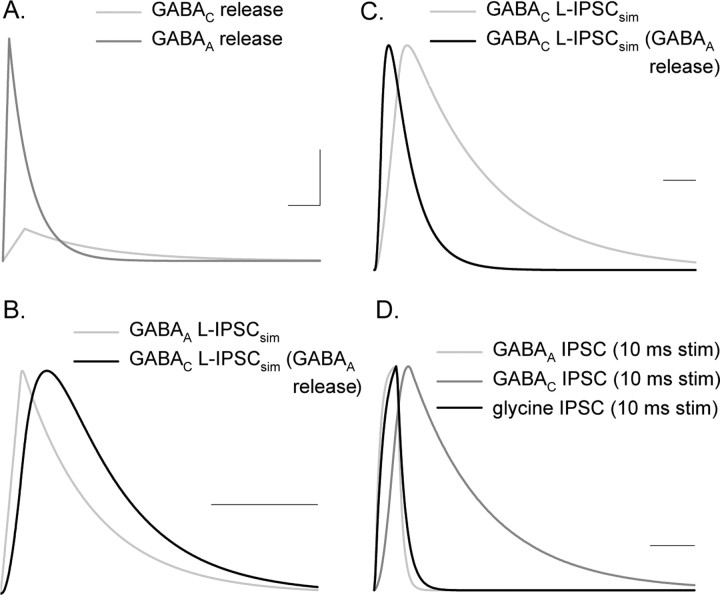
Therefore, to determine whether inhibitory receptors are activated by distinct transmitter release time courses, we estimated the transmitter release time courses that mediated glycine, GABAA, and GABAC receptor-mediated L-IPSCs by deconvolving L-IPSCs and sIPSCs, mediated by each receptor subtype (see Materials and Methods, Eq. 2). Because deconvolution analysis requires a low noise signal, we used computed sIPSCs and L-IPSCs reflecting the average values measured for each receptor type instead of the raw data (see Methods and Methods) (Table 1) (supplemental Fig. S1A,B, available at www.jneurosci.org as supplemental material). The calculated release function for the GABAAR-mediated L-IPSC had a larger amplitude and more rapid decay compared with that estimated for the GABACR-mediated L-IPSC (Fig. 6A). The differences in release magnitude were likely attributable to GABAA and GABAC receptor properties. Fewer large sIPSCs comprised the GABAC receptor-mediated L-IPSCs compared with GABAAR-mediated L-IPSCs. It is also possible that the high sensitivity GABAC receptors (Amin and Weiss, 1994) were saturated and unable to respond to additional neurotransmitter release. Additionally, the GABAC receptor release function showed a prolonged tail that was not observed in GABAA receptor release function, which could contribute to longer-lasting L-IPSCs mediated by GABAC receptors. Because this tail of neurotransmitter release is small and prolonged, it could reflect the spillover activation of GABAC receptors that has been reported in salamander bipolar cells (Ichinose and Lukasiewicz, 2002). A similar tail of release has been attributed to spillover onto AMPA receptors at the mossy fiber–granule cell synapse (Sargent et al., 2005).
Figure 6.
GABAA and GABAC receptor-mediated L-IPSCs have distinct apparent release functions and receptor kinetics, both of which contribute to L-IPSC kinetics. A, Release functions computed by deconvolving idealized GABAA and GABAC receptor-mediated L-IPSCs (supplemental data, available at www.jneurosci.org as supplemental material). GABAA receptor-mediated L-IPSCs have a much larger release function than GABAC receptors, likely because of the much smaller Q of GABAA sIPSCs versus GABAC sIPSCs. The GABAC release function has a prolonged tail not shown by the GABAA release function. Calibration: 0.05 quanta/ms, 200 ms. B, To determine how much of the difference between GABAA and GABAC L-IPSC kinetics was attributable to receptor properties, we used the GABAA calculated release function to simulate L-IPSCs with the GABAA and GABAC sIPSCs. Shown is the normalized GABAC L-IPSC, using the GABAA release function, that had a slower rise and decay time than the normalized GABAA L-IPSC, as a result of the slower kinetics of the GABAC sIPSCs. The L-IPSC simulated with the GABAA release function and sIPSC matched the properties of the average GABAA L-IPSC from Table 1. Calibration, 200 ms. C, Shown are the normalized GABAC L-IPSCs using the calculated GABAA and GABAC release rates. The prolonged tail of the estimated GABAC release rate slows the kinetics of the GABAC L-IPSCs. Again, the L-IPSC simulated with the GABAC release function and sIPSC matched the properties of the average GABAC L-IPSC from Table 1. Calibration, 200 ms. D, If GABAA, GABAC, and glycine receptor sIPSCs are activated by a brief (square wave, lasting 10 ms) burst of neurotransmitter, then the distinct receptor properties filter the response, as shown by these normalized responses. Calibration, 200 ms.
These distinct apparent release time courses suggest that either GABAA and GABAC receptors receive different inputs or they receive a common input but their distinct receptor properties, such as saturation and spillover activation, distort the release estimates. If GABAC and GABAA receptors are activated by the same GABA release function, then receptor kinetics should also shape L-IPSCs. To test whether receptor kinetics contributed to L-IPSC time courses, we simulated GABAA and GABAC receptor-mediated L-IPSCs by convolving each sIPSC waveform, which reflects distinct receptor kinetics (Fig. 5), with the same release function (Eq. 1), computed from GABAAR-mediated L-IPSCs (Fig. 6B). The normalized, simulated GABACR-mediated L-IPSC was significantly slower than the GABAAR-mediated L-IPSC (half-width, 180 vs 116 ms), demonstrating that receptor properties shaped the L-IPSCs when evoked by the same release function. However, the differences between GABAC and GABAA receptor-mediated simulated L-IPSCs were significantly smaller than the more than threefold difference in decay time seen in recorded GABAA and GABAC receptor-mediated L-IPSCs (compare Figs. 6B, 2A1; Table 1), suggesting that differences in receptor kinetics cannot account for these differences when the release time course is similar. To determine how release time course shaped L-IPSCs, we compared the L-IPSCs obtained by convolving the GABACR-mediated sIPSC with the different release time courses sensed by GABAA and GABAC receptors. Using the GABACR release function, which contained the prolonged tail of release (Fig. 6A), the normalized simulated GABAC L-IPSC (Fig. 6C, gray trace) was significantly slower (half-width of 459 ms) than normalized L-IPSC obtained using the faster GABAA release function (half-width of 180 ms) (black trace). Similar comparisons of the factors that shape GABAA and glycine receptor-mediated L-IPSCs suggested that both release and receptors properties contribute to the kinetics of these L-IPSCs (supplemental Fig. S1C, available at www.jneurosci.org as supplemental material).
The roles of receptor properties in shaping L-IPSCs may be obscured by the asynchronous nature of light-evoked transmitter release, given that the L-IPSC durations were much longer than the 30 ms light stimulus (Fig. 6A) (supplemental Fig. S1C, available at www.jneurosci.org as supplemental material). To better determine how receptor properties might shape L-IPSCs, we convolved the sIPSCs with an idealized 10 ms, synchronous release function. When release was synchronous, even more dramatic differences between glycine, GABAA, and GABAC receptor-mediated simulated L-IPSCs became apparent (Fig. 6D). These findings suggest that, when release is more synchronous, receptor properties play a larger role in shaping L-IPSCs.
GABAC receptor-mediated L-IPSCs are selectively enhanced by increased spillover
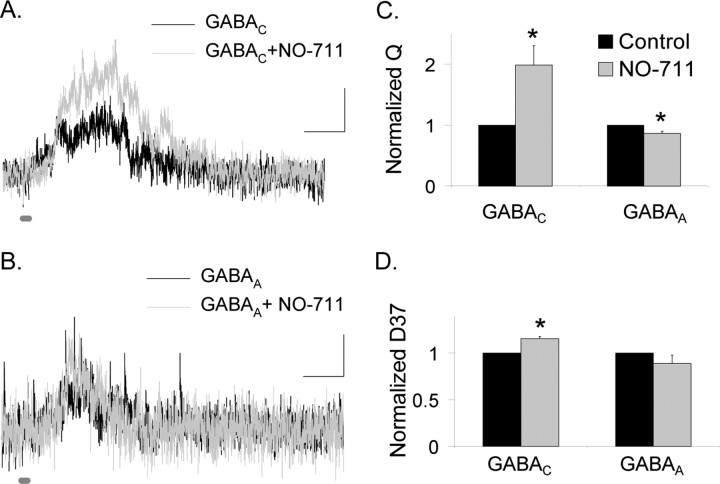
Our simulations suggest that there are differences in the neurotransmitter release that are sensed by GABAA and GABAC receptors (Fig. 6A). A possible explanation for the more prolonged release sensed by GABAC receptors is that GABA spills over from neighboring synapses activating the higher sensitivity GABAC receptors, as reported for glutamate currents receptors in the cerebellum (Sargent et al., 2005). If spillover selectively activates GABAC receptors, then increasing spillover by blocking GABA uptake with NO-711 should enhance GABAC but not GABAA receptor-mediated L-IPSCs. Figure 7 shows that NO-711 (5 μm) enhanced GABAC receptor-mediated L-IPSCs (98.8 ± 31.7%; p < 0.05; n = 6) (Fig. 7C) but did not enhance GABAA receptor-mediated L-IPSCs (13.2 ± 3.2% decrease; p < 0.01; n = 6), suggesting that spillover only activated GABAC receptors. NO-711 also prolonged the decay (D37) of GABAC receptors by 15.3 ± 2.4% (p < 0.01) (Fig. 7D) but left the GABAA D37 unchanged (11.1 ± 8.6% decrease; NS, p = 0.3), consistent with the spillover activation of GABAC receptors. Together, these results suggest that GABA spillover between synapses prolonged the time course and enhanced the magnitude of GABAC receptor-mediated L-IPSCs.
Figure 7.
GABAC receptor-mediated L-IPSCs are enhanced by blocking GABA uptake, but GABAA receptor-mediated L-IPSCs are not. A, GABAC receptor-mediated L-IPSCs (30 ms light) are enhanced by NO-711 (5 μm), which inhibits the GAT-1 GABA transporter. Calibration: 2 pA, 200 ms. B, GABAA receptor-mediated L-IPSCs (30 ms light) are minimally changed by the addition of NO-711. C, GABAC L-IPSCs Q is significantly increased by NO-711 (*p < 0.05), whereas the GABAA L-IPSC Q is decreased by a small amount (*p < 0.01). D, The D37 of GABAC receptor-mediated L-IPSCs was increased by NO-711 (*p < 0.01), whereas the D37 of GABAA receptor-mediated L-IPSCs was unchanged (p = 0.3).
Increased GABA release preferentially activates GABAC receptors
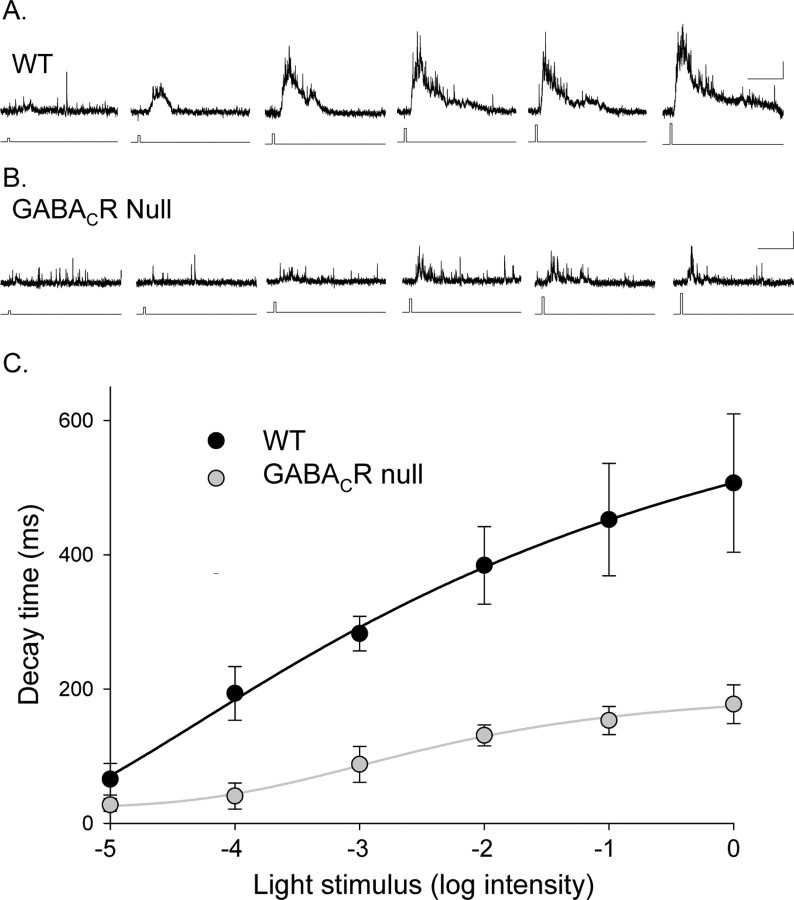
If spillover of GABA preferentially activates GABAC receptors, then we would expect that increasing GABA release would selectively increase GABAC receptor-mediated currents. We tested how the GABACR contribution to the L-IPSCs changed with enhanced neurotransmitter release by increasing the intensity of the light stimulus. Because the L-IPSC decay is determined by GABAC receptors, we compared the D37 for L-IPSCs evoked by different light intensities (30 ms duration) in GABACR null and WT mice. In both WT (Fig. 8A) and GABACR null (Fig. 8B) mice, the D37 values increased as a function of light intensity (Fig. 8C). Except for the dimmest intensity, the WT L-IPSCs (n = 10) were always significantly more prolonged than the GABACR null L-IPSCs (p < 0.01; n = 6). The maximum decay observed in GABACR null mice was only 35% of the maximum decay in WT mice (p < 0.05), reflecting the absence of GABAC receptors. The dynamic ranges (L20–80, the intensity range for 20–80% of maximal D37) were similar in WT and GABACR null mice (WT, 2.1 ± 0.8; null, 2.4 ± 0.8; p = 0.8), but the light sensitivity (L50, intensity for half-maximal D37) was reduced in mice that lacked GABAC receptors (L50 WT, −3.4 ± 0.2; L50 null, −2.7 ± 0.2; p < 0.05). The reduced light sensitivity in GABACR null mice is consistent with the notion that GABAC receptors are more sensitive to GABA than GABAA receptors (Qian and Dowling, 1993; Amin and Weiss, 1994; Feigenspan and Bormann, 1994). Our results show that stronger light stimuli resulted in more prolonged responses that were attributable to the activation of GABAC receptors to a greater extent than GABAA and glycine receptors. This suggests that stronger stimuli increase the probability of GABA release that results in the preferential activation of GABAC receptors, attributable, in part, to spillover transmission (Fig. 7).
Figure 8.
Eliminating GABAC receptors reduced the decay time of rod bipolar cell L-IPSCs as a function of light intensity. A, L-IPSCs from a WT rod bipolar cell in response to increasing intensities of light (30 ms light stimulus). Calibration: 5 pA, 500 ms. B, L-IPSCs from a GABACR null rod bipolar cell in response to increasing intensities of light. The increases in the magnitude and decay of the response are smaller for the GABACR null mice. C, The average L-IPSC D37 values from WT (n = 10) and GABACR null (n = 6) mice are plotted as a function of light intensity (log relative intensity). The curves are the best fits to a logistic function. The maximum D37 of the GABACR null curve was 35% of the WT maximum. The L20–80 values of the fits were not significantly changed (WT, 2.1 ± 0.8; null, 2.4 ± 0.8), but the GABACR null curve had a lower EC50 (WT, −3.4 ± 0.2; null, −2.7 ± 0.2).
GABAC receptors are poor temporal encoders of inhibition
We have shown that both receptor and release properties can shape L-IPSCs evoked by brief stimuli, but how do these properties shape L-IPSCs evoked by more prolonged light stimuli? Because GABAC receptors respond sluggishly to brief spontaneous and light-evoked GABA release, they are poor sensors of temporal changes in synaptic GABA concentration. In contrast, GABAA and glycine receptors, which respond briskly to brief, spontaneous GABA release, are better sensors of temporal changes of inhibitory inputs. Also, GABAC receptors are more sensitive to GABA (Amin and Weiss, 1994) and unbind GABA slowly (Chang and Weiss, 1999), suggesting that they may be saturated and ill equipped to resolve a brief light stimulus. Given these limitations of GABACR-mediated signaling, responses to more prolonged stimuli may not differ substantially from those to briefer stimuli.
To test how GABAC receptor contributions vary with light stimulus duration, we compared L-IPSCs evoked by brief (10 ms) and prolonged (1000 ms) stimuli from WT and GABACR null mice. For brief stimuli, GABAC receptors determine the duration of the L-IPSC, as shown by comparing the prolonged L-IPSC recorded from WT mice (Fig. 9A, gray trace) with the shorter L-IPSC recorded from GABACR null mice (Fig. 9B, gray trace). When the stimulus was increased to 1000 ms, the L-IPSC charge transfer in WT mice (Fig. 9A) was only marginally increased (173 ± 12%; n = 12). However, responses in GABACR null mice (Fig. 9B) were dramatically increased, compared with WT mice (414 ± 73% of the 10 ms stimulus; WT vs GABACR null, p < 0.01; n = 7) (Fig. 9C). These data suggest that the lower sensitivity to changes in stimulus duration in WT mice was attributable to slow temporal response properties and/or saturation of GABAC receptors. The enhanced sensitivity to changes in stimulus duration observed in GABACR null mice reflects the faster, temporally responding GABAA and glycine receptor abilities to respond with better fidelity to changes in transmitter release.
Figure 9.
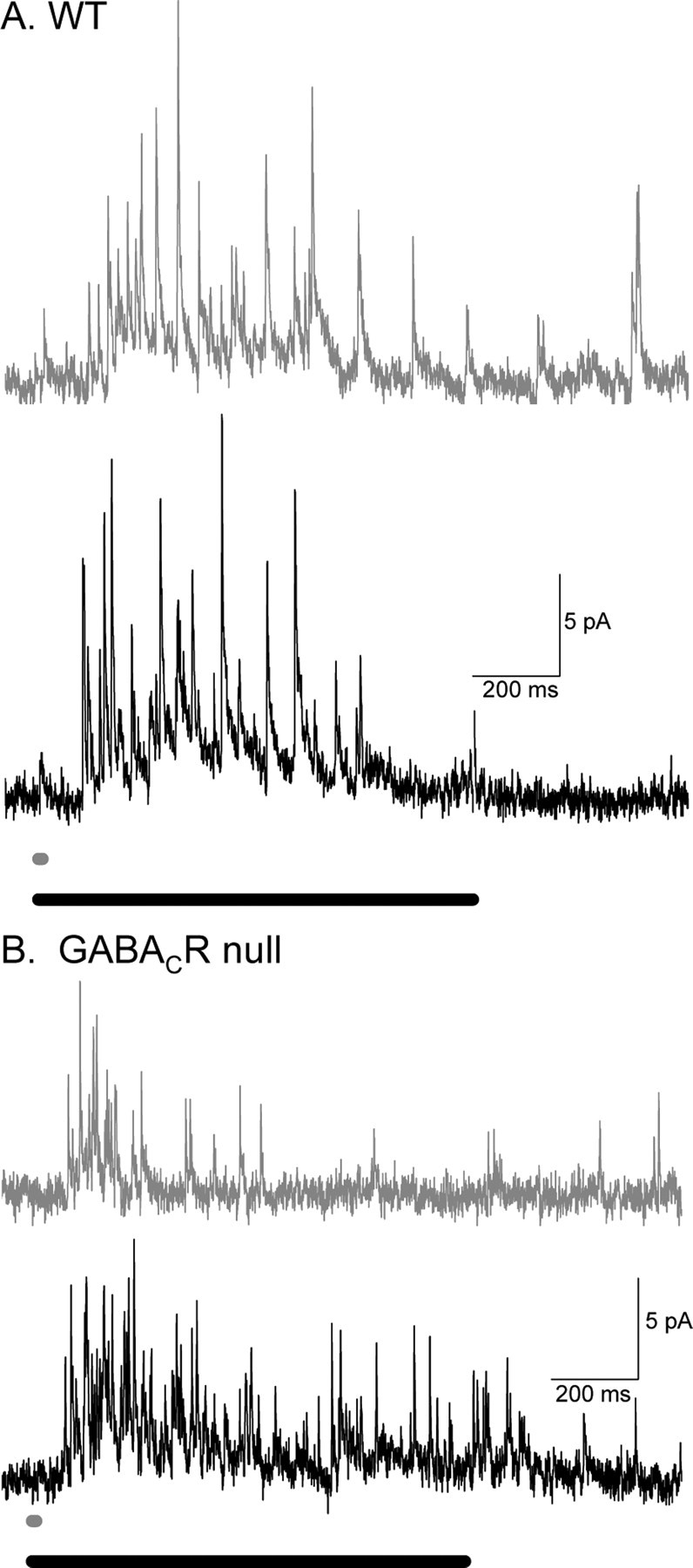
WT and GABACR null mice L-IPSCs respond differently to increasing light duration. A, Example L-IPSCs from WT rod bipolar cells in response to 10 ms (dark gray trace) and 1000 ms (black trace) light stimulation. The two L-IPSCs do not have drastically different charge transfers. B, Example L-IPSCs from GABACR null rod bipolar cells in response to 10 ms (dark gray trace) and 1000 ms (black trace) light stimulation. The 10 ms L-IPSC is much smaller than that 1000 ms L-IPSC. GABACR null L-IPSCs increase significantly more when the light duration is increased (p < 0.01).
Distinct inhibitory inputs to rod bipolar cells affect different aspects of glutamate release
How is glutamate release affected when the L-IPSCs are altered by lengthening the light stimulus? Because excitatory inputs to rod bipolar cells increase with light stimulus duration (Berntson et al., 2004; Pang et al., 2004), glutamate release is likely to increase with increased stimulus duration. However, the glutamate release from rod bipolar cells is also shaped by presynaptic inhibitory inputs, which may change with brief and prolonged light stimuli. We assessed how these two factors interact by recording L-EPSCs from A17 amacrine cells. Figure 10A shows that, when the light stimulus duration was increased from 10 to 1000 ms, the L-EPSC charge transfer in WT mice was increased to 248 ± 7% of control (n = 7). When the stimulus duration was increased, both the excitatory output (Fig. 10A) and presynaptic inhibition (Fig. 9A) to rod bipolar cells were enhanced. In WT mice, the increase in L-EPSC charge transfer with stimulus duration demonstrates that glutamate release was enhanced, consistent with our findings (Fig. 9) that presynaptic inhibition was only modestly enhanced with increased stimulus duration.
Figure 10.
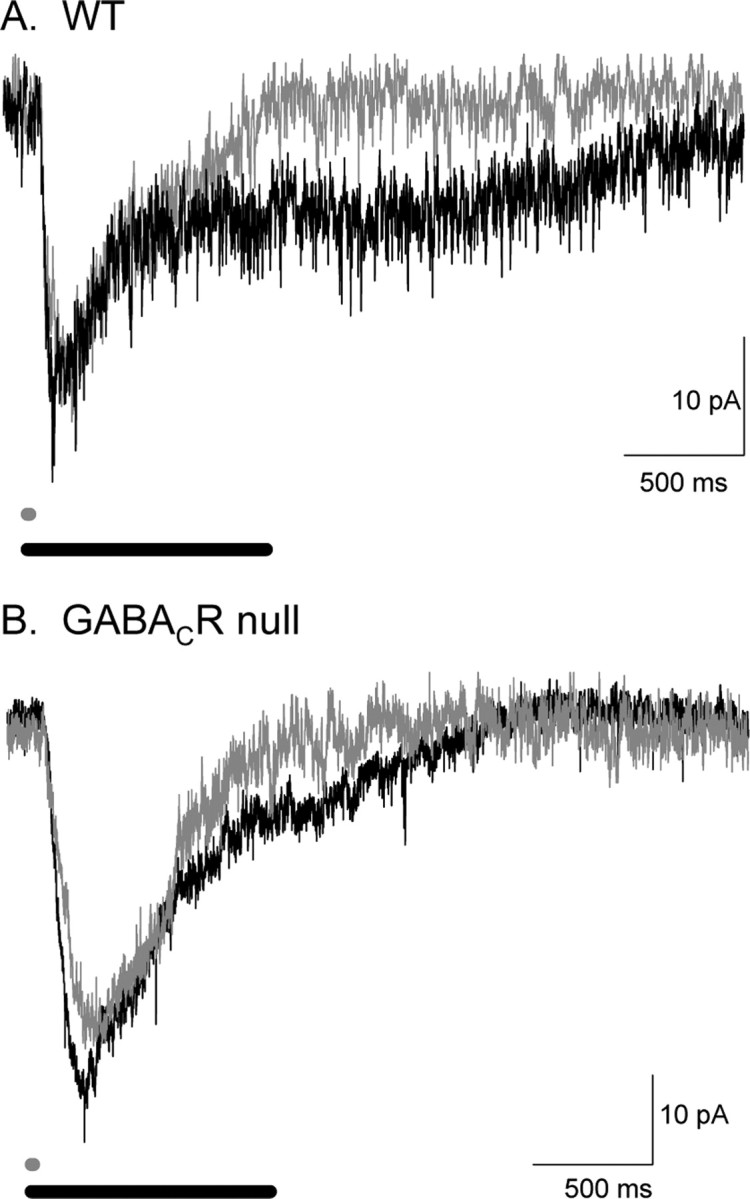
L-IPSCs differentially shape A17 L-EPSCs in WT and GABACR null mice. A, Example L-EPSCs from WT A17 amacrine cells in response to 10 ms (dark gray traces) and 1000 ms (black traces) light stimulation. The 10 ms L-EPSC is smaller than that 1000 ms L-EPSC. B, Example L-EPSCs from GABACR null A17 amacrine cells in response to 10 and 1000 ms light stimulation. The two L-EPSCs do not have as large a difference in charge transfers as the WT L-EPSCs. WT L-EPSCs increase significantly more when the light duration is increased (p < 0.01).
To better understand how GABAC receptors control the duration of glutamate release, we tested the effects of light stimulus duration in mice that lacked GABAC receptors. As noted above, glutamate release is enhanced in GABACR null mice (Fig. 3). In addition, the magnitude of presynaptic inhibition is more sensitive to stimulus duration in GABACR null mice (Fig. 9), suggesting that inhibition evoked by prolonged stimuli will suppress glutamate release more, making release less sensitive to stimulus duration. Figure 10B shows that increasing stimulus duration resulted in a smaller increase in L-EPSCs in GABACR null mice compared with WT mice (190 ± 12%; n = 10; WT vs null, p < 0.01) (Fig. 10A). Together, our findings show that amacrine cell L-EPSCs reflect a balance between glutamate release and presynaptic inhibition. Presynaptic GABAC receptors limit glutamate release, but they also allow the magnitude of glutamate release to be more sensitive to increases in stimulus duration.
Discussion
GABAC, GABAA, and glycine receptor-mediated inputs to rod bipolar cell terminals differentially shape the time course of inhibition that modulates glutamate release. Slow GABAC receptor-mediated inputs govern the extent of presynaptic inhibition, limiting the duration of glutamate release. In contrast, fast glycine and GABAA receptor-mediated inputs determine the initial peak magnitude of presynaptic inhibition, limiting the early phase of glutamate release. These differences reflect differences in receptor properties, transmitter release, and spillover that shape individual inhibitory inputs.
Receptor properties tune inhibition
Our results suggest that several aspects of receptor properties contribute to temporal tuning of inhibition in the retina. sIPSCs, which solely reflect receptor properties, showed that glycine, GABAA, and GABAC receptors have distinct kinetics, in agreement with previous reports (Euler and Wässle, 1998; Lukasiewicz and Shields, 1998; Shields et al., 2000; Frech and Backus, 2004; Vigh et al., 2005). Our data (Fig. 2) and simulations (Fig. 6) suggest that receptor kinetics determine part of the differences between L-IPSCs. Similar temporal tuning of inhibitory responses with distinct receptor types has been shown previously (Brickley et al., 1996; Hajos and Mody, 1997; Jonas et al., 1998; O’Brien and Berger, 1999; Hamann et al., 2002), but functional roles have not been assigned to these distinct inputs.
Receptor properties may also determine how L-IPSCs change with increasing stimulus duration. GABA and glycine rapidly unbind from GABAA and glycine receptors (Amin and Weiss, 1994; Legendre, 1998), whereas GABAC receptors lock onto GABA, unbinding it slowly (Chang and Weiss, 1999). Additionally, GABAC receptors are 10-fold more sensitive to GABA than GABAA receptors (Amin and Weiss, 1994) and are also more sensitive to GABA than glycine receptors are to glycine (Singer and Berger, 1999; Grudzinska et al., 2005). This prolonged, high-affinity binding of GABA could saturate GABAC receptors after a brief light stimulus, making them unresponsive to additional stimuli, unlike glycine and GABAA receptors. GABAC receptors show minimal desensitization (Amin and Weiss, 1994) compared with GABAA receptors and thus remain activated in the sustained presence of neurotransmitter. These GABAC receptor properties allow them to be preferentially activated by increased spillover (Fig. 7) and increased light-evoked release (Fig. 8), as reported in salamander (Ichinose and Lukasiewicz, 2002). In other parts of the CNS, transient and more sustained inhibition are also mediated by distinct subtypes of GABAA receptors, which have similar differences in affinity for GABA and desensitization as GABAA and GABAC receptors (Brickley et al., 1996, 2001; Wei et al., 2003; Prenosil et al., 2006).
Spillover activation of GABAC receptors may shape L-IPSCs
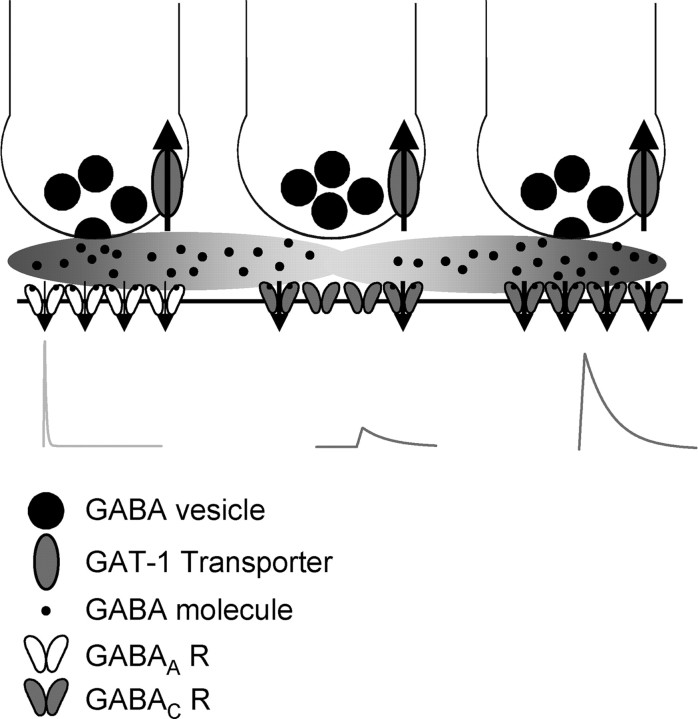
Spillover depends on the amount of neurotransmitter release (Diamond, 2001; Pankratov and Krishtal, 2003; Sola et al., 2004; Christie and Jahr, 2006), the efficacy of transmitter uptake (Chen and Diamond, 2002), and the presence of high-affinity receptors that respond to low levels of neurotransmitter (Rossi and Hamann, 1998; Diamond, 2001; Lozovaya et al., 2004). Consistent with the spillover activation, we show that the GABAC receptor-mediated component of L-IPSCs was enhanced and prolonged by reducing uptake (Fig. 7). Also, when uptake remained intact, there was a tail in the release time course sensed by GABAC receptors that was not observed with GABAA receptors (Fig. 6). Is this tail attributable to spillover?
To address this, we estimated whether the quantal content in the tail of release sensed by GABAC receptors was consistent with the magnitude of spillover observed at other synapses. We estimated the total number of released quanta from the area under the calculated release. From these areas (Fig. 6), we estimated that 31 quanta activated GABAA receptors and 16 quanta activated GABAC receptors, for a total of 47 quanta of GABA. The tail attributed to spillover and sensed by GABAC receptors represents six quanta or ∼13% of the total quantal content released at both GABAA- and GABAC receptor-containing synapses. Because synaptic GABAC receptor may be saturated as a result of their high affinity for GABA (Amin and Weiss, 1994), the calculated, total quanta of GABA released may be an underestimate of the actual release, leading to an overestimate of the percentage of GABA release attributable to spillover. The extent of spillover has been estimated for glutamatergic synapses in hippocampus (Rusakov and Kullmann, 1998; Barbour, 2001). Although hippocampal and rod bipolar cell synapse densities are similar (two glutamate synapses per cubic micrometer and three GABAC synapses per cubic micrometer, respectively) (Fletcher et al., 1998; Rusakov and Kullmann, 1998), other factors that affect spillover likely vary in the two systems, such as transporter density and synaptic geometry. Although these caveats may influence the relative magnitudes of spillover, we find that estimates of transmission attributable to spillover are similar. At hippocampal synapses, glutamate spillover to NMDA receptors is estimated to range from 1.4 to 19% of the total release (0.03–0.4% of glutamate per vesicle and 47 released vesicles, as in our calculations). The general agreement between these estimates suggests that our spillover model is reasonable (Fig. 11) and predicts that spillover from both GABAA and GABAC receptor-containing synapses activate GABAC receptors at neighboring synapses.
Figure 11.
The properties of inhibitory synaptic transmission are controlled by many independent factors. Shown is an example of three amacrine cell terminals releasing GABA onto synapses on a bipolar cell. The terminal on the left is activating GABAA receptors that have a short time course, and the terminal on the right is activating GABAC receptors that have a prolonged time course. The terminal in the middle has not released GABA, but the GABAC receptors underlying it are being activated by spillover from neighboring synapses, which respond with a small, delayed current. GABA is taken up by GAT-1 transporters, which control the amount of GABA that leaves the synapse.
Transmitter release properties shape inhibition
Our transmitter release estimates suggested that glycine, GABAA, and GABAC receptor-mediated inputs were also shaped by distinct release time courses. However, as discussed above, distinct release time courses may reflect the differences between GABAA and GABAC receptor properties, such as saturation and spillover activation. Still, GABAA and GABAC receptors are clustered at distinct synapses (Fletcher et al., 1998; Koulen et al., 1998), suggesting that distinct release from separate inputs may occur, but it is not known whether these receptors receive input from the same or separate amacrine cells. Because glycine and GABA receptors are activated by release from distinct populations of amacrine cells (Pourcho and Goebel, 1983; Vaney, 1990; Menger et al., 1998), it is likely that the different release time courses we measured reflect activation by separate release transients. This adds another dimension of control into synaptic transmission. Similar differences in neurotransmitter release have been shown to mediate distinct response kinetics in many systems, including the hippocampus (Hefft and Jonas, 2005) and the photoreceptor to bipolar cell synapse (Cadetti et al., 2005).
Roles of inhibition on signal processing
The slow GABAC receptors that dominated rod bipolar L-IPSCs are well matched to the time course of rod bipolar cell excitation, which is significantly slower than the cone pathway excitation (Schnapf and Copenhagen, 1982; Cadetti et al., 2005). Prolonged GABAC receptor-mediated inhibition is optimized to control the slow glutamate release from rod bipolar cells, as suggested by a recent report on feedback inhibition in fish bipolar cells (Vigh et al., 2005). Similar matching of IPSC and EPSC time courses has been reported in development, because the kinetics of inhibitory currents (Brickley et al., 1996; Dunning et al., 1999; Okada et al., 2000) decrease in parallel with the kinetics of excitatory currents (Takahashi, 2005). Thus, a role of GABAC receptors may be to match inhibition to slow rod-mediated excitation.
Additionally, the distinct forms of light-evoked presynaptic inhibition mediated by GABAA, GABAC, and glycine receptors differentially shape excitatory signaling to A17 amacrine cells. We found that GABAC receptors limit glutamate release from rod bipolar cells most effectively, because L-EPSCs transferred more charge and were more prolonged in mice lacking GABAC receptors, in agreement with previous studies (Lukasiewicz et al., 1994; Dong and Werblin, 1998; Bloomfield and Xin, 2000). Similarly, our light stimulus duration results suggested that the limited increase of GABAC receptor-mediated inhibition with stimulus duration serves to increase the temporal sensitivity of excitatory outputs of the retina. However, we also found that GABAC receptor-mediated inhibition was sensitive to changes in stimulus intensity, potentially compensating for the activation of more excitatory inputs with greater stimulus intensities. Thus, GABAC receptors played two roles: regulating the magnitude of glutamate release in response to brief stimuli and allowing the increase of glutamate release with increasing stimulus duration. Thus, GABAC receptors could be important in determining the timing and magnitude of excitatory signaling in the retina.
Although GABAA and glycine receptors transfer less charge than GABAC receptors in rod bipolar cells, our findings demonstrate that their more rapid response properties contributed to shaping the rod bipolar cell output by decreasing the initial peak of the A17 amacrine cell L-EPSCs. A larger amacrine cell L-EPSC peak suggests that it will reach its spike threshold faster, speeding its communication with downstream neurons. Consequently, modulating glycine and GABAA receptor-mediated presynaptic inputs to rod bipolar cells may influence the speed of A17 amacrine cell communication. Thus, the distinct inhibitory receptors on rod bipolar cell terminals shape the flow of information to A17 amacrine cells in separate ways. GABA and glycine signals originate in separate types of amacrine cells, which potentially have different spatial extents (Pourcho and Goebel, 1983; Vaney, 1990; Menger et al., 1998; O’Brien et al., 2003), but is not known whether GABAA and GABAC receptor-mediated signals originate from the same or separate amacrine cells. These separate signaling pathways could be independently modulated, suggesting an additional diversity for controlling glutamate release from rod bipolar cells.
Footnotes
This work was supported by National Institutes of Health Grants T32 EY13360 and F32 EY15629 (E.D.E.), EY08922 (P.D.L.), and EY02687 (Washington University Department of Ophthalmology), Research to Prevent Blindness, and The M. Bauer Foundation. We thank Drs. Tomomi Ichinose, Botir T. Sagdullaev, and Steve Mennerick for helpful discussion and comments on this manuscript and James Debrecht for technical assistance.
References
- Amin and Weiss, 1994.Amin J, Weiss DS. Homomeric rho 1 GABA channels: activation properties and domains. Receptors Channels. 1994;2:227–236. [PubMed] [Google Scholar]
- Barbour, 2001.Barbour B. An evaluation of synapse independence. J Neurosci. 2001;21:7969–7984. doi: 10.1523/JNEUROSCI.21-20-07969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson et al., 2004.Berntson A, Smith RG, Taylor WR. Postsynaptic calcium feedback between rods and rod bipolar cells in the mouse retina. Vis Neurosci. 2004;21:913–924. doi: 10.1017/S095252380421611X. [DOI] [PubMed] [Google Scholar]
- Bloomfield and Xin, 2000.Bloomfield SA, Xin D. Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. J Physiol (Lond) 523. 2000;3:771–783. doi: 10.1111/j.1469-7793.2000.t01-1-00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley et al., 1996.Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley et al., 2001.Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Cadetti et al., 2005.Cadetti L, Tranchina D, Thoreson WB. A comparison of release kinetics and glutamate receptor properties in shaping rod-cone differences in EPSC kinetics in the salamander retina. J Physiol (Lond) 2005;569:773–788. doi: 10.1113/jphysiol.2005.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang and Weiss, 1999.Chang Y, Weiss DS. Channel opening locks agonist onto the GABAC receptor. Nat Neurosci. 1999;2:219–225. doi: 10.1038/6313. [DOI] [PubMed] [Google Scholar]
- Chen and Diamond, 2002.Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of the rat retina. J Neurosci. 2002;22:2165–2173. doi: 10.1523/JNEUROSCI.22-06-02165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu et al., 2005.Chiu CS, Brickley S, Jensen K, Southwell A, McKinney S, Cull-Candy S, Mody I, Lester HA. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J Neurosci. 2005;25:3234–3245. doi: 10.1523/JNEUROSCI.3364-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie and Jahr, 2006.Christie JM, Jahr CE. Multivesicular release at Schaffer collateral–CA1 hippocampal synapses. J Neurosci. 2006;26:210–216. doi: 10.1523/JNEUROSCI.4307-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen et al., 1981.Cohen I, van der Kloot W, Attwell D. The timing of channel opening during miniature end-plate currents. Brain Res. 1981;223:185–189. doi: 10.1016/0006-8993(81)90821-0. [DOI] [PubMed] [Google Scholar]
- Cui et al., 2003.Cui J, Ma YP, Lipton SA, Pan ZH. Glycine receptors and glycinergic synaptic input at the axon terminals of mammalian retinal rod bipolar cells. J Physiol (Lond) 2003;553:895–909. doi: 10.1113/jphysiol.2003.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, 2001.Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J Neurosci. 2001;21:8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond and Jahr, 1995.Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Dong and Werblin, 1998.Dong C, Werblin FS. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J Neurophysiol. 1998;79:2171–2180. doi: 10.1152/jn.1998.79.4.2171. [DOI] [PubMed] [Google Scholar]
- Dunning et al., 1999.Dunning DD, Hoover CL, Soltesz I, Smith MA, O’Dowd DK. GABAA receptor-mediated miniature postsynaptic currents and alpha-subunit expression in developing cortical neurons. J Neurophysiol. 1999;82:3286–3297. doi: 10.1152/jn.1999.82.6.3286. [DOI] [PubMed] [Google Scholar]
- Eggers and Lukasiewicz, 2006.Eggers ED, Lukasiewicz PD. GABAA, GABAC and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol (Lond) 2006;572:215–225. doi: 10.1113/jphysiol.2005.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler and Wässle, 1998.Euler T, Wässle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol. 1998;79:1384–1395. doi: 10.1152/jn.1998.79.3.1384. [DOI] [PubMed] [Google Scholar]
- Feigenspan and Bormann, 1994.Feigenspan A, Bormann J. Differential pharmacology of GABA-A and GABA-C receptors on rat retinal bipolar cells. Eur J Pharmacol. 1994;288:97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Fletcher et al., 1998.Fletcher EL, Koulen P, Wässle H. GABAA and GABAC receptors on mammalian rod bipolar cells. J Comp Neurol. 1998;396:351–365. doi: 10.1002/(sici)1096-9861(19980706)396:3<351::aid-cne6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Frech and Backus, 2004.Frech MJ, Backus KH. Characterization of inhibitory postsynaptic currents in rod bipolar cells of the mouse retina. Vis Neurosci. 2004;21:645–652. doi: 10.1017/S0952523804214134. [DOI] [PubMed] [Google Scholar]
- Grudzinska et al., 2005.Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Hajos and Mody, 1997.Hajos N, Mody I. Synaptic communication among hippocampal interneurons: properties of spontaneous IPSCs in morphologically identified cells. J Neurosci. 1997;17:8427–8442. doi: 10.1523/JNEUROSCI.17-21-08427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann et al., 2002.Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hefft and Jonas, 2005.Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Ichinose and Lukasiewicz, 2002.Ichinose T, Lukasiewicz PD. GABA transporters regulate inhibition in the retina by limiting GABAC receptor activation. J Neurosci. 2002;22:3285–3292. doi: 10.1523/JNEUROSCI.22-08-03285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas et al., 1998.Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Keros and Hablitz, 2005.Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol. 2005;94:2073–2085. doi: 10.1152/jn.00520.2005. [DOI] [PubMed] [Google Scholar]
- Koulen et al., 1996.Koulen P, Sassoe-Pognetto M, Grunert U, Wässle H. Selective clustering of GABAA and glycine receptors in the mammalian retina. J Neurosci. 1996;16:2127–2140. doi: 10.1523/JNEUROSCI.16-06-02127.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen et al., 1998.Koulen P, Brandstätter JH, Enz R, Bormann J, Wässle H. Synaptic clustering of GABAC receptor r-subunits in rat retina. Eur J Neurosci. 1998;10:115–127. doi: 10.1046/j.1460-9568.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- Legendre, 1998.Legendre P. A reluctant gating mode of glycine receptor channels determines the time course of inhibitory miniature synaptic events in zebrafish hindbrain neurons. J Neurosci. 1998;18:2856–2870. doi: 10.1523/JNEUROSCI.18-08-02856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya et al., 2004.Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape “superslow” afterburst EPSC in rat hippocampus. J Physiol (Lond) 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz and Shields, 1998.Lukasiewicz P, Shields C. Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J of Neurophysiol. 1998;79:3157–3167. doi: 10.1152/jn.1998.79.6.3157. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz and Roeder, 1995.Lukasiewicz PD, Roeder RC. Evidence for glycine modulation of excitatory synaptic inputs to retinal ganglion cells. J Neurosci. 1995;15:4592–4601. doi: 10.1523/JNEUROSCI.15-06-04592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz et al., 1994.Lukasiewicz PD, Maple BR, Werblin FS. A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J Neurosci. 1994;14:1202–1212. doi: 10.1523/JNEUROSCI.14-03-01202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall et al., 2002.McCall MA, Lukasiewicz PD, Gregg RG, Peachey NS. Elimination of the r1 subunit abolishes GABAC receptor expression and alters visual processing in the mouse retina. J Neurosci. 2002;22:4163–4174. doi: 10.1523/JNEUROSCI.22-10-04163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger et al., 1998.Menger N, Pow DV, Wässle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol. 1998;401:34–46. doi: 10.1002/(sici)1096-9861(19981109)401:1<34::aid-cne3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- O’Brien et al., 2003.O’Brien BJ, Richardson RC, Berson DM. Inhibitory network properties shaping the light evoked responses of cat alpha retinal ganglion cells. Vis Neurosci. 2003;20:351–361. doi: 10.1017/s0952523803204016. [DOI] [PubMed] [Google Scholar]
- O’Brien and Berger, 1999.O’Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol. 1999;82:1638–1641. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- Okada et al., 2000.Okada M, Onodera K, Van Renterghem C, Sieghart W, Takahashi T. Functional correlation of GABAA receptor α subunits expression with the properties of IPSCs in the developing thalamus. J Neurosci. 2000;20:2202–2208. doi: 10.1523/JNEUROSCI.20-06-02202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang et al., 2004.Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol (Lond) 2004;558:897–912. doi: 10.1113/jphysiol.2003.059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov and Krishtal, 2003.Pankratov YV, Krishtal OA. Distinct quantal features of AMPA and NMDA synaptic currents in hippocampal neurons: implication of glutamate spillover and receptor saturation. Biophys J. 2003;85:3375–3387. doi: 10.1016/S0006-3495(03)74757-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcho and Goebel, 1983.Pourcho RG, Goebel DJ. Neuronal subpopulations in cat retina which accumulate the GABA agonist, [3H]muscimol: a combined Golgi and autoradiographic study. J Comp Neurol. 1983;219:25–35. doi: 10.1002/cne.902190104. [DOI] [PubMed] [Google Scholar]
- Prenosil et al., 2006.Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Qian and Dowling, 1993.Qian H, Dowling JE. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993;361:162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- Qian and Dowling, 1995.Qian H, Dowling JE. GABAa and GABAc receptors on hybrid bass retinal bipolar cells. J Neurophysiol. 1995;74:1920–1928. doi: 10.1152/jn.1995.74.5.1920. [DOI] [PubMed] [Google Scholar]
- Rossi and Hamann, 1998.Rossi DJ, Hamann M. Spillover-mediated transmission at inhbibitory synapses promoted by high affinity a6 subunit GABAA receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Rusakov and Kullmann, 1998.Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci. 1998;18:3158–3170. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent et al., 2005.Sargent PB, Saviane C, Nielsen TA, DiGregorio DA, Silver RA. Rapid vesicular release, quantal variability, and spillover contribute to the precision and reliability of transmission at a glomerular synapse. J Neurosci. 2005;25:8173–8187. doi: 10.1523/JNEUROSCI.2051-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf and Copenhagen, 1982.Schnapf JL, Copenhagen DR. Differences in the kinetics of rod and cone synaptic transmission. Nature. 1982;296:862–864. doi: 10.1038/296862a0. [DOI] [PubMed] [Google Scholar]
- Shields et al., 2000.Shields CR, Tran MN, Wong RO, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. J Neurosci. 2000;20:2673–2682. doi: 10.1523/JNEUROSCI.20-07-02673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer and Berger, 1999.Singer JH, Berger AJ. Contribution of single-channel properties to the time course and amplitude variance of quantal glycine currents recorded in rat motoneurons. J Neurophysiol. 1999;81:1608–1616. doi: 10.1152/jn.1999.81.4.1608. [DOI] [PubMed] [Google Scholar]
- Sola et al., 2004.Sola E, Prestori F, Rossi P, Taglietti V, D’Angelo E. Increased neurotransmitter release during long-term potentiation at mossy fibre-granule cell synapses in rat cerebellum. J Physiol (Lond) 2004;557:843–861. doi: 10.1113/jphysiol.2003.060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, 2005.Takahashi T. Postsynaptic receptor mechanisms underlying developmental speeding of synaptic transmission. Neurosci Res. 2005;53:229–240. doi: 10.1016/j.neures.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Takahashi et al., 1992.Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992;9:1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- Van der Kloot, 1988.Van der Kloot W. Estimating the timing of quantal releases during end-plate currents at the frog neuromuscular junction. J Physiol (Lond) 1988;402:595–603. doi: 10.1113/jphysiol.1988.sp017224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney, 1990.Vaney DI. The mosaic of amacrine cells in the mammalian retina. Prog Retin Eye Res. 1990;9:49–100. [Google Scholar]
- Vigh et al., 2005.Vigh J, Li GL, Hull C, von Gersdorff H. Long-term plasticity mediated by mGluR1 at a retinal reciprocal synapse. Neuron. 2005;46:469–482. doi: 10.1016/j.neuron.2005.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei et al., 2003.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin, 1978.Werblin FS. Transmission along and between rods in the tiger salamander retina. J Physiol (Lond) 1978;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]