Abstract
Central congenital hypoventilation syndrome is caused by mutations of the gene that encodes the transcription factor Phox2b. The syndrome is characterized by a severe form of sleep apnea attributed to greatly compromised central and peripheral chemoreflexes. In this study, we analyze whether Phox2b expression in the brainstem respiratory network is preferentially associated with neurons involved in chemosensory integration in rats. At the very rostral end of the ventral respiratory column (VRC), Phox2b was present in many VGlut2 (vesicular glutamate transporter 2) mRNA-containing neurons. These neurons were functionally identified as the respiratory chemoreceptors of the retrotrapezoid nucleus (RTN). More caudally in the VRC, many fewer neurons expressed Phox2b. These cells were not part of the central respiratory pattern generator (CPG), because they were typically cholinergic visceral motor neurons or catecholaminergic neurons (presumed C1 neurons). Phox2b was not detected in serotonergic neurons, in the A5, A6, and A7 noradrenergic cell groups nor within the main cardiorespiratory centers of the dorsolateral pons. Phox2b was expressed by many solitary tract nucleus (NTS) neurons including those that relay peripheral chemoreceptor information to the RTN. These and previous observations by others suggest that Phox2b is expressed by an uninterrupted chain of neurons involved in the integration of peripheral and central chemoreception (carotid bodies, chemoreceptor afferents, chemoresponsive NTS neurons projecting to VRC, RTN chemoreceptors). The presence of Phox2b in this circuit and its apparent absence from the respiratory CPG could explain why Phox2b mutations disrupt breathing automaticity during sleep without causing major impairment of respiration during waking.
Keywords: respiration, central congenital hypoventilation syndrome, retrotrapezoid nucleus, central chemoreceptors, medulla oblongata, pons, central autonomic pathways
Introduction
The central congenital hypoventilation syndrome (CCHS) is caused by mutations of the homeobox gene PHOX2B (Amiel et al., 2003; Weese-Mayer et al., 2005b). The defining characteristic of CCHS is a disabling central sleep apnea, although the syndrome also includes a diffuse pathology of the autonomic nervous system (Spengler et al., 2001; Gaultier et al., 2004; Weese-Mayer et al., 2005b). Daytime breathing is modestly impaired and is still activated normally by arousal, cognitive activity or aerobic exercise (Paton et al., 1993; Shea et al., 1993), but respiratory stimulation by hypoxia and, most particularly, by hypercapnia is severely attenuated during both waking and sleep (Spengler et al., 2001). The severely depressed respiratory stimulation by hypercapnia denotes a particularly marked deficit of central respiratory chemoreception (Spengler et al., 2001), the mechanism by which brain pCO2 drives respiration via acidification of the extracellular fluid (Feldman et al., 2003; Putnam et al., 2004; Nattie, 2006; Smith et al., 2006).
Central respiratory chemoreception is not fully understood. The prevalent view is that the phenomenon relies on a finite number of pH-modulated neuronal clusters located in the medulla oblongata (Okada et al., 2002; Feldman et al., 2003; Richerson et al., 2005; Nattie, 2006). Other authors consider that central respiratory chemoreception could be an emergent property of the respiratory network resulting from the uniform pH sensitivity of its contributing neurons (Kawai et al., 1996).
In the present study, we analyzed the pattern of expression of Phox2b in the adult rat and found that this transcription factor is present in the adult pons and medulla in a pattern that is generally consistent with its prenatal expression (Brunet and Pattyn, 2002; Dauger et al., 2003). The persistence of Phox2b expression in the adult rat allowed us to perform anatomical and physiological studies designed to identify the nature and function of the Phox2b-expressing neurons located in regions that are essential to respiratory rhythm and pattern generation (Feldman et al., 2003, 2006). Here, we report that Phox2b is expressed by the central chemoreceptors located in the retrotrapezoid nucleus (RTN) [for an anatomical definition of this nucleus, see Ellenberger and Feldman (1990), Cream et al. (2002), and Feldman and Del Negro (2006)] but is virtually absent in the rest of the ventral respiratory column and in the respiratory-related regions of the dorsolateral pons. We also report that RTN neurons receive inputs from the carotid bodies via a projection from the solitary tract nucleus (NTS) that also expresses Phox2b. We conclude that Phox2b expression by the pontomedullary respiratory network is restricted to a specific group of central chemoreceptor neurons in the RTN and to an uninterrupted chain of neurons that relay information from peripheral chemoreceptors to the ventral respiratory column. This pattern of expression is therefore consistent with the nature of the respiratory deficits observed in CCHS.
Materials and Methods
Anatomical and whole animal physiological experiments were done in adult male Sprague Dawley rats (250–350 g; Taconic; Germantown, NY). Procedures were in accordance with National Institutes of Health Animal Care and Use Guidelines and were approved by the University of Virginia Animal Care and Use Committee.
Physiology.
The experimental procedures (surgery, injections in RTN, hypoxia protocol, nerve recordings, single unit recording, and single neuron labeling in vivo) were described in previous publications (Mulkey et al., 2004; Guyenet et al., 2005b; Takakura et al., 2006). All recordings were made in halothane-anesthetized rats (1%), paralyzed with pancuronium, ventilated with pure oxygen, and maintained at 37.5°C. The recorded physiological variables were blood pressure, end-expiratory CO2, phrenic nerve discharge (PND), single-unit activity, and current passed through the electrode to deliver biotinamide by the juxtacellular labeling method (2–5 nA; 400 ms; 2.5 Hz). RTN neurons were recorded in rats in which the vagus nerves were cut to uncouple the central respiratory pattern generator from the ventilation cycle. Bötzinger neurons and bulbospinal inspiratory augmenting neurons were recorded in rats with intact vagus nerves as described previously (Kanjhan et al., 1995; Schreihofer et al., 1999; Stornetta et al., 2003).
All analog data were stored on a microcomputer via a micro-1401 digitizer from Cambridge Electronics Design (CED) (Cambridge, UK) and were processed off-line using version 5 of the Spike 2 software (CED). Processing included action potential discrimination and binning, neuronal discharge rate measurement, and PND “integration” (iPND) consisting of rectification and smoothing (τ, 0.015 s). Neural minute · volume (mvPND) (a measure of the total phrenic nerve discharge per unit of time) was determined by averaging iPND over 50 s (vagotomized rats) and normalizing the result by assigning a value of 0 to the dependent variable recorded at low levels of end-expiratory CO2 (below the central apneic threshold) and a value of 1 at the highest level of pCO2 investigated (between 9.5 and 10%). The CED software was also used for acquisition of perievent histograms of neuronal activity and perievent averages of iPND or tracheal CO2. The perievent histograms of neuronal single-unit activity were triggered either on iPND or on the tracheal CO2 traces and represented the summation of at least 100 respiratory cycles (350–800 action potentials per histogram). The steady-state relationship between RTN neuronal activity and end-expiratory CO2 was obtained by stepping the inspired CO2 level to various values for a minimum of 3 min and up to 5 min. The mean discharge rate of the neuron was measured during the last 30 s of each step at which time end-expiratory CO2 and the discharge of the neuron appeared to have reached equilibrium. End-expiratory CO2 was measured by averaging the maximum values recorded from 10 consecutive breaths at the midpoint of the time interval sampled.
Histology.
All histology was performed using brain tissue from rats deeply anesthetized with pentobarbital and perfused transcardially with 100 ml of buffered saline followed by 500 ml of freshly prepared 4% paraformaldehyde in 100 mm phosphate buffer. The histochemical procedures were done using 30-μm-thick free-floating sections cut in the coronal or parasagittal plane with all rinses done between all antibody incubations in Tris-buffered saline, pH 7.4. Vesicular glutamate transporter 2 (VGlut2) mRNA and glutamic acid decarboxylase 67 (GAD67) mRNA were detected by nonradioactive in situ hybridization using digoxigenin-labeled cRNA probes as described previously (Stornetta and Guyenet, 1999; Stornetta et al., 2003). Tyrosine hydroxylase (TH) was identified using a mouse monoclonal antibody (1:1000; Chemicon, Temecula, CA) followed by a Cy3-tagged goat anti-mouse IgG (1:200; Jackson ImmunoResearch, West Grove, PA). Choline acetyltransferase (ChAT) was identified using a goat antibody (1:50; Chemicon) followed by a Cy3-tagged donkey anti-goat IgG (1:200; Jackson ImmunoResearch). c-Fos was detected with a goat antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) followed by a Cy3-tagged donkey anti-goat IgG (1:200; Jackson ImmunoResearch). Tryptophan hydroxylase (TrypOH) was identified with a mouse antibody (1:1000; Sigma-Aldrich, St. Louis, MO) followed by an Alexa 488-tagged goat anti-mouse IgG (1:200; Invitrogen, Carlsbad, CA). The antibodies against c-Fos, TH, ChAT, and TrypOH have been used previously, and their specificity has been established in rat brain in previous studies from this laboratory (Wang et al., 2001; Weston et al., 2003; Mulkey et al., 2004; Takakura et al., 2006). Phox2b was detected using a rabbit polyclonal antibody raised against the 14 aa C-terminal sequence of the Phox2b protein (Pattyn et al., 1997), a sequence identical in rats and mice. The specificity was originally ascertained by the perfect match between the expression patterns detected by immunohistochemistry and in situ hybridization (Pattyn et al., 1997) and confirmed by the complete absence of immunoreactivity in Phox2b knock-out mice (A. Pattyn and J.-F. Brunet, unpublished data). For the present study, we resynthesized the C-terminal tetradecapeptide and verified that this peptide dose-dependently suppressed Phox2b-like immunoreactivity detected in rat brain with our experimental protocols (complete elimination of immunoreactivity at 3.7 μg/ml of the peptide).
Phox2b immunoreactivity was detected by primary antibody incubation at a dilution of 1:800 for 48–72 h followed by a Cy3-tagged donkey anti-rabbit IgG (1:200; Jackson ImmunoResearch). This method was used when Phox2b detection was combined with in situ hybridization for VGlut2 or GAD67 mRNA. It was also used in three of eight cases testing whether neurons labeled with biotinamide in vivo expressed Phox2b. In the other five cases for in vivo labeling, sections were incubated in a more dilute concentration of primary antibody (1:8000) followed by a goat anti-rabbit IgG (1:150; Covance Research Products, Denver, PA) followed by rabbit peroxidase–anti-peroxidase (1:150; Covance) followed by tyramide-Cy3 (1:75; PerkinElmer, Boston, MA). In cases in which Phox2b immunodetection was combined with c-Fos, TH, or ChAT immunodetection, a similar protocol using tyramide amplification was followed. Sections were incubated in Phox2b antibody (1:8000) followed by a biotinylated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch). This step was followed by streptavidin HRP (1:200; PerkinElmer) and tyramide-Cy3 (1:200; PerkinElmer) or a biotinylated tyramide (1:75; PerkinElmer) followed by avidin-Alexa 488 (1:200; Invitrogen).
A conventional multifunction Zeiss (Oberkochen, Germany) Axioskop 2 microscope (bright-field, dark-field, and epifluorescence) was used for all observations. The computer-assisted mapping technique made use of a motor-driven microscope stage controlled by the Neurolucida software and has been described in detail previously (Stornetta and Guyenet, 1999). The Neurolucida files were exported to the NeuroExplorer software (MicroBrightField, Williston, VT) to count the various types of neuronal profiles within a defined area of the reticular formation. In all experiments, brain sections were arranged on slides in sequential order. Alignment of coronal sections between brains was done relative to a reference section selected as the most caudal section containing an identifiable cluster of facial motor neurons. This level was identified in each brain and assigned the level 11.6 mm caudal to bregma (bregma, −11.6 mm) according to the atlas of Paxinos and Watson (1998). Levels rostral or caudal to this reference section were determined by adding a distance corresponding to the interval between sections multiplied by the number of intervening sections. The nomenclature of the various subdivisions of the ventral respiratory column (VRC) is after Alheid et al. (2002).
Data are reported as means ± SEM.
Results
Phox2b is expressed by the central respiratory chemoreceptor neurons of the RTN
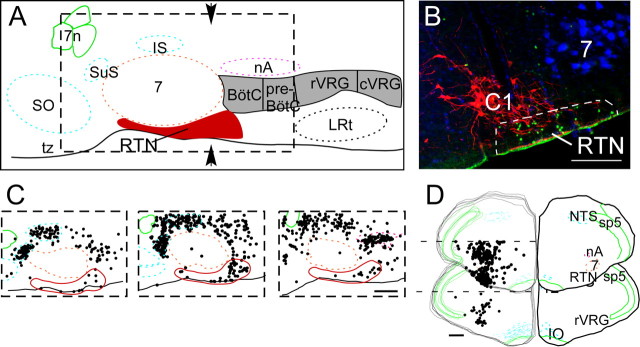
The region of the RTN is schematically outlined in Figure 1 A [redrawn from Feldman and Del Negro (2006)]. As shown in a coronal plane corresponding to the arrows in Figure 1 A, the RTN contains numerous Phox2b-immunoreactive (-ir) nuclei [Fig. 1 B, labeled by Alexa 488 (green)]. A minority of these nuclei, typically more weakly labeled, are TH-ir neurons of the C1 adrenergic group [Fig. 1 B, tagged with Cy-3 (red)] [for definition and background on C1 cells, see Guyenet (2006)]. However, the bulk of the Phox2b-ir nuclei of the RTN region are not catecholaminergic and are tightly clustered in a location near the brain surface ventrolateral to the C1 neurons and below the facial motor nucleus. The facial motor neurons in Figure 1 B were retrogradely labeled by an intraperitoneal injection of FluoroGold, which fluoresces in the blue spectrum (Leong and Ling, 1990; Mulkey et al., 2004). Computer-assisted mapping of Phox2b-ir nuclei in the RTN region was performed in parasagittal and coronal sections. The large collection of Phox2b-ir cells located rostral and dorsal to the facial motor nucleus (Fig. 1 C) is contained in the salivatory nuclei. Dorsal and caudal to the facial motor nucleus, another dense collection of Phox2b-ir cells corresponds to nucleus ambiguus (Fig. 1 C). The Phox2b-ir cells of the RTN region are located in close proximity to the ventral medullary surface both rostral and caudal to the caudal edge of the facial motor nucleus (Fig. 1 C), the bulk of the cells being within 400–500 μm on either side of this landmark. The portion of the RTN that lies under the rostral one-half of the facial motor nucleus contains very few Phox2b-ir neurons, almost all located in the marginal layer (lower 50 μm) of the medulla oblongata. The top stack of five coronal sections represented in Figure 1 D illustrates that Phox2b-ir nuclei form a distinct cluster of cells centered 2 mm lateral to the midline at the ventral surface of the rostral ventrolateral medulla. This cluster resides ∼1.5 mm ventral to a second collection of Phox2b-ir neurons that corresponds to the nucleus ambiguus. This ventral group of Phox2b-ir neurons (RTN) is no longer observed at more caudal levels of the ventrolateral medulla (Fig. 1 D, bottom stack of coronal sections).
Figure 1.
Phox2b expression in the retrotrapezoid region. A, Schematic parasagittal section through the rostral medulla and caudal pons redrawn from Feldman and Del Negro (2006). The RTN is shown in red, and the other subdivisions of the ventral respiratory column are shown in gray (BötC, Bötzinger region; preBötC, pre-Bötzinger complex; rVRG, rostral ventral respiratory group; cVRG, caudal ventral respiratory group). B, Phox2b-ir nuclei (Alexa 488; green) in the ventrolateral medulla in a coronal section at the level indicated by the arrows in A. Tyrosine hydroxylase immunoreactivity (Cy3; red) indicates the location of the C1 group of adrenergic neurons. Facial motor neurons (7) are retrogradely labeled with FG (fluoresces in the blue spectrum) after an intraperitoneal injection of FG in rats 3–7 d before perfusion. A few of the TH-ir neurons have weakly Phox2b-ir nuclei; this double label is not visible at this resolution. The RTN neurons are TH-negative and located within the region identified by the dotted line. C, Location of Phox2b-ir neurons in parasagittal sections (from left to right: 2.4, 2.2, and 1.9 mm lateral to the midline). Each box represents the region delineated by the dotted line in A. The dense clusters of Phox2b-ir neurons located dorsal and rostral to the facial motor nucleus are the parasympathetic neurons of the inferior and superior salivatory nuclei (IS and SuS in A). The motor neurons of nucleus ambiguus (nA in A) are also labeled. D, Top left side is a stack of five coronal sections (90 μm apart) straddling the region from bregma −11.4 to −11.9 mm according to Paxinos and Watson (1998) and is therefore centered on the caudal-most end of the facial motor nucleus. The bottom left stack of five sections spans the region from bregma −12.6 to −13.1 mm and encompasses the preBötC and rVRG regions. Each dot in C and D represents a single Phox2b-ir neuron placed on the section by computer-assisted plotting. The right-hand side of each section in D is traced as an overlay of the left-hand stack to illustrate the specific brain areas. Other abbreviations: 7, facial motor nucleus; 7n, facial nerve; IO, inferior olive; LRt, lateral reticular nucleus; py, pyramidal tract; sp5, spinal trigeminal tract; SO, superior olive; tz, trapezoid body. Scale bars: B, 100 μm; C, D, 500 μm.
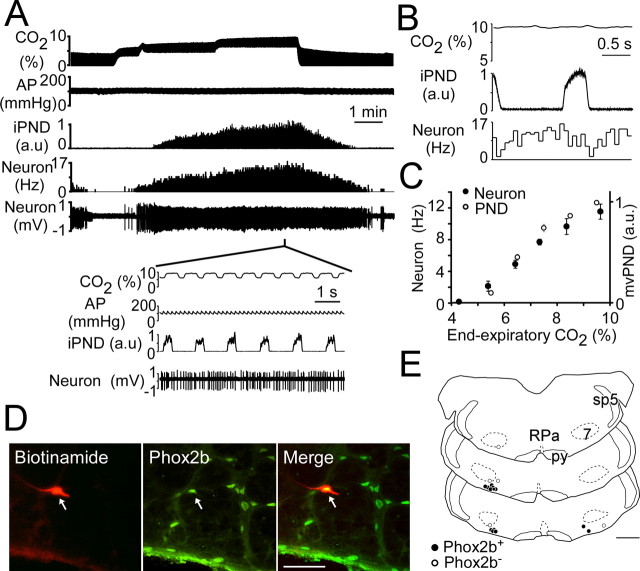
The ventral surface cluster of Phox2b-ir neurons coincides with the region in which we have previously identified central chemoreceptor neurons (Mulkey et al., 2004; Guyenet et al., 2005b). To ascertain whether the RTN chemoreceptor neurons contain Phox2b, we recorded from these cells in anesthetized rats, labeled them with biotinamide, and tested whether the labeled cells had a Phox2b-ir nucleus. The characteristic discharge pattern of an RTN chemoreceptor neuron is illustrated in Figure 2 A–C. The cell was silent at low levels of CO2 and activated by hypercapnia in proportion to the level of end-expiratory CO2 (Fig. 2 A,C). Its discharge displayed a modest synchronization with the PND at high levels of CO2 (Fig. 2 A, inset). Perievent averaging of the unit activity of the cell revealed a lower firing probability during early inspiration and at the transition between inspiration and expiration (Fig. 2 B). This pattern is the most commonly encountered in this cell group (Guyenet et al., 2005b). This neuron had a lower CO2 threshold than the PND (Fig. 2 A), a feature that was consistent from cell to cell as illustrated in Figure 2 C. This neuron was successfully labeled with biotinamide and was found to have a Phox2b-ir nucleus (Fig. 2 D). The recording and labeling procedure was repeated on 18 RTN neurons with very similar discharge characteristics (eight rats). Thirteen of the labeled neurons had a Phox2b-ir nucleus. Their location is shown in Figure 2 E. Five neurons were negative for Phox2b (Fig. 2 E). These cells tended to be located slightly dorsal to the immunoreactive ones on average, but their physiological properties could not be distinguished from those of the Phox2b-ir cells. The lack of Phox2b immunoreactivity in these cells could thus have been a false-negative result. Indeed, the first five animals tested showed 7 of 12 recorded neurons with Phox2b-ir nuclei by using the peroxidase–anti-peroxidase method, but when Phox2b was detected with the higher concentration of primary antibody and direct tagged secondary method, 6 of 6 recorded neurons with the appropriate physiological responses had Phox2b-ir nuclei.
Figure 2.
RTN chemoreceptors express Phox2b. A, Identification of a putative RTN chemoreceptor. This neuron was recorded below the caudal end of the facial motor nucleus in vivo. Graded levels of CO2 were added to the breathing mixture to quantify the steady-state relationship between the firing rate of the cell and end-expiratory CO2. The inset illustrates the activity of the cell at a higher resolution. Note the slight tendency of the cell to slow during inspiration and postinspiration. AP, Arterial pressure. B, Perievent activity histogram triggered on the integrated phrenic discharge (iPND). This analysis reveals that the cell is only mildly entrained by the central respiratory network. The averaged CO2 trace is flat, because the vagus nerves were cut, and this procedure uncoupled the central respiratory rhythm from the ventilation cycle. C, Mean relationship between RTN neuronal discharge and end-expiratory CO2 at steady state (18 neurons). The graph also illustrates the relationship between the average PND per unit of time (neural equivalent of minute · volume ventilation, mvPND, normalized for the maximum discharge observed at 10% CO2) and end-expiratory CO2 at steady state (8 rats). D, Example of one RTN chemoreceptor neuron (small arrows) labeled in vivo with biotinamide [Phox2b immunoreactivity revealed with Alexa 488 (green), biotinamide with Cy-3 (red); colocalization shown in yellow]. E, Computer-assisted mapping of the sites at which biotinamide-labeled chemoreceptor neurons were found (N = 18). Black dots represent the cells that were Phox2b-ir, and open circles signify Phox2b-negative cells. Abbreviations: RPa, Raphe pallidus; 7, facial motor nucleus; py, pyramidal tract; sp5, spinal trigeminal tract. The three coronal sections represent from top to bottom bregma levels −11.0, −11.3, and −11.6. Scale bars: D, 50 μm; E, 1 mm.
We have previously demonstrated that the RTN neurons with the above-described discharge properties and location contain VGlut2 mRNA and are therefore probably glutamatergic (Mulkey et al., 2004). To further test that RTN chemoreceptors uniformly contain Phox2b, we determined whether this protein is confined to the glutamatergic neurons of the RTN region. This was achieved by combining the immunohistochemical detection of Phox2b with in situ hybridization for VGlut2 or GAD67 mRNA. As illustrated in Figure 3, the Phox2b-ir neurons of the RTN region contained VGlut2 mRNA, but they did not contain GAD67 mRNA. In fact, the group of Phox2b-ir neurons in the RTN resides in a region of the reticular formation that is strikingly poor in GABAergic neurons (Fig. 3 G,H). Neurons that were Phox2b-ir were mapped and counted within the RTN (defined by the triangle comprised of nucleus ambiguus, medial ventral corner of the spinal trigeminal tract, and the lateral edge of the pyramidal tract as illustrated in Fig. 3 C) in sections from three rats reacted for VGlut2 mRNA and Phox2b immunoreactivity or in adjacent sections reacted for GAD67 mRNA and Phox2b immunoreactivity. All but 2 of the 272 Phox2b-ir neurons counted in RTN contained VGlut2 mRNA, whereas none of the 382 Phox2b-ir neurons counted in the same area contained GAD67 mRNA. It should be pointed out that the C1 neurons in the vicinity of the RTN chemoreceptors also express VGlut2 (Stornetta et al., 2002). Therefore, a small percentage of the Phox2b-ir neurons that were found to contain VGlut2 in the above experiment could have been C1 cells.
Figure 3.
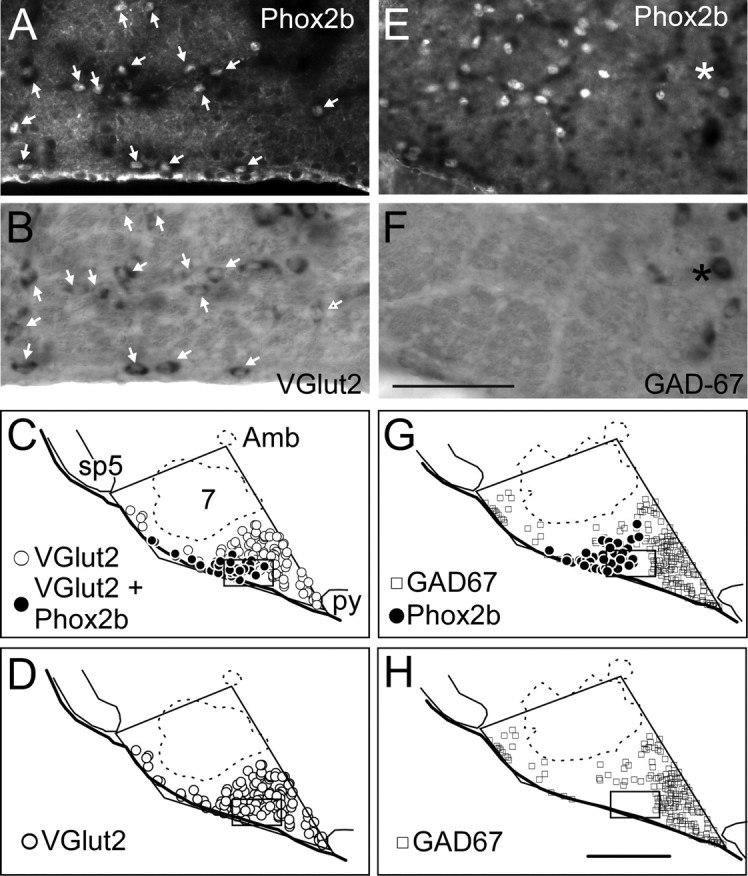
The Phox2b-ir neurons of RTN are glutamatergic. A, The RTN under fluorescence illumination in a section that was reacted for VGlut2 mRNA detection by in situ hybridization and for immunolabeling of Phox2b. The fluorescent nuclei are Phox2b-ir labeled with Cy3. B, Same field viewed under tungsten light reveals alkaline phosphatase reaction product in VGlut2 mRNA-expressing cells. Every Phox2b-ir nucleus was also positive for VGlut2 mRNA as indicated by arrowheads. C, Computer-assisted mapping of VGlut2 and Phox2b neurons in a representative section through RTN. All of the Phox2b-ir neurons also contained VGlut2 mRNA. Abbreviations: Amb, nucleus ambiguus; 7, facial motor nucleus; py, pyramidal tract; sp5, spinal trigeminal tract. D, Replot of C in which all VGlut2-positive neurons are assigned the same symbol regardless of whether they contain Phox2b. E, The RTN region under fluorescence illumination in a section that was reacted for detection of Phox2b (Cy3) and GAD67 mRNA by in situ hybridization. F, The same field viewed under tungsten light reveals that the region contains very few GAD67 mRNA-positive cells (asterisk shows one GAD67 mRNA-positive neuron). No Phox2b-ir nucleus belonged to a GAD67-positive neuron. G, Computer-assisted mapping of GAD67 mRNA-positive and Phox2b-ir neurons in a representative section through the RTN. The two populations were entirely separate. H, Replot of G in which only the GAD67 mRNA-positive neurons are shown. Scale bars: (in F) A, B, E, F, 100 μm; (in H) C, D, G, H, 500 μm. The locations of the photomicrographs are indicated by the rectangular boxes in the bottom panels.
Because serotonergic neurons may function as central respiratory chemoreceptors (Richerson, 2004), we determined whether this type of neuron expresses Phox2b. Our results show a complete absence of Phox2b immunoreactivity in medullary serotonergic neurons as demonstrated by double immunohistochemistry. A total of 2665 tryptophan-hydroxylase-ir neurons were plotted throughout the brainstem and pontine serotonergic groups (raphe obscurus, raphe pallidus, magnocellular raphe, parapyramidal area, pontine raphe, median and dorsal raphe) in three rats, and none was Phox2b-ir (Fig. 4).
Figure 4.
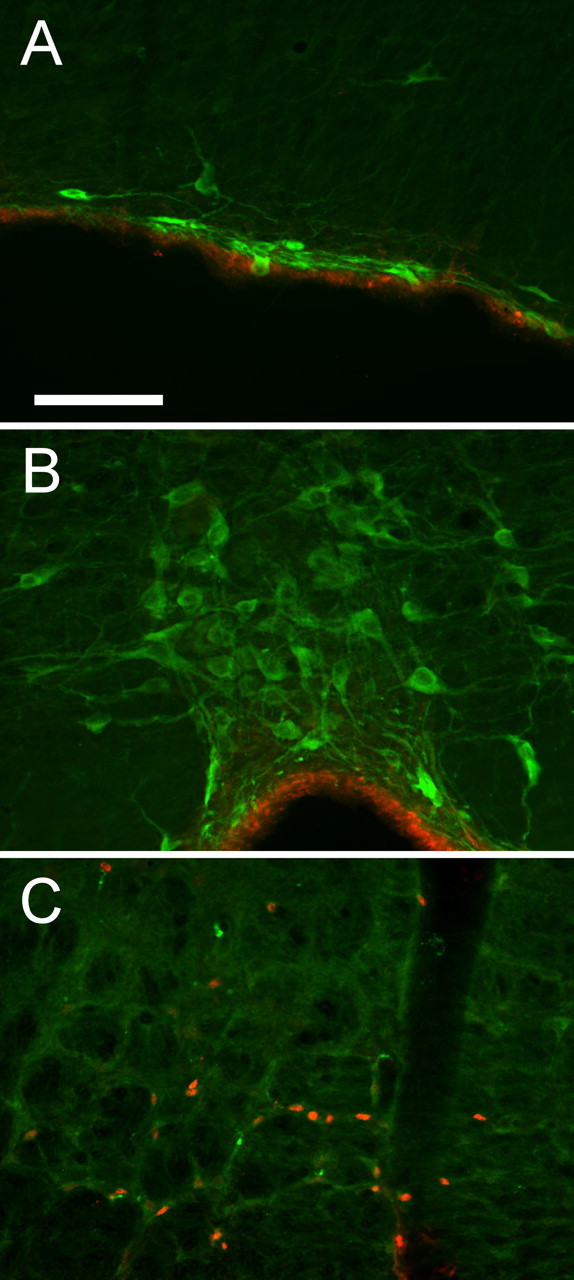
Serotonergic neurons do not contain Phox2b. Each panel is the superposition of tryptophan hydroxylase immunoreactivity (Alexa 488; green fluorescence) and Phox2b immunoreactivity (Cy3; red fluorescence). A, Parapyramidal area. B, Raphe pallidus. C, RTN. Phox2b-ir nuclei are present in the RTN but absent from all serotonergic neurons. Red immunofluorescence in A and B is an edge artifact at the ventral medullary surface. Scale bar: (in A) A–C, 100 μm.
Phox2b is expressed by NTS neurons that are activated by hypoxia and that innervate the RTN
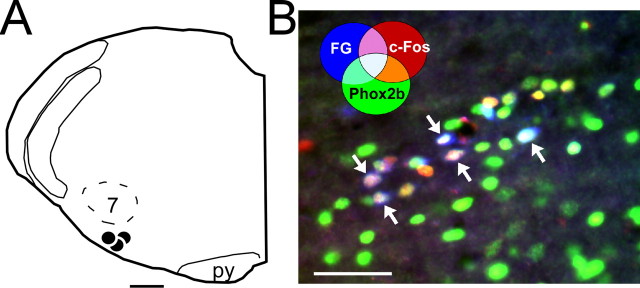
The central chemoreceptors of the RTN also receive an excitatory input from the carotid bodies (Takakura et al., 2006). The pathway from the carotid bodies to the RTN involves a direct excitatory connection from neurons located in commissural NTS (commNTS) (Takakura et al., 2006). The next experiments were designed to test whether the latter neurons also express Phox2b. NTS neurons with projections to the RTN were labeled by injecting the retrograde marker FluoroGold (FG) into their terminal field (N = 3; 2% FG in saline; injection sites shown in Fig. 5 A). One week later, the awake animals were exposed to hypoxia (8% O2) to activate peripheral chemoreceptors and their downstream central pathways. Hypoxia-activated NTS neurons were identified by the presence of c-Fos-ir nuclei (Sagar et al., 1988; Takakura et al., 2006). Figure 5 B illustrates the commNTS after immunohistochemical detection of c-Fos (Cy3, red) and Phox2b (Alexa 488, green) as well as the fluorescence in the blue spectrum emitted by the retrogradely transported FG. Note the large number of aqua/white-colored nuclei denoting the simultaneous presence of all three markers. Triple labeling indicates that these neurons were activated by stimulation of peripheral chemoreceptors, projected to the RTN and also expressed Phox2b. c-Fos was virtually absent from the NTS of control rats subjected to the same surgery to inject FG into the RTN and later exposed to room air in the same environment as the experimental animals (N = 3). Cell counts from the commNTS in the three rats exposed to hypoxia revealed that 65 ± 14% of the cells projecting from commNTS to RTN (FG-positive neurons) were activated by hypoxia (c-Fos-ir), and most of the latter neurons (88 ± 1% of the c-Fos- and FG-positive neurons) expressed Phox2b. However, the number of NTS neurons that were Phox2b-ir was vastly greater than the number of neurons that responded to hypoxia by expressing c-Fos.
Figure 5.
Hypoxia-responsive NTS neurons with projections to RTN express Phox2b. A, NTS neurons with axonal projections to the RTN were prelabeled with the retrograde tracer FG in three rats. The FG injections (large black dots) were considered to be correctly placed because they were centered below the caudal and medial edge of the facial motor nucleus (7). py, Pyramidal tract. B, Awake rats were subjected to hypoxia and hypoxia-activated NTS neurons were identified by the presence of c-Fos-ir nuclei. The brain tissue was processed for simultaneous detection of FG (native blue fluorescence), Phox2b immunoreactivity (Alexa 488 fluorescence; green), c-Fos immunoreactivity (Cy3; red). The photomicrograph taken within the commissural portion of the NTS shows multiple aqua colored neurons (arrows) with RTN projections, activated by carotid body stimulation and expressing Phox2b. The color coding for other combinations of markers is indicated on the figure. Scale bars: A, 500 μm; B, 50 μm.
In summary, the vast majority of the neurons that convey peripheral chemoreceptor information from the NTS to the RTN express Phox2b, but these neurons represent a small fraction of all the Phox2b-expressing neurons present in this structure.
Phox2b is not expressed by the ventral respiratory column caudal to the RTN
In CCHS, breathing is relatively normal under circumstances when respiration depends only to a minor degree on chemical drive (i.e., on central and peripheral chemoreceptor activity). This is the case during waking hours in general and during physical exercise in particular (Paton et al., 1993; Shea et al., 1993; Spengler et al., 2001). These observations suggest that mutations of PHOX2B do not disrupt the respiratory rhythm and pattern generator and therefore that Phox2b may not be expressed by the core of the respiratory CPG. To test this hypothesis, we examined the distribution of Phox2b in the regions of the brainstem currently identified as essential for respiratory rhythm and pattern generation, namely the parabrachial region and the VRC caudal to RTN (Feldman and Del Negro, 2006). Finally, we recorded from two specific types of VRC interneurons (Bötzinger neurons and bulbospinal inspiratory premotor neurons) in vivo, labeled them with biotinamide, and tested them for Phox2b expression.
A detailed anatomical survey of the distribution of Phox2b-ir neurons in the pons of the adult rat will be presented elsewhere. The essential point is that the parabrachial region (pneumotaxic center), notably the lateral parabrachial and Kölliker–Fuse nuclei was not among the Phox2b-expressing regions (results not illustrated). However, the ventrolateral medullary region that encompasses the VRC did contain Phox2b-ir neurons. The VRC network that generates the respiratory rhythm and pattern consists primarily of glutamatergic or GABA/glycine interneurons, whereas, according to previous developmental studies (Brunet and Pattyn, 2002; Dauger et al., 2003), the Phox2b-expressing cells of the ventrolateral medulla may consist primarily of visceral motor neurons or catecholaminergic cells. Therefore, we first determined whether every Phox2b-ir neuron located in the VRC region caudal to RTN was either cholinergic or catecholaminergic. If this were the case, the result would demonstrate that the CPG interneurons do not express this protein.
At caudal levels of the VRC, many Phox2b-ir cells contained either TH or ChAT immunoreactivity (Fig. 6 A, top panels) and indeed very few neurons were neither TH nor ChAT immunoreactive (Fig. 6 A, bottom left). The reverse was found at the RTN level (Fig. 6 A, bottom right). For quantitative purposes, a series of sections from three rats were immunolabeled for Phox2b (Alexa 488, green) and for TH and ChAT using Cy-3-tagged secondary antibodies for TH and ChAT. The Phox2b-ir neurons devoid of either TH or ChAT (i.e., Cy-3-negative) and located in the ventral one-half of the medulla oblongata were systematically plotted in a series of coronal sections 180 μm apart (Fig. 6 B). The Phox2b-expressing neurons devoid of TH or ChAT were counted within the VRC defined as the triangular shape illustrated in Figure 6 B. This triangle encompasses more territory than the VRC proper, but it has the advantage of joining three clearly defined landmarks (medial edge of spinal trigeminal tract, nucleus ambiguus, and lateral edge of inferior olive or pyramidal tract where inferior olive was absent). As shown in Figure 6 B, very few Phox2b-expressing neurons devoid of TH or ChAT were present at caudal levels of the VRC. The average number of cells found in the VRC of three rats counted in this manner is summarized in Figure 6 C. The result indicates that the Phox2b-ir neurons that are neither cholinergic nor catecholaminergic are confined to the RTN level of the VRC. This population of glutamatergic neurons presumably identifies the RTN chemoreceptors.
Figure 6.
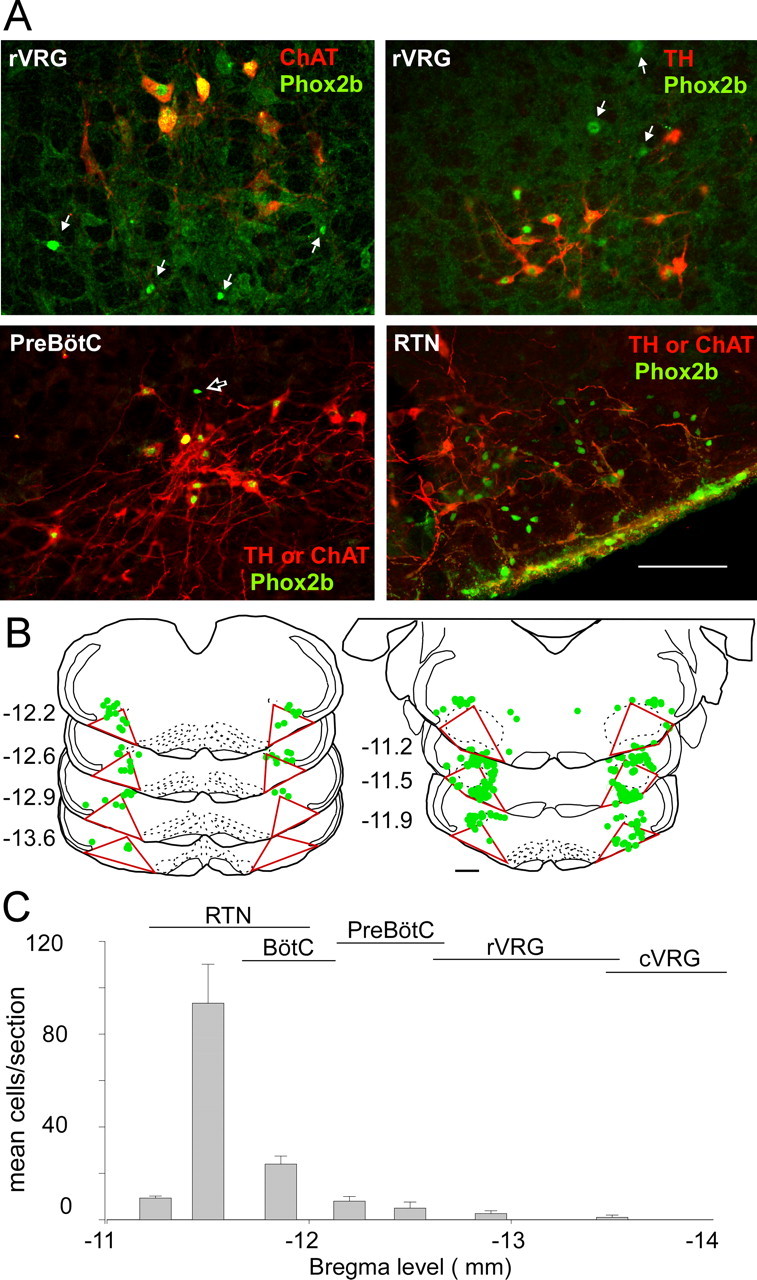
VRC neurons that express Phox2b but neither TH nor ChAT are confined to the RTN. A, Top left, VRC at bregma level −13.3 mm (rVRG) in a section reacted for detection of Phox2b (Alexa 488; green) and ChAT (Cy3; red). All ChAT-ir neurons have Phox2b-ir nuclei. White arrows point to Phox2b-ir nuclei that are not associated with cholinergic neurons. Top right, VRC (rVRG; bregma level, −13.3) in a section reacted for detection of Phox2b (Alexa 488; green) and TH-ir (Cy3; red). Many TH-ir neurons have Phox2b-ir nuclei. White arrows point to Phox2b-ir nuclei that are not associated with catecholaminergic neurons but likely belong to cholinergic motor neurons (based on location and nuclear size). Bottom left, VRC at bregma −12.6 mm (pre-Bötzinger complex level) in a section reacted for simultaneous detection of Phox2b (Alexa 488; green), TH, and ChAT. In this experiment, TH and ChAT are both labeled with Cy3-tagged secondary antibodies, and therefore both appear in red. Black arrow points to the only Phox2b-ir neuron that lacks ChAT or TH. Bottom right, RTN region in a section reacted as in the bottom left panel (TH and ChAT both appear in red). In the RTN, the vast majority of Phox2b-ir neurons are neither cholinergic nor catecholaminergic and this population probably identifies the putative central chemoreceptors. Scale bar: top panels, 100 μm; bottom panels, 200 μm. B, Computer-assisted plot of noncatecholaminergic and noncholinergic Phox2b-ir neurons in the lower medulla of one rat. The numbers at the left of the sections refer to the level behind bregma. The VRC is delineated by the triangles drawn on each section. This triangle joins three objective landmarks: the bottom of nucleus ambiguus, the edge of the pyramidal tract, and the edge of the trigeminal tract. C, Rostral–caudal distribution of the putative central chemoreceptors identified as noncatecholaminergic, noncholinergic Phox2b-expressing neurons of the VRC. The approximate rostrocaudal extent of the various subdivisions of the VRC [after Alheid et al. (2002)] are indicated. Error bars indicate SEM. For abbreviations, see Figure 1.
To test more thoroughly whether some of the few noncholinergic and noncatecholaminergic neurons of the rostral VRC might be respiratory interneurons, we recorded from two specific types of rostral VRC interneurons in vivo and labeled them with biotinamide to test for the presence of Phox2b. Bötzinger neurons were defined as described previously (Kanjhan et al., 1995; Schreihofer et al., 1999). These cells have an expiratory incrementing discharge (Fig. 7 B,D, note increase in neuron firing rate toward the end of expiration). They have myelinated axons (antidromic latency, 0.7–1.5 ms), and they project to the contralateral VRC [diagram of stimulating electrode in the right rostral ventral respiratory group (rVRG) with a recording electrode in the left (contralateral) Bötzinger area in Fig. 7 A; contralateral projection confirmed by collision test in Fig. 7 C]. The presence of contralateral axonal projections allows Bötzinger neurons to be distinguished from motor neurons that may have similar discharge patterns and serves to identify these contralateral-projecting cells as respiratory interneurons. As expected, biotinamide-labeled Bötzinger neurons were found below the compact division of nucleus ambiguus between 100 and 500 μm caudal to the caudal edge of the facial motor nucleus (Schreihofer et al., 1999) (Fig. 7 E,F). Bulbospinal inspiratory augmenting neurons were recorded in the ventrolateral medulla ∼1.5 mm caudal to the caudal edge of the facial motor nucleus (Fig. 7 K) (Stornetta et al., 2003). These cells were defined by their characteristic inspiratory augmenting pattern (Fig. 7 I,J, note increase in neuron firing toward the end of inspiration defined by the phrenic nerve activity) and the fact that they possess a contralateral myelinated axonal projection to the lower cervical cord (diagram in Fig. 7 G shows stimulating electrode in spinal cord contralateral to recording electrode in rVRG; collision test confirming spinal projection in Fig. 7 H).
Figure 7.
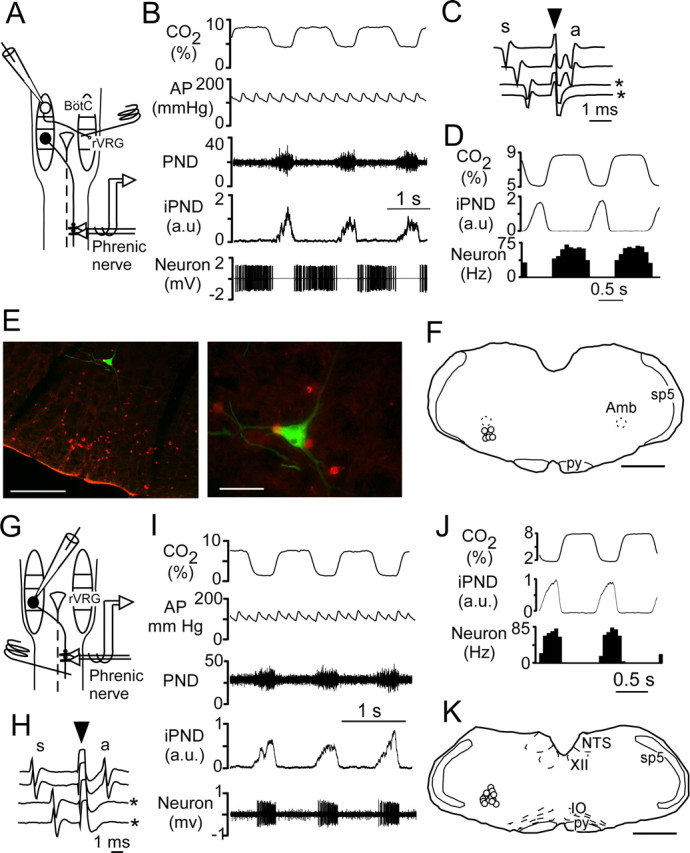
Respiratory interneurons of the VRC do not express Phox2b. A, Schematic of the experiment used to characterize expiratory-augmenting Bötzinger neurons. The stimulating electrode is represented by a coiled wire inserted in the right rVRG region. The single-unit recording electrode is inserted in the left (contralateral) Bötzinger (BötC) region. A typical premotor neuron in rVRG is represented on the right side projecting to the phrenic motor nucleus in the cervical spinal cord. The phrenic nerve activity is recorded on the side contralateral to the unit activity. B, Typical recording of a Bötzinger neuron showing simultaneous recording of CO2, arterial pressure (AP), PND, iPND, and neuronal action potentials (bottom trace). C, Positive collision test demonstrating that the neuron shown in B has an axon projecting to or through the contralateral rVRG (s, spontaneous spike; arrow down, stimulation artifact; a, antidromic spike; asterisk, two sweeps when collision occurred because of the insufficient delay between the spontaneous spike and the stimulus). D, Perievent histogram of the unit triggered on the phrenic discharge showing the expiratory incrementing discharge of the neuron. E, Biotinamide-labeling of the neuron shown in B–D. The section was also reacted for Phox2b immunoreactivity (Cy3 in red) revealing that the recorded neuron (Alexa 488; green) was Phox2b negative. Surrounding Phox2b-ir neurons are probably motor neurons or catecholaminergic neurons. In the left photomicrograph, note the cluster of Phox2b-ir neurons located in the RTN ventral to the recorded neuron. Scale bar: (in E) left, 500 μm; right, 50 μm. F, Location of seven Bötzinger neurons that were labeled with biotinamide and recovered histologically (Amb, nucleus ambiguus, compact part; the level represented is bregma −11.8 mm; scale bar, 1 mm). py, Pyramidal tract; sp5, spinal trigeminal tract. G, Schematic of the experimental design to characterize inspiratory-augmenting rVRG neurons. The stimulating electrode is represented by a coiled wire inserted in the right side of the cervical cord in the area of the phrenic motor nucleus (C4–C5). The single-unit recording electrode is inserted in the left (contralateral) rVRG. A typical inspiratory–augmenting premotor neuron in the rVRG is represented on the left side projecting to the phrenic motor nucleus in the contralateral cervical spinal cord. The phrenic nerve activity is recorded on the side contralateral to the rVRG neuron. H, Collision test demonstrating that the neuron shown in I has an axon projecting to or through the contralateral rVRG (for details, see C). I, Electrophysiological identification of an inspiratory–augmenting bulbospinal neuron. J, Perievent histogram of the unit shown in I triggered on the phrenic discharge. The histogram shows the inspiratory augmenting discharge of the neuron. K, Location of the 13 inspiratory–augmenting bulbospinal neurons that were labeled with biotinamide and recovered histologically (the coronal level represented is bregma −13.3 mm; scale bar, 1 mm). IO, Inferior olive.
Both types of respiratory interneurons were negative for Phox2b (7 of 7 Bötzinger neurons and 13 of 13 bulbospinal inspiratory neurons). An example of one Bötzinger neuron is illustrated in Figure 7 E. Note that this neuron was located close to Phox2b-ir neurons that were probably C1 adrenergic cells or visceral motor neurons.
Discussion
The present study demonstrates that Phox2b is expressed by a group of chemosensitive glutamatergic interneurons located in the RTN (Feldman et al., 2003; Mulkey et al., 2004; Guyenet et al., 2005b). We also show that Phox2b delineates an unbroken chain of neurons involved in the integration of peripheral and central chemoreception. This circuit includes previously known components [the carotid bodies and chemoreceptor afferents (Dauger et al., 2003)] to which we add the chemoresponsive projections of the NTS to the ventrolateral medulla (VLM) (Takakura et al., 2006) and the central chemoreceptors located in the RTN (Guyenet et al., 2005a). The prevalence of Phox2b in this circuit and its scarcity in regions involved in respiratory rhythm and pattern generation may account for the fact that PHOX2B mutations in humans selectively impair the chemical drive to breathe, while leaving normal daytime breathing relatively intact.
Phox2b is present in RTN chemoreceptors
The RTN region is chemosensitive and regulates breathing (Okada et al., 2002; Feldman et al., 2003; Mulkey et al., 2004; Putnam et al., 2004; Nattie and Li, 2006). The chemosensitive cells of RTN are glutamatergic and selectively innervate the pontomedullary regions that generate the respiratory motor outflows (Mulkey et al., 2004; Rosin et al., 2006; Takakura et al., 2006). The present study demonstrates that Phox2b is present in these functionally identified RTN chemoreceptors. Additionally, within the RTN region, Phox2b could only be detected in VGlut2 mRNA-containing neurons, consistent with previous evidence that the CO2-responsive neurons of RTN are glutamatergic (Mulkey et al., 2004).
The RTN Phox2b-ir cells include the previously described glutamatergic neurons located within the marginal layer of the medulla oblongata (Weston et al., 2004) and neurons located more dorsally within and just above the ventral spinocerebellar tract (Mulkey et al., 2004). Others have proposed on the basis of in vitro experiments that the marginal layer may contain different types of chemosensitive neurons than the region above it (Okada et al., 2002). We have not been able to confirm this hypothesis to date.
The ventral medullary cluster of VGlut2 and Phox2b cells extends at least 400 μm caudal to the facial motor nucleus, which indicates that a substantial fraction of the chemosensitive neurons of RTN reside at the same rostrocaudal level as the Bötzinger neurons (for example, see Fig. 7 E). Conversely, Phox2b-ir neurons are rarely found underneath the rostral two-thirds of the facial motor nucleus consistent with our previous observation that this region contains very few VGlut2-expressing neurons in the adult (Guyenet et al., 2005b). In brief, the tentative outline of the RTN recently proposed by Feldman and Del Negro (2006) and adopted in Figure 1 A matches reasonably well with the present definition of RTN based on the Phox2b and VGlut2 expression pattern. However, >90% of the Phox2b RTN neurons reside in the caudal one-half of the outlined region and about one-half of that number reside caudal to the facial motor nucleus.
Onimaru and colleagues have defined a parafacial respiratory group (pfRG) in the neonate rat consisting of neurons with periinspiratory discharges (Onimaru and Homma, 2003, 2006; Kawai et al., 2006). The location of the pFRG as defined by electrophysiological sampling has varied slightly in previous studies but is now considered to be capping the caudal end of the facial motor nucleus and extending into the Bötzinger region of the VRC (Kawai et al., 2006; Onimaru et al., 2006). Because pfRG neurons are also pH sensitive and reside in approximately the same location as the RTN of the adult (Onimaru and Homma, 2003, 2006), RTN and pfRG neurons could conceivably be two developmental stages of the same cells. The confirmed presence of both VGlut2 and Phox2b in RTN neurons may provide the means to test the validity of this hypothesis.
Central respiratory chemoreception, RTN, and CCHS
The presence of Phox2b in chemosensory pathways and its absence in the neurons that generate the respiratory rhythm and pattern is consistent with the nature of the respiratory deficits in the CCHS. However, a limitation of the study is that the effect of Phox2b mutations on the neurons investigated presently is still unknown. Furthermore, Phox2b mutations could conceivably have deleterious consequences on the development of neurons that do not express this gene either during development or later in life but are merely innervated by such neurons. Also, the function of homeobox genes in the adult brain is still speculative, ranging from a role in neuronal survival to the specification or maintenance of neuronal connections (Prochiantz and Joliot, 2003). The existence of an adult-onset CCHS associated with PHOX2B mutations suggests that this gene may still have a role in adulthood (Weese-Mayer et al., 2005a).
Given the apparently straightforward genetic basis of CCHS (Amiel et al., 2003; Trang et al., 2005), the most influential central respiratory chemoreceptor neurons are likely to be cells whose development or postnatal survival is under the control of Phox2b. The pattern of expression of Phox2b in the brainstem respiratory network suggests that the chemoreceptors responsible for the respiratory deficits of CCHS are probably neither in the raphe nor in the VRC but more likely in the RTN. Clearly, the selective expression of Phox2b by RTN neurons reinforces previous evidence that these excitatory neurons are important for respiratory chemoreception.
NTS, RTN, and chemoreflex integration
The early differentiation of the carotid bodies, glossopharyngeal sensory afferents, and the dorsal vagal complex is under the control of Phox2b (Dauger et al., 2003). As shown here, Phox2b is still abundantly expressed in the NTS of the adult. Over 90% of the hypoxia-sensitive NTS neurons that innervate the RTN region are glutamatergic, and these neurons are primarily responsible for the excitation of the chemosensitive neurons of the RTN during carotid body stimulation (Takakura et al., 2006). Thus, RTN neurons detect brain pCO2 via their intrinsic sensitivity to pH and they also detect alterations in blood pCO2 and pO2 via an oligosynaptic excitatory input from the carotid bodies that relays in commNTS (Takakura et al., 2006). The ventral surface location of RTN neurons further suggests that they could be the final common pathway for peripheral and central chemoreception postulated by Millhorn and Eldridge (1986).
Additional central chemoreceptors probably reside in the region of the pre-Bötzinger complex and within the NTS (Dean et al., 1990; Solomon et al., 2000; Feldman et al., 2003; Nattie and Li, 2006). The widespread expression of Phox2b by NTS neurons suggests that, if this structure does indeed contain central respiratory chemoreceptors, developmental abnormalities of these cells could also contribute to a reduction in central respiratory chemosensitivity. The varying level of residual central chemosensitivity present in CCHS may stem from the fact that RTN and NTS chemoreceptors are dysfunctional but not absent. It could also be attributable to the persistence of a normal chemosensitivity within the pre-Bötzinger complex or the raphe.
Catecholaminergic neurons, serotonergic neurons, and CCHS
In the adult, Phox2b was undetectable in pontine noradrenergic neurons (groups A5–A7) and could be identified only in selected populations of TH-expressing neurons including a subset of presumed C1 neurons. The lack of Phox2b in the locus ceruleus of the adult (A6) is consistent with the transient nature of the expression of this gene in these neurons during development (Coppola et al., 2005). Phox2b expression is nonetheless critical to the differentiation of all lower brainstem catecholaminergic neurons (Pattyn et al., 2000), suggesting that some of these cell groups could also be dysfunctional in CCHS. Although brainstem catecholaminergic neurons are not core components of the CPG, some of the A5, A6, and C1 neurons regulate the breathing network at multiple levels (Li and Nattie, 2006; Zanella et al., 2006). Furthermore, A5 and C1 neurons are activated by chemoreceptor stimulation (Koshiya and Guyenet, 1994, 1996) and A6 is activated by hypercapnia in vivo and mildly acid sensitive in vitro (Putnam et al., 2004). Because lesions of pontomedullary catecholaminergic systems reduce respiration and chemoreflexes in a manner that is not state dependent (Li and Nattie, 2006), a dysfunction of these systems is unlikely to be the primary cause of the sleep apnea in CCHS. However, developmental abnormalities of catecholaminergic neurons could exacerbate the nighttime respiratory deficits caused by the dysfunction of probably more important central chemoreceptors such as the RTN.
Serotonergic neurons modulate respiration at many levels and are pH responsive, at least in vitro (Putnam et al., 2004; Richerson, 2004). The absence of Phox2b in adult serotonergic neurons conforms to expectations, because the development of these neurons does not require Phox2b expression (Pattyn et al., 2003). However, serotonergic neurons derive from progenitor cells in which Phox2b acts as a molecular repressor of the serotonergic lineage that must be turned off for serotonergic neurons to develop. Accordingly, one cannot totally exclude that Phox2b mutations might also cause some developmental abnormality of serotonergic neurons.
In conclusion, the present study and previous experiments by others (Brunet and Pattyn, 2002; Dauger et al., 2003) reveal that Phox2b delineates an uninterrupted chain of sensors and neurons involved in the integration of peripheral and central chemoreception. This circuit includes the carotid bodies, chemoreceptor afferents, chemoresponsive NTS projections to the VLM, and RTN central chemoreceptors. The prevalence of Phox2b in this circuit and its rarity in regions involved in respiratory rhythm and pattern generation is consistent with the respiratory pathology caused by PHOX2B mutations as seen in CCHS.
Footnotes
This work was supported by National Institutes of Health Grants HL28785 and HL74011 (P.G.G.).
References
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A. Phox2 genes—from patterning to connectivity. Curr Opin Genet Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- Coppola E, Pattyn A, Guthrie SC, Goridis C, Studer M. Reciprocal gene replacements reveal unique functions for Phox2 genes during neural differentiation. EMBO J. 2005;24:4392–4403. doi: 10.1038/sj.emboj.7600897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cream C, Li A, Nattie E. The retrotrapezoid nucleus (RTN): local cytoarchitecture and afferent connections. Respir Physiol Neurobiol. 2002;130:121–137. doi: 10.1016/s0034-5687(01)00338-3. [DOI] [PubMed] [Google Scholar]
- Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience. 1990;36:207–216. doi: 10.1016/0306-4522(90)90363-9. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaultier C, Amiel J, Dauger S, Trang H, Lyonnet S, Gallego J, Simonneau M. Genetics and early disturbances of breathing control. Pediatr Res. 2004;55:729–733. doi: 10.1203/01.PDR.0000115677.78759.C5. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005a;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005b;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjhan R, Lipski J, Kruszewska B, Rong WF. A comparative study of pre-sympathetic and Botzinger neurons in the rostral ventrolateral medulla (RVLM) of the rat. Brain Res. 1995;699:19–32. doi: 10.1016/0006-8993(95)00814-7. [DOI] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Muckenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem—spinal cord preparation of the neonatal rat. J Physiol (Lond) 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A, Onimaru H, Homma I. Mechanisms of CO2/H+ chemoreception by respiratory rhythm generator neurons in the medulla from newborn rats in vitro. J Physiol (Lond) 2006;572:525–537. doi: 10.1113/jphysiol.2005.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. A5 noradrenergic neurons and the carotid sympathetic chemoreflex. Am J Physiol. 1994;267:R519–R526. doi: 10.1152/ajpregu.1994.267.2.R519. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. J Physiol (Lond) 1996;491:859–869. doi: 10.1113/jphysiol.1996.sp021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SK, Ling EA. Labelling neurons with fluorescent dyes administered via intravenous, subcutaneous or intraperitoneal route. J Neurosci Methods. 1990;32:15–23. doi: 10.1016/0165-0270(90)90067-p. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol (Lond) 2006;570:385–396. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL. Role of ventrolateral medulla in regulation of respiratory and cardiovascular systems. J Appl Physiol. 1986;61:1249–1263. doi: 10.1152/jappl.1986.61.4.1249. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie E. Why do we have both peripheral and central chemoreceptors? J Appl Physiol. 2006;100:9–10. doi: 10.1152/japplphysiol.01097.2005. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception 2005: a brief review. Auton Neurosci. 2006;126–127:332–338. doi: 10.1016/j.autneu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Okada Y, Chen Z, Jiang W, Kuwana S, Eldridge FL. Anatomical arrangement of hypercapnia-activated cells in the superficial ventral medulla of rats. J Appl Physiol. 2002;93:427–439. doi: 10.1152/japplphysiol.00620.2000. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Point:counterpoint: the parafacial respiratory group (pFRG)/pre-Botzinger complex (preBotC) is the primary site of respiratory rhythm generation in the mammal. Point: the PFRG is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol. 2006;100:2094–2095. doi: 10.1152/japplphysiol.00119.2006. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Paton JY, Swaminathan S, Sargent CW, Hawksworth A, Keens TG. Ventilatory response to exercise in children with congenital central hypoventilation syndrome. Am Rev Respir Dis. 1993;147:1185–1191. doi: 10.1164/ajrccm/147.5.1185. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Goridis C, Brunet JF. Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol Cell Neurosci. 2000;15:235–243. doi: 10.1006/mcne.1999.0826. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Vallstedt A, Dias JM, Samad OA, Krumlauf R, Rijli FM, Brunet JF, Ericson J. Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 2003;17:729–737. doi: 10.1101/gad.255803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Prochiantz A, Joliot A. Can transcription factors function as cell-cell signalling molecules? Nat Rev Mol Cell Biol. 2003;4:814–819. doi: 10.1038/nrm1227. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1330. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Bötzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol. 1999;407:583–597. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Shea SA, Andres LP, Shannon DC, Banzett RB. Ventilatory responses to exercise in humans lacking ventilatory chemosensitivity. J Physiol (Lond) 1993;468:623–640. doi: 10.1113/jphysiol.1993.sp019792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100:13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, O'Neill MH. CO2/H+ chemoreception in the cat pre-Botzinger complex in vivo. J Appl Physiol. 2000;88:1996–2007. doi: 10.1152/jappl.2000.88.6.1996. [DOI] [PubMed] [Google Scholar]
- Spengler CM, Gozal D, Shea SA. Chemoreceptive mechanisms elucidated by studies of congenital central hypoventilation syndrome. Resp Physiol. 2001;129:247–255. doi: 10.1016/s0034-5687(01)00294-8. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Guyenet PG. Distribution of glutamic acid decarboxylase mRNA-containing neurons in rat medulla projecting to thoracic spinal cord in relation to monoaminergic brainstem neurons. J Comp Neurol. 1999;407:367–380. [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Inspiratory augmenting bulbospinal neurons express both glutamatergic and enkephalinergic phenotypes. J Comp Neurol. 2003;455:113–124. doi: 10.1002/cne.10486. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol (Lond) 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang H, Dehan M, Beaufils F, Zaccaria I, Amiel J, Gaultier C. The French Congenital Central Hypoventilation Syndrome Registry: general data, phenotype, and genotype. Chest. 2005;127:72–79. doi: 10.1378/chest.127.1.72. [DOI] [PubMed] [Google Scholar]
- Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol. 2001;434:128–146. doi: 10.1002/cne.1169. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L. Adult identified with congenital central hypoventilation syndrome–mutation in PHOX2b gene and late-onset CHS. Am J Respir Crit Care Med. 2005a;171:88. doi: 10.1164/ajrccm.171.1.950. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Marazita ML. In pursuit (and discovery) of a genetic basis for congenital central hypoventilation syndrome. Respir Physiol Neurobiol. 2005b;149:73–82. doi: 10.1016/j.resp.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Weston M, Wang H, Stornetta RL, Sevigny CP, Guyenet PG. Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine-induced hypertension in rats. J Comp Neurol. 2003;460:525–541. doi: 10.1002/cne.10663. [DOI] [PubMed] [Google Scholar]
- Weston MC, Stornetta RL, Guyenet PG. Glutamatergic projections from the marginal layer of the rostral ventral medulla to the respiratory centers in rats. J Comp Neurol. 2004;473:73–85. doi: 10.1002/cne.20076. [DOI] [PubMed] [Google Scholar]
- Zanella S, Roux JC, Viemari JC, Hilaire G. Possible modulation of the mouse respiratory rhythm generator by A1/C1 neurones. Respir Physiol Neurobiol. 2006;153:126–138. doi: 10.1016/j.resp.2005.09.009. [DOI] [PubMed] [Google Scholar]