A recent alternative to the storage and retrieval interpretations of reconsolidation (Nader, 2003; Millin et al., 2001) posits that postreactivation amnesia may, in fact, reflect a disruption of processes activated during training and persist beyond the time windows classically ascribed to consolidation (Dudai and Eisenberg, 2004). This “lingering consolidation” hypothesis rests on the observation that younger memories, when reactivated, appear more susceptible to consolidation-blocking agents than older traces.
These observations suggest that processes ongoing from training may endure beyond the close of traditional consolidation (usually determined via protein synthesis inhibitors) and participate in the restorage process occurring after recall. Although this idea provides an attractive explanation of the time-dependent appearance of disruption-resistant reconsolidation, experiments identifying the persistent components of consolidation invoked by this theory have not been forthcoming. In a recent article in The Journal of Neuroscience, G. Kemenes et al. (2006) reported data that could help advance our understanding of this fascinating notion.
Using in vitro kinase activity assays and pharmacological inhibitors, the authors show that retrieval events differentially trigger a distinct cellular signaling cascade depending on the age of the memory. The kinase chosen, protein kinase A (PKA), has a firmly established role in the consolidation of both behavioral memory and synaptic plasticity. Moreover, a recent paper indicates that amygdala PKA exerts bidirectional control over the reconsolidation of fear memories in mice (Tronson et al., 2006). The authors studied the role of PKA after recall by examining conditioned stimulus (CS)-related activation of this enzyme in the CNS of Lymnaea stagnalis. Lymnaea provides advantages for cell and molecular biological studies of reconsolidation because it provides a well characterized network within which sites of learning-related plasticity have been described recently (I. Kemenes et al., 2006). The basic paradigm involves an association between solutions of amyl acetate (the CS) and sucrose (the unconditioned stimulus), with memory expressed as an increase in CS-elicited feeding responses.
Initially, the authors examined the role of protein synthesis after recall of 6- and 24-h-old memories [G. Kemenes et al. (2006), their Figure 1A,B (http://www.jneurosci.org/cgi/content/full/26/23/6298/F1)]. Importantly, these CS tests were scheduled to occur beyond the initial period of consolidation for this appetitive long-term memory (Fulton et al., 2005). Injection of anisomycin 10 min after CS presentation, at both the early and later recall points, led to subsequent impairments in performance, suggesting that Lymnaea exhibits protein synthesis-dependent reconsolidation and that within this time frame, translation is equally important regardless of when the trace is triggered.
Figure 1.
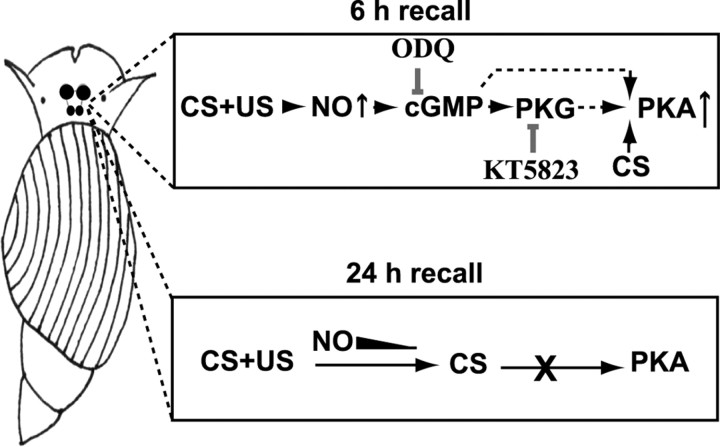
Training-related NO–cGMP signaling, in the CNS of Lymnaea, may play a role in mediating time-limited activation of PKA after recall. In the model shown here, CS presentation 6 h after conditioning enhances PKA activation through an interaction with training-stimulated cGMP/PKG. After conditioning, NO signaling diminishes with time (filled triangle in bottom box) so that by 24 h, recall is no longer able to activate PKA. Pharmacological inhibitors, such as 1H-(1,2,4)oxadiazole(4,3-a)quinoxalin-1-one (ODQ) and KT5823, as shown (top box), could be used to test the role of cGMP and PKG signaling in memory reconsolidation. US, Unconditioned stimulus.
Having detected sensitivity to a general protein synthesis inhibitor, the authors then turned their attention toward a more specific molecular target. PKA activity was measured in CNS extracts obtained either 6 or 24 h after appetitive conditioning. Intriguingly, when the memory was reactivated 6 h after training, a robust CS-specific activation of PKA was observed that was not apparent when the CS was applied 24 h after conditioning [G. Kemenes et al. (2006), their Figure 2Ci–Cii (http://www.jneurosci.org/cgi/content/full/26/23/6298/F2)]. After establishing that memory reactivation at 6 but not 24 h is associated with increased PKA activity, the authors reasoned that blockade of PKA at these two time points would produce distinct effects on postreactivation performance. In accordance with this prediction, long-term memory was blocked by injection of the PKA inhibitor KT5720 10 min after 6 h recall but not when the inhibitor was applied after a 24 h reactivation [G. Kemenes et al. (2006), their Figure 3A,B (http://www.jneurosci.org/cgi/content/full/26/23/6298/F3)].
By examining the function of both general protein synthesis and a specific signaling pathway across different time points, G. Kemenes et al. (2006) demonstrate that postretrieval events are not all alike. Indeed, the molecular machinery engaged by recall varies with the age of the memory, a finding that is in agreement with assumptions central to the hypothesis advanced by Dudai and Eisenberg (2004). These data also indicate that what may be true for a general process, such as translation, may not be true for a specific signaling molecule. This latter point highlights the necessity of examining specific pathways in the regulation of postretrieval events.
The failure to detect a progressive increase in resistance to anisomycin is not necessarily surprising; experimenters who have successfully detected translation-independent reconsolidation have looked days to weeks, rather than hours, after training (Dudai and Eisenberg, 2004). Additional testing of the effects of anisomycin could be achieved readily in Lymnaea because this form of associative memory persists for up to 3 weeks (Alexander et al., 1984), providing ample chance to study this effect over the appropriate time span.
This time-dependent activation of PKA could suggest that training-initiated signals interact with recall-related signals during earlier reconsolidation processes. What could these enduring echoes of consolidation be? Data from a recent article cited by the authors indicate that appetitive conditioning in Lymnaea involves activation of the nitric oxide (NO)–cGMP pathway for up to 5 h after training (Kemenes et al., 2002). Both cGMP and its downstream effector protein kinase G (PKG) are capable of activating PKA, so if CS-related PKA activation were timed to occur during the extended period of NO signaling, an enhanced activation of PKA could result. An examination of the effect of cGMP and PKG inhibitors on CS-induced PKA activation would provide a direct test of the involvement of the NO system in these events (Fig. 1). Indeed, studies aimed at unraveling the relationship between systems engaged by consolidation and reactivation will be essential for judging the validity of the lingering consolidation hypothesis.
The time-dependent role of PKA might also reflect processes of retrieval rather than restorage. In this interpretation, recall at specific time points would render the mechanism of retrieval sensitive to disruption by specific pharmacological inhibitors. Accordingly, if the memory is recalled a second time, the experimenter observes a deficit in performance because retrieval has been blocked by the preceding manipulation. Under this interpretation, the data presented by G. Kemenes et al. (2006) indicate that the retrieval processes revealed after 6 h memory reactivation have a specific requirement for PKA activity, whereas those connnected with 24 h memory recall do not. Can we distinguish between these two theoretical views? Spontaneous recovery and reinstatement after postreactivation memory impairments have been reported (Millin et al., 2001) and are generally thought to support retrieval-based interpretations. Investigating these questions in Lymnaea would significantly strengthen the authors' interpretations.
In conclusion, this report provides the first demonstration of a time-limited contribution for a specific molecular pathway in the processes activated by recall. In doing so, these findings serve to further elucidate the temporal nature of postrecall processing and, as such, lend weight to the belief that the processes involved in reconsolidation may, in fact, reflect an ongoing process of consolidation.
Footnotes
Editor's Note: These short reviews of a recent paper in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to mimic the journal clubs that exist in your own departments or institutions. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
References
- Alexander J, Jr, Audesirk TE, Audesirk GJ. One-trial reward learning in the snail Lymnaea stagnalis. J Neurobiol. 1984;15:67–72. doi: 10.1002/neu.480150107. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Fulton D, Kemenes I, Andrew RJ, Benjamin PR. A single time-window for protein synthesis-dependent long-term memory formation after one-trial appetitive conditioning. Eur J Neurosci. 2005;21:1347–1358. doi: 10.1111/j.1460-9568.2005.03970.x. [DOI] [PubMed] [Google Scholar]
- Kemenes G, Kemenes I, Michel M, Papp A, Muller U. Phase dependent molecular requirements for memory reconsolidation: differential roles for protein synthesis and protein kinase A activity. J Neurosci. 2006;26:6298–6302. doi: 10.1523/JNEUROSCI.0890-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenes I, Kemenes G, Andrew RJ, Benjamin PR, O'Shea M. Critical time-window for NO–cGMP-dependent long-term memory formation after one-trial appetitive conditioning. J Neurosci. 2002;22:1414–1425. doi: 10.1523/JNEUROSCI.22-04-01414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenes I, Straub VA, Nitkin ES, Staras K, O'Shea M, Kemenes G, Benjamin PR. Role of delayed nonsynaptic neuronal plasticity in long-term associative memory. Curr Biol. 2006;16:1269–1279. doi: 10.1016/j.cub.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Millin PM, Moody EW, Riccio DC. Interpretations of retrograde amnesia: old problems redux. Nat Rev Neurosci. 2001;2:68–70. doi: 10.1038/35049075. [DOI] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]