Abstract
The effects of PD 176252 [3-(1H-indol-3-yl)-N-[1-(5-methoxy-pyridin-2-yl)-cyclohexylmethyl]-2-methyl-2-[3-(nitro-phenyl)ureido]propionamide], a nonpeptide bombesin (BB) BB1/BB2 receptor antagonist, were assessed in rats using several ethologically relevant tests of anxiety. Consistent with a role for the bombesin family of peptides in subserving anxiety behaviors, the antagonist increased social interaction (3.75 and 7.5 mg/kg, i.p.), dose-dependently attenuated the number of vocalizations emitted by guinea pig pups separated from their mother (1–30 mg/kg, i.p.), reduced latency to approach a palatable snack in an anxiogenic (unfamiliar) environment, and reduced the fear-potentiated startle response (5 and 10 mg/kg, i.p., and 100–200 ng per rat, i.c.v.). When administered directly to the dorsal raphé nucleus (DRN), PD 176252 (20–500 ng) increased social interaction under aversive conditions, as did the 5-HT1A receptor agonist 8-hydroxy-2(di-n-propylamino)tetralin (50 ng). Furthermore, intra-DRN microinfusion of the peptide antagonist (PD 176252) suppressed, whereas its agonist [neuromedin B (NMB)-30] promoted, the in vivo release of 5-HT in the ventral hippocampus. In parallel, the suppressed social interaction elicited by intra-DRN administration of NMB was attenuated by a systemically administered 5-HT2C (but not 5-HT1A) receptor antagonist. Together, these findings suggest that endogenous BB-like peptides at the DRN evoke the release of 5-HT from the limbic nerve terminals originating from the raphé, specifically at the ventral hippocampus, resulting in anxiogenesis. The finding that this action was attenuated by BB receptor (BB1 and/or BB2) antagonists suggests that these compounds may represent a novel class of anxiolytic agents.
Keywords: anxiety, 5-hydroxytryptamine, neuromedin B, dorsal raphé nucleus, social interaction, fear potentiated startle, guinea pig pup vocalizations
Introduction
The diminished toxicity of benzodiazepines relative to barbiturates represented a major advance in the treatment of anxiety. However, benzodiazepines have the propensity to induce tolerance, dependence, and motor side effects, fostering the search for alternative treatments (File, 1990a,b). In this regard, considerable interest has focused on the contribution of 5-hydroxytryptamine (5-HT) in mediating anxiety. This interest was prompted by the clinical success of antidepressants and by buspirone, which binds to inhibitory 5-HT1A receptors, reducing 5-HT release from nerve terminals originating in the dorsal raphé nucleus (DRN) (Dourish, 1987). However, 5-HT1A receptors are present both presynaptically and postsynaptically, and direct injection of 5-HT1A agonists into areas containing presynaptic (DRN) and postsynaptic (hippocampus and amygdala) 5-HT1A receptors elicited opposing effects, namely anxiolytic and anxiogenic behaviors, respectively (Higgins et al., 1988, 1992; Andrews et al., 1994). In addition, other 5-HT receptor subtypes (e.g., 5-HT2C) present in the 5-HT projection areas (e.g., hippocampus and amygdala) appear to be involved in anxiogenesis (Whitton and Curzon, 1990; Clemett et al., 2000; Alves et al., 2004; Millan, 2005). Because the anxiety state may reflect a balance between anxiogenic versus anxiolytic actions of 5-HT in various brain regions, development of pharmacological tools with selectivity for specific 5-HT projections may yield anxiolytic agents with a better therapeutic profile.
Bombesin (BB), an amphibian peptide, and its mammalian counterparts [various forms of neuromedin B (NMB) and gastrin-releasing peptide (GRP)], elicit their effects through various BB receptor subtypes (Battey and Wada, 1991). Whereas NMB binds preferentially to the BB1 receptors, GRP (GRP1–27) or neuromedin C [NMC (GRP18–27)] have a greater affinity for the BB2 receptors. Interestingly, BB and NMB increased the firing rate of 5-HT cells in the DRN (Pinnock and Woodruff, 1991). Because reduced 5-HT release has been linked to reduced anxiety, antagonism of the excitatory action of BB-like peptides on DRN 5-HT neurons might be expected to decrease anxiety.
In view of the potential role of NMB and GRP in mediating anxiety, the effects of BB agonists and antagonists were assessed in several anxiety-related behavioral paradigms. Specifically, we assessed the influence of systemic administration of PD 176252 [3-(1H-indol-3-yl)-N-[1-(5-methoxy-pyridin-2-yl)-cyclohexylmethyl]-2-methyl-2-[3-(nitro-phenyl)ureido]propionamide], a nonpeptide antagonist of the BB1 receptor (with modest effects on BB2 receptors) (Ashwood et al., 1998), on anxiety-like behaviors in ethologically relevant tests, including social interaction (File, 1980), approach to a familiar palatable snack in a novel (anxiogenic) environment (Merali et al., 2004), and in the guinea pig pup vocalization test (Molewijk et al., 1996). Effects of PD 176252 on fear-potentiated startle were also assessed to determine the impact on learned fear responses (Lee and Davis, 1997). Given the potential role of 5-HT in anxiety, we examined the effects of 5-HT lesions (using 5,7-DHT) on BB1/BB2 receptor binding at the DRN and determined whether PD 176252, administered systemically or intra-DRN, influenced in vivo 5-HT release at the ventral hippocampus, a projection site of 5-HT neurons emanating from the DRN. Finally, we assessed whether the anxiogenic effects of BB1 receptor agonist (NMB; injected intra-DRN) were attenuated by serotonin (5-HT1A or 5-HT2C) receptor antagonists.
Materials and Methods
Animals
Rats were group housed in a holding room with lights on from 7:00 A.M. to 7:00 P.M. for at least 2 weeks before testing. Rats in the microdialysis experiments were housed in a holding room lit on a reversed light cycle (lights on from 7:00 P.M. to 7:00 A.M.). Sprague Dawley rats (300–375 g; Charles River, St. Constant, Quebec, Canada) were used in the Canadian studies, whereas the Cambridge studies used male hooded Lister rats (300–375 g; Harlan Olac, Bicester, UK). Female Dunkin Hartley guinea pigs (Dave Hall, UK), with litters of 1- to 3-d-old pups, were used for the vocalization studies. They were housed in mother plus litter groups on a 12 h light/dark cycle (lights on at 7:00). All animals received food and water ad libitum, and the temperature (22°C) and humidity (63%) were kept constant. All procedures were approved by the appropriate Local Ethics Committees and met the guidelines set out by the Canadian Council on Animal Care and the Home Office Guidelines in the United Kingdom. All attempts were made to minimize the number of animals used considering the variability in responses associated with the treatments.
Apparatus
Social interaction under aversive conditions (experiments 1–3) was assessed in a cylindrical white Perspex arena (70 cm diameter and 30 cm high walls), lit by a bright light source (350 lux) located directly above the arena. Social interaction under nonaversive conditions (experiments 4, 5) was assessed in a rectangular gray Perspex arena (60 × 60 cm; 30-cm-high walls), illuminated by a diffuse overhead dim-light source (30 lux). A camera linked to a video recorder in an adjacent room was located directly above the arenas to permit remote monitoring/scoring and recording of the test sessions.
The test cage for the guinea pig vocalization assessment consisted of a sound-attenuated box with a white interior where the vocalizations were recorded by means of a microphone and a digital audio tape recorder.
To assess the behavioral responses associated with the presentation of a palatable snack, animals were tested in either a familiar (home) or unfamiliar (novel) cage. The home cage consisted of a shoebox-style clear Plexiglas container (24 × 30 × 18 cm) with the bottom lined with bedding material (β chips) to a depth of ∼1 cm and a removable grilled top. The novel environment (test cage) was identical to the home cage but was freshly cleaned and devoid of the wood chip floor bedding.
For the fear-potentiated startle experiments, animals were trained and tested using acoustic startle response monitoring systems (MED Associates, St. Albans, VA), which were located in individual, ventilated, sound-attenuated chambers (30 × 55 × 50 cm). Each chamber was equipped with a wideband speaker (1–16 kHz), which provided the acoustic startle stimuli, as well as background noise. During the training and testing sessions, the rats were placed in individual cages (19 × 9 × 8.5 cm) with conductive floors (made up of six stainless steel rods of 4.9 mm diameter) that were placed on top of the startle sensor platform. Each sensor platform (25 × 11.5 cm) was linked to a signal transducer and load cell amplifier. The downward pressure exerted by the animal's startle response was converted to an analog signal that was amplified (by the load cell amplifier) and then digitized on a scale of 0–2047 arbitrary units. The presentation and ordering of all stimuli were controlled by startle reflex software (MED Associates).
Surgery
Microinjection and microdialysis experiments.
Rats were anesthetized using halothane (3% in oxygen) inhalation and positioned in a stereotaxic frame (Kopf Instruments, Tujunga, CA). For cannulation of the DRN (social interaction and microdialysis experiments), 11-mm-long stainless steel guide cannulas were positioned at 7.4 mm posterior to bregma, 2.1 mm lateral to the midline, and 6.5 mm ventral from the dura at an angle of 19°, thus sitting it 1 mm above the target area. The cannulas were secured in place using skull screws and dental acrylic cement. For microdialysis at the ventral hippocampus, guide cannulas (BAS, West Lafayette, IN) containing removable obturators were positioned 5.3 mm posterior to bregma, 4.85 mm lateral to the midline, and 3.0 mm ventral from the dura. For cannulation of the third ventricle (fear-potentiated startle experiments), rats were anesthetized with pentobarbital (65 mg/kg, i.p.), and guide cannulas were positioned 4.3 mm posterior to bregma, 0.0 mm lateral to the midline, and 4.3 mm ventral to the skull surface. All cannulated rats were housed individually and allowed a minimum of 7 d for recovery before testing. The cannulas were kept patent using stainless steel obturators, which were manipulated daily by gently wrapping the rat in a cloth and rotating the obturators.
5,7-DHT lesioning
Rats were injected with desmethylimipramine (25 mg/kg, i.p.), and 1 h later they were anesthetized and positioned in the stereotaxic instrument. A hole was drilled 1 mm posterior to bregma and 1.4 mm to the right of the midline, and an injector (connected to a glass syringe via polyethylene tubing) was lowered 3.5 mm below the dura, positioning it directly into the lateral ventricle. An injection of either vehicle [5 μl of 0.2% ascorbic acid in artificial CSF (aCSF); n = 10] or 5,7-DHT (150 μg/5 μl vehicle; n = 10) was made over 30 s, and the needle was left in place for an additional 30 s period to allow for drug diffusion. The wound was sealed with dental cement, and the rats were housed singly and allowed to recover for 13 d, during which they were monitored for adverse reactions to the treatment (none were evident).
In addition, autoradiographic analyses were undertaken to confirm whether BB1 receptor binding was present at the DRN and whether this was affected by neurochemical lesioning of DRN 5-HT cells.
Behavioral testing: social interaction
In the initial set of three experiments, social interaction was assessed under aversive (high illumination, unfamiliar environment) conditions. Rats were allocated to a partner on the basis of body weight, such that members of a pair did not differ by >10 g. In the first experiment, both rats of each pair were systemically (intraperitoneally) injected with either vehicle (n = 10) or PD 176252 (3.75 and 7.5 mg/kg; n = 10 per group) 60 min before being placed into the arena for a 5 min period. Thereafter, time spent in active social investigation (comprising sniffing, following, and grooming the partner) was recorded by an observer blind to drug treatment. Testing was performed between 10:00 A.M. and 2:00 P.M. in an order randomized for drug treatment, and the arena was thoroughly cleaned between each trial.
In experiment 2, which assessed the effects of DRN microinfusion, the cannulated rats were gently wrapped in a cloth and injected with either vehicle (50% cyclodextrin in aCSF; n = 12) or PD 176252 (100 ng/0.5 μl; n = 14) using an injection cannula that extended 1 mm below the tip of the indwelling guide cannula. Injections of 0.5 μl were made over a period of 30 s; the needle was left in position for an additional 30 s to allow for drug diffusion. Three minutes later, the cannulated rat and an unfamiliar, noncannulated partner were placed together in the arena, and their behavior was observed for 7 min as described in the preceding experiment.
After DRN microinjection, the rats were allowed a 7-d drug “washout” period. They were then randomly assigned to a retest condition with a novel partner. Rats received DRN microinfusion, as described previously, of either vehicle (aCSF; n = 10) or the 5-HT1A receptor agonist 8-hydroxy-2(di-n-propylamino)tetralin (8-OH DPAT; 50 ng/0.5 μl; n = 9). The social interaction test was conducted using the same procedure as that used 1 week previously.
In experiment 3, which involved a naive cohort, rats were assigned to one of three conditions, wherein they received DRN microinfusion of vehicle (50% cyclodextrin in aCSF; n = 12) or PD 176252 at low (20 ng; n = 9) or high (500 ng/0.5 μl, n = 11) doses and assessed in the social interaction tested as described in the preceding study.
In the next series of experiments, social interaction was assessed in a relatively nonaversive familiar, low-level illumination condition. To create a familiar, low-light testing condition, rats of experiment 4 were habituated to the social interaction arena for 2 d before testing in low lighting (30 lux). They were then assigned to one of four conditions wherein they received the following: (1) intra-DRN vehicle (0.5 μl) plus a systemic injection of vehicle (1 ml/kg, i.p.; n = 12), (2) intra-DRN NMB (50 ng/0.5 μl) plus systemic injection of vehicle (1 ml/kg, i.p.; n = 7), (3) intra-DRN vehicle (0.5 μl) plus systemic injection of a 5-HT2C receptor antagonist, 6-chloro-5-methyl-1-[(2-[2-methylpyrid-3-yloxy]pyrid-5yl)carbamoyl]indoline (SB 242084; 0.2 mg/kg, i.p.; n = 9), or (4) intra-DRN NMB (50 ng/0.5 μl) plus systemic injection of SB 242084 (0.2 mg/kg, i.p.; n = 7). On habituation day 1, each rat was placed with its unoperated partner in the arena for 5 min, whereas on habituation day 2, rats were placed individually in the test arena for 5 min. On test day (day 3), rats received their designated systemic drug injection and, 27 min later, were microinjected intra-DRN with the test drug and then placed (3 min later) into the test arena together with their unoperated partner. Their social interaction behavior was assessed over the subsequent 7 min, as described in the preceding studies. Locomotor activity in the arena was assessed by counting the number of squares crossed by the rat.
In experiment 5, rats were assigned to one of four treatment conditions wherein they received the following: (1) intra-DRN vehicle (0.5 μl) plus systemic vehicle injection (1 ml/kg, i.p.; n = 14), (2) intra-DRN NMB (50 ng/0.5 μl) plus systemic vehicle injection (1 mg/kg, i.p.; n = 9), (3) intra-DRN vehicle (0.5 μl) and systemic injection of the 5-HT1A receptor antagonist N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide (WAY 100635; 0.2 mg/kg, i.p.; n = 5), or (4) intra-DRN NMB (50 ng/0.5 μl) and systemic injection of WAY 100635 (0.2 mg/kg, i.p.; n = 5). Rats were assessed in the social interaction test as described in experiment 4.
Guinea pig pup vocalization
In experiment 6, guinea pig pups (n = 8–9 per group) were selected for the test groups using the criterion that they emit a minimum of 500 vocalizations after separation from the dam, in a 5 min test period on each of three consecutive days before the test day. On the test day, pups were submitted to a pretest (baseline) that took place 30 min after a sham injection. Subjects were then injected with either vehicle or PD 176252 (1–30 mg/kg, i.p.) and tested again 30 min later for 5 min.
Approach to a palatable snack in a novel environment
When presented with a familiar snack in a novel (anxiogenic) environment, rodents are reluctant to approach and consume the treat. The reluctance to approach the snack is attenuated by anxiolytic agents and has been validated as a test of anxiety (Merali et al., 2004). Rats in experiment 7 (n = 8 per group) were habituated to a novel, highly palatable snack (Christie HoneyMaid Graham Crumbs) presented in their home cage (15 min snack access) for eight consecutive days. The snack was presented in a 6-cm-diameter ceramic dish placed in the center of the cage. During the last 3 d (of the 8 d) of this habituation period, when the approach and consumption parameters had stabilized (varying <20%) (Merali et al., 2004), baseline measures of the latency to initiate consumption as well as the amount consumed were recorded. On the test day, rats received either PD 176252 (10 mg/kg, i.p.) or vehicle (50% cyclodextrin control condition) and returned to their home cage. Twenty minutes after treatment, rats were transferred to the novel cage (freshly cleaned clear Plexiglas test cage without bedding) and presented with the now familiar palatable snack. The latency to initiate snack consumption and the amount consumed (over 15 min) were monitored.
Fear-potentiated startle
Training/conditioning.
Rats of experiment 8 were first acclimatized to the darkened startle test chamber (with 50 dB ambient white noise) for 5 min. They were then presented with 20 light-shock pairings (per day) at an average of 60 s intervals (range, 45–75 s) on each of two consecutive days. On each trial, a 0.65 mA shock was delivered through the grid floor (using a shock source and scrambler, ENV-410B and ENV-412S, respectively; MED Associates) during the last 0.5 s of the 3.7 s conditioned stimulus (CS) (light).
Premanipulation fear test.
Rats were placed in the same startle cages (where they were trained) and 5 min later presented with 20 startle-eliciting auditory stimuli (110 dB; habituation session). Subsequently, 10 acoustic startle stimuli were presented, half of which were presented alone (unconditioned stimulus; noise-alone trials) and the other half presented 3.2 s after the onset of the 3.7 s light (CS − noise trials). Those rats that failed to show CS-elicited potentiation (i.e., <40% increase in the CS-paired startle response compared with matched non-CS associated responses) were omitted from subsequent studies (∼25%). The selected rats were then placed into groups based on similar fear levels determined from the difference scores in the pretest [(CS + noise) − (noise-alone)]. The test procedure was identical to that of the premanipulation fear test, except that the animals were first injected with the test drug or appropriate vehicle (control) 20 min before the test. The effects of central (100 and 200 ng) and systemic PD 176252 administration (5 and 10 mg/kg) relative to vehicle were tested using separate groups of animals (n = 9 per group).
In vivo microdialysis.
An initial microdialysis study (experiment 9) assessed 5-HT release after the systemic injection of PD 176252. A microdialysis probe (BAS, West Lafayette, IN) was lowered into the preimplanted guide cannula and secured such that the active portion of the membrane (4 mm long) extended into the ventral hippocampus. While the animals (n = 6 per group) recovered from surgery in the test chamber, the probe was connected via polyethylene tubing (protected by a metal tether connected to a swivel hooked onto a balance arm) to a precision pump (Harvard Apparatus, Endenbridge, UK) and perfused overnight at 0.1 μl/min with aCSF consisting of the following (in mm): 2.7 K+, 145 Na+, 1.35 Ca2−, 1.0 Mg2+, 150 Cl−, 0.05 ascorbate, pH 7.4. Approximately 18 h after surgery, dialysate samples were collected every 20 min at a flow rate of 1.5 μl/min. After a 1 h period [previously shown to be sufficient for stabilization (Merali et al., 1998)] and a stable baseline was established, the rats were injected systemically with either vehicle (50% hydroxypropyl-β-cyclodextrin in saline) or PD 176252 (10 mg/kg, i.p.), and samples were collected for an additional 3 h. In a second microdialysis study (experiment 10), rats (n = 6–8 per group) under halothane anesthesia were stereotaxically implanted with guide cannula aimed at the DRN. Once the cannula was set firmly in place, a 4 mm microdialysis probe was lowered into the ventral hippocampus and held in position using the second arm of the dual arm stereotaxic apparatus.
An injector was lowered through the guide cannula such that it protruded 1 mm beyond the tip of the guide cannula and into the DRN. The probes were perfused with aCSF at a flow rate of 1.5 μl/min, and sample collection was initiated after a 1 h stabilization period. Samples were collected every 20 min for a maximum of 5 h. After collecting five baseline samples, animals were injected with vehicle (50% cyclodextrin in aCSF or aCSF; 0.5 μl), and five more samples were collected. Animals were then injected with NMB-30, GRP1–27 (10 ng/0.5 μl; Peninsula Laboratories, Belmont, CA) or PD 176252 (200 ng/0.5 μl), and five additional samples were collected.
Sample analysis
Dialysate samples.
After collection, samples were immediately analyzed ex vivo for their 5-HT content using HPLC (Agilent Technologies, Walbronn, Germany). A 50 μl volume of each dialysate was injected via an autoinjector (1100 series Autosampler; Agilent, Walbronn, Germany) into the HPLC system equipped with a single cell electrochemical detector (model Intro; Antec Leyden, Montreal, Quebec, Canada) with an applied potential of 0.650 nA, a filter of 1 s, and a range of 0.1 nA/V. Separation of these analytes was achieved by their passage through an ESA, 4.6 × 150 mm, 5 μm analytical column (Zorbax Eclipse XDB-C18; Agilent). The column was equilibrated at a flow rate of 1 ml/min with mobile phase consisting of the following (in mm): 90 sodium dihydrogen phosphate (monobasic), 1.7 1-octane sulfonic acid (sodium salt), 50 citric acid (monohydrate), 5 KCl, 50 mm EDTA, and 10% acetonitrile, final pH 2.4. The quantification of the analytes was performed by comparing their area under the curve to those of known external standards (calibrated at 1.25 pg/50 μl, 5 pg/50 μl, and 50 pg/50 μl) using computerized Agilent ChemStation chromatography data acquisition system (Agilent).
Tissue sectioning and preparations.
The effectiveness of the neurochemical lesions (for the autoradiography study) was confirmed by measuring various catecholamine and indoleamine concentrations at specific brain sites. Thirteen days after intracerebral injection, rats were stunned and killed by cervical dislocation. The brains were removed and immediately immersed for 20 s in n-pentane, cooled to approximately −35°C. The brains were then wrapped in aluminum foil and stored at −70°C until required for sectioning or for HPLC analysis. Brains for sectioning were mounted and sectioned (10 μm thickness) using a cryostat microtome. The sections were thaw-mounted onto gelatin-coated glass microscope slides and stored at −70°C until required for autoradiography. Slides were prepared with sections for total binding and sections for nonspecific binding being taken alternately as they were cut from the brain. Brains for HPLC analysis of neurotransmitter content were thawed, and the dorsal and ventral hippocampus, striatum, and frontal cortex were dissected. Each separate brain area was placed in an Eppendorf (Eppendorf Scientific, Westbury, NY) tube with 500 μl of 0.1 m perchloric acid (containing 1 ng of an internal standard, dihydroxybenzylamine) homogenized using ultrasound and centrifuged at 8000 × g for 15 min. The supernatant was analyzed for 5-HT, 5-hydroxyindole-3-acetic acid (5-HIAA), and norepinephrine levels using HPLC analysis.
Histology
Cannula placement verification.
After the experiments, animals were killed, and their brains were removed and frozen. Location of the third ventricle cannulas (fear-potentiated startle experiment), microdialysis probes, and the microinjectors aimed at the DRN (microdialysis and social interaction experiments) were verified histologically after thionin staining of the sections. For the microdialysis experiment, only animals with both the injector and the microdialysis probe correctly positioned were included in the analyses.
Autoradiography studies
Sections were slowly thawed and dried under a stream of cold air before being placed in Tris HCl (pH 7.4 at room temperature) buffer for 20 min. They were then placed in either a “total binding” or “nonspecific binding” incubation mixture for 90 min. The total binding incubation mixture was composed of Tris HCl buffer containing bovine serum albumin (0.5%), bacitracin (40 μg/ml), MnCl2 (3 mm), leupeptin (4 μg/ml), phosphoramidon (2 μm), chymostatin (2 μg/ml), d-Phe6-ethylester (10 μm), and 125I-BB (0.1 nm; specific acitivity, 2000 Ci/mmol). Nonspecific binding incubation mixture was the same as for total binding except that cold BB (1 μm) was included. After the incubation period, sections were washed three times in fresh, ice-cold Tris HCl, pH 7.4, at 4°C) for 10 s and finally washed twice in ice-cold, HPLC grade water and dried under a cold stream of air. Autoradiograms were generated by laying the labeled tissues against [3H]Hyperfilms (GE Healthcare, Amersham, UK). Standards (radiolabeled polymer strips obtained from GE Healthcare) were also laid down at the same time (one per film) to enable quantification of the binding. The films were developed in Kodak (Rochester, NY) D-19 solution after 3 d of exposure at room temperature.
Drugs
The nonpeptide BB1/BB2 receptor antagonist PD 176252 was dissolved in 50% hydroxypropyl-β-cyclodextrine in saline using a heated sonicating water bath and administered intraperitoneally at the appropriate doses in a 1 ml/kg volume. A 50% cyclodextrin injection was used as a control (vehicle) condition. For intracranial administration, PD 176252 was dissolved in 50% cyclodextrin and then diluted accordingly in aCSF. 8-OH DPAT (Sigma/Aldrich, Poole, UK) was dissolved in aCSF. For social interaction (experiments 4, 5), NMB-30 (Bachem, Torrance, CA), WAY 100635, and SB 242084 (Organon Laboratories, Lanarkshire, Scotland) were dissolved in 10% cyclodextrin in aCSF and 20 mmol/L citric acid. Desmethylimipramine, 5,7-DHT, and all standards for HPLC were obtained from Sigma (St. Louis, MO), whereas SCH50911 [(+)-(2S)-5,5-dimethyl-2-morpholineacetic acid] was obtained from Tocris Cookson (Ellisville, MO). For the microdialysis experiment, NMB-30 and GRP1–27 were obtained from Bachem and dissolved in aCSF. Chemicals for the autoradiography and also for the mobile phase for the HPLC were obtained from Sigma and were of HPLC grade. 125I-BB (specific activity, 2000 Ci/mmol) was obtained from PerkinElmer (Boston, MA).
Data analysis
Data from the social interaction experiments were analyzed by unpaired t tests when only two groups were tested. When more than two groups were used, significance was determined through one-way ANOVA, followed by Newman–Keuls multiple comparisons (α = 0.05). For the vocalization experiments, the difference in the number of calls emitted before and after treatment was counted using Spike2 software. The reduction in the number of calls (as a percentage of baseline) was analyzed using a Kruskal–Wallis test followed by Mann–Whitney U tests between vehicle and different doses. Data from the potentiated startle experiments and from the approach to a snack in the familiar and novel environments were analyzed by mixed-measures ANOVA, where drug dose served as the between-group factor, and stimulus condition was the within-group factor.
In the first microdialysis experiment, 5-HT content was analyzed as a percentage change from baseline (mean of three samples) taken at 20 min intervals. Samples collected after treatments were compared with the mean basal value. For the second microdialysis experiment, 5-HT content was expressed as a percentage change from baseline (mean of three sample) and analyzed using a mixed-measures ANOVA with treatment condition (vehicle, PD 176252, or NMB-30) as the between-group factor and samples as the within-group factor. Follow-up comparisons were conducted using Newman–Keuls multiple comparisons (α < 0.05). A similar analysis was conducted to measure 5-HT content after local infusion of GRP at the DRN. For the 5,7-DHT lesioning studies, each of the neurotransmitter peaks from the HPLC was converted into values representing ng/mg wet weight tissue based on external neurotransmitter standards of that day. A Student's t test was used to analyze between-group differences.
For the autoradiography studies, a computerized image analysis system (microcomputer imaging device) was used to construct a standard curve of radioactivity from the standard polymer strip for each film. The optical density of selected brain regions was then measured and converted to nCi/mg protein using the standard curve. Three sections from each brain region were measured, and the values were averaged for each animal. The values for each region for each animal were then analyzed by Student's t tests for differences between control and lesioned rats.
Results
Effects of systemically administered PD 176252 on social interaction
In experiment 1, as shown in Figure 1, systemic treatment with PD 176252 significantly influenced levels of social interaction of the rats (F(2,27) = 7.20; p < 0.01). The follow-up comparisons indicated that both the 3.75 and 7.5 mg/kg doses (p < 0.01 and p < 0.05, respectively) of the antagonist significantly increased social interaction, although the effects of the two doses did not differ from one another. In contrast to social interaction, the drug treatment did not affect locomotor activity (data not shown).
Figure 1.
Effect of PD 176252 on rat social interaction. Each column represents time spent in social interaction during a 5 min test period (mean ± SEM) after injection of vehicle (open column; n = 10), PD 176252 (3.5 mg/kg, i.p.; hatched column; n = 10), or PD 176252 (7.5 mg/kg, i.p.; solid column; n = 10). *p < 0.05 and **p < 0.01 from vehicle condition.
Effect of intra-DRN PD 176252 on social interaction
Direct injection of PD 176252 into the DRN, as shown in Table 1 (experiments 2, 3), significantly increased social interaction scores (t(22) = 4.10; p < 0.05). Because the drug did not affect locomotor activity, the altered social interaction could not be attributed to motor effects. In the subsequent study, using both a higher and a lower dose of PD 176252, it was found that the 500 ng provoked an anxiolytic effect, whereas the 20 ng was not effective. Finally, as shown in Table 1, administration of 8-OH DPAT (50 ng) significantly increased social interaction without affecting locomotor activity (t(25) = 27.77; p < 0.01).
Table 1.
Effect of intra-DRN administration of PD 176252 and 8-OH DPAT on rat social interaction (seconds; mean ± SEM) and locomotor activity (beam breaks; mean ± SEM) under aversive conditions
| Social interaction score | Locomotor activity | |
|---|---|---|
| Experiment 2 | ||
| Control (vehicle; n = 12) | 39.1 ± 4.6 | 144.3 ± 8.8 |
| PD 176252 (100 ng; n = 14) | 50.1 ± 2.6* | 152.5 ± 6.1 |
| Control (vehicle; n = 10) | 47.5 ± 2.7 | 158.8 ± 9.9 |
| 8-OH DPAT (50 ng; n = 9) | 67.5 ± 3.4** | 159.2 ± 7.8 |
| Experiment 3 | ||
| Control (vehicle; n = 12) | 35.3 ± 3.3 | 172.5 ± 10.8 |
| PD176252 (20 ng; n = 9) | 36.0 ± 4.4 | 169.8 ± 14.6 |
| PD 176252 (500 ng; n = 11) | 47.4 ± 5.4* | 153.2 ± 10.4 |
*p < 0.05 and
**p < 0.01 respective vehicle controls (aCSF); post hoc Duncan's test after one-way ANOVA.
Effect of intra-DRN NMB and of systemic 5-HT1A and 5-HT2C receptor antagonists on social interaction
As seen in Table 2 (experiments 4, 5), direct injection of NMB (50 ng/0.5 μl) into the DRN significantly decreased social interaction scores, and this effect was completely blocked by pretreatment with the 5-HT2C receptor antagonist (SB 242084; 0.2 mg/kg, i.p.; F(3,31) = 7.43, p < 0.0007). In contrast, pretreatment with the 5-HT1A receptor antagonist (WAY 100635; 0.2 mg/kg, i.p.) did not affect the suppression of social interaction ordinarily induced by NMB (F(3,29) = 8.44; p < 0.0003). At the doses used, neither NMB nor WAY 100635 affected locomotor activity.
Table 2.
Effect of intra-DRN administration of NMB and/or systemic administration of SB 242084 or WAY 100635 on rat social interaction (seconds; mean ± SEM) and locomotor activity (squares crossed ± SEM) under nonaversive conditions
| Social interaction score | Locomotor activity | |
|---|---|---|
| Experiment 4 | ||
| Control (vehicle; n = 12) | 79.3 ± 5.2 | 171.2 ± 13.4 |
| NMB (50 ng/ 0.5 μl; n = 7) | 46.9 ± 8.1*† | 186.3 ± 15.8 |
| SB 242084 (0.2 mg/kg; n = 9) | 76.3 ± 5.4 | 171.3 ± 16.4 |
| SB 242084 (0.2 mg/kg) plus NMB (50 ng/0.5 μl; n = 7) | 88.7 ± 5.6 | 202.0 ± 9.0 |
| Experiment 5 | ||
| Control (vehicle; n = 14) | 81.4 ± 4.7 | 165.0 ± 11.6 |
| NMB (50 ng/ 0.5 μl; n = 9) | 47.5 ± 6.3* | 179.1 ± 15.1 |
| WAY 100635 (0.2 mg/kg; n = 5) | 74.5 ± 8.0† | 170.0 ± 27.1 |
| WAY 100635 (0.2 mg/kg) plus NMB (50 ng/ 0.5 μl; n = 5) | 47.8 ± 7.9* | 176.0 ± 26.6 |
*p ≤ 0.05 relative to vehicle control rats (aCSF).
†p ≤ 0.05 relative to rats systemically injected with the 5-HT receptor antagonist (either SB 242084 or WAY 100635) in combination with intra-DRN administration of NMB.
Effects of systemically administered PD 176252 on guinea pig pup vocalization after separation
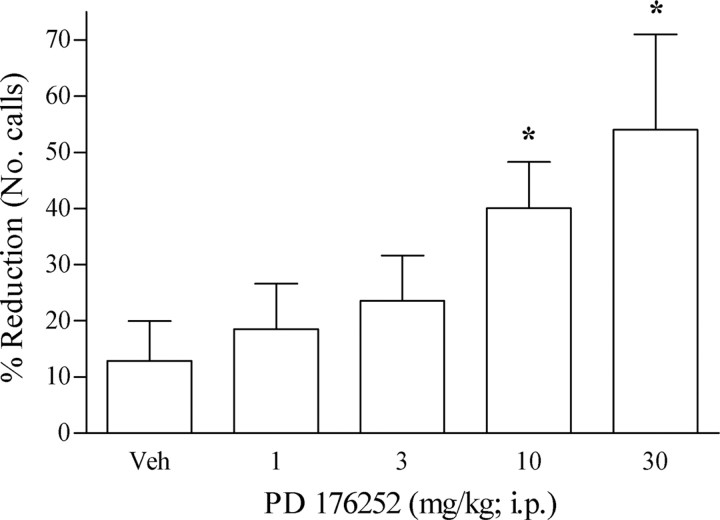
After separation from the dam, guinea pig vocalization occurred at a high level, such that after sham injection, the number of vocalizations was 560 ± 42.6. Administration of PD 176252 (30 min before the test) dose-dependently (1–30 mg/kg, i.p.) diminished the number of calls ordinarily emitted, with an MED of 10 mg/kg. At the highest dose (30 mg/kg), a fourfold change was observed relative to vehicle-treated animals (Fig. 2).
Figure 2.
Effect of PD 176252 on guinea pig pup vocalizations after separation from the dam during a 5 min test period. Each column represents percentage reduction in the number of vocalizations (mean ± SEM) made after PD 176252 (1–30 mg/kg; i.p.; n = 8 per group) or vehicle (n = 9) injection relative to the number of calls (No. calls) made after sham injection (number of vocalizations, 560 ± 42.6). *p < 0.05 and **p < 0.01 from vehicle condition.
Effects of systemically administered PD 176252 on approach and consumption of a palatable snack in a novel environment
Repeated-measures ANOVA of the amount of snack consumed revealed a significant test condition (home vs novel cage) × drug treatment (PD 176252 vs vehicle) interaction (F(1,14) = 4.82; p < 0.05). Follow-up tests of the simple effects comprising this interaction indicated that in the home cage, there were no significant group differences in the amount of snack consumed. However, as depicted in Figure 3, a marked reduction in the amount of snack consumed was evident in the novel cage condition, and PD 176252 significantly attenuated this effect. Similarly, analysis of the latency to consume snack food revealed a significant test condition × drug treatment interaction (F(1,14) = 29.38; p < 0.0001), which was attributable to a novelty-induced increase in latency to eat in the controls, and this was significantly attenuated in the PD 176252-treated rats.
Figure 3.
Effect of PD 176252 on snack consumption (grams) and on the latency to approach the snack (seconds) in the home cage and novel cage conditions. Each column depicts snack consumption (mean ± SEM over 15 min) or approach latency (mean ± SEM) under the home cage or the novel cage conditions after PD 176252 (10 mg/kg; i.p.; solid column; n = 8) or vehicle (open column; n = 8) injection. *p < 0.0.5 and **p < 0.01 from respective home cage baselines; †p < 0.05 from condition-matched control.
Effects of systemically administered PD 176252 on the fear-potentiated startle response
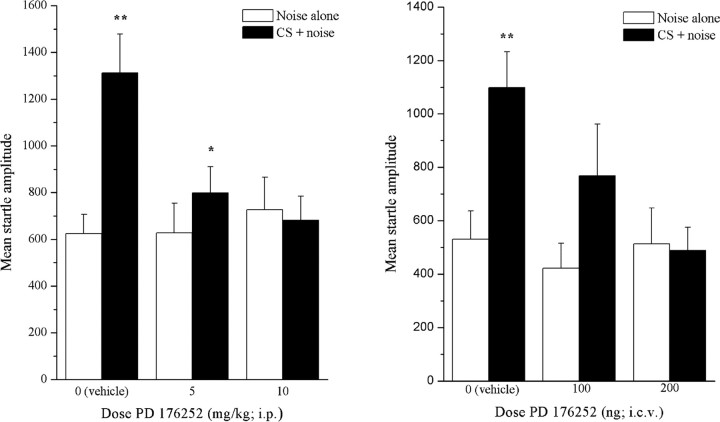
Analysis of the startle and fear-potentiated startle responses revealed that responsivity varied as a function of the treatment (doses) × stimulus condition (CS+ or CS−) interaction (F(2,24) = 7.94; p < 0.005). Comparisons of the means comprising the simple effects revealed that in the control (vehicle-treated) group, the startle amplitude in the presence of the CS (fear-related cue) (Fig. 4, solid columns) was markedly higher than that noted in the absence of CS (Fig. 4, left, open columns). Intraperitoneal administration of PD 176252 did not alter the startle response evident in the noise alone condition (CS−); however, the lower dose (5 mg/kg, i.p.) of the drug attenuated the CS+-induced potentiation of the startle response, and the higher dose (10 mg/kg, i.p.) entirely eliminated the potentiation.
Figure 4.
Effect of PD 176252 on fear-potentiated startle amplitude after intraperitoneal (left) and intracerebroventricular (right) administration. Each column depicts startle amplitude (mean ± SEM) under the noise alone (open columns) and conditioned stimulus plus noise (CS + noise; solid columns) situations after intraperitoneal (left) injections of vehicle (n = 9), PD 176252 (5 mg/kg; n = 9), or PD 176252 (10 mg/kg; n = 9) or intracerebroventricular (right) injections of vehicle (n = 9), PD 176252 (100 ng/3 μl; n = 9), or PD 176252 (200 ng/3 μl; n = 9). *p < 0.05 and **p < 0.01 (CS + noise vs noise alone).
The effects of centrally administered PD 176252 on fear-potentiated startle response
Analyses of the effects of centrally administered PD 176252 revealed that responsivity varied significantly as a function of the interaction between the stimulus condition (CS+ or CS−) and treatment condition (F(2,24) = 4.533; p < 0.05). As shown in Figure 4 (right), the startle amplitude of the control animals that received vehicle (50% cyclodextrin; 3 μl, i.c.v.) increased markedly in the presence of the fear cue (CS+). However, this fear-potentiated startle response was significantly attenuated in rats pretreated with the lower dose of PD 176252 (100 ng, i.c.v.) and completely blocked in rats that received the higher dose (200 ng, i.c.v.) of the antagonist.
Effects of 5,7-DHT lesioning on 5-HT levels
Large reductions of 5-HT and 5-HIAA levels (∼90%) in the dissected regions of brains from 5,7-DHT-treated rats indicated that the majority of the 5-HT system had been destroyed by the neurotoxin (Table 3). In addition, the fact that norepinephrine levels were not different between control and lesioned rats demonstrated the selectivity of the lesion for the 5-HT neurons.
Table 3.
Effects of 5,7-DHT lesioning on 5-HT, 5-HIAA, and norepinephrine levels in the frontal cortex, striatum, and dorsal and ventral hippocampus
| 5-HT |
5-HIAA |
Norepinephrine |
||||
|---|---|---|---|---|---|---|
| Vehicle | 5,7-DHT | Vehicle | 5,7-DHT | Vehicle | 5,7-DHT | |
| Frontal cortex | 0.94 ± 0.02 | 0.10 ± 0.03* | 0.28 ± 0.01 | 0.04 ± 0.01* | 0.39 ± 0.07 | 0.29 ± 0.04 |
| Striatum | 0.52 ± 0.03 | 0.10 ± 0.03* | 0.22 ± 0.02 | 0.05 ± 0.01* | 0.22 ± 0.02 | 0.26 ± 0.02 |
| Dorsal hippocampus | 0.40 ± 0.04 | 0.04 ± 0.009* | 0.26 ± 0.02 | 0.02 ± 0.009* | 0.24 ± 0.02 | 0.20 ± 0.02 |
| Ventral hippocampus | 0.61 ± 0.09 | 0.03 ± 0.008* | 0.31 ± 0.009 | 0.02 ± 0.009* | 0.50 ± 0.10 | 0.43 ± 0.04 |
Mean (± SEM) levels of 5-HT, 5-HIAA, and norepinephrine (nanograms per milligram of wet weight tissue) in frontal cortex, striatum, and dorsal and ventral hippocampus of rats, 2 weeks after intracerebroventricular administration (5 μl volume) of either vehicle (0.2% ascorbic acid; n = 3) or 5,7-DHT (150 μ g; n = 3).
*p < 0.01 relative to vehicle.
Regional distribution of specific 125I-BB binding to BB1 binding sites
In general agreement with previous findings (Battey and Wada, 1991; Ohki-Hamazaki, 2000; Moody and Merali, 2004), the highest densities of BB1 binding sites were found in olfactory and thalamic nuclei. Other regions displaying relatively high BB1 binding included the ventromedial hypothalamus, striatum, septum, piriform cortex, and amygdalo-hippocampal area. Binding in the hippocampus was mainly in the dentate gyrus, with diffuse binding present throughout. Binding was not strongly marked in CA1, CA2, or CA3 regions. BB1 sites were also observed in the midbrain raphé nuclei. In the DRN, the binding sites were found to be located along the midaxis running dorsoventral [i.e., low levels of binding being visible in the lateral “wings,” whereas the binding in the median raphé nucleus (MRN) was distributed throughout the nucleus]. Binding was found to be particularly marked in the rhabdoid nuclei, which lie just dorsal of the MRN (Fig. 5).
Figure 5.
Representative autoradiographs depicting coronal sections of rat brain taken from rats injected intracerebroventricularly either with 5,7-DHT (n = 7) or vehicle (n = 7) before they were killed. For ease of comparison, sections on the left are from vehicle-treated rats, and sections on the right are from rats treated with 5,7-DHT. DR, Dorsal raphé; MR, median raphé; VH, ventral hippocampal region.
Binding of 125I-BB to BB1 receptors in the DRN was reduced by ∼50% in the 5,7-DHT-treated rats (vehicle, 14.9 ± 2.9 nCi/mg protein; lesion, 7.24 ± 0.49 nCi/mg protein; p < 0.01). However, as shown in Figure 5, the binding was unaffected in the MRN. Likewise, the lesion did not appear to influence BB1 binding at the frontal cortex, olfactory nuclei, or dorsal and ventral hippocampus (data not shown).
The effects of systemically administered PD 176252 on ventral hippocampal 5-HT release
The average basal (preinjection) level of 5-HT in dialysates recovered from the ventral hippocampus of all rats was 14.2 ± 1.4 fmol/20 min (three basal samples from each of 12 rats). Intraperitoneal injection of vehicle caused a transient increase in the levels of 5-HT (10–15% of basal values). However, in rats injected with PD 176252 (10 mg/kg), release of 5-HT was markedly reduced, such that 5-HT output was ∼60% of basal levels (Fig. 6). The effect was maximal 60 min after injection and remained significantly decreased for an additional 80 min. The effect appeared not to be caused by local inhibition of 5-HT in the hippocampus, because PD 176252 (10 μm) did not alter 5-HT levels when perfused directly into the ventral hippocampus by reverse dialysis (≤10% reduction in basal levels over 60 min) (data not shown).
Figure 6.
Effect of systemically administered PD 176252 (10 mg/kg; closed squares; n = 6) or vehicle (open squares; n = 6) on extracellular levels of 5-HT [expressed as a percentage change from baseline (mean ± SEM)] as measured by in vivo microdialysis in the ventral hippocampus of the freely moving rat. *p < 0.05 and **p < 0.01 relative to baseline.
Effects of intra-DRN PD 176252, NMB-30, and GRP1–27 on release of 5-HT at the ventral hippocampus
The average basal (preinjection) level of 5-HT in dialysates recovered from the ventral hippocampus of all rats was 9.1 fmol/20 min (three basal samples from each of 24 rats). Extracellular levels of 5-HT after vehicle, PD 176252, and NMB-30 injection are depicted in Figure 7. ANOVA revealed a significant treatment effect for extracellular levels of 5-HT (F(2, 27) = 5.72; p < 0.008) with follow-up comparisons indicating that levels of 5-HT were reduced immediately after intra-DRN infusion of PD 176252, an effect that became significant by the third sample after injection (p < 0.05). As observed with systemic injection of PD 176252, intra-DRN infusion of the antagonist resulted in a reduction of 5-HT output to ∼60% of basal levels, an effect that was maximal 60 min after injection. In contrast, intra-DRN infusion of the agonist, NMB-30, resulted in a rapid and pronounced increase of 5-HT output (∼200% of basal levels), reaching statistical significance by the second postinjection sample (p < 0.05). Intra-DRN infusion of GRP was without effect on extracellular levels of 5-HT from the ventral hippocampus (data not shown).
Figure 7.
Effects of intra-DRN infusion of vehicle, PD 176252 (200 ng/0.5 μl; n = 8), or NMB-30 (10 nmol/0.5 μl; n = 6) on extracellular levels of 5-HT [expressed as a percentage change from baseline (mean ± SEM)] as measured in the ventral hippocampus of anesthetized rats using in vivo microdialysis. *p < 0.05 from time-matched sample after vehicle injection.
Discussion
Although the BB peptide family was initially implicated in the mediation of peripheral satiety signals (Gibbs et al., 1979; Kulkosky et al., 1982; Merali et al., 1999), more recent studies suggested that these peptides may be active neuromodulators/neuromediators affecting several brain mechanisms and altering behaviors associated with stress and anxiety (Merali et al., 1998, 2002). The phylogenetic conservation of so-called “gut-brain” peptides (e.g., CCK and BB-like peptides) suggests their importance from an evolutionary perspective. Although the developmental mechanisms leading to the recruitment of various peptidergic systems in the mediation of diverse behaviors is not well understood (Fink et al., 1998; Strand, 1999), in the context of danger signals, suppression of nondefensive (e.g., feeding) behaviors would be a highly adaptive response, and hence overlapping or interacting neural circuits for appetitively and aversively motivated behaviors might be expected.
The present study hinges on the working hypothesis that mammalian BB-peptides that had been implicated in satiety processes, namely GRP and NMB, may also contribute to the integration and/or mediation of stress/anxiety responses (Merali et al., 2002). Indeed, it seems that many aversive and appetitive events activate some of the same neuronal circuits (Merali et al., 1998, 2004). In this respect, BB-related peptides and their receptors are highly concentrated in brain regions associated with stress and appetitive responses, including hypothalamic nuclei (Wada et al., 1990; Battey and Wada, 1991) as well as the nucleus of the solitary tract (NTS), the bed nucleus of the stria terminalis, amygdaloid nuclei, and the hippocampus (Moody et al., 1981; Panula et al., 1982; Chronwall et al., 1985; Wolf and Moody, 1985; Shumyatsky et al., 2002). Furthermore, stressors altered endogenous levels of hypothalamic BB-related peptides and the density of BB binding sites at the NTS, arcuate nucleus, and paraventricular nucleus (PVN) (Kent et al., 1997), and in vivo analyses revealed that stressors provoked the release of BB-like peptides from the central amygdala (Merali et al., 1998, 2004). Paralleling the effects of stressors, BB administration increased hypothalamic–pituitary–adrenal functioning, and the variations of ACTH elicited by BB could be attenuated by the corticotropin-releasing hormone (CRH) antagonist α-helical CRH (Kent et al., 2001a,b).
Despite the data indicating BB involvement in the central neurochemical stress response, limited data are available concerning the behavioral effects of BB manipulations. Distinct neuronal circuits may be activated by diverse stressors (Merali et al., 2004), and aspects of the stress response (fear vs anxiety) may involve different brain structures (Lee and Davis, 1997; LeDoux, 2000) and evoke distinct anxiety characteristics (Merali et al., 2004; Anismam and Matheson, 2005). Thus, the present investigation included a variety of ethologically relevant challenges.
It has been demonstrated previously that central administration of BB dose-dependently enhanced locomotor activity in a familiar situation but reduced exploration in a novel (presumably stressful) environment (Merali et al., 1983; Schulz et al., 1984; Itoh et al., 1994). In the present investigation, we extended these findings demonstrating that antagonism of BB1 activity attenuated anxiety across a range of situations, including those that likely involved prewired circuitry (vocalization after removal of the dam, social interaction, approach to a snack in a novel environment), and those that involve conditioned fear (fear-potentiated startle). In the latter instance, the increased reactivity was restricted to the fear-potentiated response, because startle intensity was unaffected in the absence of the fear cues. Although these findings clearly support a role for BB processes in anxiety, this should not be misconstrued to suggest that BB-like peptides are necessarily involved in all anxiety-related behavioral outcomes. It remains to be established whether BB1 manipulations affect anxiety in other common paradigms (i.e., elevated plus maze, open-field emergence, conflict situations) as well as whether BB differentially influences situation- or cue-specific anxiety versus generalized anxiety or influences behaviors elicited by neurogenic stressors. Indeed, it has been argued that anxiety associated with psychogenic versus neurogenic stressors, as well as learned versus innate responses, may involve different processes, some of which may be unrelated to BB mechanisms (Merali et al., 2004).
It was shown that the BB1 and BB2 receptors are differentially distributed across brain regions (Battey and Wada, 1991; Ladenheim et al., 1992). For instance, whereas the PVN, lateral amygdala, and hippocampal and suprachiasmatic nuclei primarily express the BB2 receptor subtype, the DRN appears to have a preponderance of the BB1 receptor subtype (Battey and Wada, 1991; Wada et al., 1991; Ladenheim et al., 1992; Pinnock et al., 1994; Ohki-Hamazaki, 2000). Indeed, intracellular recording studies revealed that the majority of DRN neurons were selectively sensitive to BB1 (and not the BB2) ligands (Pinnock et al., 1994). The results of the present investigation suggested that these receptors are located on 5-HT neurons, because lesioning of the 5-HT system (using 5,7-DHT) markedly reduced BB1 receptor binding in DRN (cell body/dendrite region). Furthermore, DRN microinjection of BB1 (NMB), but not BB2 (GRP), provoked the release of 5-HT at the ventral hippocampus (terminal region). Conversely, microinfusion of the BB1/BB2 antagonist caused a diminution of extracellular 5-HT levels at the ventral hippocampus. Thus, it seems that at least a portion of the BB1 receptors in the DRN are located on the 5-HT cell bodies of the neurons that project to the ventral hippocampus.
As alluded to previously, anxiety has been associated with monoamine functioning, and particularly 5-HT and the 5-HT1A and/or 5-HT2C receptors (Lesch et al., 2003; Millan, 2003, 2005; Gordon and Hen, 2004). Consistent with this view, in the present investigation, localized infusion of PD 176252 into the DRN, which attenuated anxiety, provoked a marked reduction of 5-HT release (60% of baseline release) from the ventral hippocampus. However, infusion of PD 176252 directly into the hippocampus did not alter 5-HT release, suggesting that the 5-HT variations were not related to the local hippocampal inhibition of 5-HT neurons. In addition, microinjection of the BB1 agonist (NMB) stimulated the release of 5-HT at this site and, in parallel, increased anxiety (reduced social interaction), an effect that was blocked by a specific 5-HT2C (but not 5-HT1A) receptor antagonist.
Similar to our observations with NMB and its antagonist, elevated 5-HT expression at the DRN was reported in mice with targeted disruption of the BB1 receptor gene, and that the restraint-enhanced 5-HT expression noted in the wild-type mice was not evident in mice lacking the BB1 receptor (Yamano et al., 2002). These data support the view that these receptors are important in fine-tuning subsets of 5-HT neurons. In this respect, 5-HT1A-receptor gene expression appears to be downregulated in BB1-deficient mice, although these analyses did not assess the impact on 5-HT2C receptors and involved whole brain analyses (Yamada et al., 2002).
Unlike BB1 receptors, it seems that in the lateral amygdala, BB2 receptors are localized on GABAergic interneurons (Shumyatsky et al., 2002). Moreover, manipulation of this peptidergic receptor subtype not only influenced long-term potentiation but also affected the acquisition and performance of a conditioned fear response. Thus, although the present investigation did not support BB2 involvement in anxiety processes, it is premature to dismiss a role for this receptor subtype within the lateral amygdala in affecting fear and/or anxiety (Shumyatsky et al., 2002). Because GABA subunits have been implicated in anxiety processes, and inter-relations exist between GABA and 5-HT functioning (Sibille et al., 2000), the possibility exists that BB2 manipulations indirectly influence 5-HT functioning and anxiety through actions on GABAergic processes. This contrasts with the effects of BB1 receptors present on the DRN, because their manipulation appears to directly regulate ventral hippocampal 5-HT functioning and its effect on anxiety.
Together, it appears that the antagonism of NMB receptors by a compound that affects both the BB1 and BB2 receptor subtypes is effective as an anxiolytic in several behavioral paradigms. Moreover, consistent with the view that 5-HT processes involving the DRN may contribute to anxiety (Higgins et al., 1988, 1992; Andrews et al., 1994), social interaction was increased by intra-DRN administration of the BB1 antagonist, and this effect was as pronounced as that induced by the 5-HT agonist 8-OH DPAT. It is posited that this effect of PD 176252 may occur via an action in the DRN to reduce 5-HT levels in areas such as the hippocampus, which are innervated by the serotonergic fibers originating in the DRN, especially those endowed with 5-HT2C receptors. Indeed intra-DRN NMB decreased social interaction, an effect blocked by systemic administration of a 5-HT2C but not a 5-HT1A receptor antagonist. It remains to be determined whether localized infusion of various 5-HT receptor antagonists at the ventral hippocampus would attenuate the anxiogenic effect provoked by intra-DRN administration of NMB. Additional studies may reveal whether the treatments of anxiety through this class of compounds will provide an alternative to the benzodiazepines. However, it may be important to use agents that differentially affect BB1 and BB2 receptors given that BB2 knock-out mice exhibited an exaggerated fear-conditioned response (Shumyatsky et al., 2002). In effect, it may be that the two receptor subtypes play different roles in mediating anxiety (or different types of anxiety), and the development of pharmacotherapeutic intervention strategies for various anxiety-related disorders ought to consider their relative contributions.
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research and by the Parke Davis Neuroscience Research Centre, United Kingdom. H.A. is a Canada Research Chair in Neurosciences. Technical contributions from Christian Cayer and Jonathan James are gratefully acknowledged.
References
- Alves SH, Pinheiro G, Motta V, Landeira-Fernandez J. Anxiogenic effects in the rat elevated plus-maze of 5-HT(2C) agonists into the ventral but not the dorsal hippocampus. Behav Pharmacol. 2004;15:37–43. doi: 10.1097/00008877-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Andrews N, Hogg S, Gonzalez LE, File SE. 5-HT1A receptors in the median raphe nucleus and dorsal hippocampus may mediate anxiolytic and anxiogenic behaviours respectively. Eur J Pharmacol. 1994;264:259–264. doi: 10.1016/0014-2999(94)00473-0. [DOI] [PubMed] [Google Scholar]
- Anismam H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Ashwood V, Brownhill V, Higginbottom M, Horwell DC, Hughes J, Lewthwaite RA, McKnight AT, Pinnock RD, Pritchard MC, Suman-Chauhan N, Webb C, Williams SC. PD 176252–the first high affinity non-peptide gastrin-releasing peptide (BB2) receptor antagonist. Bioorg Med Chem Lett. 1998;8:2589–2594. doi: 10.1016/s0960-894x(98)00462-4. [DOI] [PubMed] [Google Scholar]
- Battey J, Wada E. Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends Neurosci. 1991;14:524–528. doi: 10.1016/0166-2236(91)90005-f. [DOI] [PubMed] [Google Scholar]
- Chronwall BB, Pisano JJ, Bishop JF, Moody TW, O'Donohue TL. Biochemical and histochemical characterization of ranatensin immunoreactive peptides in rat brain: lack of coexistence with Bombesin/GRP. Brain Res. 1985;338:97–113. doi: 10.1016/0006-8993(85)90252-5. [DOI] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KCF. Immunohistochemical localization of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Dourish CT. Brain 5-HT1A receptors and anxiety. In: Dourish CT, Ahlenius S, Hutson PH, editors. Brain 5-HT1A receptors. Chichester: Ellis Horwood; 1987. pp. 261–277. [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE. The history of benzodiazepine dependence: a review of animal studies. Neurosci Biobehav Rev. 1990a;14:135–146. doi: 10.1016/s0149-7634(05)80214-3. [DOI] [PubMed] [Google Scholar]
- File SE. New strategies in the search for anxiolytics. Drug Des Deliv. 1990b;5:195–201. [PubMed] [Google Scholar]
- Fink H, Rex A, Voits M, Voigt J-P. Major biological actions of CCK–a critical evaluation of research findings. Exp Brain Res. 1998;123:77–83. doi: 10.1007/s002210050546. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Fauser DJ, Rowe EA, Rolls BJ, Rolls ET, Maddison SP. Bombesin suppresses feeding in rats. Nature. 1979;282:208–210. doi: 10.1038/282208a0. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Hen R. The serotonergic system and anxiety. NeuroMol Med. 2004;5:27–40. doi: 10.1385/NMM:5:1:027. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Bradbury AJ, Jones BJ, Oakley NR. Behavioural and biochemical consequences following activation of 5HT1-like and GABA receptors in the dorsal raphe nucleus of the rat. Neuropharmacology. 1988;27:993–1001. doi: 10.1016/0028-3908(88)90058-5. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Jones BJ, Oakley NR. Effect of 5-HT1A receptor agonists in two models of anxiety after dorsal raphe injection. Psychopharmacology. 1992;106:261–267. doi: 10.1007/BF02801982. [DOI] [PubMed] [Google Scholar]
- Itoh S, Takashima A, Itoh T, Morimoto T. Open-field behavior of rats following intracerebroventricular administration of neuromedin B, neuromedin C, and related amphibian peptides. J Physiol (Japan) 1994;44:271–281. doi: 10.2170/jjphysiol.44.271. [DOI] [PubMed] [Google Scholar]
- Kent P, Anisman H, Merali Z. Are bombesin-like peptides involved in the mediation of stress response? Life Sci. 1997;62:103–114. doi: 10.1016/s0024-3205(97)01057-6. [DOI] [PubMed] [Google Scholar]
- Kent P, Anisman H, Merali Z. Central bombesin activates the hypothalamic-pituitary-adrenal axis: effects on regional levels and release of corticotropin-releasing hormone and arginine vasopressin. Neuroendocrinology. 2001a;73:203–214. doi: 10.1159/000054637. [DOI] [PubMed] [Google Scholar]
- Kent P, Bedard T, Kahn SE, Anisman H, Merali Z. Bombesin-induced HPA and sympathetic activation requires CRH receptors. Peptides. 2001b;22:57–65. doi: 10.1016/s0196-9781(00)00355-7. [DOI] [PubMed] [Google Scholar]
- Kulkosky PJ, Gibbs J, Smith GP. Feeding suppression and grooming repeatedly elicited by intraventricular bombesin. Brain Res. 1982;242:194–196. doi: 10.1016/0006-8993(82)90511-x. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Jensen RT, Mantey SA, Moran TH. Distinct distributions of two bombesin receptor subtypes in the rat central nervous system. Brain Res. 1992;593:168–178. doi: 10.1016/0006-8993(92)91305-x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle response. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Zeng Y, Reif A, Gutknecht L. Anxiety-related traits in mice with modified genes of the serotonergic pathway. Eur J Pharmacol. 2003;480:185–204. doi: 10.1016/j.ejphar.2003.08.106. [DOI] [PubMed] [Google Scholar]
- Merali Z, Johnston S, Zalcman S. Bombesin-induced behavioral changes: antagonism by neuroleptics. Peptides. 1983;4:693–697. doi: 10.1016/0196-9781(83)90020-7. [DOI] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Anisman H. Role of bombesin-related peptides in the control of food intake. Neuropeptides. 1999;33:376–386. doi: 10.1054/npep.1999.0054. [DOI] [PubMed] [Google Scholar]
- Merali Z, Kent P, Anisman H. Role of bombesin-related peptides in the mediation or integration of the stress response. Cell Mol Life Sci. 2002;59:272–287. doi: 10.1007/s00018-002-8422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Khan S, Michaud DS, Shippy SA, Anisman H. Does amygdaloid corticotropin-releasing hormone (CRH) mediate anxiety-like behaviors? Dissociation of anxiogenic effects and CRH release. Eur J Neurosci. 2004;20:229–239. doi: 10.1111/j.1460-9568.2004.03468.x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious state: focus on novel therapeutic strategies. Therapie. 2005;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- Molewijk HE, Hartog K, van der Poel AM, Mos J, Olivier B. Reduction of guinea pig pup isolation calls by anxiolytic and antidepressant drugs. Psychopharmacology. 1996;128:31–38. doi: 10.1007/s002130050106. [DOI] [PubMed] [Google Scholar]
- Moody TW, Merali Z. Bombesin-like peptides and associated receptors within the brain: distribution and behavioral implications. Peptides. 2004;25:511–520. doi: 10.1016/j.peptides.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Moody TW, O'Donohue TL, Jacobowitz DM. Biochemical localization and characterization of bombesin-like peptides in discrete regions of rat brain. Peptides. 1981;2:75–80. doi: 10.1016/s0196-9781(81)80014-9. [DOI] [PubMed] [Google Scholar]
- Ohki-Hamazaki H. Neuromedin B. Prog Neurobiol. 2000;62:297–312. doi: 10.1016/s0301-0082(00)00004-6. [DOI] [PubMed] [Google Scholar]
- Panula P, Yang HYT, Costa E. Neuronal location of the bombesin-like immunoreactivity in the central nervous system of the rat. Regul Pept. 1982;4:275–283. doi: 10.1016/0167-0115(82)90120-3. [DOI] [PubMed] [Google Scholar]
- Pinnock RD, Woodruff GN. Bombesin excites a subpopulation of 5-hydroxytryptamine-sensitive neurones in the rat dorsal raphe nucleus in vitro. J Physiol (Lond) 1991;440:55–65. doi: 10.1113/jphysiol.1991.sp018695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock RD, Reynolds T, Woodruff GN. Different types of bombesin receptors on neurons in the dorsal raphe nucleus and the rostral hypothalamus in rat brain slices in vitro. Brain Res. 1994;653:119–124. doi: 10.1016/0006-8993(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Kalivas PW, Nemeroff CB, Prange AJ., Jr Bombesin-induced locomotor hyperactivity: evaluation of the involvement of the mesolimbic dopamine system. Brain Res. 1984;304:377–382. doi: 10.1016/0006-8993(84)90343-3. [DOI] [PubMed] [Google Scholar]
- Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- Sibille E, Pavlides C, Benke D, Toth M. Genetic inactivation of the serotonin(1A) receptor in mice results in downregulation of major GABAA receptor alpha subunits, reduction of GABAA receptor binding, and benzodiazepine-resistant anxiety. J Neurosci. 2000;20:2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand FL. Evolution of neuropeptides. In: Stevens CF, editor. Neuropeptides: regulators of physiological processes. Cambridge, MA: MIT; 1999. pp. 3–17. [Google Scholar]
- Wada E, Way J, Lebacq-Verheyden AM, Battey JF. Neuromedin B and gastrin-releasing peptide mRNAs are differentially distributed in the rat nervous system. J Neurosci. 1990;10:2917–2930. doi: 10.1523/JNEUROSCI.10-09-02917.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Way J, Shapira H, Kusano K, Lebacq-Verheyden A-M, Coy D, Jensen R, Battey J. cDNA cloning, characterization and brain region-specific expression of neuromedin-B preferring bombesin receptor. Neuron. 1991;6:421–430. doi: 10.1016/0896-6273(91)90250-4. [DOI] [PubMed] [Google Scholar]
- Whitton P, Curzon G. Anxiogenic-like effect of infusing 1-(3-chlorophenyl) piperazine (mCPP) into the hippocampus. Psychopharmacology. 1990;100:138–140. doi: 10.1007/BF02245806. [DOI] [PubMed] [Google Scholar]
- Wolf SS, Moody TW. Receptors for GRP/bombesin-like peptides in the rat forebrain. Peptides. 1985;6:111–114. doi: 10.1016/0196-9781(85)90018-x. [DOI] [PubMed] [Google Scholar]
- Yamada K, Wada E, Yamano M, Sun YJ, Ohara-Imaizumi M, Nagamatsu S, Wada K. Decreased marble burying behavior in female mice lacking neuromedin-B receptor (NMB-R) implies the involvement of NMB/NMB-R in 5-HT neuron function. Brain Res. 2002;942:71–78. doi: 10.1016/s0006-8993(02)02696-3. [DOI] [PubMed] [Google Scholar]
- Yamano M, Ogura H, Okuyama S, Ohki-Hamazaki H. Modulation of 5-HT system in mice with a targeted disruption of neuromedin B receptor. J Neurosci Res. 2002;68:59–64. doi: 10.1002/jnr.10194. [DOI] [PubMed] [Google Scholar]