Abstract
The developmental processes leading to the differentiation of mechanosensory hair cells and statoacoustic ganglion neurons from the early otic epithelium remain unclear. Possible candidates include members of the Pax–Six–Eya–Dach (paired box–sine oculis homeobox–eyes absent–dachshund) gene regulatory network. We cloned zebrafish six1 and studied its function in inner ear development. Gain- and loss-of-function experiments show that six1 has opposing roles in hair cell and neuronal lineages. It promotes hair cell fate and, conversely, inhibits neuronal fate by differentially affecting cell proliferation and cell death in these lineages. By independently targeting hair cells with atoh1a (atonal homolog 1a) knockdown or neurons with neurog1 (neurogenin 1) knockdown, we showed that the remaining cell population, neurons or hair cells, respectively, is still affected by gain or loss of six1 function. six1 interacts with other members of the Pax–Six–Eya–Dach regulatory network, in particular dacha and dachb in the hair cell but not neuronal lineage. Unlike in mouse, six1 does not appear to be dependent on eya1, although it seems to be important for the regulation of eya1 and pax2b expression in the ventral otic epithelium. Furthermore, six1 expression appears to be regulated by pax2b and also by foxi1 (forkhead box I1) as expected for an early inducer of the otic placode. Our results are the first to demonstrate a dual role for a member of the Pax–Six–Eya–Dach regulatory network in inner ear development.
Keywords: six1, zebrafish, inner ear, hair cell, neuron, development
Introduction
The inner ear of vertebrates is a complex organ for hearing and maintaining balance consisting of a nonsensory epithelium housing the 6–9 sensory organs, containing the mechanosensory hair cells (Fritzsch et al., 2002). These are innervated by neurons of the VIIIth cranial or statoacoustic ganglion (SAG) (Rubel and Fritzsch, 2002). All inner ear cell types, including SAG neurons, derive from an ectodermal thickening adjacent to the hindbrain, the otic placode (Hudspeth, 1989; Haddon and Lewis, 1996; Fritzsch et al., 1998). The lineage relationship between these different cell types are just beginning to be understood (Fekete and Wu, 2002). Single-cell lineage studies have demonstrated that hair cells and neurons share a common progenitor in chickens (Satoh and Fekete, 2005), although it has yet to be demonstrated in zebrafish (Riley and Phillips, 2003).
Although otic placode induction has been the focus of extensive research (Noramly and Grainger, 2002), the mechanisms and underlying molecules controlling the formation of SAG neurons and hair cells remain unclear (Fekete, 1999; Fekete and Wu, 2002). Members of the Pax–Six–Eya–Dach (paired box–sine oculis homeobox–eyes absent–dachshund) gene regulatory network, involved in the development of numerous organs and tissues in both Drosophila (Bui et al., 2000) and vertebrates (Heanue et al., 1999; Wawersik and Maas, 2000; Li et al., 2003; Brodbeck and Englert, 2004) have been proposed to play an important role in inner ear development (Baker and Bronner-Fraser, 2001; Whitfield et al., 2002). The vertebrate Six genes, homologous to Drosophila sine oculis and optix (Seo et al., 1999; Kawakami et al., 2000), are of particular interest. Not only can they act on cell differentiation by activating cell type-specific genes (Kawakami et al., 2000), recent studies have shown that they directly interact with the cell cycle machinery (Ford et al., 1998; Coletta et al., 2004). Two Six genes, Six1 and Six4, are expressed in the developing mouse inner ear. Whereas Six4 null mutant mice do not have any ear phenotype and the adults hear normally (Ozaki et al., 2001), Six1 null mutants have severe inner ear defects (Li et al., 2003; Zheng et al., 2003; Ozaki et al., 2004). These defects include an increase in programmed cell death and a decrease in cell proliferation, resulting in a lack of development much beyond the otocyst stage.
We studied the function of the recently described zebrafish six1 (Bessarab et al., 2004) during inner ear development. In the inner ear, its expression is restricted to the ventral otocyst in which the first hair cells differentiate and prospective SAG neurons delaminate. Gain- and loss-of-function studies showed that six1 promotes formation of hair cells by increasing cell proliferation and independently inhibits neuronal development by inducing apoptosis. Furthermore, epistasis experiments and gene expression analyses are consistent with six1 acting before neurog1 (neurogenin 1) in the neuronal lineage. In the hair cell lineage, six1 likely acts via two dachshund genes. We also showed that six1 expression in the inner ear is controlled by pax2b, pax8, and foxi1 (forkhead box I1) and that six1 promotes pax2b and inhibits eya1 expression.
Materials and Methods
Cloning of zebrafish six1.
A 19 hours post-fertilization (hpf) embryonic cDNA library (gift from B. Appel, Vanderbilt University, Nashville, TN) was screened by PCR using a high-fidelity thermostable polymerase, Pwo polymerase (Roche Applied Science, Indianapolis, IN). We used a sequence directed against the 3′ end of the zebrafish expressed sequence tag similar to the human Six1 gene (GenBank accession number AI965224) as a target. The full-length open reading frame (ORF) (GenBank accession number AY254196) was amplified and subcloned into the expression vector pCS2+ (Turner and Weintraub, 1994).
Whole-mount in situ hybridization.
Whole-mount in situ hybridization was performed as described previously (Thisse et al., 1993). Experimental and control embryos were developed for the same amount of time in the final colorimetric reaction for every probe used. The neuroD (Korzh et al., 1998), dacha, dachb, dachc (Hammond et al., 2002), pax2a, pax2b (Pfeffer et al., 1998), atoh1a (atonal homolog 1) (Kim et al., 1997), and eya1 (Sahly et al., 1999) probes were described previously.
Morpholino-modified oligonucleotides and mRNA injections.
The method for injecting RNA and morpholinos (MO) into zebrafish embryos has been described previously (Westerfield, 1995; Nasevicius and Ekker, 2000). The morpholinos (Gene Tools, Philomath, OR) used were as follows (complementary bases to the predicted start codon are underlined): six1, 5′-ACCCGAAAGAAGGCAACATTGACAT-3′; dacha, 5′-CGGAGGAGTTGCAGATACGGCCATA-3′; dachb, 5′-GCCATAGTGGTCATTGAACTTAAAG-3′; dachc, 5′-CGACATCGGAGGCGCGTGGGCCATC-3′; pax2a, 5′-GGTCTGCTTTGCAGTGAATATCCAT-3′; pax2b, 5′-GGTCTGCCTTACAGTGAATATCCAT-3′; eya1, 5′-AGCTAGATCCTGCATTTCCATAGAC-3′; foxi1, 5′-TAATCCGCTCTCCCTCCAGAAACAT-3′; neurog1, 5′-TATACGATCTCCATTGTTGATAACC-3′; atoh1a, 5′-TCTCTTGTATCCGTGCTCATTCCAT-3′; pax8.1, 5′-GTTCACAAACATGCCTCCTAGTTGA-3′; pax8.2/3, 5′-GACCTCGCCCAGTGCTGTTGGACAT-3′; STD, Gene Tools standard control oligo.
As an additional control, the obtained phenotypes were confirmed by comparison with those already described in mutants when available. In the eya1 experiments, a splice-blocking MO (Kozlowski et al., 2005) gave the same results as the eya1 morpholino mentioned above.
Whole-mount immunofluorescence.
Whole-mount immunofluorescence was performed as described previously (Bricaud et al., 2001). After staining, the embryos were mounted and documented with a laser scanning confocal microscope (LSM 410; Zeiss, Oberkochen, Germany), by collecting and projecting confocal z-series. The antibody directed against HCS-1 (hair cell soma 1) (used at a concentration of 1:50; gift from J. T. Corwin, University of Virginia, Charlottesville, VA) has been shown to specifically recognize otoferlin (Cyr et al., 2006) and label hair cells in fish, amphibians, birds, and mammals (Finley et al., 1997; Gale et al., 2000). The antibody directed against HuC (used at a concentration of 1:1000; Invitrogen, Carlsbad, CA) has been shown previously to label the neurons of cranial ganglia (Marusich et al., 1994; Andermann et al., 2002). To confirm the phenotype of hair cells and neurons in our experiments, rhodamine–phalloidin and Islet-1 were also used as hair cell and neuronal markers, respectively (data not shown). As a cell proliferation marker, we used an antibody directed against human phospho-histone H3 (used at a concentration of 1:100; EMD Biosciences, San Diego, CA). Programmed cell death was studied with an antibody against the human activated caspase-3 (used at a concentration of 1:100; R & D Systems, Minneapolis, MN). The cell death and proliferation findings were also confirmed with terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (data not shown) and bromodeoxyuridine (BrdU) labeling, respectively.
BrdU Incorporation.
Two BrdU pulses of 30 min each separated by a 30 min recovery period at 28°C in egg water (Westerfield, 1995) were done starting at 28 hpf. For each pulse, dechorionated embryos were incubated in 10 mm BrdU (Invitrogen), made up in egg water with 15% DMSO in a Petri dish resting on top of ice. After the final pulse, the embryos were allowed to develop at 28°C in egg water for up to 3 days post-fertilization (dpf). After fixation in 4% paraformaldehyde at 4°C overnight, the embryos were subsequently dehydrated in methanol for 30 min at −20°C and permeabilized with a 10 μg/μl Proteinase K treatment. The embryos were then processed for cryosectioning as described below.
Detection of SAG neurons after BrdU incorporation.
To label SAG neurons, we used the mouse anti-HuC antibody described above. Because anti-HuC is an IgG2b and the mouse anti-BrdU is an IgG1 (at a concentration of 1:20; Invitrogen), we were able to perform the double immunostainings as described previously (Marusich et al., 1994). Briefly, we first performed the anti-HuC immunostaining as described above, using an anti-mouse IgG2b secondary antibody coupled to Alexa 568 (at a concentration of 1:200; Invitrogen). After the first immunostaining was completed, we incubated the sections in 2N HCl for 30 min to denature the DNA and make the BrdU detectable. BrdU immunostaining was then performed using the mouse anti-BrdU antibody described above and an anti-mouse IgG1 secondary antibody coupled to Alexa 488 (at a concentration of 1:200; Invitrogen). The sections were then mounted in Fluoromount-G (Southern Biotechnology, Birmingham, AL) and documented with our laser scanning confocal microscope as described above.
Detection of hair cells after BrdU incorporation.
To detect hair cells, we had to use the mouse monoclonal antibody Zn1 (at a concentration of 1:50; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) (Kornblum et al., 1990) because its epitope was less vulnerable than HCS-1 to the subsequent acid treatment necessary for BrdU detection. Because Zn1 and the anti-BrdU are both mouse IgG1 antibodies, we had to modify the double-immunostaining protocol as follows. Zn1 immunostaining was performed as described above using an anti-mouse IgG1 secondary antibody coupled to Alexa 488 (at a concentration of 1:200; Invitrogen). After completing the first immunostaining, sections were treated with 2N HCl for 30 min. BrdU immunostaining was then done using the mouse anti-BrdU antibody, previously described. To detect the anti-BrdU antibody without interfering with the previous immunostaining, we directly coupled the anti-BrdU antibody to Zenon 568 according to the recommendations of the manufacturer (Invitrogen). Labeled sections were then mounted in Fluoromount-G (Southern Biotechnology) and documented with our laser scanning confocal microscope.
Cryosections.
Cryosectioning was done as described previously (Link et al., 2000). Briefly, after overnight fixation in 4% paraformaldehyde at 4°C, the embryos were infiltrated with a graded series of sucrose, up to 30%, in PBS. They were then incubated in 30% sucrose in tissue freezing media (Triangle Biomedical Sciences, Durham, NC) for several hours until they sank to the bottom of the well. Embryos were then transferred into tissue freezing media overnight. After incubation, they were embedded in cryomolds, frozen on dry ice, and sectioned at 20 μm. Antibody staining was then performed as described above.
Administration of caspase inhibitor.
The method for administrating caspase-3 inhibitors has been described previously (Williams and Holder, 2000). Briefly, the caspase inhibitor zVADfmk [benzyloxycarbonyl Val-Ala-Asp (O-methyl) fluoromethyl ketone] (Sigma, St. Louis, MO) was applied to live embryos at a concentration of 300 μg/ml. To ensure both penetration and that the inhibition would be effective during the period hair cells and neurons are developing, embryos were incubated in the presence of the inhibitor from 15 hpf (before when hair cells and neurons form) to 3 dpf. In other experiments, the inhibitor was added at correspondingly later time points. The chemically similar cathepsin B inhibitor zFAfmk [benzyloxycarbonyl Phe-Ala (O-methyl) fluoromethyl ketone] (Sigma, St. Louis, MO) was used as a negative control for zVADfmk at a concentration of 300 μg/ml.
Cell counts.
To assess cell numbers accurately, multiple focal planes (confocal z-sections) encompassing all labeled cells around the inner ear were collected with a Zeiss LSM 410 laser scanning confocal microscope. By careful comparison with the previous and following sections, we made sure to only count each cell once. Sample sizes listed for the cell counts represent individual embryos because, for each embryo, only the cells on one side have been scored. Statistical analyses were done using Student’s t test and the Smith’s Statistical Package (Pomona College, Claremont, CA) with p < 0.05 considered as statistically significant.
Results
Cloning and expression of the zebrafish six1 gene
We isolated a new zebrafish member of the Six gene family related to vertebrate six1 by PCR screening a zebrafish 15–19 hpf library (Appel and Eisen, 1998). The longest clone we obtained is 1088 bp long and contains a full-length ORF of 284 amino acids. The sequence of the ORF contained in this clone is identical to one recently published (Bessarab et al., 2004).
We subsequently studied its expression with a focus on the developing inner ear by in situ hybridization. At 16.5 hpf, six1 mRNA is expressed in three distinctive domains at the periphery of the neural plate (Fig. 1A) that correspond to the sensory placodes (Sahly et al., 1999). It is initially expressed throughout the otic placode (Fig. 1B), but, by 19 hpf, six1 expression becomes restricted to the ventral half of the otic vesicle (Fig. 1C–F). This is the region from which the first sensory hair cells and neurons will arise between 24 and 48 hpf and 22–42 hpf, respectively (Haddon and Lewis, 1996). An anteroposterior expression gradient begins to emerge with stronger six1 expression anteriorly (Fig. 1F). Between 24 hpf (Fig. 1F) and 32 hpf (Fig. 1G,H), six1 expression becomes more restricted to the anterior ventral part of the otic epithelium (Fig. 1G, arrow). This expression appears to be stronger in the medial wall of the ventral otic epithelium (Fig. 1H, arrow). Six1 expression is also detectable in the prospective SAG neurons (Fig. 1G,I, stars) and in cells that appear to be migrating out of the otic epithelium (Fig. 1F–I, arrowheads), possibly corresponding to delaminating SAG neuroblasts. At 48 hpf, six1 is still expressed in the ventral epithelium of the otic vesicle and in the developing otic ganglion (Fig. 1I). After 48 hpf, six1 expression is no longer detectable in the inner ear but remains visible in lateral line neuromasts (Fig. 1J).
Figure 1.
Expression of six1 mRNA during zebrafish embryogenesis. A–J, Whole-mount in situ hybridization of six1 full-length cDNA probe in zebrafish embryos at 16.5 hpf (A, B), 19 hpf (C, D), 24 hpf (E, F), 32 hpf (G, H), 48 hpf (I), and 72 hpf (J). Position of otic placode or vesicle is noted by a white arrow in A, C, E, and J and surrounded by a dashed line in B, D, and F–I. Dotted lines in B, D, and F indicate the lumen of the otic vesicle. Arrows in F–I point to the six1-expressing cells in the ventral otic epithelium. Note that six1 expression is stronger in the anteriormost (G) and medialmost (H) parts of the ventral otic epithelium. White arrowhead in E points to the developing otic ganglion. Arrowheads in F–I point to cells delaminating from the ventral otic epithelium. Stars in G and I indicate the position of the otic ganglion. The arrowhead in C and the black arrow in E point to the anteroventral edge of the head, possibly the pituitary. The black arrowhead in E points to six1 expression in the posterior lateral line primordium. The arrowheads in J point to lateral line neuromasts. The inset in J represents a larger magnification of a neuromast (surrounded by a dashed line), with the centralmost cells (possibly the hair cells) expressing six1. All panels are lateral views with anterior to the left, except for A, which is an anterodorsal view, and H, which is a dorsal view with medial to the top. a, Anterior lateral line; o, olfactory placodes; p, posterior lateral line; t, trigeminal placode. Scale bars: B, D, F–I, 30 μm; A, E, 400 μm; C, 350 μm; J, 450 μm.
Although our main focus has been to study six1 expression in the developing inner ear, we confirmed that most of the other cranial placode-derived sensory systems expressed various levels of six1 mRNA, including the olfactory, trigeminal, and lateral line placodes (Fig. 1E). Weak expression in the anteroventral edge of the head (Fig. 1C,E) could represent six1 expression in the pituitary as described recently (Bessarab et al., 2004). However, we were unable to detect any six1 expression in the muscle and pectoral fin as described previously (Bessarab et al., 2004). This discrepancy could be attributable to differences in the probes used to detect six1 mRNA. We used the full-length cDNA, which includes a large fragment of 3′ untranslated region (UTR), whereas Besarrab and colleagues used a 598 bp fragment corresponding to the evolutionarily conserved six domain and homeodomain, generated by reverse transcription-PCR from embryonic total RNA. By using a smaller probe that only included the 3′ UTR, we observed an even more restricted pattern, mainly to just the inner ear (data not shown). This leads us to think that six1 could have several splice variants, as described for Six3 in the mouse retina (Kawakami et al., 1996) or a closely related paralog as reported for six4 (Kobayashi et al., 2000).
Six1 loss-of-function results in fewer hair cells and more SAG neurons
To study the role of six1 in inner ear development, we scored for changes in number of hair cells and neurons after six1-MO injection (Figs. 2, 3). The timing and number of hair cells forming in the first two macula, as well as the timing of neuron delamination from the otocyst, have been well studied in developing zebrafish and are relatively stereotyped (Haddon and Lewis, 1996). At 3 dpf, the anterior (or utricular) macula of a wild-type zebrafish larva contains ∼30 hair cells (Figs. 2, 3A). When six1-MO is injected, the number of hair cells is reduced by half to ∼14 (Figs. 2, 3F). Reduction in the number of hair cells is detectable as early as when the first hair cells start differentiating. At 28 hpf, in six1-MO-injected embryos, either one or no hair cell is detected (Fig. 3G) where normally two would be present (Fig. 3B).
Figure 2.
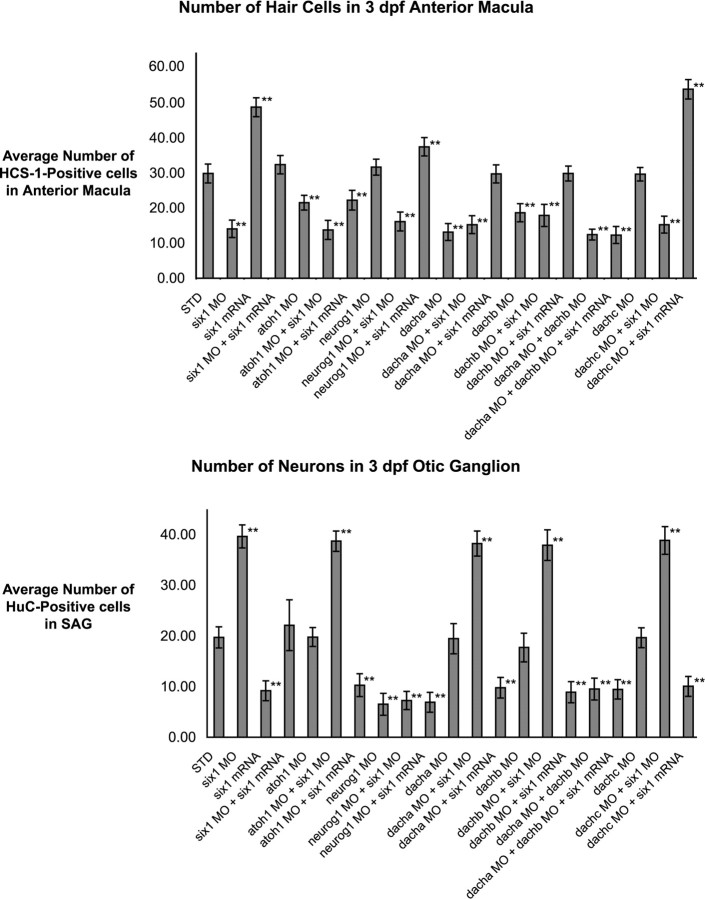
Average number of hair cells and neurons in 3 dpf anterior maculae or SAG. Hair cells and neurons have been detected by HCS-1 and HuC, respectively, using confocal microscopy. Values represent mean ± SD cell counts with a sample size of 20 anterior maculae for the hair cell counts and 15 SAG for the neuron counts. Statistical analysis was performed with Student’s t test; all comparisons were made with embryos injected with standard MO control (STD). **p < 0.005.
Figure 3.
Effects of six1 loss- and gain-of-function in the developing inner ear are complementary, differentially affecting hair cells and neurons. HCS-1 immunostaining of hair cells in 3 dpf embryos injected with standard MO control (A), six1-MO (F), and six1 mRNA (K) and 28 hpf embryos with standard MO control (B), six1-MO (G), and six1 mRNA (L) is shown. HuC immunostaining of neurons in 3 dpf embryonic otic ganglia injected with standard MO control (C), six1-MO (H), and six1 mRNA (M) is shown. The white boxes in C, H, and M outline otic ganglion neurons magnified in D, I, and N, respectively. Whole-mount in situ hybridization to neuroD in 32 hpf embryos injected with standard MO control (E), six1-MO (J), and six1 mRNA (O) is shown. Whole-mount in situ hybridization to neurog1 in 26 hpf embryos injected with standard MO control (P), six1-MO (Q), and six1 mRNA (R) is shown. All panels are lateral views with anterior to the right, except A, F, and K, which are dorsal views. Arrows point to hair cells in A, B, F, G, K, L and to SAG neurons in C, D, H, I, M, N. White dashed lines in B, C, E, G, H, J, L, M, O, P, Q, and R outline the otic vesicle. The area surrounded by a black line in E, J, and O is the same in all three panels and shows the difference in the size of neuroD expression. The changes in expression levels have been confirmed in at least 30 embryos for each marker and time point. ad, Anterodorsal lateral line ganglion; av, anteroventral lateral line ganglion; f, facial epibranchial ganglion; g, glossopharyngeal epibranchial ganglion; o, octaval/statoacoustic ganglion precursors; p, posterior lateral line placode/ganglion; t, trigeminal ganglion; v, vagal epibranchial placode/ganglion. Scale bar, 10 μm. t/ad/av/f, Trigeminal/anterodorsal lateral line/anteroventral lateral line/facial epibranchial ganglion complex
We also scored for number of neurons in the otic ganglion at two different time points. At 3 dpf in six1-MO-injected embryos, there are more HuC-positive cells (Fig. 3C,H). At this stage, the otic ganglion in a wild-type larva contains ∼20 neurons (Fig. 2), whereas, when six1 is knocked down, the number of SAG neurons doubles to ∼40 (Fig. 2). Furthermore, the expression domain of neuroD, an early marker of neuroblasts, at 32 hpf is expanded when six1 is knocked down (Fig. 3E,J).
To test for the specificity of our morpholino, we rescued the six1-MO phenotype by separately injecting six1 mRNA (Fig. 2), titrating the morpholino and demonstrating that the six1 mRNA itself was not toxic. As negative controls, we used a sense six1 morpholino and a morpholino with four mismatches. Both result in a wild-type phenotype (data not shown). A second, overlapping morpholino targeting the ATG region but centered on it also had the same effect as our experimental morpholino (data not shown).
Six1 gain-of-function results in more hair cells and fewer SAG neurons
Overexpression of six1 during inner development was achieved by injecting a synthetic six1 mRNA at early stages. Such gain-of-function experiments gave the opposite phenotype to that seen after six1 loss-of-function. At 3 dpf, more hair cells (Figs. 2, 3A,K) are present. This overproduction of hair cells is detectable as early as 28 hpf, with an average of four hair cells observed (Fig. 3L) instead of the two in wild-type embryos (Fig. 3B). We assayed for the presence of differentiated neurons at 3 dpf (Fig. 3C,D,M,N) and neural precursors at 32 hpf (Fig. 3E,O) with the neuronal markers HuC and neuroD, respectively. At 32 hpf in the six1 overexpressing embryos, fewer neuroD-positive cells are detectable in the otic ganglion (Fig. 3O) than in control embryos (Fig. 3E), suggesting that fewer neural progenitors are formed when six1 is overexpressed. At 3 dpf, the decrease in number of SAG neurons versus controls (Figs. 2, 3C,D) is even more dramatic. In extreme cases, SAG neurons are completely eliminated (Fig. 3M,N). These results indicate that, when six1 is overexpressed, not only are fewer neural progenitors formed but many of these progenitors do not go on to differentiate into neurons. In conclusion, these, together with the six1 loss-of-function results, suggest that six1 is necessary and sufficient for the normal formation of hair cells in the anterior macula, although it inhibits neuronal fate in the developing inner ear.
Six1 loss- and gain-of-function appears to only affect hair cells of the anterior macula and SAG neurons
To determine whether the role of six1 in hair cell development was restricted only to the anterior macula, we counted hair cells stained by HCS-1 in the posterior macula in either STD-MO-, six1-MO-, or six1 mRNA-injected embryos. At 36 hpf, we scored, respectively, 6.1 ± 1.29, 6.3 ± 1.49, or 6.0 ± 1.15 hair cells for each of the three conditions (10 embryos were counted for each condition). Statistical analysis with Student’s t test shows that the number of hair cells in the posterior macula under these three conditions were not significantly different (p = 0.7), suggesting that the role of six1 in sensory organ formation is restricted to the anterior macula.
Six1 is also expressed in most of the cranial ganglia. To determine whether neurogenesis in these ganglia could be inhibited or induced by six1 perturbation, we counted the number of HuC-positive cells in all of the major cranial ganglia (Fig. 4) at 3 dpf (20 embryos for each condition) in embryos injected with STD-MO, six1-MO, or six1 mRNA. No significant differences (p values ranged from 0.1 to 0.92) in the number of HuC-positive cells have been found in any of the cranial ganglia except for the otic ganglion (Fig. 4). Therefore, it seems that six1 is only involved in the formation of neurons in the SAG. Nonetheless, we cannot rule out that six1 plays a role in other aspects of cranial ganglia formation, such as neuronal identity. Also, because we could not always individually distinguish the overlapping trigeminal, facial, anterodorsal, or anteroventral lateral line ganglia, lumping all of their neurons into one category, it is possible that the number of neurons in one or more of these ganglia is being reduced or increased. However, this would have to be balanced by corresponding increases or reductions in other ganglia to keep the total number the same.
Figure 4.
Average number of neurons in 3 dpf cranial ganglia after six1 loss-of-function (six1 MO) or gain-of-function (six1 mRNA). Neurons labeled with HuC were scored using confocal microscopy. Values represent mean ± SD cell counts with a sample size of 20 embryos, except for the values for the otic ganglion, which have been taken from Figure 2. All comparisons were made with embryos injected with standard (STD) MO control. o, Otic ganglion; t/ad/av/f, trigeminal/anterodorsal lateral line/anteroventral lateral line/facial epibranchial ganglion complex; g, glossopharyngeal epibranchial ganglion; v, vagal epibranchial ganglion; m, middle lateral line ganglion; p, posterior lateral line ganglion. **p < 0.005.
Six1 affects hair cell and neuronal number through differential cell proliferation and death in these lineages
Although as a transcription factor six1 is known to play a role in cell type-specific differentiation (Spitz et al., 1998; Heanue et al., 1999), better characterized is its role in promoting proliferation and inhibiting programmed cell death (Ford et al., 1998; Zheng et al., 2003; Ozaki et al., 2004). We, therefore, determined the number of cells undergoing cell proliferation and death in the otocyst, at 24, 32, and 48 hpf of six1-MO-injected and six1-overexpressing embryos, using phospho-histone H3 and activated caspase-3, respectively, as markers (Fig. 5). Interestingly, under both conditions, we observed significant increases in both cell proliferation and death compared with control embryos. These extra cells seem to be mainly localized to the ventral otic epithelium at these time points. This is where six1 is normally expressed and is a hot spot for cell division (Lang et al., 2000) and cell death (Bever and Fekete, 1999) during inner ear development. However, some of the extra dividing and dying cells are observed in more dorsal positions and suggest that six1 could have a non-cell autonomous function, although we cannot rule out the possibility of cell mixing, as has been shown in the developing Xenopus inner ear (Kil and Collazo, 2001). Another caveat is, because we only count cells within the otocyst itself, we are missing cell proliferation and death occurring in delaminated neuroblasts and differentiating otic ganglion neurons, especially at 36 and 48 hpf.
Figure 5.
A–F, Analysis of cell proliferation (A–C) and cell death (D–F) in the otocyst of 32 hpf embryos injected with standard-MO control (A, D), six1-MO (B, E), and six1 mRNA (C, F). Lateral views of otocyst immunostained for the mitosis marker phospho-histone H3 (A–C) and the cell death marker activated caspase-3 (D–F). Otic vesicle is outlined by a dashed line. Scale bar, 30 μm. G, Quantification of cell proliferation and death in injected and non-injected otocysts at 24, 32, and 48 hpf. Values are mean ± SD cell counts of cells labeled with either phospho-histone H3 or activated caspase-3, sample size of 10 embryos for each experiment. Statistical analysis was with Student’s t test; all comparisons were made with embryos injected with standard MO control. For all three time points, the number of cells counted in both six1-MO- and six1 mRNA-injected embryos are significantly higher than controls (p < 0.05). Only labeled cells within the otocyst were counted.
To determine the identity of the supernumerary-dividing cells, we performed two BrdU pulses at 28 hpf. Normally, at this time, most of the cells in the otic epithelium are not dividing (Fig. 5). Consistent with this observation, our control embryos injected with STD-MO had very few BrdU-positive hair cells (Fig. 6A,J). Although more neurons were labeled, it was still far from all (Fig. 7A). When six1 is knocked down, most of the SAG neurons (86% in nine embryos scored) were BrdU positive (Fig. 7B), showing that they were derived from cells dividing at the time of the pulses, whereas the few hair cells were not (Fig. 6B,K). When six1 is overexpressed, most of the hair cells (84% in nine embryos scored) were BrdU positive (Fig. 6C,L), whereas the few SAG neurons formed were BrdU negative (Fig. 7C). We conclude that the extra cells observed undergoing proliferation when six1 function is perturbed mainly represent cells fated to form SAG neurons in the case of six1 loss-of-function and hair cells of the anterior macula or their progenitors in the case of six1 gain-of-function.
Figure 6.

Effects of six1 perturbation on hair cell proliferation. Zn1 (hair cell marker in green) and BrdU (in red) staining in the anterior macula of 3 dpf zebrafish injected with standard-MO control (A, D, G, J, M, P), six1-MO (B, E, H, K, N, Q), and six-1 mRNA (C, F, I, L, O, R). Arrows point to Zn1-positive cells that have incorporated BrdU. Arrowheads point to Zn1-positive cells that have not incorporated any BrdU. A–I, Transverse cryosections. J–R, Frontal cryosections through six different embryos. The orientation and the planes of section are shown on the schematic of a 3 dpf embryo in S. The dashed lines in A–I outline the lumen of the otic vesicle (o). am, Anterior macula; Ant., anterior; Dors., dorsal; Lat., lateral; Med., median; Post., posterior. Scale bar, 5 μm.
Figure 7.

Effects of six1 perturbation on neuronal proliferation. HuC (neural marker in red) and BrdU (in green) coimmunostaining in the SAG of 3 dpf zebrafish injected with standard-MO control (A, D, G), six1-MO (B, E, H), and six1 mRNA (C, F, I) on transverse cryosections. Both are nuclear markers. Arrows and arrowheads in A, B, D, E, G, and H point to BrdU-labeled SAG neurons and to SAG neurons that have not incorporated BrdU, respectively. Arrows and arrowheads in C, F, and I point to SAG neurons that have not incorporated BrdU and to a BrdU-positive cell that is not an SAG neuron, respectively. The orientation and plane of section are shown on the schematic of a 3 dpf embryo in J. The dashed lines outline the outer part of the otic vesicle (ov). Ant., Anterior; Dors., dorsal; Med., median; sag, statoacoustic ganglion. Scale bar, 10 μm.
Because we also observed an increase in the number of cells undergoing apoptosis in the otic epithelium when six1 is knocked down or overexpressed, we assayed whether inhibiting apoptosis could rescue the phenotype of six1 loss- or gain-of-function in sensory and neuronal lineages. We allowed the embryos to develop in the presence of a caspase-3 inhibitor, zVADfmk, and subsequently assayed for number of hair cells (Fig. 8). When six1 is knocked down in the presence of zVADfmk, the number of hair cells observed at 3 dpf (Fig. 8C) is comparable with the number of hair cells observed in wild-type larvae (Fig. 8A). A similar rescue of neurons is observed when six1 is overexpressed, and caspase-3-dependent apoptosis is inhibited (Fig. 8F). The increased numbers of hair cells or neurons observed when six1 is overexpressed or knocked down, respectively, are not affected by the presence of zVADfmk (Fig. 8G,H and data not shown). The phenotypes of either six1 loss-of-function (Fig. 8B) or gain-of-function (Fig. 8E) remain unaffected in controls treated with the chemically similar cathepsin B inhibitor zFAfmk (Williams and Holder, 2000) (25 embryos each). These results suggest that different cell populations undergo cell death after six1 loss-of-function versus six1 gain-of-function; the former population consists of cells fated to form hair cells, whereas the latter consists of cells fated to form SAG neurons.
Figure 8.
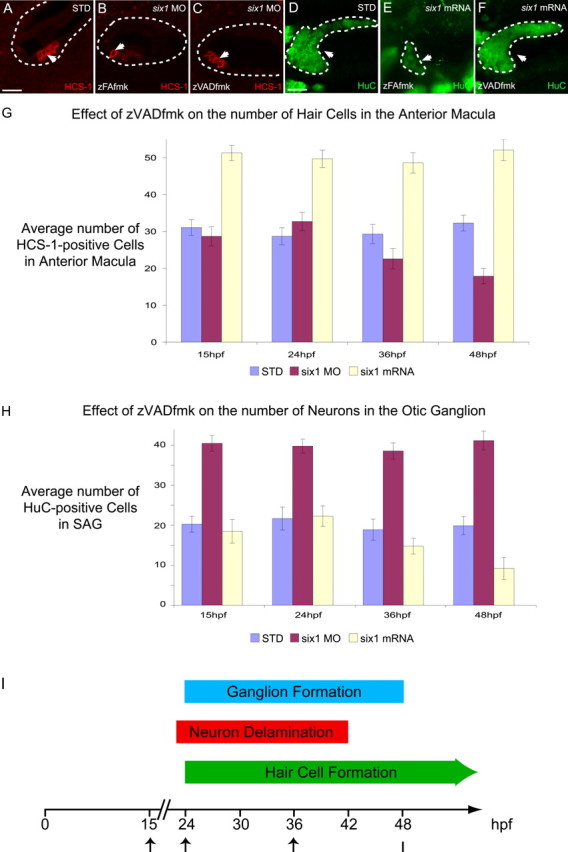
Caspase-3 inhibitor can rescue the phenotype of six1 loss- and gain-of-function. A–C, HCS-1 immunostaining of hair cells in 3 dpf embryos, untreated standard-MO control (A), treated with zFAfmk and six1-MO (B), and treated with zVADfmk and six1-MO (C). D–F, HuC immunostaining of SAG neurons in 3 dpf embryos, untreated standard-MO control (D), treated with zFAfmk and overexpressing six1 (E), treated with zVADfmk and overexpressing six1 (F). The zVADfmk compound is a caspase-3 inhibitor, whereas zFAfmk, a structurally similar cathepsin B inhibitor, was used as a negative control. The otocyst is outlined by a dashed line in A–C. Arrows are pointing to anterior macula hair cells in A–C (confocal optical section through macula) and to SAG neurons, which are also surrounded by a white dashed line in D–F (confocal projection). Scale bars: A–C, 40 μm; D–F, 30 μm. G, H, Mean number of HCS-1-positive hair cells in 3 dpf anterior macula (G) or HuC-positive neurons in 3 dpf SAG (H) of STD, six1 MO, and six1 mRNA-injected embryos treated with zVADfmk until 72 hpf beginning at 15, 24, 36, or 48 hpf with a sample size of 15 for each condition. I, Summary timeline of ganglion formation, neuron delamination, and hair cell formation in the zebrafish inner ear. The arrows mark the time points at which zVADfmk application was begun in our experiments.
Six1 acts early in both hair cell and neuronallineages
The developmental events (hair cell differentiation, neuroblast delamination, and ganglion formation) leading to the formation of the anterior macula and the SAG all happen concomitantly in the ventral otic epithelium (Fig. 8I). The lack of suitable markers for hair cell or SAG neuronal precursors means that assaying the identity of the dividing cells before they actually differentiate is currently not possible. Therefore, to begin determining when six1 perturbation may be inducing programmed cell death, we used the ability of the caspase-3 inhibitor zVADfmk to rescue six1 loss- and gain-of-function phenotypes. We assayed what was the latest time point we could begin applying zVADfmk and still see rescue when scored at 3 dpf (Fig. 8G,H). No rescue is observed in hair cell or neuronal lineages when zVADfmk is added at 48 hpf (Fig. 8G,H). In contrast, zVADfmk application beginning at 24 hpf produced results similar to application beginning at 15 hpf, almost complete rescue. When the caspase-3 inhibitor is added at an intermediate time point (36 hpf), the rescue is only partial. The timing for this rescue coincides with the initial wave of hair cell and neuronal differentiation between 24 and 48 hpf observed during inner ear development (Haddon and Lewis, 1996). These results suggest that the promotion by six1 of programmed cell death is required early in both sensory and neuronal lineages, possibly in their common precursor (Satoh and Fekete, 2005).
To begin to understand the relationship between six1 and early proneural genes, we studied the expression of neurog1 in the otic epithelium in embryos in which six1 has been either knocked down or overexpressed at 26 hpf, near the onset of neurogenesis (Fig. 3P–R). When six1 is knocked down, neurog1 expression is increased within the ventral wall of the otic epithelium (Fig. 3Q). Conversely, neurog1 expression is dramatically reduced when six1 is overexpressed (Fig. 3R). These results are consistent with six1 being necessary for neuroblast formation before the onset of neurog1 expression in the ventral otic epithelium.
To further determine whether six1 acts before or after neurogenesis, we studied the epistatic relationship between six1 and neurog1. The transcription factor neurog1 has been shown to be crucial for the formation of SAG neurons in the otic epithelium, before the neuroblasts delaminate (Andermann et al., 2002). When neurog1 is knocked down, fewer neurons are formed (Andermann et al., 2002), a phenotype that is the opposite of that observed after six1 knockdown. We therefore knocked down both six1 and neurog1 together and scored for number of SAG neurons (Fig. 2). The number of neurons (7.27 ± 1.8; n = 15) in the double knockdown is statistically the same as that observed after neurog1 knockdown (6.53 ± 2.2; n = 15; p = 0.3) but opposite to that seen after six1 loss-of-function (39.67 ± 2.0; n = 15; p < 0.005); therefore, neurog1 is likely to be epistatic to six1. This means that six1 is not downstream of neurog1 and is consistent with six1 acting upstream of neurog1, early in the neuronal lineage. Because neither of these loss-of-function phenotypes has been shown to be null, the epistatic relationship between six1 and neurog1 proposed here has to be taken as tentative. However, these results, together with the dependency of neurog1 expression on six1 function (Fig. 3P–R), lead us to think that six1 acts before neurog1, early in the neuronal lineage.
Six1 likely acts in both hair cell and neuronallineages
Because it seems that six1 function is required very early in otic development, we cannot rule out that six1 is acting on a common precursor cell whose asymmetrical division or survival results in the increase of one cell population at the expense of the other. Another possibility, which is not mutually exclusive, is that six1 is acting after hair cell and neuronal precursors separate but its effects are restricted to one lineage whose absence or promotion then results in increases or decreases, respectively, in the other cell lineage. If so, one prediction would be that six1 loss- or gain-of-function would not have any effect when the cell type in which six1 is supposed to be acting is missing. We therefore decided to study the effects of six1 loss- and gain-of-function on one lineage when the other is missing or decreased.
We impaired the formation of hair cells by knocking down atoh1a (Itoh and Chitnis, 2001; Whitfield et al., 2002), the zebrafish homolog of MATH-1 (mouse atonal homolog), which has been shown to be necessary for hair cell formation in mice (Bermingham et al., 1999). In the developing zebrafish inner ear, loss-of-function of atoh1a leads to fewer hair cells (21.6 ± 2.1; n = 20; p < 0.005), but the number of neurons remains unaffected (19.8 ± 1.9; n = 15; p = 0.9) (Fig. 2). The number of SAG neurons is still decreased by six1 overexpression, even when hair cell formation is inhibited by atoh1a loss-of-function (10.3 ± 2.2 neurons; n = 15; p < 0.005) (Fig. 2). Conversely, the number of SAG neurons is still increased by six1 loss-of-function when hair cell formation is prevented (38.73 ± 2.0 neurons; n = 15; p < 0.005) (Fig. 2). These results suggest that six1 function in the neuronal lineage is independent of the presence of hair cells.
Interestingly, in embryos in which atoh1a has been knocked down and six1 overexpressed, the number of hair cells in the anterior macula is reduced to a level comparable with that observed in the atoh1a knockdown alone (22.3 ± 2.8 hair cells; n = 20; p = 0.4) (Fig. 2). Conversely, the number of hair cells in the anterior macula of embryos in which both six1 and atoh1a have been knocked down (13.8 ± 2.7; n = 20) (Fig. 2) is reduced to a level statistically closer to that observed in six1 knockdown alone (14.1 ± 2.5; n = 20; p = 0.7) (Fig. 2) than to that observed in atoh1a knockdown alone (21.6 ± 2.1; n = 20; p < 0.005) (Fig. 2).
To decrease the neuronal population, we knocked down neurog1 as described above (Fig. 2), which results in fewer neurons. Even so, six1 gain-of-function still results in an increase in hair cell numbers even when neurog1 function is reduced (37.5 ± 2.6, n = 18 vs 29.9 ± 2.7, n = 20 in the STD-MO-injected embryos; p < 0.005) (Fig. 2). Conversely, the six1 loss-of-function phenotype of fewer hair cells is still seen, even in the absence of SAG neurons (16.2 ± 2.7; n = 20; p < 0.005) (Fig. 2). These results suggest that the role of six1 in the hair cell lineage is independent of the presence of SAG neurons.
Because atoh1a and neurog1 are likely acting later in hair cell and neuronal lineages, respectively, than six1, these results do not rule out the possibility that six1 could be acting only in one lineage after they separate but before atoh1a or neurog1 acts, with consequences on the other lineage. However, these data do suggest that, just as neurons and hair cells are differentiating, loss of one population does not necessarily affect the formation of the other.
Six1 likely acts via dacha and dachb genes in the sensory lineage
In the developing compound eye of the fly, dachshund expression is directly dependent on sine oculis (Halder et al., 1995; Chen et al., 1997; Shen and Mardon, 1997). Three dachshund genes have been shown to be expressed in the developing zebrafish inner ear (Hammond et al., 2002). In an effort to begin to understand which genes six1 is acting through, we assayed the expression of dacha, dachb, and dachc after six1 loss-of-function (Fig. 9A–F). When six1 is knocked down, the expression of both dacha and dachb is reduced in the ventral otic epithelium (Fig. 9A,D and B,E), whereas the expression of dachc remains unaffected (Fig. 9C,F). Moreover, in the dorsal otic epithelium, in which six1 is not expressed, dacha expression is still present after six1-MO injection (Fig. 9A,D, arrows).
Figure 9.
six1 acts via two of the three dach genes to promote the proliferation of hair cell progenitors but not SAG neurons. A–F, Whole-mount in situ hybridization to dacha (A, D), dachb (B, E), and dachc (C, F) in otocysts of STD (A–C) and six1-MO (D–F) injected embryos. All stainings have been performed at 28 hpf except B and E, which were performed at 32 hpf. Arrowheads point to ventral expression of dacha (A, D), dachb (B, E), and dachc (C, F). The changed expression was confirmed in at least 21 of 25 embryos scored. Arrows in A and D point to dorsal expression of dacha. G–M, HCS-1 immunostaining of hair cells in STD (G), six1-MO (H), dacha-MO (I), dachb-MO (J), six1-MO/dacha-MO (K), six1-MO/dachb-MO (L), and dachc-MO (M) injected 3 dpf embryos. N–Q, Whole-mount in situ hybridization to six1 in otocysts of STD control (N), dacha-MO (O), dachb-MO (P), and dachc-MO (Q) injected 28 hpf embryos. Arrowheads point to six1 expression in SAG; arrows point to six1 expression in the ventral otic epithelium. At least 30 embryos for each condition have been scored for six1 expression. All panels are lateral views with anterior to left and dorsal up, except for G–M, which are dorsal views with medial to the top. The otocyst is outlined by a dashed line in A–F and N–Q. Scale bars: A–F, N–Q, 15 μm; G–M, 5 μm.
Consistent with the expression data, only dacha or dachb, but not dachc, loss-of-function lead to a reduction in the number of hair cells in the anterior macula at 3 dpf, as observed with six1 loss-of-function (Fig. 9G–J,M). Dach loss-of-function has no effect on the number of SAG neurons (Fig. 2). We also scored hair cell numbers at two earlier time points (32 hpf and 2 dpf) after knocking down dacha or dachb (data not shown). Already by 32 hpf, we were able to observe fewer hair cells. Reduced numbers of hair cells are also observed at 2 dpf. Based on these results, it is likely that both dacha and dachb act early in the hair cell lineage, possibly in their progenitors, as proposed for six1.
Given that their expression is reduced after six1 knockdown and that their knockdown results in a similar reduction in hair cell number to that seen after six1 loss-of-function, it is likely that dacha and dachb act downstream of six1 in the hair cell lineage. That six1 expression is not affected by either dacha or dachb loss-of-function (Fig. 9N–Q) further supports this conclusion. Another possibility that is not mutually exclusive is that the two dach genes act together with six1. All three genes appear to act early in the developing hair cell lineage. In mouse, it has been shown that Six1 and Dach form a transcriptional complex important for organogenesis (Li et al., 2003).
To test for synergistic interactions between dacha/dachb and six1 in the hair cell lineage, we scored for numbers of hair cells in the anterior macula at 3 dpf after dacha and/or dachb knockdown(s) in either a six1 loss- or gain-of-function background (Figs. 2, 9K,L). SAG neuron counts and dachc knockdowns in similar backgrounds served as negative controls (Fig. 2). When dacha, dachb, or both were knocked down in conjunction with six1 loss-of-function, the loss of hair cells seen was indistinguishable from that observed in six1, dacha, or dachb morphants alone (Figs. 2, 9K,L). When either dacha or dachb is knocked down in a six1 gain-of-function background, the number of hair cells in the anterior macula is similar to that observed in wild-type embryos (Fig. 2). However, when both dacha and dachb are knocked down and six1 overexpressed, the number of hair cells seen at 3 dpf in the anterior macula is similar to that observed in individual six1, dacha, or dachb morphants (Fig. 2). Although the dacha and dachb knockdowns in an embryo overexpressing six1 are consistent with these two dach genes acting downstream of six1 in the hair cell lineage, these experiments do not rule out the reverse relationship. The difference in phenotypes seen after knocking down one versus both dach genes suggests that there are interactions between dacha and dachb that remain to be studied. We propose that dacha and dachb both act downstream of or possibly with six1 to promote hair cell fate in the anterior macula (summarized in supplemental Fig. S1, available at www.jneurosci.org as supplemental material).
The formation of hair cells and neurons in the inner ear requires a complex network of transcriptional interactions
We studied the interactions between six1 and other members of the Pax–Six–Eya–Dach gene regulatory network, specifically pax2a, pax2b, and eya1 (Fig. 10). The loss-of-function of six1 does not affect pax2a expression at early stages (24 hpf; data not shown), but later (32 hpf), when pax2a is expressed in the developing hair cells, its expression in the developing otocyst is reduced, notably in the utricular hair cells (Fig. 10A,B). At 28 hpf, six1 loss-of-function significantly reduces pax2b expression (Fig. 10C,D) and expands the expression domain of eya1 (Fig. 10E,F). These results suggest that six1 activates pax2b expression but inhibits eya1 expression and are consistent with the functional data on these two genes: pax2b loss-of-function leads to a loss of hair cells (Whitfield et al., 2002), whereas the eya1 mutation leads to a loss of neurons in the otic ganglion (Kozlowski et al., 2005). Less consistent is the loss of hair cells observed in the eya1 mutant (Kozlowski et al., 2005). Although the loss of hair cells is more dramatic in the cristae that develop later, half of the hair cells in the anterior macula are lost in the eya1 mutant [dog (dog-eared)]. Because of the large number of apoptotic cells observed within the otic vesicle of dog mutants, it has been proposed that eya1 could act as a suppressor of apoptosis (Kozlowski et al., 2005). Therefore, both eya1 and six1 could be required to prevent apoptosis in the hair cell lineage, whereas they could have opposite actions in the neuronal lineage.
Figure 10.
Effect of six1 loss-of-function on the expression of pax2a, pax2b, eya1, and atoh1a in the developing inner ear. A–H, Whole-mount in situ hybridization to pax2a (A, B), pax2b (C, D), eya1 (E, F), and atoh1a (G, H) in otocysts of embryos injected with standard-MO control (A, C, E, G) and six1-MO (B, D, F, H). All stainings have been performed on 28 hpf embryos except for A and B, which are 36 hpf embryos, and G and H, which are 32 hpf embryos. Otic vesicle is outlined by a dashed line. Arrows point to pax2a (A, B) and pax2b (C, D) expression sites in the ventral epithelium. The arrowheads in A and B point to pax2a expression in the dorsal epithelium. The arrow in E points to same part of otocyst as in F, indicating the posterior limit of eya1 expression in a wild-type otic vesicle. The arrows in G and H indicate the normal site of atoh1a expression in the developing anterior macula. The changed expression was confirmed in at least 28 of 32 embryos scored. All panels are lateral views with anterior to the left. Scale bar, 10 μm.
Because MATH-1 has been shown to be necessary for hair cell formation in mouse (Bermingham et al., 1999), we assayed whether six1 could regulate the expression of its homolog, atoh1a, in the zebrafish inner ear (Fig. 10G,H). When six1 is knocked down, atoh1a expression is dramatically reduced in the anterior ventral otic epithelium, in which the utricular macula hair cells are going to arise (Fig. 10H).
The Pax–Six–Eya–Dach network has been shown to rely on a complex series of feedback regulatory loops (Pignoni et al., 1997). Therefore, we studied whether any of the genes belonging to this pathway had an effect on the expression of six1 (Fig. 11). It appears that neither pax2a nor eya1 loss-of-function has an effect on the normal expression of six1 (Fig. 11B,D). These results have been confirmed by studying six1 expression pattern in noi (no isthmus) and dog (kindly provided by D. Kozlowski, Medical School of Georgia, Augusta, GA) mutants (Brand et al., 1996; Lun and Brand, 1998). Conversely, pax2b loss-of-function reduces the level of six1 expression (Fig. 11C), demonstrating that pax2b can regulate, directly or indirectly, six1 transcription. Pax8, a third member of the Pax gene family, has been shown to be important for inner ear development in zebrafish (Hans et al., 2004; Mackereth et al., 2005). Three different pax8 variants are thought to be expressed in zebrafish because of alternative splicing and two transcription initiation sites (Mackereth et al., 2005). We knocked down all three variants by injecting a mixture of two MOs, each targeting one of the two transcription initiation sites as performed previously (Mackereth et al., 2005). This results in a dramatic effect on inner ear morphology (Hans et al., 2004; Mackereth et al., 2005). Although six1 expression is still detectable in the ventral otic epithelium, it is greatly reduced (Fig. 11E), although not to the level seen after pax2b knockdown. The forkhead family member, foxi1 is an important player not only in the induction of the otic placode (Solomon et al., 2003) but also in the proper activation of differentiation pathways in the inner ear (Hans et al., 2004). We therefore studied whether six1 was dependent on foxi1 function in the zebrafish inner ear. When foxi1 is knocked down, the ear anlagen is either entirely missing or greatly reduced (Solomon et al., 2003) and no expression of six1 is detectable (Fig. 11F). Because, at 28 hpf, the lack of six1 expression could be secondary to the overall absence of the otic placode attributable to foxi1 loss-of-function, we studied six1 expression at either 28 hpf in embryos with a less severe phenotype (data not shown) or at 16.5 hpf when the placode just arises (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). In both cases, no expression of six1 is detectable (data not shown). In conclusion, six1 may be activated by foxi1 and subsequently may activate pax2b, dacha, and dachb, leading to the formation of hair cells; in contrast, six1 may inhibit neuronal fate in part by repressing the expression of eya1 in this lineage. The genetic relationships between all of these genes and six1 as well as their proposed roles in the formation of hair cells and neurons are summarized in supplemental Figure S1 (available at www.jneurosci.org as supplemental material).
Figure 11.
Effect of pax2a, pax2b, eya1, pax8, and foxi1 loss-of-function on the expression of six1. A–F, Whole-mount in situ hybridization of six1 in otocysts of 28 hpf embryos injected with STD (A), pax2a-MO (B), pax2b-MO (C), eya1-MO (D), pax8-MO (E), and foxi1-MO (F). Arrows point to six1 expression in the ventral epithelium of the otic vesicle. The star in F indicates the position where the ear should form. The changed expression was confirmed in at least 25 of 30 embryos scored for each condition. All panels are lateral views with anterior to the left. Otocysts are outlined by a dashed line. Scale bar, 10 μm.
Discussion
Six1 may have a more specific role in zebrafish than in mouse to balance hair cell and neuron numbers
Our results demonstrate that six1 plays a pivotal role in controlling the balance between utricular hair cells and SAG neurons during zebrafish inner ear development. Its mode of action involves controlling both cell proliferation and programmed cell death in the sensory and neuronal lineages. In zebrafish, genes belonging to the Pax–Six–Eya–Dach network have either the same or similar roles in both lineages, such as eya1 (Kozlowski et al., 2005), or a unique role in only one lineage, such as dacha and dachb (this study). Our study is the first report of a gene within the developing inner ear that acts in opposite ways in these two lineages.
Although the expression of Six1 in the ventral otic epithelium appears to be highly conserved across vertebrates (Pandur and Moody, 2000; Xu et al., 2003; Bessarab et al., 2004), there are some striking differences between the phenotype of the Six1 knockouts in mouse (Li et al., 2003; Zheng et al., 2003; Ozaki et al., 2004) and its loss-of-function in zebrafish. Six1-null mutant mice inner ears never develop much beyond the otocyst stage (Li et al., 2003; Zheng et al., 2003; Ozaki et al., 2004). The developmental arrest is thought to be caused by increased apoptosis and decreased cell proliferation. Sensory organs and the SAG fail to form or, if they do, undergo cell death. Although none of these studies scored for presence or absence of differentiated hair cells, two reported a dramatic reduction in the expression of prospective sensory organ markers such as Bmp4 (bone morphogenic protein 4) and Lunatic fringe (Zheng et al., 2003; Ozaki et al., 2004), consistent with a loss of hair cell precursors.
We show here that, in zebrafish, six1 loss-of-function has a less severe but more specific phenotype. The number of hair cells is reduced but the number of SAG neurons is increased. Although the less severe phenotype could be attributable to the MO knockdown not being a complete null, this does not account for the opposite phenotype seen in regards to SAG neurons. Such differences between phenotypes in zebrafish and mouse have been documented for other genes, such as eya1 (Kozlowski et al., 2005). One explanation for this less severe phenotype is a duplication of either the whole genome or gene families in the evolutionary lineage leading to zebrafish (Postlethwait et al., 2000; Robinson-Rechavi et al., 2001) that would have resulted in yet another unknown six1 gene.
Interactions between Six1 and other members of the Pax–Six–Eya–Dach gene network, such as Eya1, also seem to differ between mouse and zebrafish. Zebrafish six1 inhibits eya1 expression, although its own expression is independent of the function of eya1. In mouse, Eya1 positively regulates Six1 expression (Xu et al., 1999), although its own expression is Six1 independent (Li et al., 2003; Zheng et al., 2003). Not only may interactions between six1 and eya1 differ in zebrafish relative to mouse but so might the interactions between six1 and the pax2 genes. In mouse, Pax2 expression is dependent on Six1 in the kidneys but not the developing ear (Xu et al., 2003; Zheng et al., 2003), whereas in zebrafish, pax2b is directly or indirectly under the control of six1 in the inner ear. Six1 expression in the developing mouse inner ear is not dependent on Pax2 (Xu et al., 2003; Zheng et al., 2003), whereas in zebrafish, it appears that six1 expression is dependent on pax2b but not pax2a.
In the inner ear, relatively few genes have been found that affect the development of one sensory organ but not another. Although six1 is expressed throughout most of the ventral otic epithelium at early stages of otic development, our data suggest that its function is only required for the formation of the hair cells of the anterior but not posterior macula. Hair cells of the anterior macula may require different genes for their development from those of other sensory organs because they arise closer to the main neurogenic region of the otocyst (Haddon and Lewis, 1996; Whitfield et al., 2002) and are the only hair cells that appear to share a common progenitor with SAG neurons (Satoh and Fekete, 2005). In contrast to six1 perturbations, vgo (van gogh) mutants, defective for the tbx1 (T-box 1) gene, are missing all sensory organs but the anterior macula (Piotrowski et al., 2003).
six1 function seems restricted to the otic ganglia even though it is expressed in other ganglia. However, we cannot rule out more subtle effects of six1 in other cranial ganglia, such as controlling the type of receptors or neurotransmitters expressed by these neurons. The neural crest contribution to other placodes (Collazo et al., 1994; Baker and Bronner-Fraser, 2001) could also make six1 function less obvious than in the SAG. For instance, it has been shown that, in the nodose ganglion, loss of neurons arising from the placode could be rescued by neural crest-derived neurons and vice versa (Kirby, 1988a,b; Harrison et al., 1995).
How can six1 act differently in two celllineages?
Six genes have been shown to be involved in three different processes during development: (1) cell differentiation (Kawakami et al., 2000); (2) cell proliferation (Ford et al., 1998; Coletta et al., 2004); and (3) cell death (Li et al., 2003; Xu et al., 2003; Zheng et al., 2003). These three roles are not mutually exclusive, as has been demonstrated for Six3 in eye development (Del Bene et al., 2004).
The mechanism by which six1, through or with dacha and dachb, promotes proliferation of hair cell precursors could be similar to the one in mammals. During mammalian organogenesis, a Six1–Dach complex can regulate both cell proliferation and programmed cell death. Its phosphorylated form acts as a transcriptional repressor, but, after dephosphorylation by Eya1, this complex positively regulates genes controlling cell proliferation and cell survival, such as c-Myc and Gdnf, respectively (Li et al., 2003). However, six1 could be acting in other ways. Six proteins can positively regulate certain cell cycle genes, such as CyclinA1 (Coletta et al., 2004). Furthermore, Six genes can also promote proliferation without acting as transcription factors. In eye development, it has been shown that Six3 can prevent the formation of a Geminin–Cdt1 (cdc10-dependent transcript 1) complex (by binding to Geminin), leaving free Cdt1 to play its role in the pre-replication complex, allowing proliferation (Del Bene et al., 2004).
Six proteins can either activate or repress the same genes depending on the presence of certain cofactors. This would suggest that cofactors are differentially localized in the developing inner ear to restrict six1 action to the anterior macula and SAG neurons, even when six1 is ectopically overexpressed as in our gain-of-function experiments. Gro (Groucho), a transcriptional repressor, can bind Six genes through their two eh1 (engrailed homolog) domains (Kobayashi et al., 2001; Zhu et al., 2002; Lopez-Rios et al., 2003; Silver et al., 2003). The model proposed in fly (Silver et al., 2003) supposes that, in the presence of sufficient levels of EYA, known to bind SINE OCULIS (SO), EYA displaces GRO. This newly formed complex acts as a transcriptional activator, whereby in the absence of high levels of EYA, GRO remains bound to SO, leading to repression of target genes. Thus, SO may function both as a transcriptional activator and repressor depending on the context-specific expression levels of particular cofactors. six1 could act in a similar manner during zebrafish inner ear development. In the neuronal lineage, Six1 could interact with Gro1, a Gro homolog expressed in the developing ear (Wulbeck and Campos-Ortega, 1997), repressing the expression of its target genes, whereas, in the sensory lineage, Six1–Gro1 interactions are somehow prevented by another cofactor, leading to the activation of six1 targets.
Our data are consistent with six1 acting as a transcriptional activator in the hair cell lineage and a repressor in the neuronal lineage. However, other lineage-specific differences could explain our results as well. The Six1 protein may be differentially expressed in these two cell types or dephosphorylated by Eya1 in one cell type but not the other. Finally, different combinations of cofactors in these two lineages could lead to the different effects of six1. It has been shown in Xenopus ectoderm that six1 can promote the formation of the placodal field by repressing the transcription of ectodermal and neural crest genes and concomitantly by activating the transcription of placode-specific genes (Brugmann et al., 2004). Our results suggest that six1, by playing different roles, regulates the formation of sensory versus neuronal cell types, balancing their numbers in the developing inner ear.
Footnotes
This work was supported by a grant (RO1) from the National Institutes of Health and National Institute for Deafness and Other Communication Disorders. This work was also supported by the Oberkotter Foundation. We thank the members of Andy K. Groves, Neil Segil, and Andres Collazo laboratories for their stimulating discussions of the data presented here.
References
- Andermann P, Ungos J, Raible DW. Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev Biol. 2002;251:45–58. doi: 10.1006/dbio.2002.0820. [DOI] [PubMed] [Google Scholar]
- Appel B, Eisen JS. Regulation of neuronal specification in the zebrafish spinal cord by Delta function. Development. 1998;125:371–380. doi: 10.1242/dev.125.3.371. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes. I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bessarab DA, Chong SW, Korzh V. Expression of zebrafish six1 during sensory organ development and myogenesis. Dev Dyn. 2004;230:781–786. doi: 10.1002/dvdy.20093. [DOI] [PubMed] [Google Scholar]
- Bever MM, Fekete DM. Ventromedial focus of cell death is absent during development of Xenopus and zebrafish inner ears. J Neurocytol. 1999;28:781–793. doi: 10.1023/a:1007005702187. [DOI] [PubMed] [Google Scholar]
- Brand M, Heisenberg CP, Jiang YJ, Beuchle D, Lun K, Furutani-Seiki M, Granato M, Haffter P, Hammerschmidt M, Kane DA, Kelsh RN, Mullins MC, Odenthal J, van Eeden FJ, Nusslein-Volhard C. Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development. 1996;123:179–190. doi: 10.1242/dev.123.1.179. [DOI] [PubMed] [Google Scholar]
- Bricaud O, Chaar V, Dambly-Chaudiere C, Ghysen A. Early efferent innervation of the zebrafish lateral line. J Comp Neurol. 2001;434:253–261. doi: 10.1002/cne.1175. [DOI] [PubMed] [Google Scholar]
- Brodbeck S, Englert C. Genetic determination of nephrogenesis: the Pax/Eya/Six gene network. Pediatr Nephrol. 2004;19:249–255. doi: 10.1007/s00467-003-1374-z. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Pandur PD, Kenyon KL, Pignoni F, Moody SA. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development. 2004;131:5871–5881. doi: 10.1242/dev.01516. [DOI] [PubMed] [Google Scholar]
- Bui QT, Zimmerman JE, Liu H, Bonini NM. Molecular analysis of Drosophila eyes absent mutants reveals features of the conserved Eya domain. Genetics. 2000;155:709–720. doi: 10.1093/genetics/155.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Amoui M, Zhang Z, Mardon G. Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila. Cell. 1997;91:893–903. doi: 10.1016/s0092-8674(00)80481-x. [DOI] [PubMed] [Google Scholar]
- Coletta RD, Christensen K, Reichenberger KJ, Lamb J, Micomonaco D, Huang L, Wolf DM, Muller-Tidow C, Golub TR, Kawakami K, Ford HL. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci USA. 2004;101:6478–6483. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo A, Fraser SE, Mabee PM. A dual embryonic origin for vertebrate mechanoreceptors. Science. 1994;264:426–430. doi: 10.1126/science.8153631. [DOI] [PubMed] [Google Scholar]
- Cyr JL, Warchol ME, Richardson GP, Corwin JT. Identification of the hair cell soma antigen HCS-1 as otoferlin. Assoc Res Otolaryngol Abstr. 2006;29:97. doi: 10.1007/s10162-010-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene F, Tessmar-Raible K, Wittbrodt J. Direct interaction of geminin and Six3 in eye development. Nature. 2004;427:745–749. doi: 10.1038/nature02292. [DOI] [PubMed] [Google Scholar]
- Fekete DM. Development of the vertebrate ear: insights from knockouts and mutants. Trends Neurosci. 1999;22:263–269. doi: 10.1016/s0166-2236(98)01366-6. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Finley JR, Xia B, Corwin JT. Monoclonal antibodies raised as markers for supporting cells and hair cells. Assoc Res Otolaryngol Abstr. 1997;20:134. [Google Scholar]
- Ford HL, Kabingu EN, Bump EA, Mutter GL, Pardee AB. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc Natl Acad Sci USA. 1998;95:12608–12613. doi: 10.1073/pnas.95.21.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Barald KF, Lomax MI. Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editors. Development of the auditory system. New York: Springer; 1998. pp. 80–145. [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Meyers JR, Corwin JT. Solitary hair cells are distributed throughout the extramacular epithelium in the bullfrog’s saccule. J Assoc Res Otolaryngol. 2000;1:172–182. doi: 10.1007/s101620010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365:113–128. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Hammond KL, Hill RE, Whitfield TT, Currie PD. Isolation of three zebrafish dachshund homologues and their expression in sensory organs, the central nervous system and pectoral fin buds. Mech Dev. 2002;112:183–189. doi: 10.1016/s0925-4773(01)00637-2. [DOI] [PubMed] [Google Scholar]
- Hans S, Liu D, Westerfield M. Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development. 2004;131:5091–5102. doi: 10.1242/dev.01346. [DOI] [PubMed] [Google Scholar]
- Harrison TA, Stadt HA, Kumiski D, Kirby ML. Compensatory responses and development of the nodose ganglion following ablation of placodal precursors in the embryonic chick (Gallus domesticus) Cell Tissue Res. 1995;281:379–385. doi: 10.1007/BF00583407. [DOI] [PubMed] [Google Scholar]
- Heanue TA, Reshef R, Davis RJ, Mardon G, Oliver G, Tomarev S, Lassar AB, Tabin CJ. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev. 1999;13:3231–3243. doi: 10.1101/gad.13.24.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. How the ear’s works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Itoh M, Chitnis AB. Expression of proneural and neurogenic genes in the zebrafish lateral line primordium correlates with selection of hair cell fate in neuromasts. Mech Dev. 2001;102:263–266. doi: 10.1016/s0925-4773(01)00308-2. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Ohto H, Takizawa T, Saito T. Identification and expression of six family genes in mouse retina. FEBS Lett. 1996;393:259–263. doi: 10.1016/0014-5793(96)00899-x. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes—structure and function as transcription factors and their roles in development. BioEssays. 2000;22:616–626. doi: 10.1002/1521-1878(200007)22:7<616::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Kil SH, Collazo A. Origins of inner ear sensory organs revealed by fate map and time-lapse analyses. Dev Biol. 2001;233:365–379. doi: 10.1006/dbio.2001.0211. [DOI] [PubMed] [Google Scholar]
- Kim CH, Bae YK, Yamanaka Y, Yamashita S, Shimizu T, Fujii R, Park HC, Yeo SY, Huh TL, Hibi M, Hirano T. Overexpression of neurogenin induces ectopic expression of HuC in zebrafish. Neurosci Lett. 1997;239:113–116. doi: 10.1016/s0304-3940(97)00908-7. [DOI] [PubMed] [Google Scholar]
- Kirby ML. Nodose placode provides ectomesenchyme to the developing chick heart in the absence of cardiac neural crest. Cell Tissue Res. 1988a;252:17–22. doi: 10.1007/BF00213821. [DOI] [PubMed] [Google Scholar]
- Kirby ML. Nodose placode contributes autonomic neurons to the heart in the absence of cardiac neural crest. J Neurosci. 1988b;8:1089–1095. doi: 10.1523/JNEUROSCI.08-04-01089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Osanai H, Kawakami K, Yamamoto M. Expression of three zebrafish Six4 genes in the cranial sensory placodes and the developing somites. Mech Dev. 2000;98:151–155. doi: 10.1016/s0925-4773(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Nishikawa K, Suzuki T, Yamamoto M. The homeobox protein Six3 interacts with the Groucho corepressor and acts as a transcriptional repressor in eye and forebrain formation. Dev Biol. 2001;232:315–326. doi: 10.1006/dbio.2001.0185. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Corwin JT, Trevarrow B. Selective labeling of sensory hair cells and neurons in auditory, vestibular, and lateral line systems by a monoclonal antibody. J Comp Neurol. 1990;301:162–170. doi: 10.1002/cne.903010203. [DOI] [PubMed] [Google Scholar]
- Korzh V, Sleptsova I, Liao J, He J, Gong Z. Expression of zebrafish bHLH genes ngn1 and nrd defines distinct stages of neural differentiation. Dev Dyn. 1998;213:92–104. doi: 10.1002/(SICI)1097-0177(199809)213:1<92::AID-AJA9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kozlowski DJ, Whitfield TT, Hukriede NA, Lam WK, Weinberg ES. The zebrafish dog-eared mutation disrupts eya1, a gene required for cell survival and differentiation in the inner ear and lateral line. Dev Biol. 2005;277:27–41. doi: 10.1016/j.ydbio.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Lang H, Bever MM, Fekete DM. Cell proliferation and cell death in the developing chick inner ear: spatial and temporal patterns. J Comp Neurol. 2000;417:205–220. doi: 10.1002/(sici)1096-9861(20000207)417:2<205::aid-cne6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Link BA, Fadool JM, Malicki J, Dowling JE. The zebrafish young mutation acts non-cell-autonomously to uncouple differentiation from specification for all retinal cells. Development. 2000;127:2177–2188. doi: 10.1242/dev.127.10.2177. [DOI] [PubMed] [Google Scholar]
- Lopez-Rios J, Tessmar K, Loosli F, Wittbrodt J, Bovolenta P. Six3 and Six6 activity is modulated by members of the groucho family. Development. 2003;130:185–195. doi: 10.1242/dev.00185. [DOI] [PubMed] [Google Scholar]
- Lun K, Brand M. A series of no isthmus (noi) alleles of the zebrafish pax2.1 gene reveals multiple signaling events in development of the midbrain-hindbrain boundary. Development. 1998;125:3049–3062. doi: 10.1242/dev.125.16.3049. [DOI] [PubMed] [Google Scholar]
- Mackereth MD, Kwak SJ, Fritz A, Riley BB. Zebrafish pax8 is required for otic placode induction and plays a redundant role with Pax2 genes in the maintenance of the otic placode. Development. 2005;132:371–382. doi: 10.1242/dev.01587. [DOI] [PubMed] [Google Scholar]
- Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25:143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene “knockdown” in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Noramly S, Grainger RM. Determination of the embryonic inner ear. J Neurobiol. 2002;53:100–128. doi: 10.1002/neu.10131. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Watanabe Y, Takahashi K, Kitamura K, Tanaka A, Urase K, Momoi T, Sudo K, Sakagami J, Asano M, Iwakura Y, Kawakami K. Six4, a putative myogenin gene regulator, is not essential for mouse embryonal development. Mol Cell Biol. 2001;21:3343–3350. doi: 10.1128/MCB.21.10.3343-3350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Nakamura K, Funahashi JI, Ikeda K, Yamada G, Tokano H, Okamura HO, Kitamura K, Muto S, Kotaki H, Sudo K, Horai R, Iwakura Y, Kawakami K. Six1 controls patterning of the mouse otic vesicle. Development. 2004;131:551–562. doi: 10.1242/dev.00943. [DOI] [PubMed] [Google Scholar]
- Pandur PD, Moody SA. Xenopus Six1 gene is expressed in neurogenic cranial placodes and maintained in the differentiating lateral lines. Mech Dev. 2000;96:253–257. doi: 10.1016/s0925-4773(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Pfeffer PL, Gerster T, Lun K, Brand M, Busslinger M. Characterization of three novel members of the zebrafish Pax2/5/8 family: dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development. 1998;125:3063–3074. doi: 10.1242/dev.125.16.3063. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Piotrowski T, Ahn DG, Schilling TF, Nair S, Ruvinsky I, Geisler R, Rauch GJ, Haffter P, Zon LI, Zhou Y, Foott H, Dawid IB, Ho RK. The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development. 2003;130:5043–5052. doi: 10.1242/dev.00704. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Woods IG, Ngo-Hazelett P, Yan YL, Kelly PD, Chu F, Huang H, Hill-Force A, Talbot WS. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res. 2000;10:1890–1902. doi: 10.1101/gr.164800. [DOI] [PubMed] [Google Scholar]
- Riley BB, Phillips BT. Ringing in the new ear: resolution of cell interactions in otic development. Dev Biol. 2003;261:289–312. doi: 10.1016/s0012-1606(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Robinson-Rechavi M, Marchand O, Escriva H, Bardet PL, Zelus D, Hughes S, Laudet V. Euteleost fish genomes are characterized by expansion of gene families. Genome Res. 2001;11:781–788. doi: 10.1101/gr.165601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Sahly I, Andermann P, Petit C. The zebrafish eya1 gene and its expression pattern during embryogenesis. Dev Genes Evol. 1999;209:399–410. doi: 10.1007/s004270050270. [DOI] [PubMed] [Google Scholar]
- Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132:1687–1697. doi: 10.1242/dev.01730. [DOI] [PubMed] [Google Scholar]
- Seo HC, Curtiss J, Mlodzik M, Fjose A. Six class homeobox genes in Drosophila belong to three distinct families and are involved in head development. Mech Dev. 1999;83:127–139. doi: 10.1016/s0925-4773(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Shen W, Mardon G. Ectopic eye development in Drosophila induced by directed dachshund expression. Development. 1997;124:45–52. doi: 10.1242/dev.124.1.45. [DOI] [PubMed] [Google Scholar]
- Silver SJ, Davies EL, Doyon L, Rebay I. Functional dissection of eyes absent reveals new modes of regulation within the retinal determination gene network. Mol Cell Biol. 2003;23:5989–5999. doi: 10.1128/MCB.23.17.5989-5999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003;130:929–940. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- Spitz F, Demignon J, Porteu A, Kahn A, Concordet JP, Daegelen D, Maire P. Expression of myogenin during embryogenesis is controlled by Six/sine oculis homeoproteins through a conserved MEF3 binding site. Proc Natl Acad Sci USA. 1998;95:14220–14225. doi: 10.1073/pnas.95.24.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Maas RL. Vertebrate eye development as modeled in Drosophila. Hum Mol Genet. 2000;9:917–925. doi: 10.1093/hmg/9.6.917. [DOI] [PubMed] [Google Scholar]
- Westerfield M. Ed 3. Eugene, OR: University of Oregon; 1995. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) [Google Scholar]
- Whitfield TT, Riley BB, Chiang MY, Phillips B. Development of the zebrafish inner ear. Dev Dyn. 2002;223:427–458. doi: 10.1002/dvdy.10073. [DOI] [PubMed] [Google Scholar]
- Williams JA, Holder N. Cell turnover in neuromasts of zebrafish larvae. Hear Res. 2000;143:171–181. doi: 10.1016/s0378-5955(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Wulbeck C, Campos-Ortega JA. Two zebrafish homologues of the Drosophila neurogenic gene groucho and their pattern of transcription during early embryogenesis. Dev Genes Evol. 1997;207:156–166. doi: 10.1007/s004270050103. [DOI] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130:3085–3094. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CC, Dyer MA, Uchikawa M, Kondoh H, Lagutin OV, Oliver G. Six3-mediated auto repression and eye development requires its interaction with members of the Groucho-related family of co-repressors. Development. 2002;129:2835–2849. doi: 10.1242/dev.129.12.2835. [DOI] [PubMed] [Google Scholar]