Abstract
The importance of the lateral hypothalamus in the regulation of reward and motivation has long been recognized. However, the neuronal network involved in such a hypothalamic regulation of reward remains essentially unknown. Recently, hypocretin-containing neurons, a group of hypothalamic neurons known to be associated with the stability of arousal, have emerged as important structures in the control of brain reward function. This review summarizes a Mini-Symposium presented at the 2006 Annual Meeting of the Society for Neuroscience.
Keywords: hypocretin, orexin, dopamine, MCH, reinstatement, cocaine-seeking
The hypocretins (hcrt), also known as orexins (de Lecea et al., 1998; Sakurai et al., 1998), are two neuropeptides derived from a single precursor, produced in a few thousand cells restricted to the lateral hypothalamus (LH). Hypocretin neurons project widely throughout the brain, with the strongest innervation to midbrain structures, including monoaminergic nuclei such as dorsal raphe and locus ceruleus and, to a lesser extent, the ventral tegmental area (VTA). A main function of the hypocretins came to light when their absence was found to be associated with narcolepsy (Chemelli et al., 1999; Lin et al., 1999), a sleep disorder characterized by intrusions of rapid eye movement (REM) sleep into wakefulness. Recent models suggest that one of the main functions of the hypocretinergic system is to provide stability to a “sleep switch,” which has the property of making distinct state transitions but produces an inherent instability in those transitions (Lu et al., 2006).
For illuminating how the hypocretin neurons can normally maintain waking, Barbara Jones examines studies aimed at recording and identifying hypocretin neurons in association with natural sleep–wake states. Previous studies using extracellular single-unit recordings of hypothalamic neurons in the LH had revealed diverse cell types that discharge maximally during waking (W), slow-wave sleep (SWS), and REM sleep or W and REM sleep (Alam et al., 2002; Koyama et al., 2003). Hypocretin neurons, which comprise ∼5% of all neurons in the LH, could represent one or contingents of one or more cell types in this area. Lee et al. (2005) applied juxtacellular labeling with Neurobiotin (Nb) in head-fixed rats and subsequent immunohistochemical staining for hcrt to identify each recorded cell as hcrt positive or negative (Lee et al., 2005). The Nb-labeled/hcrt-positive neurons discharged at a relatively low average rate during active W (mean of ∼3 Hz for six cells), a lower rate during quiet W, and minimally during SWS and REM sleep. Their discharge rate was only weakly correlated with EEG gamma activity across states but highly correlated with EMG amplitude. These results show that hypocretin neurons discharge maximally during active W and cease discharge during sleep, including REM sleep. Because they recommence firing before full awakening, they may also be responsible for initiating waking. These results, along with recordings obtained in unrestrained animals by Mileykovskiy et al. (2005), suggest that hypocretin neurons play a key role in engaging arousal circuits.
The finding that hypocretin neurons show phasic activity raises the important question as to which signals activate hypocretin cells to initiate and stabilize wakefulness. In addition to metabolic cues, hypocretin neurons are sensitive to different arousal-promoting transmitters, including corticotrophin-releasing factor (CRF), a peptide that triggers the acute response to stress in the brain (Winsky-Sommerer et al., 2004) (Fig. 1). Recently, it was suggested that increased activity of hypocretin neurons could also lead to a state of hyperarousal and excitement propitious to drug craving or, at least, could contribute to the vulnerability to relapse for drug seeking during protracted abstinence. In this perspective, Boutrel et al. (2005) first showed that intracerebroventricular infusions of hcrt-1, although ineffective on cocaine intake in rats, led to a dose-related reinstatement of a previously extinguished cocaine-seeking behavior. Interestingly, hcrt-1 also reinstated an extinguished food-seeking behavior, which suggests that the hypocretin system is involved in more general consummatory behaviors and also indicates that the previously reported increased food intake induced by hcrt needs to be reevaluated. The same dose of hcrt-1 elevated intracranial self-stimulation (ICSS) thresholds, indicating a decrease in excitability of brain reward systems. This effect was in sharp contrast to the well known cocaine-induced lowering of ICSS thresholds that is considered to reflect an increased sensitivity that underlies, or at least contributes to, the positive affective state associated with drug consumption. In contrast, this long-lasting reward deficit was similar to that observed after intracerebroventricular infusion of CRF or after drug withdrawal (Macey et al., 2000). These data provide strong evidence suggesting that hcrt-1 reinstates cocaine seeking by mechanisms different from increased dopamine release only. Indeed, the blockade of hcrt-induced reinstatement of cocaine seeking by CRF/norepinephrin antagonism rather suggests that hypocretin and stress systems may closely interact to regulate cocaine-seeking behaviors. This hypothesis was later confirmed using a hcrt-1 receptor antagonist, SB334867 [1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl-urea hydrochloride], to prevent footshock-induced reinstatement of a previously extinguished cocaine-seeking behavior. These results complement a report by Harris et al. (2005) showing the involvement of the hypocretin system in the reinstatement of an extinguished conditioned place preference for morphine. Overall, these findings identify a new mechanism by which cues related to consummatory behaviors or stress could influence relapse to drug seeking.
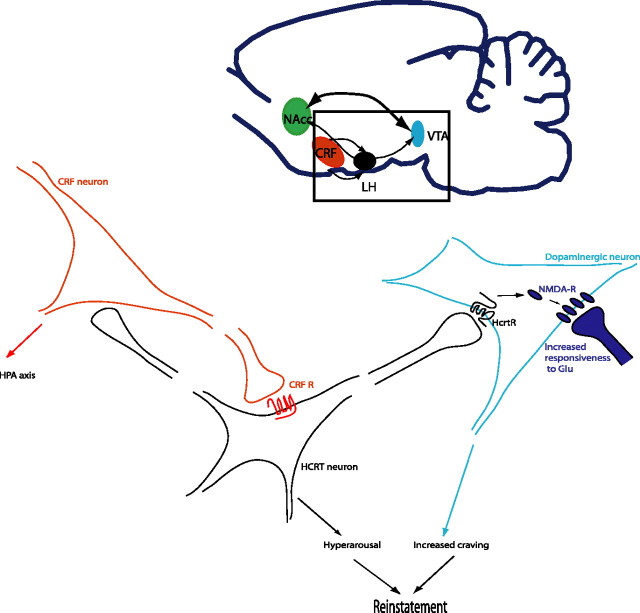
Figure 1.
Hypocretin neurons play a key role in reinstatement of drug-seeking behavior. Stressful conditions turn on CRF and activate hypocretin neurons, which results in increased alertness. In an animal with a history of drug abuse, sustained activation of hypocretin neurons recruits NMDA receptors in VTA synapses (Borgland et al., 2006) that results in increased response to glutamatergic neurons. These synaptic changes are “memorized” in the VTA neurons, in such a way that reactivation of hcrt neurons after extinction by stress (Boutrel et al., 2005) or context (Harris et al., 2005) results in reinstatement of drug-seeking behavior. Blockade of these signaling pathways by CRF receptor antagonists (Shaham et al., 1998) or hcrt receptor antagonists (Boutrel et al., 2005; Harris et al., 2005) also blocks reinstatement of drug seeking. Supporting a role of hcrt neurons in dopaminergic function, recent data has revealed that hcrt-deficient animals show decreased dopaminergic response to morphine (Narita et al., 2006). MCH and other hypothalamic cell groups may further regulate reward by interacting with nucleus accumbens neurons (NAcc) (data not shown). HPA, Hypothalamic–pituitary–adrenal.
A key question derived from these studies is which circuit is affected by the hypocretinergic system in addicted compared with naive animals. Looking into the mechanism of such neuroadaptation, Borgland et al. (2006) recorded EPSCs in VTA slices using whole-cell voltage-clamp electrophysiology. In vitro application of hcrt-1 potentiated NMDA receptor (NMDAR) neurotransmission via phospholipase C/PKC-dependent insertion of NMDARs in VTA dopamine neuron synapses. In this study, the irreversible, activity-dependent NMDA antagonist MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate], which only blocks synaptic NMDARs, was used to assess whether hcrt-1-mediated potentiation of NMDARs was attributable to NMDAR recruitment to the synapse or altered function of existing synaptic NMDARs. Hcrt-1 increased the NMDAR current after washout of MK-801, suggesting translocation of new NMDARs to the synapse from intracellular or extrasynaptic sources. Hcrt-1-mediated acute potentiation of NMDARs resulted in a late-phase long-term potentiation of AMPA receptor (AMPAR)-mediated synaptic transmission. Enhanced AMPAR signaling was not observed acutely after administration of hcrt-1. However, it was apparent hours after a short hcrt-1 application and was dependent on NMDAR activation during the hcrt-1 application. The net result of these changes is a delayed but prolonged increase of AMPARs relative to NMDARs, reflected by an increased AMPAR/NMDAR ratio similar to that observed after in vivo cocaine exposure (Ungless et al., 2001; Borgland et al., 2004). Interestingly, inhibiting hcrt receptor 1 prevented the potentiation of the AMPAR/NMDAR ratio after daily treatment with cocaine, suggesting that activation of VTA hcrt receptors may be critical for the development of glutamatergic plasticity associated with in vivo cocaine administration.
To assess whether hcrt signaling is necessary for the development of addictive behaviors, Borgland et al. (2006) used locomotor sensitization, a behavioral paradigm that involves a progressive increase in the locomotor-activating effects of cocaine. Cocaine sensitization requires NMDAR activation in the VTA (Kalivas and Volkow, 2005). The hcrt receptor 1 antagonist SB334867, administered either systemically or intra-VTA, abolished the development of locomotor sensitization after repeated cocaine administration. NMDARs are necessary for the induction of long-term potentiation as well as the promotion of burst firing in dopaminergic neurons, which may enhance dopamine output in target regions of the VTA (Heien and Wightman, 2006). Therefore, potentiation of glutamatergic synaptic transmission by hcrt in the VTA likely increases dopamine output and could enhance the reinforcing properties of drugs of abuse (Fig. 1).
Given these pharmacological effects of hcrt on dopaminergic neurons, it becomes important to determine the anatomical and functional interactions between hypocretin neurons in the lateral hypothalamus and dopamine neurons of the ventral tegmental area. Michael Bubser and colleagues showed that the LH receives a moderately dense innervation by both coarse- and fine-caliber dopamine axons, most of which derive from the VTA and its dorsal extension into the raphe nuclei. These dopaminergic afferents establish close contacts with hcrt neurons (Fadel and Deutch, 2002). Dopamine-releasing amphetamines, the mixed D1/D2 dopamine receptor agonist apomorphine, and selective D1 or D2 receptor agonists all activate hcrt cells as shown by increased expression of the immediate-early gene product, c-Fos. These effects of apomorphine are blocked by a combination of D1 and D2 receptor antagonists. Despite the marked activation of hcrt cells by dopamine agonists, only few LH cells were found that express even a low abundance of D2 dopamine receptor transcript and none that contained any other dopamine receptor transcripts (D1, D3, D4, D5) (Bubser et al., 2005). The lack of significant dopamine receptor expression in the LH raises the question as to how dopaminergic drugs alter hcrt cell activity. One possibility is through activation of a noncognate receptor; another could be the trans-synaptic activation of hcrt cells. This would be consistent with electrophysiological recordings of genetically identified hypocretin cells, showing that most of the excitatory input to these cells comes from local glutamatergic interneurons (Li et al., 2002).
One of the most significant derivations from these studies is that selective hypocretin receptor 1 antagonists could be used as a therapy to prevent relapse of drug-seeking behavior. Seiji Nishino's data support the idea that inhibition of hcrt signaling results in decreased preference for reinforcing drugs. Amphetamine-like psychostimulants have been prescribed for the treatment of narcolepsy for more than 60 years. Considering the prevalence of narcolepsy (1 in 2000) and the fact that ∼95% of narcoleptic patients are currently treated with pharmacological compounds, the total cumulative number of narcoleptic patients receiving psychostimulants is large. Nevertheless, psychostimulant abuse in narcoleptic subjects is extremely rare (Guilleminault et al., 1974; Parkes et al., 1975). It is therefore reasonable to hypothesize that hypocretin-deficient narcolepsy may be resistant to psychostimulant abuse. Amphetamine-induced locomotor sensitization and place preference tests were conducted in the hypocretin/orexin-deficient mice narcoleptic models [i.e., orexin knock-out (KO) and orexin/ataxin-3 transgenic (TG) mice]. Both KO and TG narcoleptic mice are less responsive in developing amphetamine-induced locomotor sensitization. Amphetamine-induced place preference is attenuated in KO mice. This effect could be attributable on the one hand to the lack of hcrt-induced activation of dopaminergic neurons, or on the other hand the loss of hcrt neurons eliminates dopamine-mediated modulation hcrt activity (thus affecting arousal, etc.). These results may explain the resistance of psychostimulant abuse in hypocretin-deficient narcoleptics and that hypocretin neurotransmission may be one of the critical factors for development of stimulant abuse.
Often regarded as the counterbalance of the hypocretin system, neurons containing the neurotransmitter melanin-concentrating hormone (MCH) localize in the same region and are reciprocally connected with hypocretin cells but do not overlap (Broberger et al., 1998). It is therefore important to determine whether MCH-containing neurons are also involved in the brain reward circuitry. Georgescu et al. (2005) examine data indicating that this cell group is also involved in motivated behaviors. Considering the highly enriched expression of MCH1 receptor (MCH1R) in the shell region of the nucleus accumbens (NAcSh), it is possible that MCH–MCH1R signaling mediates a reverse hypothalamic → limbic circuit that allows for integration of information that is relevant to food intake and related behaviors. Administration of MCH to the NAcSh increases food intake, and blockade of MCH1R attenuates feeding in sated rats (Georgescu et al., 2005). Moreover, MCH1R is expressed on most medium spiny neurons. Because pharmacological manipulations of both dopamine and glutamate in the NAcSh can alter feeding, DiLeone's group sought to better understand how MCH1R signaling interacts with these pathways via analysis of phosphoprotein signaling pathways. Stimulation of D1 receptor results in PKA-dependent increase in phosphorylation of serine 845 of glutamate receptor type 1 (GluR1). MCH treatment attenuates D1 receptor-mediated GluR1 effects, and this action requires calcineurin activity. MCH1R signaling also modulates the phosphorylation state of dopamine and cAMP-regulated phosphoprotein-32 in the nucleus accumbens. Importantly, analysis of MCH mutant and overexpressing mice demonstrated phosphoprotein changes consistent with the acute effects of MCH on brain slices, suggesting that the chronic state of MCH changes in animals may also result in a similar modification of signaling pathways. By modulating these phosphoproteins, MCH may play a critical role integrating dopamine and glutamate signaling to result in changes in feeding behavior. The biochemical analysis, together with data on MCH effects on the electrical properties of medium spiny neurons, give a more complete picture of MCH action in the NAcSh.
Together, the data presented in this mini-review sets the groundwork of the role of hypothalamic peptide transmitters on arousal, consummatory behaviors, and reward. The hypocretinergic system is now viewed as an integrating cellular network with multiple inputs (metabolic, emotional, and circadian) and outputs (arousal stability and brain reward). Overactivation of the hypocretinergic system by stress may induce neuroadaptive changes in dopaminergic circuits that may lead to increased susceptibility to drug addiction and reinstatement. An increased understanding of this system will likely lead to therapeutic interventions to improve sleep quality and prevent relapse.
References
- Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol (Lond) 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, de Lecea L, Sutcliffe JG, Hökfelt T. Hypocretin/orexin- and melanin-concentrating hormone expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to neuropeptide Y innervation. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. Eur J Neurosci. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilleminault C, Carskadon M, Dement WC. On the treatment of rapid eye movement narcolepsy. Arch Neurol. 1974;30:90–93. doi: 10.1001/archneur.1974.00490310092014. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Jones GA. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Heien ML, Wightman RM. Phasic dopamine signaling during behavior, reward, and disease states. CNS Neurol Disord Drug Targets. 2006;5:99–108. doi: 10.2174/187152706784111605. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–1219. doi: 10.1016/s0306-4522(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani O, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res. 2000;866:82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes JD, Baraitser M, Marsden CD, Asselman P. Natural history, symptoms and treatment of the narcoleptic syndrome. Acta Neurol Scand. 1975;52:337–353. doi: 10.1111/j.1600-0404.1975.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]