Abstract
Recent Parkinson's disease research has focused on understanding the function of the cytosolic protein, α-synuclein, and its contribution to disease mechanisms. Within neurons, α-synuclein is hypothesized to have a role in regulating synaptic plasticity, vesicle release, and trafficking. In contrast, glial-expressed α-synuclein remains poorly described. Here, we examine the consequence of a loss of α-synuclein expression on microglial activation. Using a postnatal brain-derived culture system, we defined the phenotype of microglia from wild-type and knock-out α-synuclein mice (Scna−/−). Scna−/− microglia displayed a basally increased reactive phenotype compared with the wild-type cells and an exacerbated reactive phenotype after stimulation. They also exhibited dramatic morphologic differences compared with wild-type, presenting as large, ramified cells filled with vacuole-like structures. This corresponded with increased protein levels of activation markers, CD68 and β1 integrin, in the Scna−/− cells. More importantly, Scna−/− microglia, after stimulation, secreted elevated levels of proinflammatory cytokines, TNFα (tumor necrosis factor α) and IL-6 (interleukin-6), compared with wild type. However, despite the reactive phenotype, Scna−/− cells had impaired phagocytic ability. We demonstrate for the first time that α-synuclein plays a critical role in modulating microglial activation state. We suggest that altered microglial α-synuclein expression will affect their phenotype as has already been demonstrated in neurons. This has direct ramifications for the contribution of microglia to the pathophysiology of disease, particularly in familial cases linked to altered α-synuclein expression.
Keywords: microglia, TNFα, α-synuclein, phagocytosis, inflammation, Parkinson
Introduction
Overexpression and mutations in α-synuclein are associated with early-onset Parkinson's disease (PD) (Polymeropoulos et al., 1997; Kruger et al., 1998; Singleton et al., 2003). It is aggregated intracellularly as a component of Lewy bodies characteristic of Parkinson's as well as other neurodegenerative diseases collectively referred to as synucleinopathies (Perry et al., 1990; Spillantini and Goedert, 2000). α-Synuclein belongs to a family of three proteins and is highly conserved among vertebrates (for review, see Clayton and George, 1998). It is highly expressed in the brain in both glia and neurons, in areas including the thalamus, substantia nigra, caudate nucleus, hippocampus, amygdala, and subthalamic nucleus (Shibayama-Imazu et al., 1993; Ueda et al., 1993, 1994). It is found in presynaptic terminals of neurons (Maroteaux et al., 1988; McLean et al., 2000), but also in other regions of neurons as well as within astrocytes and oligodendroglia (Richter-Landsberg et al., 2000; Mori et al., 2002). α-Synuclein binds a variety of proteins (Jenco et al., 1998; Peng et al., 2005) and lipid vesicles (Cole et al., 2002), and is involved in lipid metabolism (Cabin et al., 2002; Castagnet et al., 2005; Golovko et al., 2005, 2006). Absence of α-synuclein expression is associated with striatal dysfunction (Abeliovich et al., 2000). These data support the hypothesis that α-synuclein may regulate vesicle trafficking and release by altering lipid membrane stability, intracellular lipid uptake, and metabolism.
Whereas the function of neuronal α-synuclein continues to be characterized, the role of α-synuclein in regulating glial physiology remains poorly defined. Recent studies in human astrocytes and macrophages have demonstrated an increase in β-and α-synuclein expression, respectively, after stimulation with lipopolysaccharide (LPS) and interleukin-1 (IL-1), suggesting a role for α-synuclein in the inflammatory process (Tanji et al., 2001, 2002). Few studies have reported the expression and function of this protein within microglia. Although microglial activation in vivo correlates with fibrillar α-synuclein deposition and fibrillar α-synuclein is capable of activating microglia in vitro, it is unclear what role its expression plays in regulating microglial behavior (Croisier et al., 2005; Zhang et al., 2005). We hypothesized that expression of the protein itself may also alter microglia reactivity. Here, we present results using an in vitro microglia culture system from wild-type α-synuclein-expressing mice and α-synuclein gene-ablated mice, Scna−/−, to characterize the phenotype differences caused by lack of α-synuclein expression.
Materials and Methods
Materials
The anti-β1 integrin, extracellular signal-regulated kinase 2 (ERK2), and horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). LPS and anti-α-tubulin, a mouse monoclonal IgG2B antibody (catalog #T 6793) were purchased from Sigma (St. Louis, MO). The anti-CD68 antibody was purchased from Serotec (Raleigh, NC). The anti-α-synuclein (Syn-1) antibody is a mouse IgG1 antibody purchased from BD Transduction Laboratories (San Diego, CA) (catalog #610786). The 4D6 anti-α-synuclein is a mouse monoclonal IgG antibody and was a gift from Signet Laboratories (Dedham, MA) (catalog #9720-02). FITC-labeled Escherichia coli (K-12 strain) Bioparticles were purchased from Invitrogen (Eugene, OR).
Mice.
Synuclein gene-ablated mice (129/SvEv), Scna−/−, were generated and characterized as described previously (Cabin et al., 2002). Scna−/− and Scna+/+ (wild-type) mice were derived by crossing Scna+/− heterozygotes to create appropriate genotypes (Ellis et al., 2005).
Tissue culture.
Microglia were derived from postnatal day 1 (P1) to P2 wild-type and Scna−/− mouse brains as described previously (Floden et al., 2005). Briefly, meninges-free cortices are isolated, trypsinized, and plated onto tissue culture flasks with feeding every fifth day. At 14 d in vitro, microglia were harvested from the mixed culture by rapid shaking (120 rpm; 30 min) and plated for use. Microglial purity is routinely verified at >98% by CD68 immunoreactivity.
Quantitation of secreted tumor necrosis factor α and IL-6.
Microglia were plated at a density of 40,000 cells/well in serum-free DMEM/F-12 onto 96-well tissue culture plates for 24 h with and without 25 ng/ml LPS. After stimulation, medium was removed from the cultures and concentrations of secreted tumor necrosis factor α (TNFα) and IL-6 were determined using commercially available mouse TNFα and IL-6 colorimetric sandwich ELISA plates purchased from R & D Systems (Minneapolis, MN). Experiments were performed with eight replicates per condition and repeated a minimum of three times. Data are presented as mean values (±SD), and statistical significance was determined by unpaired one-way ANOVA with Tukey-Kramer's post hoc comparison (p < 0.05).
Phagocytosis assay.
Phagocytosis was quantified by measuring the uptake of FITC-labeled bioparticles. Briefly, microglia were plated into 96-well plates (40,000 cells/well) and incubated with or without FITC-labeled bioparticles (0.25 mg/ml) for 3 h. To quench the signal from extracellular or outer plasma membrane-associated bioparticles, the medium was removed and the cells were rinsed with 0.25 mg/ml trypan blue in PBS. Intracellular fluorescence was read via fluorescent plate reader (Bio-Tek, Winooski, VT) at 480 nm excitation and 520 nm emission. Experiments were performed with eight replicates per condition and repeated a minimum of three times. Data are presented as mean values (±SD), and statistical significance was determined by unpaired ANOVA with Tukey–Kramer's post hoc comparison (p < 0.05).
Immunocytochemistry.
To perform culture immunocytochemistry, microglia were plated in serum-free DMEM/F-12 for 24 h, fixed in 4% paraformaldehyde (37°C; 30 min), immunostained using anti-CD68, and visualized using Vector VIP as the chromagen (Vector Laboratories, Burlingame, CA). Immunofluorescent double labeling was performed using anti-CD68 and Syn-1 antibodies with FITC- and Texas Red-conjugated secondary antibodies.
Western blot.
To perform Western blot analyses, microglia were plated in serum-free DMEM/F-12 for 24 h with or without LPS (50 ng/ml). Cells were collected, and lysates were quantified. Proteins were resolved by 10% SDS-PAGE, transferred to polyvinylidene difluoride membranes and Western blotted using anti-β1 integrin, α-tubulin, CD68, ERK2 (loading control), or α-synuclein (4D6) antibodies. Blots were probed with affinity-purified horseradish peroxidase-conjugated secondary antibodies, and antibody binding was visualized by enhanced chemiluminescence. Optical density of protein bands were quantitated using Adobe Photoshop software. Quantitation data are presented as mean values (±SD), and statistical significance was determined by unpaired ANOVA with Tukey–Kramer's post hoc comparison (*p < 0.05; **p < 0.001).
Results
Microglia from Scna−/− mice are morphologically different from wild-type microglia
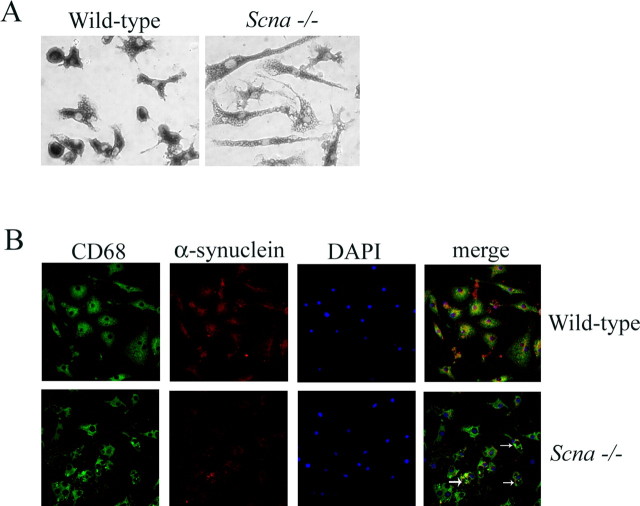
To begin defining the role of α-synuclein expression in modulating microglial activation, we first compared the morphological phenotype between Scna−/− and wild-type microglia. We chose to use the common postnatal brain-derived microglia culture system for these studies based on our previous experience with this model (Sondag and Combs, 2004; Floden et al., 2005). Microglia were cultured from P1–P2 mouse brains from both Scna−/− and wild-type animals for 14 d in vitro in parallel, and then isolated and plated for use in identical conditions. Although both Scna−/− and wild-type microglia were robustly immunoreactive for the microglial marker protein, CD68, the morphologies dramatically differed (Fig. 1A,B). The Scna−/− microglia were flat and ramified and heavily burdened with vacuole-like structures compared with their wild-type counterparts. To confirm the purity of our cultures and microglial expression of α-synuclein, we fluorescently labeled the cultured cells with CD68, Syn-1, and 4′,6′-diamidino-2-phenylindole (DAPI) (Fig. 1B). Together, these data confirm microglial expression of α-synuclein and suggest that the basal activation state of the two cell types in vitro is different.
Figure 1.
Microglia from Scna−/− mice display a ramified, heavily vacuole-laden phenotype in vitro compared with wild-type microglia. Microglia were isolated from 14 d in vitro mixed glia cultures and plated onto tissue culture plastic for 24 h. A, Cells were fixed in 4% paraformaldehyde and immunostained with an anti-CD68 antibody. Antibody binding was visualized using Vector VIP as the chromagen. B, Cells were double-labeled using anti-CD68 and anti-α-synuclein antibodies with FITC- and Texas Red-conjugated secondary antibodies, respectively. Nuclei were stained with DAPI before mounting for confocal imaging. Contrast of merged images was increased to better illustrate double label. The arrows indicate large vacuole-like structures present in Scna−/− microglia.
Microglia from Scna−/− mice displayed increased levels of reactive marker proteins
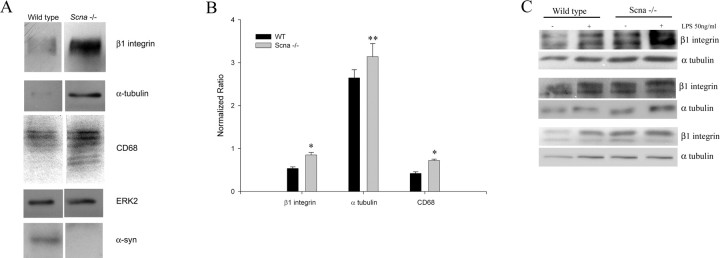
To verify the hypothesized difference in basal activation state, we next compared protein levels of well characterized microglia-reactive marker proteins, CD68 and β1 integrin, in Scna−/− and wild-type cells. Microglia were isolated from mixed glia cultures after 14 d in vitro and analyzed by Western blot. Increased basal levels of both CD68 and β1 integrin were observed in the Scna−/− microglia compared with wild-type cells consistent with the idea that the Scna−/− microglia may be basally activated (Fig. 2A). Scna−/− microglia also displayed an increase in protein levels of the cytoskeletal protein α-tubulin compared with wild-type cells, consistent with the dramatic, ramified morphology we observed (Figs. 1, 2A). Quantitative analysis of the Western blots from multiple cultures showed statistically higher reactive protein levels in the Scna−/− microglia than wild-type microglia (Fig. 2B). These data support the idea that Scna−/− microglia are basally reactive compared with wild-type cells.
Figure 2.
Microglia from Scna−/− mice display increased levels of reactive marker proteins compared with wild-type microglia. Microglia from P1–P2 Scna−/− and wild-type mice were cultured for 14 d in vitro as mixed glia cultures. At 14 d in vitro, microglia were isolated and plated for 24 h. A, Cells were lysed, and proteins were resolved by 10% SDS-PAGE. Lysates were Western blotted using anti-β1 integrin, α-tubulin, and CD68 antibodies. Equal protein loading was verified using an anti-ERK2 antibody (loading control). B, Optical density of β1 integrin, α-tubulin, and CD68 protein bands from five independent culture preparations derived from different litters were normalized against respective ERK2 levels and averaged (±SD) (**p < 0.001 and *p < 0.05 from respective control). C, Microglia isolated at 14 d in vitro were stimulated with or without 50 ng/ml LPS for 24 h, and then lysed, and proteins were resolved by 10% SDS-PAGE. Lysates from three independent culture preparations from different litters were immunoblotted using anti-β1 integrin and α-tubulin antibodies. Blots were visualized via enhanced chemiluminescence.
It is known that microglia, after stimulation, increase their expression of a number of proteins, including β1 integrins examined above (Milner and Campbell, 2003). To determine whether stimulation further differentiated the Scna−/− microglia from wild-type cells, we stimulated them with a well characterized, potent proinflammatory stimulus, bacterial endotoxin, LPS, before again performing Western blots for β1 integrin and α-tubulin. As expected, wild-type microglia showed increased levels of β1 integrin and α-tubulin after stimulation indicative of their activation (Fig. 2C) and to approximately the level seen in the unstimulated Scna−/− microglia (Fig. 2C). Stimulation of the Scna−/− microglia also resulted in additional increases in both protein levels (Fig. 2C). These data suggest that the Scna−/− cells become hyperreactive compared with wild-type cells after proinflammatory stimulation.
Microglia from Scna−/− mice secreted increased amounts of proinflammatory cytokines, TNFα and IL-6, after stimulation
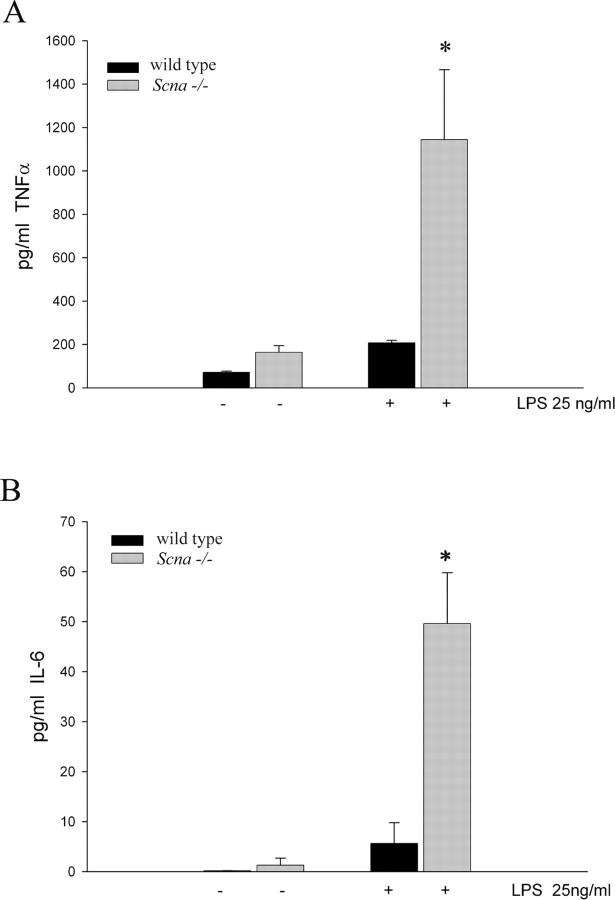
To quantitatively assess the basal and stimulated reactivity of the Scna−/− cells, we measured secretion of two cytokines, TNFα and IL-6, from wild-type and Scna−/− microglia under unstimulated and LPS-stimulated conditions. Consistent with our morphologic and Western blot assays, Scna−/− and wild-type microglia basally secreted both cytokines, although no significant differences were seen (Fig. 3A,B). More importantly, after stimulation, Scna−/− microglia secreted significantly larger amounts of both cytokines (Fig. 3A,B). These data demonstrated that Scna−/− cells have a hyperreactive phenotype after proinflammatory stimulation.
Figure 3.
Microglia from Scna−/− mice secrete increased levels of proinflammatory cytokines after stimulation compared with wild-type microglia. Microglia were isolated from Scna−/− and wild-type mixed glia cultures at 14 d in vitro and plated in the absence or presence of 25 ng/ml LPS for 24 h. Conditioned medium was collected from the cells and secreted TNFα (A) and IL-6 (B) concentrations were determined using commercial ELISA. Graphs are representative of four independent experiments. Each experiment was performed with eight replicates per condition, and values were averaged (±SD). *p < 0.001 from respective control.
Microglia from Scna−/− mice displayed decreased phagocytic ability
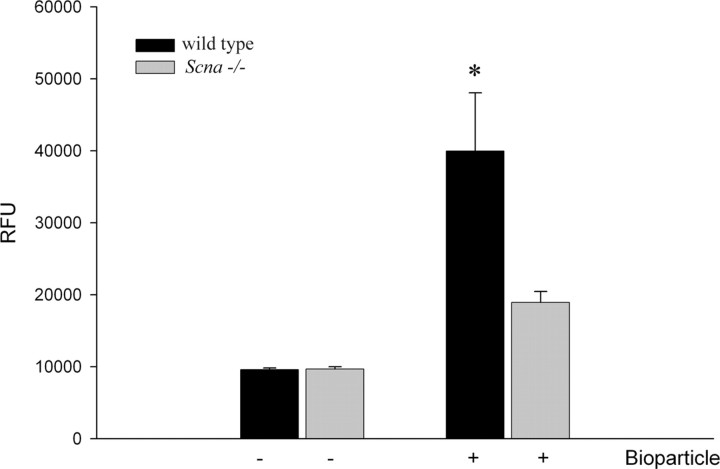
Although cytokine secretion and morphologic changes are typical assays for defining microglial activation states, we further defined the differences between cells by using a more functional measure of reactivity, phagocytic ability. Just as there are multiple activation states in microglia, many phagocytic pathways exist as well. We examine one of these phagocytic pathways here; others may be unaffected. We compared phagocytic ability between the two cell types as a final assay of activation state differences by quantitating uptake of FITC-conjugated Escherichia coli bioparticles. Wild-type microglia phagocytosed a significantly greater amount of bioparticle compared with the Scna−/− cells (Fig. 4). Therefore, although knock-out cells secrete elevated levels of cytokines after stimulation and present with a reactive morphology, they are deficient in phagocytic ability. These data demonstrate the complexity of the activation phenotype induced by the loss of α-synuclein expression.
Figure 4.
Microglia from Scna−/− mice have decreased phagocytic ability compared with wild-type microglia. Microglia from Scna−/− and wild-type control mice were cultured as mixed glia for 14 d in vitro. Microglia were isolated at 14 d and plated with or without FITC-labeled E. coli bioparticles (0.25 mg/ml) for 3 h. After the incubation, the medium was removed and the signal from unphagocytosed or extracellular membrane-associated FITC-labeled bioparticles was quenched by rinsing with 0.25 mg/ml trypan blue solution. Fluorescence intensity [relative fluorescence units (RFU)] of phagocytosed bioparticles was measured via a fluorescent plate reader (480 nm excitation and 520 nm emission) and averaged (±SD). Each condition was performed with eight replicates, and the graph is representative of three independent experiments. *p < 0.001 from respective control.
Discussion
After stimulation, microglia undergo several changes to adopt a reactive phenotype. Activated microglia alter expression of a variety of proteins, including cell adhesion molecules, such as ICAM-1 (intercellular adhesion molecule 1) and β1 integrin, cytokines, such as TNFα and IL-6, and costimulatory molecules, such as MHC II (major histocompatibility complex II) (Frohman et al., 1991; Boka et al., 1994; McGeer et al., 1988, 2001). Microglia use these proteins to increase their adhesion and migration ability, to restructure their cytoskeleton to assist in phagocytosis and migration, and to stimulate surrounding cells via secretion (Thomas, 1992). Although this is a typical phenotype displayed by active microglia, it is not the only activation state these cells acquire. It is now appreciated that a spectrum of microglial activation states exist (Mantovani et al., 2002; Gordon, 2003).
The microglial phenotype induced by α-synuclein deficiency represents one such atypical activation state. We have observed that Scna−/− microglia have a ramified, vacuolar morphology with increased cytokine secretion and decreased phagocytic ability. Although disparate characteristics, they are all a consequence of the absence of α-synuclein expression. These observations demonstrate that Scna−/− microglia have a somewhat reactive phenotype basally compared with wild-type cells. In addition, Scna−/− microglia are capable of even further increased activation after stimulation compared with their wild-type counterparts. Despite this, however, knock-out microglia have decreased phagocytic ability. Perhaps this is not entirely surprising given the fact that heightened phagocytic activity of microglia is generally associated with an ameboid morphology similar to that of our wild-type cells (Smith and Hoerner, 2000). More importantly, increased phagocytic ability is not an integral characteristic of an activated phenotype. Certainly, alternative activation states describe microglia with a typical proinflammatory phenotype but a reduced ability to phagocytose (Mantovani et al., 2002; Gordon, 2003).
Although we have not yet determined the mechanism by which α-synuclein regulates microglial activation, it is intriguing to speculate that the mechanism may involve alteration in lipid-mediated signal transduction. Lipid-dependent signaling pathways are an integral component of many stimuli leading to microglia activation. For example, phospholipase D (PLD) activity is required for macrophage activation in response to a variety of proinflammatory stimuli including LPS and TNFα (De Valck et al., 1993). PLD activity also contributes to cytoskeletal restructuring and phagocytosis ability of microglia and macrophage (Iyer et al., 2004). α-Synuclein interacts with PLD isozymes, which results in inhibition of enzymatic activity (Jenco et al., 1998; Ahn et al., 2002), and overexpression of α-synuclein potently inhibits PLD (Outeiro and Lindquist, 2003). We have demonstrated increased palmitic acid turnover in phosphatidylcholine in the α-synuclein-deficient mouse brains, indicating increased PLD activity resulting from α-synuclein deficiency (Golovko et al., 2005). In addition, our recent work suggested that α-synuclein expression also modulates PLA2 (phospholipase A2)-dependent signaling events. We have linked α-synuclein to 20:4n-6 metabolism via its impact on endoplasmic reticulum localized acyl-CoA synthetases (Golovko et al., 2006). Mutant forms of α-synuclein (A30P, E46K, A53T) fail to modulate acyl-CoA synthetase activity, suggesting potential derangement of arachidonic acid metabolism and eicosanoid-dependent signaling in the brain (Golovko et al., 2006). Together, these data support the idea that α-synuclein may modulate microglial activation by regulating proinflammatory lipid-dependent signaling events.
It will also be important to define the consequences of α-synuclein overexpression and mutant expression on microglial phenotype, because these conditions represent the situations responsible for familial PD. This is particularly relevant given the possibility that disease mutations result in loss of function. This would indicate that microglial activation from mutant synuclein expression plays a direct role in the pathophysiology of disease. An active role for microglia during selective dopaminergic loss certainly correlates with the fact that the substantia nigra represents a brain region particularly concentrated with microglia (Kim et al., 2000). Moreover, PD brains have a maintained presence of reactive microglia (McGeer and McGeer, 2004; Teismann and Schulz, 2004) that increase in number with disease duration (Croisier et al., 2005). Finally, positron emission tomography has shown that early-stage PD brains have increased microglial reactivity positively correlating with disease motor severity (Ouchi et al., 2005). These data demonstrate that altered microglial phenotype is an early induced, yet maintained disease histopathology and likely an important contributor to disease pathophysiology. Although microglial activation in vivo correlates with fibrillar α-synuclein deposition and that fibrillar α-synuclein is capable of activating microglia in vitro (Croisier et al., 2005; Zhang et al., 2005), our work suggests that altered or mutant microglial α-synuclein expression may result in a direct contribution by these cells to the proinflammatory state and neuron loss characteristic of PD.
Footnotes
This work was supported by National Institutes of Health (NIH)–National Center for Research Resources Grant 1 P20 RR17699-01 (C.K.C., E.J.M.) and NIH–National Institute of Neurological Disorders and Stroke Grant 1R21-NS043697-01A (E.J.M.). We thank Dr. Robert Nussbaum for his useful discussion and critical review of this manuscript. We also thank Joe Bertelsen of Signet Laboratories, Inc., for the generous gift of the α-synuclein 4D6 antibody.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho W-H, Castillo PE, Shinsky N, Verdugo JMG, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Ahn BH, Rhim H, Kim SY, Sung YM, Lee MY, Choi JY, Wolozin B, Chang JS, Lee YH, Kwon TK, Chung KC, Yoon SH, Hahn SJ, Kim MS, Jo YH, Min do S. Alpha-synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells. J Biol Chem. 2002;277:12334–12342. doi: 10.1074/jbc.M110414200. [DOI] [PubMed] [Google Scholar]
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnet PI, Golovko MY, Barcelo-Coblijn GC, Nussbaum RL, Murphy EJ. Fatty acid incorporation is decreased in astrocytes cultured from alpha-synuclein gene-ablated mice. J Neurochem. 2005;94:839–849. doi: 10.1111/j.1471-4159.2005.03247.x. [DOI] [PubMed] [Google Scholar]
- Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J Biol Chem. 2002;277:6344–6352. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valck D, Beyaert R, Van Roy F, Fiers W. Tumor necrosis factor cytotoxicity is associated with phospholipase D activation. Eur J Biochem. 1993;212:491–497. doi: 10.1111/j.1432-1033.1993.tb17686.x. [DOI] [PubMed] [Google Scholar]
- Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC, Nussbaum RL. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking α-synuclein. Mol Cell Biol. 2005;25:10190–10201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Li S, Combs CK. β-Amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor α and NMDA receptors. J Neurosci. 2005;25:2566–2575. doi: 10.1523/JNEUROSCI.4998-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman EM, Frohman TC, Gupta S, de Fougerolles A, van den Noort S. Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer's disease. J Neurol Sci. 1991;106:105–111. doi: 10.1016/0022-510x(91)90202-i. [DOI] [PubMed] [Google Scholar]
- Golovko MY, Faergeman NJ, Cole NB, Castagnet PI, Nussbaum RL, Murphy EJ. Alpha-synuclein gene deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of alpha-synuclein palmitate binding. Biochemistry. 2005;44:8251–8259. doi: 10.1021/bi0502137. [DOI] [PubMed] [Google Scholar]
- Golovko MY, Rosenberger TA, Faergeman NJ, Feddersen S, Cole NB, Pribill I, Berger J, Nussbaum RL, Murphy EJ. Acyl-CoA synthetase activity links wildtype but not mutant a-synuclein to brain arachidonate metabolism. Biochemistry. 2006;45:6956–6966. doi: 10.1021/bi0600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Barton JA, Bourgoin S, Kusner DJ. Phospholipases D1 and D2 coordinately regulate macrophage phagocytosis. J Immunol. 2004;173:2615–2623. doi: 10.4049/jimmunol.173.4.2615. [DOI] [PubMed] [Google Scholar]
- Jenco JM, Rawlingson A, Daniels B, Morris AJ. Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by α- and β-synucleins. Biochemistry. 1998;37:4901–4909. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminals. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism Relat Disord. 2004;10:S3–S7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Yasojima K, McGeer EG. Inflammation in Parkinson's disease. Adv Neurol. 2001;86:83–89. [PubMed] [Google Scholar]
- McLean PJ, Ribich S, Hyman BT. Subcellular localization of alpha-synuclein in primary neuronal cultures: effect of missense mutations. J Neural Transm Suppl. 2000:53–63. doi: 10.1007/978-3-7091-6284-2_5. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol. 2003;170:3850–3858. doi: 10.4049/jimmunol.170.7.3850. [DOI] [PubMed] [Google Scholar]
- Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118:3523–3530. doi: 10.1242/jcs.02481. [DOI] [PubMed] [Google Scholar]
- Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK. Senile dementia of Lewy body type. A clinically and neuropathologically distinct form of Lewy body dementia in the elderly. J Neurol Sci. 1990;95:119–139. doi: 10.1016/0022-510x(90)90236-g. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C, Gorath M, Trojanowski JQ, Lee VM. alpha-Synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J Neurosci Res. 2000;62:9–14. doi: 10.1002/1097-4547(20001001)62:1<9::AID-JNR2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Shibayama-Imazu T, Okahashi I, Omata K, Nakajo S, Ochiai H, Nakai Y, Hama T, Nakamura Y, Nakaya K. Cell and tissue distribution and developmental change of neuron specific 14 kDa protein (phosphoneuroprotein 14) Brain Res. 1993;622:17–25. doi: 10.1016/0006-8993(93)90796-p. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Smith ME, Hoerner MT. Astrocytes modulate macrophage phagocytosis of myelin in vitro. J Neuoroimmunol. 2000;154:154–162. doi: 10.1016/s0165-5728(99)00218-0. [DOI] [PubMed] [Google Scholar]
- Sondag CM, Combs CK. Amyloid precursor protein mediates proin flammatory activation of monocytic lineage cells. J Biol Chem. 2004;279:14456–14463. doi: 10.1074/jbc.M313747200. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Ann NY Acad Sci. 2000;920:16–27. doi: 10.1111/j.1749-6632.2000.tb06900.x. [DOI] [PubMed] [Google Scholar]
- Tanji K, Mori F, Nakajo S, Imaizumi T, Yoshida H, Hirabayashi T, Yoshimoto M, Satoh K, Takahashi H, Wakabayashi K. Expression of beta-synuclein in normal human astrocytes. NeuroReport. 2001;12:2845–2848. doi: 10.1097/00001756-200109170-00018. [DOI] [PubMed] [Google Scholar]
- Tanji K, Mori F, Imaizumi T, Yoshida H, Matsumiya T, Tamo W, Yoshimoto M, Odagiri H, Sasaki M, Takahashi H, Satoh K, Wakabayashi K. Upregulation of alpha-synuclein by lipopolysaccharide and interleukin-1 in human macrophages. Pathol Int. 2002;52:572–577. doi: 10.1046/j.1440-1827.2002.01385.x. [DOI] [PubMed] [Google Scholar]
- Teismann P, Schulz JB. Cellular pathology of Parkinson's disease: astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318:149–161. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: evaluation of microglia and their functions. Brain Res Rev. 1992;1:61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo K, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer's disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Saitoh T, Mori H. Tissue-dependent alternative splicing of messenger-RNA for NACP, the precursor of non-A-β component of Alzheimer's disease. Biochem Biophys Res Commun. 1994;205:1366–1372. doi: 10.1006/bbrc.1994.2816. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]