Abstract
Previous studies have shown that cortical stimulation selectively activates extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation and immediate early gene expression in striatal GABAergic enkephalinergic neurons. In the present study, we demonstrate that blockade of adenosine A2A receptors with caffeine or a selective A2A receptor antagonist counteracts the striatal activation of cAMP–protein kinase A cascade (phosphorylation of the Ser845 residue of the glutamate receptor 1 subunit of the AMPA receptor) and mitogen-activated protein kinase (ERK1/2 phosphorylation) induced by the in vivo stimulation of corticostriatal afferents. The results indicate that A2A receptors strongly modulate the efficacy of glutamatergic synapses on striatal enkephalinergic neurons.
Keywords: caffeine, adenosine A2A receptor, striatum, phosphorylation, ERK1/2, AMPA receptor
Introduction
The striatum is the main input and information processing structure of the basal ganglia. Cortico-limbic-thalamic glutamatergic and mesencephalic dopaminergic systems converge in the GABAergic medium-sized spiny neurons, which constitute >90% of the striatal neuronal population (Gerfen, 2004). These are the striatal efferent neurons, which can be classified into GABAergic enkephalinergic and GABAergic dynorphinergic neurons. Enkephalinergic neurons predominantly express adenosine and dopamine receptors of the A2A and D2 subtype, respectively, whereas dynorphinergic neurons predominantly express adenosine and dopamine receptors of the A1 and D1 subtype, respectively (Ferré et al., 1997, 2005; Gerfen, 2004).
The dopamine D1 receptor is a Gs/olf-coupled receptor, whose main signaling pathway is the cAMP–protein kinase A (PKA) cascade. The D1 receptor modulates neuronal excitability and glutamatergic neurotransmission in the GABAergic dynorphinergic neuron by inducing PKA-mediated phosphorylation of different substrates, such as L-type voltage-dependent calcium channels, DARPP-32 (the dopamine and cyclic adenosine 3′, 5′-monophosphate-regulated phosphoprotein, 32 kDa), the NMDA and AMPA glutamate receptors (GluRs), and the nuclear constitutive transcription factor cAMP response element binding protein (for review, see Greengard et al., 1999). Furthermore, the D1 receptor can potentially activate mitogen-activated protein kinase (MAPK) [extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation] by PKA-dependent and -independent mechanisms (Zanassi et al., 2001), which seem to require a concomitant stimulation of NMDA receptors (Valjent et al., 2005).
The adenosine A2A receptor is functionally very similar to the D1 receptor. It is also a Gs/olf-coupled receptor whose activation stimulates the cAMP–PKA cascade. A2A receptor forms heteromers with D2 receptor, which antagonistically modulates A2A receptor function by inhibiting adenylyl cyclase activation (Kull et al., 1999; Hillion et al., 2002). Furthermore, stimulation of A2A receptor decreases the ability of dopamine to bind the D2 receptor by means of an intramembrane A2A–D2 receptor interaction (Ferré et al., 1991). The same as with the D1 receptor, A2A receptor-mediated PKA activation can potentially phosphorylate different substrates and induce MAPK activation. However, under basal conditions, there is a strong tonic activation of striatal D2 receptors, which impairs the ability of A2A receptors to signal through the cAMP–PKA cascade (for review, see Ferré et al., 2004, 2005).
Both enkephalinergic and dynorphinergic neurons receive corticostriatal afferents and display excitatory postsynaptic responses to corticostriatal stimulation (Kawaguchi et al., 1990). Nevertheless, corticostriatal stimulation selectively activates MAPK and immediate early gene (IEG) expression in the enkephalinergic neurons without activation in dynorphinergic neurons (Berretta et al., 1997; Gerfen et al., 2002). In fact, MAPK activation is responsible for the induction of striatal IEG expression after corticostriatal stimulation (Sgambato et al., 1998). In the present study, we demonstrate that blockade of A2A receptors with caffeine or a selective A2A receptor antagonist counteracts the striatal activation of cAMP–PKA cascade (phosphorylation of the Ser845 residue of the GluR1 subunit of the AMPA receptor) and MAPK (ERK1/2 phosphorylation) induced by the in vivo stimulation of corticostriatal afferents. The results support our hypothesis that strong cortico-limbic-thalamic input to the GABAergic enkephalinergic neuron allows the A2A receptor to override the inhibitory effects of the D2 receptor with significant activation of the cAMP–PKA cascade and MAPK (Ferré et al., 2004, 2005).
Materials and Methods
Electrical cortical stimulation.
Male Sprague Dawley rats (250–300 g; Charles River Laboratories, Wilmington, MA) were used in all experiments. Animals were maintained in accordance with the guidelines of the Institutional Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health. The animals were implanted unilaterally under Equithesin (National Institute on Drug Abuse Pharmacy, Baltimore, MD) anesthesia with bipolar stainless steel electrodes, 0.15 mm in diameter (Plastics One, Roanoke, VA), into the orofacial area of the lateral agranular motor cortex (3 mm anterior, 3 mm lateral, and 4.2 mm below bregma). The electrodes and a head holder (connected to a swivel during stimulation) were fixed on the skull with stainless steel screws and dental acrylic resin. Five days after surgery, rats were placed in individual bowl chambers, and the implanted electrodes were attached to an electrical stimulator (Grass S88K; Grass Instruments, West Warwick, RI). Ten minutes before cortical stimulation, the animals were given an intraperitoneal administration of saline, the A2A receptor antagonist MSX-3 (3,7-dihydro-8-[(1E)-2-(3-methoxyphenyl)ethenyl]-7-methyl-3-[3-(phosphonooxy)propyl-1-(2-propynyl)-1H-purine-2,6-dione]disodium salt hydrate) (3 mg/kg), the A1 receptor antagonist 8-cyclopentyl-1,3-dimethylxanthine (CPT) (4.8 mg/kg), or the nonselective adenosine antagonist caffeine (anhydrate base; 10 mg/kg). All drugs were purchased from Sigma (St. Louis, MO). All drugs were dissolved in sterile saline (with a few drops of 0.1N NaOH for MSX-3, final pH 7.4) and administered in a volume of 3 ml/kg of body weight. The doses of the adenosine antagonists were previously shown to provide motor activation by selectively antagonizing A1 receptors (CPT), A2A receptors (MSX-3), or both A1 and A2A receptors (caffeine) (Karcz-Kubicha et al., 2003). The parameters of stimulation were the same as those shown previously to induce selective phosphorylation of ERK1/2 in enkephalinergic cells of the projecting striatal area, the lateral caudate–putamen (Gerfen et al., 2002). After 10 min of habituation, biphasic current pulse trains (pulse of 0.1 ms; 150–200 μA, 100 Hz, 160 ms trains repeating once per second) were delivered using two coupled constant current units (Grass PSIU6; Grass Instruments). The intensity was 150 μA for most cases or it was increased up to 200 μA, until small jaw movements were observed. The cases that failed to show visible somatic movements were excluded from additional analysis. In no case did animals display evidence of seizure activity from the electrical stimulation. Stimulation was applied for 20 min, and the animals were killed immediately after the stimulation offset.
Immunohistochemistry.
Immediately after the offset of cortical stimulation, rats were deeply anesthetized with Equithesin and perfused transcardially with 0.1 m PBS, followed by 4% formaldehyde in 0.1 m sodium phosphate monobasic buffer, pH 7.4. Brains were postfixed in the same fixative for 2 h and immersed in 20% sucrose/0.1 m sodium phosphate, pH 7.4, solution for 48 h at 4°C. Twenty-micrometer-thick coronal sections were cut at the anteroposterior (AP) level of bregma 0.0 ± 1.0 mm in a Leica (Nussloch, Germany) CM3050S cryostat at −20°C, collected in PBS, and stored in antifreeze-buffered solution (20% ethylene glycol, 10% glycerol, and 10% sucrose in PBS) at −80°C until processing. The slices were then rinsed with PBS, incubated with 100 mm sodium borohydride and 0.1% hydrogen peroxide in PBS for 10 min, rinsed, and incubated in blocking buffer (PBS/0.1% Triton X-100/5% bovine serum albumin) for 2 h before incubation with rabbit polyclonal anti-phospho-Thr202/Tyr204 ERK1/2 (1:2000 dilution; Cell Signaling Technology, Danvers, MA) in blocking buffer for 24 h at 4°C. Sections were washed and incubated in 1:200 biotinylated goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA) for 2 h, then washed and incubated for 2 h in ABC reagent (PK-6100; Vector Laboratories), washed again, treated with 0.33 mg/ml 3,3′-diaminobenzidine, 50 mm ammonium nickel sulfate, and 0.003% peroxide until development (5–10 min), transferred to PBS, mounted onto chrom-alum gelatin-coated slides, air-dried, dehydrated through graded ethanols, cleared in xylene, and coverslipped with DPX (mixture of xylene and dibutyl phthalate; Fisher Scientific, Tustin, CA). Images were acquired using a digital camera (HRC; Zeiss, Gottingen, Germany) coupled to an Axioimager A1 microscope (Zeiss).
Western blotting.
Immediately after the offset of cortical stimulation, the animals were decapitated, and the brains were rapidly extracted, frozen in dry ice-cold isopentane, and stored at −80°C. Subsequently, unilateral tissue punches of the lateral striatum (16 gauge) at the AP level of bregma 0.0, corresponding to the area with maximal expression of phosphorylated ERK1/2 (Fig. 1), were obtained from ∼1 mm-thick coronal sections cut in the cryostat at −20°C. The rostral side of the coronal sections was localized approximately between bregma 0.5 mm and bregma −0.5 mm. For Western blot assays, tissue punches were sonicated for 10–15 s in sonication buffer (1% SDS in deionized sterile water). The volume of sonication buffer was 200 μl. The protein concentrations of all samples were determined using a bicinchoninic acid assay kit (Pierce, Rockford, IL) and were further diluted with 1% SDS to equalize the protein concentrations in each sample. Loading buffer (16% glycerol, 20% mercaptoethanol, and 0.05% bromophenol blue) was added to each sample (3:1, sample to loading buffer ratio) before heating at 95°C for 10 min. The proteins in the samples were separated by SDS-PAGE (10% acrylamide/0.27% N,N′-methylenebisacryalamide resolving gel) for 3–4 h at 200 V. For each electrophoresis run, 20 μl of sample (containing 8–10 μg of total protein depending on each particular run) were loaded in each well, and increasing amounts of protein pooled from the samples were also loaded and electrophoresed to produce a standard curve. Proteins were transferred electrophoretically to polyvinylidene fluoride Immobilon-P membranes (Millipore, Bedford, MA) at 0.3 A for 4 h. Membranes were washed four times for 15 min in blocking buffer: 2% polyvinylpyrrolidone in PBST (phosphate-buffered saline plus 0.05% Tween 20, pH 7.4) for phospho-ERK1/2 and phospho-GluR1 or 2% nonfat dry milk in PBST for total ERK1/2 and total GluR1. Membranes were then incubated overnight at 4°C with their primary antibody diluted in the respective blocking buffers plus 0.01% sodium azide. The antibodies used were rabbit polyclonal anti-GluR1 (1:2000 dilution; Chemicon, Temecula, CA), rabbit polyclonal anti-phospho-Ser845 GluR1 (1:2000 dilution; Upstate, Charlottesville, VA), rabbit polyclonal anti-ERK1/2 (1:2000 dilution; Cell Signaling Technology), and rabbit polyclonal anti-phospho-Thr202/Tyr204 ERK1/2 (1:5000 dilution; Cell Signaling Technology). After four washes for 15 min in blocking buffer, the blots were incubated for 2 h at room temperature with horseradish peroxidase-conjugated secondary goat anti-rabbit IgG antibody in blocking buffer (1:2000 dilution, PI-1000; Vector Laboratories). Finally, the blots were washed six times for 10 min in PBS and incubated during 60 s in the reagents of the enhanced chemiluminescence procedure of Amersham Biosciences (Piscataway, NJ). Luminescence from the blots was detected by exposing the membranes to Amersham Biosciences Hyperfilm during 30 s to 5 min, followed by digital scanning of the developed film in transparency mode. The scanned image of the membranes and band intensities were calibrated and quantified using NIH ImageJ software (version 1.34). The amount of the protein of interest in each sample was interpolated from the band intensities of the standard curves. The standard curves run with the Western blots in our assays indicate that the band intensities for each of the test samples quantified were within linear range of detection. For each animal, the values obtained from the experiments with phosphorylated and total ERK1/2 corresponded to the addition of the bands intensities of both ERK1 and ERK2 (also called p44 and p42 MAP kinases, in relation to their molecular weights, 44 and 42 kDa, respectively). For each animal, the values of phosphorylated ERK1/2 and phosphorylated GluR1 were normalized (as percentage of control) to total ERK1/2 and total GluR1 subunit of the AMPA receptor, respectively. In each Western blot, all values were normalized (as percentage of control) with respect to the average of the values from two vehicle-treated animals included in the same blot.
Figure 1.
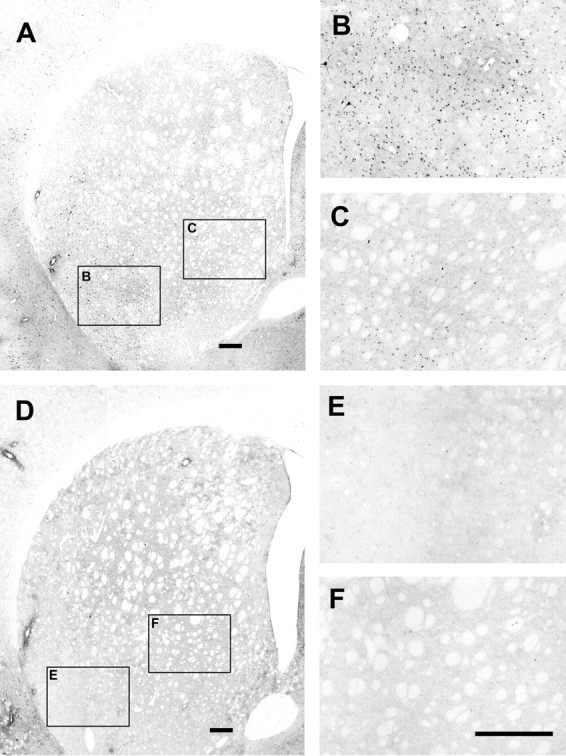
Striatal ERK1/2 phosphorylation induced by cortical electrical stimulation. A, D, Immunohistochemical localization of phosphorylated ERK1/2 in coronal sections (AP level of bregma 0.0) through the striatum ipsilateral to the implanted electrode from stimulated (A) and sham nonstimulated (D) animals. B, C, E, F, Higher magnification of the respective fields indicated in A and D, which shows cells displaying immunoreactivity to phospho-ERK1/2 antibody in the lateral caudate–putamen of the stimulated animals. Scale bars, 250 μm.
Results
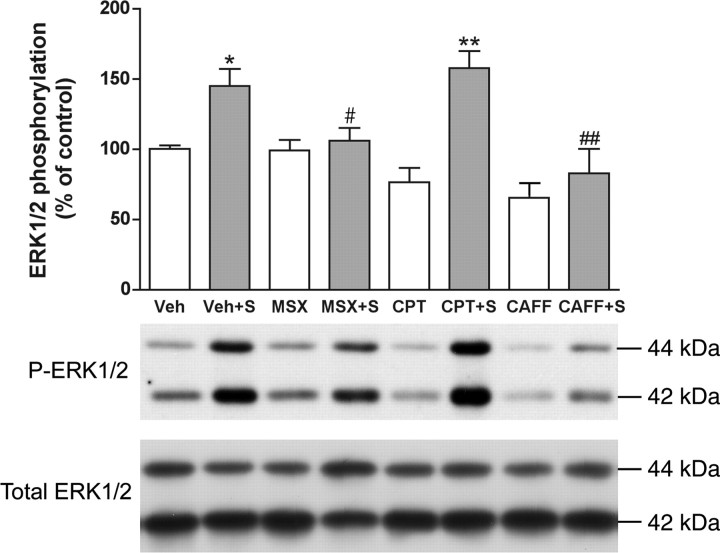
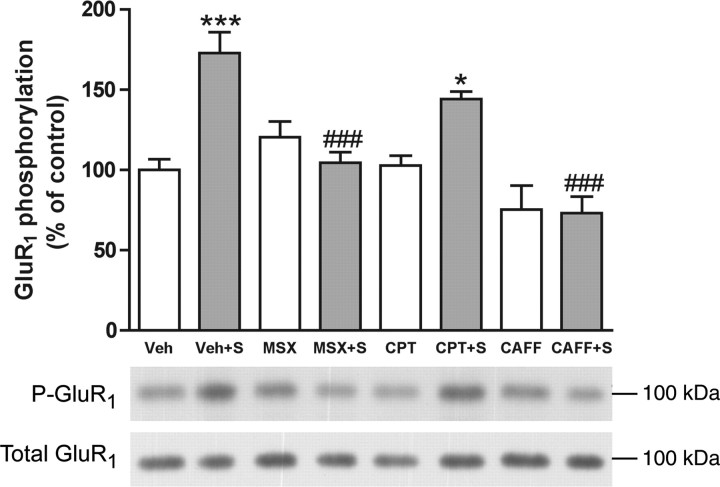
As reported previously (Sgambato et al., 1998; Gerfen et al., 2002), cortical stimulation in the orofacial area of the lateral agranular motor cortex (demonstrated by the selective elicitation of jaw movements) induced a predominant phosphorylation of ERK1/2 in the lateral caudate–putamen at the AP level of bregma 0.0 ± 1.0 mm (Fig. 1). Also as reported previously (Sgambato et al., 1998), ERK1/2 phosphorylation could also be observed in the contralateral striatum (data not shown). Western blot assays were therefore performed from punches of the lateral striatum corresponding to the area with maximal ERK1/2 phosphorylation. Cortical stimulation induced a significant 50% increase in ERK1/2 phosphorylation in the ipsilateral striatum compared with electrode-implanted nonstimulated controls (Fig. 2). This effect was significantly counteracted by the nonselective adenosine receptor antagonist caffeine and by the selective A2A receptor antagonist MSX-3 but not by the A1 receptor antagonist CPT (Fig. 2). None of the adenosine receptor antagonists produced any significant effect in sham nonstimulated animals (Fig. 2). Corticostriatal stimulation was also found to significantly increase phosphorylation of the Ser845 residue of the GluR1 subunit of the AMPA receptor in the ipsilateral striatum (∼75% increase compared with controls) (Fig. 3). Striatal AMPA receptor phosphorylation was also completely counteracted by caffeine or MSX-3, whereas CPT did not significantly modify AMPA receptor phosphorylation induced by cortical stimulation. Again, none of the adenosine receptor antagonists produced any significant effect in sham nonstimulated animals (Fig. 3). There were not significant differences in the values of total ERK1/2 and GluR1 subunit of the AMPA receptors between the different sham and stimulated groups.
Figure 2.
Striatal ERK1/2 phosphorylation induced by cortical electrical stimulation. Caffeine and the A2A receptor antagonist MSX-3, but not the A1 receptor antagonist CPT, counteract ERK1/2 phosphorylation induced by cortical electrical stimulation. Results are shown in means ± SEM (n = 6–10 per group) of representative Western blots. *p < 0.05 and **p < 0.01, significantly different compared with the vehicle (Veh)-treated group; #p < 0.05 and ##p < 0.01, significantly different compared with the stimulated vehicle-treated group (Veh+S) (ANOVA with Tukey–Kramer tests).
Figure 3.
Striatal GluR1 phosphorylation induced by cortical electrical stimulation. Caffeine and the A2A receptor antagonist MSX-3, but not the A1 receptor antagonist CPT, counteract GluR1 phosphorylation induced by cortical electrical stimulation. Results are shown in means ± SEM (n = 5–13 per group) and representative Western blots. *p < 0.05 and ***p < 0.001, significantly different compared with the vehicle (Veh)-treated group; ###p < 0.001, significantly different compared with the stimulated vehicle-treated group (Veh+S) (ANOVA with Tukey–Kramer tests).
Discussion
It can be assumed that there are two major distinct types of striatal glutamatergic synapses, the D1 receptor-regulated and the D2 (and A2A) receptor-regulated glutamatergic synapses, mostly localized at the heads of the dendritic spines of the GABAergic dynorphinergic and GABAergic enkephalinergic neurons, respectively (Ferré et al., 2005). Dopamine afferents make preferential synaptic contact with the neck of the dendritic spines (Gerfen, 2004). This arrangement allows mesencephalic dopaminergic inputs to modulate cortico-limbic-thalamic glutamatergic excitatory inputs. In the GABAergic dynorphinergic neuron, dopamine stimulates D1 receptors, which modulate neuronal excitability and glutamatergic neurotransmission by inducing PKA-mediated phosphorylation of different substrates, which include NMDA and AMPA receptors. PKA-mediated phosphorylation of NMDA receptors (in the C terminus of the NR1–1 subunit) potentiates NMDA receptor-mediated currents (Greengard et al., 1999), and there is also evidence for a tight crosstalk between the second-messenger pathways of D1 and NMDA receptors (Dudman et al., 2003). These functional interactions depend on the existence of physical interactions, heteromerization, between the D1 receptor and specific subunits of the NMDA receptor (Lee et al., 2002; Woods and Ferré, 2005). Coactivation of D1 and NMDA receptors induces MAPK activation (Valjent et al., 2005), although a significant phosphorylation of ERK1/2 can be obtained with selective stimulation of D1 receptors under conditions of chronic dopamine depletion (Gerfen et al., 2002; Kim et al., 2006). D1 receptor-mediated PKA activation can also induce phosphorylation at Ser845 of the GluR1 subunit of the AMPA receptor (Wolf et al., 2003), which, together with phosphorylation of ERK1/2, are critical initial steps in the establishment of plastic changes in excitatory synapses, involving recruitment of AMPA receptors to the postsynaptic density (Malinow and Malenka, 2002; Lee et al., 2003; Thomas and Huganir, 2004). In fact, D1 receptor-mediated AMPA receptor phosphorylation (at Ser845 of the GluR1 subunit) has been shown to be involved in the dopamine-mediated modulation of glutamate-dependent striatal synaptic plasticity (Wolf et al., 2003).
The D1 receptor-mediated mechanisms by which dopamine can potentiate the function and plasticity of glutamatergic synapses in the GABAergic dynorphinergic neuron cannot operate in the D2–A2A receptor-regulated glutamatergic synapse of the GABAergic enkephalinergic neurons, in which dopamine, by acting on D2 receptors, inhibits the cAMP–PKA cascade and MAPK (Ferré et al., 1997, 2004, 2005; Gerfen et al., 2002). In the GABAergic enkephalinergic neurons, it is adenosine, by acting on A2A receptors, that mimics the role of dopamine and D1 receptors on the glutamatergic synapses of GABAergic dynorphinergic neurons. A2A receptors are more concentrated in the striatum than anywhere else in the brain, and they are preferentially located both presynaptically and postsynaptically at glutamatergic synapses, in a strategic position to modulate corticostriatal input to the GABAergic enkephalinergic neurons (Hettinger et al., 2001; Ciruela et al., 2006). At the postsynaptic site, A2A receptors form heteromeric complexes with D2 and metabotropic glutamate mGlu5 receptors localized in the perisynaptic ring outside the synaptic cleft (Ferré et al., 2005). Costimulation of A2A and mGlu5 receptors exerts a synergistic effect on their ability to inhibit dopamine binding to the D2 receptor and on MAPK activation (Popoli et al., 2001; Ferré et al., 2002; Nishi et al., 2003). At the presynaptic site and inside the synaptic cleft, A2A receptors form heteromeric complexes with adenosine A1 receptors, which provide a switch mechanism by which low and high concentrations of adenosine inhibit and stimulate, respectively, glutamate release (Ciruela et al., 2006).
In the GABAergic enkephalinergic neurons, A2A receptor-mediated PKA activation can potentially phosphorylate the different substrates involved in synaptic plasticity. However, under basal conditions, endogenous dopamine exerts a strong tonic activation of D2 receptors in the striatum, which impairs the ability of A2A receptors to signal through the cAMP–PKA cascade. For instance, A2A receptor blockade does not produce a decrease in the basal PKA-mediated phosphorylation of GluR1 (at Ser845 of the GluR1 subunit), although it counteracts GluR1 phosphorylation induced by D2 receptor blockade (Hakansson et al., 2006). We have postulated that a strong cortico-limbic-thalamic glutamatergic input produces a sufficient amount of intrasynaptic glutamate and adenosine (most probably derived from ATP coreleased with glutamate), with a significant stimulation of presynaptic A2A receptors and postsynaptic perisynaptic A2A and mGlu5 receptors. Then, under conditions of strong glutamatergic input, adenosine and A2A receptors can provide a mechanism of facilitation of plastic changes in the excitatory synapses of the GABAergic enkephalinergic neurons (Ferré et al., 2005). The present results strongly support this hypothesis, because phosphorylation of ERK1/2 and GluR1 at Ser845 induced by stimulation of corticostriatal afferents was completely dependent on A2A receptor function. The results also indicate that caffeine, the most consumed psychoactive drug in the world, can potentially alter this form of striatal plasticity, which suggests that caffeine may be deleterious for the normal development of striatal function.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse, Department of Health and Human Services.
References
- Berretta S, Parthasarathy HB, Graybiel AM. Local release of GABAergic inhibition in the motor cortex induces immediate-early gene expression in indirect pathway neurons of the striatum. J Neurosci. 1997;17:4752–4763. doi: 10.1523/JNEUROSCI.17-12-04752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman JT, Eaton ME, Rajadhyaksha A, Macias W, Taher M, Barczak A, Kameyama K, Huganir R, Konradi C. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem. 2003;87:922–934. doi: 10.1046/j.1471-4159.2003.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci USA. 1991;88:7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA, Casado V, Fuxe K, Goldberg SR, Lluis C, Franco R, Ciruela F. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci USA. 2002;99:11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Canals M, Marcellino D, Burgueno J, Casado V, Hillion J, Torvinen M, Fanelli F, Benedetti Pd P, Goldberg SR, Bouvier M, Fuxe K, Agnati LF, Lluis C, Franco R, Woods A. Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism Relat Disord. 2004;10:265–271. doi: 10.1016/j.parkreldis.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Ferré S, Borycz J, Goldberg SR, Hope BT, Morales M, Lluis C, Franco R, Ciruela F, Cunha R. Role of adenosine in the control of homosynaptic plasticity in striatal excitatory synapses. J Integr Neurosci. 2005;4:445–464. doi: 10.1142/s0219635205000987. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Basal ganglia. In: Paxinos G, editor. The rat nervous system. Amsterdam: Elsevier Academic; 2004. pp. 445–508. [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Hakansson K, Galdi S, Hendrick J, Snyder G, Greengard P, Fisone G. Regulation of phosphorylation of the GluR1 AMPA receptor by dopamine D2 receptors. J Neurochem. 2006;96:482–488. doi: 10.1111/j.1471-4159.2005.03558.x. [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferré S, Fuxe K. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Muller CE, Fuxe K, Goldberg SR, Popoli P, Ferré S. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990;10:3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Palmiter RD, Cummins A, Gerfen CR. Reversal of supersensitive striatal dopamine D1 receptor signaling and extracellular signal-regulated kinase activity in dopamine-deficient mice. Neuroscience. 2006;137:1381–1388. doi: 10.1016/j.neuroscience.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Kull B, Ferré S, Arslan G, Svenningsson P, Fuxe K, Owman C, Fredholm BB. Reciprocal interactions between adenosine A2A and dopamine D2 receptors in Chinese hamster ovary cells co-transfected with the two receptors. Biochem Pharmacol. 1999;15:1035–1045. doi: 10.1016/s0006-2952(99)00184-7. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Nishi A, Liu F, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P. Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc Natl Acad Sci USA. 2003;100:1322–1327. doi: 10.1073/pnas.0237126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli P, Pezzola A, Torvinen M, Reggio R, Pintor A, Scarchilli L, Fuxe K, Ferré S. The selective mGlu5 receptor agonist CHPG inhibits quinpirole-induced turning in 6-hydroxydopamine-lesioned rats and modulates the binding characteristics of dopamine D2 receptors in the rat striatum: interactions with adenosine A2a receptors. Neuropsychopharmacology. 2001;25:505–513. doi: 10.1016/S0893-133X(01)00256-1. [DOI] [PubMed] [Google Scholar]
- Sgambato V, Pages C, Rogard M, Besson MJ, Caboche J. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci. 1998;18:8814–8825. doi: 10.1523/JNEUROSCI.18-21-08814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann NY Acad Sci. 2003;1003:241–249. doi: 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]
- Woods AS, Ferré S. Amazing stability of the arginine-phosphate electrostatic interaction. J Proteome Res. 2005;4:1397–1402. doi: 10.1021/pr050077s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanassi P, Paolillo M, Feliciello A, Avvedimento EV, Gallo V, Schinelli S. cAMP-dependent protein kinase induces cAMP-response element-binding protein phosphorylation via an intracellular calcium release/ERK-dependent pathway in striatal neurons. J Biol Chem. 2001;276:11487–11495. doi: 10.1074/jbc.M007631200. [DOI] [PubMed] [Google Scholar]