Abstract
Functional magnetic resonance imaging was used to study patients with chronic neuropathic pain involving the maxillary region (V2) of the trigeminal nerve in patients with spontaneous pain and evoked pain to brush (allodynia). Patients underwent two functional scans (2–3 months apart) with mechanical and thermal stimuli applied to the affected region of V2 and to the mirror site in the unaffected contralateral V2 region, as well as bilaterally to the mandibular (V3) division. Patients were stimulated with brush, noxious cold, and noxious heat. Significant changes were observed in regions within and outside the primary trigeminal sensory pathway. Stimulation to the affected (neuropathic) side resulted in predominantly frontal region and basal ganglia activation compared with the control side. The differences were consistent with the allodynia to brush and cold. A region of interest-based analysis of the trigeminal sensory pathway revealed patterns of activation that differentiated between the affected and unaffected sides and that were particular to each stimulus. Activation in the spinal trigeminal nucleus was constant in location for all pain stimuli. Activation in other brainstem nuclei also showed differences in the blood oxygenation level-dependent signal for the affected versus the unaffected side. Thus, sensory processing in patients with trigeminal neuropathic pain is associated with distinct activation patterns consistent with sensitization within and outside of the primary sensory pathway.
Keywords: brainstem, caudate, dorsal horn, facial, neuropathology, noxious, pain, peripheral nerve, phenotype, prefrontal cortex, somatosensory cortex, trigeminal
Introduction
Although the incidence of chronic facial neuropathic pain in the United States is not known, it is estimated to affect at least 5–10 individuals of 100,000 (Lipton et al., 1993; Marbach, 1999; Kitt et al., 2000). One study found that almost 22% of the adult (≥18 years old) population in the United States reported experiencing orofacial pain (face/cheek pain or burning mouth pain) more than once in the previous 6 months (Lipton et al., 1993). After a third molar tooth extraction (estimated to be >10 million/year in the United States, according to the American Dental Association), there is a 0.5–3% rate of phantom tooth pain (Marbach, 1993). Studies indicate that the incidence of persistent pain after endodontic treatment is ∼5% (Vickers and Cousins, 2000). Other studies indicate that this surgery may produce acute (3.57%) or persistent (0.91%) dysesthesias, or outright injury of the lingual nerve (2.1%) (Gulicher and Gerlach, 2000). A high incidence of paresthesias (>70%) after damage to branches of the trigeminal nerve (inferior alveolar, mental or lingual) has also been reported, and 20% of these patients reported suffering pain in the affected area (Sandstedt and Sorensen, 1995).
Facial pain encompasses a number of disorders, including those affecting the trigeminal nerve directly (trigeminal neuralgia, invasion by cancer, acute herpes zoster, postherpetic neuralgia, multiple sclerosis, cosmetic surgery); disorders affecting the sinuses (sinusitis), teeth, and gums; temporomandibular; vascular and headache disorders; and idiopathic pain. These conditions may be considered in three categories: (1) acute pain after injury; (2) chronic inflammatory pain (e.g., sinusitis); and (3) neuropathic pain (e.g., trigeminal neuralgia) (Eide and Rabben, 1998). Neuropathic facial pain has similarities to other neuropathic diseases and can be further classified into cases with a known cause (e.g., surgery or disease) and those with an unknown cause (e.g., classic trigeminal neuralgia, also known as tic doloureux). Trigeminal neuralgia, although technically a neuropathy, is characterized by little or no pain at rest, with intermittent paroxysms of shooting pain (Cheshire, 2005).
A few studies have reported changes in CNS processing in patients with neuropathic pain. Among the first were positron emission tomography (PET) studies in patients with postherpetic neuralgia (Iadarola et al., 1995). Subsequent PET studies looked at processing during brush-induced allodynia after nerve injury (Witting et al., 2006) and heat allodynia in a patient with neuropathic pain (Casey et al., 2003). Functional magnetic resonance imaging (fMRI) reports include investigations of complex regional pain syndrome (Maihofner and Handwerker, 2005), central pain from syringomyelia (Ducreux et al., 2006), mechanical allodynia in patients with neuropathic pain of central and peripheral origin (Peyron et al., 2004; Hofbauer et al., 2006), and phantom pain (Borsook et al., 1998).
Understanding changes in the CNS processing in human neuropathic pain should contribute to a better understanding of the neurobiology of the disease process, improve evaluation of surrogate models of neuropathic pain (Klein et al., 2005), and potentially offer methods for objective evaluation of pharmacological and other interventions. Imaging the trigeminal system allows for evaluation of activation in the trigeminal pathway, including the peripheral nerve input to the dorsal horn (spinal nucleus), projections from the spinal nucleus to the thalamus, and thalamocortical projections (DaSilva et al., 2002; Borsook et al., 2003). This methodology may be applied to patients with clinical conditions such as migraine, trigeminal neuralgia, or trigeminal neuropathy (Borsook et al., 2004).
In this study, we recruited patients with right-sided trigeminal neuropathy affecting the maxillary or V2 division of the trigeminal nerve. We evaluated the fMRI blood oxygenation level-dependent (BOLD) response in the trigeminal system and higher brain regions to mechanical (brush) and thermal (cold and warm/hot) stimulation to the affected and unaffected areas of the face. Our hypothesis was that painful trigeminal neuropathy is associated with sensitization in the trigeminal sensory pathway that would be observed as distinct alterations in the activation pattern in the trigeminal sensory and other CNS pathways.
Materials and Methods
Patient recruitment and consent
Subject recruitment and experimental protocols were approved by the Institutional Review Board at McLean Hospital. All subjects were recruited through advertisements placed in the local newspaper or at physicians' offices. In the latter case, subjects completed a form that provided permission to contact them. After a phone interview, subjects attended a screening session where they were explained the nature of the study, the subject's time commitment, and the types of painful stimuli that were going to be used. A full medical examination, medical history, and compliance with inclusion/exclusion criteria were performed before enrollment into the study. Subjects on pain medications were asked to discontinue use of their medication before their scheduled scanning sessions. Specifically, medications were discontinued for one dosing interval [i.e., the number of hours they were off depended on the longevity of their particular drug and dosage, e.g., 12 h for long-acting morphine sulfate (MS Contin), 6 h for oxycodone plus acetominophen (Percocet) or gabapentin (Neurontin)]. The subjects were asked to abstain from consuming any caffeinated beverages or foods containing caffeine 12 h before their scheduled scanning session(s) and to refrain from eating up to 6 h before their scanning session(s). Informed consent was explained and obtained by the study staff.
Facial pain mapping and testing
Each subject completed a figure showing the frontal and lateral aspects of a face to map spontaneous and evoked pain (see Fig. 1). To determine individual sensitivity to thermal stimuli, each subject participated in quantitative sensory testing (QST) before scanning. During the QST testing period, thermal pain thresholds were measured on the affected and the corresponding mirror V2 region on the unaffected side of the face. The pain thresholds to cold and heat for the affected side were used to determine the temperature of the stimuli the subject would receive in the scanner (see below, Imaging paradigm). Brush stimulation was also applied to the affected side of the face to measure pain response (brush allodynia).
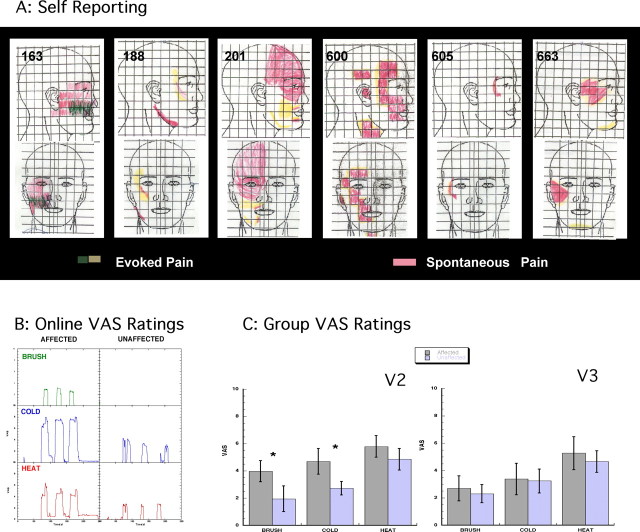
Figure 1.
A, Facial maps. Subjects were asked to draw on divided frontal and lateral views of the face the areas in which they experience spontaneous (red) and evoked (yellow/green) pain. This information was used to define the area for stimulation in the scanner. B, VAS on-line ratings. On-line VAS ratings for one subject in response to brush, cold, and heat applied to the affected and unaffected V2 areas are shown. The response to thermal stimuli in the affected area seemed to last longer and produced lingering pain not observed in the ratings of the unaffected area. C, Group results. VAS group results obtained from the on-line ratings for all subjects for V2A versus V2U (left) and all visits to brush, cold, and heat are shown. Brush and cold achieved statistically significant differences between sides (t test, *p < 0.05), but heat did not. V3 affected versus unaffected (right) stimulation produced ratings that were not significantly different to V2 unaffected. Error bars indicate SEM.
Experimental paradigm
The experimental paradigm (see supplemental Fig. 1, available at www.jneurosci.org as supplemental material) comprised two MRI scanning visits, with the second visit occurring 2–3 months after the first. There were two scan sets during each visit: the first set consisted of a series of anatomical scans, followed by functional scans. Four areas of the face were selected for sensory stimulation: the maxillary division of the trigeminal nerve of the pain-affected facial location (V2A), the mirror location on the unaffected contralateral side (V2U), and the mandibular division on both the affected (V3A) and unaffected (V3U) sides of the face. Sensory stimulation included brush, cold, and heat. One functional scan was acquired for each sensory stimulation applied to each selected area [i.e., a total of 12 functional scans (4 areas × 3 types of stimuli) were acquired during the second set]. After completion of the functional scanning, subjects were given the option of receiving a dose of their own prescribed pain medications to relieve some of the potential discomfort arising from sensory stimulation or received an intramuscular 10–30 mg dose of ketorolac (Toradol) or a lidocaine patch (Lidoderm), if the study physician determined it necessary. None of the subjects requested rescue medicines for any of the scanning sessions.
Imaging paradigm
Before stimuli administration, the affected region of the face (V2A), determined at prescreening, was marked with a water-soluble pen. This region was mirrored on the opposite unaffected side of the face. The V3 regions, nonoverlapping with the affected areas, were also marked on both sides (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). All regions on the affected and the unaffected side were tested with the stimuli before entering the scanner. A specially designed module that allowed for placement of thermal probes and the ability to apply brush stimuli to the specific regions was used. While in the scanner, subjects rated continuously the intensity of stimulus-evoked pain using a visual analog scale (VAS; 0 = no pain, 10 = maximum pain) as described previously (Borras et al., 2004).
Mechanical stimulation.
Brush stimuli, applied by a Velcro-topped (soft side) stick, were administered at 1–2 Hz (one to two strokes per second) within the marked regions. Stimuli were given three times, each for a period of 25 s separated by 30 s of no stimulus.
Thermal stimulation.
A Federal Drug Administration-approved Thermal Sensory Analyzer (MEDOC, Haifa, Israel) was used to deliver heat/cold stimuli through a probe that has been adapted to rest on the face. The probe is 1.6 × 1.6 cm, or about one-half the size of the thumb. For heat, stimuli were given at +1°C above their pain threshold value (see above, Facial pain mapping and testing) on the affected area of the face. For cold, stimuli were given at −1°C below their threshold value (see above, Facial pain mapping and testing). Stimuli were given three times, each for a period of 25 s separated by 30 s of no stimulus.
Imaging parameters
fMRI was performed in a 3.0 T Siemens (Erlangen, Germany) Trio scanner with a phased array coil. For anatomical localization, a magnetization-prepared rapid gradient echo sequence was used (1 × 1 × 1.6 mm resolution) (Ruggieri and Najm, 2001). Functional scans were acquired using a gradient echo echo-planar imaging sequence (Ruggieri and Najm, 2001) with an isotropic resolution of 3.5 mm; 41 slices (no-gaps) were prescribed along the brainstem axis, repetition time/echo time of 2.5 s/30ms were used, and 128 volumes were acquired per functional scan (see supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
Data analysis
The image analysis package fsl 3.2 (FMRIB, University of Oxford, UK; www.fmrib.ox.ac.uk/fsl) was used for most data processing. Head-motion correction, data smoothing (5 mm Gaussian kernel), and prewhitening was done using fsl tools. For comparison of affected versus unaffected sides, the unaffected images were flipped left–right to match images from the affected side. The general linear model was used (fsl) for statistical analysis; we combined all subjects and both visits to report aggregate results using a fixed-effects model. A fixed-effects model was used because of the small cohort (Friston et al., 1999; Worsley, 2001)
Statistical maps were inspected for each individual to rule out results biased by a small number of highly active subjects. Thresholds were determined from a partition of the statistical map distribution using a generalized mixture model approach (Pendse et al., 2006) that deconstructs the overall statistical map into several optimized Gaussian distributions. Each voxel is associated with a vector of probabilities of association with each of the modeled Gaussian distributions. The map for the distribution associated with activation (or deactivation) was thresholded at a probability of p > 0.5 (meaning that the chances of a voxel belonging to that cluster was >0.5); the corresponding z-value in the original z distribution was obtained from matching the center of the activation distribution and used as threshold for determining activated voxels. Tables 2–4 depict activation with coordinates of the most active voxel in Montreal Neurological Institute (MNI) space.
Table 2.
Brush V2 Affected versus Unaffected
| Region | Zmax | Coordinates |
||
|---|---|---|---|---|
| x | y | z | ||
| Positive activation | ||||
| MFG | ||||
| BA46 | 3.38 | 38 | 48 | 28 |
| BA 45 | 3.45 | 48 | 34 | 28 |
| IFG (BA 47) | 3.21 | −30 | 28 | −4 |
| ACG | ||||
| BA 32 | 2.56 | 4 | 36 | 14 |
| BA 32 | 2.88 | 4 | 44 | 26 |
| BA 24 | 2.33 | 2 | 16 | 30 |
| Ins | 3.44 | 42 | 10 | 6 |
| STG (BA 22) | 3.22 | 60 | 6 | −4 |
| SI (BA 2) | 3.22 | 50 | −28 | 42 |
| Th | 2.55 | 18 | −22 | 14 |
| GP | 2.65 | −22 | −6 | −4 |
| Put | 2.50 | −18 | 8 | −4 |
| Negative activation | ||||
| Parahip | −3.28 | −24 | −8 | −28 |
| ITG (BA 19) | −3.34 | −32 | −6 | −46 |
| Caudate | −3.26 | −6 | 8 | 12 |
| Th | −3.13 | −4 | −16 | 0 |
| Ins | −2.92 | 42 | −10 | −2 |
| −3.58 | −36 | −32 | 20 | |
| PCG (BA 23) | −3.19 | 16 | −52 | 26 |
Zmax, Maximum Z value; BA, Brodmann's areas; Ins, insula; STG, superior temporal gyrus; Th, thalamus; PCG, posterior cingulate gyrus; ACG, anterior cingulate gyrus; Put, putamen; ITG, inferior temporal gyrus; Parahip, parahippocampal gyrus.
Table 3.
Cold V2 affected versus unaffected
| Region | Zmax | Coordinates |
||
|---|---|---|---|---|
| x | y | z | ||
| Positive activation | ||||
| MFG (BA 10) | 3.91 | −40 | 46 | −14 |
| 3.17 | 38 | 44 | 14 | |
| ACG (BA 32) | 3.25 | 10 | 44 | 4 |
| 3.06 | 10 | 26 | 30 | |
| 3.84 | −8 | 14 | 36 | |
| STG (BA 38) | 3.08 | 56 | 12 | −12 |
| 2.67 | −50 | 16 | −12 | |
| GP | 3.39 | −24 | −8 | −4 |
| 3.91 | 20 | 4 | −2 | |
| Th | 2.89 | 14 | −16 | 4 |
| IPL (BA 40) | 3.28 | 58 | −40 | 24 |
| 3.19 | −56 | −48 | 28 | |
| Cereb | 3.21 | 24 | −66 | −30 |
| 3.47 | −18 | −60 | −28 | |
| Negative activation | ||||
| GOb (BA 11) | −3.43 | −24 | 40 | −10 |
| Caudate | −2.59 | 14 | 20 | 10 |
| −2.61 | −18 | 20 | 10 | |
| Hip | −2.41 | 26 | 6 | −24 |
Zmax, Maximum Z value; BA, Brodmann's areas; ACG, anterior cingulated gyrus; STG, superior temporal gyrus; Th, thalamus; IPL, inferior parietal lobe; Hip, hippocampus; Cereb, cerebellum.
Table 4.
Heat V2 affected versus unaffected
| Region | Zmax | Coordinates |
||
|---|---|---|---|---|
| x | y | z | ||
| Positive activation | ||||
| SFG (BA 8) | 3.18 | 4 | 30 | 62 |
| PrCG (BA 6) | 2.61 | −56 | 0 | 16 |
| STG (BA 38) | 2.74 | 34 | 22 | −36 |
| 3.41 | 52 | 14 | −26 | |
| IPL (BA 40) | 3.24 | 58 | −28 | 20 |
| Ins | 3.38 | 40 | 8 | −12 |
| Negative activation | ||||
| IFG (BA 47) | −3.74 | −34 | 34 | −16 |
| MFG (BA 8) | −3.29 | 28 | 24 | 46 |
| Caudate | −3.37 | −8 | 14 | 8 |
| Parahip (BA 36) | −3.01 | 30 | −10 | −36 |
| −2.99 | −36 | −6 | −48 | |
| −2.62 | −24 | −12 | −18 | |
| PCG (BA 31) | −3.37 | 10 | −46 | 38 |
| −3.21 | −6 | −46 | 36 | |
Zmax, Maximum Z value; BA, Brodmann's areas; STG, superior temporal gyrus; Ins, insula; IPL, inferior parietal lobe; Parahip, parahippocampal gyrus; PCG, posterior cingulate gyrus; SFG, superior frontal gyrus; PrCG, precentral gyrus.
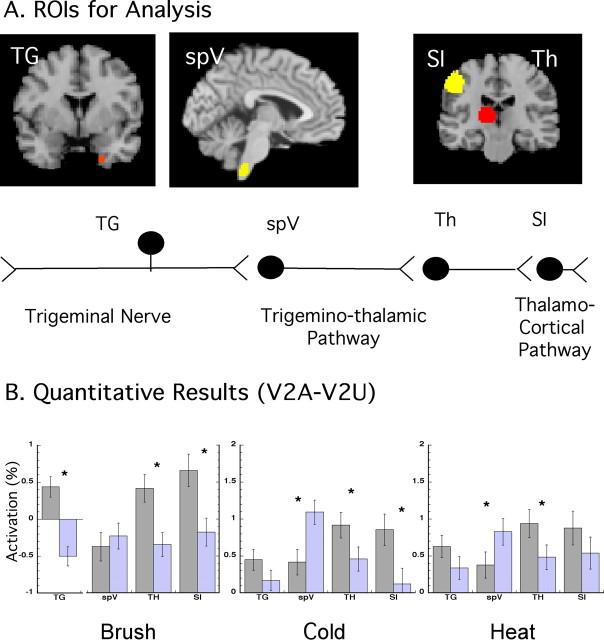
Region of interest-based analysis.
In addition, region of interest (ROI) analysis was performed on each subject for the sensory pathway including the trigeminal ganglion (TG), spinal trigeminal nucleus (spV), thalamus (Th), and somatosensory cortex (SI). All individual structural and functional statistical sets were transformed into the MNI template. ROIs were drawn individually on each subject's anatomical scan and registered to their functional scans. Registration was inspected to assure proper alignment, especially at the level of the medulla. The TG was localized using an approach we have used previously (Borsook et al., 2004). The spV was identified as described by us previously (DaSilva et al., 2002), and the thalamus and SI were found after standard brain landmarks. ROIs were used to mask average percentage signal change images. For this report only, two stimulus sites were considered for analysis, V2A and V2U. Percentage signal changes for ROIs were then averaged across the ROI, and its SEM was calculated. t tests were used to determine significant differences.
Brainstem activation.
Activation in the brainstem comparing brush, cold, and heat was determined for each stimulus by inspection of thresholded statistical maps and visual localization with the atlas (Duvernoy et al., 1995). The resulting activations were displayed on three orthogonal planes (coronal, sagittal, and axial); for clarity, cerebrum activation (shown in other figures) was masked out.
Results
Patients and psychophysical results
Patients
We screened 31 potential subjects (by phone and physical examination), of which 6 subjects with right-sided V2 (maxillary) neuropathy were recruited to the study. All patients had evoked pain to brush stimuli of >4/10 and spontaneous pain of >4/10. Each subject was scanned in two separate sessions. Subject demographics are listed in Table 1. Note that most of the subjects were women, and all premenopausal women were scanned during the midfollicular phase (days 5–7) of their menstrual cycle (Table 1). Each subject underwent a physical examination before the study. An example of data collected on each subject is provided in the supplemental material (supplemental Clinical Case History and Evaluation, available at www.jneurosci.org). Figure 1A shows self-drawn maps of each patient's pain in frontal and lateral views. Note that although all subjects had involvement of the V2 region, some also had pain in other regions of the trigeminal system ipsilateral to the affected V2. Only the V2 area with allodynia was stimulated.
Table 1.
Patient demographics
| Subject number | Age | Sex | Affected side | Origin of pain | Diagnosis | Pain medications |
|---|---|---|---|---|---|---|
| 163 | 54 | F | Right V2 | Face pain after antibiotic treatment for strep throat (2000) | Idiopathic facial neuropathy | No meds for pain |
| 188 | 39 | Fa | Right V2 | Face pain after car accident (2002) | Traumatic facial neuropathy | Tylenol |
| 201 | 57 | F | Right V1/V2/V3 | Face pain after herpes zoster (2003) | Post-herpetic neuralgia | Acyclovir, Neurontin |
| 600 | 48 | Fa | Right V1/V2 | Face pain after ski accident (1994) | Traumatic facial neuropathy | Buproprion, sertraline, Tylenol #3 |
| 605 | 54 | M | Right V2 | Face pain after herpes zoster (2003) | Post-herpetic neuralgia | No meds for pain |
| 663 | 41 | Fa | Right V2/V3 | Pain after car accident (1997) | Traumatic facial neuropathy | Vicodin, Paroxetine, Klonopin |
F, Female; M, male.
aPremenopausal females scanned on days 5–9 (follicular phase) of their menstrual cycles.
Psychophysical measures
Spontaneous and evoked pain ratings at screening.
Pain was rated on a VAS. The group average spontaneous pain rating was 7.7 ± 0.6 (mean ± SEM), and the reported previous history of pain evoked by stimuli (e.g., touching the area, clothing, etc.) was 7.2 ± 0.42. During screening on the initial visit, the average pain to a brush stimulus (mechanical allodynia) to the affected V2 was 4.8 ± 0.62.
Threshold temperature testing.
At the beginning of each visit, thresholds for cold and heat pain were determined on the affected V2 side (V2A) and the mirror site on the unaffected V2 side (V2U). For cold pain threshold, the group (mean ± SEM) temperature on the affected side was 28.5 ± 0.8°C (scan 1) and 27.8 ± 2.7°C (scan 2); on the unaffected side, the values were 18.2 ± 2.7°C (scan 1) and 18.0 ± 3.2°C (scan 2). Similarly, for heat pain, the thresholds on the affected side were 37.7 ± 0.6°C (scan 1) and 39.2 ± 0.5°C (scan 2), and 43.7 ± 1.2°C (scan 1) and 44.0 ± 1.0°C (scan 2) on the unaffected side. Temperature differences were significant between affected and unaffected sides for cold and heat. Furthermore, no significant differences in cold or heat thresholds were found for either V2A or V2U between visits 1 and 2.
VAS pain ratings.
An example of on-line VASs recorded during the experiments for one subject (188) is presented in Figure 1B. Brush allodynia in the affected V2 was clearly reported by the subject, whereas no pain was reported during brush stimulation of the contralateral side. Similarly, for this subject, increased sensitivity was observed for cold and heat on the affected side but not on the unaffected side. Note that after the heat stimulus to the affected area, the VAS score indicated that some pain persisted after each stimulus; this was more pronounced in some subjects. In contrast, stimulation of the unaffected area elicited responses that coincided with the duration of the stimulus.
In Figure 1C, the average maximum response (i.e., maximum VAS for at least 5 s during the stimulation for each stimulus was obtained) for the three stimuli for all patients and visits is presented. Brush and cold produced significantly different VAS scores on the affected (V2A) and unaffected (V2U) sides, but there was no significant difference between the two sides in the response to heat. There was no significant difference in reported pain when brush versus cold and cold versus heat on the affected side were compared, but reported pain on the affected side was significantly higher after the heat stimulus compared with the brush stimulus (Fig. 1C). There were no significant differences between the two sides in the VAS scores reported after brush, cold, or heat applied to the V3 region. These results indicate our ability to perform brush, cold, and heat stimulation of the face in the scanner to examine the allodynic response with imaging.
Imaging results
The imaging results are presented below in five sections: Activation in the trigeminal pathway (group results and ROI-based results), Activation outside the trigeminal pathway (brainstem and other higher brain regions), Modality (stimulus)-specific activation, Differences (contrast) in activation, and Reproducibility of results between visits.
Activation in the trigeminal pathway
Activation in the trigeminal sensory pathway (TG→spV→thalamus→SI) after mechanical and thermal stimuli was evaluated using two approaches: inspection of the group results and an ROI-based approach (see Materials and Methods for details).
Group results
Figure 2 shows averaged statistical maps of activation along the trigeminal pathway after stimulation on the V2A. Note that activation was present in the TG, spV, thalamus, and SI as noted by the squares and circles in the figure. All stimuli produced painful responses, and the activation patterns were similar at all levels of the trigeminal pathway regardless of stimulus, although brush appeared to produce greater activation in all areas. This parallels the VAS scores for brush-induced allodynia. Significant activation in the TG and spV was induced after V2A stimulation (p < 0.05, uncorrected) (Fig. 2), similar to that previously reported after V2 stimulation in healthy subjects (DaSilva et al., 2002; Borsook et al., 2003). Similar regions along the trigeminal pathway showed activation after V2U stimulation (data not shown). Quantitative differences revealed by ROI analysis are discussed in the next section.
Figure 2.
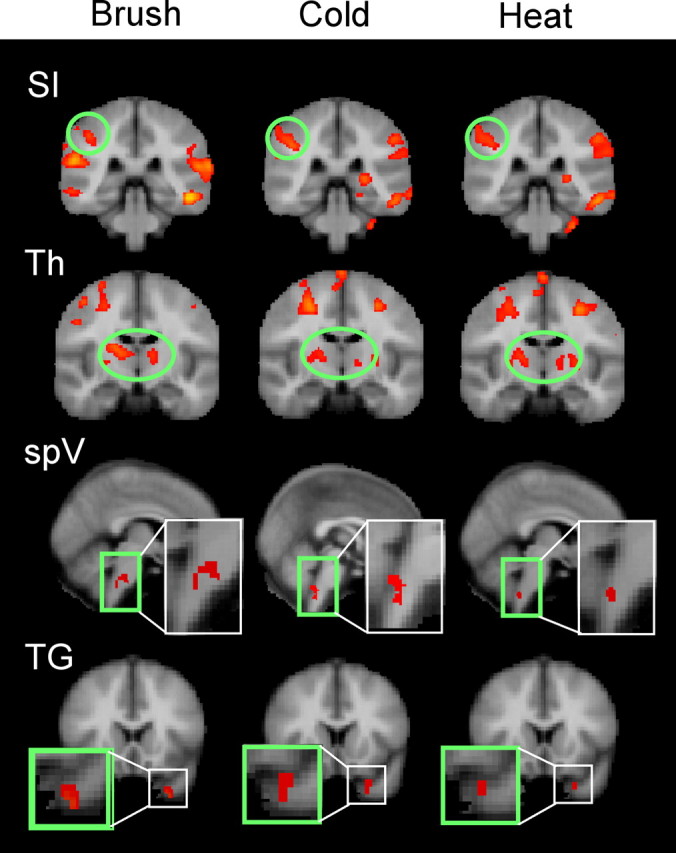
Group analysis for the trigeminal pathway. Specific activations along the trigeminal pathway (SI, thalamus, spV, and TG) are presented for the three stimuli. SI displayed similar activation across the three stimuli; the thalamus seemed to have a more medial activation with brush compared with heat and cold. Aggregate image analysis of the TG and trigeminal nuclei did not meet statistical threshold. Subthreshold activation was seen for spV in brush, but only spV for heat and cold. The TG activated similarly for the three stimuli. Quantified analysis through an ROI-based analysis is shown in Figure 3. Th, Thalamus.
After stimulation to the V2 area, only ipsilateral activation was observed in either the TG or spV as reported previously (Borsook et al., 2003). Contralateral TG or spV did not activate, suggesting that artifacts from blood vessels or autonomic changes did not contribute to the observed activations. Bilateral activation was seen in the thalamus to both V2A and V2U for brush and cold but only to V2A for heat. Contrast analysis (V2A vs V2U) revealed greater thalamic activation on the contralateral side (Tables 2, 3). SI activation was observed in V2A for all of the stimuli, but significantly only for brush applied to V2U. In the V2A versus V2U analysis, only SI activation to brush appeared significantly different.
ROI-based results
To quantify differences, an ROI approach was implemented as described in Materials and Methods. Figure 3A shows the ROIs used in the analysis. Figure 3B displays the results for each ROI expressed as percentage signal change (±SEM) for affected (gray bars) and unaffected (light purple bars) areas. Greater activation (or a trend toward greater activation) after stimulation of the affected side was seen for most stimuli in all areas except the spV. In the spV, significant differences between affected and unaffected sides were only observed after the thermal stimuli, which induced less activation after stimulation of the affected side than the unaffected side. In the TG, significant changes were only observed for brush, although the pattern of activation was similar for cold and heat (V2A > V2U). Brush stimulation to the affected V2 produced greater activation in the TG than brush to the unaffected side as observed previously (decreased activation) (Borsook et al., 2003).
Figure 3.
ROI-based analysis for trigeminal pathway. A, ROI-based approach analysis. The location of the ROIs from which z values were extracted to perform an ROI-based analysis (see Materials and Methods) is shown. B, The bar graphs indicate mean differences in percentage signal change (with SEM) for each ROI for the three stimuli. TG was significantly more active in V2A for the three stimuli. The spV was more active in the unaffected area, and the SI and thalamus were more active on V2A than V2U not achieving significance SI for heat. See ROI-based results. Th, Thalamus.
Activation outside the trigeminal sensory pathway
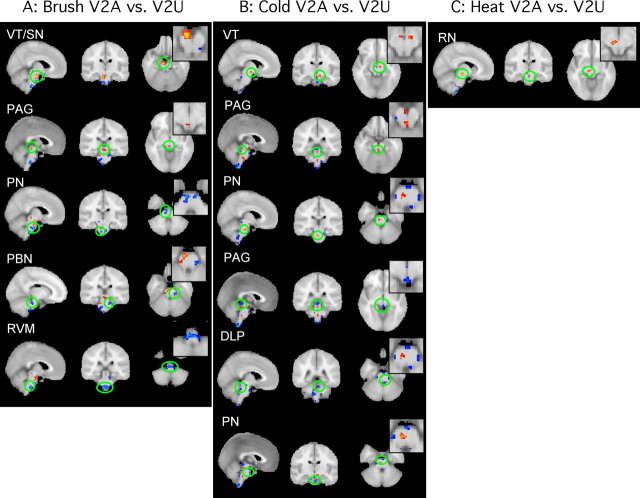
Brainstem activation
Figure 4A–C shows contrast maps for activation for each stimulus [brush (A), cold (B), and heat (C)] for V2A versus V2U. Red indicates that activation was greater after stimulation to V2A than V2U, and blue indicates that activation after stimulation to V2A was less than V2U. Activations in the brainstem were focal. Significant differences between V2A versus V2U in brainstem nuclei were observed for each stimulus. After brush (Fig. 4A), increased activation was observed in the ventral tegmentum/substantia nigra (VT/SN) and the periaqueductal gray (PAG), whereas decreased activation was observed in the pontine nuclei (PN), the parabrachial nuclei, and the rostroventral medulla. After cold (Fig. 4B), a larger number of areas were activated compared with brush. Increased activation was seen in the VT and PN (the latter was opposite to that observed for brush). Activation in the PAG was increased in the more rostral portion and decreased in a more caudal location. We have previously observed both increased and decreased activation in the PAG (Becerra et al., 2001) to a noxious heat stimulus. Decreases were observed in the dorsolateral pons and PN, but in a different location from the regions of increased activation. The V2A versus V2U comparison revealed that relatively few areas were differentially activated on the two sides by heat stimuli (Fig. 4C), with changes only seen in the red nucleus (RN).
Figure 4.
Brainstem activation. Brainstem activation differences of V2A versus V2U for the three stimuli are shown. Images are presented in three planes with a zoom-in image for axial slices. A, Activation differences to brush stimulation. B, Activation differences to cold stimulation, C, Activation differences to heat stimulation. Red-yellow, V2A > V2U; blue, V2A < V2U; PBN, parabrachial nucleus; RVM, rostral ventral medulla; DLP, dorsolateral pons.
Higher brain regions
Results are shown for activated regions and maximum activations and coordinates are presented for brush (supplemental Table 1A,B and Table 2), cold (supplemental Table 2A,B and Table 3), and heat (supplemental Table 3A,B and Table 4) for the affected side (supplemental Tables 1A, 2A, 3A), unaffected side (supplemental Tables 1B, 2B, 3B), and differences (Tables 2–4) after identical stimulation of affected and unaffected sides with each of the three stimuli (supplemental material is available at www.jneurosci.org).
Modality-specific differences in activation
Activation in affected and unaffected regions
Supplemental Tables 1A,B, 2A,B, and 3A,B show areas of the brain with significant changes in activation after brush, cold, and heat stimuli, respectively, applied to the V2A and V2U sides of the face. Figure 5 is a summary of the results.
Figure 5.
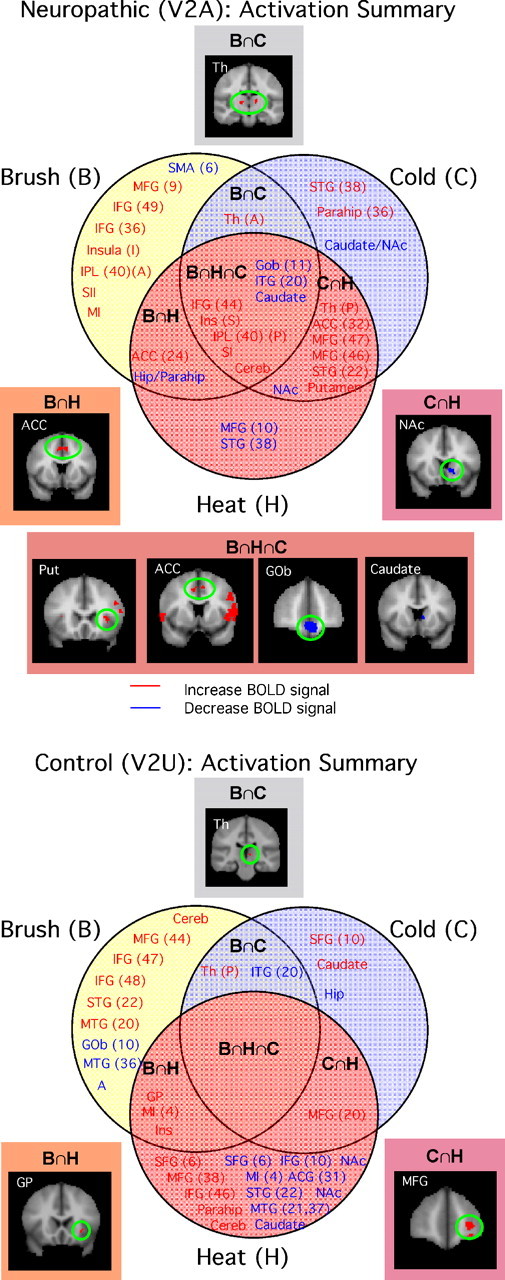
Summary of regions activated by brush, cold, and heat. A summary of the aggregate image results for V2A and V2U according to a Venn diagram is shown. Top, Neuropathic: significant activation was found in common for the three stimuli. Note also that significant commonality between heat and cold stimuli was observed. See Activation in affected and unaffected regions. Bottom, Control: activation in V2U to the three stimuli resulted in very little overlap between stimuli and not all among the three of them. See text. STG, Superior temporal gyrus; IPL, inferior parietal lobe; SII, secondary somatosensory cortex; MI, motor cortex; Th, thalamus; Parahip, parahippocampal gyrus; ITG, inferior temporal gyrus; MTG, medial temporal gyrus; PCG, posterior cingulate cortex; A, amygdala; Hip, hippocampus; Cereb, cerebellum; Put, putamen. Letters in parentheses after structure label: S, superior; I, inferior; A, anterior; P, posterior.
After stimulation of the affected side, regional activation could be segregated into seven groups of activation (Fig. 5, top): activations that were only present for each specific stimulus [brush (B), cold (C), and heat (H)], for brush and heat (B∩H), for brush and cold (B∩C), for cold and heat (C∩H), and for areas that were common to all three stimuli (B∩C∩H). In the same figure, examples of activations for specific regions [e.g., nucleus accumbens (NAc), anterior cingulate cortex (ACC), and thalamus (Th)] within each cluster are shown. Note that with V2A stimulation, there were more unique activations after brush than after the thermal stimuli (eight regions, compared with three unique regions for cold and two unique regions for heat). Overlap was most prominent for cold and heat stimulation [seven regions, compared with one region for cold and brush (B∩C) and two regions for heat and brush (B∩H)], and eight regions were activated after any of the three stimuli (B∩H∩C) (Fig. 6A, bottom left). Additional segregation was seen for increased activation shown in red and decreased activation shown in blue. These results indicate differences in CNS sensory processing based on the modality of the stimulus (i.e., dynamic mechanical vs thermal).
Figure 6.
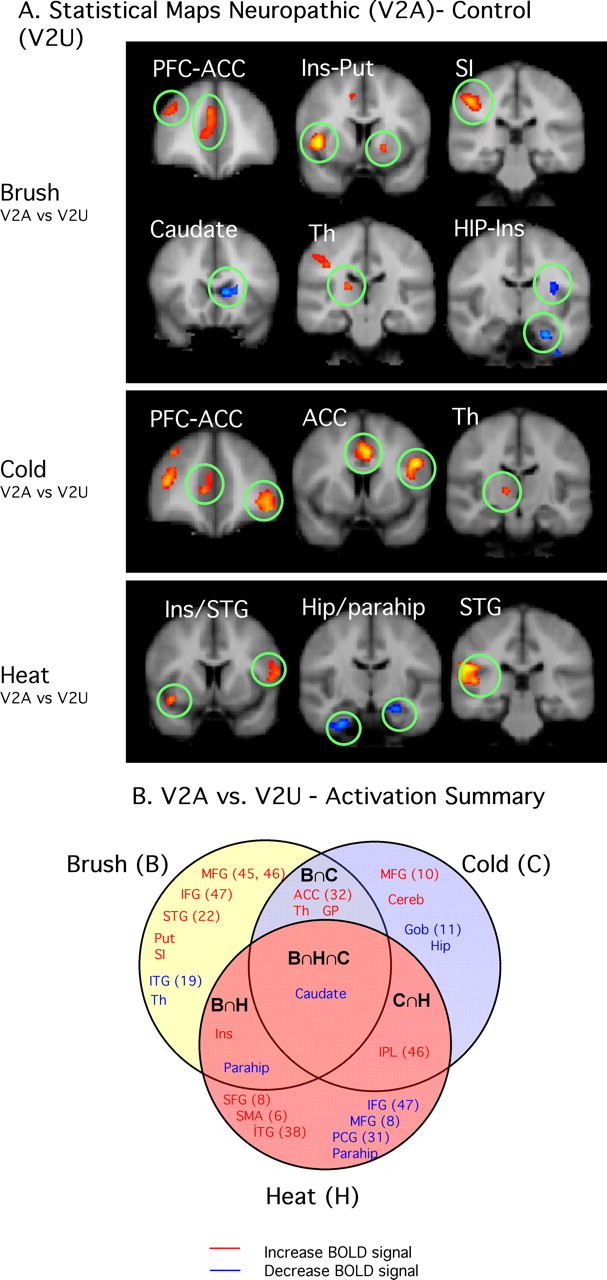
Summary of regions showing differences between neuropathic and control sides. A, Contrast maps for the three stimuli applied to the affected area (V2A vs V2U). Brush displayed significant differences in sensory/discriminative and emotional/cognitive areas, whereas cold and heat seem to have main differences in nonsensory pathways. See text. B, Venn diagram depicting commonalities of contrasts for the three stimuli. Caudate was found to commonly activate more in the affected versus the unaffected area. See the legend to Figure 5 for the list of abbreviations.
A similar summary of the data were produced for the unaffected side for the three stimuli. Brush to V2U uniquely activated nine brain regions, all different from those activated by V2A stimulation. Note that cold stimulation of the unaffected side produced only six regions of activation in the brain (Fig. 5, bottom, and supplemental Table 2B, available at www.jneurosci.org as supplemental material). Only two regions were activated by both cold and brush (B∩C). Heat stimulus to the unaffected side induced activation uniquely in 15 regions (the same stimulus on the neuropathic side induced activation in just 2 regions). Also note that some regions were consistently activated (e.g., decreased activation in the NAc to heat occurred with stimulation to either side). When stimuli were applied to the unaffected side, there were no regions that were activated commonly by each of the three stimuli (B∩H∩C). It is unclear whether these changes reflect the baseline state (i.e., healthy) or activation on a sensitized brain because of the ongoing chronic pain from the peripheral trigeminal injury.
Differences in activation in affected versus unaffected V2
Tables 2–4 show differences in activation in brain regions after brush, cold, and heat stimuli, respectively, applied to the V2 affected (neuropathic) versus the V2 unaffected (control) sides of the face. This is summarized in Figure 6. Examples of activation differences are shown in Figure 6A, and the Venn diagram (Fig. 6B) summarizes regional activation according to each specific sensory stimulus. As seen in the diagram, brush activated eight regions uniquely, two regions common with heat (B∩H), three regions with cold (B∩C), and one region with both heat and cold (B∩H∩C); cold activated four regions uniquely and one with heat; heat activated seven regions uniquely. Brush and cold showed significant differences in VAS scores at the time of scanning. The areas activated can be grouped into cortical regions including the frontal lobe [middle frontal gyrus (MFG), inferior frontal gyrus (IFG), and orbital gyrus (GOb)], ACC, insula, superior temporal gyrus, and hippocampus and subcortical regions including the basal ganglia [putamen, caudate nucleus, globus pallidus (GP)] and thalamus These regions/circuits differentiate the neuropathic state for cold and brush. The major differences were in the frontal lobe, where brush activated more inferior regions, whereas cold activated the orbitofrontal cortex. Both brush and cold activated the more rostral component of the cingulate cortex, the more affective region of the structure. In the basal ganglia, the caudate was activated by both brush and cold, whereas brush activated the putamen as well. The inferior temporal gyrus was uniquely activated by brush in the neuropathic state versus control. Although heat showed no difference in VAS for neuropathic versus control in the on-line measures, there were significant differences in pain thresholds. In terms of activation differences after heat for affected versus unaffected, a number of regions were differentially activated (Fig. 6B), mainly frontal (superior frontal gyrus, IFG, and MFG).
Test–retest psychophysical and imaging results
Reproducibility of psychophysical and imaging results between imaging visit 1 and 2 are shown in Figure 7A, in which VAS reports to brush, cold, and heat are compared between visits 1 and 2. No statistical differences were found between the two visits for any area of the face stimulated by any of the three types of stimuli used. Similarly, imaging data did not indicate significant differences between visits 1 and 2 when comparing the response to thermal and mechanical stimuli in the V2 affected area (Fig. 7B). The only areas exhibiting statistically significant differences in activation between visits were located mostly in white matter or ventricles and hence have no functional significance. Although patients reported some differences in their spontaneous pain (background pain), VAS scores for evoked pain did not vary significantly from one visit to the next one (see above, Psychophysical measures).
Figure 7.
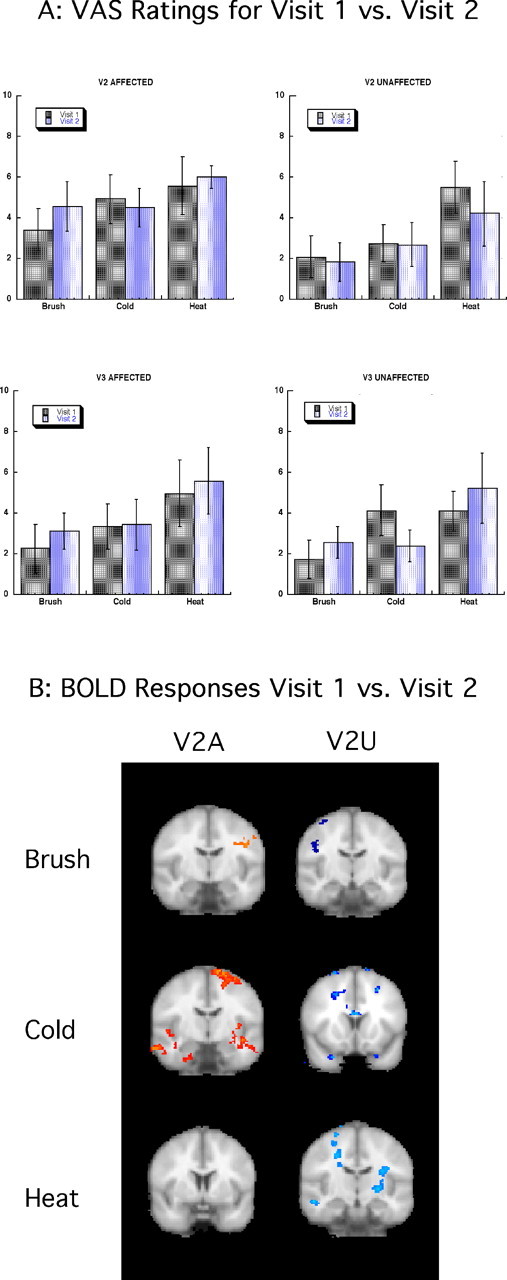
Reproducibility of results: visit 1 versus visit 2. A, VAS ratings for visits 1 and 2. VAS scores for all the stimulated areas for all stimuli are presented indicating no significant differences for on-line VAS scores between sessions. Error bars indicate SEM. B, BOLD responses for visits 1 and 2. fMRI results showed no significant differences between sessions. Activations are located, for the most part, in white-matter tracts.
Discussion
The results indicate that fMRI can detect significant changes in activation along the trigeminal sensory pathway, in the brainstem, and in higher brain regions in the neuropathic pain state. The patient cohort included individuals with a very similar focal neuropathic pain problem, albeit of different etiologies, that was regionally localized and with at least two common underlying mechanistic pain pathologies: ongoing (spontaneous) chronic pain with minimal intensity and mechanical allodynia to brush (evoked pain) (Woolf et al., 2003). The responses to brush, cold, and heat were analyzed at two levels: changes in the trigemino–thalamic–cortical pathway and changes in extra trigeminal sensory pathways including brainstem and higher centers (cortical, subcortical).
Activation in the primary trigeminal sensory pathway
TG
The three stimuli we used are known to activate distinct sets of sensory fibers: brush activates Aβ fibers, cold allodynia activates Aδ fibers, and noxious heat activates both C and Aδ fibers. Thus, despite the fact that all three stimuli can elicit subjective pain from the same stimulus site, they may well show distinct patterns of activation. Brush to the V2A region induced a significant increase in signal in the TG and a decreased signal in the V2U as observed previously in healthy volunteers (Borsook et al., 2003). Changes in V2A may be a result of abnormal input from Aβ fibers or cutaneous mechano-nociceptors as a consequence of nerve damage affecting V2 input (Koltzenburg and Handwerker, 1994). Both thermal stimuli also showed a trend toward greater activation in the TG after stimulation to the V2A field than to the mirror V2U field, but it did not reach significance in this sample size. These changes may also reflect alterations in fiber function after nerve damage, because afferent input for both cold and heat produced consistent central changes in other areas (see below).
Trigeminal Nucleus (spV)
We observed no significant changes in signal in the spV after brush to the affected or unaffected side, but noxious cold and heat produced significant changes with greater activation in spV after stimulation of V2U than of V2A. Brush clearly produced significant changes in the thalamus and cortex, and the lack of changes observed in spV may indicate that input to the thalamus and cortex occurs through alternate pathways including the main sensory nucleus. The observed changes in activation induced in the ipsilateral medulla by noxious cold or heat occurs in the location of V2 representation in the brainstem, as reported previously for a similar stimulus site for noxious heat in healthy subjects (DaSilva et al., 2002).
Thalamus and cortex (SI)
The observed increases in activation after brush stimulation to V2A versus V2U correlates well with the psychophysical responses indicating allodynia in V2A, the predominant presenting symptom in this group of patients. Cold and heat both produced activation in the thalamus and SI cortex that was significantly greater after stimulation of V2A (with the exception of activation in the SI after heat to V2A, which trended toward an increase over V2U, but not significantly). The increases are consistent with the psychophysical results indicating allodynia for cold but not for heat (as noted in Results, VAS scores after heat did not reveal significant heat allodynia) (Fig. 1C). The lack of heat allodynia and of significant changes in the SI after heat may be attributable to loss of fibers or may be because the stimulus temperature was too low to elicit thermal allodynia. The trends observed suggest that a higher temperature may be required to produce significant differences in the response to heat stimulation to the two sides. Alternatively, “after sensations” [after discharges presumably from central sensitization (Eide and Rabben, 1998)], only seen after heat to the V2A, may have resulted in a smaller difference in the BOLD response between baseline and activated state.
Bilateral activation, more pronounced contralaterally, was observed in the thalamus (see Results). Bilateral activation after stimuli applied to one side of the body has been reported in a number of brain structures including the thalamus, to either experimental pain (Coghill et al., 1999; Witting et al., 2001; Bingel et al., 2002) or clinical pain (Albuquerque et al., 2006) conditions.
Activation outside the primary trigeminal sensory pathway: brainstem
Previous work reported changes in the brainstem after painful electrical stimuli in healthy subjects (Dunckley et al., 2005). Here, a number of brainstem regions showed differences in activation to stimuli that may differentiate CNS processing of neuropathic pain from processing of normal pain. Four structures showed activation that was greater after stimulation to V2A than to V2U: the VT and PAG after brush, the PN after cold, and the RN after heat. The VT, previously reported in other imaging studies of noxious heat (Becerra et al., 2001), contains dopamine neurons and projects to multiple brain regions including the hypothalamus, nucleus accumbens, amygdala, and prefrontal regions (Oades and Halliday, 1987). Neuropathic pain may drive dopaminergic-related functions such as reward; thus, the observed sensitization may reflect ongoing dopaminergic drive that produces a diminished effect on normal reward function, similar to the tachyphylaxis seen with some dopaminergic drugs (Ozaki et al., 2002; Narita et al., 2004). Both brush and cold activated the PAG, a structure known to be involved in descending modulation of pain (Willis and Westlund, 1997; Ren and Dubner, 2002). The increased signal to brush allodynia and cold hyperalgesia may indicate a decreased threshold of activation in the structure, although no differences were seen between unaffected and affected sides when the heat stimulus was used. Again, the lack of differences in VAS reports by the subjects during the heat stimulus suggests simply that a higher temperature is necessary to see heat hyperalgesia in these patients. Activation in PN increased in response to cold only. Involvement in these areas has been observed in rat models of neuropathic pain (Mao et al., 1993) and in human imaging studies of healthy subjects (Dunckley et al., 2005). Finally, we observed significant unilateral activation in the RN after heat stimulus to the V2A (Fig. 4), similar to that reported after experimental visceral and somatic pain in healthy subjects (Bingel et al., 2002; Dunckley et al., 2005) and in paroxysmal hemicrania (Matharu et al., 2006). A role for the RN in neuropathic pain is unknown, but it has been suggested to be involved in withdrawal behavior or execution of movements (Keifer and Houk, 1994) and the sensorimotor integration of nociceptive information involves via a spino-rubral circuit (Steffens et al., 2000).
Activation outside the primary trigeminal sensory pathway: higher centers
A number of salient differences were observed in the activity seen in CNS regions after stimulation of the V2A and V2U. The first is that in the neuropathic state, the main differences are changes in the frontal lobes, basal ganglia for brush and cold allodynia evoked through stimulation of V2A specifically. PET studies of heat allodynia in a model of capsaicin-induced pain sensitization in humans reported frontal lobe changes (Lorenz et al., 2002), suggesting that the brain may recognize unique features of pain. Our findings indicate frontal lobe activation for allodynia, with some segregation depending on the modality of the stimulus (i.e., cold, heat, or brush). In a similar study, capsaicin-induced heat hyperalgesia induced activation in the frontal (MFG, IFG) cortices (Maihofner and Handwerker, 2005). Although the latter group also evaluated punctate hyperalgesia, we are unaware of any study evaluating brush, cold, and heat in either a surrogate model of hyperalgesia/allodynia or in patients with neuropathic pain. One recent PET study of neuropathic pain reported differences in CNS activation to brush-induced allodynia compared with the contralateral normal skin (Witting et al., 2006). They reported no changes in the thalamus or cingulate but did report changes in the GOb, which we observed after brush, cold, and heat stimulation (Fig. 5, Tables 2–4). The differences in the studies may relate to differences in the experimental paradigm (e.g., PET vs fMRI) and the site of the stimulus.
A surprising finding was our observation of activation in the basal ganglia in the neuropathic versus the control group. As shown in Results, brush, cold, and heat produced decreased activation in the caudate nucleus, brush increased activation in the putamen, and both brush and cold increased activation in the GP. No such activations have been reported in the capsaicin models (Maihofner and Handwerker, 2005), nor in complex regional pain syndrome (Maihofner et al., 2006). However, we and others have reported activation in the nucleus accumbens and/or putamen in response to noxious heat (Becerra et al., 2001; Aharon et al., 2006) and noxious cold (Tracey et al., 2000) in healthy subjects. The role of the basal ganglia in pain processing has been reviewed (Barker, 1988; Bernard et al., 1992; Chudler and Dong, 1995) but is not well understood. One fMRI study has indicated somatotopic mapping in the putamen using laser-evoked pain stimuli (Bingel et al., 2004), which suggests that such systems may be involved in motor responses to pain information. Our data suggest that activation across subdivisions of these structures may further differentiate the neuropathic pain condition from controls. Based on their afferent and efferent connections, particularly with thalamic and cortical regions, the basal ganglia are thought to be involved in sensorimotor, associative, and limbic functions (Rolls, 1994; Nakano et al., 2000; Herrero et al., 2002; Afifi, 2003; McHaffie et al., 2005). Thus, this system may play a role in the regulation of responses to cortical information, reinforcement of behavior, and attention (Flaherty and Graybiel, 1994; Herrero et al., 2002).
Caveats
There are a number of caveats to consider including sensory differences in etiology of pain, ongoing spontaneous pain, and medication issues. These are discussed in detail in the supplemental material (Supplemental caveats, available at www.jneurosci.org).
Conclusions
Our results indicate that both trigeminal sensory and emotional circuits are altered in the neuropathic state. In addition to changes observed along the trigeminal pathway, other regions including frontal areas, insula, parahippocampal regions, and the anterior cingulate (affective component) are differentially activated after stimulation to the neuropathic (V2A) versus control (V2U) sides of the face. These changes appear stable because they can be reproduced within each subject on repeated assessments. The results suggest that it may be possible to define an fMRI “fingerprint” that could be used as an objective diagnostic for neuropathic pain.
Footnotes
This work was supported by an unrestricted grant from GlaxoSmithKline (GSK) and by National Institutes of Health (NIH) Grant NS042721 (D.B.). In addition, portions of this work were presented at the Society for Neuroscience 2005 Annual Meeting. We thank Leanne Nasser, Megan Wardrop, and Perry Renshaw (NIH Grant NS1R01DA14178) for their help. We are grateful to Pauline Williams (GSK) for supporting this study.
References
- Afifi AK. The basal ganglia: a neural network with more than motor function. Semin Pediatr Neurol. 2003;10:3–10. doi: 10.1016/s1071-9091(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Aharon I, Becerraa L, Chabris CF, Borsook D. Noxious heat induces fMRI activation in two anatomically distinct clusters within the nucleus accumbens. Neurosci Lett. 2006;392:159–164. doi: 10.1016/j.neulet.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Albuquerque RJ, de Leeuw R, Carlson CR, Okeson JP, Miller CS, Andersen AH. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: an fMRI study. Pain. 2006;122:223–234. doi: 10.1016/j.pain.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Barker RA. The basal ganglia and pain. Int J Neurosci. 1988;41:29–34. doi: 10.3109/00207458808985739. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99:313–321. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Bingel U, Glascher J, Weiller C, Buchel C. Somatotopic representation of nociceptive information in the putamen: an event-related fMRI study. Cereb Cortex. 2004;14:1340–1345. doi: 10.1093/cercor/bhh094. [DOI] [PubMed] [Google Scholar]
- Borras MC, Becerra L, Ploghaus A, Gostic JM, DaSilva A, Gonzalez RG, Borsook D. fMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. J Neurophysiol. 2004;91:2723–2733. doi: 10.1152/jn.00249.2003. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Fishman S, Edwards A, Jennings CL, Stojanovic M, Papinicolas L, Ramachandran VS, Gonzalez RG, Breiter H. Acute plasticity in the human somatosensory cortex following amputation. NeuroReport. 1998;9:1013–1017. doi: 10.1097/00001756-199804200-00011. [DOI] [PubMed] [Google Scholar]
- Borsook D, DaSilva AF, Ploghaus A, Becerra L. Specific and somatotopic functional magnetic resonance imaging activation in the trigeminal ganglion by brush and noxious heat. J Neurosci. 2003;23:7897–7903. doi: 10.1523/JNEUROSCI.23-21-07897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Burstein R, Becerra L. Functional imaging of the human trigeminal system: opportunities for new insights into pain processing in health and disease. J Neurobiol. 2004;61:107–125. doi: 10.1002/neu.20085. [DOI] [PubMed] [Google Scholar]
- Casey KL, Lorenz J, Minoshima S. Insights into the pathophysiology of neuropathic pain through functional brain imaging. Exp Neurol. 2003;184([Suppl 1]):S80–S88. doi: 10.1016/j.expneurol.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Cheshire WP. Trigeminal neuralgia: diagnosis and treatment. Curr Neurol Neurosci Rep. 2005;5:79–85. doi: 10.1007/s11910-005-0003-6. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain. 1995;60:3–38. doi: 10.1016/0304-3959(94)00172-B. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, Borsook D. Somatotopic activation in the human trigeminal pain pathway. J Neurosci. 2002;22:8183–8192. doi: 10.1523/JNEUROSCI.22-18-08183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducreux D, Attal N, Parker F, Bouhassira D. Mechanisms of central neuropathic pain: a combined psychophysical and fMRI study in syringomyelia. Brain. 2006;129:963–976. doi: 10.1093/brain/awl016. [DOI] [PubMed] [Google Scholar]
- Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM, Bourgouin P, Cabanis EA, Cattin F, Guyot J, Iba-Zizen MT, Maeder P, Parratte B, Tatu L, Vuillier F, Vannson JL. The human brain stem and cerebellum. In: Duvernoy HM, editor. Surface, structure, vascularization, and three-dimensional anatomy with MRI. Ed 2. New York: Springer/Wein; 1995. pp. 35–40. [Google Scholar]
- Eide PK, Rabben T. Trigeminal neuropathic pain: pathophysiological mechanisms examined by quantitative assessment of abnormal pain and sensory perception. Neurosurgery. 1998;43:1103–1110. doi: 10.1097/00006123-199811000-00055. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Input–output organization of the sensorimotor striatum in the squirrel monkey. J Neurosci. 1994;14:599–610. doi: 10.1523/JNEUROSCI.14-02-00599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? NeuroImage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Gulicher D, Gerlach KL. Incidence, risk factors and follow-up of sensation disorders after surgical wisdom tooth removal. Study of 1,106 cases. Mund Kiefer Gesichtschir. 2000;4:99–104. doi: 10.1007/s100060050178. [DOI] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Hofbauer RK, Olausson HW, Bushnell MC. Thermal and tactile sensory deficits and allodynia in a nerve-injured patient: a multimodal psychophysical and functional magnetic resonance imaging study. Clin J Pain. 2006;22:104–108. doi: 10.1097/01.ajp.0000149798.93498.7c. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Max MB, Berman KF, Byas-Smith MG, Coghill RC, Gracely RH, Bennett GJ. Unilateral decrease in thalamic activity observed with positron emission tomography in patients with chronic neuropathic pain. Pain. 1995;63:55–64. doi: 10.1016/0304-3959(95)00015-K. [DOI] [PubMed] [Google Scholar]
- Keifer J, Houk JC. Motor function of the cerebellorubrospinal system. Physiol Rev. 1994;74:509–542. doi: 10.1152/physrev.1994.74.3.509. [DOI] [PubMed] [Google Scholar]
- Kitt CA, Gruber K, Davis M, Woolf CJ, Levine JD. Trigeminal neuralgia: opportunities for research and treatment. Pain. 2000;85:3–7. doi: 10.1016/s0304-3959(99)00310-3. [DOI] [PubMed] [Google Scholar]
- Klein T, Magerl W, Rolke R, Treede RD. Human surrogate models of neuropathic pain. Pain. 2005;115:227–233. doi: 10.1016/j.pain.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Handwerker HO. Differential ability of human cutaneous nociceptors to signal mechanical pain and to produce vasodilatation. J Neurosci. 1994;14:1756–1765. doi: 10.1523/JNEUROSCI.14-03-01756.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115–121. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Cross D, Minoshima S, Morrow T, Paulson P, Casey K. A unique representation of heat allodynia in the human brain. Neuron. 2002;35:383–393. doi: 10.1016/s0896-6273(02)00767-5. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO. Differential coding of hyperalgesia in the human brain: a functional MRI study. NeuroImage. 2005;28:996–1006. doi: 10.1016/j.neuroimage.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO, Birklein F. Functional imaging of allodynia in complex regional pain syndrome. Neurology. 2006;66:711–717. doi: 10.1212/01.wnl.0000200961.49114.39. [DOI] [PubMed] [Google Scholar]
- Mao J, Mayer DJ, Price DD. Patterns of increased brain activity indicative of pain in a rat model of peripheral mononeuropathy. J Neurosci. 1993;13:2689–2702. doi: 10.1523/JNEUROSCI.13-06-02689.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbach JJ. Is phantom tooth pain a deafferentation (neuropathic) syndrome? Part I: Evidence derived from pathophysiology and treatment. Oral Surg Oral Med Oral Pathol. 1993;75:95–105. doi: 10.1016/0030-4220(93)90413-x. [DOI] [PubMed] [Google Scholar]
- Marbach JJ. Medically unexplained chronic orofacial pain. Temporomandibular pain and dysfunction syndrome, orofacial phantom pain, burning mouth syndrome, and trigeminal neuralgia. Med Clin North Am. 1999;83:691–710. doi: 10.1016/s0025-7125(05)70130-9. [DOI] [PubMed] [Google Scholar]
- Matharu MS, Cohen AS, Frackowiak RS, Goadsby PJ. Posterior hypothalamic activation in paroxysmal hemicrania. Ann Neurol. 2006;59:535–545. doi: 10.1002/ana.20763. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J Neurol. 2000;247:V1–V15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Imai S, Narita M, Ozaki S, Kishimoto Y, Oe K, Yajima Y, Yamazaki M, Suzuki T. Molecular mechanism of changes in the morphine-induced pharmacological actions under chronic pain-like state: suppression of dopaminergic transmission in the brain. Life Sci. 2004;74:2655–2673. doi: 10.1016/j.lfs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Narita M, Iino M, Sugita J, Matsumura Y, Suzuki T. Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: implication of the reduction in mu-opioid receptor functions in the ventral tegmental area. J Neurochem. 2002;82:1192–1198. doi: 10.1046/j.1471-4159.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- Pendse G, Borsook D, Becerra L. A generalized mixture modeling approach applied to the problem of thresholding fMRI statistical maps. Soc Neurosci Abstr. 2006;32:492–5. [Google Scholar]
- Peyron R, Schneider F, Faillenot I, Convers P, Barral FG, Garcia-Larrea L, Laurent B. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63:1838–1846. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Neurophysiology and cognitive functions of the striatum. Rev Neurol (Paris) 1994;150:648–660. [PubMed] [Google Scholar]
- Ruggieri PM, Najm IM. MR imaging in epilepsy. Neurol Clin. 2001;19:477–489. doi: 10.1016/s0733-8619(05)70027-x. [DOI] [PubMed] [Google Scholar]
- Sandstedt P, Sorensen S. Neurosensory disturbances of the trigeminal nerve: a long-term follow-up of traumatic injuries. J Oral Maxillofac Surg. 1995;53:498–505. doi: 10.1016/0278-2391(95)90055-1. [DOI] [PubMed] [Google Scholar]
- Steffens H, Rathelot JA, Padel Y. Effects of noxious skin heating on spontaneous cell activity in the magnocellular red nucleus of the cat. Exp Brain Res. 2000;131:215–224. doi: 10.1007/s002219900279. [DOI] [PubMed] [Google Scholar]
- Tracey I, Becerra L, Chang I, Breiter H, Jenkins L, Borsook D, Gonzalez RG. Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2000;288:159–162. doi: 10.1016/s0304-3940(00)01224-6. [DOI] [PubMed] [Google Scholar]
- Vickers ER, Cousins MJ. Neuropathic orofacial pain part 1–prevalence and pathophysiology. Aust Endod J. 2000;26:19–26. doi: 10.1111/j.1747-4477.2000.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting N, Kupers RC, Svensson P, Arendt-Nielsen L, Gjedde A, Jensen TS. Experimental brush-evoked allodynia activates posterior parietal cortex. Neurology. 2001;57:1817–1824. doi: 10.1212/wnl.57.10.1817. [DOI] [PubMed] [Google Scholar]
- Witting N, Kupers RC, Svensson P, Jensen TS. A PET activation study of brush-evoked allodynia in patients with nerve injury pain. Pain. 2006;120:145–154. doi: 10.1016/j.pain.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Borsook D, Koltzenburg M. Mechanistic approach to the diagnosis of pain. In: Bountra C, Munglani R, Schmidt WK, editors. Pain, current understanding, emerging therapies, and novel approaches to drug discovery. New York: Dekker; 2003. pp. 1–8. [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. Oxford: Oxford UP; 2001. [Google Scholar]