Abstract
Neurons in the middle temporal visual area (MT) have been implicated in the perception of visual motion, whereas prefrontal cortex (PFC) neurons have been linked to temporary storage of sensory signals, attentional and executive control of behavior. Using a task that placed demands on both sets of neurons, we investigated their contribution to working memory for visual motion. Monkeys compared the direction of two moving random-dot stimuli, sample and test, separated by a brief memory delay. Neurons in both areas showed robust direction-selective activity during all phases of the task. During the sample, ∼60% of task-related PFC neurons were direction selective, and this selectivity emerged 40 ms later than in MT. Unlike MT, the PFC responses to sample did not correlate with behavioral choices, but their selectivity was modulated by task demands and diminished on error trials. Reliable directional signals were found in both areas during the memory delay, but these signals were transient rather than sustained by neurons of either area. Responses to the test in both areas were modulated by the remembered sample direction, decreasing when the test direction matched the sample. This decrease arose in the PFC 100 ms later than in MT and was predictive of the forthcoming decision. Our data suggest that neurons in the two regions are functionally connected and make unique contributions to different task components. PFC neurons reflect task-related information about visual motion and represent decisions that may be based, in part, on the comparison in MT between the remembered sample and test.
Keywords: extrastriate cortex, prefrontal cortex, same/different task, single-cell recordings, random dots, direction discrimination, working memory, delay activity
Introduction
As we interact with our environment, the features of objects in the visual scene are not consistently present on the retina. As a result, sensory cues used to guide visual behavior are not always available. Thus, continuity of visual experience requires a short-term storage mechanism. This type of storage used to guide subsequent action is often referred to as working memory (Baddeley, 1986). Despite the temporal proximity of sensory processing and storage and the fact that they deal with the same sensory signals, they have often been treated as separate processes. In recent years, the evidence has emerged that the two processes are closely intertwined and that the elemental sensory attributes are represented by segregated memory systems that include cortical areas processing these attributes (Fuster, 1997; Pasternak and Greenlee, 2005; Postle, 2006).
The work presented here is aimed at identifying and characterizing the components of the circuitry underlying processing and retention of one of the fundamental visual attributes: visual motion. To that end, we compared the behavior of neurons in the dorsolateral prefrontal cortex (PFC), a region often linked to temporary storage of sensory signals (Goldman-Rakic, 1995; Miller and Cohen, 2001), with the behavior of motion processing neurons in the middle temporal (MT) area, during a task requiring identification, retention, and retrieval of information about motion direction. Although PFC neurons have been shown to represent complex visual patterns (Miller et al., 1996; Rainer and Miller, 2002), little is known about the way they handle information about behaviorally relevant visual motion (Kim and Shadlen, 1999). On the other hand, the role of area MT in processing of visual motion and signaling it to the animal is well established (Newsome et al., 1989; Salzman et al., 1992; Britten et al., 1996; Bisley et al., 2001). Furthermore, there is recent evidence that its neurons may also participate in the circuitry subserving retention of visual motion (Bisley and Pasternak, 2000; Bisley et al., 2001, 2004). Because the PFC and MT are anatomically interconnected (Barbas, 1988; Schall et al., 1995; Burman et al., 2006), they may be functionally linked, and this relationship is likely to be revealed in the comparison of their activity during the performance of the same behavioral task.
We found that a high proportion of PFC neurons showed direction-selective responses to behaviorally relevant motion. These responses strongly resembled those recorded in MT, although they were weaker and occurred relatively late, suggesting their bottom-up origins. During the memory delay, both areas displayed transient periods of directionality reflecting the preceding sample direction. Although these directional signals decreased with time of delay in both areas, they were more prevalent in the PFC. During the comparison test, strong modulation by the remembered sample direction was present in both areas. Although this modulation in MT preceded that in the PFC by 100 ms, only the latter was predictive of the monkey's decisions. These results suggest a strong functional link between MT and the PFC during task performance and place important constraints on the individual contributions made by neurons in both areas.
Materials and Methods
Subjects
We recorded from the PFC of two adult male macaque monkeys (Macaca nemestrina and Macaca mulatta) and from area MT of two other adult male macaque monkeys (Macaca nemestrina). Water was restricted for 22 h before each daily experiment, and the daily liquid rations were provided in the form of fruit drink during the testing sessions. At the end of each testing day, the monkeys were given fresh fruit and vitamins. No testing occurred on weekends, and the monkeys received 100 ml/kg allotments of water. Food was available continually in the home cage, and body weights were measured on a daily basis to monitor health and growth. Experiments were performed in accordance with the guidelines published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (revised 1996) and were approved by the University of Rochester Committee for Animal Research.
Visual stimuli and behavioral procedures
The stimuli and the behavioral tasks were similar to those used in previous studies from this laboratory (Bisley and Pasternak, 2000; Bisley et al., 2001, 2004).
Visual stimuli
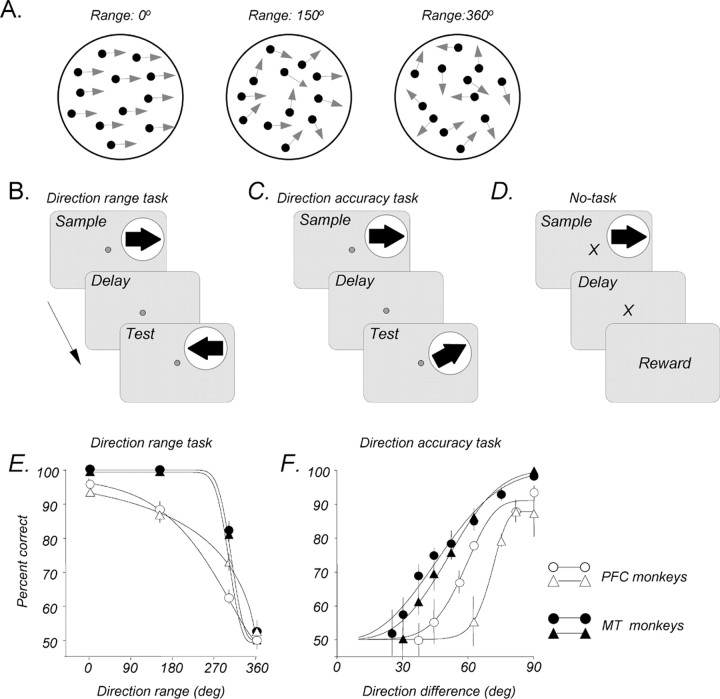
Stimuli were presented on a video monitor (17 inch Nanao FlexScan T560i or 19 inch IIyama Vision Master Pro 513, both running at 1152 × 870 pixel resolution and a 75 Hz refresh rate), placed 42 or 57 cm in front of the monkeys. They consisted of random dots placed in a circular aperture and having a constant translational step size (Δx) and temporal interval (Δt = 13 ms) (Rudolph and Pasternak, 1999; Bisley and Pasternak, 2000; Bisley et al., 2004). The dots were 0.03° of visual angle in diameter with a luminance of 15 cd/m2, shown on a dark background of 0.1 cd/m2. Each dot persisted for the entire duration of the stimulus. On each video frame, the range of directions in the stimulus could be specified, and each dot was assigned a direction chosen randomly from a specified distribution (Fig. 1A).
Figure 1.
Visual stimuli, behavioral tasks, and discrimination performance. A, Random-dot stimuli. The stimuli consisted of random dots displaced in directions chosen from a predetermined distribution. The width of this distribution determined the range of directions within which individual dots move and was varied between 0° (all dots moving in the same direction) and 360° (dots moving in all directions). B, Direction range task. During this task, sample and test stimuli, separated by a 1500 ms delay, moved either in the same or in opposite directions. Monkeys were required to fixate a small spot at the center of the display throughout the trial and pressed the right or the left button to report whether the directions of the two stimuli were the same or different. During each recording session, the direction range in the sample was varied, whereas the test always contained coherent motion (0° range). C, Direction accuracy task. During this task, the random-dot stimuli presented as the sample or the test always moved coherently, and the difference in direction between sample and test was varied. D, No-task condition. During each trial, a coherent stimulus, followed by a 1500 ms delay, and a reward were presented while the monkey maintained fixation on a cross-shaped stimulus. No button press was required of the animal. E, Direction range task. Average psychometric functions for the two monkeys participating in MT recording sessions (filled symbols) and for the two monkeys participating in the PFC recording sessions (open symbols) are shown. The data were fitted with a Weibull function. Error bars indicate ±1 SEM. The data for each monkey were collected during 5–10 behavioral sessions, each consisting of 250–400 trials, and the points were fitted with a Weibull function. F, Direction accuracy task. Average psychometric functions for the MT and PFC monkeys relating performance to the angle of difference between sample and test directions are shown. The data were collected during 5–10 sessions, each consisting of 120–300 trials. Other details are as in E.
Behavioral tasks
The monkeys performed two variants of the direction discrimination task in which they compared the directions of two 500 ms moving stimuli separated by a 1500 ms delay. They indicated whether those directions were same or different by pressing one of two adjacent response buttons. Monkeys were required to register their response immediately after the offset of the test stimulus, and every correct response was rewarded with a drop of juice.
Direction range task
During the sample, a direction range was selected at random and without replacement (method of constant stimuli) from a set of four (0, 150, 300, and 360°). The test stimulus moved either in the same or 180° opposite to the sample direction (Fig. 1B) and was always coherent (0° direction range). Responses on trials in which the sample had 360° of direction range were rewarded at random.
Direction accuracy task
Both sample and test stimuli consisted of coherently moving dots, and on each trial, the difficulty of the discrimination was increased by decreasing the angle of difference between sample and test directions (Bisley and Pasternak, 2000) (Fig. 1C). A staircase procedure was used to control the overall performance level with three consecutive correct responses resulting in smaller direction difference between sample and test, whereas one incorrect response resulted in a larger difference between the two directions. This approach was used because, in contrast to the direction range task, the performance on the direction accuracy task was strongly affected by target eccentricity, which in the case of MT recordings changed during each recording session. Thus, we could not preselect a set of values bracketing the threshold, as was the case with the direction range task. The use of two direction discrimination tasks allowed us to compare neural activity in response to the same motion stimuli under conditions of different behavioral demands. The direction range task stressed the ability to integrate local motion in the sample, rather than the subsequent “same–opposite” comparison. On the other hand, the direction accuracy task did not challenge the ability to extract the correct sample direction, but performance depended on the ability to accurately compare, during the test, two similar directions.
Overall performance in both tasks was generally on the order of 75–85% correct. Figure 1, E and F, shows the average psychometric functions, in each task, for the two monkeys used for PFC recordings and two other monkeys used for MT recordings. The range and accuracy thresholds were taken as values, derived from Weibull fits of the data, corresponding to 75% correct performance. Direction range thresholds were on the order of 250–330°, whereas direction accuracy thresholds were on the order of 40–70°. As seen in Figure 1, E and F, the two monkeys used for MT recordings generally performed somewhat better in both tasks. It is important to note that despite this difference in thresholds, both pairs of monkeys performed at a similar overall percentage correct. In addition, much of the analysis presented here uses trials that began with a coherent sample, on which all four monkeys showed near-perfect performance.
No-task condition
In this condition (Fig. 1D), the fixation target was an “X,” rather than a small square, to cue the monkey that the reward will be obtained at the end of the delay and no response will be required. Each trial began after the monkey acquired the fixation target, followed by a stimulus and delay. The coherent random-dot stimulus moved either in the preferred or the anti-preferred direction and was identical in appearance and duration to the sample stimulus used during the task. The reward was delivered if the monkey maintained fixation throughout the trial, with no button-press requirement. This task was used as a control to evaluate task-related aspects of neuronal activity, and it was run on a subset of recording sessions in both MT and the PFC.
Block design
During each recording session, the trials were run in blocks according to the following sequence. Once the waveform of a single neuron was isolated, 40–64 trials were used to determine the presence or properties of its direction selectivity (procedure detailed below). The main behavioral task, consisting of 250–400 trials (direction range) or 120–300 trials (direction accuracy), was then introduced. The session ended with a block of 40–120 “no-task” trials.
Fixation control
In both tasks, the monkey initiated a trial by fixating a small spot on the monitor within a 1.5° window. Successful fixation for 1000 ms brought on the presentation of the sample and the rest of the trial. Fixation was maintained for the duration of each trial. Eye position was monitored through the use of implanted scleral search coils (Remmel, 1984), or with infrared video eye-tracking (ETL-200; ISCAN, Burlington, MA).
Surgical procedures
For all surgical procedures, anesthesia was induced with ketamine hydrochloride (10 mg/kg) and diazepam (0.25 mg/kg) and maintained with 1.5–3% isoflurane. Monkeys were implanted with head-restraint devices and scleral search coils for monitoring eye position. The PFC and area MT were localized by examining brain scans of each monkey obtained by magnetic resonance imaging [T2-weighted, 1.5T General Electric (Chalfont St. Giles, UK) and 3T Siemens (Iselin, NJ) magnets, small surface coil; echo time/repetition time, 5000/90 or 3000/85; 1.5-mm-thick slices]. The arcuate and principal sulci were identified from the structural scans to guide PFC recording, and the craniotomies were centered on the posterior end of the principal sulcus. Likewise, the superior temporal sulcus (STS) was identified from coronal scans, and a craniotomy was placed in each animal at a location from which MT could be accessed with a dorsal-to-ventral electrode path. Cilux recording chambers (19 mm internal diameter; Crist Instruments, Hagerstown, MD) were implanted over all craniotomies using bone cement and titanium screws.
Electrophysiological recordings
Data acquisition
Recording procedures were similar to those used previously (Bisley et al., 2004; Zaksas and Pasternak, 2005). During each session, a single tungsten microelectrode [1.5–3 MΩ (Alpha Omega Engineering, Alpharetta, GA) or 1.5–5 MΩ (Frederick Haer Company, Bowdoinham, ME)] was lowered into cortex through a steel guide tube positioned in a cilux grid (Crist et al., 1988). For the PFC recording, the guide tube touched the dura but did not penetrate it. For recording in MT, which is located deep in the STS, dura-penetrating guide tubes were used. Waveforms from single neurons were isolated and recorded using either a dual window discriminator (BAK Electronics, Mount Airy, MD) or a Multichannel Acquisition Processor system (Plexon, Dallas, TX).
Confirming prefrontal location
We aimed at recording in the region of the PFC interconnected with MT (Barbas, 1988; Schall et al., 1995; Burman et al., 2006). Because this region is located anterior to the frontal eye fields (FEFs), we were guided by the location of the FEF by identifying the region, anterior to the arcuate sulcus, where neuronal activity was generated predominantly in concert with the monkeys' eye movements. In addition, in one monkey, the location of the FEF was determined by intracortical microstimulation (Bruce et al., 1985). Eye position was recorded during a task in which the monkey was instructed to saccade from a central fixation point to one of four possible target locations (one in each visual quadrant). The target fixation spot was extinguished 100 ms after fixation was acquired, at which point stimulation was applied on 50% of the trials and the monkey received a juice reward on every trial. Current was delivered through the recording electrodes as a 70 ms train of biphasic pulse pairs (0.2 ms positive pulse, 0.1 ms delay, 0.2 ms negative pulse, 200 Hz, 25–80 μA).
Receptive field mapping
MT.
The procedure for receptive field (RF) mapping was identical to that used previously (Bisley et al., 2004; Zaksas and Pasternak, 2005). Briefly, the borders of the RF of each neuron (part of visual space in which dot motion elicited a robust response) were mapped initially by hand, using a joystick-controlled patch of dots, while the monkey passively fixated a small cross on the screen. Once the RF was outlined, it was fitted with a patch of coherently moving dots. Speed and density of the moving dots were optimized by varying them systematically to obtain a maximal response.
PFC.
There is some evidence for spatial structure of the visual responses in the PFC (Suzuki and Azuma, 1983). To determine whether spatial location of the stimuli could significantly impact response properties, we recorded from a subset of PFC neurons with task-related activity while the monkeys performed short blocks of trials (24–40) with the stimuli placed in each of the four visual quadrants (4° stimulus, 5.7° eccentricity), as well as in the fovea. We found that only 22% (11 of 50) of neurons had a significant effect of spatial location on motion response rates (p < 0.05, ANOVA). For these neurons, the average difference in firing rates between the locations with highest and lowest responses was only, on average, 4.1 spikes per second (SD, 2.8). Importantly, not a single neuron failed to respond altogether as a function of spatial location. Thus, the effect of spatial location of visual stimulation on responses of PFC neurons recorded during our task was limited. To maximize consistency in experimental design with the recordings from MT neurons, we generally chose the final stimulus location to be eccentric (5.7°) and contralateral to the hemisphere with the implanted recording chamber. In a minority of recording sites (29%), the stimuli were presented in the fovea. Stimulus size was maintained at 4° of visual angle. Dot density was set to 2.5 dots/degree2, an average value derived from optimal stimulation of MT RFs. Dot speed was 5 or 10°/s (Δx = 0.065 or 0.13°, Δt = 13 ms).
Evaluating responsivity and directional preference
MT.
The activity of each recorded neuron was analyzed to determine whether it responded to visual motion. A 100 ms window was slid along the spike train in 50 ms increments for the entire duration of the stimulus (500 ms). A neuron was classified as having responded to the stimulus if at least 100 ms of activity were significantly different from baseline (p < 0.0056, unpaired t test with Bonferroni correction; baseline defined as the firing rate during 200 ms immediately preceding the sample onset). After the RF of an MT neuron was identified, a monkey fixated passively while the RF was stimulated by coherent motion in eight possible directions, 45° apart. At least five trials were presented for each direction of the stimulus. The preferred direction was determined by computing a vector average of the mean firing rates in response to each stimulus direction. The opposite direction was termed anti-preferred.
PFC.
Unlike in MT, prefrontal neurons were not assumed to be responsive during passive viewing conditions. Therefore, responsivity and direction selectivity were evaluated during the monkeys' performance of a direction discrimination task. This was, in essence, the direction range task, but the sample and test were always coherent and the sample could move in one of eight directions. Neurons were classified as task related if at least 100 ms of activity either in the sample, delay, or test significantly deviated from the 200 ms period immediately preceding sample onset (Bonferroni corrected, p < 0.0056 in sample or test, p < 0.0017 in delay). At least five trials were presented for each of eight possible sample directions, and the preferred and anti-preferred directions were determined as above.
Data analysis
General methods
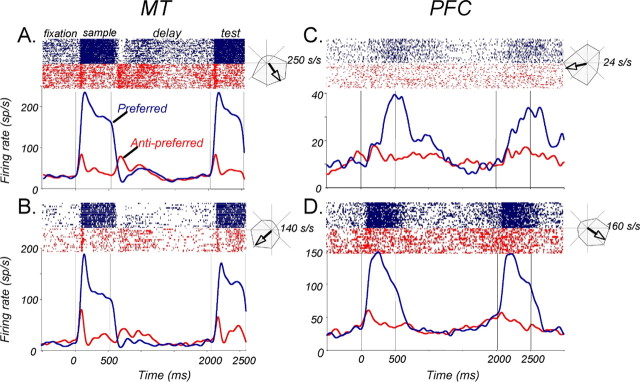
Analysis of spike data and statistical tests were performed using MATLAB (Mathworks, Natick, MA), JMP (SAS Institute, Cary, NC), and Excel (Microsoft, Redmond, WA). For the purposes of visual inspection, as seen in the example plots in Figure 3, the activity of each neuron during each behavioral session was plotted as a spike density function, generated by convolving the spike train with a Gaussian probability function (1 ms steps, σ = 30 ms). The firing rate at different stages of the task was analyzed by computing the mean number of action potentials over a given epoch in repeated presentations.
Figure 3.
Activity of example neurons during the tasks. Timing of sample and test presentation is indicated by the black bars along the timeline and vertical lines indicating onset and offset. Only trials with coherent sample (0° direction range) are shown. Rasters and spike density functions (1 ms step, 30 ms Gaussian envelope) are color-coded with respect to preferred (blue) and anti-preferred (red) directions. Polar plots to the right of each activity plot show the direction tuning of the neuron (response to motion in 8 directions) and the calculated vector (arrow) indicating the preferred direction. A, Direction-selective MT neuron during the direction range task. Note the typical short response latency and early directionality during the delay. B, MT neuron during the direction accuracy task. C, Direction-selective neuron in the PFC during the direction accuracy task. Note the late maximal response to the sample and somewhat higher activity throughout much of the delay after the preferred sample. D, Direction-selective neuron in the PFC during the direction accuracy task. Note the rapid onset and strong selectivity of responses during visual stimulation and an apparent absence of direction-selective activity during the delay. sp/s and s/s, Spikes per second.
Response latency
Response latencies of neurons in both cortical regions were calculated as the time at which activity during the stimulus first exceeded baseline firing rates. Baseline was defined as the activity during the last 200 ms of the fixation period immediately preceding stimulus onset. Starting at the time of sample onset, a 25 ms window was slid along the activity spike train in 1 ms increments, and the latency was taken as the midpoint of the first of 20 consecutive bins to differ from baseline by >2 SD. This measure, similar to ones used by other studies (Freedman et al., 2003), provided a reliable estimate of latency that closely matched the start of activity seen in individual trial rasters.
Direction selectivity
Responses were defined as direction selective if firing rates during preferred and anti-preferred motion were statistically different for at least 100 ms of the stimulus (p < 0.0056, Bonferroni corrected unpaired t test). The magnitude of directionality was calculated using a conventional directionality index [DI = (preferred − anti-preferred)/(preferred + anti-preferred)].
Receiver operator characteristic analysis
To quantify the reliability of directional bias in the neuronal activity, we used a receiver operator characteristic (ROC) measure (Britten et al., 1992, 1996). An ROC value is the probability with which, on the basis of firing rates, an ideal observer can reliably classify stimulus direction as preferred or anti-preferred. A value of 0.5 indicates that a given firing rate could have been elicited with equal probability by the preferred or anti-preferred stimulus. A value of 1.0 indicates that responses to the preferred stimulus were always greater than responses to the anti-preferred stimulus. Conversely, 0 indicates that anti-preferred responses were always greater than preferred responses. To determine the time at which direction selectivity emerges in the population response (see Fig. 5C), ROC values for individual neurons were calculated for a 25 ms window slid in 10 ms increments along the spike train. For all other analyses, the ROC values were calculated for a 100 ms window slid in 10 ms increments along the spike train. The 100 ms window is shorter than periods used by other studies in similar analyses (Romo et al., 1999; Amemori and Sawaguchi, 2006). A wider window can incorporate more spikes and decrease the likelihood of falsely detecting transient signals, but increasing the width also makes an assumption that neurons are capable of integration across extended periods of time. The 100 ms window allowed us to minimize that assumption and more closely approximate the instantaneous spike rates as observed in individual trial rasters. The significance of each ROC value was established by a permutation test by randomly redistributing firing rates for all the trials into “preferred” and “anti-preferred” groups, regardless of the actual sample direction associated with each trial. An ROC value was then calculated from the redistributed groups, and the process was repeated 2000 times, creating a distribution of ROC values. The real ROC value was deemed significant if it fell in the top or bottom 0.5% of the distribution. The 99% confidence level, a criterion more stringent than the commonly used 95% (Amemori and Sawaguchi, 2006), was determined by analyzing activity recorded during the fixation period before sample onset and finding that this criterion prevented an excessive amount (>5%) of false positives.
Figure 5.
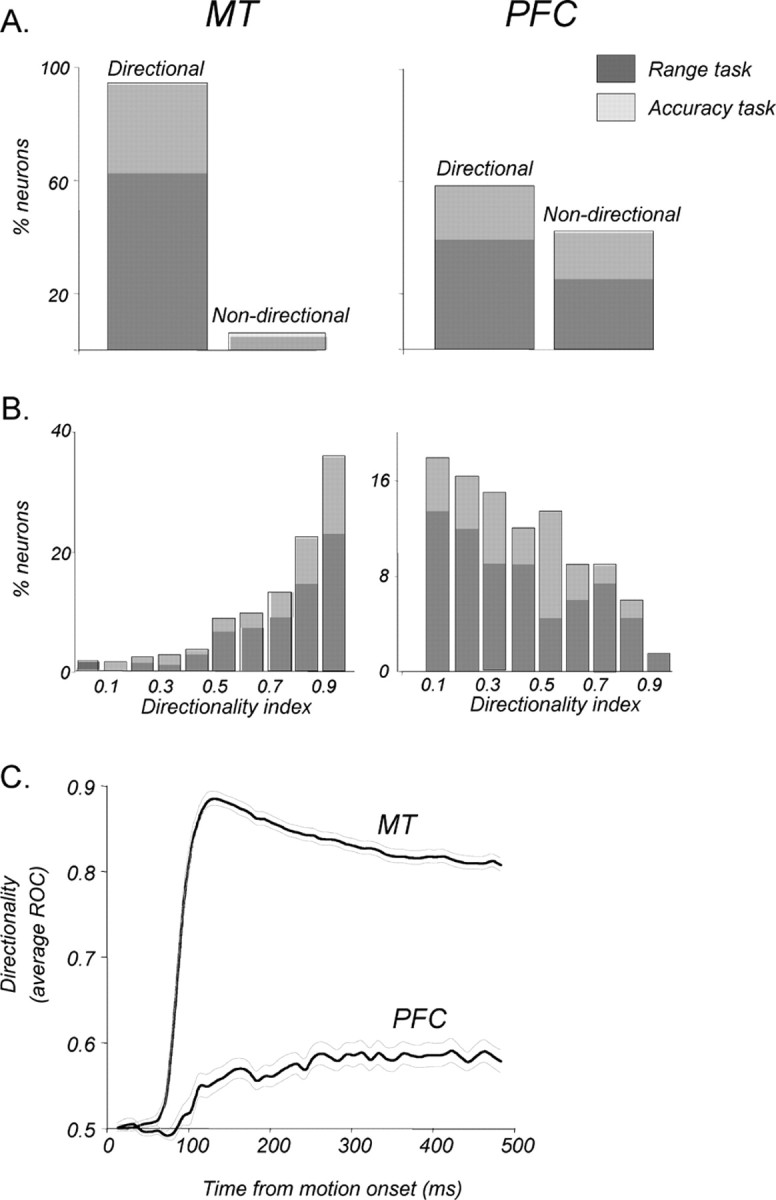
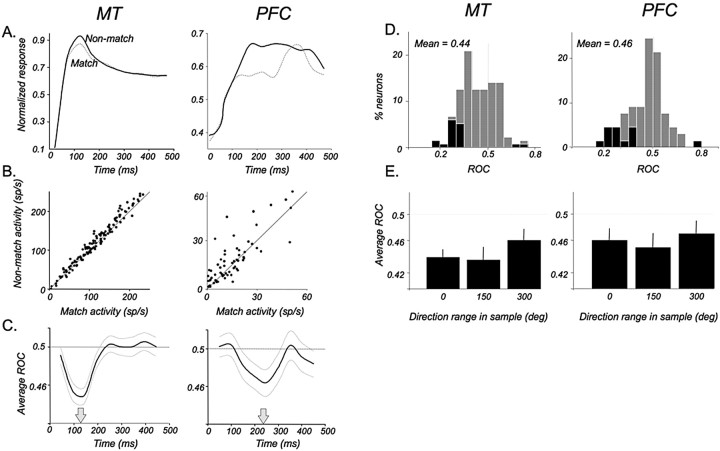
Direction selectivity in MT and in the PFC. A, Proportions of direction-selective neurons among those recorded in MT and in PFC areas. Ninety-four percent (288 of 305) of all recorded MT neurons and 58% (67 of 115) of PFC neurons with task-related activity were direction selective (see Materials and Methods). Relative contributions to these proportions by neurons recorded during the direction range (dark gray) and direction accuracy (light gray) tasks are shown for each area. B, Distributions of maximal DIs showing the magnitude of selectivity for neurons in each area (MT, n = 288; PFC, n = 67). Neurons recorded during the two tasks are shown as in A. C, Emergence and reliability of the directional signals in each area expressed through ROC analysis. Directional signals in MT were substantially more reliable and occurred significantly earlier than in the PFC (p < 0.001, bootstrap hypothesis test; see Materials and Methods for details). The thin gray curves indicate ±1 SD.
Emergence of direction selectivity
To evaluate the relative time course of directional signals in both areas, we determined the time at which reliable selectivity arose in the MT and PFC neuronal populations. To that end, we calculated the time at which the average ROC index significantly exceeded 0.5 (see Fig. 5C). Variance of this population mean was calculated with bootstrap analysis (Efron and Tibshirani, 1993). For neurons in each cortical area, the arrays of ROC values were sampled without replacement, and an average time of directional signal emergence was calculated. This procedure was repeated 4000 times, and the SD of the resulting distribution was taken as measure of error in the population mean.
We also used a two-sample bootstrap hypothesis test to determine whether the time at which directional signal emerged was different between neurons in area MT and PFC (see Fig. 6C). This was done by first pooling the bootstrap distributions of the population latencies of the two neuronal groups (calculated as above). Two data sets, equal in size to the original groups, were then randomly selected without replacement, and their means were compared. After 1000 iterations of this procedure, the level of significance was indicated by the proportion of iterations that produced a difference of means greater than the difference between the real means.
Figure 6.
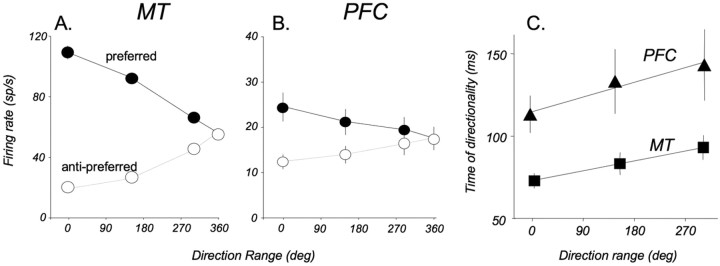
Effect of direction range on responses in direction-selective neurons in MT and in the PFC. A, MT neurons. The firing rate of each neuron was calculated for all range values during a 100 ms window at the time of its maximal direction selectivity. Coherence strongly modulates responses to both preferred and anti-preferred motion. n = 186. B, PFC neurons. Note a similar pattern of modulation by direction range to that in MT. n = 40. C, Emergence of directionality as a function of direction range. The latencies for each range level were computed from an average ROC index for the population of all direction-selective neurons in each area (see Fig. 5C) as the time at which the ROC index significantly deviated from 0.5. Linear fits of the data recorded in MT (y = 0.07x + 73) and in the PFC (y = 0.1x + 115) indicate that delays in the emergence of directionality increase in both areas at a very similar rate as a function of decreasing stimulus coherence. sp/s, Spikes per second.
Choice probability
We were interested whether the activity recorded in both areas was predictive of perceptual decisions made by the monkeys on a trial-by-trial basis. This relationship was explored by calculating choice probability (CP). The procedure was identical to that of the ROC analysis described above, except that the trials were grouped not according to the stimulus direction, but rather by the direction indicated by the monkey's decision at the end of the trial (Britten et al., 1996). For example, if the coherent test stimulus moved in the preferred direction and the monkey's response was “same,” this decision indicated that the remembered sample direction had also been preferred. The significance of CP for individual neurons was tested with the permutation test as above. As in the case of the ROC analysis, CP analysis was always performed and reported separately for trials at each coherence level.
Results
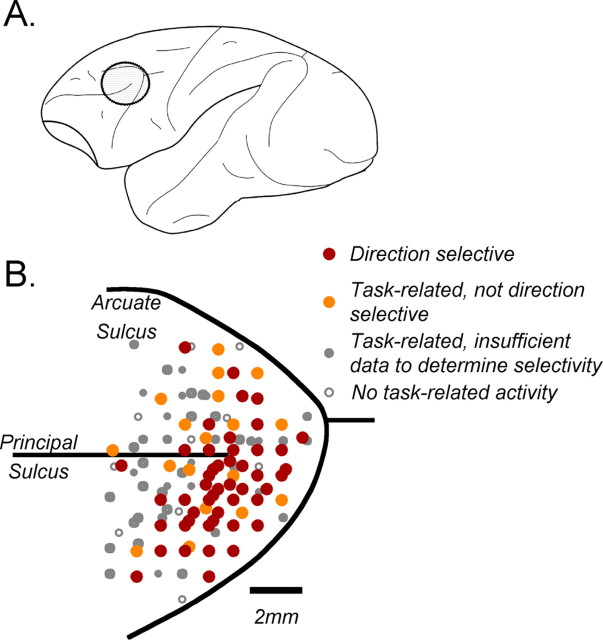
We sampled 313 neurons in area MT and 483 neurons in the PFC. Of the sampled neurons in MT, 306 provided sufficient data for analysis, 205 recorded during the direction range task and 101 during the direction accuracy task. Data from 197 of the MT neurons from the same two monkeys were presented previously (Bisley et al., 2004). In the PFC, 204 (42%) neurons showed task-related activity (see Materials and Methods), and of those, 115 had sufficient data for analysis presented here, 74 recorded during the direction range task and 41 during the direction accuracy task. Because this was the first study to examine the PFC neurons in the context of working memory for visual motion, the region of interest was estimated from anatomical studies showing likely projection sites from motion processing area MT (Barbas, 1988; Schall et al., 1995; Burman et al., 2006). The distribution of recording sites in the PFC is shown in Figure 2.
Figure 2.
Locations of PFC recordings. A, Diagram of macaque cortex showing the location of recording chambers over the PFC. B, A magnified image of the region in A, showing locations of electrode penetrations and the properties of recorded neurons. Penetrations were made in four hemispheres (2 monkeys), and their maps were overlaid in the diagram. Each point is coded in accordance with the most experimentally relevant level of response found at the site. Therefore, any sites labeled as direction selective may have also contained neurons of any other response type. By extension, any sites labeled with filled symbols may have also contained neurons with no task-related activity.
Most responsive neurons were found within area 8Ar and the dorsolateral part of area 46, just anterior to the FEFs. Among sites where the neurons showed task-related activity, many were modulated by motion direction during one or more time periods of the task (Fig. 2, red), and those appeared to concentrate in the more posterior and lateral portions of the principal sulcus, in front of the arcuate. In a number of sites, neurons showed task-related activity, but this activity had no directional bias (Fig. 2, orange symbols). Kim and Shadlen (1999), who first used visual motion stimuli while recording from the PFC, also reported the presence of neurons modulated by stimulus direction in the same regions. We cannot be certain of the extent to which direction-selective responses may be found in more anterior portions of the PFC. However, as seen in Figure 2, such response properties were relatively rare in the most anterior part of the recorded region of interest. This suggests that, for the most part, direction-selective motion responses may be anatomically constrained to the region with direct access to relevant bottom-up input.
Examples of neuronal activity in MT and the PFC during the task are shown in Figure 3. The two example MT neurons (Fig. 3A,B), recorded during the direction range and accuracy tasks, respectively, show a pattern of activity discussed in a recent paper from this laboratory (Bisley et al., 2004). Specifically, robust direction-selective responses to motion presented during the sample and test and a characteristic pattern of early direction-specific activation during early delay, followed by weak suppression and a slight anticipatory increase in activity shortly before the appearance of the test.
The activity of example PFC neurons, shown in Figure 3, C and D, illustrates a number of similarities to MT and some important differences. As in MT, these cells show direction selectivity during the sample and the test. However, one neuron took several hundred milliseconds to reach maximal response (Fig. 3C), whereas the other (Fig. 3D) was activated almost as rapidly as the example MT neurons. In addition, the pattern of delay activity is different in the two example PFC neurons. One showed greater activity after the preferred direction for a substantial portion of the delay, a difference that disappeared shortly before the test. The other showed very limited directional bias. Below, we will characterize and compare responses of neurons in the two regions during each phase of the task: sample, delay, and test.
Responses to visual motion
Response latencies
The time at which neurons in a given cortical area begin responding to sensory stimuli may provide insight into the relative level these neurons occupy in the hierarchy of visual processing. To that end, we computed the response latencies of all neurons that responded to the coherent sample. In direction-selective cells, the analysis was performed on responses to the preferred direction. The comparison of response latencies to visual stimulation in MT and PFC neurons revealed substantial differences between the two areas, as illustrated by the latency distributions shown in Figure 4. MT responses began, on average, 62.9 ms (SD, 22.4) after stimulus onset, a value consistent with those reported in other studies (Schmolesky et al., 1998; Raiguel et al., 1999). The latencies of responses to motion presented during the direction range task and during the direction accuracy task, shown by dark and light gray shading (Fig. 4), were not statistically different (p > 0.3, Kolmogorov–Smirnov test). We also found no difference in response latencies between the two monkeys (p > 0.5, Kolmogorov–Smirnov test).
Figure 4.
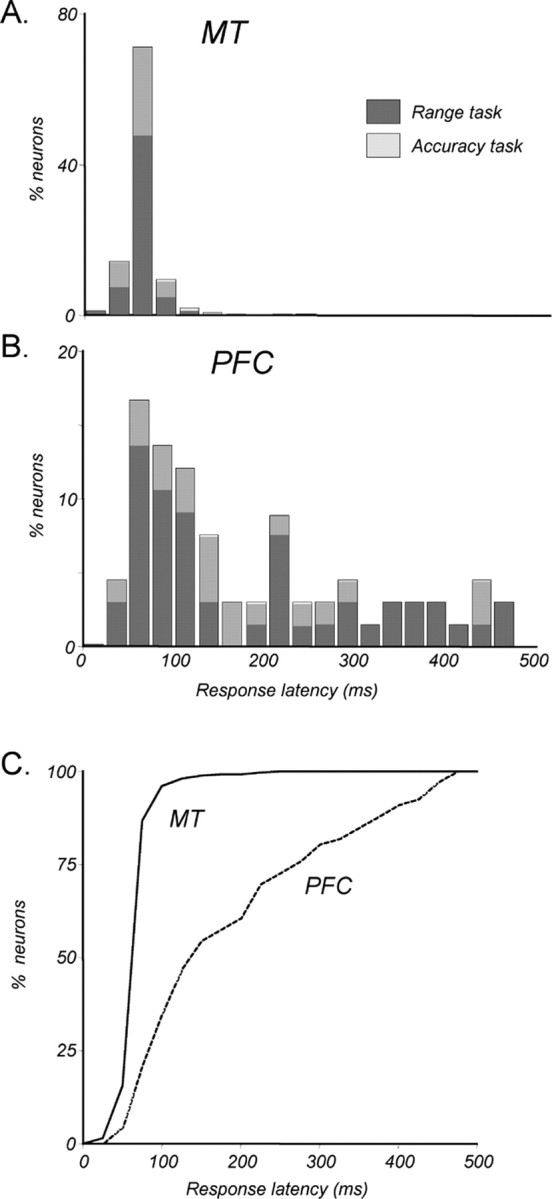
Response latencies. A, Distribution of response latencies in MT to sample onset (n = 256). Latency is defined as the time at which the response to a preferred sample exceeded baseline (measured during 200 ms before onset) by at least 2 SDs. Proportions are shown separately for neurons recorded during direction range (dark gray) and direction accuracy (light gray) tasks. The distributions for the two tasks are not significantly different (p > 0.3, unpaired t test). B, Distribution of response latencies in the PFC (n = 66). Details are as in A. C, Recruitment of MT and PFC neurons during sample presentation. A cumulative proportion of neurons in each area that respond by a given time in the sample is shown. By ∼60 ms after sample onset, one-half of the recorded MT neurons began responding. In contrast, it took ∼140 ms for one-half of the recorded PFC neurons to begin responding.
Response latencies in the PFC were significantly longer than those in MT (p ≪ 0.01, unpaired t test), averaging 184.4 ms (SD, 125.4), and were not affected by the nature of the direction discrimination task or inter-animal differences (p > 0.05 in each case, Kolmogorov–Smirnov test). Unlike latencies in MT that were tightly distributed around the mean, PFC latency distribution was broader and asymmetric, with the distribution skewed toward shorter latencies. The median latency, corresponding to the 50% level in the recruitment plot in Figure 4C, is 140 ms, substantially shorter than the mean of 184.4 ms. This value is consistent with previous reports of visual responses in the PFC (Funahashi et al., 1990; Chafee and Goldman-Rakic, 1998). A number of the recording sites were located close to the arcuate sulcus, a region often considered to be the FEF. However, the motion-responsive neurons not only lacked activity related to eye movements but also had the long latencies reported above. Thus, the recorded cells likely constitute a different class than the commonly reported FEF neurons, because the latter are known to respond twice as fast, on the order of 50–75 ms (Schmolesky et al., 1998; Pouget et al., 2005). Note that a number of PFC neurons began their responses as late as 200–500 ms after sample onset. Such long latencies suggest that this group of neurons were unlikely to receive direct inputs from sensory areas like MT. The delayed nature of these responses suggests that they were either gated through a multisynaptic circuit, or possibly recruited by the faster-responding neurons in the PFC local network. The latter possibility was supported by the finding that the strength of direction selectivity did not correlate with response latency (discussed below).
Direction selectivity
In response to coherently moving random dots (0° range), 288 of 306 MT neurons (94%) were direction selective (Fig. 5A, left), consistent with previous reports (Maunsell and Van Essen, 1983). This incidence of direction selectivity did not depend on the nature of the direction discrimination task (direction range task: 191 of 205, 93%; direction accuracy task: 97 of 101, 97%; p > 0.05, χ2 test). Of the 115 PFC neurons with task-related activity, 67 (58%) had direction-selective responses (Fig. 5A, right). Like in MT, the proportions of direction-selective neurons recorded during the two direction discrimination tasks were statistically indistinguishable (p > 0.05, χ2 test; direction range: 45 of 74, 61%; direction accuracy task: 22 of 41, 54%).
More than 40% of MT neurons reached maximal selectivity at ∼100 ms, with a minority achieving maximal directionality several hundred milliseconds into the stimulus (median, 200 ms; mean, 256 ms; SEM, 7.8). In the PFC, the timing of maximal directionality was highly variable, with approximately one-half of neurons reaching their maximum direction selectivity 300 ms into the response (median, 300 ms; mean, 313 ms; SEM, 14.7), regardless of the nature of the direction discrimination task (p > 0.05, unpaired t test). Overall, PFC neurons reached maximal selectivity significantly later than MT neurons (p < 0.005, unpaired t test). The magnitude of this selectivity was quantified by computing a DI for each neuron. The distributions of DIs (Fig. 5B) illustrate a commonly reported high degree of direction selectivity in MT (Baker et al., 1981; Maunsell and Van Essen, 1983; Bisley et al., 2004), with the average DI of 0.78 (SD, 0.21) and 50% of the neurons having DIs of ≥0.85. The DIs for PFC neurons were substantially lower, averaging 0.45 (SD, 0.23), with 50% of the neurons having a DI of ≥0.42. Like with other measures, these values did not depend on the nature of the direction discrimination task (p > 0.05, unpaired t test). Because both the onset latencies and the magnitude of directionality were highly variable in the PFC, we tested for a relationship between the two. Interestingly, there was no correlation between the DIs of the neurons and how quickly they responded to motion (r2 = 0.001; p > 0.8, regression analysis).
We further characterized the relationship of direction-selective signals in MT and the PFC by examining the relative time at which reliable direction selectivity of coherent sample responses emerged in both sets of neurons (Fig. 5C). Because we found no differences in response properties recorded during the two direction discrimination tasks, the data were combined. For greater power, rather than estimating the latencies for individual neurons, we computed a population average of the ROC indices of all direction-selective neurons as a function of time and used a bootstrap method (Efron and Tibshirani, 1993) to estimate the latency at which this index significantly deviated from 0.5 (see Materials and Methods for details). In MT, a significant directional signal first emerged 73 ms (SD, 4.5) after motion onset (p < 0.0025, Bonferroni corrected t test), the value consistent with a previous finding that maximal directional information is acquired by MT neurons within the first 100 ms of stimulus onset (Osborne et al., 2004). In contrast, in the PFC, directional signals first appeared 113 ms (SD, 11.3) after motion onset (p < 0.0025, Bonferroni corrected t test). The 40 ms delay between the onset of directionality in MT and the PFC was significant (p < 0.001, two-sample bootstrap hypothesis test) (Efron and Tibshirani, 1993).
Direction range
In response to random-dot stimuli containing a range of local directions, MT neurons displayed a characteristic pattern of activity (Fig. 6A) reflecting their ability to extract the mean direction even at low coherence (Bisley et al., 2004). We were interested to know whether PFC neurons showed similar response patterns, reflecting an accurate representation of bottom-up motion signals.
Responses of 186 MT and 40 PFC neurons as a function of range to the preferred and anti-preferred directions are shown in Figure 6, A and B. These responses were calculated for a 100 ms period centered on the time of maximal directionality during the coherent sample. The relationship between responses and direction range was similar in neurons in the two areas: the firing rates decreased as the direction range in the preferred stimulus increased. Conversely, responses to the anti-preferred direction increased with increasing direction range. This resulted in a response to the noncoherent stimulus (360° range) that was lower than that to the preferred 0° stimulus and higher than the response to the anti-preferred 0° stimuli (MT, p ≪ 0.001; PFC, p < 0.05; paired t test). In both areas, responses to preferred stimuli were, on average, significantly higher than to anti-preferred stimuli at the 0, 150, and 300° range (MT: p ≪ 0.001, paired t test; PFC: p ≪ 0.001 for 0 and 150°, p < 0.05 for 300°). The similarity in the relationship between the neuronal response and the direction range in the two areas suggests that the product of motion integration likely to be taking place in MT (Movshon et al., 1985) is being transmitted to the PFC. This idea is supported by the constant difference between the two areas in the time at which direction selectivity emerged, with the PFC lagging 50 ms behind MT at all direction ranges (Fig. 6C). Note that this latency of selectivity in both areas increased systematically and in parallel with increasing direction range, further supporting the idea that the direction-selective responses recorded in PFC may represent bottom-up motion signals arriving from MT.
Activity during the memory delay
To successfully complete the trial, the monkeys must retain the information about sample direction during the 1500 ms delay and use it for subsequent comparison with the test direction. Thus, we focused on the activity of MT and PFC neurons during the memory delay to determine whether the firing rates during this period carried information about the sample direction and whether this information is reflected in the monkey's choices.
To determine the presence of reliable directional signals, we used ROC analysis, an approach that calculates the probability with which firing rates reflect the direction of the preceding sample (see Materials and Methods). If, at any time during the response of a neuron this probability significantly differs from chance, then the neural activity at that time was reliably indicative of the preceding sample direction. Chance is designated by a value of 0.5, and a significant ROC value between 0.5 and 1 indicates a reliable correlation of higher firing rates with motion in the preferred direction of the neuron. Conversely, a value between a 0.5 and 0 indicates that a higher firing rate correlates with motion in the anti-preferred direction of the neuron. The results of this analysis for MT neurons are shown in Figure 7A–C and for PFC neurons are shown in Figure 7D–F. The presence of a predictive relationship between firing rates and sample direction (blue and red thick line segments) during the sample and delay is shown for all direction-selective neurons in both areas (Fig. 7A,D). Each horizontal line represents the results of the ROC analysis, as a function of time, for a single neuron recorded during a block of trials. The blue line segments indicate the presence of a significant positive correlation (higher firing rates associated with the preferred sample), whereas the red line segments indicate the presence of a significant negative correlation (higher firing rates associated with the anti-preferred direction). Figure 7, B and E, shows the incidence of these directional signals, as a function of time in both areas, following the two sample directions, and Figure 7, C and F, shows the duration of directional signals encountered throughout the delay.
Figure 7.
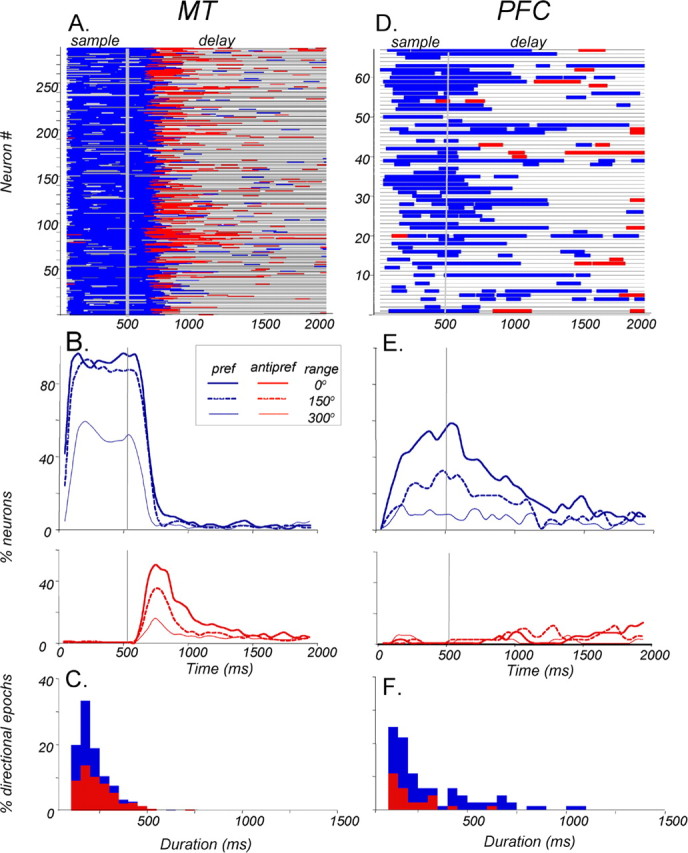
Directional signals in the PFC and MT during the memory delay. ROC analysis was applied to the sample and delay activity of each neuron (100 ms window, 10 ms step). A, Time course of directional signals in individual MT neurons (n = 288). Each horizontal line is the timeline of a single neuron through the sample and delay periods. Blue segments indicate times at which activity associated with a preferred sample was reliably higher than that associated with an anti-preferred sample. Red segments indicate the opposite relationship, whereas gray shows the times at which the signal was not significantly indicative of sample direction. Note that reliable direction selectivity seen during the sample generally disappears ∼200–250 ms into the delay and is often replaced by an anti-preferred-dominated signal. In the last third of the delay, directional activity is rare regardless of sign. B, The incidence of MT neurons with significant directional signals dominated by the preferred (pref; blue, top) and anti-preferred (antipref; red, bottom) directions, as a function of time in the sample and delay. Interrupted curves show the incidence for trials with a higher direction range (150 and 300° ranges). As expected from the data in Fig. 6A, directionality is less common at higher range levels. C, Distribution of durations of significant directional periods in MT occurring during the delay activity for preferred-dominated (blue) and anti-preferred-dominated (red) signals. Note that the anti-preferred signals were, on average, significantly longer than the preferred signals (p < 0.001, unpaired t test). D, Time course of directional signals in individual PFC neurons (n = 67). The pattern here also shows a high degree of transience in the signals but differs from that in A, because the directional signals in the delay are dominated by the preferred direction. Signals driven by the anti-preferred direction are relatively uncommon. E, The incidence of PFC neurons with significant directional signals. Preferred-dominated directional signals are less common at higher range levels, both during the sample and most of the delay. Overall, directional signals during the latter half of the delay are more common in the PFC than they are in MT. F, In the PFC, the preferred direction-dominated signals lasted longer than those dominated by the anti-preferred signals (p < 0.05, unpaired t test).
Area MT
The ROC analysis revealed strong positive correlation between firing rates and stimulus direction (Fig. 7A, blue line segments) during the sample. This is expected because all MT neurons subjected to this analysis responded more strongly to the preferred direction of the sample. In the first few hundred milliseconds of the delay, the directional signals continued to be dominated by the preferred direction. In the subsequent period, the correlation between the activity and sample direction became mostly negative (red line segments), followed by brief periods of directionality of both types, appearing with decreasing frequency at different times in different neurons. These data are summarized in Figure 7B, which shows the incidence of directional signals during the sample and delay associated with the preferred (top graph, solid blue curve) and the anti-preferred (bottom graph, solid red curve) sample. During the first 500 ms of the delay, the number of neurons firing more after the preferred direction decreased and the number of neurons dominated by the anti-preferred direction increased. Both types of signals become less frequent with time, and toward the end of the delay, only ∼5% of MT neurons carried reliable signals reflecting sample direction. The data in Figure 7B also show that the number of neurons representing sample direction during the sample and during the delay decreased with direction range in the sample.
The length of the directional periods, shown in Figure 7A, illustrates an important feature of the delay activity in MT. Specifically, in the vast majority of recorded neurons, the representation of sample direction during the delay was transient rather than sustained and could appear and disappear more than once in the same neuron. We estimated the duration of these transient signals by calculating the length of each significant period of activity after a coherent (0° range) sample. The distribution of these durations is shown in Figure 7C (color-coded as above) by whether higher activity followed the preferred (blue) or anti-preferred (red) sample. On average, signals dominated by the preferred direction lasted 156 ms (SD, 77), whereas those dominated by the anti-preferred direction were significantly longer (p ≪ 0.001, Kolmogorov–Smirnov test), lasting, on average, 205 ms (SD, 121). In summary, during the memory delay, MT neurons carried reliable signals reflecting the direction of the preceding sample. These signals were transient, dominated by the anti-preferred direction, and become rare at the end of the delay.
PFC
The analysis of PFC activity also revealed directional signals during the delay. However, these signals, starting in the sample and carrying well into the delay, were dominated by the preferred direction (Fig. 7D, blue segments). The data in Figure 7D illustrate the transient nature of these directional signals, their appearance at different times in different neurons, and their relative paucity at the end of the delay. The incidence plots (Fig. 7E) show that after the preferred sample, early delay activity represented the preceding sample direction in ∼60% of neurons. The number of neurons carrying this information gradually decreased, dropping down to ∼10% at the end of the delay. After the anti-preferred sample, significant delay activity was minimal during the first few hundred milliseconds of the delay, increasing to ∼10% toward its end. Thus, just before the appearance of the comparison test, ∼20% of neurons carried directional signals regardless of sign. The analysis of durations of directional signals occurring in the delay (Fig. 7F) revealed that, like in MT, the majority of significant directional epochs were quite brief, rarely extending beyond 500 ms and none persisting throughout the delay. The average duration of signals dominated by the preferred direction (Fig. 7F, blue) was 303 ms, whereas the less frequent, anti-preferred-dominated signals were significantly shorter with the mean of 177 ms (p < 0.05, Kolmogorov–Smirnov test). Thus, although sample direction is not represented consistently in the activity of individual neurons throughout the memory delay, it is represented within the population of recorded PFC neurons. Surprisingly, this representation is weakest at the end of the delay, at the time when the information is most needed.
Comparison of delay activity in MT and PFC
The pattern of delay activity was notably different between the two areas. After just one-third (500 ms) of the delay, the preferred-dominated signal in MT during 0° range trials was only seen in 4% of the neurons, whereas the analogous signal in the PFC was seen in 24% of the neurons, a significantly higher proportion (p < 0.001, χ2 test). On the other hand, the anti-preferred-dominated signal was found only in 7% of the PFC neurons but in significantly more MT neurons (22%; p < 0.025, χ2 test). It is interesting to note that proportions of neurons with opposite directionality were so similar between the two areas. Regardless of sign, 26% of MT neurons and 31% of PFC neurons were direction predictive at this time in the delay, and these proportions are not significantly different (p > 0.05, χ2 test). Signals in this early period of the delay are of particular interest. It is a time associated with “loading” sensory information into maintenance circuitry, and the time when masking or electrical stimulation maximally disrupts performance (Compte et al., 2000; Wang, 2001; Harris et al., 2002; Pasternak and Zaksas, 2003). The high proportions of neurons in both areas representing sample direction in the early delay may be related to the importance of this period in working memory. However, by the very end of the delay, just before test onset, only 5% of MT neurons were predictive of sample direction, regardless of sign, whereas 20% of the PFC neurons were direction predictive, a significantly higher proportion (p < 0.01, χ2 test). Thus, despite the absence of sustained predictive signals in the activity of individual neurons, the PFC population appeared to carry such signals with higher frequency than MT later in the memory delay. In addition, directional signals in the PFC (mean, 268 ms; SD, 219; median, 175) lasted slightly but significantly longer than in MT (mean, 183 ms; SD, 106; median, 150 ms; p = 0.001, Kolmogorov–Smirnov test). Considered alone, this result would be consistent with a role of PFC in maintenance. However, on trials with a less discriminable sample (Fig. 7B,E, 300° range, lightest curves), the directional signals in the PFC occurring late in delay were negligible. Given the significantly above-chance performance under these conditions (64% correct, on average), it is doubtful that the delay activity in PFC is sufficient to support successful performance. Nevertheless, it is conceivable that confidence in the available signal at the 300° range would increase if the information was pooled across a much larger number of neurons than those recorded here. It is also possible that the conservative criterion of a 99% confidence level could have lead to an underestimated number of neurons with significant ROC indices. However, we believe that this more stringent criterion provides a more reliable estimate of the data (see Materials and Methods).
Responses to the test
Responses to the test were of particular interest because they represent the time of comparison and decision leading to the completion of the trial. At the time of the test, neurons relevant to the task must have access to the information about the remembered sample. Thus, we asked whether test responses were modulated by the previously seen sample direction. This analysis was only performed on data collected during the direction range task, because during this task, the test moved in the preferred direction on trials in which it matched the sample and on trials in which it was preceded by the anti-preferred sample (i.e., non-match trials). This analysis could not be performed in the direction accuracy task because the structure of this task was such that the test moved in the preferred direction only when it followed the preferred sample. Figure 8A shows average test responses of 169 neurons in MT (left plot) and 60 neurons in the PFC (right plot) to the preferred coherently moving test sorted by whether the preceding sample had been a match (also preferred) or a non-match (anti-preferred). We found that during the test response, neurons in both areas had a period of lower firing rate if the preceding sample had been a match rather than a non-match. Individual neuron firing rates, at the time of the maximal effect in both areas, are shown in the scatter plots of Figure 8B. Firing rates were significantly lower in both areas when the preferred test was a match to a preferred sample (MT, p ≪ 0.001; PFC, p < 0.05; paired t test in each case). Because MT responses to the sample did not differ from those to a non-matching test (p = 0.35, paired t test), it is likely that the lower firing rates on match trials represent active suppression. It is not clear whether this mechanism may be similar in the PFC. The average response to the sample, at the time of the maximal difference between the matching and non-matching test, was intermediate but not significantly different from neither type of the test responses (non-match, p = 0.46; match, p = 0.49; paired t test).
Figure 8.
Modulation of test responses by sample direction. A, Average normalized responses to the test in MT (n = 169) and the PFC (n = 60) shown for trials in which the preferred test was a match (dotted line) or a non-match (solid line) to the preceding sample. B, Comparison of firing rates of individual neurons contributing to the averages in A to the preferred test during match and non-match trials. Rates were calculated for a 100 ms window centered on the time when the match suppression was most reliable (130 and 230 ms after test onset for MT and the PFC, respectively). C, Average reliability of the differences in responses during the test that matched and did not match the sample, computed by ROC analysis. ROC values <0.5 indicate lower firing rates during the matching test. In both areas, the effect is transient. However, it appears in MT 100 ms earlier than in the PFC. Thin gray curves indicate ±1 SEM. The mean of each distribution is indicated by an arrow. D, Distributions of ROC values for individual neurons at the time of maximal effect (MT, 130 ms; PFC, 230 ms). Black bars represent ROC values significantly different from 0.5 (95% confidence level, permutation test). E, Effect of direction range in sample on the match effect in test. The average ROC for each group of neurons, at the respective times of maximal effect, is shown as a function of preceding sample coherence. Test responses in both areas were reliably modulated by sample direction regardless of sample coherence (MT: p < 0.01, t test, at each range level; PFC: p < 0.01, t test, at 0 and 150° range; p < 0.05, t test, at 300° range). Error bars are ±1 SEM. sp/s, Spikes per second; deg, degree.
We used ROC analysis to establish the reliability of differences between responses to matching and non-matching test stimuli. Here, in the match condition, values >0.5 correspond to higher firing rates, whereas in the non-match condition, values <0.5 correspond to higher firing rates. In MT (Fig. 8C, left), reliable modulation of the test response by preceding direction was relatively rapid and transient, becoming maximal ∼130 ms from stimulus onset. The distribution of ROC values for individual neurons computed at that time are shown in Figure 8D (left). The mean for the population was 0.439, a value significantly different from 0.5 (p ≪ 0.001, t test).
A similar effect and a significant ROC value of 0.46 was seen in the PFC (Fig. 8C,D) (p < 0.01, t test), although it reached its maximum 100 ms later, 230 ms after stimulus onset. Thus, both MT and PFC responses to the test reliably indicate whether the preceding sample direction had been the same or different, although this correlate arises earlier in MT. We also examined the presence of the match/non-match effect when the sample contained a greater range of local directions (Fig. 8E). In MT, the test response was reliably modulated by 150 and 300° sample (p ≪ 0.001, t test). Similarly, test responses of PFC neurons also modulated significantly by 150 and 300° sample direction (p < 0.01 and p < 0.05, t test).
It was important to consider whether this effect, particularly in the sensory MT neurons, could simply be caused by the adaptation of neurons to a preferred sample. Van Wezel and Britten (2002) showed that MT responses to motion stimuli decrease after a 3.0 s adaptation and a brief 0.5 s delay. Our paradigm had a substantially shorter (0.5 s) sample and a longer (1.5 s) delay period, making the detection of such an adaptation effect less likely. In addition, Van Wezel and Britten (2002) showed that magnitude of the adaptation effect was directly proportional to the firing rate during the first stimulus and independent of its coherence level. In our data, decreasing coherence of the sample led to a decrease in the size of the effect. Importantly, even at relatively high coherence (150° range), in trials during which the match suppression effect was maximal (Fig. 8E), we found no significant correlation between the sample firing rate and the magnitude of match suppression (r = 0.09; p > 0.3, regression analysis). This evidence suggests that although classic adaptation cannot be ruled out as a potential source of the match effect, it is unlikely to be a major contributor.
In summary, there are important similarities in the behavior of neurons in area MT and in the PFC during the test, suggesting a potential functional link between them. After the onset of the matching test, MT neurons decrease their firing rates, reaching maximal suppression around 130 ms. PFC neurons also lower their firing rates during such stimuli, but this effect occurs ∼100 ms later. The presence of modulation by sample direction presented >1500 ms earlier suggests that both MT and PFC have access to the information about the remembered direction. However, each region may play a different role in the ongoing process of comparison between the test and the remembered sample. The earlier appearance in MT of the match signals point to a scenario in which MT neurons would perform the initial comparison, passing the differential signal to PFC.
Behavioral relevance of directional activity in MT and the PFC
Task dependence
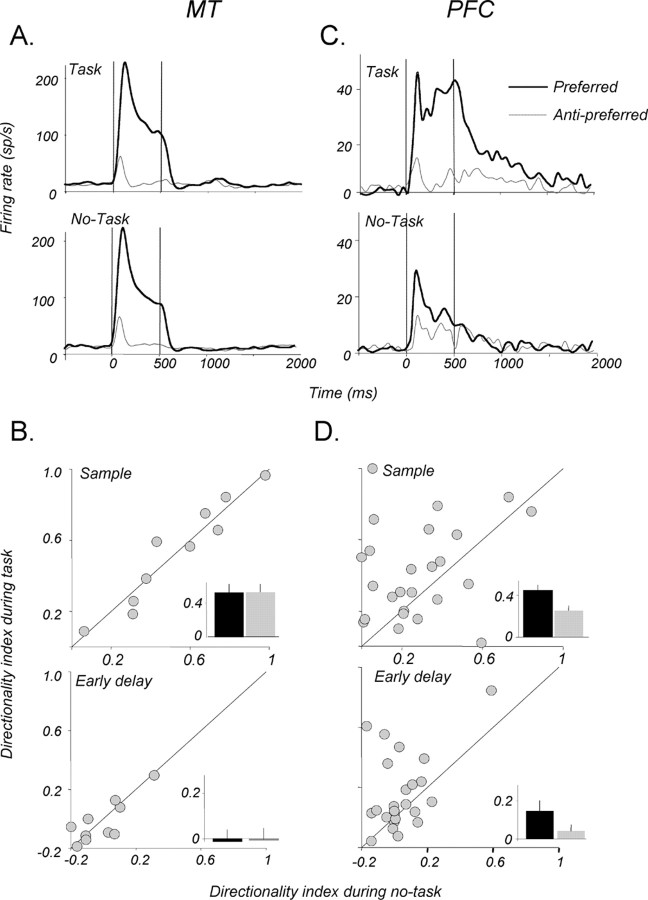
Because our goal was to determine whether MT and PFC neurons contribute to behavioral performance, we examined whether the direction selectivity encountered in the two areas depends on the behavioral context in which visual motion is presented. We recorded the activity of neurons in each area while the monkeys performed the memory for motion task and during a no-task block of trials (Fig. 1D), in which the stimuli, their location, and the length of the delay were identical to those used in the discrimination task. The behavior of two example neurons, one from MT and the other from the PFC during the direction range task and the no-task condition is shown in Figure 9, A and C. The inspection of the example MT neuron revealed no obvious differences under the two conditions. Indeed, for the small number of MT neurons we examined (Fig. 9B), we found no significant changes in directionality (expressed as the DI) during the sample (p > 0.05, paired t test) or during the first 500 ms period of the delay (p > 0.05, paired t test). Similarly, there were no significant differences in activity in the middle or late 500 ms of the delay after the preferred or the anti-preferred direction (data not shown; p > 0.05, paired t test in each case). Thus, direction selectivity in MT did not appear to be affected by the demands of the behavioral task, at least during the no-task paradigm used here. In light of several reports that activity in MT can be modulated by attention (Treue and Maunsell, 1996; Cook and Maunsell, 2004), the absence of modulation by task demands observed here may be a result of the monkey's attention not being actively directed away from the RF.
Figure 9.
Task dependence of directional signals. A, Activity of an example MT neuron during the direction range task (top) and the no-task condition (bottom). The behavioral demands did not change either the response magnitude or the strong direction selectivity. B, Comparison of the directionality indices for a subset of neurons recorded in MT during task performance and the no-task condition. The task requirement did not significantly alter direction selectivity in either the sample (top) or the early period (first 500 ms) of the memory delay (p > 0.05, paired t test in each case). C, Activity of an example PFC during the direction range task (top) and during the no-task condition (bottom). When performance was not required, the directionality of the neuron was greatly diminished both during the sample and in the early delay. D, Directionality indices for PFC neurons recorded during the task and during the no-task condition. Note the significant decrease in directionality, both in the sample and the early delay, when the performance requirement is removed (p < 0.05, paired t test in each case). Insets, Bar plots show the average DI for the two conditions. Error bars are ±1 SEM.
The behavior of PFC neurons during the no-task condition was markedly different from that observed in MT. The example neuron (Fig. 9C) displayed strong direction-selective activity during the sample and the early delay but became much less directional during the no-task trials. This task dependence of directional sample response and early delay activity was present, on average, in the group of 25 PFC neurons for which no-task data were collected (Fig. 9D) (p < 0.05, paired t test for each epoch). As in MT, the no-task effect was detected in the middle and late epochs of the delay (data not shown; p > 0.05, paired t test in each case). Thus, direction selectivity of PFC neurons was strongly affected by task demands, suggesting that stimulus selectivity in the PFC may be actively gated out (or suppressed) when stimulus direction is not relevant to the task. This result is consistent with previous reports of behavioral context affecting stimulus selectivity in prefrontal neurons (Asaad et al., 2000) providing support for the adaptive coding model of PFC activity (Duncan, 2001), whereby the “function” of PFC neurons is determined less by an intrinsic specialization than a flexibility of adapting their selectivity to a currently relevant feature. On the other hand, direction selectivity decreased but was not eliminated when the monkeys were not required to perform the task. One plausible explanation is that monkeys may have simply reduced, but not eliminated, the attentional allocation toward the motion stimuli. Alternatively, the residual direction selectivity in the PFC during the no-task condition may simply reflect motion signals made available by direct bottom-up inputs.
Activity on error trials
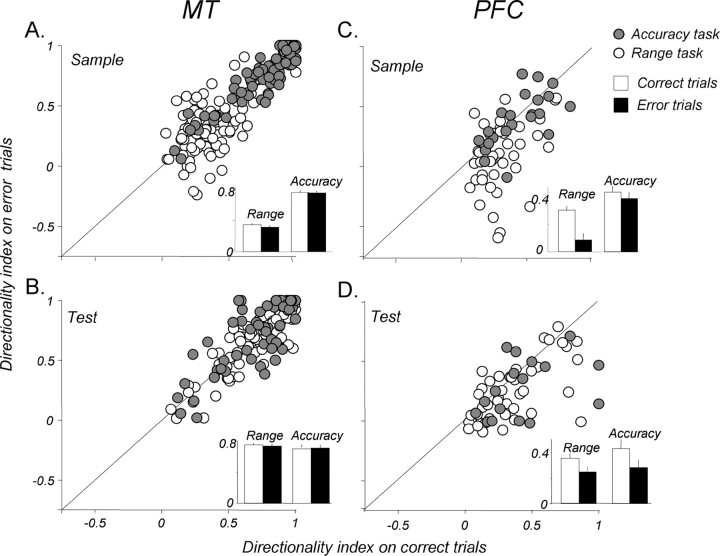
We also evaluated the behavioral relevance of directional signals by examining activity on trials in which the monkeys made errors. It is difficult to determine the causes of errors, but it is likely that they correlate with changes in the animal's attentional state. Allocating attention has been shown to increase stimulus selectivity in visual neurons (for review, see Reynolds and Chelazzi, 2004). We therefore reasoned that direction selectivity, particularly in the PFC, given the no-task result above, may decrease on error trials. As seen in Figure 10, A and B, the strength of directionality in MT during the sample and test was similar on correct and error trials both during the direction range and direction difference tasks (p > 0.05, paired t test in each case).
Figure 10.
Direction selectivity on error trials. A, Directionality of MT responses to sample during correct and error trials. Open circles show DI from the direction range task, and filled circles show DI from the direction accuracy task. Insets, Bar plot shows the average DIs for the two conditions, with no significant difference (p > 0.05, paired t test for both tasks). B, Directionality of MT responses to the test on correct and error trials. The average DI is not significantly different during correct and error trials in either task (p > 0.05, paired t test for both tasks). C, Directionality of PFC responses to sample on correct and error trials. There is a significant decrease in directionality on error trials in the direction range task (p ≪ 0.001, paired t test), but not in the accuracy task (p > 0.05, paired t test). D, Directionality of PFC responses to the test. Average DIs on error trials were significantly lower than that on correct trials in both tasks (range: p < 0.005, paired t test; accuracy: p < 0.05, paired t test). Error bars are ±1 SEM.
This was not the case in the recorded PFC neurons. Here, during the range task, direction selectivity of both sample and test responses was significantly diminished on error trials (Fig. 10C,D, open circles) (sample: p ≪ 0.001, paired t test; test: p < 0.005, paired t test), with a significantly larger decrease in DI during the sample (72% lower) than during the test (30% lower) (p < 0.05, unpaired t test). During the accuracy task (Fig. 10C,D, gray circles), no error effect was seen in the sample, but the directionality was significantly lower during the test (p < 0.05, paired t test). Because direction selectivity on error trials did not decrease in MT, the effects in the PFC are unlikely to be attributable to a change in bottom-up input. Rather, this pattern of effects may be related to the differences between behavioral strategies and attentional demands of the two behavioral tasks. Performance of the direction range task is limited mainly by the ability to integrate sample direction rather than the subsequent same–opposite comparison. Conversely, the direction accuracy task performance is limited by the ability to correctly perform the comparison, during the test, of two similar directions. Thus, it is likely that attentional demands are generally greater during the sample in the direction range task and during the test in the accuracy task. Given this distinction, a link between errors and a change in the animals' attentional state is strongly supported by a greater decrease in sample directionality during the direction range task and the decrease in test directionality during the accuracy task. Analysis of three consecutive 500 ms epochs of delay activity (early, middle, and late) revealed no effect of errors on direction selectivity in either area or task (data not shown; p > 0.05, paired t test in each case). This absence of error effects could be attributable to the possibility that such effects may be difficult to detect because of the variable and the weak nature of direction-selective signals recorded during the delay (Fig. 7).
Relating neuronal activity to behavioral choices
Sample and delay
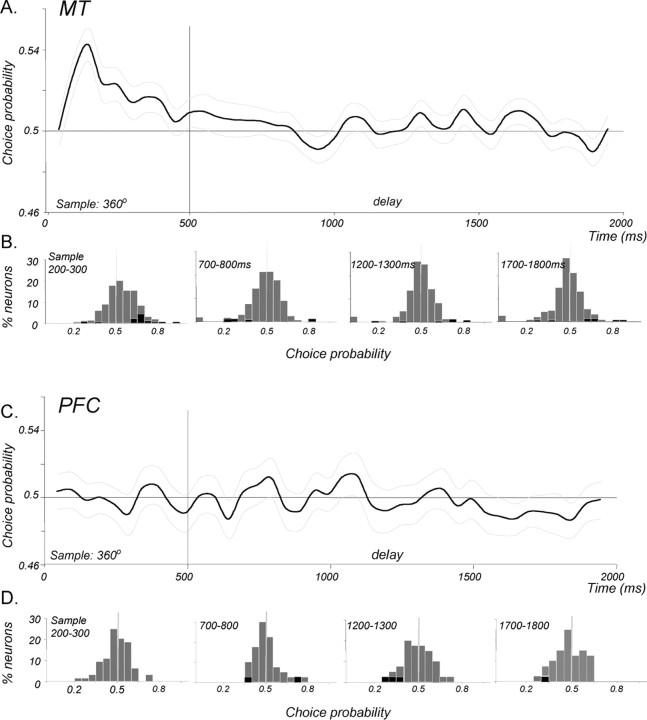
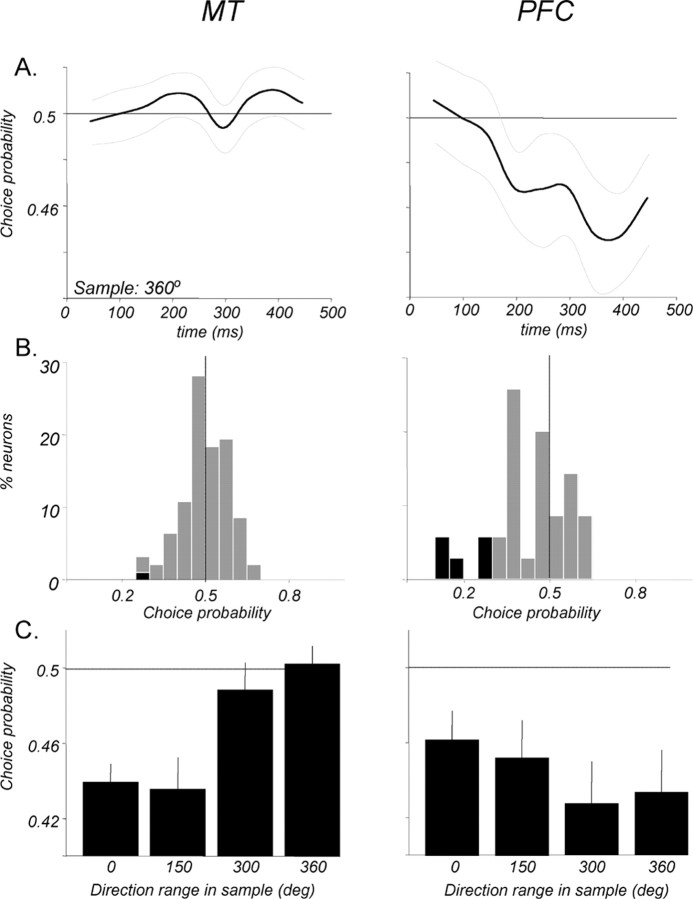
To gain insight into the link between these neurons and behavior, we asked whether signals available in MT and the PFC during the sample and memory delay might contribute to the monkey's upcoming decision. To this end, we calculated CP, an ROC-based measure that correlates the trial-by-trial variability of the neuronal response with the behavioral outcome of those trials (Britten et al., 1996). The analysis was applied to trials in the direction range task in which the sample contained only random motion (360° range), thus having no net direction. The use of the random motion sample removed the potential confound of relating firing rates to the real rather than the perceived sample direction indicated exclusively by the monkey's choice. The perceived direction of such a sample could be gleaned from the choice because the monkey always reported it to be the same or “different” as a test that had coherent motion with an easily identifiable direction. For example, a same report that followed the test moving in the preferred direction, or a different report that followed the test moving in the anti-preferred direction, would both indicate that the monkey had perceived the preceding 360° sample to move in the preferred direction. The average CP for each population as a function of time is shown in Figure 11, A and C, and Figure 11, B and D, shows the distributions of individual neuron CPs during 100 ms windows in the sample (centered 150 ms after stimulus onset) and delay (centered 750, 1250, and 1750 ms after sample onset). MT responses to the sample were significantly correlated with the monkeys' indication of perceived direction, having an average CP of 0.542 (p ≪ 0.001, t test; n = 170). These perception-related signals began early and were transient, disappearing by ∼400 ms into the sample response. No such signals were seen during any part of the memory delay.
Figure 11.
CP during the sample and delay. The relationship between firing rates and the monkeys' choices was evaluated with ROC analysis on trials when the sample contained no net direction (360° range). A, Average CP for 170 MT neurons during the sample and memory delay. A positive significant CP (p < 0.001, t test) was seen during the sample response, correlating higher firing rates with choices that indicated the sample as having been preferred. No such correlation was present from the end of the sample response through the entire memory delay. Light gray curves indicate ±1 SEM. B, Distributions showing CPs of individual neurons during the sample and three representative epochs of the delay. Significant individual CPs (95% confidence, permutation test) are indicated by black bars. C, Average CP for 40 PFC neurons during the sample and memory delay. No significant correlation between firing rates and choices (p > 0.05, t test) was seen at any time during the sample or memory delay. D, Distributions showing CPs of individual PFC neurons during the sample and three representative epochs of the delay. Details are as in B.
It is important to note that the although activity correlated with the monkeys' decisions on a trial-by-trial basis, this correlation was likely to be related to the perception of the sample direction rather than to the anticipation a specific motor response. The structure of the trials ensured that before the test onset the monkey could never predict or anticipate either the test direction or its relationship to the sample.
Surprisingly, PFC responses did not significantly correlate with the perceived sample direction as indicated by the monkeys' decisions, with the average sample CP of 0.496 (p > 0.05, t test). There were also no signals related to perceived sample direction detectable at any time during the memory delay. The presence of such signals in MT during the sample implies that monkeys' perception of direction can be linked to MT activity during the task. It also necessitates that the representation of perceived sample direction must be carried in some form, somewhere in the brain, continuously through the duration of the delay. The lack of significant CP in MT and in the PFC during the delay suggests that the relevant signals used by the monkey may be provided by other cortical regions. Alternatively, the mechanism through which these signals are maintained in the delay may be different from the rate code that expressed them during the sample.
Test: representation of same–different decision
Although the analysis of test responses (Fig. 8) revealed strong modulation by sample direction, it did not address whether this modulation is linked to the same–different decision made by the monkeys. Because the monkeys made a number of errors, there were trials in which, for example, the sample may have been a match to the preferred test, but the monkey reported it as different. The link between test responses and behavior can therefore be established by analyzing the trials based on choices rather than the actual stimulus directions. On trials in which the sample moved coherently, the virtual absence of errors ensured that the probability of a same choice (Fig. 12C) is almost identical to the probability of the sample having actually been a match (see above and Fig. 8). Indeed, the average CPs calculated for MT and PFC neurons at the time of maximal match/non-match effect (Fig. 8 C) were nearly identical to those computed in the ROC analysis (0.439, p ≪ 0.001 and 0.462, p < 0.05, respectively; t test). Measuring CP, however, proved more useful in the analysis of the 360° range trials, in which the monkey made approximately an equal number of same and different choices given a sample with no net direction (Fig. 12C).
Figure 12.
Representation of choice in the test response. A, Average CP in the test response, relating preferred test response rates to whether it was reported as the same as or different from the noncoherent (360° range) sample. Test responses in MT (left; n = 92) show no correlation with the decision (left), whereas test responses in the PFC (right; n = 35) show a significant decision correlate evolving with time. B, Distributions of CPs of individual neuron in both areas. Data for MT (left) were calculated for a 100 ms window covering the 100–200 ms epoch of the test response. Data for the PFC (right) were calculated for a 100 ms window centered at the time of the maximal CP, 380 ms after test onset. Significant CPs are indicated by black bars. C, Average CPs in the test response in each area as a function of sample direction range. When sample coherence was high (0 and 150° range), monkeys made very few errors, and thus the average CPs in both regions were similar to the average ROCs (see Fig. 8E) relating firing rates to the stimulus directions. However, at low or no sample coherence (300 and 360° range), test responses in MT did not correlate with decisions, whereas responses in the PFC did so with high reliability (p < 0.01 in both cases, t test). Error bars are ±1 SEM. deg, Degree.
This analysis revealed that test responses in MT did not correlate with the monkey's decision (130 ms from test onset; mean CP, 0.502; p > 0.05, t test), whereas test responses in the PFC did, late in the response (380 ms from test onset; mean CP, 0.434; p < 0.01, t test). The average CP data calculated at the time of the maximal match/non-match effect are summarized in Figure 12C. In MT, when the sample contains the 300 or 360° range, the correlation of the test response with the monkey's choices breaks down. In the same set of 300° range trials, MT responses correlate with the direction of the preceding sample (Fig. 8D), but not with the monkeys' decisions regarding that direction (Fig. 12C). In contrast, PFC responses show significant choice probabilities at all levels of direction range and thus correlate with the decisions regardless of the sample coherence. Together, the data suggest that soon after the onset of the test, MT neurons are reliably modulated by stored sensory evidence of the sample direction (Fig. 8). However, this signal, a correlate of the comparison, is not predictive of the upcoming response. On the other hand, once the same correlate of the comparison process appears in the PFC, the signal is reliably predictive of the monkey's same/different decision.
Discussion
We found that direction-selective activity, common in MT neurons, was also expressed by neurons in the PFC, although in a task-related manner and emerging significantly later after stimulus onset. During the memory delay, the directional signals, present in both areas, were transient, becoming less common with time. In contrast to MT, PFC delay activity was dominated by the preferred direction, lasted, on average, ∼100 ms longer, and was more likely to carry directional signals late in the delay. During the sample, MT activity correlated with the monkeys' choice, but the correlation did not persist into the delay or the test. PFC neurons, on the other hand, did not have choice-related activity during the sample or the delay, but a decision correlate did arise during the test.
Use of motion representation in MT and the PFC
Decades of research have cemented a central role for MT neurons in the processing of visual motion (Maunsell and Van Essen, 1983; Movshon et al., 1985), and their activity has been linked closely to the perception of motion (Newsome et al., 1989; Salzman et al., 1990; Bisley et al., 2001). Can any direction-selective neurons be linked to motion perception, or do areas like MT contribute more specifically to this vital function? A part of the answer is gleaned from the present experiment. We found that sample responses in MT correlated with the monkeys' report of sample direction, but sample responses of direction-selective neurons in the PFC did not. Significant choice-related activity in MT has been reported previously by a number of investigators (Britten et al., 1996), and the finding of activity predictive of the monkey's choice in the context of a more complex same/different task provides additional support for a close link between direction-selective activity in MT and motion perception.
The contrast in this relationship between directional activity in MT and the PFC and perceptual decisions is significant because it suggests that a reliable directional signal does not translate into equivalent use across cortical areas. Despite the absence of a direct link between prefrontal activity during the sample and perceptual decisions, the behavioral relevance of this activity was revealed by reduced direction selectivity during the no-task condition and, during the task, on error trials. Such results are consistent with the PFC playing a role in directing attention to behaviorally relevant sensory stimuli (Rowe and Passingham, 2001; Curtis and D'Esposito, 2003). This modulation of direction selectivity by the animal's behavioral state suggests the presence of an active gating mechanism, consistent with that postulated by previous work (Seidemann et al., 1998; Bisley et al., 2001). It, likewise, supports the model of the PFC as a substrate for executive control in which neuronal activity and stimulus selectivity are highly adaptable to current task demands (Duncan, 2001). Although their directional responses to the sample do not correlate with perception, PFC neurons may still contribute to task performance. For instance, use of these responses may lie in transmitting relevant sensory input to the circuitry underlying working memory and the ensuing comparison to the test.
Mechanism of maintenance
Classic physiological studies of visual working memory have identified the PFC as an important region for sensory signal maintenance (Goldman-Rakic, 1996; Compte et al., 2003). However, a number of recent studies linked the PFC to attention and response selection, rather than sensory maintenance (Curtis and D'Esposito, 2003; Postle et al., 2003; Lebedev et al., 2004; Passingham and Sakai, 2004). Meanwhile, converging evidence supports extensive involvement of sensory areas in short-term storage of the stimulus dimensions they encode (Fuster, 1997; Pasternak and Greenlee, 2005; Postle, 2006).
The precise mechanisms for maintenance of sensory information during a memory delay are not known. Studies of inferotemporal and prefrontal cortices originally proposed that maintenance could be expressed through persistent elevated activity that was selective for the remembered object property or location (Fuster and Alexander, 1971; Kubota and Niki, 1971; Funahashi et al., 1989; Goldman-Rakic, 1990). More recently, a number of studies reported that much of the delay activity is dynamic rather than simply sustained, and the remembered information is often represented by the firing rate only for a portion of the delay (Romo et al., 1999; Rainer and Miller, 2002; Baeg et al., 2003). These observations are supported by the finding that in both areas the direction of motion is represented by firing rates in an intermittent manner, inconsistent with sustained activation. Although at any time during the delay there were neurons with firing rates reflecting the sample direction, virtually no neurons in either area appeared to host sustained mnemonic activity. Even more surprising, the delay activity in neither area correlated with the monkeys upcoming choices, despite the fact that such a correlation did exist in MT during the sample, immediately preceding the delay.
There is a possibility that sustained, directional, and choice-related delay activity is not a feature of MT or PFC neurons but is characteristic of other brain regions not yet studied in the context of the current behavioral task. A plausible candidate are regions in the parietal cortex, where signals related to maintenance of spatial locations for saccades, similar to those observed in the PFC, have been found previously (Chafee and Goldman-Rakic, 1998). However, this is less likely, because unlike in the PFC, parietal delay activity can be disrupted by visual distracters unrelated to task performance (Constantinidis and Steinmetz, 1996; Powell and Goldberg, 2000). We cannot rule out the participation in maintenance of other dorsal stream areas because they have not been studied in the context of memory for motion tasks.
The absence of sustained delay activity in MT and the PFC needs to be reconciled with vast data implicating sensory areas, and certainly the PFC, in sensory storage. The answer may require that we re-evaluate the contribution of sustained activation to the mechanism of maintenance. Intermittent representation of motion in the delay, as observed here, may be indicative of a storage mechanism that operates on a network level and would not require sustained activation of single neurons. One possibility is that maintenance is achieved through a temporal rather than a rate code. There is little evidence for temporal structure of spikes in the PFC on a single neuron level (Compte et al., 2003). Nevertheless, it has been shown with computational modeling and recording of local field potentials that temporally irregular spiking of individual neurons can be effectively harnessed by a regularly oscillating network during a memory delay (Pesaran et al., 2002; Brunel, 2003; Brunel and Wang, 2003; Lee et al., 2005). Although it is difficult to assess the functional significance of transient delay activation, another possible mechanism for storage could include circuitry in which groups of single neurons are recruited sequentially rather than in a sustained manner (Batuev et al., 1979). A recent study of delay activity in rodent PFC supports the possibility of such mechanism (Baeg et al., 2003).
In all, the absence of persistent, rate-coded, choice-related delay activity in MT and the PFC does not necessarily exclude them from the maintenance circuitry. Instead, the similarity of the temporal characteristics of delay representation (brief durations of selectivity) between the two areas suggest that they are probably linked in a shared storage circuitry, the modus operandi of which has yet to be fully described. In fact, the immediate presence of stored signal in MT is supported by the rapid emergence of modulation of test responses by the preceding sample direction.
Comparison, decisions, and the representation of behavioral outcome
The successful execution of our task required the comparison of test direction with that of the preceding sample, leading to a decision expressed by a motor response. The PFC has been linked to decision-making circuitry (Kim and Shadlen, 1999; Romo and Salinas, 2003), whereas a lesion study provided evidence that MT is necessary for optimal execution of the retrieval/comparison phase of the task identical to that used here (Bisley and Pasternak, 2000). Thus, we were particularly interested in the behavior of neurons in both areas at the time of comparison and decision.
We found that in both areas, the response to the test was transiently lower when its direction matched the preceding sample. This result is in line with a number of previous studies that showed lower activity in multiple cortical areas in response to familiar rather than novel stimuli (Ranganath and Rainer, 2003). A plausible interpretation of the match effect is that it represents a process of sensory comparison between the test and the remembered sample. Compared with the PFC, MT neurons express the match effect much earlier, almost immediately after starting to respond. Such rapid access to the sample direction supports the idea of direct connectivity or participation in the maintenance circuitry. It also suggests that MT neurons may represent the first site of the comparison process. A similar comparison correlate was recently observed in the secondary somatosensory cortex, thus supporting the role of sensory neurons as a logical substrate of comparison along the stimulus dimension they specialize in processing (Romo et al., 2002).
Although our data revealed a reliable match effect in both areas, only the PFC activity appeared to reliably predict the monkeys' choices (Fig. 12A). How, or whether, this decision correlate first arises in the PFC neurons is unclear. It may, as suggested by a model proposed by Mazurek et al. (2003), reflect the integration of evidence from earlier stages of processing. Alternatively, it could be at the core of the goal-directed decision-making process (Koene and Hasselmo, 2005) or could represent the behavioral output of the decision in the form of a motor plan.
Concluding remarks
Together, our results show that successful execution of our task is likely to depend on a network of functionally linked cortical areas that include MT and the PFC. They also discern some of the respective contributions of the two regions. During visual stimulation, both areas represent motion direction and have access to the stored signals at the time of the comparison and decision. The properties and timing of these signals point to MT as their likely source, whereas the unique ability of PFC neurons to gate these signals suggest they may play an important executive role during the task. One of the more surprising observations was that, despite reliable directional signals in both areas during the memory delay, there was no detectable correlation between this activity and behavioral choice, raising a question about the role of neurons in area MT and the PFC in maintenance. It remains to be seen whether the apparent absence of behaviorally relevant delay signals in the two areas is indicative of an alternative mechanism of maintenance not revealed by the analysis of firing rates of individual neurons.
Footnotes
This work was supported by National Eye Institute Grants RO1 EY11749 (T.P.), T32 EY07125 (D.Z.), and P30 EY01319 (Center for Visual Science). We thank Nicholas P. LaMendola for help with data collection and analysis, Marc Mancarella for excellent technical assistance, and Martin Gira for help with electronics. Cory Hussar contributed to some of the data analysis. We also thank Mike Shadlen, John Assad, Dominic Barraclough, and Larry Abbott for comments on this manuscript.
References
- Amemori K-i, Sawaguchi T. Rule-dependent shifting of sensorimotor representation in the primate prefrontal cortex. Eur J Neurosci. 2006;23:1895–1909. doi: 10.1111/j.1460-9568.2006.04702.x. [DOI] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol. 2000;84:451–459. doi: 10.1152/jn.2000.84.1.451. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Oxford: Clarendon/Oxford UP; 1986. Working memory. [Google Scholar]
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 2003;40:177–188. doi: 10.1016/s0896-6273(03)00597-x. [DOI] [PubMed] [Google Scholar]
- Baker JF, Petersen SE, Newsome WT, Allman JM. Visual response properties of neurons in four extrastriate visual areas of the owl monkey (Aotus trivirgatus): a quantitative comparison of medial, dorsomedial, dorsolateral, and middle temporal areas. J Neurophysiol. 1981;45:397–416. doi: 10.1152/jn.1981.45.3.397. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- Batuev AS, Pirogov AA, Orlov AA. Unit activity of the prefrontal cortex during delayed alternation performance in monkey. Acta Physiol Acad Sci Hung. 1979;53:345–353. [PubMed] [Google Scholar]
- Bisley JW, Pasternak T. The multiple roles of visual cortical areas MT/MST in remembering the direction of visual motion. Cereb Cortex. 2000;10:1053–1065. doi: 10.1093/cercor/10.11.1053. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Zaksas D, Pasternak T. Microstimulation of cortical area MT affects performance on a visual working memory task. J Neurophysiol. 2001;85:187–196. doi: 10.1152/jn.2001.85.1.187. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Zaksas D, Droll J, Pasternak T. Activity of neurons in cortical area MT during a memory for motion task. J Neurophysiol. 2004;91:286–300. doi: 10.1152/jn.00870.2003. [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Brunel N. Dynamics and plasticity of stimulus-selective persistent activity in cortical network models. Cereb Cortex. 2003;13:1151–1161. doi: 10.1093/cercor/bhg096. [DOI] [PubMed] [Google Scholar]
- Brunel N, Wang X-J. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J Neurophysiol. 2003;90:415–430. doi: 10.1152/jn.01095.2002. [DOI] [PubMed] [Google Scholar]
- Burman K, Palmer S, Gamberini M, Rosa M. Cytoarchitectonic subdivisions of the dorsolateral frontal cortex of the marmoset monkey (Callithrix jacchus), and their projections to dorsal visual areas. J Comp Neurol. 2006;495:149–172. doi: 10.1002/cne.20837. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79:2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- Compte A, Constantinidis C, Tegner J, Raghavachari S, Chafee MV, Goldman-Rakic PS, Wang X-J. Temporally irregular mnemonic persistent activity in prefrontal neurons of monkeys during a delayed response task. J Neurophysiol. 2003;90:3441–3454. doi: 10.1152/jn.00949.2002. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal activity in posterior parietal area 7a during the delay periods of a spatial memory task. J Neurophysiol. 1996;76:1352–1355. doi: 10.1152/jn.1996.76.2.1352. [DOI] [PubMed] [Google Scholar]
- Cook EP, Maunsell JHR. Attentional modulation of motion integration of individual neurons in the middle temporal visual area. J Neurosci. 2004;24:7964–7977. doi: 10.1523/JNEUROSCI.5102-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist CF, Yamasaki DSG, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods. 1988;26:117–122. doi: 10.1016/0165-0270(88)90160-4. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J Neurosci. 2003;23:5235–5246. doi: 10.1523/JNEUROSCI.23-12-05235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Visuospatial coding in primate prefrontal neurons revealed by oculomotor paradigms. J Neurophysiol. 1990;63:814–831. doi: 10.1152/jn.1990.63.4.814. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Network memory. Trends Neurosci. 1997;20:451–459. doi: 10.1016/s0166-2236(97)01128-4. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325–335. doi: 10.1016/s0079-6123(08)62688-6. discussion 335–326. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Miniussi C, Harris IM, Diamond ME. Transient storage of a tactile memory trace in primary somatosensory cortex. J Neurosci. 2002;22:8720–8725. doi: 10.1523/JNEUROSCI.22-19-08720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- Koene RA, Hasselmo ME. An integrate-and-fire model of prefrontal cortex neuronal activity during performance of goal-directed decision making. Cereb Cortex. 2005;15:1964–1981. doi: 10.1093/cercor/bhi072. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol. 1971;34:337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Messinger A, Kralik JD, Wise SP. Representation of attended versus remembered locations in prefrontal cortex. PLoS Biol. 2004;2:1919–1935. doi: 10.1371/journal.pbio.0020365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, Rainer G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron. 2005;45:147–156. doi: 10.1016/j.neuron.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol. 1983;49:1127–1147. doi: 10.1152/jn.1983.49.5.1127. [DOI] [PubMed] [Google Scholar]
- Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. A role for neural integrators in perceptual decision making. Cereb Cortex. 2003;13:1257–1269. doi: 10.1093/cercor/bhg097. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon JA, Adelson EH, Gizzi MS, Newsome WT. The analysis of moving visual patterns. In: Chagas C, Gattas R, Gross CG, editors. Pattern recognition mechanisms. Vatican City: Ponticifica Academia Scientiarum; 1985. pp. 117–151. [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- Osborne LC, Bialek W, Lisberger SG. Time course of information about motion direction in visual area MT of macaque monkeys. J Neurosci. 2004;24:3210–3222. doi: 10.1523/JNEUROSCI.5305-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham D, Sakai K. The prefrontal cortex and working memory: physiology and brain imaging. Curr Opin Neurobiol. 2004;14:163. doi: 10.1016/j.conb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Greenlee M. Working memory in primate sensory systems. Nat Rev Neurosci. 2005;6:97–107. doi: 10.1038/nrn1603. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Zaksas D. Stimulus specificity and temporal dynamics of working memory for visual motion. J Neurophysiol. 2003;90:2752–2757. doi: 10.1152/jn.00422.2003. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Druzgal TJ, D'Esposito M. Seeking the neural substrates of visual working memory storage. Cortex. 2003;39:927–946. doi: 10.1016/s0010-9452(08)70871-2. [DOI] [PubMed] [Google Scholar]
- Pouget P, Emeric EE, Stuphorn V, Reis K, Schall JD. Chronometry of visual responses in frontal eye field, supplementary eye field, and anterior cingulate cortex. J Neurophysiol. 2005;94:2086–2092. doi: 10.1152/jn.01097.2004. [DOI] [PubMed] [Google Scholar]
- Powell KD, Goldberg ME. Response of neurons in the lateral intraparietal area to a distractor flashed during the delay period of a memory-guided saccade. J Neurophysiol. 2000;84:301–310. doi: 10.1152/jn.2000.84.1.301. [DOI] [PubMed] [Google Scholar]
- Raiguel SE, Xiao DK, Marcar VL, Orban GA. Response latency of macaque area MT/V5 neurons and its relationship to stimulus parameters. J Neurophysiol. 1999;82:1944–1956. doi: 10.1152/jn.1999.82.4.1944. [DOI] [PubMed] [Google Scholar]
- Rainer G, Miller EK. Timecourse of object-related neural activity in the primate prefrontal cortex during a short-term memory task. Eur J Neurosci. 2002;15:1244–1254. doi: 10.1046/j.1460-9568.2002.01958.x. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Remmel RS. An inexpensive eye movement monitor using the scieral search coil technique. IEEE Trans Biomed Eng BME. 1984;31:388–390. doi: 10.1109/TBME.1984.325352. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Romo R, Salinas E. Flutter discrimination: neural codes, perception, memory and decision making. Nat Rev Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernandez A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399:470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A, Lemus L, Brody CD. Neuronal correlates of decision-making in secondary somatosensory cortex. Nat Neurosci. 2002;5:1217–1225. doi: 10.1038/nn950. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. NeuroImage. 2001;14:77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- Rudolph K, Pasternak T. Transient and permanent deficits in motion perception after lesions of cortical areas MT and MST in the macaque monkey. Cereb Cortex. 1999;9:90–100. doi: 10.1093/cercor/9.1.90. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Britten KH, Newsome WT. Cortical microstimulation influences perceptual judgements of motion direction. Nature. 1990;346:174–177. doi: 10.1038/346174a0. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Murasugi CM, Britten KH, Newsome WT. Microstimulation in visual area MT: effects on direction discrimination performance. J Neurosci. 1992;12:2331–2355. doi: 10.1523/JNEUROSCI.12-06-02331.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Seidemann E, Zohary E, Newsome WT. Temporal gating of neural signals during performance of a visual discrimination task. Nature. 1998;394:72–75. doi: 10.1038/27906. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Azuma M. Topographic studies on visual neurons in the dorsolateral prefrontal cortex of the monkey. Exp Brain Res. 1983;53:47–58. doi: 10.1007/BF00239397. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Van Wezel RJA, Britten KH. Motion adaptation in area MT. J Neurophysiol. 2002;88:3469–3476. doi: 10.1152/jn.00276.2002. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24:455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- Zaksas D, Pasternak T. Area MT neurons respond to visual motion distant from their receptive fields. J Neurophysiol. 2005;94:4156–4167. doi: 10.1152/jn.00505.2005. [DOI] [PubMed] [Google Scholar]