Abstract
Cocaine abuse increases the risk of life-threatening neurological complications such as strokes and seizures. Although the vasoconstricting properties of cocaine underlie its cerebrovascular effects, the mechanisms underlying its neurotoxicity remain incompletely understood. Here, we use optical techniques to measure cerebral blood volume, hemoglobin oxygenation (StO2), and intracellular calcium ([Ca2+]i) to test the hypothesis that cocaine increases [Ca2+]i in the brain. The effects of cocaine were compared with those of methylphenidate, which has similar catecholaminergic effects as cocaine (except for serotonin increases) but no local anesthetic properties, and of lidocaine, which has similar local anesthetic effects as cocaine but is devoid of catecholaminergic actions. To control for the hemodynamic effects of cocaine, we assessed the effects of cocaine in animals in which normal blood pressure was maintained by infusion of phenylephrine, and we also measured the effects of transient hypotension (mimicking that induced by cocaine). We show that cocaine induced significant increases (∼10–15%) in [Ca2+]i that were independent of its hemodynamic effects and of the anesthetic used (isofluorance or α-chloralose). Lidocaine but not methylphenidate also induced significant [Ca2+]i increases (∼10–13%). This indicates that cocaine at a dose within the range used by drug users significantly increases the [Ca2+]i in the brain and its local anesthetic, but neither its catecholaminergic nor its hemodynamic actions, underlies this effect. Cocaine-induced [Ca2+]i increases are likely to accentuate the neurotoxic effects from cocaine-induced vasoconstriction and to facilitate the occurrence of seizures from the catecholaminergic effects of cocaine. These findings support the use of calcium channel blockers as a strategy to minimize the neurotoxic effects of cocaine.
Keywords: cocaine, cerebrovascular, calcium, blood volume, oxygenation, neurotoxicity
Introduction
The abuse of cocaine can lead to transient cerebral ischemia, stroke, and hemorrhages, which are believed to reflect the vasoconstricting effects of cocaine (Volkow et al., 1988; Martin et al., 1994; Johnson et al., 2001; Buttner et al., 2003; Bartzokis et al., 2004; Bolouri et al., 2004; Wilson et al., 2004). The vasoactive properties of cocaine are well known clinically and are taken advantage of when it is used as a local anesthetic (De et al., 2003). Indeed, studies have shown that cocaine reduces cerebral blood flow (CBF) and blood volume in human subjects and in laboratory animals (Volkow et al., 1988; Pearlson et al., 1993; Wallace et al., 1996; Schmidt et al., 2006). Cocaine has multiple pharmacological targets including blockade of the dopamine, serotonin, and norepinephrine transporters (Ritz et al., 1987) that give it its sympathomimetic effects and blockade of sodium channels, which gives it its local anesthetic actions (Gissen et al., 1980). Cocaine-induced reductions in CBF could reflect (1) direct vasoconstrictive effects elicited via increases in intracellular calcium ([Ca2+]i) in vascular smooth muscle (Zhang et al., 1996), (2) indirect vasconstriction secondary to increases of sympathomimetic amines, or (3) an indirect consequence of reduced neural activity and metabolic demand attributable to blockade of sodium channels. Additionally, one must also consider the impact of the peripheral hemodynamic effects of cocaine. For example, cocaine elicits an increase in cerebrovascular resistance and a decrease in carotid blood flow (Stankovic et al., 1998) and an increase in blood pressure. In addition, repeated cocaine administration has been shown to increase voltage-sensitive calcium currents in response to membrane depolarization in prefrontal cortex pyramidal neurons (Uchimura and North, 1990; White and Kalivas, 1998; Trantham-Davidson and Lavin, 2004; Nasif et al., 2005). These data combined with the well known role of intracellular calcium as a “final common pathway to cell death” (Schanne et al., 1979) led us to hypothesize that the toxic effects of cocaine in the brain may in part be related to changes in [Ca2+]i.
Noninvasive imaging techniques such as positron emission tomography (London et al., 1990; Volkow et al., 1992, 1996) and magnetic resonance imaging (MRI) (Breiter et al., 1997; Breiter and Rosen, 1999; Mandeville et al., 2001; Lee et al., 2003) have been used to investigate the effects of cocaine in the human brain and in animals. Optical techniques have also been used to monitor the cerebrovascular and functional effects of cocaine in animals (Stankovic et al., 1998; Devonshire et al., 2004; Berwick et al., 2005). One advantage of optical technology is that it can concurrently detect oxyhemoglobin and deoxyhemoglobin, thereby distinguishing changes in the total hemoglobin concentration and oxygenation (Jobsis, 1977; Chance et al., 1988; Cope and Delpy, 1988). In addition, fluorescence techniques can directly access [Ca2+]i in the brain. Fluorescent indicators (e.g., Rhod2) are administered as acetoxymethyl esters that readily penetrate cell membranes and inside the cell are hydrolyzed to form the calcium-binding indicator, which is membrane impermeable (Grynkiewicz et al., 1985; Minta et al., 1989). Exposure to light of the appropriate wavelength induces fluorescence emission that depends on [Ca2+]i (Del Nido et al., 1998; Du et al., 2001a,b). Most optical experiments have used either isolated cells or brain slices (Kudo et al., 1992; Takahashi et al., 1993; Helmchen and Waters, 2002). We recently developed a fiber optic-based optical catheter that enables simultaneous detection of changes in [Ca2+]i as well as hemoglobin concentrations and oxygenation from the cortex of the living rat (Du et al., 2005).
Here, we test the hypothesis that the toxic effects of cocaine are not just the result of its cerebrovascular effects but also its ability to increase [Ca2+]i. For this purpose, we use our new optical technique to measure changes in the concentration and oxidation states of hemoglobin, concurrently with Rhod2-Ca fluorescence (representing [Ca2+]i) in the living brain during administration of cocaine. To assess whether the effects of cocaine on [Ca2+]i were attributable to its sympathomimetic and/or local anesthetic properties, we compared the response to that of methylphenidate [a drug with sympathomimetic effects similar to cocaine, except for serotonin increases, but with no local anesthetic properties (Volkow et al., 1999)] and lidocaine [a local anesthetic devoid of catecholaminergic actions (Woodward et al., 1995)], respectively. In parallel, we also measured cerebral blood volume (CBV), blood pressure, and heart rate to assess whether the increases of cocaine in [Ca2+]i in the brain occurred independently of its peripheral hemodynamic changes.
Materials and Methods
Subjects.
All experimental procedures were approved by the Institutional Animal Care and Use Committee. Thirty-three female Sprague Dawley rats (250–350 g) were divided into experimental groups as shown in Table 1.
Table 1.
Animal groups and experimental design
| Group | Intravenous drug challenge | Anesthetic | Hemodynamic intervention |
|---|---|---|---|
| 1 (n = 4) | Vehicle (0.9% NaCl, 0.1 cc/100 mg) | Isoflurane | No |
| 2a (n = 6) | Cocaine hydrochloride (1 mg/kg) | Isoflurane | No |
| 2b (n = 3) | Cocaine hydrochloride (1 mg/kg) | α-Chloralose | No |
| 3 (n = 5) | Methylphenidate hydrochloride (1 mg/kg) | Isoflurane | No |
| 4 (n = 6) | Lidocaine hydrochloride (1 mg/kg) | Isoflurane | No |
| 5 (n = 4) | No | Isoflurane | Yes, blood withdrawal to maintain MABP at 40–50 mmHg for 4 min |
| 6 (n = 5) | Cocaine hydrochloride (1 mg/kg) | Isoflurane | Yes, intravenous phenylephrine to maintain MABP within a normal range (70–90 mmHg) during the cocaine challenge |
Animal preparation.
All animals were induced with 3% isoflurane, intubated, and mechanically ventilated (Inspira asv; Harvard Apparatus, Holliston, MA). Anesthesia was maintained with 1.5–2% isoflurane in a 60–70% O2/air mixture. The femoral artery was cannulated for continuous arterial blood pressure monitoring, and the femoral vein was catheterized for administration of drugs. The anesthetized rat was then positioned in a stereotaxic frame (frame number 9; Kopf Instruments, Tujunga, CA), and an ∼3 mm left craniotomy was made above the area of the parietal cortex, which corresponds in part to the hindlimb somatosensory area (the craniotomy center positioned ≈2 mm behind and 2 mm lateral to bregma). The electrocardiogram, intra-arterial blood pressure, respiratory rate, and body temperature were continuously recorded (module 224002; Small Animal Instruments, Stony Brook, NY). Blood gases were monitored regularly to keep PaCO2 in the range of 30–45 mmHg during the experiments. Figure 1A illustrates the schematic of the experimental animal setup, and Fig. 1B shows an example of the physiological monitoring output in real time. Except for group 2b, all animals were maintained with isoflurane anesthesia at 1.8–2% during the experimental protocol. In group 2b rats, the anesthesia was switched from isoflurane to α-chloralose with careful attention to anesthetic depth and hemodynamics at the time of Rhod2 loading (see below). The α-chloralose was delivered through the venous catheter using an initial dose of 40 mg/kg/h, followed by a constant infusion of 27 mg/kg/h. We included this group of animals to ensure that the findings were not attributable to the hemodynamic effects of cocaine in isoflurane-anesthetized animals (see below).
Figure 1.
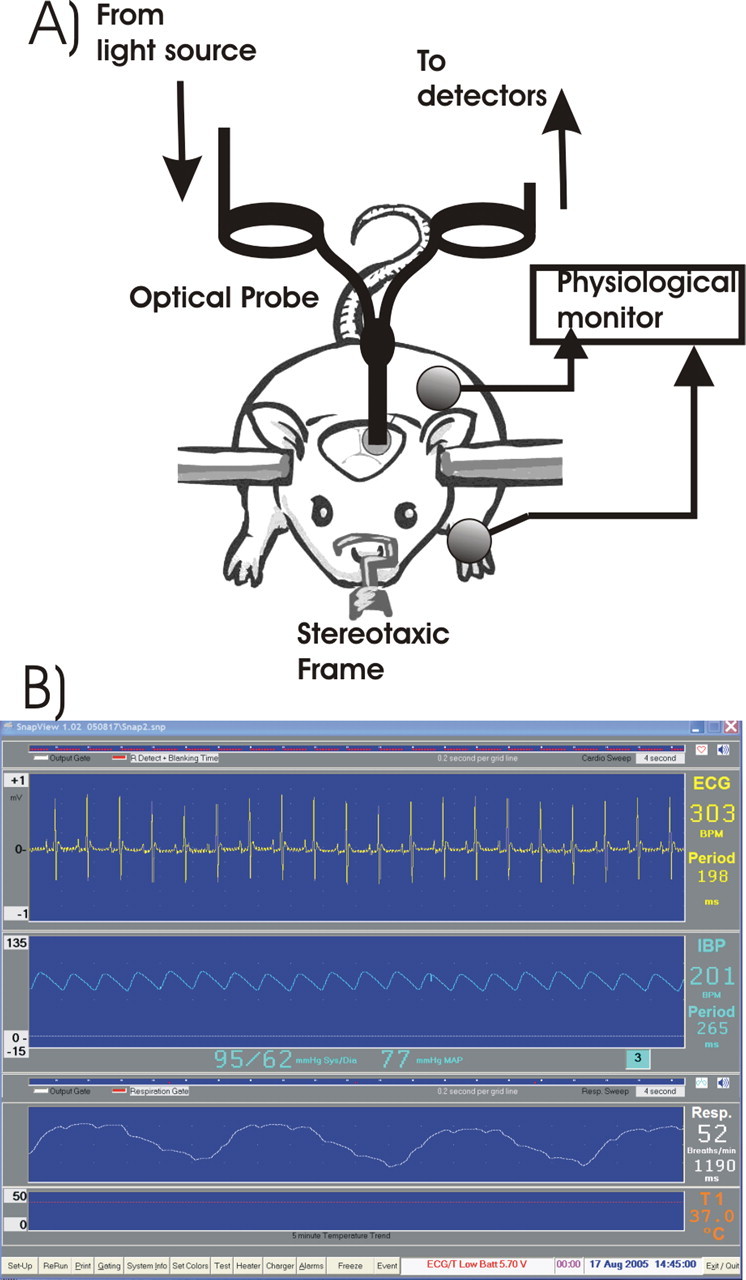
A, Schematic illustration of the optical diffusion fluorescence experimental setup used for all studies. B, Example of a real-time physiological monitoring acquired during experiments.
Rhod2 loading.
Fifty micrograms of the fluorescence calcium indicator Rhod2 acetoxymethyl ester (Rhod2/AM) (R-1243; Invitrogen, Eugene, OR) were dissolved in 2 μl of dimethylsulfoxide and 440 μl of distilled water at room temperature. A 30 g needle attached to a stereotaxic micromanipulator was inserted into the somatosensory cortex 1.2–1.5 mm below the surface at an angle of ∼45° to the surface with the needle tip starting at the center of the craniotomy. The Rhod2 solution was infused into the brain at a perfusion flow rate of 3 μl/min using a microinjection pump (CMA/100; Carnegie Medicine, Stockholm, Sweden). A total volume of 100 μl of the Rhod2 solution was infused. After Rhod2 loading, the optical probe was positioned onto the exposed cortex area (Fig. 1A), and baseline recordings were made for 60–80 min.
Experimental protocol.
After the optical baseline recordings (to allow for Rhod2 diffusion and hydrolysis), vehicle (group 1), cocaine hydrochloride (groups 2a and 2b), methylphenidate hydrochloride (group 3), or lidocaine hydrochloride (group 4) was quickly injected (<10 s) via the femoral vein. The intravenous line was flushed with 1 cc of 0.9% NaCl before and after the injection (Fig. 2B), which induced transient, small optical signal increases that were later used as temporal “landmarks” to coregister the experiments from individual animals for statistical analysis (see below, Optical data acquisition and analysis). In group 5, exsanguination of 2–3 cc of blood from the arterial line was performed to transiently (≈4 min) reduce the mean arterial blood pressure (MABP) to 40–50 mmHg. This experimental group was added to mimic the hemodynamic changes (transient mild hypotension to 40–50 mmHg) that occurred in the isoflurane-anesthetized group 2a rats during the cocaine challenge. Group 6 rats received phenylephrine (0.1–0.3 mg/h) after intravenous cocaine to maintain the MABP within normal limits.
Figure 2.
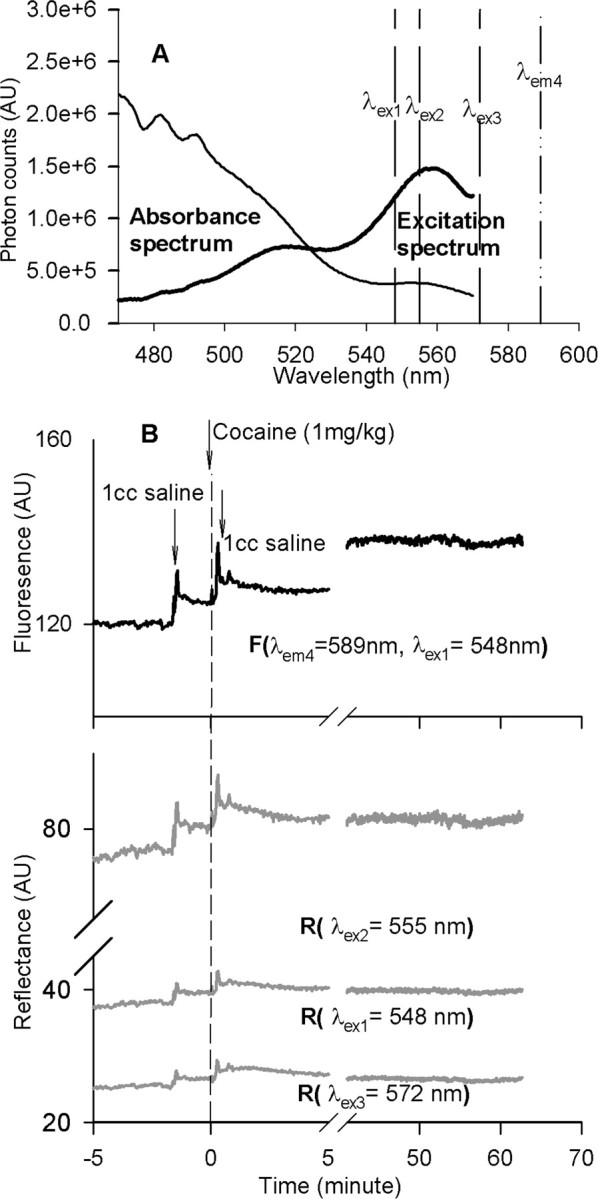
A, Hemoglobin absorbance spectrum and excitation spectra of calcium fluorescence indicator Rhod2 obtained from the surface of rat brain cortex. The vertical lines illustrate the center wavelengths of the excitation (λex1, λex2, and λex3) and fluorescence emission (λem4). B, Example of calcium-dependent fluorescence recording along with the reflectance of the excitations from the cortex of the brain simultaneously at those wavelengths in response to drug challenges. AU, Arbitrary units.
Optical diffusion and fluorescence detection instrument.
We used a catheter-based optical diffusion and fluorescence instrument that has been described previously (Du et al., 2005). Briefly, it consisted of a 150 W xenon lamp, a fast excitation monochromator, and the photo-counting detectors for fluorescence (PMT-F) and diffuse reflectance (PMT-A). The lamp was connected to the computer-controlled monochromator to select the incident lights of 548, 555, and 572 nm by timesharing to sequentially deliver the selected lights onto the brain surface through one arm of a Y-shaped bifurcated fiber-optic bundle (Fig. 1A). The fluorescence and the diffuse-reflected light re-emitted from the brain tissue were collected by the fiber optic tip of the common leg and transferred through the outgoing leg of the bundle. After passing through a beam splitter, 5% of the signal intensity was reflected by a dichroic cube for detection by PMT-A, whereas 95% was delivered to PMT-F for fluorescence detection. A bandpass filter centered at 589 nm with ±10 nm bandwidth is placed in front of PMT-F to define the fluorescence emission at 589 nm. A filter cube in front of PMT-F was synchronized with the monochromator to pass the fluorescence emission through while being excited at 548 nm but block the incident light at 555 and 572 nm. The scattered re-emission (i.e., diffuse photon reflection) at 548, 555, and 572 nm from the brain tissue were detected by PMT-A. The signals were digitized and stored in a personal computer for data processing.
Optical data acquisition and analysis.
To detect the calcium fluorescence and diffuse reflectance from the cortex, the optical fiber tip was placed in contact with the cortical surface as shown in Figure 1A. The interface between the fiber optic and the exposed brain surface was filled with gel (Surgical Lubricant Sterile Bacteriostatic; E. Fougera, Melville, NY) to reduce the mismatch in refractive index between optical fiber, air, and brain tissue, thus minimizing the interface specular reflection from the surface of the brain. Figure 2A illustrates the absorbance and Rhod2 excitation spectra obtained simultaneously from the cortex. The center wavelengths of excitations (referred to as λex1, λex2, and λex3 by dashed lines) and fluorescence emission (referred to as λem4 by a dashed dotted line) to be used for time trace acquisitions (Fig. 2B) were superimposed on the spectra. As shown in Figure 2A, these wavelengths were remote from the major absorption peaks of hemoglobin (∼480–500 nm), allowing a stronger fluorescence emission because of less attenuation by hemoglobin, thus providing a better signal-to-noise ratio for signal acquisition in vivo.
After the 60 min post-loading Rhod2 period, time traces were recorded for both fluorescence (emitted at λem4 = 589 nm while excited at λex1 = 548 nm) and the diffuse reflectance at multiple wavelengths (λex1 = 548 nm, λex2 = 555 nm, and λex3 = 572 nm). The excitation and diffuse reflectance spectra were monitored periodically. Figure 2B shows an example of the data acquisition of the fluorescence and the reflectance signals before, during, and after intravenous administration of vehicle and drugs (e.g., cocaine or methylphenidate). Recording was continued for ∼60 min after drug administration. The two parameters, CBV and hemoglobin oxygenation (StO2), can be separately distinguished from the reflectance obtained from the cortical surface at the wavelengths of 555 and 572 nm. As has been described previously (Du et al., 2005), the summation and subtraction of the optical signal densities between these two wavelengths reflected the changes of the hemoglobin concentration (i.e., referring to the change in blood volume) and hemoglobin oxygenation, respectively, as follows:
where ΔOD = ODafter − ODbefore is the change in optical density before and after drug administration that was associated with the changes in hemoglobin concentrations; λex2 = 555 nm and λex3 = 572 nm; εHb and εHbO are the extinction coefficients of the deoxygenated and oxygenated hemoglobin, which are constant; B is a pathlength factor that accounts for changes in the photon pathlength caused by tissue scattering; and L is the distance between where the light enters the tissue and where the detected light exits the tissue. B and L are assumed not to be changed during the experiments. Therefore, Δ[BV] and Δ[HbO] represent the changes in blood volume and oxygenated hemoglobin concentration induced by a given drug (e.g., cocaine, methylphenidate) or other hemodynamic manipulation (e.g., hypotension by exsanguination). Because both wavelengths of 548 nm (for Rhod2 excitation) and 589 nm (for Rhod2-Ca fluorescence emission) are the isosbestic wavelengths of tissue oxygenation as described previously (Du et al., 2001a,b, 2005), the influence of the variation in the oxygenation state of tissue on the fluorescence signal was eliminated. Furthermore, to minimize the interference of physiological changes (e.g., blood volume and possible cell swelling) on the Rhod2–calcium fluorescence, a ratio of fluorescent emission at 589 nm over reflected excitation at 548 nm was used as follows:
Thus, the ratio of Fλem4 over Rλex1 represented intracellular calcium-dependent fluorescence changes in response to drug challenges or exsanguination. Fλem4 and Rλex1 represent Rhod2 emission at 589 nm (λem4) and reflectance at 548 nm (λex1), respectively.
The average signals of the diffuse reflectance and calcium-dependent Rhod2 fluorescence before (∼10 min) the vehicle, cocaine, methylphenidate, and lidocaine challenges or exsanguination insult were defined as baseline (100%). All values were expressed as a percentage of baseline ± SEM. Intragroup differences were analyzed with a paired Student's t test, and intergroup differences were analyzed with ANOVA and a post hoc unpaired Student's t test; p < 0.05 was considered significant.
Results
Effects of cocaine on the MABP, heart rate, and body temperature
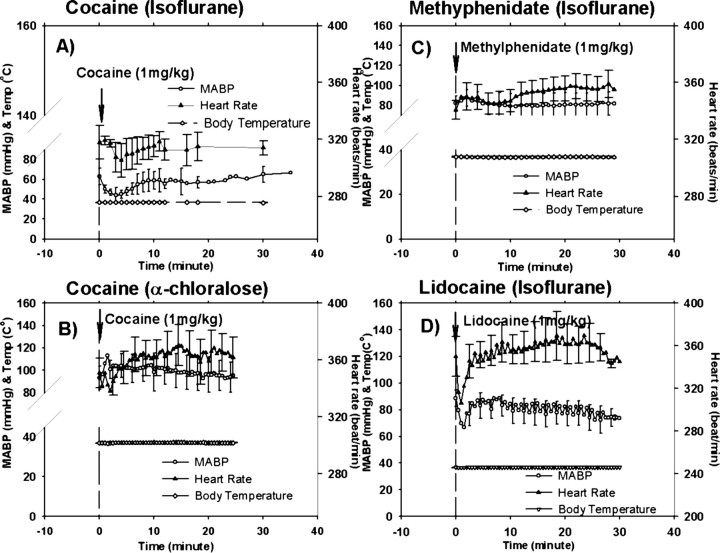
In the isoflurane-anesthetized rats (group 2a), cocaine induced brief (3–4 min) and transient mild hypotension; the MABP decreased from ∼62.8 ± 8.5 to ∼43.8 ± 8.1 mmHg (p < 0.01) and also reduced heart rate (318 ± 2.4 to 307 ± 2.1 beats/min (bpm); p < 0.01) (Fig. 3A). Both parameters returned to baseline levels by 8–10 min after injection and remained within normal range for the rest of the recording period. No significant changes in body temperature were observed (Fig. 3A). In the α-chloralose-anesthetized (group 2b) rats, cocaine increased MABP from 93.1 ± 10.3 mmHg (baseline) to 113.2 ± 7.9 mmHg within 2 min after injection (p < 0.01), and it rapidly (<5–6 min) recovered to ∼100 mmHg; the heart rate decreased slightly (not statistically significant; p > 0.05) from 351 ± 26.7 to 338 ± 29.7 bpm within the initial 2 min of the injection and increased to 370.3 ± 17.9 bpm at ∼15 min.
Figure 3.
MABP, heart rate (electrocardiogram), and body temperature (Temp) as a function of time in response to 1 mg/kg cocaine (isoflurane-anesthetized rats; A), 1 mg/kg cocaine (α-chloralose-anesthetized rats; B), 1 mg/kg methylphenidate (C), and 1 mg/kg lidocaine (D). Data are presented as a mean ± SEM.
Effects of cocaine on CBV, oxygenation (StO2), and intracellular calcium (Rhod2-Ca2+)
Isoflurane-anesthetized rats
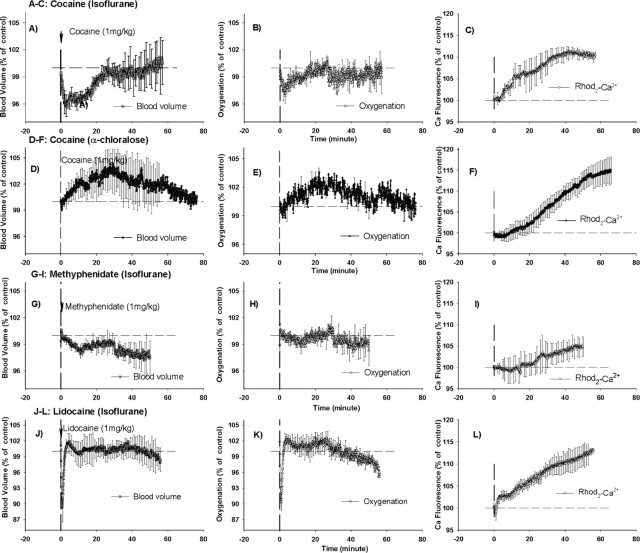
Cocaine induced transient decreases in CBV (−4.2 ± 1.2%; p < 0.05) and StO2 (−3.1 ± 0.9%; p < 0.05) 3–4 min after its injection (Fig. 4A,B). CBV and StO2 returned to baseline levels at 25 and 16 min after injection, respectively. In contrast, CBV and StO2 did not change in response to vehicle challenge in control rats (group 1) (Fig. 5 A,B). A comparison of the temporal course for the effects of cocaine in CBV (Fig. 4A) with those in MABP (Fig. 3A) reveals that the decrease in CBV only coincides with the decrease in MABP over the first 3–4 min (e.g., MABP rapidly normalizes whereas CBV persists at a low level of 95% until ∼15 min when it starts to recover).
Figure 4.
Optical diffusion and fluorescence recordings of CBV, tissue oxygenation (StO2), and intracellular calcium ([Ca2+]i) from rat cortex after 1 mg/kg cocaine in isoflurane-anesthetized rats (A–C), 1 mg/kg cocaine in α-chloralose-anesthetized rats (D–F), 1 mg/kg methylphenidate in isoflurane-anesthetized rats (G–I), and 1 mg/kg lidocaine in isoflurane-anesthetized rats (J–L). The data are presented as relative changes from baseline (100%). The vertical dashed lines in each graph represent the time of intravenous drug administration.
Figure 5.
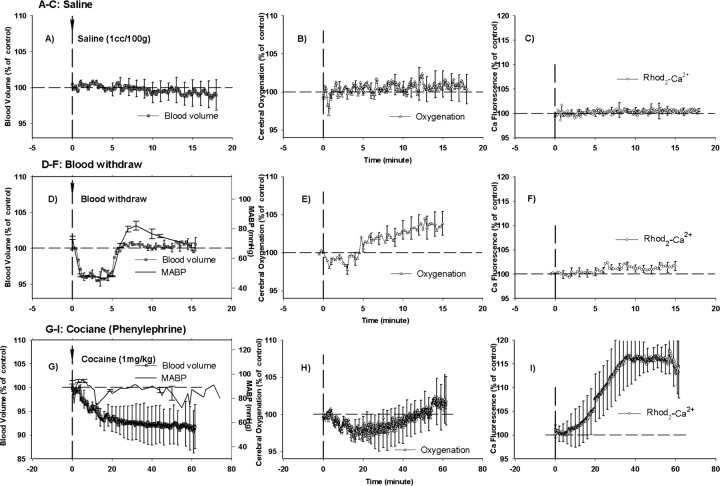
Optical diffusion and fluorescence recordings of CBV, tissue oxygenation (StO2), and intracellular calcium ([Ca2+]i) from rat cortex after intravenous administration of vehicle/saline (A–C), blood exsanguination (D–F), and 1 mg/kg cocaine (G–I). In the experiment illustrated in G–I, the MABP was maintained normal with phenylephrine. The vertical lines represent the beginning of the injection or hemodynamic challenge.
Intracellular calcium, as measured by Rhod2-Ca fluorescence (Fig. 4C), was unchanged for 4–5 min after the cocaine challenge and gradually increased to a maximum of ∼10.3 ± 1.3% at 42 min after injection (Table 2). The increase in fluorescence of Rhod2-Ca trended toward a slow recovery after 42 min. In control rats (group 1), Rhod2-Ca fluorescence did not change over time in response to the vehicle challenge (Fig. 5C).
Table 2.
Effect of the drug administration on the intracellular calcium in the living rat brain
| Group 2a (cocaine, isoflurane) | Group 2b (cocaine, α-chloralose) | Group 3 (methylphenidate) | Group 4 (lidocaine) | Group 6 (cocaine, phenylephrine) | |
|---|---|---|---|---|---|
| Time from cocaine injection to [Ca2+]i increase (min) | 6.2 ± 1.7 | 8.8 ± 0.7 | 15.3 ± 2.7* | 2.2 ± 0.2 | 7.3 ± 1.5 |
| Maximal [Ca2+]i increase (% from baseline) | 10.3 ± 1.3 | 14.7 ± 2.3 | 4.2 ± 0.7* | 13.3 ± 2.4 | 16.3 ± 2.3 |
*p < 0.05, group 3 versus group 2a and group 3 versus group 4.
α-Chloralose-anesthetized rats
In rats anesthetized with α-chloralose (group 2b), cocaine elicited a slow and prolonged increase in CBV and StO2, which reached a peak of ≈2–3% above baseline levels at ≈25–30 min after the cocaine injection and gradually recovered over 1 h (Fig. 4D,E). Similarly to the isoflurane-anesthetized rats (group 2a) (Fig. 4C), [Ca2+]i also increased excessively in the α-chloralose-anesthetized rats after the cocaine challenge, although with a delay of ≈10 min (Fig. 4F, Table 2); the cocaine-induced [Ca2+]i increase continued for 1 h and reached a plateau (Table 2).
Cocaine, intracellular calcium, and changes in MABP
To clarify the potential interdependency between the intracellular calcium changes and the systemic hypotension observed in the isoflurane rats, we examined the effect of transient hypotension induced by blood withdrawal on the optical recordings. First, the decrease in MABP induced by blood withdrawal was similar to that induced by cocaine in the isoflurane-anesthetized rats (−36.4 ± 5.8 vs −30.3 ± 8.0%) (Fig. 5D). Second, in response to the mild hypotension, CBV and StO2 decreased −3.2 ± 0.6 and −0.7 ± 0.5%, respectively (Fig. 5D,E). However, unlike cocaine, there was no significant Rhod2-Ca fluorescence increase (Fig. 5F) along with the decrease in the MABP (Fig. 5D). This experiment suggests that the intracellular calcium increase observed after the cocaine challenge in the isoflurane-anesthetized rats is neither attributable to the transient hypotensive episode nor to the changes in CBV or StO2. This conclusion is also supported by the consistent cocaine-induced [Ca2+]i increases in the α-chloralose-anesthetized rats in which MABP is slightly elevated after systemic cocaine (Fig. 3B).
We also tested the effect of maintaining the MABP within normal range by administering phenylephrine at the time of cocaine exposure to circumvent the transient hypotension observed with cocaine in the isoflurane-anesthetized rats (Fig. 5G). Interestingly, despite normotension, the cocaine administration still resulted in a decrease in CBV (maximal change, −8.2 ± 1.3%; p < 0.05) and an increase in the Rhod2-Ca fluorescence (maximal change, 16.3 ± 2.3%; p < 0.05) (Fig. 5G,I). This result also suggests that the changes in intracellular calcium observed during a cocaine challenge are independent of changes in MABP, CBV, and StO2 changes.
Physiological changes and cerebral responses induced by methylphenidate
Methylphenidate induced increases in the heart rate that persisted for the 35 min recording period after its administration (Fig. 3C). In addition, it induced a transient increase in MABP. Peak percentage increases in heart rate and MABP corresponded to 4.0 ± 1.6 and 11.0 ± 6.1%, respectively (Fig. 3C). The body temperature was unaffected.
Methylphenidate caused a mild decrease in CBV (−2.0 ± 0.8% at 11 min) but no significant change in StO2 (−1.9 ± 1.2%) (Fig. 4G,H). The methylphenidate-induced CBV decrease was lower than that induced by cocaine in the isoflurane-anesthetized rats (group 2a) and persisted for >40 min (p < 0.02). Importantly, the Rhod2-Ca fluorescence was unchanged for ∼15 min, increased slightly, and reached a peak of 4.2 ± 0.7% (Fig. 4I), which was significantly lower (p < 0.003) than for cocaine (i.e., 10.3 ± 1.3% as shown in Fig. 4C and Table 2).
Physiological changes and cerebral and [Ca2+]i responses induced by lidocaine
Intravenous lidocaine briefly (2–3 min) decreased the MABP from 88.6 ± 6.1 to 66.9 ± 11.5 mmHg and the heart rate from 349.8 ± 18.5 to 306.2 ± 18.6 bpm within ≈1 min (Fig. 3D). In parallel, CBV and StO2 briefly and transiently decreased ≈10% (CBV, 10.7 ± 4.3%; StO2, 9.7 ± 4.0%) (Fig. 4, compare J, K). Both parameters recovered with a minor overshoot within 3 min. The fluorescence-dependent Ca signal started to increase within 2 min and rose significantly to a maximum of 13.3 ± 2.4% at ∼50 min after the lidocaine injection (Fig. 4L), which was similar to the [Ca2+]i increases observed with cocaine (p > 0.05).
Discussion
This study provides evidence for the first time in vivo that acute cocaine at a dose used by cocaine abusers for recreational purposes significantly increases the intracellular calcium concentration ([Ca2+]i) in the rodent brain and that these effects are independent of changes in peripheral and central hemodynamics. It also shows that methylphenidate at a dose that is approximately double in potency to that used for cocaine in blocking dopamine and norepinephrine transporters (Han and Gu, 2006) did not increase [Ca2+]i, suggesting that cocaine-induced increases in [Ca2+]i are not caused by its dopaminergic or its noradrenergic actions. Finally, it documents that intravenous lidocaine induced increases in intracellular [Ca2+]i similar to those observed with cocaine, suggesting that the mechanism whereby cocaine evokes [Ca2+]i increases is via its local anesthetic effects and not by its catecholaminergic actions.
The effects of cocaine on intracellular calcium concentration ([Ca2+]i)
Using a novel optical diffusion fluorescence device, we demonstrated for the first time in vivo that an acute cocaine challenge induced increases in [Ca2+]i in the brain. We examined the effect of cocaine on the [Ca2+]i response in both isoflurane- and α-chloralose-anesthetized animals because of the cocaine-induced differences in the hemodynamic profiles with the two anesthetic regimens (see below). As described, in the isoflurane-anesthetized animals, the [Ca2+]i started to increase ≈6 min after cocaine and gradually increased to ≈10–11% above the baseline at ∼40 min. In the α-chloralose-anesthetized animals, cocaine induced an ≈14% [Ca2+]i increase although it occurred slightly later (≈9 min). It is noteworthy that the cocaine-induced [Ca2+]i increase, regardless of anesthetic, was quantitatively larger than that observed in transient (5 min) global ischemia in which it only increases ∼8.5 ± 1.7% compared with baseline (Du et al., 2005). In transient cerebral ischemia, there is a clear pathophysiological link between ischemia, calcium transients, and ensuing selective neuronal death (Benveniste et al., 1988). For example, selective neuronal necrosis that occurs in the brain after short-term (5–15 min) transient ischemia is associated with extracellular calcium decreases (Benveniste et al., 1988) or intracellular calcium increases (Silver and Erecinska, 1990). Furthermore, inhibition of calcium transients prevents neuronal death after a 10 min ischemic insult (Benveniste et al., 1988). However, the cocaine-induced [Ca2+]i increases observed in this study occur under non-ischemic conditions [ischemia defined here as a CBF of <20 cc/100 g/min (Astrup et al., 1977; Symon et al., 1977)] and may explain why direct cell damage has not been documented after acute cocaine administration.
To clarify whether the cocaine-induced increases in [Ca2+]i were dependent on hemodynamic changes (i.e., MABP), we measured Rhod2-Ca fluorescence changes under different conditions, (1) during two different anesthesia regimens and (2) in response to exsanguinations, and we also measured CBV, StO2, and Rhod2-Ca changes while we maintained stable blood pressure during the cocaine administration with phenylephrine in isoflurane-anesthetized animals in which cocaine induced brief, transient hypotension. The fact that there was no significant change in Rhod2-Ca fluorescence in response to the transient decrease in blood pressure (Fig. 5F) suggests that the Rhod2-Ca fluorescence increase was not caused by the peripheral hemodynamic effects of cocaine. Moreover, with stable blood pressures in both α-chloralose-anesthetized animals (Fig. 4F) and in isoflurane-anesthetized animals in which MABP was maintained normal with phenylephrine (Fig. 5G), cocaine still induced an increase in Rhod2-Ca fluorescence, corroborating the fact that the cocaine-induced [Ca2+]i increase occurs independent of changes in peripheral hemodynamics.
An interesting finding from our study is that the increases in [Ca2+]i persisted for >60 min after the cocaine injection, which is a time when the concentration of cocaine in plasma is negligible (Carmona et al., 2005). Indeed, the half-life for the concentration of cocaine in plasma in the rat is ∼26 min (Carmona et al., 2005), which suggests that the increases in [Ca2+]i are attributable to a cocaine metabolite that is detectable a long time after cocaine in plasma is no longer present (Burke and Ravi, 1990; Burke et al., 1990), rather than to cocaine itself. Interestingly, the blockade of cocaine of norepinephrine transporters in the primate heart persists hours after its administration (Fowler et al., 1994) even when the half-life of cocaine in myocardium is ∼10 min (Volkow et al., 1996).
In the adult organism, cocaine is metabolized mostly through hydrolysis and enzymatically into its major metabolites, ecgonine methyl ester, benzoylecgonine, and to N-demethylation by cytochrome P-450 enzymes to produce norcocaine (Inaba, 1989). In humans, the half-life of benzoylecgonine is known to be longer than in rats (Dempsey et al., 1999) and can be detected in the urine for >96 h (Hamilton et al., 1977). Like cocaine, benzoylecgonine passes the blood–brain barrier (Spiehler and Reed, 1985), and both compounds can constrict cerebral arteries (Madden and Powers, 1990). In fact, benzoylecgonine constricts cerebral arteries more than cocaine (Madden and Powers, 1990). Thus, given the pharmacokinetic profiles, a potential cocaine metabolite candidate eliciting the [Ca2+]i increases observed in this study could be benzoylecgonine. Alternatively, we cannot rule out the possibility that cocaine may induce conformational changes in ion channels that persist after the drug is no longer present in the tissue. Future studies will clarify this issue.
The effects of methylphenidate on [Ca2+]i
Unlike cocaine, acute methylphenidate did not elicit a large increase in Rhod2-Ca-dependent fluorescence (methylphenidate, 4.2 ± 0.7%; cocaine, 10.3 ± 1.3%), and it occurred significantly later (15 vs 6 min) (Table 2). Methylphenidate, like cocaine, is a dopamine transporter and a norepinephrine transporter inhibitor but differs from cocaine in that it is devoid of local anesthetic actions and does not bind to the serotonin transporter (Ritz et al., 1987). Thus, the differences between cocaine and methylphenidate in the induction of increases [Ca2+]i could be attributable to either the differences in their serotonergic effects or their local anesthetic properties. The fact that lidocaine, which is devoid of catecholamienrgic effects, has similar effects to those of cocaine in increasing [Ca2+]i suggests that it is the local anesthetic and not the serotonergic effects of cocaine that underlie its [Ca2+]i increases (see below).
The effects of lidocaine on [Ca2+]i
Intravenous lidocaine in a clinical relevant dose elicited similar (10–13%) [Ca2+]i increases to that of cocaine, although they occurred more rapidly (2.2 vs 6.2 min), providing strong evidence that cocaine elicits [Ca2+]i increases via its local anesthetic action. It has been known for decades that local anesthetics have the potential to permanently damage the spinal cord (Ferguson and Watkins, 1937; Macdonald and Watkins, 1937); however, the mechanism behind their neurotoxic action is still unresolved. Our study demonstrates for the first time in vivo that a local anesthetic (lidocaine) causes [Ca2+]i increases in the brain that may explain its potential neurotoxicity, especially when local anesthetics are combined with vasoactive agents such as epinephrine, which is often administered in conjunction with the local anesthetic when used clinically. Interestingly, Gold et al. (1998) have demonstrated in vitro that lidocaine in cultured dorsal root ganglion cells also causes increases in the free intracellular concentration of calcium (see also Kirihara et al., 2003; Sakura et al., 2005) and have suggested that this is involved in its toxic effects. Our in vivo data strongly corroborates the data of Gold et al. (1998), and future studies with our new optical approach will confirm whether the lidocaine-induced [Ca2+]i increases in the brain are also observed in the peripheral nervous system.
The effects of cocaine on CBV and StO2
In the isoflurane-anesthetized animals, the initial cocaine-induced decrease in CBV occurred in close temporal correspondence to the decrease in blood pressure (Figs. 3A, 4A). However, the decrease in CBV persisted after the MABP had recovered. This mismatch between CBV and MABP (i.e., after 8 min) was not confirmed in animals anesthetized with α-chloralose in which cocaine induced an increase in both CBV and StO2, whereas MABP was stable and slightly elevated. Therefore, the CBV and StO2 response to cocaine is dependent on the anesthetic used. The conflicting hemodynamic responses to cocaine with different anesthetic regimens could explain reported discrepancies in the literature using MRI to investigate the effects of systemic cocaine. For example, using 0.7% halothane, 1 mg/kg cocaine was found to elicit widespread increases in regional CBV using contrast and functional MRI (fMRI) (Marota et al., 2000). In another study, urethane was used as an anesthetic, and cocaine was found to cause widespread and dose-depended early decreases and later increases in the blood oxygenation level-dependent (BOLD) fMRI signal with intravenous cocaine challenges in the rat brain (Luo et al., 2003). Recently, isoflurane-anesthetized rats were used, and intravenous cocaine (1 mg/kg) was found to elicit a negative BOLD fMRI signal on the cortical surface (Schmidt et al., 2006), which is especially relevant for our study because our optical probe only records changes in the cortical surface (1–2 mm) (Du et al., 2005).
In humans, abusing intravenous cocaine has been shown to reduce regional glucose metabolism in neocortical areas, basal ganglia, and the hippocampus (London et al., 1990) and to reduce global and regional CBF (Johnson et al., 2005). Studies on the effects of acute cocaine on the BOLD responses of the human brain (cocaine abusers) have been inconsistent with reports of increases in limbic and cortical regions (Breiter et al., 1997) as well as decreases (Risinger et al., 2005). Our data would suggest that the fMRI signal is negative in the isoflurane-anesthetized rats and positive in the α-chloralose-anesthetized rats. However, all of our data are acquired in cocaine-naive rats and cannot be directly compared with the human data acquired in subjects regularly abusing cocaine. It is, however, important to emphasize that the [Ca2+]i increases in response to cocaine occur regardless of the anesthetic and hemodynamic response and we are hypothesizing therefore that this would also occur in the human brain contributing to the neurotoxic potential of cocaine.
Optical method used
This study also demonstrates the feasibility of using optical imaging to simultaneously assess CBV, StO2, and [Ca2+]i in the rodent brain. Moreover, the optical fluorescence technique used to measure [Ca2+]i was not affected by the vascular changes in CBV and StO2 attributable to cocaine. Considering the strong optical effects of changing CBV and StO2 in the previous ischemia experiments (Du et al., 2005), it is clear that the strategy to use appropriate wavelengths to measure Rhod2-Ca fluorescence enables reliable measurements of changes (Del Nido et al., 1998; Du et al., 2001a,b).
Clinical significance
It is well recognized that cocaine abuse is associated with neurological deficits that can be mild and transient such as facial paralysis to severe and irreversible such as permanent tetraplegia (Spivey and Euerle, 1990). In as much as increases in [Ca2+]i are associated with cell death (Schanne et al., 1979), cocaine-induced increases in [Ca2+]i are likely to be clinically relevant, particularly when considering that they occur in parallel to cocaine-induced decreases in MABP, blood volume, tissue oxygenation, and CBF. The increases in [Ca2+]i would make the tissue more vulnerable to ischemia secondary to decreases in CBF that would otherwise not induce ischemia and/or cell damage. Cocaine-induced increases in [Ca2+]i in brain are also likely to contribute to the pathogenesis of seizure activity, which is one of the most frequent complications associated with cocaine overdoses (Kaye and Darke, 2004). Indeed, studies have shown that intracellular calcium overload is a hallmark pathological finding after seizure activity (Meldrum, 1986a,b).
In summary, we show that acute cocaine at a dose used by cocaine abusers for recreational purposes induced large increases in [Ca2+]i in the cortex of the rat brain that are independent of its decreases in CBV and in tissue oxygenation. We also document that the mechanism behind the cocaine-induced [Ca2+]i increases are related to the local anesthetic actions of cocaine and not its sympathomimetic effects. These findings are clinically relevant because they suggest that the neurotoxic effects of cocaine are directly related to [Ca2+]i and support the use of calcium channel blockers as a strategy to minimize brain damage after cocaine abuse.
Footnotes
This work was supported by Laboratory Directed Research and Development Grants 02-08 (H.B.) and 04-066 (C.D.), Brookhaven National Laboratory, Department of Energy Office of Science and Biological Research, and New York State Office of Science, Technology, and Academic Research. We thank Dr. P. Thanos for valuable discussions regarding the pharmacokinetics and pharmacodynamics of cocaine and methylphenidate.
References
- Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8:51–57. doi: 10.1161/01.str.8.1.51. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Bridge P, Mintz J. Cortical gray matter volumes are associated with subjective responses to cocaine infusion. Am J Addict. 2004;13:64–73. doi: 10.1080/10550490490265352. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Jorgensen MB, Diemer NH, Hansen AJ. Calcium accumulation by glutamate receptor activation is involved in hippocampal cell damage after ischemia. Acta Neurol Scand. 1988;78:529–536. doi: 10.1111/j.1600-0404.1988.tb03697.x. [DOI] [PubMed] [Google Scholar]
- Berwick J, Devonshire IM, Martindale AJ, Johnston D, Zheng Y, Kennerley AJ, Overton PG, Mayhew JE. Cocaine administration produces a protracted decoupling of neural and haemodynamic responses to intense sensory stimuli. Neuroscience. 2005;132:361–374. doi: 10.1016/j.neuroscience.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Bolouri MR, Small GA. Neuroimaging of hypoxia and cocaine-induced hippocampal stroke. J Neuroimaging. 2004;14:290–291. doi: 10.1177/1051228404265751. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann NY Acad Sci. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Burke WM, Ravi NV. Urinary excretion of cocaine. Ann Intern Med. 1990;112:548–549. doi: 10.7326/0003-4819-112-7-548. [DOI] [PubMed] [Google Scholar]
- Burke WM, Ravi NV, Dhopesh V, Vandegrift B, Maany I. Prolonged presence of metabolite in urine after compulsive cocaine use. J Clin Psychiatry. 1990;51:145–148. [PubMed] [Google Scholar]
- Buttner A, Mall G, Penning R, Sachs H. The neuropathology of cocaine abuse. Leg Med (Tokyo) 2003 Mar 5;(Suppl 1):S240–S242. doi: 10.1016/s1344-6223(02)00122-0. [DOI] [PubMed] [Google Scholar]
- Carmona GN, Schindler CW, Greig NH, Holloway HW, Jufer RA, Cone EJ, Gorelick DA. Intravenous butyrylcholinesterase administration and plasma and brain levels of cocaine and metabolites in rats. Eur J Pharmacol. 2005;517:186–190. doi: 10.1016/j.ejphar.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Chance B, Leigh JS, Miyake H, Smith DS, Nioka S, Greenfeld R, Finander M, Kaufmann K, Levy W, Young M, Cohen P, Yoshioka H, Boretsky R. Comparison of time-resolved and -unresolved measurements of deoxyhemoglobin in brain. Proc Natl Acad Sci USA. 1988;85:4971–4975. doi: 10.1073/pnas.85.14.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope M, Delpy DT. System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med Biol Eng Comput. 1988;26:289–294. doi: 10.1007/BF02447083. [DOI] [PubMed] [Google Scholar]
- De R, Uppal HS, Shehab ZP, Hilger AW, Wilson PS, Courteney-Harris R. Current practices of cocaine administration by UK otorhinolaryngologists. J Laryngol Otol. 2003;117:109–112. doi: 10.1258/002221503762624530. [DOI] [PubMed] [Google Scholar]
- Del Nido PJ, Glynn P, Buenaventura P, Salama G, Koretsky AP. Fluorescence measurement of calcium transients in perfused rabbit heart using rhod 2. Am J Physiol. 1998;274:H728–H741. doi: 10.1152/ajpheart.1998.274.2.H728. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Jacob P, III, Partridge JC, Jones RT, Panganiban K, Rowbotham MC. Cocaine metabolite kinetics in the newborn. J Anal Toxicol. 1999;23:24–28. doi: 10.1093/jat/23.1.24. [DOI] [PubMed] [Google Scholar]
- Devonshire IM, Berwick J, Jones M, Martindale J, Johnston D, Overton PG, Mayhew JE. Haemodynamic responses to sensory stimulation are enhanced following acute cocaine administration. NeuroImage. 2004;22:1744–1753. doi: 10.1016/j.neuroimage.2004.03.042. [DOI] [PubMed] [Google Scholar]
- Du C, MacGowan GA, Farkas DL, Koretsky AP. Calcium measurements in perfused mouse heart: quantitating fluorescence and absorbance of Rhod-2 by application of photon migration theory. Biophys J. 2001a;80:549–561. doi: 10.1016/S0006-3495(01)76037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, MacGowan GA, Farkas DL, Koretsky AP. Calibration of the calcium dissociation constant of Rhod(2)in the perfused mouse heart using manganese quenching. Cell Calcium. 2001b;29:217–227. doi: 10.1054/ceca.2000.0186. [DOI] [PubMed] [Google Scholar]
- Du C, Koretsky AP, Izrailtyan I, Benveniste H. Simultaneous detection of blood volume, oxygenation, and intracellular calcium changes during cerebral ischemia and reperfusion in vivo using diffuse reflectance and fluorescence. J Cereb Blood Flow Metab. 2005;25:1078–1092. doi: 10.1038/sj.jcbfm.9600102. [DOI] [PubMed] [Google Scholar]
- Ferguson FR, Watkins KH. Paralysis of the bladder and associated neurological sequelae of spinal anaesthesia (cauda equina syndrome) Br J Surg. 1937;25:735–752. [Google Scholar]
- Fowler JS, Ding YS, Volkow ND, Martin T, MacGregor RR, Dewey S, King P, Pappas N, Alexoff D, Shea C. PET studies of cocaine inhibition of myocardial norepinephrine uptake. Synapse. 1994;16:312–317. doi: 10.1002/syn.890160407. [DOI] [PubMed] [Google Scholar]
- Gissen AJ, Covino BG, Gregus J. Differential sensitivities of mammalian nerve fibers to local anesthetic agents. Anesthesiology. 1980;53:467–474. doi: 10.1097/00000542-198012000-00006. [DOI] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Hampl KF, Drasner K, Levine JD. Lidocaine toxicity in primary afferent neurons from the rat. J Pharmacol Exp Ther. 1998;285:413–421. [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamilton HE, Wallace JE, Shimek EL, Jr, Land P, Harris SC, Christenson JG. Cocaine and benzoylecgonine excretion in humans. J Forensic Sci. 1977;22:697–707. [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Waters J. Ca2+ imaging in the mammalian brain in vivo. Eur J Pharmacol. 2002;447:119–129. doi: 10.1016/s0014-2999(02)01836-8. [DOI] [PubMed] [Google Scholar]
- Inaba T. Cocaine: pharmacokinetics and biotransformation in man. Can J Physiol Pharmacol. 1989;67:1154–1157. doi: 10.1139/y89-184. [DOI] [PubMed] [Google Scholar]
- Jobsis FF. Non-invasive, infra-red monitoring of cerebral O2 sufficiency, blood volume, HbO2-Hb shifts and blood flow. Acta Neurol Scand Suppl. 1977;64:452–453. [PubMed] [Google Scholar]
- Johnson BA, Devous MD, Sr, Ruiz P, Ait-Daoud N. Treatment advances for cocaine-induced ischemic stroke: focus on dihydropyridine-class calcium channel antagonists. Am J Psychiatry. 2001;158:1191. doi: 10.1176/appi.ajp.158.8.1191. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Dawes MA, Roache JD, Wells LT, Ait-Daoud N, Mauldin JB, Wang Y, Lancaster JL, Fox PT. Acute intravenous low- and high-dose cocaine reduces quantitative global and regional cerebral blood flow in recently abstinent subjects with cocaine use disorder. J Cereb Blood Flow Metab. 2005;25:928–936. doi: 10.1038/sj.jcbfm.9600093. [DOI] [PubMed] [Google Scholar]
- Kaye S, Darke S. Non-fatal cocaine overdose among injecting and non-injecting cocaine users in Sydney. Addiction. 2004;99:1315–1322. doi: 10.1111/j.1360-0443.2004.00875.x. [DOI] [PubMed] [Google Scholar]
- Kirihara Y, Saito Y, Sakura S, Hashimoto K, Kishimoto T, Yasui Y. Comparative neurotoxicity of intrathecal and epidural lidocaine in rats. Anesthesiology. 2003;99:961–968. doi: 10.1097/00000542-200310000-00032. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Akita K, Nakamura T, Ogura A, Makino T, Tamagawa A, Ozaki K, Miyakawa A. A single optical fiber fluorometric device for measurement of intracellular Ca2+ concentration: its application to hippocampal neurons in vitro and in vivo. Neuroscience. 1992;50:619–625. doi: 10.1016/0306-4522(92)90451-7. [DOI] [PubMed] [Google Scholar]
- Lee JH, Telang FW, Springer CS, Jr, Volkow ND. Abnormal brain activation to visual stimulation in cocaine abusers. Life Sci. 2003;73:1953–1961. doi: 10.1016/s0024-3205(03)00548-4. [DOI] [PubMed] [Google Scholar]
- London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, Herning R, Grayson R, Jaffe JH, Wagner HN., Jr Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry. 1990;47:567–574. doi: 10.1001/archpsyc.1990.01810180067010. [DOI] [PubMed] [Google Scholar]
- Luo F, Wu G, Li Z, Li SJ. Characterization of effects of mean arterial blood pressure induced by cocaine and cocaine methiodide on BOLD signals in rat brain. Magn Reson Med. 2003;49:264–270. doi: 10.1002/mrm.10366. [DOI] [PubMed] [Google Scholar]
- Macdonald AD, Watkins KH. An experimental investigation into the cause of paralysis following spinal anaesthesia. Br J Surg. 1937;25:879–883. [Google Scholar]
- Madden JA, Powers RH. Effect of cocaine and cocaine metabolites on cerebral arteries in vitro. Life Sci. 1990;47:1109–1114. doi: 10.1016/0024-3205(90)90169-r. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Jenkins BG, Kosofsky BE, Moskowitz MA, Rosen BR, Marota JJ. Regional sensitivity and coupling of BOLD and CBV changes during stimulation of rat brain. Magn Reson Med. 2001;45:443–447. doi: 10.1002/1522-2594(200103)45:3<443::aid-mrm1058>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Marota JJ, Mandeville JB, Weisskoff RM, Moskowitz MA, Rosen BR, Kosofsky BE. Cocaine activation discriminates dopaminergic projections by temporal response: an fMRI study in rat. NeuroImage. 2000;11:13–23. doi: 10.1006/nimg.1999.0520. [DOI] [PubMed] [Google Scholar]
- Martin DH, DiCarlo RP. Recent changes in the epidemiology of genital ulcer disease in the United States. The crack cocaine connection. Sex Transm Dis. 1994;21(2 Suppl):S76–S80. [PubMed] [Google Scholar]
- Meldrum BS. Neuropathological consequences of chemically and electrically induced seizures. Ann NY Acad Sci. 1986a;462:186–193. doi: 10.1111/j.1749-6632.1986.tb51253.x. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Cell damage in epilepsy and the role of calcium in cytotoxicity. Adv Neurol. 1986b;44:849–855. [PubMed] [Google Scholar]
- Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- Nasif FJ, Hu XT, White FJ. Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci. 2005;25:3674–3679. doi: 10.1523/JNEUROSCI.0010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, Jeffery PJ, Harris GJ, Ross CA, Fischman MW, Camargo EE. Correlation of acute cocaine-induced changes in local cerebral blood flow with subjective effects. Am J Psychiatry. 1993;150:495–497. doi: 10.1176/ajp.150.3.495. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Sakura S, Kirihara Y, Muguruma T, Kishimoto T, Saito Y. The comparative neurotoxicity of intrathecal lidocaine and bupivacaine in rats. Anesth Analg. 2005;101:541–547. doi: 10.1213/01.ANE.0000155960.61157.12. [DOI] [PubMed] [Google Scholar]
- Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206:700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Schmidt KF, Febo M, Shen Q, Luo F, Sicard KM, Ferris CF, Stein EA, Duong TQ. Hemodynamic and metabolic changes induced by cocaine in anesthetized rat observed with multimodal functional MRI. Psychopharmacology (Berl) 2006;185:479–486. doi: 10.1007/s00213-006-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Intracellular and extracellular changes of [Ca2+] in hypoxia and ischemia in rat brain in vivo. J Gen Physiol. 1990;95:837–866. doi: 10.1085/jgp.95.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiehler VR, Reed D. Brain concentrations of cocaine and benzoylecgonine in fatal cases. J Forensic Sci. 1985;30:1003–1011. [PubMed] [Google Scholar]
- Spivey WH, Euerle B. Neurologic complications of cocaine abuse. Ann Emerg Med. 1990;19:1422–1428. doi: 10.1016/s0196-0644(05)82612-5. [DOI] [PubMed] [Google Scholar]
- Stankovic MR, Fujii A, Maulik D, Kirby D, Stubblefield PG. Optical brain monitoring of the cerebrovascular effects induced by acute cocaine exposure in neonatal pigs. J Matern Fetal Investig. 1998;8:108–112. [PubMed] [Google Scholar]
- Symon L, Lassen NA, Astrup J, Branston NM. Thresholds of ischaemia in brain cortex. Adv Exp Med Biol. 1977;94:775–782. doi: 10.1007/978-1-4684-8890-6_107. [DOI] [PubMed] [Google Scholar]
- Takahashi MP, Sugiyama M, Tsumoto T. Contribution of NMDA receptors to tetanus-induced increase in postsynaptic Ca2+ in visual cortex of young rats. Neurosci Res. 1993;17:229–239. doi: 10.1016/0168-0102(93)90050-z. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Lavin A. Acute cocaine administration depresses cortical activity. Neuropsychopharmacology. 2004;29:2046–2051. doi: 10.1038/sj.npp.1300482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura N, North RA. Actions of cocaine on rat nucleus accumbens neurones in vitro. Br J Pharmacol. 1990;99:736–740. doi: 10.1111/j.1476-5381.1990.tb12999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Wang GJ, Logan J, MacGregor R, Dewey SL, Schlyer D, Hitzemann R. Distribution and kinetics of carbon-11-cocaine in the human body measured with PET. J Nucl Med. 1992;33:521–525. [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ. Cocaine addiction: hypothesis derived from imaging studies with PET. J Addict Dis. 1996;15:55–71. doi: 10.1300/J069v15n04_04. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Fischman M, Foltin R, Abumrad NN, Gatley SJ, Logan J, Wong C, Gifford A, Ding YS, Hitzemann R, Pappas N. Methylphenidate and cocaine have a similar in vivo potency to block dopamine transporters in the human brain. Life Sci. 1999;65:PL7–PL12. doi: 10.1016/s0024-3205(99)00225-8. [DOI] [PubMed] [Google Scholar]
- Wallace EA, Wisniewski G, Zubal G, vanDyck CH, Pfau SE, Smith EO, Rosen MI, Sullivan MC, Woods SW, Kosten TR. Acute cocaine effects on absolute cerebral blood flow. Psychopharmacology (Berl) 1996;128:17–20. doi: 10.1007/s002130050104. [DOI] [PubMed] [Google Scholar]
- Wilson KC, Saukkonen JJ. Acute respiratory failure from abused substances. J Intensive Care Med. 2004;19:183–193. doi: 10.1177/0885066604263918. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Compton DM, Balster RL, Martin BR. In vitro and in vivo effects of cocaine and selected local anesthetics on the dopamine transporter. Eur J Pharmacol. 1995;277:7–13. doi: 10.1016/0014-2999(95)00042-j. [DOI] [PubMed] [Google Scholar]
- Zhang A, Cheng TP, Altura BT, Altura BM. Acute cocaine results in rapid rises in intracellular free calcium concentration in canine cerebral vascular smooth muscle cells: possible relation to etiology of stroke. Neurosci Lett. 1996;215:57–59. doi: 10.1016/s0304-3940(96)12925-6. [DOI] [PubMed] [Google Scholar]