Abstract
We investigated the modulating actions of the nonselective K+ channel blocker 4-aminopyridine (4-AP) on amyloid β (Aβ1–42)-induced human microglial signaling pathways and functional processes. Whole-cell patch-clamp studies showed acute application of Aβ1–42 (5 μm) to human microglia led to rapid expression of a 4-AP-sensitive, non-inactivating outwardly rectifying K+ current (IK). Intracellular application of the nonhydrolyzable analog of GTP, GTPγS, induced an outward K+ current with similar properties to the Aβ1–42-induced IK including sensitivity to 4-AP (IC50 = 5 mm). Reverse transcriptase-PCR showed a rapid expression of a delayed rectifier Kv3.1 channel in Aβ1–42-treated microglia. Aβ1–42 peptide also caused a slow, progressive increase in levels of [Ca2+]i (intracellular calcium) that was partially blocked by 4-AP. Chronic exposure of human microglia to Aβ1–42 led to enhanced p38 mitogen-activated protein kinase and nuclear factor κB expression with factors inhibited by 4-AP. Aβ1–42 also induced the expression and production of the pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor-α, the chemokine IL-8, and the enzyme cyclooxygenase-2; 4-AP was effective in reducing all of these pro-inflammatory mediators. Additionally, toxicity of supernatant from Aβ1–42-treated microglia on cultured rat hippocampal neurons was reduced if 4-AP was included with peptide. In vivo, injection of Aβ1–42 into rat hippocampus induced neuronal damage and increased microglial activation. Daily administration of 1 mg/kg 4-AP was found to suppress microglial activation and exhibited neuroprotection. The overall results suggest that 4-AP modulation of an Aβ1–42-induced IK (candidate channel Kv3.1) and intracellular signaling pathways in human microglia could serve as a therapeutic strategy for neuroprotection in Alzheimer's disease pathology.
Keywords: Alzheimer's disease, amyloid β peptide, 4-aminopyridine, microglia, inflammation, cytokines
Introduction
Alzheimer's disease (AD), a neurodegenerative disorder exhibiting a gradual decline in cognitive function, is characterized by the presence of neuritic plaques composed of neurofibrillary tangles and amyloid β (Aβ) peptide (Hardy, 1997; Selkoe, 2001). Statistically significant correlations between Aβ1–42 deposition and cognitive impairment have been established in AD brain (Naslund et al., 2000). A common finding in AD is the presence of activated microglia in the vicinity of neuritic plaques (Arends et al., 2000). Thus, Aβ deposition could stimulate microglia into a reactive state, thereby evoking release of a milieu of inflammatory mediators that in assemblage cause neurotoxicity (Akiyama et al., 2000). A host of membrane receptors have been implicated in the transduction of Aβ stimulation of microglia. These include RAGE (receptor of advanced glycation end products) (Yan et al., 1996), scavenger receptor class-A (El Khoury et al., 1996), serpin enzyme complex (Boland et al., 1996), formyl peptide receptor (Lorton et al., 2000), and a cell surface complex composed of integrin and integrin-associated protein and B-class scavenger receptor (Bamberger et al., 2003) also implicated in the phagocytosis of fibrillar peptide (Koenigsknecht and Landreth, 2004).
Specific intracellular signaling effectors are activated in microglia and THP-1 monocytes exposed to Aβ peptide including tyrosine kinase-mediated pathways (McDonald et al., 1998; Combs et al., 1999). Peptide stimulation of tyrosine kinases was coupled to mobilization of intracellular Ca2+ and activation of protein kinase C and other downstream kinase activity (Combs et al., 1999). An important finding was that pharmacological modulation of components of these signaling pathways conferred neuroprotection (Combs et al., 1999). Other microglial signaling factors have been identified in peptide-stimulated cells; for example, binding of nonsteroidal anti-inflammatory drugs to the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) in microglia and THP-1 cells has been found as an anti-inflammatory strategy relevant to AD (Combs et al., 2000) and other neurodegenerative disorders (Sundararajan et al., 2006).
The nonselective K+ channel blocker 4-aminopyridine (4-AP) has been evaluated as a putative therapeutic in AD, presumably resulting from actions to prolong duration of action potential and thereby enhance neurotransmitter release (Glover, 1982). However, results from clinical trials using 4-AP have been ambiguous; in one study, cognitive deterioration was reduced in patients receiving 4-AP (Wesseling et al., 1984), whereas in a subsequent study, no significant difference between patients receiving 4-AP and those receiving placebo were reported (Davidson et al., 1988).
In this study, we investigated the effects of 4-AP on Aβ1–42-induced intracellular signaling pathways and functional responses of human microglia. 4-AP was effective in vitro in attenuating the Aβ1–42-induced upregulation of a K+ current, Ca2+ influx pathway, p38 mitogen-activated protein kinase (MAPK), and nuclear factor κB (NF-κB) activation and expression and production of a host of pro-inflammatory mediators. Also, in the presence of 4-AP, supernatant from Aβ1–42-treated microglia was less toxic to cultured neurons. In vivo 4-AP reduced microglial activation and was neuroprotective in peptide-injected rat hippocampus. These results indicate that 4-AP exhibits a wide-spectrum anti-inflammatory activity and neuroprotection both in vitro and in vivo.
Materials and Methods
Preparation of primary cultured human microglia.
Human microglia were prepared according to procedures reported previously (Satoh et al., 1995). Briefly, embryonic brain tissues 12–18 weeks gestation were incubated in PBS containing 0.25% trypsin and DNase (40 μg/ml) for 30 min at 37°C. Enzyme-treated tissues were dissociated into single cells by gentle pipetting. Dissociated cells were then cultured into DMEM containing 5% horse serum, 5 mg/ml glucose, 20 μg/ml gentamicin, and 2.5 μg/ml amphotericin B. After 7–10 d of growth in culture flasks, freely-floating microglia were collected from a medium of mixed cell cultures. The purity of the microglial cultures was in excess of 98% as determined by immunostaining with the cell-specific markers CD11b or ricinus communis agglutinin-1. Use of embryonic human tissues was approved by the Clinical Screening Committee for Human Subjects of the University of British Columbia.
Electrophysiology.
Procedures used in whole-cell patch-clamp studies have been described previously (McLarnon et al., 1997). Briefly, 1 d post-plated coverslips were placed on the stage of an inverted microscope (TMS; Nikon, Tokyo, Japan), and an amplifier (Axopatch 200B; Molecular Devices, Foster City, CA) was used to record macroscopic currents. Patch pipettes were fabricated using Corning (Corning, NY) glass number 7052 with resistances in the range of 2–4 MΩ. Capacitance and series resistance were compensated manually on the amplifier. The whole-cell configuration was used, and data were sampled at 5 kHz with the low-pass filter set at 1 or 2 kHz. Protocols were generated by computer and consisted of applying a depolarizing step from a holding potential (VH) of −60 mV to study outward K+ currents. All voltage-clamp experiments were performed with 500 μm 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) in bath solution to remove any chloride contribution to the overall whole-cell current. Data were recorded on disk and analyzed off-line using pClamp software. All experiments were performed at room temperature (20–22°C). The bath solution contained the following (in mm): 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4. The pipette solution contained the following (in mm): 140 KCl, 10 NaCl, 1 MgCl2, 0.5 EGTA, 1 ATP, and 10 HEPES, pH adjusted to 7.4.
Calcium spectrofluorometry.
Levels of intracellular calcium ([Ca2+]i) in human microglia were monitored using the calcium spectrofluorometry technique. One day post-plated cells on glass coverslips were loaded with 1 μm fura-2/AM (Invitrogen, Eugene, OR) with the solubilizing agent 0.02% pluronic acid in standard physiological solution (PSS). The PSS contained the following (in mm): 126 NaCl, 5 KCl, 1.2 MgCl2, 1 CaCl2, 10 d-glucose, and 10 HEPES, pH 7.4. In experiments performed with no Ca2+ in the extracellular solution, a Ca2+-free PSS was used in which the CaCl2 was replaced with 1 mm EGTA. After the wash period in dye-free solution, the coverslips were mounted on the stage of a Zeiss (Oberkochen, Germany) Axiovert inverted microscope containing a 40× quartz objective lens. The cells were then exposed to alternating wavelengths of 340 and 380 nm UV light (bandwidth, ±5 nm) at intervals of 6 s, and emission light was passed through a 510 nm filter (bandwidth, ±20 nm). The signals were acquired from a digital camera (DVC-1300 camera; Photometrics, Tucson, AZ) and recorded using an imaging system (Empix Imaging, Mississauga, Ontario, Canada) as fluorescence ratios of 340/380 (F340/F380) every 6 s. Increases in [Ca2+]i were expressed as 340/380 fluorescence ratios, and a 0.1 ratio change corresponds to 90 nm Ca2+. All experiments were performed at room temperature.
Phospho-p38 MAPK, NF-κB, and COX-2 immunocytochemistry.
The effects of stimuli on phosphorylated p38 MAPK (phospho-p38 MAPK), NF-κB, and cyclooxygenase-2 (COX-2) in human microglia were determined using immunocytochemical procedures described previously (Nakajima et al., 1998; Tikka and Koistinaho, 2001; Choi et al., 2002). Briefly, after preincubation in serum-free medium for 48 h, cells were treated with Aβ1–42 (5 μm), 4-AP (2 mm), Aβ42–1, or Aβ1–42 in combination with 4-AP after a 30 min preincubation with 4-AP. Optimum treatment times with stimuli were chosen for each of the factors examined: phospho-p38 MAPK, 30 min; NF-κB, 8 h; COX-2, 24 h. Subsequent to treatment with stimuli, cells were fixed with 4% paraformaldehyde in 0.1 m PBS, washed in PBS, and permeabilized in 0.2% Triton X-100 containing 5% normal goat serum (NGS) in 0.1 m PBS/0.5% bovine serum albumin (BSA) (BPBS) solution for 25 min. The cells were then incubated with rabbit anti-phospho-p38 MAPK (1:250 dilution; Cell Signaling Technology, Beverly, MA) or rabbit anti-human NF-κB p65 (1:250 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) to target the active p65 NF-κB subunit. Phospho-p38 and p65 primary antibodies were incubated with 5% NGS in BPBS at 4°C for 48 h. To examine for COX-2, cells were incubated with rabbit anti-human COX-2 (1:200 dilution; Cayman Chemical, Ann Arbor, MI) containing 10% NGS in BPBS at 4°C for 72 h. After incubation with a primary antibody, cells were washed in PBS and subsequently incubated with Alexa Fluor-488 anti-rabbit IgG secondary antibody (1:100; Invitrogen) containing 5% NGS in BPBS at room temperature for 2 h. After a wash in PBS, cells were incubated in 4′-6′-diaminodino-2-phenylindole (DAPI; Invitrogen) at 1 μg/ml in PBS to visualize nuclei (blue) and determine cell numbers in the field of view. Cells were then washed in water and mounted onto glass slides using gelvatol, examined under a Zeiss Axioplan fluorescent microscope, and photographed using a cooled CCD camera. The ratio of positive cells to total number per field was determined in a blind manner from four representative fields in each independent experiment. Values are expressed as means ± SEM, and statistical significance was determined using one-way ANOVA and the Newman–Keuls multiple comparison post-test (p < 0.05).
Reverse transcriptase-PCR.
Human fetal microglia were seeded into poly-l-lysine-coated 12-well plates at a density of ∼5 × 104 cells/well. In experiments investigating the effects of 4-AP on Aβ1–42-induced expressions of pro-inflammatory mediators, human microglia were preincubated in serum-free conditions for 48 h to promote a resting state and subsequently treated for 8 h with Aβ1–42, 4-AP (2 mm), Aβ42–1, and Aβ1–42 in combination with 4-AP after a 30 min preincubation with 4-AP. For effects of Aβ1–42 on Kv expression, microglia were treated with Aβ1–42 for differing time periods: 10 min, 30 min, 1 h, and 2 h subsequent to incubation in serum-free medium for 48 h. Total RNA was isolated using TRIzol (Invitrogen-BRL, Gaithersburg, MD), subjected to DNase treatment, and processed for the first-strand cDNA synthesis using Moloney murine leukemia virus reverse transcriptase (RT) (Invitrogen-BRL). cDNA products were then amplified by PCR using a GeneAmp thermal cycler (Applied Biosystems, Foster City, CA) with Taq polymerase. Specific sense and antisense primers with the expected product size are summarized in Table 1. PCR consisted of an initial denaturation step of 95°C for 6 min, followed by a 30–40 cycle amplification program consisting of denaturation at 95°C for 35 s, annealing at 59°C for 1 min, and elongation at 72°C for 1 min. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used as a reaction standard. The amplified PCR products were identified by electrophoresis using 1.5% agarose gels containing ethidium bromide and visualized under UV light. The intensities of each band were measured by densitometry using the NIH ImageJ 1.24 software (National Institutes of Health, Bethesda, MD). The band intensities of PCR products in control and with stimuli were expressed as relative mRNA levels (mRNA values normalized to G3PDH).
Table 1.
Primer sequences for RT-PCR
| Product | Sequence | Size (bp) |
|---|---|---|
| COX-2 sense | 5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′ | 305 |
| COX-2 antisense | 5′-AGATCATCTCTGCCTGAGTATCTT-3′ | |
| IL-1β sense | 5′-AAAAGCTTGGTGATGTCTGG-3′ | 179 |
| IL-1β antisense | 5′-TTTCAACACGCAGGACAGG-3′ | |
| IL-6 sense | 5′-GTGTGAAAGCAGCAAAGAGGC-3′ | 159 |
| IL-6 antisense | 5′-CTGGAGGTACTCTAGGTATAC-3′ | |
| IL-8 sense | 5′-ATGACTTCCAAGCTGGCCGTG-3′ | 301 |
| IL-8 antisense | 5′-TATGAATTCTCAGCCCTCTTCAAAA-3′ | |
| TNF-α sense | 5′-CAAAGTAGACCTGCCCAGAC-3′ | 490 |
| TNF-α antisense | 5′-GACCTCTCTCTAATCAGCCC-3′ | |
| Kv1.1 sense | 5′-GTTAGGGGAACTGACGTGGA-3′ | 482 |
| Kv1.1 antisense | 5′-CTGAGCAGGAGAGGAAACCAG-3′ | |
| Kv1.2 sense | 5′-GGGACAGAGTTGGCTGAGAA-3′ | 513 |
| Kv1.2 antisense | 5′-GGAGGATGGGATCTTTGGAC-3′ | |
| Kv1.3 sense | 5′-GCGACGAGAAGGACTACCC-3′ | 513 |
| Kv1.3 antisense | 5′-TGCTGCTGAAACCTGAAGTG-3′ | |
| Kv1.5 sense | 5′-GAGGACGAGGAGGAAGAAGG-3′ | 528 |
| Kv1.5 antisense | 5′-CAAGCAGAAGGTGATGATGG-3′ | |
| Kv1.6 sense | 5′-GGGAGTCAGGAGGAAGAGGA-3′ | 569 |
| Kv1.6 antisense | 5′-ATGCTGGGAAAAAGCGAAT-3′ | |
| Kv2.1 sense | 5′-ACAGAGCAAACCAAAGGAAGAAC-3′ | 385 |
| Kv2.1 antisense | 5′-CACCCTCCATGAAGTTGACTTTA-3′ | |
| Kv3.1 sense | 5′-TCCTGAACTACTACCGCACG-3′ | 620 |
| Kv3.1 antisense | 5′-GAACTCTACCTTGTTGGGGC-3′ | |
| G3PDH sense | 5′-CCATGTTCGTCATGGGTGTGAACCA-3′ | 251 |
| G3PDH antisense | 5′-GCCAGTAGAGGCAGGGATGATGTTC-3′ |
ELISA.
ELISA kits (R & D Systems, Minneapolis, MN) were used to determine the production of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1β, and IL-8 in culture supernatants from human fetal microglia stimulated for 48 h with 5 μm Aβ1–42, Aβ1–42 in the maintained presence of 4-AP (2 mm) after a 30 min preincubation with 4-AP, 4-AP alone, or reverse peptide Aβ42–1 (5 μm). After incubation of microglia with stimuli, cell culture supernatants were collected and stored at −70°C.
Cytotoxicity of human fetal microglial supernatants on primary rat hippocampal neurons.
Human fetal microglia were seeded into 24-well plates at 1.5 × 105 cells/well. After a 48 h preincubation in serum-free medium, cells were treated for 48 h with Aβ1–42 (5 μm), 4-AP (2 mm), Aβ42–1, or Aβ1–42 in combination with 4-AP after a 30 min preincubation with 4-AP. After treatment with stimuli, cell-free supernatants were transferred to primary rat hippocampal neurons plated on glass coverslips.
Isolation of primary rat hippocampal neurons has been described previously (Sheldon et al., 2004). Briefly, 2- to 4-d-old Wistar rats were anesthetized and decapitated, and the hippocampi were removed. The hippocampi were then enzymatically treated and mechanically dissociated, and the resulting cell suspension was plated at a density of 5–7 × 105 neurons cm−2 onto glass coverslips coated with poly-d-lysine and laminin. The initial growth medium was DMEM/F-12 supplemented with 10% fetal bovine serum (Invitrogen Canada, Burlington, Ontario, Canada). After 24 h, this medium was changed to serum-free Neurobasal Medium A (Invitrogen Canada). Cultures were then fed every 4–5 d by half-changing the existing medium with fresh Neurobasal Medium A. Glial proliferation was inhibited 48 h after initial plating by adding 5–10 mm cytosine arabinoside (Sigma-Aldrich, St. Louis, MO). Neurons were used 12–15 d after plating.
Primary hippocampal neurons were treated with microglial conditioned medium or medium controls (conditioned medium not exposed to microglia) for 16 h. After treatment, cells were fixed with 4% paraformaldehyde in 0.1 m PBS. After a wash in PBS, cells were incubated in DAPI at 1 μg/ml in PBS to visualize nuclei. Cells were then washed in water and mounted onto glass slides using gelvatol, examined under a Zeiss fluorescent microscope, and photographed using a cooled CCD camera. The percentage of damaged neurons was determined by counting in a blind manner the number of fragmented/condensed nuclear-stained neurons by the overall number of positively DAPI-stained neurons. Control experiments consisted of incubating neurons with conditioned medium without microglial exposure. The effects of microglial conditioned medium and controls on neuronal viability were each determined from five independent experiments. Data are presented as mean ± SEM, and significance was determined by one-way ANOVA and the Newman–Keuls post hoc multiple comparison test (p < 0.05).
Effects of 4-AP on Aβ1–42-induced microglial activation and neurotoxicity in vivo.
The procedure used for Aβ1–42 injection in vivo has been described previously (Jantaratnotai et al., 2003; Ryu et al., 2004). Briefly, male Sprague Dawley rats (250–280 g; Charles River, St. Constant, Quebec, Canada) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and mounted in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). Aβ1–42 or Aβ42–1 (1 nmol in 2 μl) was injected slowly (0.2 μl/min) into the hippocampus [anteroposterior, −3.6 mm; mediolateral, −1.8 mm; dorsoventral, −3.2 mm; according to the atlas of Paxinos and Watson (1986)] using a 10 μl Hamilton syringe. The concentration of Aβ used in the present study was in the range of 1–2 nmol used in previous experiments with stereotaxic injection of peptide (Weldon et al., 1998; Walsh et al., 2002). Vehicle control animals were given injections of the same solutions used for dissolving the peptide. 4-AP (Sigma-Aldrich) was dissolved in 0.9% saline and administered intraperitoneally at 1 mg/kg 15 min before Aβ1–42 injection, followed by once-daily injections of 1 mg/kg for 7 d. The doses of 4-AP used in this study are based on previous studies using 4-AP in rats (Casamenti et al., 1982; Haroutunian et al., 1985).
Seven days after Aβ1–42 injection, anesthetized rats were perfused transcardially with heparinized cold saline, followed by 4% paraformaldehyde. Brains were postfixed overnight in the same fixative and placed in 30% sucrose for cryoprotection. Serial coronal sections (40 μm) through the hippocampus were cut on a cryostat. For immunohistochemistry, free-floating brain sections were permeabilized with 0.2% Triton X-100 and 0.5% BSA in 0.1 m PBS for 30 min, blocked in PBS containing 0.5% BSA and 10% NGS for 30 min, and incubated overnight at room temperature in PBS containing 5% NGS and primary antibodies. The following primary antibodies were used: mouse anti-NeuN (1:1000; Chemicon, Temecula, CA) for neurons and mouse anti-ED1 (1:500; Serotec, Oxford, UK) for activated microglia/macrophages. For controls, primary antibodies were omitted. The following day, sections were incubated with biotinylated secondary antibody (1:200; Vector Laboratories, Burlingame, CA), visualized with the ABC Elite system (1:200; Vector Laboratories), and developed in 3,3′-diaminobenzidine (Sigma-Aldrich). The number of NeuN- or ED1-positive cells in the superior blade of the dentate granule cell layer was conducted on three consecutive sections as described previously (Ryu et al., 2004). Representative photomicographs were taken, and counting was performed using a Zeiss Axioplan 2 fluorescent microscope equipped with a DVC camera (Diagnostic Instruments, Sterling Heights, MI) and Northern Eclipse software (Empix Imaging). All quantitative analyses were performed in a blinded manner with values expressed as means ± SEM. Statistical comparisons were made using one-way ANOVA, followed by the Newman–Keuls post hoc multiple comparison test (GraphPad Prism 3.0; Graph Pad, San Diego, CA) with the significance level set at p < 0.05. All animal experiments were approved by the Animal Care Committee of the University of British Columbia.
Reagents.
The Aβ peptide (Aβ1–42) and reverse peptide (Aβ42–1) were purchased from California Peptide (Napa, CA), and fresh stock solution was prepared according to a method described previously (Walker et al., 2001) with slight modifications. Briefly, Aβ1–42 was prepared by first dissolving the peptide in 35% acetonitrile (Sigma-Aldrich), diluted to 1.5 mm with sterile water and then to 500 μm with incremental additions of PBS with vortexing between additions. The Aβ solution was subsequently incubated at 37°C for 18 h to promote Aβ fibrilization and aggregation and stored at −20°C. A similar procedure was followed for preparation of reverse peptide Aβ42–1. The vehicle control was prepared as described for the preparation of Aβ1–42, however Aβ1–42 was omitted. 4-AP, GTPγS, iberiotoxin, NPPB, SKF96365, and tetraethylammonium (TEA) were purchased from Sigma-Aldrich, and apamin was a generous gift from Dr. J. Church (University of British Columbia).
Results
Acutely applied Aβ1–42 to human microglia induces a 4-AP-sensitive outward K+ current
A representative control current response to a depolarizing step (applied from a holding potential of −60 to +20 mV) is shown in Figure 1A. Current amplitudes were small in unstimulated human microglia (range, 40–70 pA), consistent with previous data from human (McLarnon et al., 1997) and rodent (Kettenmann et al., 1990; Norenberg et al., 1994) cells. Within 3 min after acute application of Aβ1–42 (at 5 μm) to human microglia, a rapidly activating outward current (labeled IK), with an amplitude of 875 pA, was observed (Fig. 1A). The current exhibited no inactivation over the duration of depolarizing steps. Overall, the amplitude of the current induced with acute application of Aβ1–42 in human microglia increased 14-fold (to 850 ± 102 pA; n = 7) from currents under unstimulated conditions (59 ± 6 pA; n = 7). Subsequent washoff of the peptide allowed the current to recover to control levels (Fig. 1A).
Figure 1.
Aβ1–42 induces an outwardly rectifying current (IK) that is attributed to K+. A, A representative recording of the Aβ1–42-induced outward K+ current (IK) in response to a depolarizing step in human microglia. The trace is a typical leak current evoked in control solution with a depolarization step to +20 mV from a holding potential of −60 mV. Aβ1–42 (5 μm) elicited a large IK with the same step depolarization. The current recovered subsequent to washoff of Aβ1–42. B, IK, induced by Aβ1–42 (5 μm), with sequential depolarizing steps applied from −60 mV to a maximum level of +20 mV in 10 mV increments. C, The I–V relationship constructed from the pulse protocol shown indicates that the outward current induced by acute Aβ1–42 was outwardly rectifying with a threshold of −40 mV. The figure is a representative recording from one cell. D, Determination of the reversal potential of the IK via analysis of tail currents. The protocol for tail current analysis consisted of applying a depolarizing step from −60 to +40 mV, followed by a secondary step to potentials varying from −100 to −20 mV. The resulting tail currents elicited with the secondary steps from −100 to −20 mV in a representative experiment are indicated by arrows. E, An I–V plot of tail current amplitudes versus step potential is shown, and results indicate that the reversal potential of the current is −78 mV, which is close to the equilibrium potential for K+.
Experiments were performed to characterize kinetic properties of IK. The threshold for activation was determined using a series of depolarizing steps (applied from −60 mV to a maximum of +20 mV in 10 mV increments) (Fig. 1B). The corresponding plot of current amplitude versus step potential (I–V plot) is shown in Figure 1C. The current was outwardly rectifying in shape; overall, the activation threshold for IK was −33.7 ± 2.4 mV (n = 4). The reversal potential for IK was determined using analysis of tail currents with a protocol consisting of depolarizing steps (from −60 to +40 mV), followed by a series of variable potentials in the range from −100 to −20 mV (Fig. 1D). The initial current for each step (indicated by solid arrows in Fig. 1D) was then measured and plotted versus voltage. A typical tail current analysis of the outward current is shown in Figure 1E. A plot of tail current amplitudes versus step potential yielded an overall reversal of −76.2 ± 3.6 mV (n = 6) close to the equilibrium potential for K+ (in this study, Erev for K+ = −84 mV). As noted in Materials and Methods, bath solutions included the compound NPPB (500 μm) to block anion currents.
The effects of 4-AP (at 2 mm) on the Aβ1–42 peptide-induced IK are shown in Figure 2A. In this experiment, IK was partially blocked to 52% of control by 4-AP; recovery of IK was observed after washoff of 4-AP (Fig. 2A). Overall, 4-AP at 2 and 5 mm reduced IK to 58.3 ± 7.3% (n = 3) and 38.4 ± 6.6% (n = 3) of control, respectively. Another nonselective K+ channel blocker, TEA, was also examined at a single concentration of 10 mm (data not shown); TEA inhibited IK to 28 ± 5.9% (n = 3) of control.
Figure 2.
A, IK is inhibited by the nonselective K+ channel inhibitor 4-AP. A representative recording from one cell is shown. The first trace is a typical current evoked in control solution with a depolarization step to +20 mV. Aβ1–42 (5 μm) elicited IK with the same step depolarization. Application of 4-AP (2 mm) in the presence of Aβ1–42 reduced IK to 52% of control. The current recovered subsequent to washoff of 4-AP with Aβ1–42 maintained in bath solution. B, Typical profile of the intracellular GTPγS-induced outward current. Intracellular application of GTPγS (10 μm) via the electrode induced an outward current within minutes of rupture of the cell membrane in the whole-cell patch-clamp mode in response to a depolarizing step from −60 to +20 mV. Extracellular application of 4-AP (2 mm) reduced the outward K+ current to 65% of the control. Washoff of 4-AP allowed the current to recover. C, Concentration-dependent inhibition of the intracellular GTPγS-induced outward K+ current by 4-AP. 4-AP was applied in the extracellular bath solution, and amplitudes of the GTPγS-induced currents were measured in the presence of 4-AP and normalized to control amplitudes (C; current amplitude before 4-AP application). Results are a summary of the following: n = 4 cells for 1 mm; n = 6 cells for 2 mm; n = 10 cells for 5 mm; n = 4 cells for 10 mm; n = 3 cells for 20 mm.
Intracellular application of GTPγS on signaling properties of human microglia
Previous work (McKinney and Gallin, 1993) has characterized a K+ current in macrophages induced by GTPγS, which showed similar kinetic behavior to IK. This prompted us to investigate intracellular GTPγS as a putative activator for IK in the present study. Small currents (near 40 pA) were elicited on initial formation of a whole-cell seal (data not shown), and after 4–5 min of dialysis of GTPγS, a steady current with amplitude exceeding 1 nA was recorded in response to the depolarizing step to +20 mV (Fig. 2B). Overall, the mean amplitude of the GTPγS-induced outward current was 1150 ± 110 pA (n = 9). The current induced with intracellular GTPγS exhibited kinetic behavior very similar to IK with rapid activation and no inactivation over the duration of depolarizing steps. The peak amplitude of this current was not significantly different from the Aβ1–42-induced IK. Overall, the threshold for activation (data not shown) was −28.1 ± 1.7 mV (n = 4) and reversal potential (data not shown) was −68.4 ± 1.1 mV (n = 4) for the GTPγS-activated outward current, which were not significantly different from the values measured for IK induced by Aβ1–42 peptide (−34 mV for activation threshold and −76 mV for reversal potential, respectively).
The effects of acute application of 4-AP (2 mm) to the GTPγS-induced K+ current are shown in Figure 2B. 4-AP reduced the current to 65% of control with partial recovery after washoff (Fig. 2B). Overall, 4-AP (2 mm) reduced the GTPγS-induced outward K+ current to 61.3 ± 9.5% of control (n = 6), close to the value determined for IK as induced by amyloid peptide (58%). The concentration-dependent effects of 4-AP on the GTPγS-induced outward K+ current were determined (range of 4-AP, 1–20 mm) with results summarized in Figure 2C. Overall, an IC50 value of 5 mm for 4-AP inhibition of the current was obtained. Several other pharmacological agents were tested for effects on the GTPγS-activated current. TEA (10 mm) blocked the K+ current to 23.8 ± 3.7% of control (n = 3; data not shown). However, both iberiotoxin (50 nm) and apamin (100 nm) were ineffective (data not shown), suggesting that the G-protein-dependent current was not attributable to activation of a BK- or SK-type (large or small conductance) KCa channel. Interestingly, the GTPγS-induced K+ current in macrophages was also insensitive to both apamin and charybdotoxin (McKinney and Gallin, 1993), an inhibitor of BK- and IK-type (large and intermediate conductance) KCa channels, further supporting the actions of intracellular GTPγS to induce a similar current in both human microglia and macrophages.
Aβ1–42 induces the expression of Kv3.1
Based on electrophysiological data, the channel induced with acute Aβ1–42 displayed the profile of a delayed rectifier type of K+ channel. We examined a series of Kv channels (Kv1.1, Kv1.2, Kv1.3, Kv1.5, Kv1.6, Kv2.1, and Kv3.1) with delayed rectifier K+ channel properties reported previously in human cells (Jiang et al., 2002). Human microglia were incubated with Aβ1–42 for 10 min, 30 min, 1 h, and 2 h, and expression of each of the Kv channels was examined using RT-PCR. Results from a representative experiment are shown in Figure 3A, indicating a rapid time-dependent increase in Kv3.1 after a 10 min incubation with Aβ1–42. Peak expression occurred at 30 min and decreased at 1 and 2 h; no expression of Kv3.1 was evident after a 4 h exposure to peptide (data not shown). All other Kv channels (Kv1.1, Kv1.2, Kv1.3, Kv1.5, Kv1.6, and Kv2.1) showed basal expression in unstimulated human microglia. Incubation with reverse peptide Aβ42–1 (5 μm) had no effect on these Kv channel expressions.
Figure 3.
A, A representative RT-PCR experiment of Aβ1–42 (5 μm) treatment for 10 min, 30 min, 1 h, and 2 h on Kv1.1, Kv1.2, Kv1.3, Kv1.5, Kv1.6, Kv2.1, and Kv3.1 channel expression from n = 3 independent experiments. G3PDH served as a reaction standard. B, Summary of relative mRNA levels of Kv channels induced by Aβ1–42. Results are expressed as mean ± SEM from n = 3 independent experiments. One-way ANOVA and the Newman–Keuls multiple comparison test was used to evaluate statistical significance. *p < 0.05 and **p < 0.001, statistical significance from control. C, PTX (100 ng/ml) before treatment (2 h) attenuated the effects of Aβ1–42 (5 μm; 30 min) to increase the expression of Kv3.1.
Densitometry analysis of PCR product band intensities demonstrated the effect of Aβ1–42 to time-dependently increase Kv3.1 channel expression with no effects of Aβ1–42 to increase Kv1.1, Kv1.2, Kv1.3, Kv1.5, Kv1.6, and Kv2.1 channel expression. Results are summarized in Figure 3B. Aβ1–42 significantly increased Kv3.1 channel expression at 10 min by 5.6-fold compared with control (p < 0.05). Incubation with Aβ1–42 for 30 min significantly increased Kv3.1 channel expression further by 13-fold compared with Kv3.1 channel expression in control (p < 0.001). Kv3.1 channel expression remained elevated at 1 and 2 h; however, these increases were not significantly different from control (p > 0.05). Relative mRNA levels of Kv1.1, Kv1.2, Kv1.3, Kv1.5, Kv1.6, and Kv2.1 channels induced with different Aβ1–42 treatment times were not significantly different from control (p > 0.05).
Because data in patch-clamp studies above indicated that the Aβ1–42-induced IK was mediated through a G-protein, the possibility that Kv3.1 expression induced with Aβ1–42 was regulated by a G-protein was investigated using RT-PCR. Results showed that pertussis toxin (PTX), an inhibitor of Gi-proteins, attenuated Aβ1–42-induced Kv3.1 expression (Fig. 3C). These results indicated that the expression of Kv3.1 was regulated by Gi-proteins.
4-AP attenuates the Aβ1–42-induced increase in [Ca2+]i in human microglia
The effects of 4-AP on Aβ1–42-induced [Ca2+]i responses in human microglia were next investigated. Application of Aβ1–42 (5 μm) evoked a slow, progressive increase in [Ca2+]i, to a plateau level (Fig. 4A). In this experiment, a peak increase in [Ca2+]i of 0.07 (F340/F380) was attained after 6 min of peptide application (n = 21 cells). In the maintained presence of peptide, the replacement of Ca2+-PSS with Ca2+-free PSS reduced the response to baseline levels. Overall, the mean amplitude of the response was 0.11 ± 0.01 (n = 98 cells) with Ca2+-free PSS decreasing responses to basal levels. The decrease in [Ca2+]i induced with Aβ1–42 in Ca2+-free solution suggests that an influx pathway mediates the increase in [Ca2+]i with Aβ1–42. This point was examined by applying Aβ1–42 in Ca2+-free solution, and exposure of human microglia to peptide had little effect to alter [Ca2+]i (n = 23 cells) (Fig. 4B); a similar result was obtained in a total of 38 cells. In standard Ca2+-containing solution, acute application of reverse peptide Aβ42–1 (5 μm) had no effect to alter levels of [Ca2+]i (data not shown).
Figure 4.

A, Acute application of Aβ1–42 induces a slow, progressive increase in [Ca2+]i. A representative trace of the increase in [Ca2+]i induced by Aβ1–42 (5 μm) in Ca2+-PSS (n = 21 cells) is shown. Subsequent application of Aβ1–42 in Ca2+-free PSS resulted in an immediate decrease in [Ca2+]i to baseline levels. B, The standard PSS was first exchanged for Ca2+-free PSS. Acute application of Aβ1–42 (5 μm) in Ca2+-free PSS did not elicit an increase in [Ca2+]i (n = 23 cells). C, A representative trace of the effect of 4-AP on the Ca2+ influx pathway induced by Aβ1–42 (n = 26 cells). Subsequent to the slow, progressive increase in [Ca2+]i induced by acute Aβ1–42 (5 μm) in Ca2+ PSS, application of 4-AP (2 mm) rapidly decreased [Ca2+]i to baseline levels.
The effects of 4-AP on [Ca2+]i increases evoked by peptide were examined at a single concentration of 2 mm. As shown in Figure 4C, Aβ1–42 induced a progressive increase in [Ca2+]i that was maintained even after removal of Aβ1–42 (n = 26 cells). Application of 4-AP to plateau [Ca2+]i decreased the response to baseline level. Overall, 4-AP (at 2 mm) inhibited the Aβ1–42-induced increase in [Ca2+]i by 95 ± 0.5% (n = 149 cells). These results indicate that 4-AP is an effective inhibitor of a Ca2+ influx pathway induced by acute Aβ1–42 in human microglia.
Previous work has reported that a primary Ca2+ influx pathway in human microglia are store-operated channels (SOCs) with cells lacking expression of voltage-gated Ca2+ channels (McLarnon et al., 1999). We investigated the possibility that SOCs could mediate the entry of Ca2+ induced by Aβ1–42 by using SKF96365, an agent demonstrated to block SOCs in human microglia (Franciosi et al., 2002; Choi et al., 2003) and in other cells (Li et al., 1999). After attainment of a plateau in [Ca2+]i with Aβ1–42, application of SKF96365 (50 μm) did not alter [Ca2+]i (data not shown). This result suggests that a SOC does not contribute to the influx pathway induced by acute Aβ1–42 in human microglia.
Modulation of Aβ1–42-induced p38 MAPK and NF-κB activation by 4-AP
We next used immunocytochemical analysis to investigate the effects of 4-AP on p38 MAPK and NF-κB. Representative photomicrographs of the effects of 4-AP on Aβ1–42-induced p38 MAPK activation in human microglia are shown in Figure 5A (left). Under basal conditions, low numbers of phospho-p38 MAPK-positive cells were detected. Phospho-p38 MAPK was increased at the earliest time studied at 10 min after application of Aβ1–42 (5 μm) (data not shown). Stimulation with peptide for 30 min induced a marked increase in expression of phospho-p38 MAPK in microglia (Fig. 5A, green staining), which was partially blocked if 4-AP (2 mm) was included with Aβ1–42 treatment. 4-AP itself showed no effect to alter phospho-p38 MAPK from control. Results from four independent experiments are summarized in Figure 5A (right). Overall, Aβ1–42 significantly increased (by 371%) the number of cells expressing activated p38 MAPK (p < 0.001). 4-AP (2 mm), in the maintained presence of Aβ1–42, resulted in a significant reduction (by 58%) in the number of cells expressing phospho-p38 MAPK compared with stimulation with Aβ1–42 alone (p < 0.001). 4-AP (2 mm) or reverse peptide Aβ42–1 (5 μm) did not alter the basal level of phospho-p38 MAPK-stained cells from levels in control (p > 0.05).
Figure 5.
Effects of 4-AP on Aβ1–42-induced p38 MAPK and NF-κB activation. A, Left, Representative photomicrographs of phosphorylated p38 (phospho-p38)-stained microglia. Green and blue indicate staining for phospho-p38 MAPK- and DAPI-positive nuclei, respectively. Under control conditions, little or no phospho-p38 MAPK expression was observed. Aβ1–42 (5 μm) treatment of microglia for 30 min induced an intense expression of phospho-p38 MAPK. Aβ1–42 in the maintained presence of 4-AP (2 mm) inhibited expression of phospho-p38 MAPK. Application of 4-AP (2 mm) alone had no effect on phospho-p38 MAPK expression. Right, The percentage of phospho-p38 MAPK-positive microglia relative to total cells is shown. Data are means ± SEM from four independent experiments. *p < 0.001, significance compared with control; **p < 0.001, significance compared with Aβ1–42. B, Left, Effects of 4-AP on Aβ1–42-induced NF-κB activation. Representative photomicrographs of p65 (the active subunit of NF-κB)-stained microglia are shown. Green and blue indicate staining for p65- and DAPI-positive nuclei, respectively. Under control conditions, little or no p65 expression was observed. Aβ1–42 (5 μm) treatment of microglia for 8 h induced an intense expression of p65. Aβ1–42 in the maintained presence of 4-AP (2 mm) inhibited expression of p65. Application of 4-AP (2 mm) alone had no effect on p65 expression. Right, The percentage of p65-positive microglia relative to total cells is shown. Data are means ± SEM from five independent experiments. *p < 0.01, significance compared with control; **p < 0.01, significance compared with Aβ1–42.
4-AP was also effective in reducing Aβ1–42-induced activation of NF-κB (Fig. 5B). A low number of cells expressed the active subunit of NF-κB, p65, under basal conditions (Fig. 5B, green staining). p65 was increased at the earliest time studied for this factor (1 h after peptide application), with subsequent increases up to 24 h (data not shown). The effects of Aβ1–42 (5 μm) to increase the expression of p65 in microglia at 8 h of peptide exposure are shown in Figure 5B (left). In the presence of Aβ1–42 (5 μm), 4-AP (2 mm) attenuated the induction of NF-κB with Aβ1–42 treatment. 4-AP itself showed no effect to alter p65 levels from control. Results from five independent experiments are shown in Figure 5B (right). Overall, Aβ1–42 induced a significant increase (by 493%) in p65-expressing cells (p < 0.01). 4-AP in the maintained presence of Aβ1–42 significantly decreased (by 60%) the number of cells expressing p65 (p < 0.01). 4-AP (2 mm) alone induced an increase in p65-positive cells; however, the increase was not significant (p > 0.05). The small trend in increased p65-expressing cells with 4-AP treatment could suggest a nonselective action of the compound. Application of reverse peptide (Aβ42–1) did not alter basal levels of p65-expressing cells (p > 0.05).
Effects of 4-AP on Aβ1–42-induced pro-inflammatory mediator expression and production in human microglia
An important component of the study was to determine the role of IK in modulation of microglial functional processes. The effects of 4-AP on Aβ1–42-induced expression of the pro-inflammatory cytokines (IL-1β, IL-6, TNF-α), chemokine [CXCL8 (IL-8)], and inducible enzyme COX-2 were determined using RT-PCR. Results from a representative experiment are shown in Figure 6A. Overall, microglia express CXCL8 (IL-8) constitutively under unstimulated conditions, whereas IL-1β, IL-6, TNF-α, and COX-2 were not expressed. After an 8 h incubation with 5 μm Aβ1–42, increased expression of all pro-inflammatory mediators was observed. In the presence of Aβ1–42, 4-AP (2 mm) decreased the expression of all pro-inflammatory mediators. 4-AP alone had little or no effect on expressions of mediators. Aβ42–1 (5 μm) was ineffective to alter expression of pro-inflammatory mediators from control.
Figure 6.
Effects of 4-AP on Aβ1–42-induced expression and production of pro-inflammatory mediators by human microglia. A, Expression of IL-1β, IL-6, IL-8, TNF-α, and COX-2 were examined in microglia incubated for 8 h with Aβ1–42, 4-AP, Aβ1–42 in the presence of 4-AP (2 mm), or medium alone. Stimulation of microglia with vehicle solution or Aβ42–1 (5 μm) alone served as control. The results shown are a representative of seven independent experiments. The expression of G3PDH served as a reaction standard. B, Summary of relative mRNA levels of inflammatory mediators induced by Aβ1–42, 4-AP, and combined Aβ1–42 and 4-AP. Results are expressed as mean ± SEM from n = 7 independent experiments. One-way ANOVA and the Newman–Keuls multiple comparison post-test were performed to evaluate statistical significance. *p < 0.05, statistically significant from control; **p < 0.05, statistically significant from Aβ1–42-stimulated levels. Effects of Aβ1–42, 4-AP, and Aβ1–42 in the maintained presence of 4-AP on pro-inflammatory cytokine secretion by human microglia using ELISA. C–F, Data are mean ± SEM of TNF-α from four independent experiments (C), IL-6 from three independent experiments (D), IL-1β from six independent experiments (E), and IL-8 from four independent experiments (F); each experiment was performed in duplicate. Human microglia were exposed to medium alone, Aβ1–42 (5 μm), 4-AP (2 mm), Aβ1–42 in the presence of 4-AP, or Aβ42–1 for 48 h. One-way ANOVA and the Newman–Keuls multiple comparison post-test were performed to evaluate statistical significance. *p < 0.001, statistical significance from control; **p < 0.001, statistical significance from Aβ1–42-stimulated levels.
Densitometry analysis was used for semiquantitative determination of PCR product band intensities. A summary of relative mRNA levels of inflammatory mediators induced with Aβ1–42 and 4-AP and of Aβ1–42 in the maintained presence of 4-AP is shown in Figure 6B (n = 7 experiments), and fold increases in relative mRNA levels of cytokines induced with the different stimuli compared with control are summarized in Table 2. Overall, Aβ1–42 (5 μm) increased relative mRNA levels of all pro-inflammatory mediators (relative to control) by (× fold increase) IL-1β (3.8), IL-6 (6.8), IL-8 (1.8), TNF-α (3.6), and COX-2 (2.9) (p < 0.05). Aβ1–42 in the presence of 4-AP (2 mm) led to a significant decrease in pro-inflammatory mediator expression relative to Aβ1–42-stimulated levels by (× fold decrease) IL-1β (0.56), IL-6 (0.60), IL-8 (0.65), TNF-α (0.61), and COX-2 (0.51) (p < 0.05). Stimulation with 4-AP alone led to a small increase in levels of pro-inflammatory mediators (relative to control) by (× fold increase) IL-1β (1.3), IL-6 (1.5), IL-8 (1.1), TNF-α (1.5), and COX-2 (1.5); however, the increases were not significant (p > 0.05). Aβ42–1 did not alter relative mRNA levels of pro-inflammatory mediators from control (p > 0.05; data not shown).
Table 2.
Fold increases in relative pro-inflammatory mediator mRNA induced by Aβ1–42 and Aβ1–42 plus 4-AP compared with relative mRNA in control using semiquantitative RT-PCR
| Aβ1–42 | Aβ1–42 plus 4-AP | |
|---|---|---|
| IL-1β | 3.8* | 2.0 |
| IL-6 | 6.8** | 4.2** |
| CXCL8 (IL-8) | 1.8* | 1.2 |
| TNF-α | 3.6* | 2.2* |
| COX-2 | 2.9* | 1.5 |
*p < 0.05;
**p < 0.01.
We also investigated the effects of 4-AP on protein levels of pro-inflammatory factors with increased expressions as a result of Aβ1–42 stimulation. The productions of TNF-α, IL-6, IL-1β, and IL-8 were investigated after 48 h stimulation with Aβ1–42 in the presence and absence of 4-AP (2 mm) using ELISA. This time point was chosen as the optimum time point to determine the modulatory actions of 4-AP on cytokine production using ELISA, because protein levels continued to accumulate through 48 h and incubations with Aβ1–42 for periods longer than 24 h could induce both direct and indirect effects of the peptide (Walker et al., 2001). A summary of cytokine production with each of the stimuli is presented in Figure 6C–F, and fold increases in cytokine production compared with control are summarized in Table 3. Aβ1–42 increased secretion of TNF-α (by 2.3-fold; n = 4 independent experiments) (Fig. 6C), IL-6 (by 46-fold; n = 3 independent experiments) (Fig. 6D), IL-1β (by 1.9-fold; n = 6 independent experiments) (Fig. 6E), and IL-8 (by 4.5-fold; n = 4 independent experiments) (Fig. 6F) and compared with basal levels in human microglia; all increases were significant (p < 0.001). Aβ1–42 in the maintained presence of 4-AP (2 mm) decreased levels of TNF-α (to 0.54-fold), IL-6 (to 0.27-fold), IL-1β (to 0.74-fold), and IL-8 (to 0.53-fold) compared with Aβ1–42 alone; all decreases were significant (p < 0.001). The changes in levels of pro-inflammatory mediators induced with 4-AP and Aβ42–1 each alone were not significantly different from basal levels (p > 0.05).
Table 3.
Fold increases in pro-inflammatory mediator production induced by Aβ1–42 and Aβ1–42 plus 4-AP compared with levels in control
*p < 0.01;
**p < 0.001.
The production of COX-2 after stimulation with Aβ1–42, 4-AP, or Aβ1–42 and 4-AP combined was determined using immunocytochemistry (Fig. 7A). Stimulation with Aβ1–42 (5 μm for 24 h) induced an increase in the number of COX-2-positive microglia (green staining) from control that was partially blocked with 4-AP (2 mm) in the maintained presence of Aβ1–42. 4-AP and Aβ42–1 each alone had no effect to alter COX-2 levels from control. Aβ1–42 significantly increased the percentage of microglia expressing COX-2 by 5.1-fold from control levels (p < 0.001) (Fig. 7B). 4-AP (2 mm) in the maintained presence of Aβ1–42 significantly decreased (by 0.43-fold) the percentage of COX-2-expressing cells (p < 0.01). 4-AP (2 mm) and Aβ42–1 (5 μm) each alone induced an increase in the percentage of COX-2-positive microglia compared with unstimulated conditions; however, the increases were not significant (p > 0.05).
Figure 7.
Effects of 4-AP on Aβ1–42-induced COX-2-expressing microglia. A, Representative photomicrographs of COX-2-stained microglia. Green and blue indicate staining for COX-2- and DAPI-positive nuclei, respectively. Under control conditions, little or no COX-2 expression was evident. Treatment of microglia for 24 h with Aβ1–42 (5 μm) induced an intense expression of COX-2. Aβ1–42 in the presence of 4-AP (2 mm) treatment inhibited production of COX-2. 4-AP alone had no effect on basal levels of COX-2 production. B, The percentage of COX-2-positive microglia relative to total cells is shown under the different experimental conditions. Data are means ± SEM from six independent experiments. *p < 0.001, significance compared with control; and **p < 0.01, significance compared with Aβ1–42.
4-AP inhibits Aβ1–42-induced microglial mediated neurotoxicity
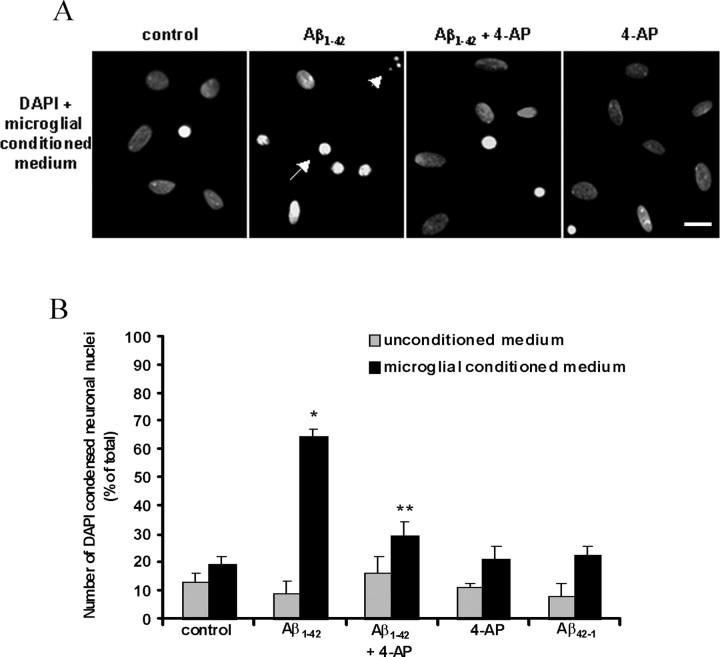
The modulatory potential of 4-AP on Aβ1–42-induced microglial neurotoxicity was investigated using DAPI staining. As shown in Figure 8A, incubation of neurons with medium from Aβ1–42-stimulated microglia (conditioned medium) resulted in increased numbers of neurons with condensed (bright fluorescent nuclei) and fragmented nuclei. 4-AP (2 mm) in the maintained presence of Aβ1–42 decreased microglial-mediated neurotoxicity. 4-AP alone had no effect to alter basal levels of neuronal viability. Unconditioned medium (medium containing stimuli but unexposed to microglia) had no effect on neuronal viability (representative figures not shown). The percentages of damaged primary hippocampal neurons induced by microglial conditioned medium as well as with unconditioned medium were obtained from n = 5 independent experiments for each of conditioned and unconditioned medium experiments, and results are summarized in Figure 8B. Overall, incubation of neurons with microglial conditioned medium induced a significant increase (by 232%) in the number of neurons with condensed nuclei compared with levels induced by unstimulated microglia (p < 0.001). 4-AP significantly reduced the amount of Aβ1–42-induced microglial neurotoxicity by 54% (p < 0.001). Conditioned medium from microglia treated with 4-AP and Aβ42–1 each separately increased the level of neuronal death by 9 and 14%, respectively, compared with basal levels; however, the increases were not significant (p > 0.05). Treatment of neurons with medium from unstimulated microglia did not alter the basal level of neuronal damage (p > 0.05). Incubation of neurons for 16 h with unconditioned medium containing Aβ1–42 (5 μm) alone and in the presence of 4-AP (2 mm), 4-AP alone, and Aβ42–1 (5 μm) did not alter the basal percentage of neuronal damage (p > 0.05).
Figure 8.
Effects of microglial conditioned medium on neuronal survival. A, Representative photomicrographs of DAPI-stained primary hippocampal neurons treated for 16 h with microglial conditioned medium [microglia stimulated for 48 h with Aβ1–42 (5 μm), 4-AP (2 mm), each alone, or in combination]. Condensed (arrow) and fragmented (arrowhead) nuclei indicate damaged neurons. Scale bar, 20 μm. B, Summary of microglial-mediated neurotoxicity results from n = 5 independent experiments and corresponding control experiments (neurons treated with unconditioned medium) from n = 5 independent experiments. *p < 0.001, statistically significant from medium of unstimulated microglia; **p < 0.001, statistically significant from conditioned medium of Aβ1–42-stimulated microglia.
Effects of 4-AP on Aβ1–42-induced microglial activation and neuronal degeneration in vivo
The results from the in vitro studies suggested 4-AP as a wide-spectrum inhibitor of inflammatory responses in microglia. An important aspect of this study was to determine the anti-inflammatory potential and neuroprotective actions of 4-AP in vivo. This was done by microinjection of Aβ1–42 (1 nmol) into the dentate gyrus of rat hippocampus, and at 7 d after injection, both microglial activation and neuronal toxicity were determined. Animals receiving vehicle were used as control. The effects of 4-AP were investigated in rats administered 4-AP (1 mg/kg) intraperitoneally daily for 7 d.
The effect of 4-AP on Aβ1–42-induced neuronal toxicity in vivo was determined using NeuN, a marker of viable neurons. Representative NeuN-positive staining results from Aβ1–42 injection into the dentate granule cell layer of rat hippocampus, in the absence and presence of 4-AP, are presented in Figure 9A. Loss of NeuN-positive granule neurons was evident at 7 d after Aβ1–42 injection compared with vehicle. As shown in Figure 9A, 4-AP was effective in protecting neurons from Aβ1–42-induced damage. Little or no loss of neurons was observed with 4-AP or with Aβ42–1 applied separately. Overall, at 7 d after Aβ1–42 injection, the number of NeuN-positive neurons in the superior blade of dentate granule cell layer decreased by 18% compared with vehicle (Fig. 9C) (n = 4; p < 0.05). Treatment with 4-AP reduced the neurotoxic effect of Aβ1–42, because the number of NeuN-positive cells was significantly increased by 16% in Aβ1–42-injected brain administered 4-AP relative to the number of NeuN-positive cells in peptide-injected brain (n = 4; p < 0.05). No significant neuronal loss was observed in Aβ42–1-injected or 4-AP-treated rat brain (Fig. 9C) (n = 4/group; p > 0.05).
Figure 9.
Effects of 4-AP on Aβ1–42-induced hippocampal neuron degeneration and microglial activation in vivo. A, Representative photographs of tissue sections stained with NeuN antibody from the superior blade of dentate granule cell layer taken 7 d after injection with vehicle, Aβ1–42 (1 nmol), Aβ1–42 plus 4-AP (1 mg/kg, i.p.), and 4-AP and Aβ42–1 (1 nmol). B, Representative photographs of tissue sections stained with ED1 from the superior blade of dentate granule cell layer taken from vehicle-injected rats, Aβ1–42 (1 nmol), Aβ1–42 plus 4-AP (1 mg/kg), and 4-AP or Aβ42–1 (1 nmol) at 7 d after injection. C, Quantification of the effects of Aβ1–42, 4-AP, and Aβ1–42 in the presence of 4-AP on NeuN-positive neurons. Data are mean ± SEM (n = 4/group). *p < 0.05 versus vehicle; **p < 0.05 versus Aβ1–42. D, Quantification of the effects of Aβ1–42, 4-AP, and Aβ1–42 in the presence of 4-AP on ED1-positive microglia. Data are mean ± SEM (n = 4/group). *p < 0.05 versus vehicle; **p < 0.05 versus Aβ1–42. Scale bars, 50 μm.
The effect of 4-AP on Aβ1–42-induced microglial responses in vivo was determined using ED1, a marker of microglial activation. Representative ED1-positive staining results from Aβ1–42 injection into the dentate granule cell layer of rat hippocampus, in the absence and presence of 1 mg/kg 4-AP, are presented in Figure 9B. The numbers of ED1-positive microglia were considerably increased with peptide relative to vehicle (Fig. 9B). 4-AP treatment attenuated the number of activated microglia in Aβ1–42-injected rat brain (Fig. 9B), and injection of 4-AP alone had no effect on the numbers of activated microglia (Fig. 9B). Aβ42–1 had a small effect to increase the numbers of ED1-positive cells (Fig. 9B). Overall, in Aβ1–42-injected brain, the numbers of microglia were significantly increased by 18-fold compared with vehicle (Fig. 9D) (n = 4; p < 0.05). In Aβ1–42-injected brain, administration of 4-AP resulted in a significant reduction in the number of ED1-positive microglia (by 68%) relative to the numbers of microglia with Aβ1–42 (Fig. 9D) (n = 4; p < 0.05). No significant increase in the number of ED1-positive microglia was found in 4-AP-treated brain compared with vehicle (Fig. 9D) (n = 4; p > 0.05). Aβ42–1 injection resulted in increased ED1-positive microglia compared with vehicle; however, the increase was not significant (Fig. 9D) (n = 4; p > 0.05).
Discussion
The overall novel finding from this work is that the nonselective K+ channel blocker 4-AP, in a broad spectrum of in vitro and in vivo assays, inhibits Aβ1–42-induced microglial activation and a diversity of cellular functional responses. The latter include outward K+ current, entry of Ca2+, production of pro-inflammatory mediators, and expression of inflammatory cell signaling factors p38 MAPK and NF-κB. As discussed below, we attribute all inhibitory 4-AP effects to blocking actions on an outwardly rectifying IK induced by Aβ1–42 stimulation of human microglia. Most importantly, our results also document 4-AP enhancement in survival of hippocampal neurons in vitro (supernatant assay using Aβ1–42 stimulation of microglia) and neuroprotection in vivo (using intrahippocampal injection of Aβ1–42).
A primary action of 4-AP is inhibition of an outwardly rectifying IK induced by acute application of Aβ1–42 peptide. This current was absent in unstimulated human microglia and was characterized by rapid kinetics of activation with little or no inactivation with depolarizing steps. The electrophysiological results indicate that upregulation of a K+ channel in response to Aβ1–42 is an early signaling event in the activation process of human microglia. A novel finding was that intracellular application of GTPγS induced an outward K+ current in human microglia (Fig. 2B) with properties similar to IK activated by peptide (Fig. 2A) including threshold for activation, current–voltage relationship, and reversal potential for tail currents. In addition, the GTPγS-dependent current was partially blocked by 4-AP (IC50 of 5 mm) and TEA, consistent with results obtained for IK. A previous study has reported induction of a 4-AP-sensitive K+ current in murine macrophages by GTPγS (McKinney and Gallin, 1993); however, the underlying channel was not identified.
RT-PCR analysis was used to determine constitutive and peptide-stimulated expressions of delayed rectifier K+ channels in human microglia (Fig. 3). Of the K+ channels studied, only Kv 3.1 showed no basal expression in control with a rapid induction after cell exposure to peptide and sensitivity to PTX (Fig. 3C). The finding of Aβ1–42 induction of Kv3.1 is novel; however, a 4-AP- and TEA-sensitive Kv3.1 channel has been reported in rodents both in proliferating T-lymphocytes (Chandy et al., 1990; Grissmer et al., 1990) and in fibroblasts (Grissmer et al., 1994). As discussed below, the rapid induction of IK in Aβ1–42-stimulated human microglia could suggest that the underlying channel was either present in the membrane but nonfunctional or rapidly inserted into the membrane.
At this time, it is not possible to conclude that the IK measured after peptide application is attributable solely, or even partially, to Kv3.1. Our results (Fig. 3) showed the constitutive expression of a series of outwardly rectifying K+ channels, Kv1.1, Kv1.2, Kv1.3, Kv1.5, Kv1.6, and Kv2.1, in resting human microglia. Although none of these Kv exhibited altered expression at any time after Aβ1–42 treatment of cells, the rapidity in appearance of IK after peptide application could suggest a process independent of nuclear transcription. In this case, IK could be attributable to a direct posttranslational insertion of protein into plasma membrane independent of the RT-PCR results. Indeed, our results from unstimulated microglia point to a discontinuity between expression of Kv and a functional IK, because the constitutive expression of the series of Kv does not lead to any measurable current from resting human cells (Fig. 1) (McLarnon et al., 1997). A lack of basal current, despite expression of mRNA for Kv channels, has been attributed to stimulus control of the translation of channel protein into plasma membrane in rodent cells (Norenberg et al., 1993; Khanna et al., 2001). In summary, the patterns of mRNA expression suggest Kv3.1 as a plausible candidate channel underlying the peptide-stimulated IK in human microglia. However, additional studies are required to determine involvement of other Kv channels in mediating IK in activated microglia.
The sensitivity of a diversity of peptide-induced cellular actions to 4-AP would suggest some degree of coupling between induction of IK and cellular responses such as increased influx of Ca2+. Although membrane potential (Vm) was not measured in this work, one possibility is that cell depolarization resulting from 4-AP inhibition of IK could block a Ca2+ entry pathway sensitive to changes in Vm. In human microglia, a candidate Ca2+ influx pathway sensitive to Vm is plasmalemmal SOC entry (McLarnon et al., 2000; Khoo et al., 2001); however, application of the SOC inhibitor SKF96365 had no effect on the Aβ1–42-induced increase in [Ca2+]i. Human or rodent microglia show little or no evidence for the presence of voltage-gated Ca2+ channels (Eder, 1998; McLarnon et al., 1999), with only a few exceptions reported (Colton et al., 1994; Silei et al., 1999). At present, the peptide-induced Ca2+ entry pathway remains to be identified.
This study is the first report of inhibitory effects of 4-AP on expression of p38 MAPK and NF-κB in microglia (Fig. 5). The results showed a rapid time course for activation of p38 MAPK (10–30 min after Aβ1–42 application). Although expression for NF-κB was measured at 8 h after Aβ1–42 treatment (Fig. 5B), we also observed upregulation of this transcription factor at much earlier times (i.e., 1 h). Thus, our measurements of rapid induction of the intracellular signaling factors with Aβ1–42 and sensitivity to 4-AP would be consistent with a coupling and dependence of the factors on activation of IK and/or influx of Ca2+. Previous work has reported Aβ activation of p38 MAPK in microglia leads to pro-inflammatory responses (McDonald et al., 1998; Pyo et al., 1998), and inhibition of p38 MAPK was found to diminish microglial reactivity (Giovannini et al., 2002). The inhibitory effects of 4-AP on Aβ1–42-induced NF-κB activation could account for the effects of this nonselective K+ channel blocker to reduce inflammatory mediators released from activated microglia (Combs et al., 2001). Although 4-AP was effective in reducing peptide-induced cytokine productions, residual levels of cytokines remained elevated relative to control (Fig. 6). In vivo, residual levels of the assemblage of cytokines with 4-AP treatment could possibly constitute a pro-inflammatory environment.
To our knowledge, this work is the first report of effects of 4-AP to block Aβ1–42-induced neurotoxicity in vivo. Peptide injection caused a significant loss of dentate granule neurons that was mainly prevented in the presence of 4-AP. Importantly, our data also showed marked upregulation of microglia in peptide-injected brain with actions of 4-AP to then reduce microgliosis. The latter result would be consistent with our in vitro results showing activated microglia acting as a source of inflammatory mediators with neurotoxic potential. In this case, neuroprotection conferred by 4-AP could be attributable to effects of the compound to block IK in activated microglia and reduce cellular production of agents such as pro-inflammatory cytokines, as measured in our study. Indeed, reduction of inflammatory mediators (McDonald et al., 1997; Combs et al., 2000) could account for the findings from the supernatant assay (Fig. 8) whereby conditioned medium containing an assemblage of inflammatory mediators from Aβ1–42-stimulated microglia induced killing of neurons with 4-AP conferring significant neuroprotection. The supernatant assay is a measure of microglial-mediated neurotoxicity with cellular secretion of an assemblage of inflammatory mediators, and microglial activation is associated with neurodegeneration through the production of a diversity of neurotoxic factors.
K+ channels in microglia contribute to cellular homeostatic mechanisms and modulate functional responses. Our results further implicate involvement of downstream intracellular factors [Ca2+]i, p38 MAPK, and NF-κB as pivotal signaling components to transduce Aβ1–42 actions in microglia. Most importantly, 4-AP inhibition of these factors was found to confer neuroprotection in vitro and in vivo. One possibility is that 4-AP partial block of IK leads to cellular depolarization and a subsequent decrease in Ca2+ entry; alterations in mobilization of [Ca2+]i could then act to modulate phosphorylation of kinases and factors involved in gene transcription and expression in an inflammatory signaling network in activated microglia. This result is relevant to previous findings whereby enhanced transient increases in [Ca2+]i and Ca2+-dependent kinase activity were measured in Aβ peptide-stimulated microglia and THP-1 monocytes (Combs et al., 1999). Interestingly, abnormalities in Ca2+-dependent signaling pathways have recently been documented in AD microglia and in cultured human microglia exposed to Aβ1–42 (McLarnon et al., 2005).
The identification of specific target sites in microglial signaling pathways such as tyrosine kinases (McDonald et al., 1997) and PPARγ (Combs et al., 2000) could serve as a rationale strategy to reduce Aβ-induced inflammation in AD brain. Our findings suggest block of IK by 4-AP as a plausible maneuver to reduce Aβ1–42-induced inflammation in AD brain. Interestingly, 4-AP has been used clinically in AD individuals (Wesseling et al., 1984; Davidson et al., 1988). At the levels used, 4-AP showed no toxic effects, and in one trial, the compound showed some effectiveness to enhance cognitive function (Wesseling et al., 1984). However, clinical use of the compound is compromised by nonselective block of K+ channels leading to side effects such as seizure activity (Luhmann et al., 2000). The development of a selective K+ channel inhibitor for Kv3.1 could serve as a potential therapeutic strategy in the treatment of AD.
Footnotes
This work was supported by a doctoral award (S.F.) and grant (J.G.M.) from the Alzheimer's Society of Canada and by an investigator initiated grant from the Alzheimer's Association (USA) (J.G.M.).
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, MacKenzie IR, McGeer PL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends YM, Duyckaerts C, Rozemuller JM, Eikelenboom P, Hauw JJ. Microglia, amyloid and dementia in Alzheimer disease. A correlative study. Neurobiol Aging. 2000;21:39–47. doi: 10.1016/s0197-4580(00)00094-4. [DOI] [PubMed] [Google Scholar]
- Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland K, Behrens M, Choi D, Manias K, Perlmutter DH. The serpin-enzyme complex receptor recognizes soluble, nontoxic amyloid-beta peptide but not aggregated, cytotoxic amyloid-beta peptide. J Biol Chem. 1996;271:18032–18044. doi: 10.1074/jbc.271.30.18032. [DOI] [PubMed] [Google Scholar]
- Casamenti F, Corradetti R, Loffelholz K, Mantovani P, Pepeu G. Effects of 4-aminopyridine on acetylcholine output from the cerebral cortex of the rat in vivo. Br J Pharmacol. 1982;76:439–445. doi: 10.1111/j.1476-5381.1982.tb09237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy KG, Cahalan MD, Grissmer S. Autoimmune diseases linked to abnormal K+ channel expression in double-negative CD4−CD8− T cells. Eur J Immunol. 1990;20:747–751. doi: 10.1002/eji.1830200406. [DOI] [PubMed] [Google Scholar]
- Choi HB, Khoo C, Ryu JK, van Breemen E, Kim SU, McLarnon JG. Inhibition of lipopolysaccharide-induced cyclooxygenase-2, tumor necrosis factor-alpha and [Ca2+]i responses in human microglia by the peripheral benzodiazepine receptor ligand PK11195. J Neurochem. 2002;83:546–555. doi: 10.1046/j.1471-4159.2002.01122.x. [DOI] [PubMed] [Google Scholar]
- Choi HB, Hong SH, Ryu JK, Kim SU, McLarnon JG. Differential activation of subtype purinergic receptors modulates Ca2+ mobilization and COX-2 in human microglia. Glia. 2003;43:95–103. doi: 10.1002/glia.10239. [DOI] [PubMed] [Google Scholar]
- Colton CA, Jia M, Li MX, Gilbert DL. K+ modulation of microglial superoxide production: involvement of voltage-gated Ca2+ channels. Am J Physiol. 1994;266:C1650–C1655. doi: 10.1152/ajpcell.1994.266.6.C1650. [DOI] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of β-amyloid and prion proteins. J Neurosci. 1999;19:928–939. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer's disease: inhibition of beta amyloid stimulated pro-inflammatory responses and neurotoxicity by PPARγ agonists. J Neurosci. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. β-Amyloid stimulation of microglia and monocytes results in TNFα-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M, Zemishlany Z, Mohs RC, Horvath TB, Powchik P, Blass JP, Davis KL. 4-Aminopyridine in the treatment of Alzheimer's disease. Biol Psychiatry. 1988;23:485–490. doi: 10.1016/0006-3223(88)90020-0. [DOI] [PubMed] [Google Scholar]
- Eder C. Ion channels in microglia (brain macrophages) Am J Physiol. 1998;275:C327–C342. doi: 10.1152/ajpcell.1998.275.2.C327. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Franciosi S, Choi HB, Kim SU, McLarnon JG. Interferon-gamma acutely induces calcium influx in human microglia. J Neurosci Res. 2002;69:607–613. doi: 10.1002/jnr.10331. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Scali C, Prosperi C, Bellucci A, Vannucchi MG, Rosi S, Pepeu G, Casamenti F. Beta-amyloid-induced inflammation and cholinergic hypofunction in the rat brain in vivo: involvement of the p38MAPK pathway. Neurobiol Dis. 2002;11:257–274. doi: 10.1006/nbdi.2002.0538. [DOI] [PubMed] [Google Scholar]
- Glover WE. The aminopyridines. Gen Pharmacol. 1982;13:259–285. doi: 10.1016/0306-3623(82)90046-5. [DOI] [PubMed] [Google Scholar]
- Grissmer S, Hanson DC, Natoli EJ, Cahalan MD, Chandy KG. CD4−CD8− T cells from mice with collagen arthritis display aberrant expression of type l K+ channels. J Immunol. 1990;145:2105–2109. [PubMed] [Google Scholar]
- Grissmer S, Nguyen AN, Alyar J, Hanson DC, Mather RJ, Gutman GA, Karmillowicz MJ, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5 and 3.1 stably expressed in mammalian cell lines. Mol Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Barnes E, Davis KL. Cholinergic modulation of memory in rats. Psychopharmacology (Berl) 1985;87:266–271. doi: 10.1007/BF00432705. [DOI] [PubMed] [Google Scholar]
- Jantaratnotai N, Ryu JK, Kim SU, McLarnon JG. Amyloid beta peptide-induced corpus callosum damage and glial activation in vivo. NeuroReport. 2003;14:1429–1433. doi: 10.1097/00001756-200308060-00005. [DOI] [PubMed] [Google Scholar]
- Jiang B, Sun X, Cao K, Wang R. Endogenous Kv channels in human embryonic kidney (HEK-293) cells. Mol Cell Biochem. 2002;238:69–79. doi: 10.1023/a:1019907104763. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hoppe D, Gottmann K, Banati K, Kreutzberg G. Cultured microglial cells have a distinct pattern of membrane channels different from peritoneal macrophages. J Neurosci Res. 1990;26:278–287. doi: 10.1002/jnr.490260303. [DOI] [PubMed] [Google Scholar]
- Khanna R, Roy L, Zhu X, Schlichter LC. K+ channels and the microglial respiratory burst. Am J Physiol Cell Physiol. 2001;280:C796–C806. doi: 10.1152/ajpcell.2001.280.4.C796. [DOI] [PubMed] [Google Scholar]
- Khoo C, Helm J, Choi HB, Kim SU, McLarnon JG. Inhibition of store-operated Ca(2+) influx by acidic extracellular pH in cultured human microglia. Glia. 2001;36:22–30. doi: 10.1002/glia.1092. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar β-amyloid through a β1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Bressler B, Prameya R, Dorovini-Zis K, Van Breemen C. Agonist-stimulated calcium entry in primary cultures of human cerebral microvascular endothelial cells. Microvasc Res. 1999;57:211–226. doi: 10.1006/mvre.1998.2131. [DOI] [PubMed] [Google Scholar]
- Lorton D, Schaller J, Lala A, De Nardin E. Chemotactic-like receptors and Abeta peptide induced responses in Alzheimer's disease. Neurobiol Aging. 2000;21:463–473. doi: 10.1016/s0197-4580(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Dzhala VI, Ben-Ari Y. Generation and propagation of 4-AP-induced epileptiform activity in neonatal intact limbic structures in vitro. Eur J Neurosci. 2000;12:2757–2768. doi: 10.1046/j.1460-9568.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- McDonald DR, Brunden KR, Landreth GE. Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. J Neurosci. 1997;17:2284–2294. doi: 10.1523/JNEUROSCI.17-07-02284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DR, Bamberger ME, Combs CK, Landreth GE. β-Amyloid fibrils activate parallel mitogen-activated protein kinase pathways in microglia and THP1 monocytes. J Neurosci. 1998;18:4451–4460. doi: 10.1523/JNEUROSCI.18-12-04451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney LC, Gallin EK. G-protein activators induce a potassium conductance in murine macrophages. J Membr Biol. 1993;130:265–276. doi: 10.1007/BF00240483. [DOI] [PubMed] [Google Scholar]
- McLarnon JG, Xu R, Lee YB, Kim SU. Ion channels of human microglia in culture. Neuroscience. 1997;78:1217–1228. doi: 10.1016/s0306-4522(96)00680-x. [DOI] [PubMed] [Google Scholar]
- McLarnon JG, Zhang L, Goghari V, Lee YB, Walz W, Krieger C, Kim SU. Effects of ATP and elevated K+ on K+ currents and intracellular Ca2+ in human microglia. Neuroscience. 1999;91:343–352. doi: 10.1016/s0306-4522(98)00491-6. [DOI] [PubMed] [Google Scholar]
- McLarnon JG, Helm J, Goghari V, Franciosi S, Choi HB, Nagai A, Kim SU. Anion channels modulate store-operated calcium influx in human microglia. Cell Calcium. 2000;28:261–268. doi: 10.1054/ceca.2000.0150. [DOI] [PubMed] [Google Scholar]
- McLarnon JG, Choi HB, Lue LF, Walker DG, Kim SU. Perturbations in calcium-mediated signal transduction in microglia from Alzheimer's disease patients. J Neurosci Res. 2005;81:426–435. doi: 10.1002/jnr.20487. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Kikuchi Y, Ikoma E, Honda S, Ishikawa M, Liu Y, Kohsaka S. Neurotrophins regulate the function of cultured microglia. Glia. 1998;24:272–289. doi: 10.1002/(sici)1098-1136(199811)24:3<272::aid-glia2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Appel K, Bauer J, Gebicke-Haerter P, Illes P. Expression of an outwardly rectifying K+ channel in rat microglia cultivated on teflon. Neurosci Lett. 1993;160:69–72. doi: 10.1016/0304-3940(93)9001-0. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Gebicke-Haerter PJ, Illes P. Voltage-dependent potassium channels in activated rat microglia. J Physiol (Lond) 1994;475:15–32. doi: 10.1113/jphysiol.1994.sp020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 2. New York: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- Pyo H, Jou I, Jung S, Hong S, Joe EH. Mitogen-activated protein kinases activated by lipopolysaccharide and beta-amyloid in cultured rat microglia. NeuroReport. 1998;9:871–874. doi: 10.1097/00001756-199803300-00020. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Franciosi S, Sattayaprasert P, Kim SU, McLarnon JG. Minocycline inhibits neuronal death and glial activation induced by beta-amyloid peptide in rat hippocampus. Glia. 2004;48:85–90. doi: 10.1002/glia.20051. [DOI] [PubMed] [Google Scholar]
- Satoh J, Lee YB, Kim SU. T cell costimulatory molecules B7–1 (CD-80) and B7–2 (CD-86) are expressed in human microglia but not astrocytes in culture. Brain Res. 1995;704:92–96. doi: 10.1016/0006-8993(95)01177-3. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Sheldon C, Cheng YM, Church J. Concurrent measurements of the free cytosolic concentrations of H+ and Na+ ions with fluorescent indicators. Pflügers Arch. 2004;449:307–318. doi: 10.1007/s00424-004-1344-8. [DOI] [PubMed] [Google Scholar]
- Silei V, Fabrizi C, Venturini G, Salmona M, Bugiani O, Tagliavini F, Lauro GM. Activation of microglial cells by PrP and beta-amyloid fragments raises intracellular calcium through L-type voltage sensitive calcium channels. Brain Res. 1999;818:168–170. doi: 10.1016/s0006-8993(98)01272-4. [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Jiang Q, Heneka M, Landreth G. PPARgamma as a therapeutic target in central nervous system diseases. Neurochem Int. 2006;49:136–144. doi: 10.1016/j.neuint.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-d-aspartate neurotoxicity by inhibiting microglia. J Immunol. 2001;166:7527–7533. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- Walker DG, Lue LF, Beach TG. Gene expression profiling of amyloid beta peptide-stimulated human post-mortem brain microglia. Neurobiol Aging. 2001;22:957–966. doi: 10.1016/s0197-4580(01)00306-2. [DOI] [PubMed] [Google Scholar]
- Walsh DT, Montero RM, Bresciani LG, Jen AY, Leclercq PD, Saunders D, EL-Amir AN, Gbadamoshi L, Gentleman SM, Jen LS. Amyloid-beta peptide is toxic to neurons in vivo via indirect mechanisms. Neurobiol Dis. 2002;10:20–27. doi: 10.1006/nbdi.2002.0485. [DOI] [PubMed] [Google Scholar]
- Weldon DT, Rogers SD, Ghilardi JR, Finke MP, Cleary JP, O'Hare E, Esler WP, Maggio JE, Mantyh PW. Fibrillar β-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J Neurosci. 1998;18:2161–2173. doi: 10.1523/JNEUROSCI.18-06-02161.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling H, Agoston S, Van Dam GB, Pasma J, DeWit DJ, Havinga H. Effects of 4-aminopyridine in elderly patients with Alzheimer's disease. N Engl J Med. 1984;310:988–989. doi: 10.1056/NEJM198404123101514. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]