Abstract
Small-object manipulation is essential in numerous human activities, although its neural bases are still essentially unknown. Recent functional imaging studies have shown that precision grasping activates a large bilateral frontoparietal network, including ventral (PMv) and dorsal (PMd) premotor areas. To dissociate the role of PMv and PMd in the control of hand and finger movements, we produced, by means of transcranial magnetic stimulation (TMS), transient virtual lesions of these two areas in both hemispheres, in healthy subjects performing a grip–lift task with their right, dominant hand. We found that a virtual lesion of PMv specifically impaired the grasping component of these movements: a lesion of either the left or right PMv altered the correct positioning of fingers on the object, a prerequisite for an efficient grasping, whereas lesioning the left, contralateral PMv disturbed the sequential recruitment of intrinsic hand muscles, all other movement parameters being unaffected by PMv lesions. Conversely, we found that a virtual lesion of the left PMd impaired the proper coupling between the grasping and lifting phases, as evidenced by the TMS-induced delay in the recruitment of proximal muscles responsible for the lifting phase; lesioning the right PMd failed to affect dominant hand movements. Finally, an analysis of the time course of these effects allowed us to demonstrate the sequential involvement of PMv and PMd in movement preparation. These results provide the first compelling evidence for a neuronal dissociation between the different phases of precision grasping in human premotor cortex.
Keywords: motor control, transcranial magnetic stimulation, hand shaping, grasping, grip–lift synergy, intrinsic hand muscles, fingers
Introduction
In primates, the progressive evolution to bipedal gait has permitted the use of the hand as an extraordinary device to manipulate objects and make use of tools. Because lesions of the corticospinal (CS) tract often impair irremediably dexterous finger movements (Forssberg et al., 1999; Duque et al., 2003; Hermsdorfer et al., 2003), the contribution of the CS system [and that of its main cortical area of origin, the primary motor cortex (M1)] to such movements has been extensively studied (Muir and Lemon, 1983; Schieber and Poliakov, 1998; Brochier et al., 1999).
However, functional imaging studies have evidenced that, in humans, precision grasping activates a large bilateral network of frontoparietal areas, including the ventral (PMv) and dorsal (PMd) premotor cortex (Binkofski et al., 1999; Ehrsson et al., 2000, 2001; Kuhtz-Buschbeck et al., 2001). In monkeys, it has been suggested that PMd and PMv are part of two independent circuits, originating from the posterior parietal cortex and controlling, respectively, the reaching and grasping components of goal-directed hand movements (Jeannerod et al., 1995; Tanne-Gariepy et al., 2002). This view is supported by the finding that selective inactivation of the monkey PMv causes severe deficits in the grasping component of hand movements, leaving unaffected the hand transport (Fogassi et al., 2001). The role of PMv in shaping the hand posture appropriately to grasp objects has been confirmed by electrophysiological studies in monkeys (Rizzolatti et al., 1988; Murata et al., 1997; Rizzolatti and Luppino, 2001). As far as PMd is concerned, cell recordings in monkeys have indicated that its caudal part mostly contains a representation of proximal forelimb muscles and that its activity correlates with the direction and amplitude of reaching movements (Scott et al., 1997; Messier and Kalaska, 2000; Cisek et al., 2003).
In humans, however, the respective contribution of PMv and PMd to hand movements, the time course of their involvement, and their hemispheric dominance are still essentially unknown. Although brain imaging studies have already provided us with a comprehensive picture of the brain regions involved in hand and finger movements, this approach does not allow making inferences about the causal relationship between these activations and the processes under investigation. Transcranial magnetic stimulation (TMS) has proved useful to overcome this limitation by producing, in healthy subjects, transient virtual lesions of restricted brain areas. Combined with a precise quantification of the deficits resulting from such virtual lesions, this approach permits to infer the contribution of the stimulated brain area to the task under investigation (Walsh and Cowey, 2000) and has already been used successfully in motor control studies (Chen et al., 1997; Chouinard et al., 2005; Tunik et al., 2005). The aim of the present study was to determine the respective contribution of PMv and PMd to precision grasping. To do so, we produced, by means of TMS, transient virtual lesions of these two areas in both hemispheres, in healthy subjects performing a standard grip–lift task with their right, dominant hand.
Materials and Methods
Ten healthy volunteers (25.8 ± 2 years) participated in the present study. All subjects were right-handed according to the Edinburgh handedness inventory (Oldfield, 1971). Their vision was normal, or corrected to normal, and none of them had neurological history. Subjects were screened for potential risk of adverse reactions to TMS by using the transcranial magnetic stimulation adult safety screen (Keel et al., 2001). All experimental procedures were approved by the Ethics Committee of the Université Catholique de Louvain, and all subjects gave their written informed consent.
Grip–lift task.
Subjects sat comfortably on a padded chair with their elbow flexed at ∼135°. The task consisted of grasping a 575 g manipulandum between the right-hand index and thumb and in lifting it (Fig. 1A); the manipulandum was positioned on a table in front of subjects. The subject’s right hand was placed on the table on its ulnar edge, with the wrist positioned midway between pronation and supination; the grip was open with both the thumb and index positioned ∼3–4 cm apart from the manipulandum grip surfaces.
Figure 1.
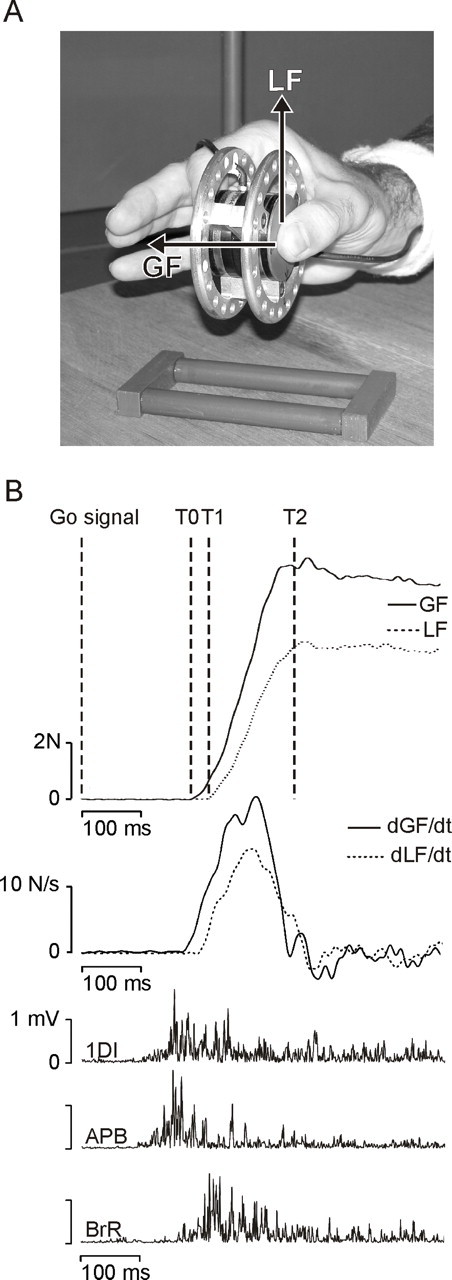
The grip–lift task. A, Picture of the manipulandum used to investigate grip–lift movements. The task was always performed with the right, dominant hand, and only the thumb and index fingertips were in contact with the manipulandum. GF and LF vectors are shown for the thumb. B, Typical control grip–lift movement gathered during a sham trial. From top to bottom, GF and LF, their first derivatives (dGF/dt and dLF/dt), and the EMG activity of the 1DI, APB, and BrR. T0–T1 and T1–T2 cursors on the GF and LF traces delimit the preloading and loading phases, respectively.
The manipulandum consisted of two parallel vertical grip surfaces of smooth brass (40 mm diameter, 30 mm apart) (Fig. 1A). The grip surfaces covered three-dimensional force-torque sensors (Mini 40 F/T transducers; ATI Industrial Automation, Garner, NC). Each sensor measured the three orthogonal forces (Fx, Fy, Fz) and torques (Tx, Ty, Tz) along the corresponding axes intersecting the center of the grip surface. The sensing ranges for Fx, Fy, and Fz were ± 40, ±40, and ± 120 N, with a 0.02, 0.02, and 0.06 N resolution, respectively. The sensing ranges for Tx, Ty, and Tz were ±2 Nm, with a 0.001 Nm resolution. The force tangential to the grip surface [load force (LF)] was computed as the vectorial sum of Fx and Fy. The force normal to the grip surface [grip force (GF)] was given by Fz.
At the beginning of each trial, an auditory Go signal was delivered to instruct the subjects to grasp the manipulandum and to lift it, at a natural speed, to a height of ∼20 cm, as indicated by an elastic band. Subjects had to maintain the manipulandum in this position for ∼3 s until another auditory signal indicated the end of the trial.
Transcranial magnetic stimulation.
Ten subjects participated in a first experiment, which allowed us to investigate the consequences of repetitive TMS (rTMS) applied over either the left PMv or PMd on the grip–lift task. TMS was delivered using a rapid model 200 stimulator (Magstim, Whitland, UK) through a 70 mm outer diameter figure-of-eight coil. The coil was held tangential to the skull with the handle pointing backward. Before each experiment, TMS was applied over the left M1, and the coil position was adjusted to optimize the motor-evoked potential (MEP) amplitude in response to a single TMS pulse in the contralateral first dorsal interosseus (1DI). Once the optimal coil position was found, the resting motor threshold, defined as the TMS intensity that induced 50 μV peak-to-peak MEPs in 5 of 10 trials, was determined, and the intensity of stimulation was set at 120% of this value. It is noteworthy that, at this intensity, rTMS applied over PMv or PMd never induced an electromyographic (EMG) response in the contralateral hand and arm muscles (see below). The rTMS train (10 Hz, 500 ms) was delivered synchronously with the Go signal; trains of rTMS were separated by at least 12 s. In a second experiment, the same procedure was repeated in a subgroup of six subjects (26.3 ± 1 years), and rTMS (500 ms, 10 Hz) was applied over either the left/right PMv or the left/right PMd (Fig. 2). As additional controls, rTMS was also applied over the left or right M1 in experiment 2. Again, rTMS was synchronized with the Go signal.
Figure 2.
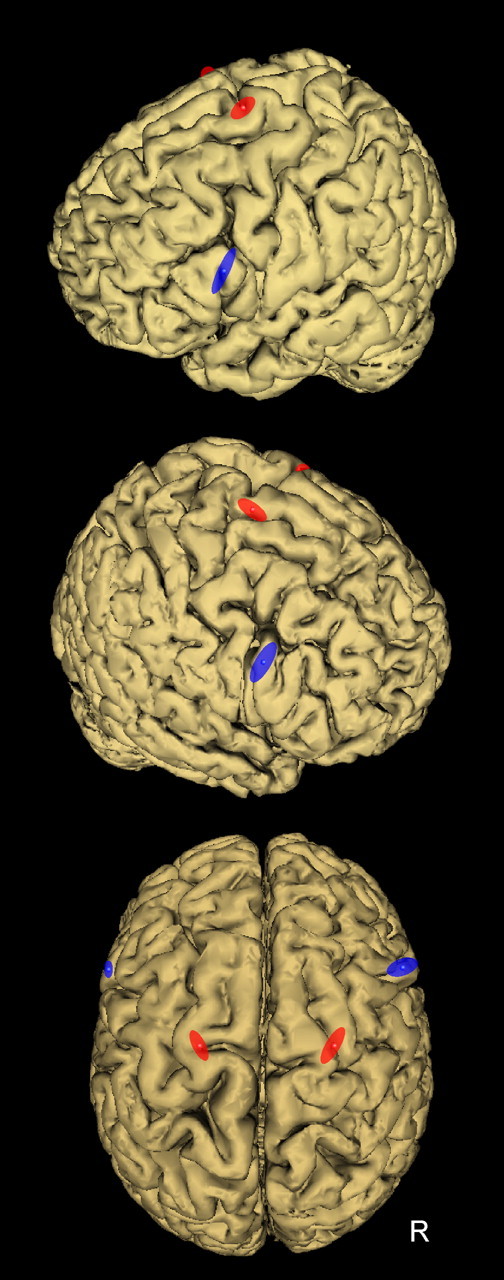
Location of the TMS coil positions to induce virtual lesion of PMv (blue) and PMd (red). To stimulate PMv, the coil was positioned over the caudal portion of the pars opercularis of the inferior frontal gyrus (BA44), corresponding to the following normalized MNI coordinates: −60 ± 2, 16 ± 3, 23 ± 9 mm (x, y, z, mean ± SD; n = 10) and 56 ± 6, 16 ± 4, 26 ± 9 mm (n = 6) for the left and right PMv, respectively. To target PMd, the coil was positioned over the superior portion of the precentral gyrus, as delimited by the superior frontal sulcus. The mean MNI coordinates of stimulation sites for the left and right PMd were, respectively, −22 ± 3, −4 ± 4, 71 ± 4 mm (x, y, z, mean ± SD; n = 10) and 24 ± 4, −5 ± 6, 72 ± 3 mm (n = 6). Each ellipse was centered on the mean MNI coordinates of PMv and PMd stimulation points, and their surface shows the 95% confidence interval of the normalized coordinates calculated for each subject.
Finally, to determine more precisely the time course of the contribution of PMv and PMd in the grip–lift task, a third experiment was performed in another subgroup of six subjects (25.2 ± 2 years). In this experiment, paired-pulse TMS (interval of 5 ms) was delivered over the left PMv or PMd at five different delays after the Go signal, namely 50, 100, 150, 200, or 250 ms. The TMS intensity was also set at 120% of the resting motor threshold determined for the left, contralateral M1.
In these three experiments, control data were gathered from sham trials. The sham stimulation consisted of applying an rTMS train over either PMv or PMd with the coil perpendicular to the scalp, so that TMS was ineffective; this procedure allowed us to cancel out possible unspecific effects of TMS on the task performance. Because control data gathered after sham stimulation of PMd or PMv in experiment 1 were undistinguishable (all F values <1), in experiments 2 and 3, to reduce the number of blocks, sham stimulations were applied at a location between PMv and PMd.
Location of stimulation sites.
For each subject, the coil position was precisely determined before each experiment by means of an original method that allows us to perform an on-line coregistration of the stimulation sites onto individual anatomical magnetic resonance imaging (MRI) (Noirhomme et al., 2004). In addition, this software permits to normalize individual coordinates of the TMS sites with respect to the Montreal Neurological Institute (MNI) brain atlas. This normalization procedure was performed a posteriori by normalizing each individual MRI onto the MNI brain template by means of an iterative algorithm that searches for the optimal projection of a given brain onto the MNI brain. This algorithm solved the best transformation parameters optimizing the mutual information metric between the individual and MNI brains (Mattes et al., 2003) by using sequentially two types of transformations: (1) rigid and scale transformations (De Craene et al., 2004) and (2) nonrigid basis-spline transformations (Spall, 1998).
To stimulate PMv, the coil was positioned over the caudal portion of the pars opercularis of the inferior frontal gyrus (BA44), a region known to be selectively activated during object manipulation and grasping movements (Binkofski et al., 1999; Ehrsson et al., 2000, 2001; Kuhtz-Buschbeck et al., 2001). In the present study (Fig. 2), the mean normalized MNI coordinates of the stimulation sites were, respectively, −60 ± 2, 16 ± 3, 23 ± 9 mm (x, y, z, mean ± SD; n = 10) and 56 ± 6, 16 ± 4, 26 ± 9 mm (n = 6) for the left and right PMv, in agreement with those reported in the aforementioned functional MRI (fMRI) studies (supplemental Table A, at www.jneurosci.org as supplemental material).
To target PMd, the coil was positioned over the superior portion of the precentral gyrus, as delimited by the superior frontal sulcus. This site was selected on the basis of previous fMRI and TMS studies performed in subjects executing comparable tasks (Binkofski et al., 1999; Ehrsson et al., 2000; Chouinard et al., 2005). In the present study (Fig. 2), the mean MNI coordinates of stimulation sites for left and right PMd were, respectively, −22 ± 3, −4 ± 4, 71 ± 4 mm (x, y, z, mean ± SD; n = 10) and 24 ± 4, −5 ± 6, 72 ± 3 mm (n = 6) and were very close to those available in the aforementioned literature for PMd.
Experimental procedure.
Before each experiment, the subjects performed two practice sessions of 12 trials each. Experiment 1 consisted of 12 blocks of 12 trials each. In half of the blocks, rTMS was applied over either the left PMv or the left PMd. In the other half of the blocks, as control, sham rTMS was applied over either the left PMv or the left PMd. These 12 blocks were randomly interleaved. Experiment 2 consisted of 24 blocks in which rTMS was applied over the left/right PMv, the left/right PMd, or the left/right M1 (three blocks for six stimulations sites); the six remaining blocks were sham TMS, delivered at an intermediate position between PMv and PMd. In experiment 3, the experimental session consisted of 15 blocks of 15 trials each. In a given block, TMS was applied randomly at the five different delays. Paired-pulse TMS was applied over the left PMv in five blocks and over the left PMd in five other blocks. The five remaining blocks consisted of sham TMS delivered at an intermediate position between PMv and PMd. All of these blocks were randomly distributed.
Data acquisition and analysis.
The signals from the force transducers were digitized on-line at 1 kHz with a 12-bit 6071E analog-to-digital converter in a PXI chassis (National Instruments, Austin, TX). The signals were then low-pass filtered (15 Hz) with a fourth-order, zero phase-lag Butterworth filter. The GF and LF onsets were determined when values exceeded the mean + 2 SDs of the premovement resting value (Flanagan and Tresilian, 1994). EMG activity was recorded with surface electrodes (Neuroline; Medicotest, Oelstykke, Denmark) from two intrinsic hand muscles, namely the abductor pollicis brevis (APB) and 1DI and from one proximal arm muscle, namely the brachio-radialis (BrR). The raw EMG signals were amplified (gain, 1000), bandpass filtered (10–500 Hz; Neurolog; Digitimer, Hertfordshire, UK), and digitized at 1 kHz. The EMG signals were then rectified off-line. The baseline was defined as the mean EMG value computed over a 200 ms time window before the Go signal; the EMG onset was determined when the EMG signal exceeded the baseline + 2 SDs.
The following temporal parameters were measured: (1) the reaction time (RT), defined as the delay between the Go signal and the onset of the first EMG activity in one of the intrinsic hand muscles; (2) the movement time (MT), i.e., the time between the first EMG activity and the first contact of a finger with the manipulandum; (3) the contact time (CT), i.e., the delay between the contact of the two fingertips on the manipulandum; (4) the delay between the APB and 1DI recruitment; (5) the delay between the 1DI and the BrR recruitment; and (6) the delay between the Go signal and the BrR onset time. In addition, we also measured (7) the preloading phase duration (T0–T1) (Fig. 1B), i.e., the delay between the contact of the second finger on the manipulandum and the onset of LF and the (8) the duration of the loading phase (T1–T2), which is the phase in which both GF and LF increased progressively until LF equaled to the weight of the object. The following dynamics parameters were also measured: (1) the GF (dGF/dt) and (2) LF (dLF/dt) rates and (3) the peak value of dGF/dt and (4) the peak value of dLF/dt during the loading phase. The grip–lift synergy was estimated by computing a cross-correlation function between dGF/dt and dLF/dt (Duque et al., 2003). For each individual trial, this method provides two values: (1) the maximum coefficient of correlation, which estimates the similarity between the profiles of dGF/dt and dLF/dt, and (2) the time shift, which quantifies the asynchrony between dGF/dt and dLF/dt; a positive value of time shift indicates that GF leads LF.
To determine the index and thumb fingertip positions on the manipulandum, the x and y coordinates of the center of pressure (CP) of each fingertip were measured with respect to the center of the grip surface (see Fig. 4). The x and y coordinates of the fingertip CP were obtained by dividing the x and y torque value (Tx and Ty) by the applied grip force (Fz), respectively. Then, the distances separating the thumb and index along the x- and y-axes were computed. In addition, an estimate of the CP variability was performed for each fingertip by measuring the area of the isodensity ellipsoid in which 95% of the CPs were located (see Fig. 4).
Figure 4.
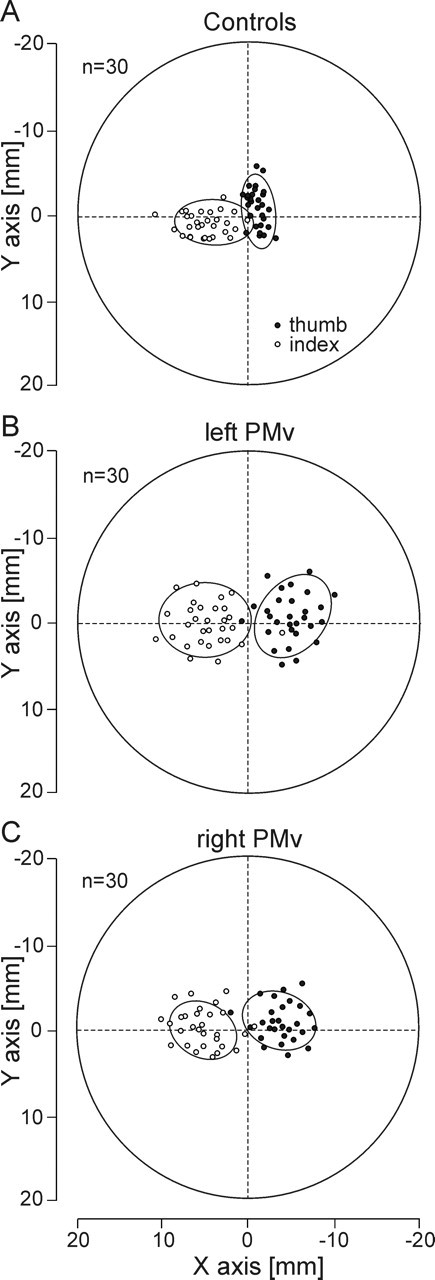
Effects of the virtual lesions of the left or right PMv on the fingertip positioning. Side view of the manipulandum showing the distribution of the fingertip positions for the thumb (black circles) and the index finger (white circles). The two graspable surfaces of the manipulandum were superimposed to represent the thumb and index fingertip positions on the same graph. The subject’s hand came from the right side of the figure. The ellipses represent the area in which 95% of the fingertip positions were found. A, Distribution of the thumb and index fingertip positions for 30 control trials in one subject. Note that the index finger contact points were always slightly farther than those of the thumb. B, A virtual lesion of the left PMv increased significantly both the horizontal distance between the thumb and index finger contact points and their variability. C, Similar effects, although less pronounced, of a virtual lesion of the right PMv on fingertip positions and distributions.
Statistical analysis.
In all three experiments, because there was no statistically significant difference between the movement parameters gathered for the different sham blocks (ANOVA; all F values <0.51; all p values >0.29), these data were pooled together and used as control values. In experiment 1, all parameters (fingertip positioning and temporal and dynamic parameters) were compared across conditions by means of one-way ANOVA, with the stimulation site (PMv, PMd, or sham) as within-subject factor. In experiment 2, a two-way ANOVA with site (PMv, PMd, M1, or sham) and side (left or right) as within-subject factors was performed. Similarly, in experiment 3, a two-way ANOVA with site (PMv, PMd, or sham) and TMS timings (50, 100, 150, 200, or 250 ms) as within-subject factors was used. Bonferroni’s corrected t tests were used for post hoc analysis when appropriate.
Results
Contribution of the left, contralateral PMv and PMd to hand movements
In experiment 1, to determine the distinct contribution of premotor areas to the control of grasping movements performed with the dominant hand, rTMS was applied over either the left PMv or left PMd. As shown in Table 1, a transient virtual lesion of PMd or PMv did not affect the RT, as indicated by an absence of site effect (all F values <1). In contrast, TMS applied over the left PMv yielded a significantly longer MT and CT when compared with PMd stimulation and control conditions (all F values >3.54; all p values <0.008). TMS applied over PMd has no effect on MT and CT when compared with control values (all p values >0.05).
Table 1.
Effects of the left PMv and PMd inactivation on the grip–lift movement parameters
| Controls | Left PMv | Left PMd | |
|---|---|---|---|
| Temporal parameters | |||
| RT (ms) | 182.3 ± 42.5 | 175.8 ± 37.2 | 172.1 ± 44.1 |
| MT (ms) | 87.4 ± 16.7 | 107.4 ± 20.4* | 85.2 ± 15.3 |
| CT (ms) | 5.2 ± 1.4 | 15.6 ± 11.4* | 5.7 ± 1.5 |
| APB–1DI (ms) | 12.4 ± 4.6 | 29.3 ± 6.7* | 11.3 ± 5.9 |
| BrR (ms) | 221.2 ± 15.7 | 220.3 ± 18.3 | 237.2 ± 17.4* |
| 1DI–BrR (ms) | 28.4 ± 8.2 | 16.3 ± 7.6 | 55.4 ± 10.3* |
| Preloading phase (ms) | 31.3 ± 14.7 | 37.1 ± 15.3 | 63.4 ± 17.3* |
| Loading phase (ms) | 181.2 ± 29.1 | 185.3 ± 30.2 | 177.2 ± 27.4 |
| Dynamic parameters | |||
| GF peak (N) | 8.7 ± 1.6 | 8.3 ± 1.5 | 8.5 ± 2.1 |
| LF peak (N) | 6.1 ± 1.2 | 6.2 ± 1.5 | 6.1 ± 1.3 |
| dGF/dt peak (N/s) | 45.2 ± 6.9 | 47.3 ± 7.1 | 44.8 ± 6.5 |
| dLF/dt peak (N/s) | 42.6 ± 6.1 | 43.4 ± 6.4 | 40.9 ± 5.8 |
| Cross-correlation coefficient | 0.85 ± 0.10 | 0.78 ± 0.12 | 0.82 ± 0.10 |
| Time shift (ms) | 23.8 ± 7.8 | 27.3 ± 8.8 | 45.7 ± 9.3* |
| Finger positioning | |||
| Ind-Th H dist (mm) | 5.2 ± 1.3 | 12.5 ± 2.0* | 6.1 ± 1.4 |
| Ind-Th V dist (mm) | 2.1 ± 1.5 | 3.1 ± 1.8 | 2.7 ± 1.3 |
| Index CP area (mm2) | 32.8 ± 8.4 | 90.3 ± 15.3* | 37.3 ± 9.3 |
| Thumb CP area (mm2) | 28.8 ± 6.3 | 79.5 ± 14.2* | 34.1 ± 9.4 |
Mean ± SD values (n = 10) of movement parameters gathered in the control condition (sham) and after rTMS over PMv and PMd. Ind-Th H dist and Ind-Th V dist, Horizontal and vertical distance between the index and thumb on the manipulandum.
*p < 0.05.
The recruitment of distal and proximal muscles was altered differentially after PMv or PMd stimulations. Only virtual lesions of left PMv had a consequence on the intrinsic hand muscle recruitment, as evidenced by a longer interval between the APB and 1DI recruitment (F = 5.76; p = 0.015). In contrast, after a virtual lesion of left PMd, the onset of the BrR recruitment was delayed significantly (F = 4.75; p = 0.002) with respect to the Go signal when compared with the control and PMv stimulation conditions. Consequently, this yielded a distinct increase in the preloading phase duration (F = 8.54; p < 0.001). This finding is further supported by the significant increase of the time shift between dGF/dt and dLF/dt after the left PMd TMS (F = 8.34; p < 0.001). The duration of the loading phase remained unchanged after TMS of either PMv or PMd (all F values <1).
Lesioning the left PMv also altered specifically the finger positions on the manipulandum, as shown by a significant increase of the horizontal distance between the thumb and index fingertip positions on the manipulandum (F = 6.12; p < 0.001); the distance between the fingers along the y-axis remained unchanged. In addition, the finger positioning on the manipulandum was found much more variable after a virtual lesion of PMv than in the PMd stimulation and control conditions, as shown by an larger area of the CP ellipses computed for both the thumb and index finger (all F values >6.84; all p values <0.006). No difference between the area of CP ellipses was found between the PMd and control conditions (all p values >0.05).
TMS applied over either PMv and PMd failed to affect the dynamic parameters and the cross-correlation coefficient between dGF/dt and dLF/dt (all F values <1).
Lateralization of hand movement control in the premotor cortex
In experiment 2, the subjects performed the same grip–lift task with their right hand, but rTMS was delivered over either the left or right PMv and PMd and also, as additional controls, over the left or right M1. In this experiment, the movement parameters significantly altered by TMS-induced lesions were the same as those reported in experiment 1, and, for the sake of clarity, only the results of the statistical analyses performed on these parameters will be described here.
As shown in Table 2, the present results confirm the key role played by the left PMv in precision grasping: only a virtual lesion of the left PMv, and not of the right PMv, led to a longer MT and CT (site × side interaction, all F values >12.1; all p values <0.001). Indeed, post hoc comparisons revealed that MT and CT significantly increased after the stimulation of the left PMv relative to all other conditions (all t values >4.34; all p values <0.05). No other significant difference was found across stimulation sites for MT and CT. Similarly, the interval between the onsets of intrinsic hand muscle contraction increased exclusively after a virtual lesion of the left, but not of the right, PMv (site × side interaction, F = 11.5; p < 0.001) (Fig. 3A). In contrast, we found that the precise positioning of the fingers on the manipulandum was under the control of both the left and right PMv. Indeed, a virtual lesion of either the left or right PMv modified the finger position on the object, as shown by an increased distance between the index and thumb along the horizontal axis of the manipulandum and a larger variability in CP distributions (site, all F values >3.42; all p values <0.021) (Figs. 3B, 4B,C). Although these effects were significant for both left and right PMv stimulation when compared with the other sites, it is worth noting that they were slightly larger when TMS was applied over the left PMv than over the right PMv, as shown by a significant site × side interaction (all F values >4.58; all p values <0.012; post hoc, all t values >5.32; all p values <0.019) (Fig. 4B,C). As expected, TMS applied over either the left or right M1 also altered the performance of grip–lift movements (Table 2), but, importantly, the movement parameters modified after PMv virtual lesions (MT, CT, APB–1DI, and finger positioning) were never found to be affected after left or right M1 stimulations (all p values >0.05).
Table 2.
Hemispheric dominance for controlling grip–lift movement parameters
| Controls | Left PMv | Left PMd | Left M1 | Right PMv | Right PMd | Right M1 | |
|---|---|---|---|---|---|---|---|
| Temporal parameters | |||||||
| RT (ms) | 180.7 ± 37.4 | 171.5 ± 35.6 | 176.2 ± 34.9 | 254.3 ± 47.2* | 185.7 ± 38.3 | 183.4 ± 37.2 | 192.1 ± 29.5 |
| MT (ms) | 89.1 ± 15.3 | 105.7 ± 17.3* | 84.3 ± 18.5 | 93.6 ± 17.4 | 87.4 ± 12.7 | 81.4 ± 14.5 | 91.2 ± 15.3 |
| CT (ms) | 5.6 ± 1.0 | 17.7 ± 12.1* | 4.7 ± 1.3 | 6.1 ± 1.9 | 5.2 ± 1.4 | 6.1 ± 1.1 | 5.3 ± 1.5 |
| APB–1DI (ms) | 11.7 ± 4.3 | 26.3 ± 7.1* | 14.8 ± 5.2 | 10.7 ± 4.3 | 13.7 ± 5.1 | 15.2 ± 6.8 | 13.2 ± 5.8 |
| BrR (ms) | 218.8 ± 16.4 | 221.5 ± 18.3 | 234.3 ± 15.8* | 293.7 ± 25.4* | 215.3 ± 17.9 | 218.9 ± 20.3 | 201.6 ± 14.8* |
| 1DI–BrR (ms) | 29.8 ± 9.4 | 22.7 ± 7.4 | 44.1 ± 10.3* | 28.8 ± 12.4 | 21.3 ± 10.2 | 20.5 ± 10.1 | 18.7 ± 10.4* |
| Preloading phase (ms) | 35.4 ± 15.4 | 41.7 ± 17.3 | 69.1 ± 18.1* | 43.6 ± 19.2 | 43.1 ± 8.7 | 34.6 ± 21.5 | 21.6 ± 9.3* |
| Loading phase (ms) | 183.2 ± 27.4 | 185.6 ± 29.4 | 181.8 ± 28.4 | 223.7 ± 31.4* | 187.3 ± 27.3 | 182.5 ± 31.4 | 157.4 ± 28.3* |
| Dynamic parameters | |||||||
| GF peak (N) | 8.5 ± 1.4 | 8.9 ± 1.5 | 8.4 ± 1.5 | 7.2 ± 3.2* | 8.8 ± 1.6 | 8.3 ± 1.4 | 8.5 ± 1.8 |
| dGF/dt peak (N/s) | 46.7 ± 7.1 | 45.7 ± 8.4 | 47.1 ± 7.3 | 31.9 ± 10.3* | 45.5 ± 9.2 | 46.3 ± 6.4 | 65.8 ± 9.5* |
| dLF/dt peak (N/s) | 42.3 ± 6.1 | 41.9 ± 7.1 | 42.8 ± 6.5 | 28.7 ± 9.4* | 40.3 ± 6.7 | 41.5 ± 6.9 | 58.3 ± 7.1* |
| Cross-correlation coefficient | 0.86 ± 0.10 | 0.81 ± 0.10 | 0.85 ± 0.11 | 0.53 ± 0.21* | 0.78 ± 0.12 | 0.83 ± 0.10 | 0.55 ± 0.15* |
| Time shift (ms) | 25.5 ± 8.7 | 28.2 ± 9.3 | 40.3 ± 8.9* | 55.4 ± 15.2* | 30.2 ± 9.5 | 23.2 ± 10.1 | 57.2 ± 19.1* |
| Finger positioning | |||||||
| Ind-Th H dist (mm) | 5.3 ± 1.9 | 11.5 ± 2.2* | 6.9 ± 1.6 | 5.5 ± 1.4 | 9.2 ± 2.0* | 5.8 ± 1.7 | 5.7 ± 2.1 |
| Index CP area (mm2) | 30.1 ± 7.6 | 85.9 ± 17.1* | 29.4 ± 8.1 | 33.2 ± 9.1 | 64.5 ± 9.6* | 33.5 ± 10.4 | 34.2 ± 10.2 |
| Thumb CP area (mm2) | 24.9 ± 5.3 | 73.8 ± 13.7* | 27.2 ± 6.3 | 27.2 ± 8.4 | 57.2 ± 12.5* | 30.4 ± 7.8 | 32.6 ± 7.1 |
Mean ± SD values (n = 6) of movement parameters gathered in the control condition (sham) and after the inactivation of the left and right PMv, PMd, and M1. Ind-Th H dist, Horizontal distance between the index and thumb on the manipulandum.
*p < 0.05.
Figure 3.
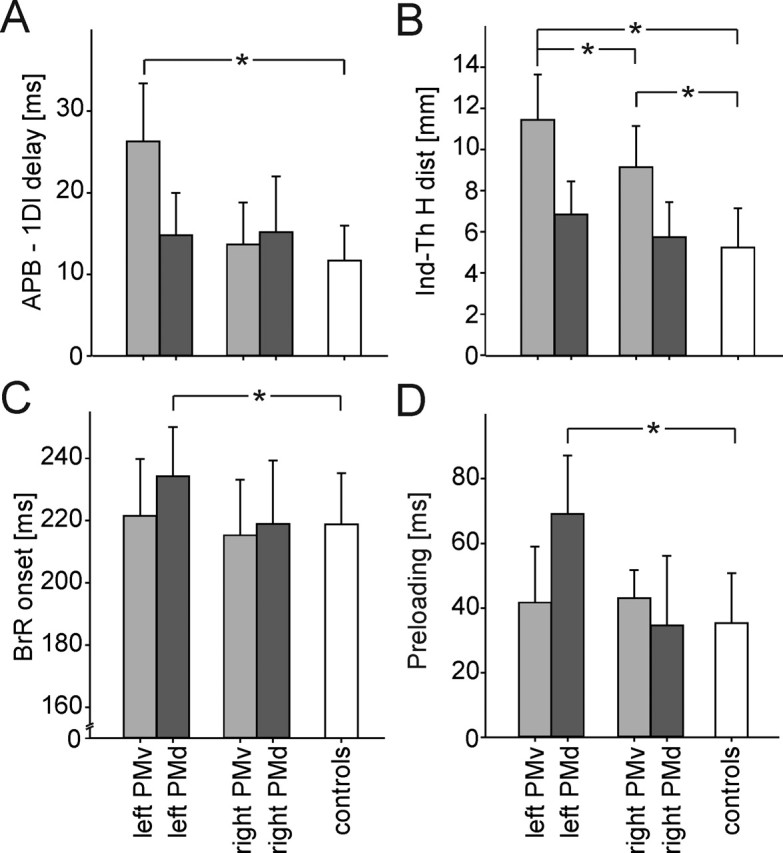
Distinctive effects of the left or right PMv or PMd inactivation on the grip–lift movement. Virtual lesions of PMv specifically altered the grasping phase of grip–lift movement, whereas lesions of the left PMd only affected the lifting phase. Histograms showing the dissociation between the effects of virtual lesions of the left and right PMv (light gray) and PMd (dark gray) when compared with the control condition (white) for four movement parameters: A, the delay between the intrinsic hand muscle recruitment, i.e., the APB and 1DI; B, the index–thumb horizontal distance (Ind-Th H dist); C, the BrR muscle onset; and D, the preloading phase duration. *p < 0.05
The critical role of the left PMd in controlling the BrR muscle recruitment was confirmed in experiment 2 because a longer 1DI–BrR recruitment delay was found exclusively after a left PMd virtual lesion (site × side interaction, F = 7.2; p = 0.023) (Table 2, Fig. 3C). Similarly, as far as the coupling between the grasping and lifting phases is concerned, only a left PMd virtual lesion yielded a longer preloading phase (site × side interaction, F = 12.1; p < 0.001) (Fig. 3D). Post hoc comparisons confirmed these effects and failed to reveal any significant difference between other stimulated sites, including the right PMd. Finally, as already described, TMS applied over the left PMd led to a longer time shift between dGF/dt and dLF/dt; similar effects on the time shift were observed after virtual lesions of the left and right M1 (all t values >3.45; all p values <0.05). However, it is noteworthy that the longer time shift resulting from a left PMd virtual lesion can be explained by an increase of the preloading phase duration (t = 6.32; p = 0.004), whereas, after TMS of the left and right M1, the longer time shift was attributable to a change in the loading phase duration (all t values >3.64; all p values <0.012). This observation suggests that the motor deficits observed after PMd stimulation were specific and did not result from a spread of induced current to M1.
The other consequences of left, contralateral M1 stimulation on grip–lift movements (Table 2) are likely attributable to the direct effects of the TMS-induced muscle twitches in the right hand. Regarding the effects of right, ipsilateral M1 stimulation on movement parameters, they have been extensively described previously and have been interpreted in terms of perturbations of transcallosal influences during movement preparation (Davare et al., 2006).
Time course of the left, contralateral PMv and PMd contribution to hand movements
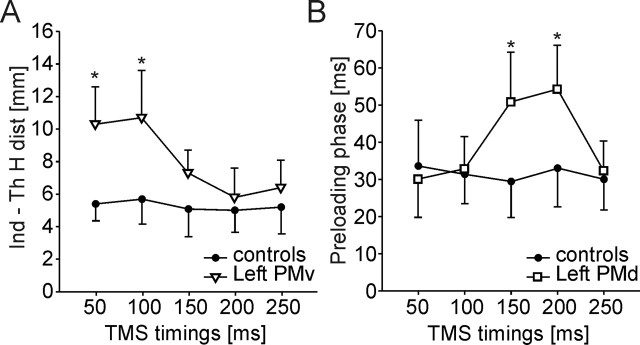
In experiment 3, the time course of the left PMv and PMd involvement in grasping movements was investigated by means of paired-pulse TMS delivered at five different timings (50, 100, 150, 200, or 250 ms) after the Go signal (Fig. 5). The paired-pulse TMS applied over the left PMv significantly altered the same movement parameters as those found modified in experiments 1 and 2, namely MT, CT, the APB–1DI delay, the horizontal distance between fingertips, and the CP ellipse areas (site × timings interaction, all F values >4.75; all p values <0.018). However, those movement parameters were affected by a left PMv virtual lesion only when TMS was delivered either 50 ms (site, all F values >4.58; all p values < 0.008) or 100 ms (site, all F values >3.25; all p values < 0.01) after the Go signal (Fig. 5A). Therefore, given the mean RT (182.3 ± 42.5 ms), MT (87.4 ± 16.7 ms), and CT (5.2 ± 1.4 ms) found in the present study, those two delays of TMS application corresponds, respectively, to ∼230 and 180 ms when expressed with respect to the finger contact with the manipulandum. This finding suggests an early involvement of PMv during hand movement preparation.
Figure 5.
Distinct time course of the effects of PMv or PMd virtual lesions. A, Time course of the effect of a left PMv virtual lesion on the horizontal index–thumb distance. This parameter was found increased after left PMv stimulation but only when paired-pulse TMS was delivered 50 or 100 ms after the Go signal (white triangles) when compared with control (black circles). B, Time course of the consequence of a left PMd virtual lesion on the preloading phase duration. A virtual lesion of the left PMd led to a longer preloading phase (white squares) but only when paired-pulse TMS was delivered 150 or 200 ms when compared with controls (black circles). *p < 0.05
The left PMd was found to intervene later in the grasping movement preparation than the left PMv. Indeed, both the preloading phase and time shift increased significantly only when paired-pulse TMS was delivered over PMd 150 and 200 ms after the Go signal (site, all F values >5.73; all p values < 0.003) (Fig. 5B). Therefore, the contribution of PMd appears to be crucial ∼130–80 ms before the fingers contact the object or, given the mean duration of the preloading phase (31.3 ± 14.7 ms), ∼160–110 ms before subjects started to lift the manipulandum.
Discussion
The present study provides compelling evidence for a dissociation between the role of PMv and PMd in controlling precision grasping in humans. Virtual lesions of the left and right PMv impaired specifically the grasping component of movements performed with the right hand, whereas left PMd lesions modified the timing of the lifting phase. The fact that TMS applied over PMv or PMd produced deficits very different from those observed after M1 stimulations validates our conclusions about the distinct contribution of premotor areas to precision grasping. Finally, we found that PMv intervenes earlier than PMd during grip–lift movement preparation.
PMv contribution to grasping movements
In the present study, to produce virtual lesions of PMv, the coil was positioned over the caudal portion of the pars opercularis of the inferior frontal gyrus, corresponding to BA44. In humans, this region has been shown repeatedly to contribute to precision grasping (Binkofski et al., 1999; Ehrsson et al., 2000, 2001; Kuhtz-Buschbeck et al., 2001). Because both the BA44 in humans and the rostral part of F5 in monkeys (F5ab, located in the caudal bank of the inferior arcuate sulcus) have been shown to be active during fine finger movements, these two areas are usually regarded as functionally equivalent (Binkofski et al., 1999; Fogassi et al., 2001; Picard and Strick, 2001; Rizzolatti et al., 2002; Grezes et al., 2003). However, the homology between the human BA44 and monkey F5 remains very controversial (Picard and Strick, 2001; Binkofski and Buccino, 2004), and this issue has been reopened recently by the finding, in the monkey, of a cortical area architectonically comparable with human BA44 and located in the fundus of the inferior arcuate sulcus (Petrides et al., 2005).
The present study constitutes the first attempt to identify, in humans, the movement parameters controlled by PMv during a grip–lift task. First, we found that lesioning the left or right PMv modifies the fingertip positions on the object and increases their dispersion. Precise fingertip positioning is a prerequisite to grasp an object properly, as shown by the dramatic consequences of inadequate finger positions on grip stability (Goodale et al., 1994). In the present study, however, because of the size of graspable surfaces of the manipulandum, this large variation in fingertip position had no consequence on the task performance per se, and all subjects always managed to lift the manipulandum. Using a smaller or a nonsymmetrical manipulandum requiring a much more precise finger positioning would have probably led to more severe deficits in the grip–lift task. The consequences on precision grasping we observed after TMS of PMv are reminiscent of those observed in monkeys after a reversible inactivation of F5ab (Fogassi et al., 2001). Altogether, those observations corroborate the view that, in primates, PMv is involved in the visuomotor transformations that allow to shape the hand posture appropriately to grasp objects (Murata et al., 1997; Rizzolatti and Luppino, 2001; Raos et al., 2006). Interestingly, we found that both the left and right PMv are responsible for coding the hand posture during grasping movements performed with the right, dominant hand. This is consistent with functional imaging studies showing an activation in both the left and right PMv when subjects manipulated (Binkofski et al., 1999) or grasped (Ehrsson et al., 2000, 2001) objects. Along the same line, it is noteworthy that, in monkeys, large injections of muscimol into F5ab also impair ipsilateral hand movements (Fogassi et al., 2001). Second, we found that the left PMv is involved in elaborating the appropriate pattern of activation of intrinsic hand muscles, a finding consistent with results from monkey experiments in which contralateral PMv inactivation has been shown to modify the timing of the agonist–antagonist muscle recruitment, resulting in impaired forearm movements (Matsumura et al., 1991).
Altogether, those results suggest that, at an early phase of movement preparation, both PMv perform the visuomotor transformations required to configure the hand posture correctly, but, once the right hand is selected, only the left, contralateral PMv contributes to the elaboration of the motor program for the appropriate hand muscle recruitment. Whether comparable control mechanisms also apply for nondominant hand movements requires additional experiments. Finally, we found that PMv is involved early during precision grasping preparation, ∼200 ms before fingers touch the object. This observation is in accordance with the early neuronal responses found in monkey F5ab when the object to grasp is displayed (Murata et al., 1997; Raos et al., 2006). Altogether, this suggests that PMv is involved very early in the visuomotor transformations required to elaborate the motor commands for precision grasping.
PMd contribution to grip–lift movements
To induce virtual lesions of PMd, the coil was positioned over BA6 and, more specifically, over the superior portion of the precentral gyrus delimited by the superior frontal sulcus. This area is probably equivalent to the caudal part of the dorsal premotor cortex (F2, PMdc) in monkeys (Fink et al., 1997; Picard and Strick, 2001). The role of F2 has never been investigated in a grip–lift task. Most data about F2 concern reaching movements and support the classical view that F2 codes for the direction and amplitude of reaching movements (Kurata, 1993; Jeannerod et al., 1995; Scott et al., 1997; Messier and Kalaska, 2000).
We found that a virtual lesion of left PMd delayed the recruitment of the proximal muscles involved in the lifting phase, leading to a longer preloading phase and therefore to less synchronized grasping and lifting movements. The deficits in proximal muscle recruitment observed in the present study after TMS of PMd are compatible with the finding that, in monkeys, F2 mainly contains a representation of proximal arm muscles (Kalaska et al., 1997; Scott et al., 1997; Wise et al., 1997). However, it is noteworthy that PMd lesions failed to modify the load force and the loading phase duration, suggesting that PMd is not directly involved in determining the accurate level of proximal muscle contraction. Instead, the present results suggest that PMd may control the correct timing of the lifting phase with respect to the grasping phase. As previously proposed by Kurata and Hoffman (1994), PMd could be involved in conditional motor behaviors, a view supported by recent studies showing that PMd contributes to movement preparation when hand movements are conditioned to either external cues (Grafton et al., 1998; Schluter et al., 1999; Chouinard et al., 2005) or internal cues, as in complex sequences of finger tapping (Harrington et al., 2000; Haslinger et al., 2002; Haaland et al., 2004). Because the lifting phase can only be initiated when finger positioning is completed and/or when the grip force has reached an adequate level, our results are compatible with such a function of PMd; the initiation of the lifting phase is, however, likely to rely more on somatosensory signals than on visual or arbitrary cues (Johansson and Westling, 1984). In accordance with the role of PMd in initiating the lifting phase, the present study shows that the PMd involvement in the grip–lift task occurs ∼100 ms after that of PMv. This sequential involvement of premotor areas suggests that PMv provides the triggering information to PMd when the grip force and/or the hand configuration are adequate, a view consistent with the existence, in monkeys, of connections between F5 and F2 (Marconi et al., 2001).
As far as the dominance of the left PMd in controlling the right, dominant hand is concerned, our observations are compatible with previous studies (Haslinger et al., 2002; Hlustik et al., 2002) showing that complex sequential movements performed with the right hand only lead to an increased activation in the left, contralateral PMd. Whether the left dominance reported here reflects an effector-independent contribution of the left PMd to the grip–lift synergy is an issue that requires to be further investigated by comparing the performance of the left and right hands.
Conclusions
The present study substantiates the view that the premotor areas, and PMv in particular, play a key role in visuomotor transformations required to generate grasping movements. Such a key position between sensory and motor systems corroborates the idea that the premotor cortex may underlie interactions between finger movements and other cognitive processes, such as, for example, counting abilities or magnitude estimate (Pesenti et al., 2000; Zago et al., 2001; Andres et al., 2004). The study of the functional relationships between finger movements and these other processes will clearly benefit from the identification of the specific contribution of PMv and PMd. Hence, the present findings constrain the possible links between action and other cognitive functions by establishing the role of PMv in the hand posture configuration and the involvement of PMd in the hand movement sequencing.
Footnotes
This work was supported by research grants from the Université Catholique de Louvain (Belgium), the Fonds de la Recherche Scientifique Médicale (Belgium), and the Fondation Médicale Reine Elisabeth (Belgium). M.D. is a research assistant supported by a grant from the Université Catholique de Louvain (Belgium). M.A. is a research assistant at the Fonds National pour la Recherche Scientifique (Belgium). We are grateful to J. Duque and A. Zenon for their comments on a previous version of this manuscript and to D. Zosso, Q. Noirhomme, and M. De Craene for their help with the coregistration and normalization software.
References
- Andres M, Davare M, Pesenti M, Olivier E, Seron X (2004). Number magnitude and grip aperture interaction. NeuroReport 15:2773–2777. [PubMed] [Google Scholar]
- Binkofski F, Buccino G (2004). Motor functions of the Broca’s region. Brain Lang 89:362–369. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H (1999). A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci 11:3276–3286. [DOI] [PubMed] [Google Scholar]
- Brochier T, Boudreau MJ, Pare M, Smith AM (1999). The effects of muscimol inactivation of small regions of motor and somatosensory cortex on independent finger movements and force control in the precision grip. Exp Brain Res 128:31–40. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Hallett M, Cohen LG (1997). Involvement of the ipsilateral motor cortex in finger movements of different complexities. Ann Neurol 41:247–254. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Leonard G, Paus T (2005). Role of the primary motor and dorsal premotor cortices in the anticipation of forces during object lifting. J Neurosci 25:2277–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, Kalaska JF (2003). Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol 89:922–942. [DOI] [PubMed] [Google Scholar]
- Davare M, Duque J, Vandermeeren Y, Thonnard JL, Olivier E (2006). Role of the ipsilateral primary motor cortex in controlling the timing of hand muscle recruitment. Cereb Cortex in press. [DOI] [PubMed]
- De Craene M, du Bois d’Aische A, Macq B, Kipfmueller F, Weisenfeld N, Haker S, Warfield S-K (2004). Multi-modal non-rigid registration using a stochastic gradient approximation. In: Presented at the IEEE International Symposium on Biomedical Imaging April Arlington, VA:.
- Duque J, Thonnard JL, Vandermeeren Y, Sebire G, Cosnard G, Olivier E (2003). Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain 126:732–747. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H (2000). Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol 83:528–536. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren E, Forssberg H (2001). Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol 85:2613–2623. [DOI] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE (1997). Multiple nonprimary motor areas in the human cortex. J Neurophysiol 77:2164–2174. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Tresilian JR (1994). Grip-load force coupling: a general control strategy for transporting objects. J Exp Psychol Hum Percept Perform 20:944–957. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G (2001). Cortical mechanism for the visual guidance of hand grasping movements in the monkey: a reversible inactivation study. Brain 124:571–586. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Eliasson AC, Redon-Zouitenn C, Mercuri E, Dubowitz L (1999). Impaired grip-lift synergy in children with unilateral brain lesions. Brain 122:1157–1168. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Meenan JP, Bulthoff HH, Nicolle DA, Murphy KJ, Racicot CI (1994). Separate neural pathways for the visual analysis of object shape in perception and prehension. Curr Biol 4:604–610. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Arbib MA (1998). Dorsal premotor cortex and conditional movement selection: a PET functional mapping study. J Neurophysiol 79:1092–1097. [DOI] [PubMed] [Google Scholar]
- Grezes J, Armony JL, Rowe J, Passingham RE (2003). Activations related to “mirror” and “canonical” neurones in the human brain: an fMRI study. NeuroImage 18:928–937. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Elsinger CL, Mayer AR, Durgerian S, Rao SM (2004). Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. J Cogn Neurosci 16:621–636. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Rao SM, Haaland KY, Bobholz JA, Mayer AR, Binderx JR, Cox RW (2000). Specialized neural systems underlying representations of sequential movements. J Cogn Neurosci 12:56–77. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Weilke F, Ceballos-Baumann AO, Bartenstein P, Grafin von Einsiedel H, Schwaiger M, Conrad B, Boecker H (2002). The role of lateral premotor-cerebellar-parietal circuits in motor sequence control: a parametric fMRI study. Brain Res Cogn Brain Res 13:159–168. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Hagl E, Nowak DA, Marquardt C (2003). Grip force control during object manipulation in cerebral stroke. Clin Neurophysiol 114:915–929. [DOI] [PubMed] [Google Scholar]
- Hlustik P, Solodkin A, Gullapalli RP, Noll DC, Small SL (2002). Functional lateralization of the human premotor cortex during sequential movements. Brain Cogn 49:54–62. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H (1995). Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci 18:314–320. [PubMed] [Google Scholar]
- Johansson RS, Westling G (1984). Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56:550–564. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Scott SH, Cisek P, Sergio LE (1997). Cortical control of reaching movements. Curr Opin Neurobiol 7:849–859. [DOI] [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM (2001). A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol 112:720. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Ehrsson HH, Forssberg H (2001). Human brain activity in the control of fine static precision grip forces: an fMRI study. Eur J Neurosci 14:382–390. [DOI] [PubMed] [Google Scholar]
- Kurata K (1993). Premotor cortex of monkeys: set- and movement-related activity reflecting amplitude and direction of wrist movements. J Neurophysiol 69:187–200. [DOI] [PubMed] [Google Scholar]
- Kurata K, Hoffman DS (1994). Differential effects of muscimol microinjection into dorsal and ventral aspects of the premotor cortex of monkeys. J Neurophysiol 71:1151–1164. [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, Lacquaniti F, Caminiti R (2001). Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex 11:513–527. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Sawaguchi T, Oishi T, Ueki K, Kubota K (1991). Behavioral deficits induced by local injection of bicuculline and muscimol into the primate motor and premotor cortex. J Neurophysiol 65:1542–1553. [DOI] [PubMed] [Google Scholar]
- Mattes D, Haynor DR, Vesselle H, Lewellen TK, Eubank W (2003). PET-CT image registration in the chest using free-form deformations. IEEE Trans Med Imaging 22:120–128. [DOI] [PubMed] [Google Scholar]
- Messier J, Kalaska JF (2000). Covariation of primate dorsal premotor cell activity with direction and amplitude during a memorized-delay reaching task. J Neurophysiol 84:152–165. [DOI] [PubMed] [Google Scholar]
- Muir RB, Lemon RN (1983). Corticospinal neurons with a special role in precision grip. Brain Res 261:312–316. [DOI] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G (1997). Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol 78:2226–2230. [DOI] [PubMed] [Google Scholar]
- Noirhomme Q, Ferrant M, Vandermeeren Y, Olivier E, Macq B, Cuisenaire O (2004). Registration and real-time visualization of transcranial magnetic stimulation with 3-D MR images. IEEE Trans Biomed Eng 51:1994–2005. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Pesenti M, Thioux M, Seron X, De Volder A (2000). Neuroanatomical substrates of arabic number processing, numerical comparison, and simple addition: a PET study. J Cogn Neurosci 12:461–479. [DOI] [PubMed] [Google Scholar]
- Petrides M, Cadoret G, Mackey S (2005). Orofacial somatomotor responses in the macaque monkey homologue of Broca’s area. Nature 435:1235–1238. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (2001). Imaging the premotor areas. Curr Opin Neurobiol 11:663–672. [DOI] [PubMed] [Google Scholar]
- Raos V, Umilta MA, Murata A, Fogassi L, Gallese V (2006). Functional properties of grasping-related neurons in the ventral premotor area F5 of the macaque monkey. J Neurophysiol 95:709–729. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G (2001). The cortical motor system. Neuron 31:889–901. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M (1988). Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp Brain Res 71:491–507. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V (2002). Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol 12:149–154. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Poliakov AV (1998). Partial inactivation of the primary motor cortex hand area: effects on individuated finger movements. J Neurosci 18:9038–9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter ND, Rushworth MF, Mills KR, Passingham RE (1999). Signal-, set-, and movement-related activity in the human premotor cortex. Neuropsychologia 37:233–243. [DOI] [PubMed] [Google Scholar]
- Scott SH, Sergio LE, Kalaska JF (1997). Reaching movements with similar hand paths but different arm orientations. II. Activity of individual cells in dorsal premotor cortex and parietal area 5. J Neurophysiol 78:2413–2426. [DOI] [PubMed] [Google Scholar]
- Spall JC (1998). An overview of the simultaneous perturbation method for efficient optimization. Johns Hopkins APL Technical Digest 19:482–492. [Google Scholar]
- Tanne-Gariepy J, Rouiller EM, Boussaoud D (2002). Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res 145:91–103. [DOI] [PubMed] [Google Scholar]
- Tunik E, Frey SH, Grafton ST (2005). Virtual lesions of the anterior intraparietal area disrupt goal-dependent on-line adjustments of grasp. Nat Neurosci 8:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V, Cowey A (2000). Transcranial magnetic stimulation and cognitive neuroscience. Nat Rev Neurosci 1:73–79. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R (1997). Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20:25–42. [DOI] [PubMed] [Google Scholar]
- Zago L, Pesenti M, Mellet E, Crivello F, Mazoyer B, Tzourio-Mazoyer N (2001). Neural correlates of simple and complex mental calculation. NeuroImage 13:314–327. [DOI] [PubMed] [Google Scholar]