Abstract
Across human cultures and mammalian species, sex differences can be found in the expression of aggression and parental nurturing behaviors: males are typically more aggressive and less parental than females. These sex differences are primarily attributed to steroid hormone differences during development and/or adulthood, especially the higher levels of androgens experienced by males, which are caused ultimately by the presence of the testis-determining gene Sry on the Y chromosome. The potential for sex differences arising from the different complements of sex-linked genes in male and female cells has received little research attention. To directly test the hypothesis that social behaviors are influenced by differences in sex chromosome complement other than Sry, we used a transgenic mouse model in which gonadal sex and sex chromosome complement are uncoupled. We find that latency to exhibit aggression and one form of parental behavior, pup retrieval, can be influenced by both gonadal sex and sex chromosome complement. For both behaviors, females but not males with XX sex chromosomes differ from XY. We also measured vasopressin immunoreactivity in the lateral septum, which was higher in gonadal males than females, but also differed according to sex chromosome complement. These results imply that a gene(s) on the sex chromosomes (other than Sry) affects sex differences in brain and behavior. Identifying the specific X and/or Y genes involved will increase our understanding of normal and abnormal aggression and parental behavior, including behavioral abnormalities associated with mental illness.
Keywords: sex chromosomes, vasopressin, aggression, parental behavior, Klinefelter syndrome, autism
Introduction
Many sex differences in vertebrate behavior are established during development by the actions of gonadal steroid hormones (Arnold and Gorski, 1984). However, some sexual dimorphisms are not completely explained by these mechanisms (Carruth et al., 2002; De Vries et al., 2002; Arnold et al., 2003, 2004). Studies of a gynandromorphic zebra finch showed a potential interaction between sex chromosome genes and hormones in the development of the sexually dimorphic song nuclei (Agate et al., 2003). Sex differences in cultured mouse mesencephalic neurons expressing tyrosine hydroxylase, are explained by the complement of sex chromosomes in cells rather than effects of gonadal hormones (Pilgrim et al., 1999; Carruth et al., 2002). Several sex chromosome genes, including the testes determining gene Sry, are expressed in the brain and, thus, may have direct sex-specific effects (Lahr et al., 1995; Mayer et al., 2000; Xu et al., 2002; Vawter et al., 2004). Sex differences in expression of some sex-linked genes in embryonic brains occur before gonadal differentiation (Dewing et al., 2003), or in adults as a result of differences in gene dosage rather than effects of sex hormones (Xu et al., 2002).
Studies using inbred and wild mice that vary in intensity of male–male aggression have suggested that aggressive behavior may be modified by genes on the Y chromosome (Selmanoff et al., 1975; Shrenker and Maxson, 1982; Roubertoux and Carlier, 1988; Carlier et al., 1990; Roubertoux et al., 1994; Van Oortmerssen and Sluyter, 1994; Maxson, 1996; Sluyter et al., 1996). The Y chromosomal testis-determining gene (Sry) and the gene encoding the enzyme steroid sulfatase (Sts), which is located within the pseudoautosomal region of the X and Y chromosomes, have been suggested as the origin(s) of these strain differences, but neither gene has been manipulated directly to test its contribution to these behavioral differences.
In the present study we used a mouse model in which testis determination and chromosomal sex are disassociated. Sry was deleted from the Y chromosome (producing the Y− chromosome) and replaced by a transgenic copy of Sry on an autosome (Lovell-Badge and Robertson, 1990; De Vries et al., 2002). This mouse model (hereafter referred to as the core cross) allowed us to compare four groups of mice in a two by two design. Mice differed along two dimensions: Sry was present or absent (these groups were therefore gonadal males or females), and they differed in the complement of non-Sry sex chromosome genes (XX vs XY−). This design permits testing the independent contributions of Sry, which are mediated largely by differences in gonadal secretions, versus non-Sry sex chromosome genes, which have been largely untested in previous studies. The cross does not allow for discrimination between any other specific sex chromosome genes, thus, effects of sex chromosome complement may be caused by either Y or X chromosome-specific genes. In addition, we tested adults under the same hormonal conditions to eliminate the potential contributions of acute differences in hormone levels on behavior at the time of testing. We found that the complement of sex chromosome genes other than Sry influenced aggression and parental behaviors, although their effects were detected only in gonadal females.
Materials and Methods
Animals.
Mice were produced at the University of Virginia School of Medicine Animal Facility in Jordan Hall. The transgenic core cross has been described previously (De Vries et al., 2002). Briefly, the cross uses males carrying a 129/SvEv-Gpi1c Y chromosome (Simpson et al., 1997) with an 11 kb deletion removing the testis-determining gene Sry (Gubbay et al., 1992). The Sry deletion is complemented by the insertion of a fully penetrant Sry transgene [derived from the transgenic line C57BL/6Ei-YARK/JTgN(Sry-129)2Ei] located on an autosome (Mahadevaiah et al., 1993, 1998). These “XY−Sry” mice possess testes and are fully fertile. The Y− chromosome and the Sry transgene segregate independently, thus, four types of offspring are produced by mating XY−Sry males to XX females: XX females, XY− females, XY−Sry males, and XXSry males. Male and female are defined here according to the gonadal phenotype, males possess testes and females ovaries. Mice were genotyped at weaning by PCR for the YMT2/B-related Ssty subfamily family present on the Y long arm, which detects the Y− chromosome (Turner et al., 2000). For the present study, the Y− chromosome and Sry transgene were crossed onto the C57BL/6J background by breeding XY−Sry males from each generation with normal C57BL/6J XX females (The Jackson Laboratory, Bar Harbor, ME). When tested, all offspring were at least in the sixth generation of these backcrosses.
Mice were housed individually from weaning on a 12 h light/dark cycle (lights off at 1:00 P.M., eastern daylight time) and received food (#7912; Harlan Teklad, Madison, WI) and water ad libitum. Stimulus mice were all from the C57BL/6J strain. Stimulus males were housed individually and females were group housed (4–5 per cage). The University of Virginia Animal Use and Care Committee approved all procedures.
For tests of social behavior and aggression, as well as for measurement of septal vasopressin, we removed the gonads of adult mice, and treated all mice with chronic testosterone implants to compare all four groups under conditions in which all individuals had equivalent circulating levels of gonadal hormones. This procedure also eliminated potential group differences in testing-induced changes in gonadal secretions. We used testosterone because this steroid increases both aggression and septal vasopressin immunoreactivity.
Surgery, hormone replacement, and brain treatment.
The mice used for the social behavior tests were gonadectomized bilaterally between 45 and 55 d of age. Surgery was conducted under ketamine-xylazine anesthesia (20 mg/2 mg per 25 g body weight). At the time of surgery each mouse received a subcutaneous SILASTIC capsule (1.02 mm inner diameter × 2.16 mm outer diameter) in the midscapular region filled to 10 mm in length with crystalline testosterone (T). These implants yield plasma T levels within the physiological range for C57BL/6J males: 1–2 ng/ml (Scordalakes and Rissman, 2003). About 4 weeks later, at the end of the behavioral testing series, mice were deeply anesthetized with a lethal dose of sodium pentobarbital. At this time, we verified that the T implant was still present and that it was not empty. Animals were decapitated and brains were rapidly removed and fixed in 5% acrolein for 4 h, then placed in 30% sucrose overnight. Cryoprotected brains were frozen and stored at −70°C until sectioning. For the study of parental behavior, gonadally intact mice were first tested between the ages of 45 and 65 d. Two to 7 d after the test, all mice were gonadectomized (as described above, but without any hormone replacement) and retested 14 d later. A third group of mice (between 45 and 65 d of age) were anesthetized with isoflurane inhalant and a small blood sample (300 μl) was taken either via suborbital or cardiac puncture, plasma was frozen and stored for subsequent hormone assay.
Social behavior tests.
In the first study, gonadectomized T-implanted mice were given three social behavior tests. Each mouse was tested for olfactory preferences, social recognition and aggression, in the same order, between 8:00 A.M. and 12:00 P.M. under regular room-lighting conditions. The groups had the following genotypes: XX (n = 15), XY− (n = 13), XXSry (n = 14), XY−Sry (n = 10). First, we assessed their preferences for olfactory cues deriving from male and female C57BL/6J mice presented in soiled bedding. This is a sexually dimorphic behavior and these procedures have been published (Dominguez-Salazar et al., 2004). During the 10 min test, we recorded the time the subject spent investigating clean, male-soiled, or female-soiled bedding. Three days later, the second social behavior, social recognition, was evaluated by recording the amount of time each subject spent investigating an ovariectomized (OVX) female. The stimulus female was introduced to the subject in its home cage for 1 min, then removed for 9 min before reintroduction. During each 1 min interaction, we recorded the amount of time the resident spent sniffing the body, head, or tail of the OVX female. At the end of the eighth introduction, a novel OVX female was introduced and again chemoinvestigation was scored (Imwalle et al., 2002). Last, 3 d after social recognition testing, we studied aggression, using a resident/intruder test paradigm. On three consecutive days, the intruder stimulus mouse was placed into the home cage of the resident subject; the same intruder was used each time. Intruders were gonad-intact (XY) males (C57BL/6J strain) in which the olfactory bulb had been surgically removed (Rowe and Edwards, 1971). This was done to ensure that all intruders were submissive and did not initiate aggressive contact (Rowe and Edwards, 1971; Miczek et al., 2001). Each aggression test lasted for up to 6 min or until the first aggressive bout (whichever occurred first). An aggressive bout was scored if the resident displayed a combination of at least two aggressive behaviors (bite, chase, wrestle, or lunge) and the latency to aggression was then recorded. Behavioral measures were used as defined previously (Selmanoff et al., 1976). Lunge was scored is the resident mouse was on its two hind feet and stretch forward toward the intruder. This behavior was typically followed by a bite directed toward the head or body of the intruder. All behavior tests were scored by direct observation by a single individual (JDG).
Vasopressin immunocytochemistry.
Brain tissue (n = 7 from each genotype) was cut in a coronal plane at 30 μm in a cryostat. Sections were collected into a series of three and stored at −20°C in antifreeze until processing. Tissues were processed for vasopressin (AVP) immunoreactivity using a rabbit anti-AVP serum (1:5000; ICN Laboratories, Costa Mesa, CA) and a protocol that has been described previously (Scordalakes and Rissman, 2004). Tissues were incubated in primary antibody for 48 h at 4°C, after which they were incubated for several hours in biotinylated goat anti-rabbit serum (1:500; Vector Laboratories, Burlingame, CA) followed by the avidin-biotin complex ABC detection system (1:1000; Vector Elite kit; Vector Laboratories). Immunoreactivity (IR) was visualized using nickel-intensified DAB as the chromogen.
The density of AVP-IR fibers and cells was quantified in a defined region within the lateral septum (LS). The portion of the LS that was quantified included the area bordering the ventricular wall [Franklin and Paxinos (1997), their Fig. 29]. This area was selected because another study using the core cross on an MF1 genetic background (De Vries et al., 2002) found a sex chromosome complement effect on the density of AVP-IR in LS. Fiber density was analyzed by computerized gray-level thresholding using MetaMorph image analysis system (Molecular Devices, West Chester, PA) (Scordalakes and Rissman, 2004). The light intensity and camera settings were kept constant across the sections to standardize measurements. Immunoreactivity was expressed as the amount of AVP-IR staining (μm2). Both the left and right sides of the LS were examined and the section in each brain with the highest density of AVP-IR was used for the statistical analysis (De Vries et al., 2002). Experimenters were blind to genotypes at the time of data acquisition.
Parental behavior tests.
Mice were housed individually and tested for parental behavior between 8:00 A.M. and 12:00 P.M. under regular room light. To start the test, three mouse pups (C57BL/6J), aged prenatal day (PN) 1, 2, or 3 (PN1 = the day of birth), were placed in the home cage on the side furthest from the test subject. Torn tissue paper was added to the cage for nest building immediately before pups were placed in the cage. Pups remained with the test animals for 15 min or until a pup was attacked. If a pup was attacked and/or bitten, all pups were removed and infanticidal behavior was recorded (Gandelman and Vom Saal, 1975). During the 15 min test, the following parental behaviors were recorded: (1) quality of nest as assessed on a scale of 0–3 (0 = no nest, 1 = covering the pups, 2 = some nest characteristics, and 3 = full nest, with bowl shaped sides and top), (2) latency to retrieve pups, (3) number of pups retrieved, (4) latency to visit pups, and (5) total time spent crouching over pups [for definitions, see Meek et al. (2001)]. Each subject was tested twice, once while gonad-intact and again 14 d after gonadectomy. The presence or absence of gonads has previously been shown to make little difference in the expression of parental behavior in mice (Svare et al., 1977; Svare and Mann, 1981). The original groups consisted of the following genotypes: XX (n = 10), XY− (n = 12), XXSry (n = 11), XY−Sry (n = 11). Four of the mice (1 XY−, 2 XXSry, and 1 XY−Sry) that were tested in the first trial died during or after gonadectomy and, thus, were not retested in trial 2. A composite parental behavior score was devised by adding points (pts) for each behavior observed; pup retrieval = 1 pt, each pup retrieved = 1 additional pt, display of crouch = 1 pt, and for nest construction, each grade was equal to one point (1–3 pts). Thus, the highest score (indicative of the best parental behavior) would be an 8; no parental behavior would be a 0 score. As was the case for the social behavior tests, JDG scored all behaviors directly.
Hormone assay.
To determine whether sex chromosome complement affects plasma levels of testosterone in gonad-intact adults from the core cross, assays for testosterone were conducted using radioimmunoassay kits from Diagnostic Products Corporation (Los Angeles, CA). Assays were performed by the University of Virginia Core Ligand and Assay Laboratory. Samples were run in duplicate, the assay ranged from 0.5 to 6.5 ng/ml, the average interassay variability was 3.2% and the average intra-assay coefficient of variation was 9.2%. Group sizes varied between 13 and 14 for each genotype.
Statistics.
Data were analyzed by appropriate statistical tests. Two-way ANOVA tests were used to evaluate all behaviors, the AVP-IR and testosterone data. The two factors were gonadal sex (Sry present or absent) and sex chromosome complement (XX vs XY−). In two cases, repeated-measures ANOVA was used. In the case of the olfactory preference tests, we used repeated measures because three scores are collected from each individual (time spent sniffing clean, female-soiled, and male-soiled bedding). For the aggression tests, each mouse is repeatedly tested, three times over a 3 d period. Planned comparisons were made with Bonferroni’s t tests. Proportions of mice displaying aggression on the first trial and infanticide in tests of parental behavior were analyzed with χ2 tests.
Results
Chemo investigatory behavior is influenced by gonadal sex
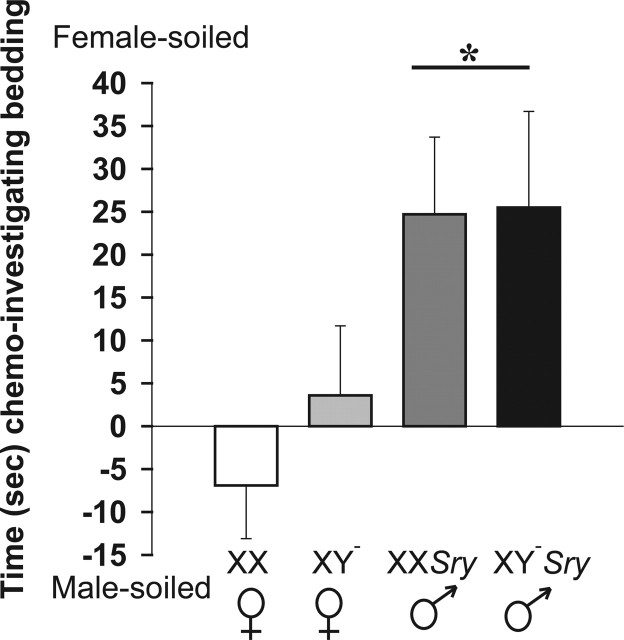
Overall, with respect to investigatory behavior, males and females from the core cross responded differently to soiled bedding but there was no influence of sex chromosome complement. In the olfactory preference tests, repeated-measures ANOVA revealed that the type of bedding had a strong effect on investigatory behavior (F(2,159) = 40.86; p < 0.001). Moreover an interaction between sex and bedding was observed (F(2,159) = 6.44; p < 0.002). No effect of sex chromosome complement was noted (F(1,159) = 1.05). Mice spent more time investigating female-soiled, followed by significantly less time investigating male-soiled, and the least amount of time sniffing clean bedding (p < 0.05) (Table 1, Fig. 1). Gonadal males spent more time investigating female-soiled bedding than any other bedding choice and more time than gonadal females spent investigating any of the three beddings (p < 0.05). When the amount of time that each individual spent investigating female-soiled minus time spent with male-soiled bedding was calculated, there was again a marked effect of sex (F(1,53)=10.78; p < 0.002) but no effect of sex chromosome complement (F(1,53)=2.66). Females had no preference for male- versus female-soiled bedding, whereas males preferred female-soiled bedding (p < 0.05).
Table 1.
Mean (±SEM) time (s) spent investigating clean, male-soiled, and female-soiled bedding
| Genotype | Clean bedding | Male-soiled bedding | Female-soiled bedding |
|---|---|---|---|
| XX | 30 ± 4* | 51 ± 7 | 44 ± 6 |
| XY− | 39 ± 6* | 74 ± 8 | 74 ± 12 |
| XXSry | 37 ± 6* | 69 ± 8 | 86 ± 10** |
| XY−Sry | 31 ± 3* | 55 ± 7 | 89 ± 10** |
*Significantly less time investigating clean bedding compared with time spent investigating male- or female-soiled bedding (p < 0.05).
**Significantly more time investigating female-soiled bedding compared with time spent investigating clean or male-soiled bedding (p < 0.05).
Figure 1.
Time (mean ± SEM) spent in seconds investigating female-soiled minus time spent investigating male-soiled bedding. Males prefer to investigate female-soiled bedding, whereas females have no preference (*p < 0.05). XX, n=15; XY−, n=13; XXSry, n = 14; XY−Sry, n = 10.
Social recognition is displayed by mice of all genotypes
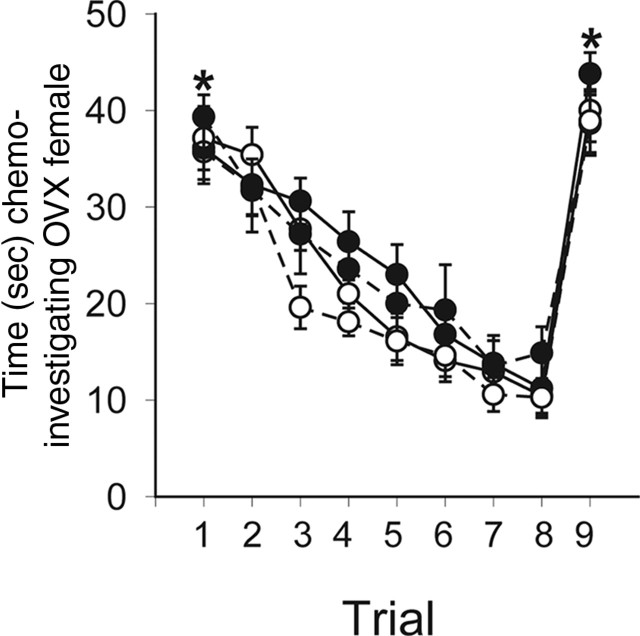
All mice, regardless of gonadal sex or sex chromosome complement, habituated to repeated presentation of the same OVX female, and the behavior was reversed by the introduction of a novel OVX female. The only significant factor was trial (F(8,418)=68.26; p < 0.001). The habituation to the first female and dishabituation to the novel female was examined by limiting the repeated measures ANOVA to the first and last trials with the original females and the final trial with the novel females. This analysis revealed an effect of trial (F(2,143)=140.27; p < 0.001) wherein chemoinvestigatory behavior was high on the first trial and significantly reduced on the last trial with the now familiar female (trial 8). When a novel female was substituted, the subjects increased their amount of chemoinvestigation (p < 0.05) (Fig. 2).
Figure 2.
Time in seconds (means ± SEM) spent investigating an ovariectomized stimulus female. The same female was presented at 9 min intervals for 1 min each time. After the eighth presentation, a novel female was substituted. All mice were adults, gonadectomized, and treated with a testosterone-filled implant (s.c.). *All mice investigated the female more on trials 1 and 9 compared with trial 8. XX, •, solid line (n=15); XY−, •, dashed line (n=13); XXSry, ○, solid line (n=14); XY−Sry, ○, dashed line (n=10).
Aggression is affected by sex chromosome complement
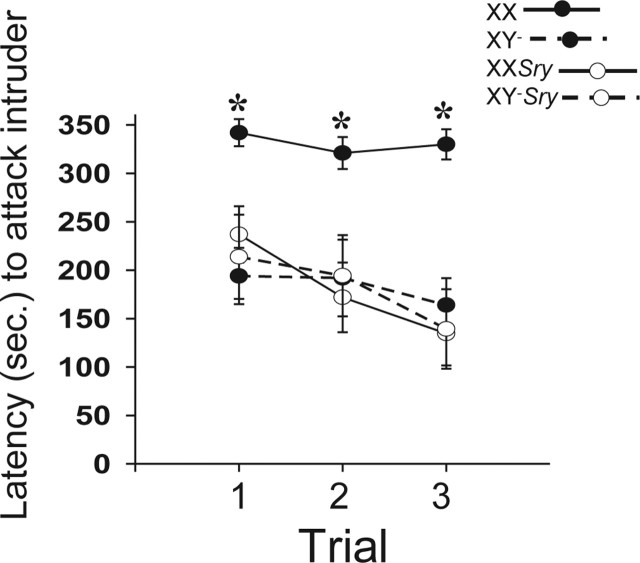
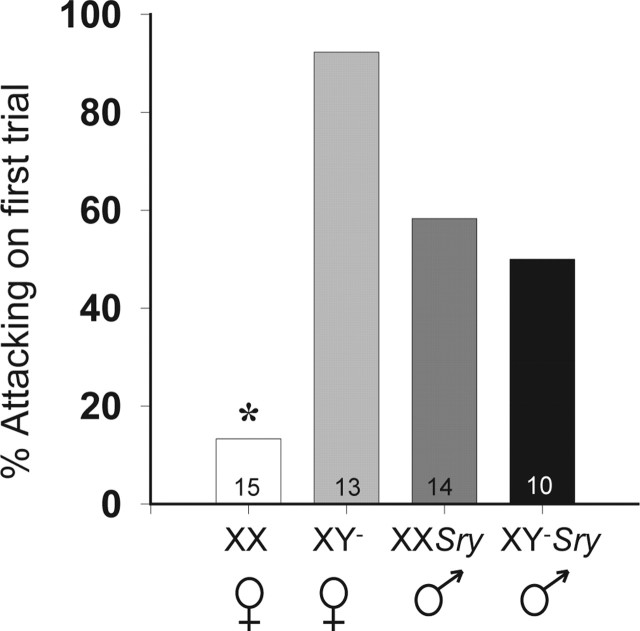
With respect to aggression, in addition to the expected sex difference, there was a marked effect of sex chromosome complement in gonadal females. Aggression latencies were significantly affected by gonadal sex (F(1,156)=7.18; p < 0.01) and sex chromosomes (F(1,156)=5.75; p < 0.02), and we noted an interaction between the two variables (F(1,156)=5.98; p < 0.02). Bonferroni’s planned comparisons showed that over all trials, XX females were slower to become aggressive toward intruders than mice in all other groups (p < 0.05). An effect of trial was evident (F(2,156)=6.82; p < 0.002) and caused by a gradual reduction in aggression latencies over days, with a significant difference between the first and the final tests (p < 0.05) (Fig. 3). An examination of the proportion of mice that displayed aggression on the first encounter with an intruder (i.e., in trial 1) show that XX females were significantly less likely to become aggressive than XY− females, XXSry males, or XY−Sry males (χ2=18.24; p < 0.001) (Fig. 4).
Figure 3.
Aggression latencies (means ± SEM) in intruder/resident tests over 3 consecutive days. All mice were adults, gonadectomized, and treated with a testosterone-filled implant (s.c.). *XX females were significantly slower to display aggression than all other groups. XX, •, solid line (n = 15); XY−, •, dashed line (n = 13); XXSry, ○, solid line (n = 14); XY−Sry, ○, dashed line (n = 10).
Figure 4.
Percentage of mice in each genotype that displayed aggressive behavior on the first test. The asterisk indicates that XX females were significantly different from all other groups. n for each group is listed in each histogram.
Vasopressin fiber density is influenced by sex chromosome complement
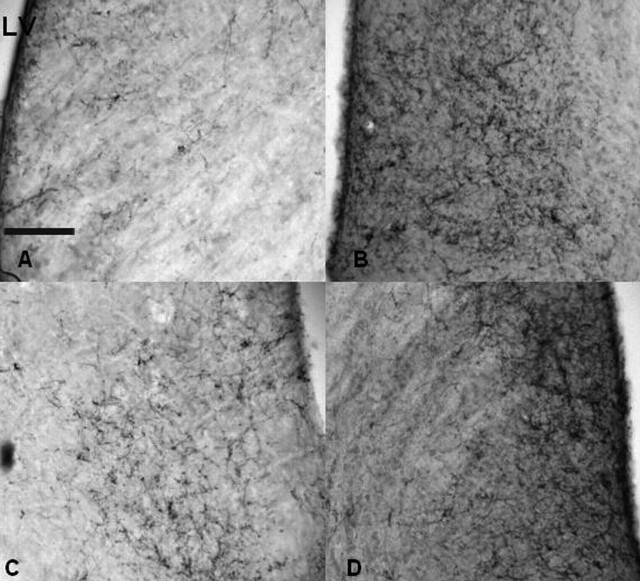
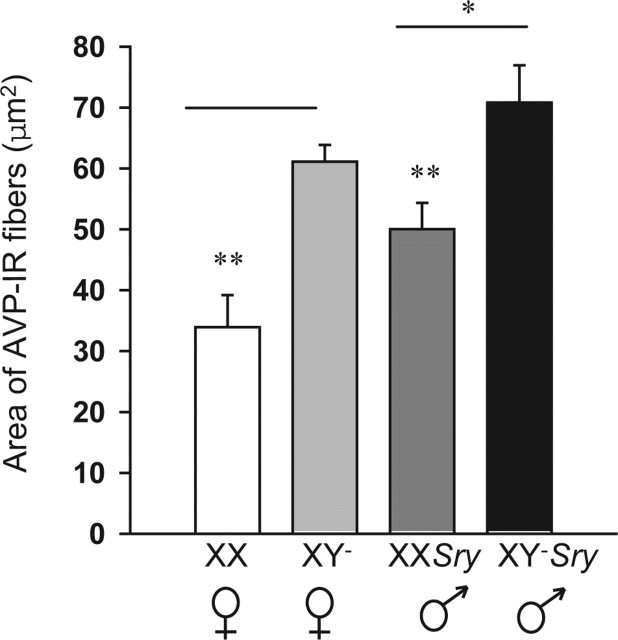
As reported previously for mice from the core cross on an outbred genetic background (De Vries et al., 2002), fiber densities in the lateral septum were significantly affected by gonadal sex (F(1,28) = 4.32; p < 0.05) and chromosomal sex complement (F(1,28) = 19.35; p < 0.001). No interaction was detected. However, our hypothesis predicted sex chromosome complement affects between mice of the same gonadal sex (XX versus XY− and XXSry versus XY−Sry). Thus, we used Bonferroni’s planned comparisons to examine these effects. The sex chromosome complement effects were in agreement with the hypothesis; XX females had less AVP-IR than did XY− females and, likewise, XXSry males had less AVP-IR than XY−Sry males (p < 0.050 (Figs. 5, 6).
Figure 5.
Images taken from the lateral septum of four representative mice. Brains were processed for immunocytochemistry with an antibody against vasopressin. The genotypes represented in each panel are XX (A), XY− (B), XXSry (C), and XY−Sry (D). Scale bar: (in A) 200 μm. LV, Lateral ventricle.
Figure 6.
Mean (±SEM) area (μm2) covered with AVP immunoreactivity in the lateral septum of seven mice from each genotype. *Males had significantly more AVP-IR than did females. **XX individuals had significantly less AVP-IR than XY− mice. p < 0.05.
Parental behaviors are affected by gonadal sex and sex chromosome complement
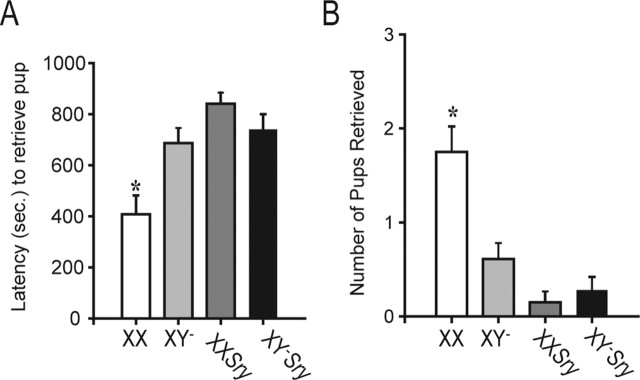
With parental behaviors, as with aggression, there was an effect of sex chromosome complement in addition to the expected sex differences. To examine parental behavior, a two-way repeated-measures ANOVA was used to assess effects of test order (which also corresponded to gonadal status), sex, and sex chromosomes for each of four independent measures of parental behavior and the composite parental score. There were no effects of trial (and, thus, of experience or gonadal status) for any of the measures. Thus, by using a repeated-measures ANOVA we were able to increase the power of our analysis. However, it should be noted that nearly all the significant effects listed below are noted when the data are subjected to two separate two-way ANOVAs for data collected from gonad-intact mice or from gonadectomized mice. Also we noted that several of the measures were noncontinuous variables: number of pups retrieved, nest score and paternal score. In these cases, nonparametric tests were also performed and the results did not differ from those reported below. A significant main effect of sex and significant interaction between sex and sex chromosome complement were discovered for latency to retrieve pups (sex, F(1,84) = 14.40, p < 0.001; interaction, F(1,84) = 11.65, p < 0.001) (Fig. 7). In addition, both factors significantly affected the number of pups retrieved and an interaction between sex and sex chromosomes was present (sex, F(1,84) = 21.53, p < 0.001; sex chromosomes, F(1,84) = 5.76, p < 0.02; interaction, F(1,84) = 9.74, p < 0.003) (Fig. 6). Finally, parental score was significant for both factors and a trend for an interaction was present (sex, F(1,84) = 29.41, p < 0.0001; sex chromosomes, F(1,84) = 4.52, p < 0.04; interaction, F(1,84) = 3.89, p = 0.0550) (Table 2). For all three measures, females performed in a more parental manner (faster to retrieve, retrieving more pups, and higher parental scores) than did males (p < 0.05). In addition, the interactions were attributable to XX females, which were superior on each measure to XY− females and all males (p < 0.05). A sex difference in latency to visit pups was observed with females visiting faster than males (F(1,84) = 5.13; p < 0.04) (Table 2). Nest construction scores were higher in females than in males (F(1,84) = 32.46; p < 0.0001) (Table 2), and latencies to crouch were faster for females than for males (F(1,84) = 4.35; p < 0.04) (Table 2). When tested with or without gonads, more than one-half of all males were infanticidal, whereas few females showed this behavior (Table 2). χ2 tests revealed a sex difference in infanticide (χ2 = 41.29; p < 0.001). We also analyzed infanticide in the two male genotypes alone and, in this case, no differences were noted.
Figure 7.
These data are averages over two parental behavior trials. Data in A show the latencies (in seconds; mean ± SEM) to retrieve the first pup. In B, the number of pups retrieved (of a total of 3 pups) is presented. *XX females significantly different from all other groups. XX, n = 10; XY−, n = 12; XXSry, n = 11; XY−Sry, n = 11.
Table 2.
Measures of parental behavior and infanticide
| Genotype | Parental score | Nest score | Latency to crouch over pups (s) | Latency to visit pups (s) | Number of trials that included infanticide |
|---|---|---|---|---|---|
| XX (20) | 4.6 ± 0.6** | 1.8 ± 0.2 | 733 ± 56 | 10 ± 2 | 1 of 20 |
| XY− (23) | 2.5 ± 0.4 | 1.3 ± 0.2 | 798 ± 46 | 13 ± 2 | 1 of 23 |
| XXSry (20) | 0.8 ± 0.3 | 0.5 ± 0.1* | 842 ± 42 | 20 ± 8 | 12 of 20 |
| XY−Sry(21) | 0.7 ± 0.3 | 0.2 ± 0.1* | 900 ± 0 | 27 ± 7 | 17 of 21 |
Because there was no trial effect data from both trials (first with and next without gonads) were averaged. The numbers (in parentheses next to genotype) refers to the number of test trials conducted on animals of each genotype.
*Sex difference.
**XX different from all other genotypes. p < 0.05.
Adult concentrations of testosterone are unaffected by sex chromosome complement
As expected in gonad-intact mice, testosterone concentrations in plasma were sexually dimorphic (F(1,54) = 4.74; p < 0.035); males had higher levels than females. However, there was no contribution of sex chromosome complement, nor was an interaction detected (Table 3).
Table 3.
Testosterone concentrations in plasma of gonad intact adult mice
| Genotype | Gonads | Testosterone (ng/ml) |
|---|---|---|
| XX (14) | Ovaries | 0.46 ± 0.23 |
| XY− (13) | Ovaries | 0.69 ± 0.29 |
| XXSry (13) | Testes | 1.37 ± 0.41* |
| XY−Sry (14) | Testes | 1.40 ± 0.37* |
Means ± SEM. Values listed in parentheses next to genotypes are the numbers per group.
*Sex difference. p < 0.05.
Discussion
Our data show that sex chromosome complement influences two sexually dimorphic social behaviors: intruder-directed aggression and pup retrieval. This is the first demonstration that sex chromosome genes have an effect on behavior using a model in which sex chromosome complement and gonadal sex are uncoupled. Because we controlled gonadal hormone levels in these mice, group differences in behavior cannot be explained by differences in the levels of gonadal hormones at the time of testing. For both aggression and pup retrieval, significant differences were noted between XX and XY− females (i.e., mice that possessed ovaries) Specifically, the XY− females behaved in a manner unlike XX females but similar to males for the following measures: latency to aggression, proportion of mice displaying aggression during the first encounter, latency to retrieve pups, and numbers of pups retrieved. All females (XX and XY−) showed similar behavioral responses to conspecific-soiled bedding, and their behavior was significantly different from gonadal males. In addition, the majority of the measures of parental behavior were dimorphic by gonadal sex. Females bearing both sex chromosome complements had faster latencies to visit and crouch over pups, build nests, and they displayed less infanticidal behavior than males of either sex chromosome complement. Thus, sex differences in these behaviors depend on the expression of Sry, in brain and/or testes, whereas the behaviors that differ between XX and XY− females are Sry independent.
The effects of sex chromosome complement described here were found in gonadal females, but not in gonadal males. This interaction of non-Sry sex chromosome genes, with Sry itself, requires explanation. One possibility is that the effects of Sry in brain, or in gonad via testicular secretions during development, are dominant such that in the brain, circuits mediating aggression are masculinized (leading to an increase in a trait typical of males) or, in the case of pup-retrieval, defeminized (leading to loss of a trait typical of females), whether or not the sex chromosome complement is XX or XY. In contrast, when testosterone levels are low during development and Sry is not expressed in brain, as is the case in gonadal females, some masculinization or defeminization occurs if the sex chromosome complement is XY rather than XX. Such interactive effects of gonadal hormones, however, must have occurred before adulthood because the groups in the present study did not differ in T levels after gonadectomy in adulthood. The most likely time for these interactive hormonal effects is during the perinatal period.
Classic behavioral genetic studies first hypothesized that genes on the Y chromosome may be responsible for dramatic differences between mouse strains in male/male aggression (Selmanoff et al., 1975; Shrenker and Maxson, 1982; Roubertoux and Carlier, 1988; Roubertoux et al., 1994; Maxson, 1996; Le Roy et al., 1999). Two candidate genes, Sts and Sry, have been suggested as the genetic factor(s) that could explain differences in behavior caused by allelic differences in the Y chromosome. Activity of the Sts enzyme correlates with differences in aggression (Roubertoux and Carlier, 1988; Carlier et al., 1990; Roubertoux et al., 1994; Guillot et al., 1995; Mortaud et al., 1996; Le Roy et al., 1999). This gene is present on the pseudoautosomal region present on both sex chromosomes and, thus, differences in our studies between XX and XY− females are unlikely to be attributed to differences in Sts, because two copies of the gene are expressed in both types of females. However, we cannot yet rule out Sts completely because its function may be regulated differently on the Y and X chromosomes, and it could escape from inactivation on the X. The other candidate gene, Sry, is unique to the Y chromosome without a homolog on X and, like Sts, it is expressed in brain (Lahr et al., 1995; Maxson, 1996; Mayer et al., 2000). Again in our study, differences were present between XX and XY− females, neither of which had Sry. Thus, neither gene is a likely candidate to explain behavioral differences noted in our experiments. It is important to note that differences in mouse strains, and parameters of the behavioral tests including length of the interaction between residents and intruders, condition of the intruders, and types of aggression measured all differ between our study and earlier reports. Thus, our findings may be limited to the specifics of our testing paradigm.
Our data do not indicate on which sex chromosomes the operational gene(s) is located. The group differences reported here can potentially be explained by the presence or absence of Y chromosome gene(s) (other than Sry), one versus two copies of X chromosome gene(s) (presumably that escapes X inactivation), or differences in parental imprinting, because XX but not XY animals have a paternal imprint on X genes. Recent studies of XO mice have identified an X dosage effect on fear reactivity (Isles et al., 2004) and an X imprinting effect on a cognitive behavioral measure (Davies et al., 2005), the latter substantiating an earlier suggestion made on the basis of studies of women with Turner syndrome (Skuse et al., 1997). Several X and Y chromosome genes are expressed in mouse and human brain (Xu et al., 2002; Dewing et al., 2003; Vawter et al., 2004) and a cluster of genes on the maternal X are imprinted (Davies et al., 2005; Raefski and O’Neill, 2005), making these prime candidates for the behavioral effects we report.
Previous experiments have used a genetic approach to test the hypothesis that sex chromosome genes have effects on behavior. In one study, parental behavior toward pups was assessed in XXSxr male mice (mice have two X chromosomes and a large segment of the Y chromosome including Sry attached to one X), XX females, and “XY” male littermates. Important here was the finding that the XXSxr males exhibited more pup retrieval and less infanticide than the “XY” males (Reisert et al., 2002). This contrasts with our finding that XXSry males did not differ from XY−Sry males. However, several factors differed between the two studies, including the use of gonad-intact versus gonadectomized mice, the testing paradigm, and genetic complications that arise from the use of the Sxr mice (Burgoyne, 1989). Two other studies asked if sex chromosome complement affects cognitive behavior. One study used the C57BL/6Jei-YPOS strain to compare spatial abilities of XX and XY females in a Morris water-maze test; the XY females were significantly faster to escape on 2 of the 5 d compared with XX females (Stavnezer et al., 2000). A mouse model for Klinefelter’s syndrome (XXY) has recently been developed and tested for cognitive ability in a pavlovian learning task (Lue et al., 2005); XXY males mastered the task more slowly than did XY control males. In both experiments, the mice were gonad-intact at the time of testing and in both models, animals of the same gonadal sex are known to have different concentrations of steroid hormones during both development and adulthood (Taketo et al., 1991; Lue et al., 2005), which confounds the interpretation of these data.
Using the same genetic cross used here but on a random bred MF1 background, we documented effects of gonadal sex on social preferences and masculine sexual behavior, but no effects of sex chromosome complement were found (De Vries et al., 2002). Here, we replicated the sex differences in social preferences using a different testing paradigm. The social recognition test, along with the olfactory preference tests, demonstrates that mice of all genotypes in the core cross are able to discriminate males from females and familiar from unfamiliar individuals. Here, we replicate the earlier observation of consistent difference in vasopressin-containing fibers in the lateral septum between XX and XY− females, and between XXSry and XY−Sry males, with an XY− sex chromosome complement correlated with denser vasopressin fibers in the lateral septum. Our data extend this observation to a second mouse background strain, C57BL/6J. Both of the behaviors that differ in XX and XY− females in the present study are influenced by vasopressin in rodents (Compaan et al., 1993; Wang et al., 1998; Bester-Meredith et al., 1999; Wersinger et al., 2002) and sex differences in AVP in males and females are correlated with these behavioral differences (Wang et al., 1998; Arnold et al., 2003). These observations make AVP a possible intermediate target because it is not expressed on a sex chromosome but may be influenced by sex chromosome genes.
In our study, at the time behaviors were assessed and brains were collected for immunocytochemistry, mice had equivalent gonadal hormone levels. One exception was the first test of parental behavior, when the mice were gonadally intact. The results in gonadal-intact mice did not differ significantly from those found several weeks later after the mice had all been gonadectomized, as reported previously (Svare et al., 1977; Svare and Mann, 1981). Moreover, T concentrations in plasma of gonad-intact mice were sexually dimorphic (females have lower testosterone levels than males) but not different in the two groups of females, or between the two groups of males. This pattern (no XX versus XY− difference, but a sex difference), with respect to T concentrations, has also been reported in this cross on another inbred (SJL) background (Palaszynski et al., 2005). Ovary intact XX and XY− females undoubtedly have differences in hormone levels because XY− females have severe oocyte depletion resulting in subfertility or sterility (Mahadevaiah et al., 1993). Although we have not controlled for any differences between XX and XY mice of the same gonadal sex in levels of gonadal hormones before gonadectomy, they do not differ on numerous other measures of sexual characters that would be influenced by global differences in gonadal hormones during development (De Vries et al., 2002; Markham et al., 2003; Wagner et al., 2004; Palaszynski et al., 2005).
Within human populations, mutations of X-linked genes affect mental capacity; in the extreme form are conditions like fragile-X, and milder cases may involve autism (Petit et al., 1996; Thomas et al., 1999; Jamain et al., 2003; Kau et al., 2004; Kaufmann et al., 2004). In humans, sex differences in both parental nurturing behavior and aggression are widely acknowledged, as well as individual variation in same-sex populations. Our data suggest that genetic variability on the sex chromosomes is worth investigating in normal and in clinical populations.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants R01 NS043196 (A.P.A.) and K02 MH0112349 (E.F.R.) and by National Institute of Child Health and Human Development (NICHD) Network on Psychosexual Differentiation Grant R21 HD44398. The Virginia Core Ligand and Assay Laboratory is supported by NICHD–NIH through a cooperative agreement (U54 HD28934). We thank Vasiliki Michopoulos and Dr. Jin Ho Park for technical assistance and Drs. Alexander Kauffman and Sheri Berenbaum for comments on previous drafts of this manuscript. We thank Drs. Geert De Vries, Amanda Swain, and Robin Lovell-Badge for numerous discussions of the issues addressed in this paper.
References
- Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP (2003). Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci USA 100:4873–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP (2004). Sex chromosomes and brain gender. Nat Rev Neurosci 5:701–708. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA (1984). Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci 7:413–442. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Rissman EF, De Vries GJ (2003). Two perspectives on the origin of sex differences in the brain. Ann NY Acad Sci 1007:176–188. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA (1999). Species differences in paternal behavior and aggression in peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav 36:25–38. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS (1989). Genetics of XX and XO sex reversal in the mouse. In: Evolutionary mechamisms of sex determination (Wachtel S, ed.) , pp. 161–169. Boca Raton, FL: CRC.
- Carlier M, Roubertoux PL, Kottler ML, Degrelle H (1990). Y chromosome and aggression in strains of laboratory mice. Behav Genet 20:137–156. [DOI] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, Arnold AP (2002). Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci 5:933–934. [DOI] [PubMed] [Google Scholar]
- Compaan JC, Buijs RM, Pool CW, De Ruiter AJ, Koolhaas JM (1993). Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice. Brain Res Bull 30:1–6. [DOI] [PubMed] [Google Scholar]
- Davies W, Isles A, Smith R, Karunadasa D, Burrmann D, Humby T, Ojarikre O, Biggin C, Skuse D, Burgoyne P, Wilkinson L (2005). Xlr3b is a new imprinted candidate for X-linked parent-of-origin effects on cognitive function in mice. Nat Genet 37:625–629. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP (2002). A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci 22:9005–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Shi T, Horvath S, Vilain E (2003). Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res Mol Brain Res 118:82–90. [DOI] [PubMed] [Google Scholar]
- Dominguez-Salazar E, Bateman HL, Rissman EF (2004). Background matters: the effects of estrogen receptor alpha gene disruption on male sexual behavior are modified by background strain. Horm Behav 46:482–490. [DOI] [PubMed] [Google Scholar]
- Franklin KBL, Paxinos G (1997). The mouse brain in stereotaxic coordinates. New York: Academic.
- Gandelman R, Vom Saal FS (1975). Pup-killing in mice: the effects of gonadectomy and testosterone administration. Physiol Behav 15:647–651. [DOI] [PubMed] [Google Scholar]
- Gubbay J, Vivian N, Economou A, Jackson D, Goodfellow P, Lovell-Badge R (1992). Inverted repeat structure of the Sry locus in mice. Proc Natl Acad Sci USA 89:7953–7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot PV, Carlier M, Maxson SC, Roubertoux PL (1995). Intermale aggression tested in two procedures, using four inbred strains of mice and their reciprocal congenics: Y chromosomal implications. Behav Genet 25:357–360. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Scordalakes EM, Rissman EF (2002). Estrogen receptor alpha influences socially motivated behaviors. Horm Behav 42:484–491. [DOI] [PubMed] [Google Scholar]
- Isles AR, Davies W, Burrmann D, Burgoyne PS, Wilkinson LS (2004). Effects on fear reactivity in XO mice are due to haploinsufficiency of a non-PAR X gene: implications for emotional function in Turner’s syndrome. Hum Mol Genet 13:1849–1855. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T (2003). Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34:27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau AS, Tierney E, Bukelis I, Stump MH, Kates WR, Trescher WH, Kaufmann WE (2004). Social behavior profile in young males with fragile X syndrome: characteristics and specificity. Am J Med Genet A 126:9–17. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, Cox C, Capone GT, Stanard P (2004). Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet A 129:225–234. [DOI] [PubMed] [Google Scholar]
- Lahr G, Maxson SC, Mayer A, Just W, Pilgrim C, Reisert I (1995). Transcription of the Y chromosomal gene, Sry, in adult mouse brain. Brain Res Mol Brain Res 33:179–182. [DOI] [PubMed] [Google Scholar]
- Le Roy I, Mortaud S, Tordjman S, Donsez-Darcel E, Carlier M, Degrelle H, Roubertoux PL (1999). Genetic correlation between steroid sulfatase concentration and initiation of attack behavior in mice. Behav Genet 29:131–136. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R, Robertson E (1990). XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 109:635–646. [DOI] [PubMed] [Google Scholar]
- Lue Y, Jentsch JD, Wang C, Rao PN, Sinha Hikim AP, Salameh W, Swerdloff RS (2005). XXY mice exhibit gonadal and behavioral phenotypes similar to Klinefelter syndrome. Endocrinology 146:4148–4154. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah SK, Lovell-Badge R, Burgoyne PS (1993). Tdy-negative XY, XXY and XYY female mice: breeding data and synaptonemal complex analysis. J Reprod Fertil 97:151–160. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah SK, Odorisio T, Elliott DJ, Rattigan A, Szot M, Laval SH, Washburn LL, McCarrey JR, Cattanach BM, Lovell-Badge R, Burgoyne PS (1998). Mouse homologues of the human AZF candidate gene RBM are expressed in spermatogonia and spermatids, and map to a Y chromosome deletion interval associated with a high incidence of sperm abnormalities. Hum Mol Genet 7:715–727. [DOI] [PubMed] [Google Scholar]
- Markham JA, Jurgens HA, Auger CJ, De Vries GJ, Arnold AP, Juraska JM (2003). Sex differences in mouse cortical thickness are independent of the complement of sex chromosomes. Neuroscience 116:71–75. [DOI] [PubMed] [Google Scholar]
- Maxson SC (1996). Ciba Found Symp. Issues in the search for candidate genes in mice as potential animal models of human aggression 194:21–30. discussion 30–35. [DOI] [PubMed] [Google Scholar]
- Mayer A, Mosler G, Just W, Pilgrim C, Reisert I (2000). Developmental profile of Sry transcripts in mouse brain. Neurogenetics 3:25–30. [DOI] [PubMed] [Google Scholar]
- Meek LR, Dittel PL, Sheehan MC, Chan JY, Kjolhaug SR (2001). Effects of stress during pregnancy on maternal behavior in mice. Physiol Behav 72:473–479. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S (2001). Aggressive behavioral phenotypes in mice. Behav Brain Res 125:167–181. [DOI] [PubMed] [Google Scholar]
- Mortaud S, Donsez-Darcel E, Roubertoux PL, Degrelle H (1996). Murine steroid sulfatase gene expression in the brain during postnatal development and adulthood. Neurosci Lett 215:145–148. [DOI] [PubMed] [Google Scholar]
- Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP, Voskuhl RR (2005). A yin-yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology 146:3280–3285. [DOI] [PubMed] [Google Scholar]
- Petit E, Herault J, Raynaud M, Cherpi C, Perrot A, Barthelemy C, Lelord G, Muh JP (1996). X chromosome and infantile autism. Biol Psychiatry 40:457–464. [DOI] [PubMed] [Google Scholar]
- Pilgrim C, Beyer C, Reisert I (1999). The effects of sex and sex hormones on the development of dopaminergic neurons. In: Development of dopaminergic neurons (Di Porzio U, Pernas-Alonso R, Perrone-Capano C, eds) , pp. 75–86. Austin, TX: R.G. Landes.
- Raefski AS, O’Neill MJ (2005). Identification of a cluster of X-linked imprinted genes in mice. Nat Genet 37:620–624. [DOI] [PubMed] [Google Scholar]
- Reisert I, Karolczak M, Beyer C, Just W, Maxson SC, Ehret G (2002). Sry does not fully sex-reverse female into male behavior towards pups. Behav Genet 32:103–111. [DOI] [PubMed] [Google Scholar]
- Roubertoux PL, Carlier M (1988). Differences between CBA/H and NZB mice on intermale aggression. II. Maternal effects. Behav Genet 18:175–184. [DOI] [PubMed] [Google Scholar]
- Roubertoux PL, Carlier M, Degrelle H, Haas-Dupertuis MC, Phillips J, Moutier R (1994). Co-segregation of intermale aggression with the pseudoautosomal region of the Y chromosome in mice. Genetics 136:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe FA, Edwards DA (1971). Olfactory bulb removal: influences on the aggressive behaviors of male mice. Physiol Behav 7:889–892. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF (2003). Aggression in male mice lacking functional estrogen receptor alpha. Behav Neurosci 117:38–45. [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF (2004). Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav 3:20–26. [DOI] [PubMed] [Google Scholar]
- Selmanoff MK, Jumonville JE, Maxson SC, Ginsburg BE (1975). Evidence for a Y chromosomal contribution to an aggressive phenotype in inbred mice. Nature 253:529–530. [DOI] [PubMed] [Google Scholar]
- Selmanoff MK, Maxson SC, Ginsburg BE (1976). Chromosomal determinants of intermale aggressive behavior in inbred mice. Behav Genet 6:53–69. [DOI] [PubMed] [Google Scholar]
- Shrenker P, Maxson SC (1982). The Y chromosomes of DBA/1Bg and DBA/2Bg compared for effects on intermale aggression. Behav Genet 12:429–434. [DOI] [PubMed] [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ (1997). Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet 16:19–27. [DOI] [PubMed] [Google Scholar]
- Skuse DH, James RS, Bishop DV, Coppin B, Dalton P, Aamodt-Leeper G, Bacarese-Hamilton M, Creswell C, McGurk R, Jacobs PA (1997). Evidence from Turner’s syndrome of an imprinted X-linked locus affecting cognitive function. Nature 387:705–708. [DOI] [PubMed] [Google Scholar]
- Sluyter F, van Oortmerssen GA, de Ruiter AJ, Koolhaas JM (1996). Aggression in wild house mice: current state of affairs. Behav Genet 26:489–496. [DOI] [PubMed] [Google Scholar]
- Stavnezer AJ, McDowell CS, Hyde LA, Bimonte HA, Balogh SA, Hoplight BJ, Denenberg VH (2000). Spatial ability of XY sex-reversed female mice. Behav Brain Res 112:135–143. [DOI] [PubMed] [Google Scholar]
- Svare B, Mann M (1981). Infanticide: genetic, developmental and hormonal influences in mice. Physiol Behav 27:921–927. [DOI] [PubMed] [Google Scholar]
- Svare B, Bartke A, Gandelman R (1977). Individual differences in the maternal behavior of male mice: no evidence for a relationship to circulating testosterone levels. Horm Behav 8:372–376. [DOI] [PubMed] [Google Scholar]
- Taketo T, Saeed J, Nishioka Y, Donahoe PK (1991). Delay of testicular differentiation in the B6.YDOM ovotestis demonstrated by immunocytochemical staining for mullerian inhibiting substance. Dev Biol 146:386–395. [DOI] [PubMed] [Google Scholar]
- Thomas NS, Sharp AJ, Browne CE, Skuse D, Hardie C, Dennis NR (1999). Xp deletions associated with autism in three females. Hum Genet 104:43–48. [DOI] [PubMed] [Google Scholar]
- Turner JM, Mahadevaiah SK, Benavente R, Offenberg HH, Heyting C, Burgoyne PS (2000). Analysis of male meiotic “sex body” proteins during XY female meiosis provides new insights into their functions. Chromosoma 109:426–432. [DOI] [PubMed] [Google Scholar]
- Van Oortmerssen GA, Sluyter F (1994). Studies on wild house mice. V. Aggression in lines selected for attack latency and their Y-chromosomal congenics. Behav Genet 24:73–78. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Evans S, Choudary P, Tomita H, Meador-Woodruff J, Molnar M, Li J, Lopez JF, Myers R, Cox D, Watson SJ, Akil H, Jones EG, Bunney WE (2004). Gender-specific gene expression in post-mortem human brain: localization to sex chromosomes. Neuropsychopharmacology 29:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, Xu J, Pfau JL, Quadros PS, De Vries GJ, Arnold AP (2004). Neonatal mice possessing an Sry transgene show a masculinized pattern of progesterone receptor expression in the brain independent of sex chromosome status. Endocrinology 145:1046–1049. [DOI] [PubMed] [Google Scholar]
- Wang Z, Young LJ, De Vries GJ, Insel TR (1998). Voles and vasopressin: a review of molecular, cellular, and behavioral studies of pair bonding and paternal behaviors. Prog Brain Res 119:483–499. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS III (2002). Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry 7:975–984. [DOI] [PubMed] [Google Scholar]
- Xu J, Burgoyne PS, Arnold AP (2002). Sex differences in sex chromosome gene expression in mouse brain. Hum Mol Genet 11:1409–1419. [DOI] [PubMed] [Google Scholar]