Abstract
Central serotonergic neurons have been implicated in numerous animal behaviors and psychiatric disorders, but the molecular mechanisms underlying their development are not well understood. Here we generated Lmx1b (LIM homeobox transcription factor 1 β) conditional knock-out mice (Lmx1bf/f/p) in which Lmx1b was only deleted in Pet1 (pheochromocytoma 12 ETS factor-1)-expressing 5-HT neurons. In Lmx1bf/f/p mice, the initial generation of central 5-HT neurons appeared normal. However, the expression of both 5-HT-specific and non-5-HT-specific markers was lost in these neurons at later stages of development. The loss of gene expression is concomitant with downregulation of Lmx1b expression, with the exception of serotonin transporter Sert and tryptophan hydroxylase TPH2, whose expression appears to be most sensitive to Lmx1b. Interestingly, the expression of Pet1 is tightly coupled with expression of Lmx1b during later stages of embryonic development, indicating that Lmx1b maintains Pet1 expression. In Lmx1bf/f/p mice, almost all central 5-HT neurons failed to survive. Surprisingly, Lmx1bf/f/p mice survived to adulthood and exhibited normal locomotor activity. These data reveal a critical role of Lmx1b in maintaining the differentiated status of 5-HT neurons. Lmx1bf/f/p mice with normal locomotor function should provide a unique animal model for examining the roles of central 5-HT in a variety of animal behaviors.
Keywords: transcription factor, Lmx1b, differentiation, serotonergic neurons, development, locomotor activity
Introduction
Serotonin [5-hydroxytryptamine (5-HT)] has been implicated in numerous physiological and behavioral activities. In this context, recent genetic studies, especially those originating from phenotypic analysis of 5-HT receptor knock-out mice, have highlighted the important role of the 5-HT system in modulating many developmental processes and psychiatric functions (Scearce-Levie et al., 1999; Gaspar et al., 2003; Gingrich et al., 2003). How the serotonergic neurons are generated and maintain their differentiation during development, however, remains essentially unknown (Goridis and Rohrer, 2002). Recent evidence suggests that a variety of transcription factors [Lmx1b (LIM homeobox transcription factor 1 β), Pet1 (pheochromocytoma 12 ETS factor-1), Mash1/Ascl1 (mammalian achaete-schute homolog 1/achaete-scute complex-like 1), Nkx2.2 (NK2 transcription factor-related 2.2), and Gata binding protein 2 (Gata2) and Gata3] are important for the development of 5-HT neurons in the CNS (van Doorninck et al., 1999; Cheng et al., 2003; Ding et al., 2003; Hendricks et al., 2003; Craven et al., 2004; Pattyn et al., 2004; Chen and Ding, 2006). In postmitotic 5-HT neurons, Lmx1b and Pet1 have been implicated as major contributors in the development of 5-HT neurons (Cheng et al., 2003; Ding et al., 2003; Hendricks et al., 2003). Mice lacking Pet1 have a loss of 70–80% of 5-HT neurons in the CNS (Hendricks et al., 2003), whereas Lmx1b knock-out mice lack all central 5-HT neurons (Ding et al., 2003), suggesting that Lmx1b and Pet1 are differentially required for the development of 5-HT neurons. During development, expression of Lmx1b precedes Pet1, and, after a transient expression, Pet1 is lost in Lmx1b knock-out mice, raising the possibility that the maintenance of Pet1 expression is dependent on Lmx1b (Cheng et al., 2003; Ding et al., 2003). However, because of difficulties in labeling Lmx1b-null cells and, thus, in distinguishing between excessive cell death and loss of gene expression in Lmx1b knock-out mice, the question of whether Lmx1b is required for maintenance of Pet1 expression remains to be clarified.
Serotonergic neurons have been thought to have important roles in multiple developmental processes (Lauder, 1993; Whitaker-Azmitia et al., 1996). Surprisingly, despite the loss of a majority of central 5-HT neurons, Pet1 knock-out mice survive and show normal brain morphology (Hendricks et al., 2003). This observation could be attributed to a compensatory effect derived from the remaining 5-HT neurons in Pet1 mutant mice. Because conventional knock-out of Lmx1b in mice leads to perinatal lethality, we hypothesized that mice lacking Lmx1b in 5-HT neurons only (Pet1-expressing neurons) would have no central 5-HT neurons. Mutant mice without central 5-HT neurons, if they survive past birth, would provide a valuable model for determining the function of 5-HT in animal behavior and physiology. Therefore, the goals of the present study were twofold: (1) to evaluate the later role of Lmx1b in the development of 5-HT neurons by taking the advantage of the finding that Lmx1b expression precedes Pet1 and (2) to assess the function of the central 5-HT neurons in the survival and locomotor activity of mice. To these ends, we generated and analyzed Lmx1b conditional knock-out mice, hereafter referred to as Lmx1bf/f/p mice, in which Lmx1b is deleted only in Pet1-expressing cells.
Materials and Methods
Generation and genotyping of Lmx1b floxed allele.
A 129 genomic clone was used to generate the Lmx1b targeting vector, and the targeting vector was electroporated into AB1 embryonic stem (ES) cells, followed by G418 selection. Correctly recombined clones were identified by Southern blot and injected into C57BL/6 blastocysts. Pups derived from chimeras mated with C57BL/6 mice were genotyped by PCR using the following primers: for floxed Lmx1b1, AGG CTC CAT CCA TTC TTC TC; floxed Lmx1b2, CCA CAA TAA GCA AGA GGC AC; and for wild-type allele, Lmx1b1-a, GAT AGG GCA TTC AAC CAG GAC GAG CAA AGA; and Lmx1b-b, AAA CAG AAG CCA CAG AGA GCC AAG GAG AAG. Southern blotting was performed using genomic DNA purified from tail DNA of wild-type and Lmx1b mutant mice.
Animals.
Lmx1bf/f/p mice, their wild-type littermates, and ePet–cre (Pet enhancer–cre recombinase) mice aged between 8 and 12 weeks were acclimated to the experimental room and were used for behavioral tests by observers blind to the genotype and the treatment of the animals. All experiments were done in accordance with the guidelines of the university, and the experimental protocols were approved by the Animal Studies Committee at Washington University School of Medicine.
5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside staining, immunocytochemical staining, and in situ hybridization.
5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) staining, immunocytochemical staining, and in situ hybridization were performed as described previously (Wang et al., 1998; Chen et al., 2001). The following primary antibodies were used: guinea pig anti-Lmx1b (1:200; a gift from T. Jessell, Columbia University, New York, NY), rabbit anti-5-HT antibody (1:5000; Immunostar, Hudson, WI), rabbit anti-Cre recombinase (1:1000; Babco, Richmond, CA), rabbit anti-tyrosine hydroxylase (TH) (1:2000; Chemicon, Temecula, CA), mouse anti-β-galactosidase (β-gal) (1:20; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA). For secondary antibodies, either secondary antibodies conjugated to cyanine 3 (Cy3) or FITC (1:200; Jackson ImmunoResearch, West Grove, PA) or biotinylated secondary antibodies of appropriate species (Vector Laboratories, Burlingame, CA) were used, followed by Cy3- or FITC-conjugated streptavidin (1:1000; Jackson ImmunoResearch). The slides were observed using a fluorescence microscopy or a laser scanning confocal microscopy. Hoechst 33258 (Sigma, St. Louis, MO) was used as the blue fluorescent nuclear counterstain.
HPLC.
Two-month-old mice of both genotypes were used for HPLC (n = 4 for each genotype). Norepinephrine (NE), dihydroxyphenyacetic acid (DOPAC), dopamine (DA), 5-hydroxyindoleacetic acid (5-HIAA), and 5-HT were measured using HPLC with electrochemical detection as described previously with several modifications (Renner and Luine, 1986). The concentrations of the amines and amine metabolites were calculated with respect to the mean peak height values obtained from standard runs set in the internal standard mode using CSW32 software (DataApex, Prague, Czech Republic). The resulting values were corrected for volume and expressed as picograms of amine per milligram of wet tissue weight.
Locomotor activity: motor function.
Motor performance was assessed on an accelerating rotarod treadmill (Ugo Basile, Comerio, Italy) as described previously (Malmberg et al., 2003).
Open-field test.
Mice were evaluated over a 1 h period in transparent (47.6 × 25.4 × 20.6 cm) polystyrene enclosures as described previously (Wozniak et al., 2004).
Statistical analysis.
Statistical comparisons were performed using GraphPad Software (San Diego, CA) Prism Software with Student's t test or one-way ANOVA followed by appropriate Fisher's post hoc analysis. Except for the mentioned exceptions, all data were expressed as the mean ± SEM, and error bars represent SEM. In all cases, p < 0.05 was considered statistically significant.
Results
Generation of a floxed Lmx1b allele and Lmx1bf/f/p mice
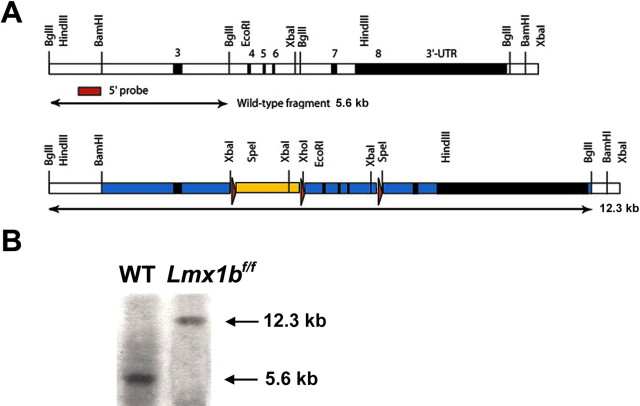
To generate a floxed Lmx1b allele, we inserted loxP sites around exons 4, 5, and 6 of the Lmx1b gene using homologous recombination (Fig. 1). The construct was electroporated into AB1 ES cells, and ∼10% of the G418/FIAU resistant clones were correctly targeted. Correctly targeted ES cells were injected into C57BL/6J blastocysts to generate chimeras that transmitted this allele through the germ line. The PGKneobpA cassette was removed by the action of Cre in vivo using deleter cytomegalovirus–cre mice (Fig. 1). To generate Lmx1b conditional knock-out mice, ePet–cre mice (Scott et al., 2005) were mated with Lmx1bfloxp/floxp mice to generate Lmx1bfloxp/+/ePet–cre mice, hereafter referred to as Lmx1bf/+/p mice. These mice were subsequently mated with Lmx1bfloxp/floxp mice to generate Lmx1bfloxp/floxp/ePet–cre mice or Lmx1bf/f/p mice.
Figure 1.
Gene targeting of Lmx1b. A, Strategy for generating floxed Lmx1b mice. Top, Wild-type locus. Exons 3–8 (black boxes) and introns (white boxes) are shown. The homeodomain is contained within exons 4, 5, and 6. Bottom, Targeting strategy. A loxP–PGKneobpA–loxP cassette (blue box with red triangles representing the loxP sites) was inserted into the first BglII site 5′ to exon 4, and an oligonucleotide containing a single loxP site was inserted into the first BglII site 3′ to exon 6 by conventional cloning methods. In both cases, the BglII site was destroyed. In the targeting construct, flanking regions were included (blue) that extended ∼3 kb 5′ to the PGKneobpA cassette and 5.5 kb 3′ to the 3′ loxP site and an MC1-TK cassette for negative selection. B, Southern blot shows a wild-type (WT) band (5.6 kb) and a floxed band (12.3 kb) after digestion with BglII.
Initial generation of central 5-HT neurons is normal in Lmx1bf/f/p mice
To determine whether central 5-HT neurons were initially generated in Lmx1bf/f/p mice, we analyzed the expression of Lmx1b and other molecular markers at embryonic day 11.0 (E11.0)–E11.25, when 5-HT neurons are first generated in the rostral part of the hindbrain (Ding et al., 2003). Between E11.0 and E11.25, the pattern of 5-HT staining in the rostral hindbrain of Lmx1bf/f/p mice exhibited characteristic bilateral organization along the midline of the hindbrain, similar to Lmx1bf/+/p or wild-type mice (Fig. 2A,B and data not shown). Tryptophan hydroxylase (TPH) and the serotonin transporter (Sert) also showed similar expression patterns in Lmx1bf/f/p mice and wild-type mice (Fig. 2C–F). In addition, Lmx1b expression detected by in situ hybridization up to E11.5 was not significantly different in the rostral part of the hindbrain of Lmx1bf/f/p mice when compared with wild-type mice (Fig. 2G,H). At this stage, Pet1 expression was also indistinguishable between Lmx1bf/f/p mice and wild-type controls (Fig. 2I,J). Examination of these markers in the caudal region of the hindbrain revealed expression pattern in Lmx1bf/f/p mice similar to the control mice (data not shown). Together, these data demonstrate that the initial generation of central 5-HT neurons was not affected in Lmx1bf/f/p mice.
Figure 2.
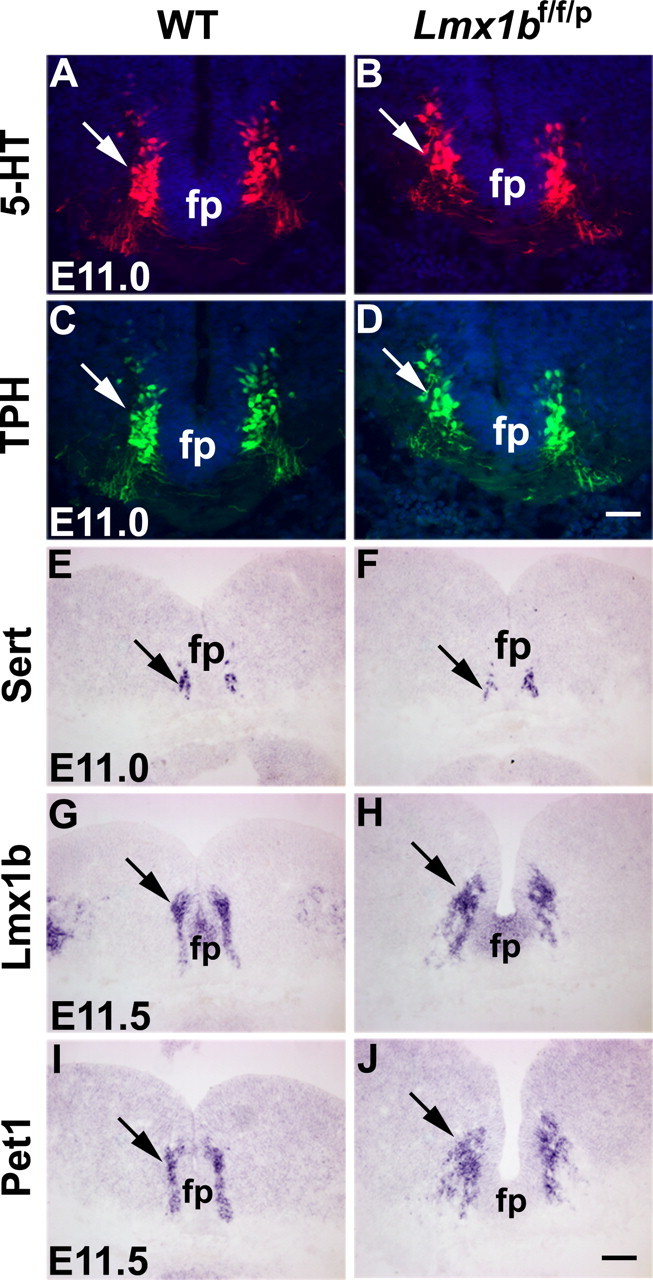
Normal expression of 5-HT, TPH, Sert, Lmx1b, and Pet1 in the rostral part of the hindbrain of Lmx1bf/f/p mice and wild-type (WT) mice. A, B, 5-HT expression detected by immunocytochemical staining in Lmx1bf/f/p mice (B, arrow) was similar compared with Lmx1bf/+/p mice (A, arrow) at E11. C, D, Normal expression of TPH detected by immunocytochemical staining in Lmx1bf/f/p mice (D, arrow) compared with the control (C, arrow) at E11. E–J, Expression of Sert, Lmx1b, and Pet1 detected by in situ hybridization was indistinguishable in Lmx1bf/f/p mice (F, H, J, arrows) compared with wild-type mice (E, G, I, arrows). fp, Floor plate. Scale bars: D, 100 μm; J, 200 μm.
Downregulation and loss of 5-HT neuron-specific gene expression in Lmx1bf/f/p mice
At E12.5, Lmx1b expression detected by immunocytochemical staining appeared weaker in Lmx1bf/f/p mice when compared with the wild-type control, suggesting that Pet1–cre had already exerted its recombination activity (Fig. 3A,B and data not shown). At this stage, despite the initial normal generation of 5-HT neurons, 5-HT staining was markedly reduced in Lmx1bf/f/p mice compared with wild-type mice (Fig. 3C,D and data not shown). Interestingly, Cre staining was also reduced in Lmx1bf/f/p mice relative to control, suggesting that expression of Pet1–cre is dependent on Lmx1b expression (Fig. 3E,F). In contrast to Lmx1b and Pet1 expression, expression of Sert and TPH2 [which is expressed only in central 5-HT neurons (Zhang et al., 2004)] detected by in situ hybridization was essentially lost by this stage (Fig. 3G–J). By E14.5, only a few 5-HT-positive (5-HT+) neurons were present (Fig. 3K,I), and Lmx1b and Pet1 expression was further reduced compared with the control (Fig. 3M–P). We also examined the expression of markers that are not related to 5-HT synthesis [secretogranin II (SCGII) (Kato et al., 2000) and calcitonin receptor (Ctr) (Nakamoto et al., 2000)] in Lmx1bf/f/p mice and found that expression of SCGII and Ctr was almost lost in Lmx1bf/f/p mice at E13.5 (Fig. 3Q–T). By E16.5, 5-HT, Lmx1b, Pet1, and other markers were rarely detected in Lmx1bf/f/p mice (data not shown). These results indicated that Lmx1b controls not only the expression of 5-HT-related markers but also non-5-HT-related markers.
Figure 3.
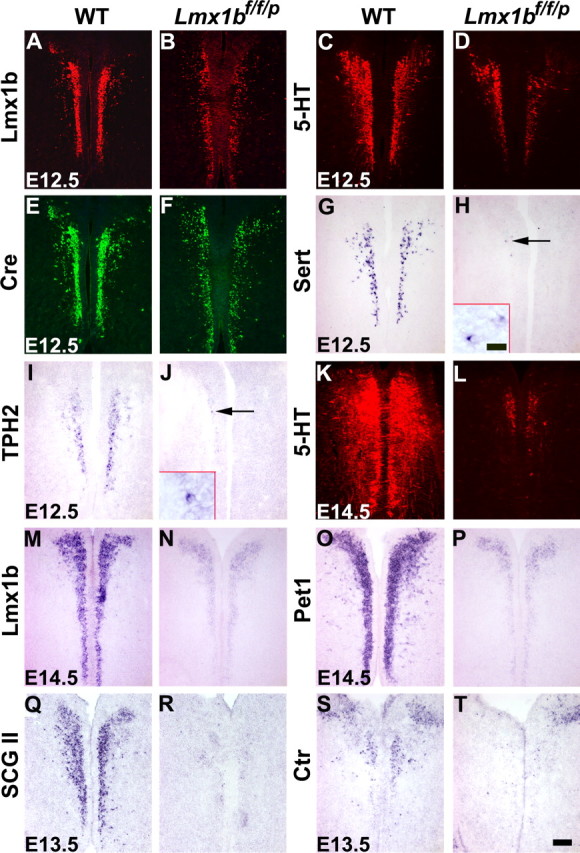
Downregulation and/or loss of molecular markers in the rostral part of the hindbrain of Lmx1bf/f/p mice compared with wild-type mice. A–F, Immunocytochemical staining showed that Lmx1b (A, B), 5-HT (C, D), and Cre (E, F) expression was downregulated in Lmx1bf/f/p mice (B, D, F) compared with the control (A, C, E) at E12.5. G–J, Expression of Sert and TPH2 detected by in situ hybridization was virtually lost in Lmx1bf/f/p mice (H, J) at E12.5. Arrows indicate a few remaining Sert+ and TPH2+ cells. Small insets in H and J showed higher magnification of Sert+ and TPH2+ cells. K, L, 5-HT staining was almost lost in Lmx1bf/f/p mice (L) by E14.5. M–P, Similar downregulation of Lmx1b and Pet1 in Lmx1bf/f/p mice (N, P) compared with wild-type mice (M, O) at E14.5. Q–T, Expression of SCGII and Ctr was almost lost in Lmx1bf/f/p mice (R, T) at E13.5. WT, Wild type. Scale bar: 100 μm; inset, 20 μm.
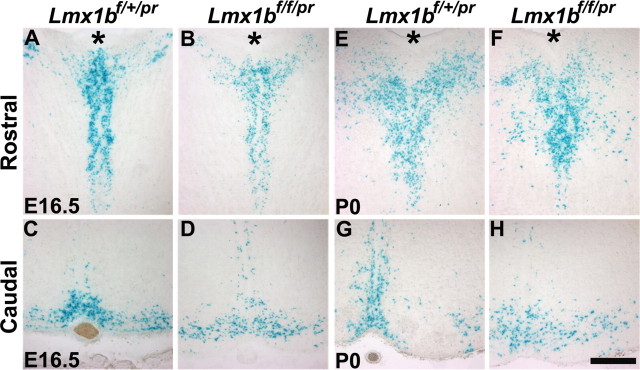
Examination of Lmx1b-null cells in the hindbrain of Lmx1bf/f/pr mice by X-gal staining
A loss and downregulation of molecular marker expression could be attributable to cell loss or abnormal cell migration. To distinguish these possibilities, we generated Lmx1b+/−/ePet–cre/ROSA26 mice and Lmx1b−/−/ePet–cre/ROSA26 mice, here referred to Lmx1bf/+/pr and Lmx1bf/f/pr, respectively. At E16.5 and postnatal day 0 (P0), X-gal staining showed that the presence of presumptive 5-HT neurons was similar in Lmx1bf/f/pr mice and control mice (Fig. 4A–H). Moreover, no obvious ectopic X-gal staining was noted in Lmx1bf/f/pr mice (Fig. 4A–H). These data indicated that the loss and downregulation of molecular markers in Lmx1b mutants were caused by gene regulation or blockage of differentiation rather than by cell loss or abnormal migration.
Figure 4.
Examination of central 5-HT neurons by X-gal staining. A–D, X-gal staining of 5-HT neurons in the rostral (A, B) and caudal (C, D) part of the hindbrain of Lmx1bf/+/pr (A, C) and Lmx1bf/f/pr (B, D) mice at E16.5. X-gal staining patterns were comparable between Lmx1bf/+/pr and Lmx1bf/f/pr mice. E–H, X-gal staining of 5-HT neurons in the rostral (E, F) and caudal (G, H) part of the hindbrain of Lmx1bf/+/pr (E, G) and Lmx1bf/f/pr (F, H) mice at P0. X-gal staining pattern was also similar in Lmx1bf/f/pr mice compared with Lmx1bf/+/pr mice. Asterisk (*) indicates the cerebral aqueduct. Scale bar, 100 μm.
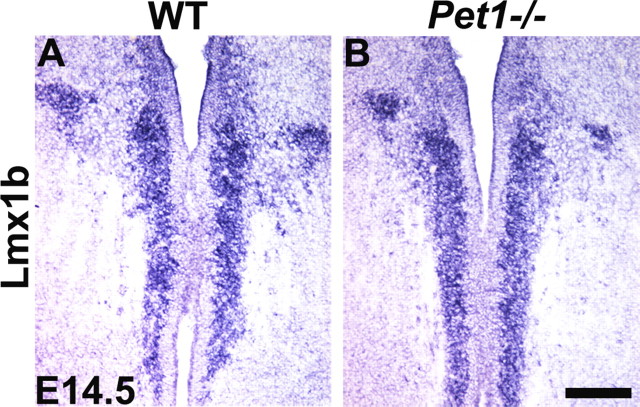
Normal expression of Lmx1b in Pet1−/− mice at later stages of embryogenesis
We previously showed that Lmx1b expression is normal in Pet1 knock-out mice at E11.5 (Ding et al., 2003). To assess whether persistent expression of Pet1 is required for maintaining Lmx1b expression, we examined Lmx1b expression in Pet1 knock-out mice at E14.5 and E16.5 before possible abnormal cell death of 5-HT neurons. At both stages examined, expression of Lmx1b was indistinguishable between Pet1 knock-out and wild-type mice (Fig. 5A,B and data not shown). These results indicate that Lmx1b does not require Pet1 for its persistent expression at least until E16.5.
Figure 5.
Normal expression of Lmx1b in Pet1 mutant mice at E14.5. A, B, There was no difference in Lmx1b expression detected by in situ hybridization in the rostral part of the hindbrain between wild-type (A) and Pet1 mutants (B) at E14.5. Scale bar, 100 μm.
Loss of 5-HT neurons in the raphe nuclei of Lmx1bf/f/p mice
Surprisingly, all Lmx1bf/f/p mice survived to adulthood without apparent deficits in motor capability. To examine whether 5-HT neurons were present in the raphe nuclei, we analyzed the 5-HT staining pattern in adult Lmx1bf/f/p and wild-type mice. Serotonergic neurons in the raphe nuclei of the brain are classified into nine groups (B1–B9) based on their anatomical architecture and location (Dahlstroem and Fuxe, 1964). In wild-type mice, 5-HT neurons were present in all B nuclei of the hindbrain (Fig. 6A,C). In marked contrast, only one or two 5-HT+ cells were occasionally found in a few sections from Lmx1bf/f/p mice (Fig. 6B,D). Furthermore, these cells, when present, appeared to be randomly distributed in the various raphe nuclei (Fig. 6B). Consistent with the near absence of 5-HT cell bodies, no ascending or descending 5-HT fibers in the rostral brain or spinal cord were detected in Lmx1bf/f/p mice, whereas wild-type mice had abundant 5-HT fiber staining in both the brain and spinal cord (data not shown). Expression of Pet1, Lmx1b, Sert, TPH and the vesicular monamine transporter VMAT2 were virtually absent in the raphe nuclei of Lmx1bf/f/p mice (data not shown).
Figure 6.
5-HT neurons are selectively missing in the raphe nuclei of Lmx1bf/f/p mice. A–D, Immunocytochemical staining of 5-HT in B7 (A, B) and the caudal part (B1–B3) (C, D) of the raphe nuclei of wild-type mice (A, C) and Lmx1bf/f/p mice (B, D). Only a few 5-HT+ cells were detected in the mutant (arrow in B). E, F, Nissl staining showed the loss of B6 in the raphe nuclei of Lmx1bf/f/pr mice (F, arrow) compared with B6 in wild-type mice (arrow pointing to dark staining of B6 in E). G, H, Immunocytochemical staining with anti-β-gal antibody in B7 nucleus of Lmx1bf/+/pr mice (G) and Lmx1bf/f/pr mice (H). Arrows in H indicate a few remaining cells in mutants. Small insets in G and H showed higher magnification of confocal images of anti-β-gal+ cells. I, HPLC analysis of 5-HT and 5-HIAA in the brain and spinal cord of Lmx1bf/f/p mice (n = 4) and wild-type littermates (n = 4). There was severe deficiency of the levels of 5-HT and its metabolite 5-HIAA in the CNS of Lmx1bf/f/p mice. Two-tailed t test. Asterisk indicates the cerebral aqueduct. WT, Wild type; wet weight, wet tissue weight. Scale bars: 100 μm; insets, 10 μm.
To examine the distribution and density of cell bodies in the raphe nuclei of wild-type and Lmx1bf/f/p mice, we used Nissl staining and found that the cytoarchitecture of B1–B9 nuclei of Lmx1bf/f/p mice was altered compared with wild-type control. For example, the cytoarchitecture of B6 (the caudal nucleus raphe dorsalis) in wild-type brainstem sections can be clearly delineated by Nissl staining (Fig. 6E). However, this nucleus was missing in the mutants (Fig. 6F).
One possible explanation for the absence of 5-HT cells in raphe nuclei is that the presumptive 5-HT neurons might have migrated to other regions. However, we did not find ectopic 5-HT neurons in the Lmx1bf/f/p mice. To further assess whether some neurons that failed to express 5-HT markers may have been mislocated, we followed the fate of Pet1–cre+ cells in Lmx1bf/f/pr mice by immunochemical staining using an anti-β-galactosidase antibody and X-gal staining and found that the pattern of β-gal+ cells in B nuclei recapitulated the typical 5-HT expression pattern (Fig. 6G). In contrast, only a few β-gal+ cells were detected in the raphe nuclei of Lmx1bf/f/pr mice (Fig. 6H). Confocal examination of single β-gal+ cells from Lmx1bf/+/pr mice showed the normal morphology of 5-HT neurons (Fig. 6G, inset), whereas the few remaining β-gal+ cells in Lmx1bf/f/pr mice had no processes or dendrites (Fig. 6H, inset). Moreover, the β-gal+ cells present in Lmx1bf/f/pr mice appeared smaller with respect to β-gal+ cells in the controls (Fig. 6H, insert). These data strongly suggest that 5-HT neurons are completely lost in the raphe system of adult Lmx1bf/f/p mice and the few remaining 5-HT+ or β-gal+ cells are unlikely to be physiologically normal 5-HT neurons. Our data also suggest that the loss of presumptive 5-HT cells in the raphe nuclei of Lmx1bf/f/p mice primarily occurred during postnatal stages.
To determine more quantitatively the level of 5-HT in Lmx1bf/f/pr mice, we performed HPLC analysis. The levels of 5-HT and its metabolite 5-HIAA were minimal or undetectable in the brain and spinal cord of Lmx1bf/f/p mice compared with wild-type mice (Fig. 6I). The residual 5-HT in the CNS of Lmx1bf/f/pr mice may have been derived from circulatory sources or, alternatively, from putative 5-HT neurons located outside of the raphe system (Frankfurt et al., 1981; Weissmann et al., 1987; Ishimura et al., 1988). Nevertheless, our data strongly indicate that the raphe 5-HT neurons are completely lost or nonfunctional in Lmx1bf/f/p mice.
Normal expression of TH and NE in the CNS of Lmx1bf/f/p mice
Pharmacological studies indicated that depletion of central serotonin may affect the level of dopamine and NE (Romaniuk et al., 1989; Koed and Linnet, 2000). To examine whether other neurotransmitter systems are affected in Lmx1bf/f/p mice, we performed HPLC studies. HPLC analysis revealed that the levels of DA, DOPAC, and NE in the brain (Fig. 7A) and spinal cord (Fig. 7B) was similar in Lmx1bf/f/p mice and wild-type mice. In addition, no major structural abnormalities outside the B nuclei were observed in Lmx1bf/f/p mice (data not shown).
Figure 7.
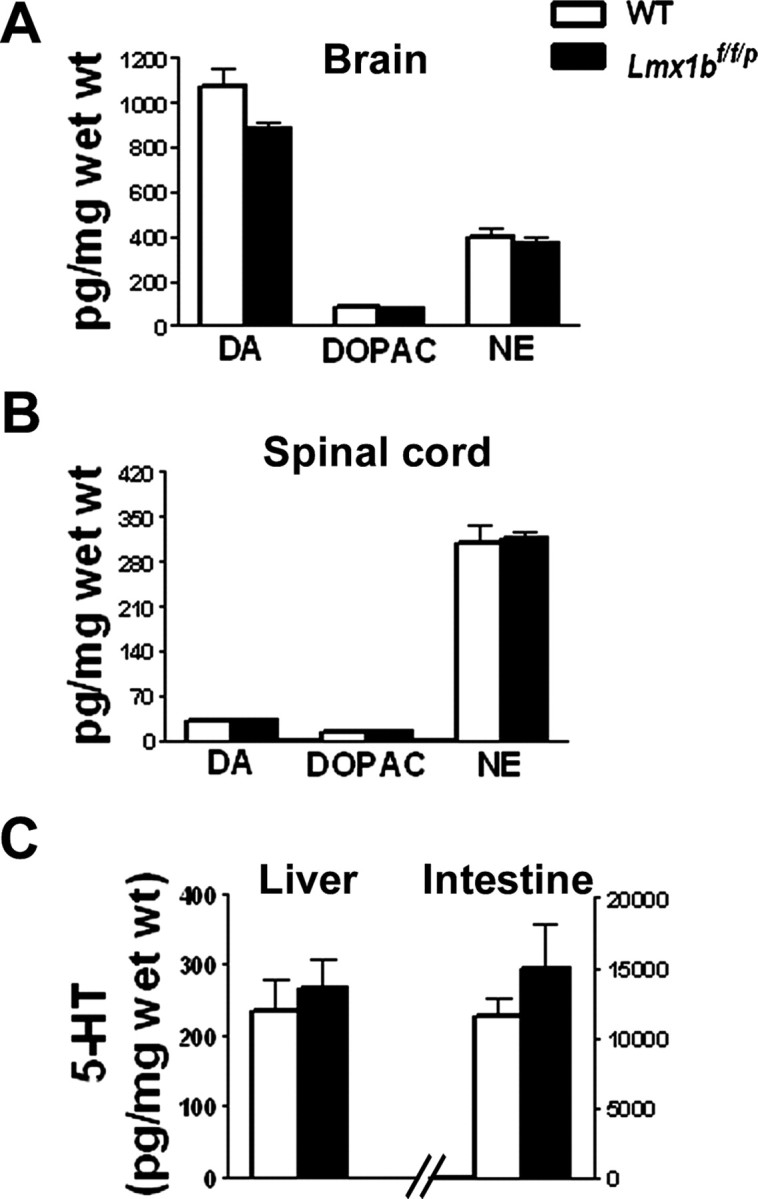
HPLC analysis of DA, DOPAC, and NE in Lmx1bf/f/p and wild-type mice. HPLC analysis of two different monoamines and their metabolites levels in the brain (A) and spinal cord (B) of 2-month-old wild-type (n = 4) and Lmx1bf/f/p (n = 4) mice. There were no significant differences in the levels of DA and its metabolite DOPAC or NE in the brain or spinal cord of Lmx1bf/f/p compared with wild-type mice. C, HPLC analysis of 5-HT levels in the peripheral tissues of wild-type (n = 4) and Lmx1bf/f/p (n = 4) mice. The levels of 5-HT were indistinguishable in the liver and intestine between Lmx1bf/f/p and wild-type mice. Wet weight, Wet tissue weight; WT, wild type.
Normal expression of 5-HT in peripheral tissues of Lmx1bf/f/p mice
We also examined whether 5-HT expression was affected in the periphery, internal organs, and the dorsal root ganglia (DRGs) of Lmx1bf/f/p mice. In the small intestines, the plantar skin (glabrous skin of the hindpaw), the adrenal glands, the sciatic nerve, and DRGs, the 5-HT staining pattern in Lmx1bf/f/p mice was indistinguishable from that in wild-type mice (data not shown). HPLC analysis showed that the levels of 5-HT in liver and intestine of Lmx1bf/f/p mice were not significantly different from those in wild-type mice, demonstrating that only 5-HT levels in the CNS were affected (Fig. 7C).
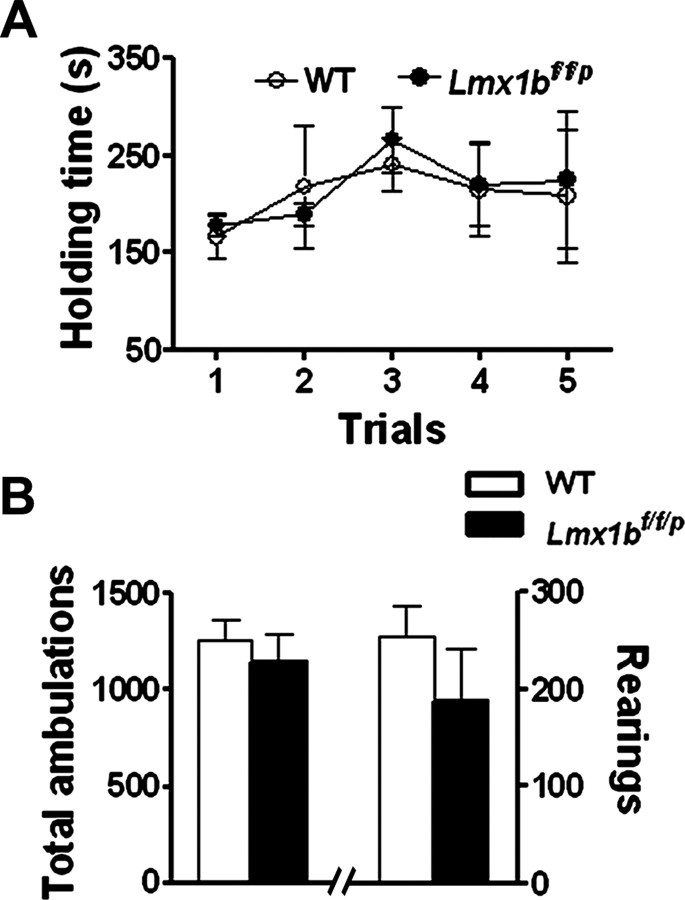
Normal locomotor activity in Lmx1bf/f/p mice
To examine motor function of Lmx1bf/f/p mice, we compared the performance of Lmx1bf/f/p and wild-type littermates on the accelerating rotarod test, which allows for the evaluation of coordinated movement and balance. In five different trials, no significant differences between wild-type and Lmx1bf/f/p mice were observed in this test (Fig. 8A). We also compared general activity levels of the mice by assessing performance on a 1 h locomotor activity/open-field test. Activity levels of wild-type and Lmx1bf/f/p mice were comparable in terms of total ambulations (whole-body movements) and the number of rearings (Fig. 8B). These data demonstrate that the locomotor activity of Lmx1bf/f/p mice is normal.
Figure 8.
Rotarod and open-field tests. A, Motor performance was tested by using the accelerating rotarod in five consecutive trials with 15 min interval. Results indicate the time (seconds) that mice remained on the rotating rod before falling. No differences were observed between Lmx1bf/f/p mice and wild-type mice. One-way ANOVA followed by Fisher's post hoc analysis. B, The general activity levels of the Lmx1bf/f/p mice were not different from wild-type mice in total ambulations (whole-body movements) or numbers of rearings quantified over a 1 h period. Two-tailed t test; n = 9–15 per genotype. WT, Wild type.
Discussion
In this study, we used a genetic approach to delete Lmx1b only in Pet1-expressing neurons in mice. We find that Lmx1b is essential for maintenance of differentiated status of central 5-HT neurons. In the absence of Lmx1b, these neurons fail to survive, and subsequently mice lack the entire central 5-HT system.
Surprisingly, these mutant mice survive to adulthood and exhibit normal motor behaviors.
Lmx1b maintains the differentiation and survival of central 5-HT neurons by differentially regulating downstream gene expression
In the hindbrain of Lmx1bf/f/p mice, the initial Lmx1b expression and initial generation of 5-HT neurons were normal. Although the time window for normal expression of Lmx1b is short, it allows us to assess the effect of the later deletion of Lmx1b in the development of 5-HT neurons. This cannot be achieved in conventional Lmx1b mutants because many 5-HT or 5-HT-related genes do not have the chance to be expressed (Ding et al., 2003). In Lmx1bf/f/p mice, the downregulation of Lmx1b after E12.5 is accompanied by a decrease in 5-HT staining and the downregulation or loss of 5-HT neuron-specific markers. Interestingly, non-5-HT neuron-specific markers are also lost, suggesting that the overall differentiation program of 5-HT neurons is blocked by the loss of Lmx1b. These data show that, although 5-HT neurons are generated initially, the program for their further differentiation depends on the persistent presence of Lmx1b. Thus, in Lmx1bf/f/p mice, a transient expression of Lmx1b is necessary but not sufficient for maintaining the serotonergic phenotype. Our data also indicate that a block of the differentiation of 5-HT neurons eventually results in the abnormal loss of central 5-HT neurons, which occurs primarily during postnatal stages. Together with previous studies (Cheng et al., 2003; Ding et al., 2003), we demonstrate that Lmx1b is essential not only for the generation of 5-HT neurons but also for maintaining their differentiation and survival during postnatal development.
Analysis of Lmx1b-null mice showed a loss of Pet1 expression. However, whether this reflects a loss of cells or gene regulation by Lmx1b remained unclear (Cheng et al., 2003; Ding et al., 2003). By conditional deletion of Lmx1b at a later stage of development, we found that expression of Pet1 is remarkably reminiscent of Lmx1b in Lmx1bf/f/p mice with respect to their downregulation compared with the control. The present study thus provides new evidence indicating that, although initiation of Pet1 is independent of Lmx1b (Cheng et al., 2003), its persistent expression is tightly coupled with and dependent on expression of Lmx1b. In this regard, Pet1 differs from other 5-HT-specific markers such as Sert and TPH2, whose expression does not mimic that of Pet1, despite the fact that Pet1 binding sites have been identified in these genes (Hendricks et al., 1999). The fast extinction of Sert and TPH2 expression suggests that these two genes are most sensitive to the level of Lmx1b expression. It is likely that Lmx1b may directly regulate Sert and TPH2, together with other transcription factors such as Pet1. Given that Lmx1b expression is not altered in central 5-HT neurons in Pet1 mutants during development, our data suggest that Lmx1b is required for Pet1 maintenance but does not require Pet1 for its own maintenance during further differentiation processes of 5-HT neurons (until E16.5 at least).
The central serotonergic system is dispensable for embryonic development and survival of mice
Lmx1bf/f/p mice lack all central 5-HT neurons in the raphe system, whereas 5-HT expression in the periphery is normal. Despite the lack of central 5-HT neurons, Lmx1bf/f/p mice survive without apparent developmental abnormalities. These results are surprising given that 5-HT has been implicated in a variety of neuronal developmental processes, including neuronal differentiation and proliferation (Lauder, 1993; Levitt et al., 1997; Azmitia, 2001). In the developing hindbrain of Lmx1bf/f/p mice, some 5-HT neurons, as indicated by 5-HT staining, are transiently present between E11.0 and E16.5. One may argue that residual and transient expression of 5-HT may be sufficient to compensate for the loss of 5-HT cells to certain degree. However, because several 5-HT-specific genes such as Sert, which is required for normal function of 5-HT neurons, are almost completely lost at a very early stage, it is unlikely that the remaining 5-HT neurons contribute significantly to neural development and survival of Lmx1bf/f/p mice. This is in contrast to Pet1 knock-out mice, in which a relatively large number of 5-HT neurons are maintained (Hendricks et al., 2003). The possibility that some subtle defects in the developing nervous tissues of Lmx1bf/f/p mice are present cannot be excluded. Nevertheless, our data indicate that the raphe nuclei 5-HT systems are dispensable for overall embryonic development and survival of animals.
The central serotonergic system is not required for normal locomotor activity
Serotonergic neurons project widely to the spinal cord, including the motor neurons in the ventral horn (Lakke, 1997). Numerous pharmacological studies suggest that 5-HT exerts both facilitatory and inhibitory modulation of locomotor activity (Jacobs and Fornal, 1993, 1997; Schmidt and Jordan, 2000). For example, depletion of central 5-HT by administration of the 5-HT synthesis inhibitor p-chlorophenylalanine indicates that 5-HT is involved in postural control and locomotor function in neonatal rats (Myoga et al., 1995; Pflieger et al., 2002). The evidence also showed that the central pattern generators for locomotion are subject to the influence of descending pathways, including 5-HT (Vinay et al., 2002). In contrast, we found that coordination and balance of motor activity are normal in adult Lmx1bf/f/p mice, indicating that the central 5-HT system is not required for acquisition of locomotor function. Several reasons may be offered to explain a discrepancy in motor behavior between genetic deletion and pharmacological perturbation. One possibility is that depletion of 5-HT by pharmacological treatments may have nonspecific effects that also interfere with other neurotransmitter pathways that are required for locomotor activity. Alternatively, other molecular pathways or neurotransmitter systems may compensate for the lack of 5-HT required for locomotor activity. A recent study showed that ablation of neuropeptide Y-expressing neurons by injection of diphtheria toxin in hypothalamic neurons of adult mice, but not in neonatal mice, caused feeding deficits (Luquet et al., 2005). A network-based compensatory mechanism has been suggested to explain this observation (Luquet et al., 2005). It is thus conceivable that similar mechanisms could be involved in such adaptive changes in the neural circuits, especially during the critical postnatal period of development, and thereby result in a normal motor behavior of adult mutant mice lacking the central 5-HT system. Future studies using temporally controlled deletion of central 5-HT system may settle this issue. Nonetheless, the fact that Lmx1bf/f/p mice have normal locomotor activity provides a unique animal model for conducting a battery of behavioral tests that are designed to examine the role of the central 5-HT system in many physiological and behavioral activities, including depression, aggression, sexual behavior, and pain perception.
Footnotes
This work was supported by National Institutes of Health grants (R.W.G., E.D., K.R., R.J., and Z.F.C.). We thank J. Yin, K. H. Zhang, and C. Xiang for technical support, and T. Jessell and Y. Q. Ding for the Lmx1b antibody. We are grateful for D. Wozniak's help with the open field test.
References
- Azmitia, 2001.Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Chen and Ding, 2006.Chen ZF, Ding YQ. Transcriptional control of the development of central serotonergic neurons. In: Thiel G, editor. Transcription factors in the nervous system. Weinheim, Germany: Wiley-VCH Verlag; 2006. pp. 143–156. [Google Scholar]
- Chen et al., 2001.Chen ZF, Rebelo S, White F, Malmberg AB, Baba H, Lima D, Woolf CJ, Basbaum AI, Anderson DJ. The paired homeodomain protein DRG11 is required for the projection of cutaneous sensory afferent fibers to the dorsal spinal cord. Neuron. 2001;31:59–73. doi: 10.1016/s0896-6273(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Cheng et al., 2003.Cheng L, Chen CL, Luo P, Tan M, Qiu M, Johnson R, Ma Q. Lmx1b, Pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J Neurosci. 2003;23:9961–9967. doi: 10.1523/JNEUROSCI.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven et al., 2004.Craven SE, Lim KC, Ye W, Engel JD, de Sauvage F, Rosenthal A. Gata2 specifies serotonergic neurons downstream of sonic hedgehog. Development. 2004;131:1165–1173. doi: 10.1242/dev.01024. [DOI] [PubMed] [Google Scholar]
- Dahlstroem and Fuxe, 1964.Dahlstroem A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62([Suppl 232]):231–255. [PubMed] [Google Scholar]
- Ding et al., 2003.Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- Frankfurt et al., 1981.Frankfurt M, Lauder JM, Azmitia EC. The immunocytochemical localization of serotonergic neurons in the rat hypothalamus. Neurosci Lett. 1981;24:227–232. doi: 10.1016/0304-3940(81)90161-0. [DOI] [PubMed] [Google Scholar]
- Gaspar et al., 2003.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gingrich et al., 2003.Gingrich JA, Ansorge MS, Merker R, Weisstaub N, Zhou M. New lessons from knockout mice: the role of serotonin during development and its possible contribution to the origins of neuropsychiatric disorders. CNS Spectr. 2003;8:572–577. doi: 10.1017/s1092852900018848. [DOI] [PubMed] [Google Scholar]
- Goridis and Rohrer, 2002.Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci. 2002;3:531–541. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- Hendricks et al., 1999.Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks et al., 2003.Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Ishimura et al., 1988.Ishimura K, Takeuchi Y, Fujiwara K, Tominaga M, Yoshioka H, Sawada T. Quantitative analysis of the distribution of serotonin-immunoreactive cell bodies in the mouse brain. Neurosci Lett. 1988;91:265–270. doi: 10.1016/0304-3940(88)90691-x. [DOI] [PubMed] [Google Scholar]
- Jacobs and Fornal, 1993.Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosci. 1993;16:346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- Jacobs and Fornal, 1997.Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Kato et al., 2000.Kato A, Kammen-Jolly K, Fischer-Colbie R, Humpel C, Schrott-Fischer A, Marksteiner J. Co-distribution patterns of chromogranin B-like immunoreactivity with chromogranin A and secretoneurin within the human brainstem. Brain Res. 2000;852:444–452. doi: 10.1016/s0006-8993(99)02229-5. [DOI] [PubMed] [Google Scholar]
- Koed and Linnet, 2000.Koed K, Linnet K. Opposing changes in serotonin and norepinephrine transporter mRNA levels after serotonin depletion. Eur Neuropsychopharmacol. 2000;10:501–509. doi: 10.1016/s0924-977x(00)00121-8. [DOI] [PubMed] [Google Scholar]
- Lakke, 1997.Lakke EA. The projections to the spinal cord of the rat during development: a timetable of descent. Adv Anat Embryol Cell Biol. 1997;135(I–XIV):1–143. doi: 10.1007/978-3-642-60601-4. [DOI] [PubMed] [Google Scholar]
- Lauder, 1993.Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Levitt et al., 1997.Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- Luquet et al., 2005.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Malmberg et al., 2003.Malmberg AB, Gilbert H, McCabe RT, Basbaum AI. Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T. Pain. 2003;101:109–116. doi: 10.1016/s0304-3959(02)00303-2. [DOI] [PubMed] [Google Scholar]
- Myoga et al., 1995.Myoga H, Nonaka S, Matsuyama K, Mori S. Postnatal development of locomotor movements in normal and para-chlorophenylalanine-treated newborn rats. Neurosci Res. 1995;21:211–221. doi: 10.1016/0168-0102(94)00857-c. [DOI] [PubMed] [Google Scholar]
- Nakamoto et al., 2000.Nakamoto H, Soeda Y, Takami S, Minami M, Satoh M. Localization of calcitonin receptor mRNA in the mouse brain: coexistence with serotonin transporter mRNA. Brain Res Mol Brain Res. 2000;76:93–102. doi: 10.1016/s0169-328x(99)00335-6. [DOI] [PubMed] [Google Scholar]
- Pattyn et al., 2004.Pattyn A, Simplicio N, van Doorninck JH, Goridis C, Guillemot F, Brunet JF. Ascl1/Mash1 is required for the development of central serotonergic neurons. Nat Neurosci. 2004;7:589–595. doi: 10.1038/nn1247. [DOI] [PubMed] [Google Scholar]
- Pflieger et al., 2002.Pflieger JF, Clarac F, Vinay L. Postural modifications and neuronal excitability changes induced by a short-term serotonin depletion during neonatal development in the rat. J Neurosci. 2002;22:5108–5117. doi: 10.1523/JNEUROSCI.22-12-05108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner and Luine, 1986.Renner K, Luine V. Analysis of temporal and dose-dependent effects of estrogen on monoamines in brain nuclei. Brain Res. 1986;366:64–71. doi: 10.1016/0006-8993(86)91281-3. [DOI] [PubMed] [Google Scholar]
- Romaniuk et al., 1989.Romaniuk A, Strzelczuk M, Wieczorek M. Serotonin depletion with P-chlorophenylalanine in the cat: effects on carbachol-induced defensive behavior and regional brain amine content. Acta Neurobiol Exp (Wars) 1989;49:130–140. [PubMed] [Google Scholar]
- Scearce-Levie et al., 1999.Scearce-Levie K, Chen JP, Gardner E, Hen R. 5-HT receptor knockout mice: pharmacological tools or models of psychiatric disorders. Ann NY Acad Sci. 1999;868:701–715. doi: 10.1111/j.1749-6632.1999.tb11350.x. [DOI] [PubMed] [Google Scholar]
- Schmidt and Jordan, 2000.Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Scott et al., 2005.Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorninck et al., 1999.van Doorninck JH, van Der Wees J, Karis A, Goedknegt E, Engel JD, Coesmans M, Rutteman M, Grosveld F, De Zeeuw CI. GATA-3 is involved in the development of serotonergic neurons in the caudal raphe nuclei. J Neurosci. 1999;19:RC12. doi: 10.1523/JNEUROSCI.19-12-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay et al., 2002.Vinay L, Brocard F, Clarac F, Norreel JC, Pearlstein E, Pflieger JF. Development of posture and locomotion: an interplay of endogenously generated activities and neurotrophic actions by descending pathways. Brain Res Brain Res Rev. 2002;40:118–129. doi: 10.1016/s0165-0173(02)00195-9. [DOI] [PubMed] [Google Scholar]
- Wang et al., 1998.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Weissmann et al., 1987.Weissmann D, Belin MF, Aguera M, Meunier C, Maitre M, Cash CD, Ehret M, Mandel P, Pujol JF. Immunohistochemistry of tryptophan hydroxylase in the rat brain. Neuroscience. 1987;23:291–304. doi: 10.1016/0306-4522(87)90290-9. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia et al., 1996.Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Wozniak et al., 2004.Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, Young C, Olney JW, Muglia LJ. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2004.Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]