Abstract
α-Synuclein (α-syn), a protein implicated in Parkinson's disease pathogenesis, is a presynaptic protein suggested to regulate transmitter release. We explored how α-syn overexpression in PC12 and chromaffin cells, which exhibit low endogenous α-syn levels relative to neurons, affects catecholamine release. Overexpression of wild-type or A30P mutant α-syn in PC12 cell lines inhibited evoked catecholamine release without altering calcium threshold or cooperativity of release. Electron micrographs revealed that vesicular pools were not reduced but that, on the contrary, a marked accumulation of morphologically “docked” vesicles was apparent in the α-syn-overexpressing lines. We used amperometric recordings from chromaffin cells derived from mice that overexpress A30P or wild-type (WT) α-syn, as well as chromaffin cells from control and α-syn null mice, to determine whether the filling of vesicles with the transmitter was altered. The quantal size and shape characteristics of amperometric events were identical for all mouse lines, suggesting that overexpression of WT or mutant α-syn did not affect vesicular transmitter accumulation or the kinetics of vesicle fusion. The frequency and number of exocytotic events per stimulus, however, was lower for both WT and A30P α-syn-overexpressing cells. The α-syn-overexpressing cells exhibited reduced depression of evoked release in response to repeated stimuli, consistent with a smaller population of readily releasable vesicles. We conclude that α-syn overexpression inhibits a vesicle “priming” step, after secretory vesicle trafficking to “docking” sites but before calcium-dependent vesicle membrane fusion.
Keywords: amperometry, catecholamine, chromaffin, exocytosis, Parkinson's disease, secretory
Introduction
α-Synuclein (α-syn) is a 14 kDa protein widely expressed in central neurons that display synaptic plasticity. The protein is initially observed in neuronal cell bodies and later in presynaptic neuronal terminals (Maroteaux and Scheller, 1991; Jakes et al., 1994; Iwai et al., 1995; Hsu et al., 1998; Bayer et al., 1999), in which its distribution changes in response to neuronal stimulation (Fortin et al., 2005).
Three α-syn mutations cause forms of familial Parkinson's disease (Polymeropoulos et al., 1997; Kruger et al., 1998; Zarranz et al., 2004), and overexpression of wild-type (WT) α-syn can lead to early-onset Parkinson's disease (Singleton et al., 2003), suggesting that its physiological function could be relevant to disease pathogenesis. Although α-syn has been suggested to be neuroprotective (Chandra et al., 2005), there are abundant data implying a role in the regulation of presynaptic secretory function. α-Syn-deficient knock-out (null) mice maintain a reduced reserve pool of synaptic vesicles in hippocampal synapses (Cabin et al., 2002), and α-syn knockdown in hippocampal cell cultures reduces reserve vesicle pools (Murphy et al., 2000) and inhibits long-term potentiation (Liu et al., 2004). Studies measuring evoked striatal dopamine (DA) release indicate an enhanced rate of presynaptic recovery in α-syn null mice (Abeliovich et al., 2000; Yavich et al., 2004). Accordingly, mice overexpressing A30P α-syn show decreased DA release in response to prolonged stimulation (Yavich et al., 2004, 2005). Together, results from studies on DA release suggest that α-syn may act presynaptically to inhibit neurosecretion.
If α-syn expression indeed inhibits evoked transmitter release, there are a variety of steps at which this could occur. Previous studies report that α-syn can interfere with catecholamine synthesis and vesicular and plasma membrane uptake (Lee et al., 2001; Lotharius et al., 2002; Perez et al., 2002; Wersinger et al., 2003; Peng et al., 2005), and regulation of each of these processes is known to affect the quantal size of transmitter release (Sulzer and Pothos, 2000). Effects of α-syn on quantal size, however, have not been studied directly. Additional possible inhibitory actions of α-syn on transmitter release include a regulation of the number of secretory vesicles, their trafficking to release sites, altering the calcium dependence of release, or direct effects on vesicle fusion with the plasma membrane.
We studied effects of α-syn on evoked transmitter secretion using adrenal chromaffin cells from α-syn-overexpressing and α-syn null mice and chromaffin cell-derived PC12 cell lines with high expression levels of WT and A30P mutant α-syn. These cells possess advantages for studies of this type because quantal catecholamine secretion can be directly recorded and because they normally express very low levels of endogenous α-syn, so that overexpression of α-syn may more effectively reveal its function. Our data confirm that α-syn overexpression inhibits evoked transmitter secretion. The inhibition of secretory transmission by α-syn does not occur via effects on quantal size, the calcium dependence of transmitter release, or the kinetics of quantal exocytosis but apparently via interfering with a step after vesicle docking and before calcium-dependent fusion.
Materials and Methods
PC12 cell lines.
PC12 cells were grown on rat tail collagen-coated dishes in RPMI 1640 media containing 5% fetal bovine serum and 10% heat-inactivated horse serum (complete media) as described previously (Greene and Tischler, 1976). Cell lines overexpressing human WT (line EP1) or mutant α-syn (lines AP2, AP3, and AM9) were generated based on the TET-On system (Clontech, Mountain View, CA). The cloning of WT and mutant α-syn inserts into a pCDNA3 vector (Invitrogen, Carlsbad, CA) has been described previously (Stefanis et al., 2001). To subclone genes into phosphorylated TET-responsive element (pTRE) vector (Clontech), a SacII site was added to the 5′ end of each DNA fragment by PCR using pCDNA3-based constructs as templates. The PCR products were inserted into the topoisomerase TOPO–TA cloning vector (Invitrogen), the obtained fragments were verified by sequencing, and the TOPO–TA vectors were restriction digested with SacII/XbaI. The resulting inserts were subcloned into pTRE vectors. PC12 cells were then cotransfected with a pTRE vector encoding either WT or A30P α-syn and a pTK–hygromycin vector encoding hygromycin resistance, which allowed to select stable transfectants. Two control (CNT) cell lines were generated: TET-On cells transfected with empty pTRE vector and cells expressing enhanced green fluorescent protein (EGFP) from pBI–EGFP vector (Clontech). Clones were selected after hygromycin treatment (400 μg/ml; Roche, Nutley, NJ), and monoclonal and polyclonal cell lines were generated. Screening of clones for α-syn overexpression was performed by Western blotting (mouse monoclonal anti-α-syn-1; BD Transduction Laboratories, Lexington, KY). For this purpose, cells were cultured using TET-free FBS (Clontech), and lysates were collected 24 h after exposure to the medium with or without doxycycline (2 ng/ml). All lines positive for overexpression of α-syn were quite leaky in that there was substantial overexpression of α-syn even in the absence of doxycycline. Therefore, in all experiments presented here, the α-syn-expressing lines were cultured in normal growth medium without doxycycline.
Western blotting of PC12 cell extracts and immunocytochemistry of PC12 cell cultures.
PC12 cells were scraped from 35 mm dishes and washed by centrifugation and resuspension in cold PBS. The cells were resuspended in a buffer containing (25 mm HEPES, 5 mm EDTA, 1 mm EGTA, 5 mm MgCl2, 5 mm DTT, 0.5% Triton X-100, and protease inhibitor mixture, pH 7.3). Protein concentrations were quantified using the Bradford method (Bio-Rad, Hercules, CA). Lysates (50–100 μg of protein) were resolved by electrophoresis on 10 or 13% SDS-polyacrylamide gels. After transfer to nitrocellulose membrane, the blot was cut into strips based on molecular weight, and the strips were probed with monoclonal rat anti-α-syn-1 antibody (1:500; BD Biosciences, Franklin Lakes, NJ) using previously described procedures (Stefanis et al., 1998). In some cases, the blots were stripped and reprobed with anti-extracellular signal-regulated kinase 2 antibodies (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA). For immunocytochemistry, cell cultures were fixed with 4% paraformaldehyde and labeled with α-syn antibody (1:500; Invitrogen–Zymed, Carlsbad, CA) and secondary antibodies conjugated to Alexa 488 (1:500; Invitrogen, Carlsbad, CA).
Dopamine release.
Depolarization-induced DA release and intracellular monoamine levels were measured by HPLC coupled with electrochemical detection as described previously (Stefanis et al., 2001). Briefly, PC12 cells from various lines were plated onto 24-well dishes and exposed to normal incubation medium (2 mm KCl) or stimulated with high potassium (80 mm KCl) for 1 min. The medium was then collected to quantify DA release, and the cells were collected to normalize for protein content. In some instances, cells were stimulated with the calcium ionophore A23187 (1 μm; Sigma, St. Louis, MO) or α-latrotoxin (20 nm; gift from Alexander Petrenko, New York University, New York, NY). Statistical comparison was performed using one-way ANOVA with post hoc Tukey's test (Prism; GraphPad Software, San Diego, CA).
Electron microscopy.
PC12 cells were plated, fixed, and embedded for electron microscopy as described previously (Stefanis et al., 2001). The proximity of each dense-core vesicle (DCV) to the nearest cell membrane was measured in individual electron micrographs by an observer blind to the treatment condition. Between 10 and 11 cell sections were analyzed for each PC12 cell line.
Chromaffin cells, obtained from the adrenal glands of control and WT α-syn-overexpressing mice, were prepared and analyzed as described previously (Chaudhry et al., 1998). Briefly, three control mice and three mice overexpressing WT α-syn (two males and one female in each group) were deeply anesthetized and fixed by transcardiac perfusion with 4% formaldehyde in 0.1 m PBS. Pieces of adrenal medulla were dissected out (∼1 mm3) and treated with 1% OsO4 in 0.1 m NaPi, followed by dehydration in graded ethanol solutions and propylene oxide and embedding in Durcupan astrocyte-conditioned medium. Ultrathin sections (<100 nm) were cut and mounted on nickel grids and lightly contrasted with 10 mg/ml uranyl acetate for 11 min and 3 mg/ml lead citrate for 70 s. The ultrathin sections were viewed in a Philips (Aachen, Germany) CM10 transmission electron microscope, and images were taken at random by an observer blind to the conditions. Images were recorded with a MegaView II CDD camera driven by the image processing software AnalySIS (Mathiisen et al., 2006). Twenty to 30 images per section were stored in a semiautomated counting system, IMGAP, an extension to AnalySIS designed by F.-M. S. Haug (University of Oslo, Oslo, Norway). Region of interest were drawn on the computer monitor by an observer blind to the conditions, and the number of large DCVs were detected by global intensity thresholding after background smoothing. The distance between the large DCVs and the intercellular membrane was measured. Data are displayed for male mice only (two male mice each in both groups), but similar results were also observed for the female mice (data not shown). Statistical comparison was performed using Kolmogorov–Smirnov test.
Western blotting and immunohistochemistry of adrenal glands from α-syn transgenic mice.
Transgenic α-syn mice were generated as described previously (Fortin et al., 2005). Two transgenic lines were used for these experiments: WT human α-syn transgenic line S222 and the A30P line P254. Adult mice (∼1 year old, 30–35 g) were anesthetized with intraperitoneal pentobarbital injections and decapitated, and the adrenal glands were rapidly dissected and homogenized in ice-cold buffer containing (in mm): 4 HEPES, pH 7.4, 32 sucrose, 1 EGTA, and protease inhibitors (complete protease inhibitor cocktail; Roche). Homogenates were centrifuged at 1000 × g for 10 min. Equal amounts of supernatant protein (15 or 20 μg) from transgenic and nontransgenic adrenal gland homogenates were separated in 12.5% denaturing acrylamide gel, transferred to nitrocellulose membrane, and immunoblotted with rat monoclonal anti-human α-syn (1:2000; Alexis Biochemicals, San Diego, CA) or with mouse monoclonal anti-syn-1 (1:1000; BD Transduction Laboratories) antibodies. α-syn bands were detected using horseradish-conjugated secondary antibodies (1:2000; Amersham Biosciences, Piscataway, NJ) and enhanced chemiluminescence (Pierce, Rockford, IL).
For immunohistochemistry, adult mice were anesthetized with intraperitoneal pentobarbital injections and perfused with 4% paraformaldehyde in PBS, and adrenal glands were removed, postfixed overnight, and then equilibrated with 30% sucrose/PBS. Tissues were frozen, and sections were cut on a cryostat at 30 μm. Adrenal gland sections were immunostained with primary antibodies to tyrosine hydroxylase (1:1000; Protos Biotech, New York, NY) and α-syn (1:500; Invitrogen–Zymed) and secondary antibodies conjugated to Alexa 488 and Alexa 594, respectively (1:500; Invitrogen).
Chromaffin cell culture.
Adrenal glands from 3- to 6-month-old mice were dissected in HBSS. The capsule and cortex were removed, and the remaining medullae were minced. The tissue was then digested at 37°C with Ca2+-free collagenase IA solution (0.2%) for 30 min, rinsed three times with HBSS, and triturated gently in a solution containing 1% bovine serum albumin and 0.02% deoxyribonuclease. After centrifugation at 300 × g for 5 min, cell pellets were suspended in medium comprising DMEM enriched with 10% fetal bovine serum and penicillin/streptomycin. Cells were plated onto poly-d-lysine/laminin-coated glass wells in 50 mm dishes.
Amperometric recordings.
Amperometric recordings from chromaffin cells were performed between days 3 and 5 after plating as described previously (Pothos et al., 2002). The recording media contained the following (in mm): 140 NaCl, 2 KCl, 1 NaH2PO4, 25 glucose, 10 HEPES, 2 MgCl2, and 1.2 CaCl2, pH 7.3. Release was evoked by high KCl stimulation (3 s duration, Picospritzer; General Valve, Fairfield, NJ) with the puff pipette at a distance of ∼20 μm from the cell. The stimulation media contained the following (in mm): 102 NaCl, 40 KCl, 25 glucose, 10 HEPES, 1.2 and CaCl2, pH 7.3. Cells were stimulated five times with a recording time of 1 min per stimulus and 2 min intervals between stimuli. Recordings were made with freshly beveled carbon-fiber electrodes (5 μm diameter; Thornel Cystic, Greenville, SC) using an Axopatch 200B amplifier (Molecular Devices, Palo Alto, CA). The output was low-pass filtered at 10 kHz, digitized at 25 kHz [NIDAQ PCI 6052 E (National Instruments, Austin, TX) and Igor Software (WaveMetrics, Lake Oswego, OR)], and recorded traces were digitally filtered (600 Hz binomial) and analyzed using a locally written Igor routine (Mosharov and Sulzer, 2005). Events smaller than 4 pA were discarded, and cells yielding fewer than 50 amperometric events for all stimuli were not included in the analyses. For statistical analyses, ANOVA with post hoc Tukey's test or Kruskal–Wallis test with post hoc Dunn's test were used (Prism; GraphPad Software). In each case, mutant and control chromaffin cells were cultured, plated, and recorded on the same day with the same experimental setup and conditions.
Results
PC12 cell lines overexpressing WT or mutant α-syn exhibited impaired DA release without altered calcium threshold or dependency
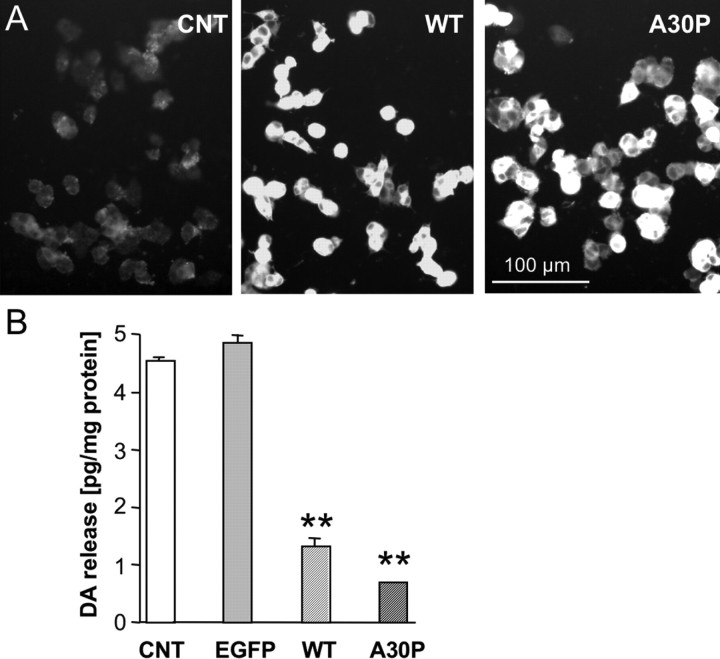
We have shown previously that high levels of α-syn overexpression in PC12 cells can induce morphological abnormalities and non-apoptotic cell death (Stefanis et al., 2001). For our current studies, we therefore generated several PC12 cell lines that overexpress either human WT or A30P mutant α-syn at lower levels. In contrast to the previously characterized cell lines, these cells did not display reduced total DA content or impaired health of the cultures because cell growth and overall cell morphology appeared normal (Fig. 1A) (Mosharov et al., 2006). Overexpression in PC12 cells of WT and A30P α-syn decreased overall stimulation-dependent catecholamine release in response to high potassium (Fig. 1B). A possible explanation of the effect of α-syn on secretion could be that it blocks stimulation-evoked Ca2+ entry. This, however, was not the cause of release inhibition, because DA secretion in WT α-syn-overexpressing cells was also depressed in response to stimulation by the Ca2+ ionophore A32187 or α-latrotoxin treatments (by 54 and 50%, respectively), both of which cause massive calcium influx independently of voltage-gated calcium channel activation (Bittner et al., 1998).
Figure 1.
α-syn-mediated reduction of stimulated dopamine release in PC12 cell lines. Clonal PC12 cell lines were generated expressing empty vector, EGFP, WT, or A30P mutant (A30P) α-syn. A, Examples of PC12 cell cultures from control, WT, and A30P-expressing cell lines immunolabeled for α-syn. B, Stimulated DA release in control and α-syn-overexpressing PC12 cell lines. Cell cultures were stimulated for 1 min with high K+ medium (80 mm), and the superfusate was analyzed by HPLC. Evoked DA release was reduced in the α-syn-overexpressing lines (n = 6 cultures for each line; means ± SEM; **p < 0.001, ANOVA with Tukey's post hoc test).
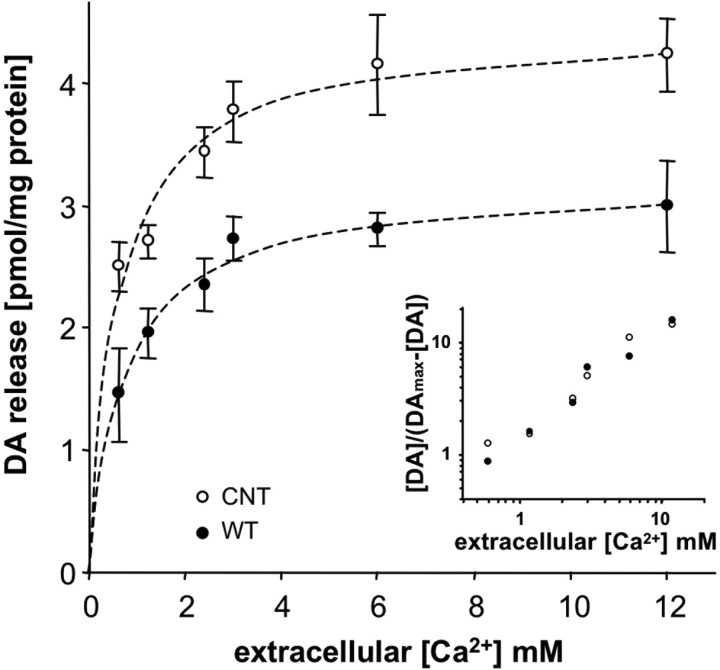
An alternate possibility is that α-syn could affect the Ca2+ dependence of neurosecretion. To test this hypothesis, we compared high potassium (80 mm) evoked DA release over a range of extracellular Ca2+ concentrations (Fig. 2). α-syn-overexpressing cells uniformly released less DA than control cells at all Ca2+ concentrations tested with an identical Ca2+ threshold and Ca2+ dependence of release (Fig. 2, inset). Thus, Ca2+ sensitivity and cooperativity of evoked transmitter release were not altered by α-syn overexpression.
Figure 2.
Calcium sensitivity of DA release from PC12 cell lines. Control and WT α-syn-overexpressing cell cultures were stimulated with high-potassium solution in the presence of varying extracellular Ca2+ concentrations. DA release was significantly reduced from the α-syn-overexpressing cell line at all Ca2+ concentrations tested (n = 6–7, 3–4 dishes from 2 independent experiments; p < 0.001, ANOVA with Tukey's post hoc test). The inset shows a double logarithmic plot of normalized DA release and Ca2+ concentration, indicating that there was no difference in Ca2+ sensitivity (threshold) or cooperativity (slope) of release between the cell lines (Sorensen et al., 2002).
α-syn overexpression enhanced the number of “docked” vesicles in PC12 cells
We next examined whether the number of “docked” vesicles potentially available for secretory fusion was reduced in α-syn-overexpressing cells. Electron micrographs of the different PC12 cell lines were analyzed for alterations in the number or distribution of DCVs. Representative electron micrographs are shown in Figure 3. The DCVs from both control and α-syn-overexpressing cells exhibited the typical morphology for theses cells, with a halo between the dense core and the vesicular membrane (Sombers et al., 2004). Surprisingly, cells overexpressing α-syn had more DCVs “morphologically docked,” i.e., close to the plasma membrane compared with controls. In controls, 34% of the vesicles (56 of 164) were located within 200 nm (∼2 DCV diameters) from the plasmalemmal membrane, whereas in WT and A30P α-syn-overexpressing cells, 75% (207 of 275) and 87% (122 of 141), respectively, were within this distance, a highly significant difference from controls (Fig. 3). The finding that more morphologically docked vesicles were present in the α-syn-overexpressing cell lines than in controls suggests that the α-syn-mediated depression of release is attributable to neither a loss of DCVs nor impaired trafficking of DCVs to the plasma membrane.
Figure 3.
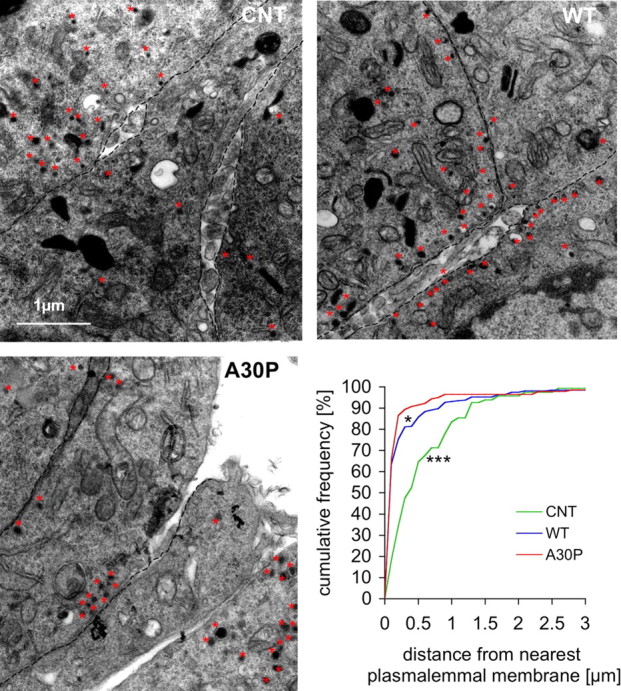
DCV distribution in PC12 cell lines. Electron micrographs of PC12 cells are shown from CNT, WT, and A30P α-syn-overexpressing lines with examples of DCVs labeled by red stars. A greater fraction of the DCVs were located in a docked position near plasmalemmal membranes (marked by dashed lines) in both overexpressing cells lines (cumulative frequency of controls was different from WT and A30P; *p < 0.05, ***p < 0.01, Kolmogorov–Smirnov test; n = 10–11 cell sections).
α-syn overexpression decreased the number of single vesicle release events
Another mechanism that could decrease evoked neurosecretion in α-syn-overexpressing cells, and one consistent with studies on effects of the protein on catecholamine synthesis, uptake, and vesicular accumulation (Lee et al., 2001; Lotharius et al., 2002; Perez et al., 2002; Wersinger et al., 2003; Peng et al., 2005), is that amount of transmitter released per vesicle fusion event (quantal size) might be decreased. To examine this possibility, we used amperometric recordings of quantal catecholamine release from primary cultures of adrenal chromaffin cells derived from α-syn-overexpressing mice, their nontransgenic littermates, and α-syn null mice. Although we and others routinely record quantal events from PC12 cells, the features of quantal secretion from this cancer cell line are unstable, depending in part on its stage in cell division and selection over generations in culture. In contrast, primary cell adrenal chromaffin cultures provide far more consistent results (Colliver et al., 2001).
For these experiments, we chose two previously characterized mouse lines (Fortin et al., 2005) in which Western immunoblots of the adrenal glands revealed ∼15-fold increase in the levels of human WT or A30P α-syn overexpression (Fig. 4A). In addition, we used a previously characterized line of α-syn null mice (Dauer et al., 2002). Immunohistochemistry performed on sections of adrenal medulla derived from nontransgenic control mice revealed strong α-syn labeling in the connective tissue that surrounded groups of chromaffin cells and in endothelial cells lining blood vessels, whereas the chromaffin cells themselves showed relatively faint staining. In contrast, both WT and A30P α-syn overexpression resulted in substantial cytoplasmic and nuclear immunostaining of chromaffin cells, as confirmed by colocalization with tyrosine hydroxylase immunolabel (Fig. 4B). Interestingly, although the Western blots demonstrated somewhat higher levels of expression for the WT α-syn (19-fold vs 12-fold for A30P α-syn), there was an apparently higher level of diffuse cytosolic and nuclear immunofluorescence associated with A30P mutant α-syn than with WT α-syn, which may result from the reduced ability of the A30P mutant protein to bind membranes (Jensen et al., 1998; Perrin et al., 2000; Cole et al., 2002; Jo et al., 2002; Outeiro and Lindquist, 2003; Fortin et al., 2004; Kubo et al., 2005). In summary, these results indicate that the endogenous α-syn level in chromaffin cells is low in nontransgenic controls, whereas robust expression is evident in both lines of α-syn-overexpressing mice, thus providing a means to test the effect of α-syn overexpression.
Figure 4.
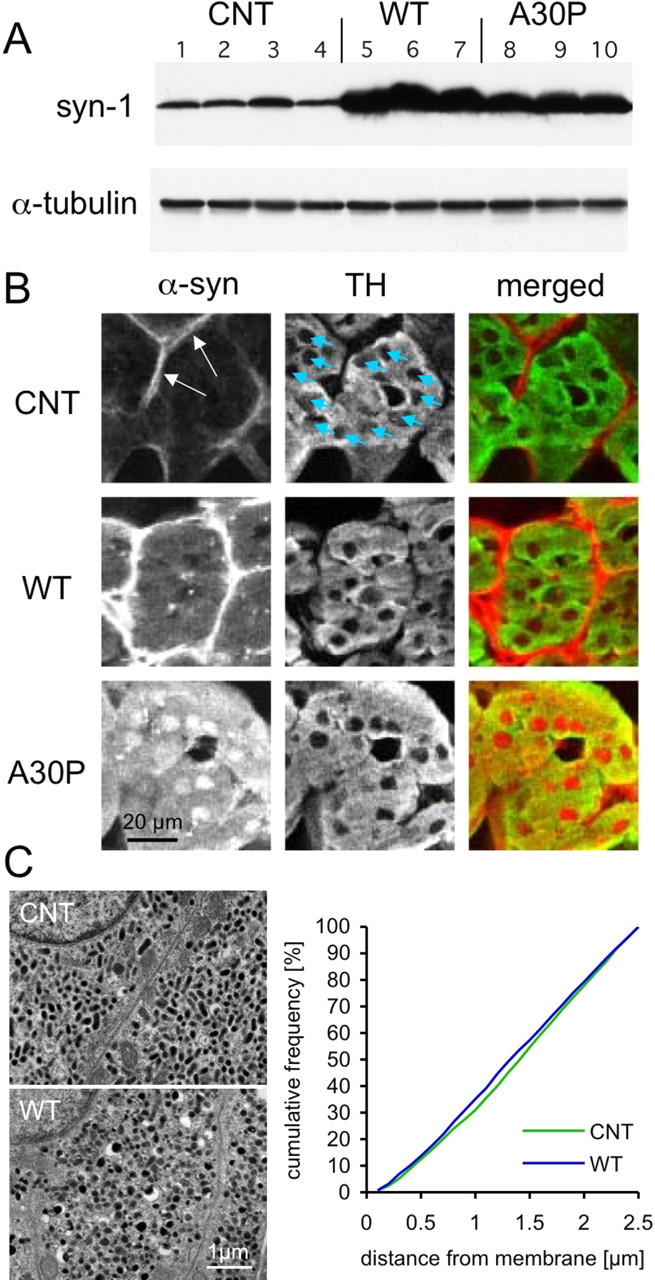
Chromaffin cells from WT and A30P α-syn transgenic mice. A, Western immunoblots of adrenal gland tissue indicate a 19-fold and 12-fold increase of α-syn level in WT and A30P transgenic mice, respectively, compared with nontransgenic littermates. Each lane corresponds to protein derived from one animal (4 nontransgenic, 3 WT, and 3 A30P α-syn transgenic mice). B, Immunohistochemistry for α-syn of adrenal medulla sections provided relatively strong labeling in the connective tissue surrounding groups of chromaffin cells (arrows) and in endothelial cells lining blood vessels, but the chromaffin cells, stained for tyrosine- hydroxylase (TH; blue arrows point to nuclei), showed very faint labeling. In contrast, both WT and A30P α-syn overexpression resulted in substantial cytoplasmic and nuclear immunostaining of chromaffin cells. C, Electron micrographs of chromaffin cells derived from CNT and WT mouse adrenal glands are shown. The cytosol of both CNT and WT is almost completely filled with DCVs. The cumulative frequency plot indicates the location of large DCVs with respect to the plasma membrane. For each condition, sections of adrenal glands from two animals were analyzed (CNT, n = 1871, 1670 vesicles; WT, n = 2574, 1420 vesicles). Because the cytosol of mature chromaffin cells are filled with large DCVs, the frequency distribution is nearly linear with no difference detected in the morphological docking or total number of DCVs between the genotypes (Kolmogorov–Smirnov test, p > 0.5).
We examined control and WT α-syn-overexpressing chromaffin cells by electron microscopy as above for PC12 cells and found no change in the number and distribution of DCVs in the overexpressors (Fig. 4C). Because the cytosol of mature chromaffin cells is nearly completely filled with DCVs [compare with cumulative frequency distribution of PC12 DCVs (Fig. 3)], it is unlikely that an alteration in the distribution of vesicles would be detected unless the overall number of DCVs was affected. The lack of effect of α-syn overexpression on the number of DCVs in close proximity to the plasma membrane provides additional evidence that α-syn-induced inhibition of exocytosis is not attributable to reduced docking.
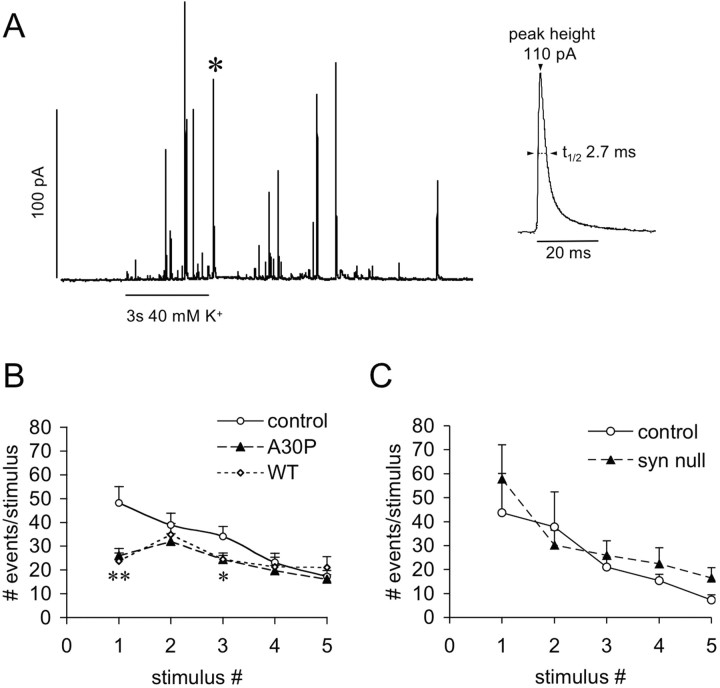
Amperometric recordings of quantal catecholamine release were performed after 3–5 d in culture. Cells were stimulated with 3 s applications of 40 mm KCl five times with 2 min intervals between the stimuli. After each stimulus, 1 min of release activity was recorded (Fig. 5A). Analysis of the shape characteristics of individual release events from chromaffin cells from both transgenic mouse lines (WT and A30P), α-syn null mice, and their nontransgenic littermates (TG control, null control) revealed no difference in the quantal size (number of molecules), height (Imax), and duration [half time (t1/2)] (Table 1). Likewise, there was no significant effect of α-syn overexpression on the presence and the shape of “feet” or the rising phase of the spikes (data not shown), which are thought to be affected by changes in the initial fusion pore formation and expansion, correspondingly (Mosharov and Sulzer, 2005). Thus, α-syn overexpression does not seem to affect release characteristics of individual fusion events.
Figure 5.
Amperometric recordings of quantal catecholamine release from cultured chromaffin cells derived from control, α-syn null, WT, and A30P α-syn-overexpressing mice. A, A representative recording trace from a control chromaffin cell. Individual events (A, inset, magnified event marked in trace by asterisk) were analyzed for peak height (Imax), duration (t1/2), and number of molecules (see Table 1). B, Chromaffin cells were stimulated with 40 mm KCl five times with a 2 min period between stimuli. Quantal release was recorded for a period of 1 min after the 3 s high KCl stimulus. The number of events was reduced twofold in cells from WT and A30P α-syn-overexpressing mice during the first stimulus (**p < 0.01), whereas for the consecutive stimuli, the number of events was close to controls (significant difference only for the third stimulus; *p < 0.05, paired ANOVA, followed by Tukey's post hoc test). C, The number of events for control and α-syn null mice at all stimuli was not different.
Table 1.
Quantal release events of chromaffin cells derived from WT, A30P, null, and normal control mice were analyzed for number of events (total for five consecutive stimuli), quantal size (number of molecules), Imax, and t1/2
| Number of events (5 stimuli) | Number of molecules | Imax (pA) | t1/2 (ms) | |
|---|---|---|---|---|
| TG controls (n = 24) | 161 ± 16 | 850,000 ± 71,000 | 30.4 ± 3.0 | 9.2 ± 0.6 |
| WT (n = 12) | 117 ± 14* | 1,000,000 ± 145,000 | 37.8 ± 2.4 | 7.4 ± 1.3 |
| A30P (n = 20) | 118 ± 7* | 925,000 ± 90,000 | 33.2 ± 3.8 | 8.7 ± 0.6 |
| Null controls (n = 7) | 125 ± 35 | 859,000 ± 215,000 | 26.9 ± 4.7 | 8.2 ± 0.9 |
| α-syn null (n = 9) | 153 ± 28 | 970,000 ± 98,000 | 23.9 ± 3.1 | 9.4 ± 0.7 |
The number of quantal events obtained from WT and A30P cells was significantly lower than their corresponding controls (*p < 0.05, ANOVA of the means with post hoc Tukey's test), with no difference in remaining parameters. There was no difference in any parameter between α -syn null mice and their corresponding wild-type littermates (null controls). In all experiments, the mutant and control chromaffin cells were cultured, plated, and recorded on the same day. TG, Transgenic.
However, cells from both transgenic mouse lines released a significantly lower total number of quanta in response to high K+ stimulation compared with cells from control mice (Fig. 5B) (Tukey's test, p < 0.01). For the first stimulus, the number of events was approximately twofold lower in the transgenic lines, whereas the number of events for consecutive stimuli was similar to controls (significant difference only for stimuli 1 and 3; Tukey's test, p < 0.05). No difference was found in the number of events released per stimulus between the α-syn null mice and nontransgenic control mice (Fig. 5C). These data are consistent with an inhibition of neurosecretion in α-syn-overexpressing cells and support the hypothesis that overexpression of either WT or mutant α-syn causes a reduction of the releasable pool of vesicles, resulting in markedly decreased release for the first stimulus, whereas release for consecutive stimuli is less affected.
Discussion
The effects of the presynaptic protein α-syn on secretory transmission have remained poorly understood. Our previous work indicated that normal α-syn expression slows the rate of synaptic recovery in DA terminals (Abeliovich et al., 2000). We now find that stimulation-dependent vesicular catecholamine release was impaired in α-syn-overexpressing PC12 cell lines and in primary cultures of chromaffin cells derived from mice overexpressing α-syn. Although it is possible that α-syn has a different role in DCV-containing secretory cells than in small synaptic vesicle-containing neurons, it is notable that the results presented here are in close agreement with the conclusions derived from studies on neuronal striatal DA release in α-syn null and α-syn-overexpressing mice, in that all suggest an inhibitory role of α-syn in evoked neurosecretion (Abeliovich et al., 2000; Yavich et al., 2004).
The cellular systems we examined in this study offer an advantage in that quantal catecholamine release can be measured directly. Previous studies suggested that quantal catecholamine release might be inhibited by impaired transmitter synthesis, impaired transmitter accumulation in vesicles, a reduction in the number of release-competent vesicles, or a combination of these mechanisms. Amperometric recordings of quantal catecholamine release can be used to distinguish between these possibilities, and we found that overexpression of either WT and A30P α-syn in chromaffin cells affected release solely by reducing the number of quanta released per stimulus compared with α-syn null and control cells. Thus, although data from several studies (Lee et al., 2001; Lotharius et al., 2002; Perez et al., 2002; Wersinger et al., 2003; Peng et al., 2005) suggest that α-syn may affect DA synthesis, vesicular transporter activity, or plasma membrane reuptake, these potential effects of α-syn overexpression did not result in an altered transmitter accumulation into releasable vesicles. This observation, however, does not directly contradict the previous findings, and it may be that compensatory physiological responses allow vesicles to accumulate transmitter normally even with altered DA synthesis or uptake: for example, whereas α-syn might disrupt vesicular pH gradients, quantal size might be maintained by enhanced cytosolic catecholamine levels (Mosharov et al., 2006).
A plausible explanation for the reduction of evoked catecholamine release induced by α-syn overexpression would be a reduced size of vesicular pools caused by impaired vesicle synthesis or transport to release sites. However, we found no alterations in the distribution of DCVs in chromaffin cells, whereas in PC12 cells overexpressing α-syn, there was an increase in the number of morphologically docked DCVs, as defined by proximity to the plasma membrane. This enhanced presence of docked DCVs is suggestive of an inhibition of exocytosis after docking and reminiscent of increased morphologically docked DCVs after exposure to botulinum toxin A, a compound that inhibits exocytosis by cleaving SNARE (soluble N-ethylmaleimide-sensitive factor attached protein receptor) proteins (Neale et al., 1999). Together, the results indicate that inhibition of catecholamine release by α-syn occurs after vesicle synthesis, trafficking to the plasma membrane, vesicular transmitter accumulation, and docking. That is, α-syn-induced inhibition occurs during a late step in exocytosis.
Such a late step could be attributable to an interference with the coupling of the fusion machinery to calcium entry. If this were the case, different levels of inhibition should exist for transmitter release from control and α-syn-overexpressing cells in response to different levels of extracellular Ca2+. In contrast, we found that the Ca2+ threshold and Ca2+ dependence of release in control and α-syn-overexpressing cells were identical. It should be further noted that kinetic parameters of quantal transmitter secretion were unchanged, indicating that vesicle fusion with the plasma membrane and degranulation and diffusion of the transmitter remained normal in mutant cells (Mosharov and Sulzer, 2005). We can further exclude that α-syn overexpression impaired endosomal vesicle recycling, as has been suggested for small synaptic vesicles from a study in a dopaminergic cell line (Lotharius et al., 2002), because this release inhibition occurred for chromaffin cell DCVs, which do not undergo endosomal recycling (Pothos et al., 2002).
Thus, an inhibition by α-syn at a step after vesicle docking and before calcium-dependent fusion, a step often referred to as “priming” (Klenchin and Martin, 2000; Nagy et al., 2004), is most consistent with our results. In addition to the points above that exclude effects at other stages, three observations support an effect on priming. First, an inhibition of priming by α-syn should elicit a similar fraction of release inhibition at every Ca2+ concentration, as observed. Second, there was an accumulation of morphologically docked DCVs in PC12 cells, suggesting a problem with priming rather than docking. Third, the number of quanta released in the α-syn-overexpressing chromaffin cells was reduced by 50% for the first stimulus, and release in response to subsequent stimuli was near control levels. This is generally considered to provide evidence for a smaller initial pool of releasable, primed vesicles, because less refilling of the pool is required (Trommershauser et al., 2003).
The extent of presynaptic inhibition was nearly identical for both WT and A30P α-syn overexpression, although A30P mutant α-syn exhibits greatly impaired phospholipid binding (Jensen et al., 1998; Perrin et al., 2000; Cole et al., 2002; Jo et al., 2002; Outeiro and Lindquist, 2003; Fortin et al., 2004; Kubo et al., 2005). This suggests that either the interaction of α-syn with membranes does not play a role in the inhibition of vesicle priming by the protein or that A30P α-syn expression levels were high enough to compensate for impaired A30P membrane-binding capacity.
In summary, our results use multiple independent techniques that together indicate that α-syn overexpression inhibits evoked transmitter release and that it does so at a late step after docking and before fusion. Although these experiments demonstrate the effects of α-syn on release of transmitter from DCVs, the findings are consistent with results on evoked DA release from small synaptic vesicles of striatonigral terminals in α-syn null mice (Abeliovich et al., 2000; Yavich et al., 2004). The present findings in conjunction with the recently identified dispersion of α-syn away from terminals with high stimulation (Fortin et al., 2005) suggests the intriguing possibility that α-syn might act as a high-pass filter for synaptic transmission, in that it would inhibit transmitter release only under low firing rates but not during burst firing that occurs in association with “incentive salience” learning (Schultz et al., 1997).
Footnotes
This work was supported by the Picower Foundation and the Parkinson's Disease Foundation (K.E.L., E.M., D.S.), National Institute of Neurological Disorders and Stroke (R.H.E., M.D.T., D.S.), National Institute on Drug Abuse (R.H.E., D.S.), and the Norwegian Research Council (A.Z.Q., F.A.C.). We thank M. Schoenebeck for performing electron microscopy and F.-M. S. Haug for technical help, A. Schmidt and L. Reichardt for pronuclear injections to generate transgenic mice, and A. Hananiya and K. Phillips for excellent technical assistance.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Jakala P, Hartmann T, Egensperger R, Buslei R, Falkai P, Beyreuther K. Neural expression profile of alpha-synuclein in developing human cortex. NeuroReport. 1999;10:2799–2803. doi: 10.1097/00001756-199909090-00019. [DOI] [PubMed] [Google Scholar]
- Bittner MA, Krasnoperov VG, Stuenkel EL, Petrenko AG, Holz RW. A Ca2+-independent receptor for α-latrotoxin, CIRL, mediates effects on secretion via multiple mechanisms. J Neurosci. 1998;18:2914–2922. doi: 10.1523/JNEUROSCI.18-08-02914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J Biol Chem. 2002;277:6344–6352. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- Colliver TL, Hess EJ, Ewing AG. Amperometric analysis of exocytosis at chromaffin cells from genetically distinct mice. J Neurosci Methods. 2001;105:95–103. doi: 10.1016/s0165-0270(00)00359-9. [DOI] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci USA. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of α-synuclein. J Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of α-synuclein. J Neurosci. 2005;25:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which responds to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LJ, Mallory M, Xia Y, Veinbergs I, Hashimoto M, Yoshimoto M, Thal LJ, Saitoh T, Masliah E. Expression patters of synculeins (non-Abeta component of Alzheimer's disease amyloid precursors protein/alpha-synuclein) during murine brain development. J Neurochem. 1998;71:338–344. doi: 10.1046/j.1471-4159.1998.71010338.x. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- Jensen PH, Nielsen MS, Jakes R, Dotti CG, Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson's disease mutation. J Biol Chem. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- Jo E, Fuller N, Rand RP, St. George-Hyslop P, Fraser PE. Defective membrane interactions of familial Parkinson's disease mutant A30P alpha-synuclein. J Mol Biol. 2002;315:799–807. doi: 10.1006/jmbi.2001.5269. [DOI] [PubMed] [Google Scholar]
- Klenchin VA, Martin TF. Priming in exocytosis: attaining fusion-competence after vesicle docking. Biochimie. 2000;82:399–407. doi: 10.1016/s0300-9084(00)00208-x. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kubo S, Nemani VM, Chalkley RJ, Anthony MD, Hattori N, Mizuno Y, Edwards RH, Fortin DL. A combinatorial code for the interaction of alpha-synuclein with membranes. J Biol Chem. 2005;280:31664–31672. doi: 10.1074/jbc.M504894200. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- Liu S, Ninan I, Antonova I, Battaglia F, Trinchese F, Narasanna A, Kolodilov N, Dauer W, Hawkins RD, Arancio O. alpha-Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J. 2004;23:4506–4516. doi: 10.1038/sj.emboj.7600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotharius J, Barg S, Wiekop P, Lundberg C, Raymon HK, Brundin P. Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem. 2002;277:38884–38894. doi: 10.1074/jbc.M205518200. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Scheller RH. The rat brain synucleins; family of proteins transiently associated with neuronal membrane. Brain Res Mol Brain Res. 1991;11:335–343. doi: 10.1016/0169-328x(91)90043-w. [DOI] [PubMed] [Google Scholar]
- Mathiisen TM, Nagelshus EA, Jouleh B, Torp R, Feydennlund DS, Mylonakou M-N, Amiry-Moghaddam M, Covolan L, Utvik JK, Riber B, Gujord KM, Knutset J, Skare O, Laake P, Davanger S, Haug F-MS, Rinvik E, Ottersen OP. Postembedding immunogold cytochemistry of membrane molecules and amino acid transmitters in the central nervous system. In: Zaborszky L, Wouterlood FG, Lanciego JL, editors. Neuroanatomical tract-tracing 3. Molecules, neurons, and systems. New York: Springer Science+Business Media; pp. 72–108. [Google Scholar]
- Mosharov EV, Sulzer D. Analysis of exocytotic events recorded by amperometry. Nat Methods. 2005;2:651–658. doi: 10.1038/nmeth782. [DOI] [PubMed] [Google Scholar]
- Mosharov EV, Staal RG, Bove J, Hananiya A, Markov D, Poulsen N, Larsen KE, Troyer MD, Edwards RH, Przedborski S, Sulzer D. α-synuclein overexpression permeabilizes secretory vesicles and increases cytosolic catecholamine. J Neurosci. 2006;26:9304–9311. doi: 10.1523/JNEUROSCI.0519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Reim K, Matti U, Brose N, Binz T, Rettig J, Neher E, Sorensen JB. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron. 2004;41:417–429. doi: 10.1016/s0896-6273(04)00038-8. [DOI] [PubMed] [Google Scholar]
- Neale EA, Bowers LM, Jia M, Bateman KE, Williamson LC. Botulinum neurotoxin A blocks synaptic vesicle exocytosis but not endocytosis at the nerve terminal. J Cell Biol. 1999;147:1249–1260. doi: 10.1083/jcb.147.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118:3523–3530. doi: 10.1242/jcs.02481. [DOI] [PubMed] [Google Scholar]
- Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for α-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-Synuclein and Parkinson's disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Mosharov E, Liu KP, Setlik W, Haburcak M, Baldini G, Gershon MD, Tamir H, Sulzer D. Stimulation-dependent regulation of the pH, volume and quantal size of bovine and rodent secretory vesicles. J Physiol (Lond) 2002;542:453–476. doi: 10.1113/jphysiol.2002.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Sombers LA, Hanchar HJ, Colliver TL, Wittenberg N, Cans A, Arbault S, Amatore C, Ewing AG. The effects of vesicular volume on secretion through the fusion pore in exocytotic release from PC12 cells. J Neurosci. 2004;24:303–309. doi: 10.1523/JNEUROSCI.1119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen JB, Matti U, Wei SH, Nehring RB, Voets T, Ashery U, Binz T, Neher E, Rettig J. The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis. Proc Natl Acad Sci USA. 2002;99:1627–1632. doi: 10.1073/pnas.251673298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis L, Troy CM, Qi H, Shelanski ML, Greene LA. Caspase-2 (Nedd-2) processing and death of trophic factor-deprived PC12 cells and sympathetic neurons occur independently of caspase-3 (CPP32)-like activity. J Neurosci. 1998;18:9204–9215. doi: 10.1523/JNEUROSCI.18-22-09204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA. Expression of A53T mutant, but not wild type, α-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001;21:9549–9560. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Pothos EN. Presynaptic mechanisms that regulate quantal size. Rev Neurosci. 2000;11:159–212. doi: 10.1515/revneuro.2000.11.2-3.159. [DOI] [PubMed] [Google Scholar]
- Trommershauser J, Schneggenburger R, Zippelius A, Neher E. Heterogeneous presynaptic release probabilities: functional relevance for short-term plasticity. Biophys J. 2003;84:1563–1579. doi: 10.1016/S0006-3495(03)74967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger C, Prou D, Vernier P, Sidhu A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003;17:2151–2153. doi: 10.1096/fj.03-0152fje. [DOI] [PubMed] [Google Scholar]
- Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of α-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L, Oksman M, Tanila H, Kerokoski P, Hiltunen M, van Groen T, Puolivali J, Mannisto PT, Garcia-Horsman A, MacDonald E, Beyreuther K, Hartmann T, Jakala P. Locomotor activity and evoked dopamine release are reduced in mice overexpressing A30P-mutated human alpha-synuclein. Neurobiol Dis. 2005;20:303–313. doi: 10.1016/j.nbd.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]