Abstract
The dorsolateral prefrontal cortex (DLPFC) has been implicated in the ability to perform complex behaviors requiring the implementation of cognitive control. A central supposition of models of prefrontal function is that the DLPFC engages control by selectively modulating the activity of target structures to which it is connected, but no studies in the primate have directly investigated DLPFC output signals. Here, we recorded the activity of DLPFC neurons identified as sending a direct projection to the superior colliculus, a midbrain oculomotor structure, while monkeys performed alternating blocks of trials in which they had to look toward a flashed peripheral stimulus (prosaccades) and trials in which they had to look away from the stimulus in the opposite direction (antisaccades). We report the first direct evidence that the primate DLPFC sends task-selective signals to a target structure. This supports the notion that the DLPFC orchestrates the activity of other brain areas in accordance with task requirements.
Keywords: prefrontal cortex, monkey, corticotectal, superior colliculus, antidromic, antisaccade
Introduction
The prefrontal cortex has been implicated in a large number of cognitive processes collectively referred to as “executive function” or “cognitive control.” Electrophysiological studies in monkeys have revealed that prefrontal neurons carry an assortment of cognitive signals, including highly selective activity related to abstract rules (Wallis et al., 2001), categories (Freedman et al., 2002), number (Nieder et al., 2002), working memory (Goldman-Rakic, 1995), attention (Rainer et al., 1998a,b; Everling et al., 2002), and decision making (Kim and Shadlen, 1999). It is assumed that the prefrontal cortex participates in this vast array of cognitive processes by a process of top-down control in which it acts to coordinate the activity of cortical and subcortical areas to which it is reciprocally connected (Miller and Cohen, 2001). Despite the emphasis of theories of prefrontal cortex function on the functional connectivity of the area, few studies have attempted to systematically investigate the relationship between prefrontal cortex activity and the activity of other brain regions (Chafee and Goldman-Rakic, 2000; Freedman et al., 2003; Wallis and Miller, 2003).
A prominent subcortical target of descending prefrontal projections is the superior colliculus (SC) (Goldman and Nauta, 1976; Leichnetz et al., 1981). The SC has been shown to be critical for the initiation of saccadic eye movements (Wurtz and Goldberg, 1972; Hikosaka and Wurtz, 1983). An eye movement task that has been widely used to investigate top-down control in both humans and primates is the antisaccade task (Hallett, 1978). In this task, a stimulus is presented in the peripheral visual field and subjects are instructed to look away from the stimulus to its mirror location. The successful performance of this task requires the suppression of an automatic saccade toward the stimulus (prosaccade) and the transformation of the stimulus location into the motor command for the antisaccade. The SC is thought to play a critical role in antisaccade performance (Munoz and Everling, 2004). In the monkey SC, activity of fixation neurons is enhanced, whereas buildup, visual, and motor responses of saccade-related neurons are markedly attenuated on antisaccade trials compared with prosaccade trials (Everling et al., 1999).
So far, the activity of prefrontal neurons that project to the SC has only been studied in one prefrontal area, the frontal eye fields (FEFs) (Segraves and Goldberg, 1987; Everling and Munoz, 2000; Sommer and Wurtz, 2000). Corticotectal saccade-related neurons in the FEF show a pattern of discharge similar to saccade-related neurons in the SC for antisaccades, i.e., a reduced activity on antisaccade trials compared with prosaccade trials (Everling and Munoz, 2000).
Another prefrontal area that has been implicated in antisaccade performance by lesion studies in human patients (Pierrot-Deseilligny et al., 1991), functional magnetic resonance imaging (fMRI) studies in normal human subjects (Curtis and D'Esposito, 2003; Ford et al., 2005), and single-neuron recording studies in monkeys (Funahashi et al., 1993; Everling and DeSouza, 2005; Johnston and Everling, 2006) is the dorsolateral prefrontal cortex (DLPFC). Based on this evidence, and the prominent anatomical connection between the two areas, the DLPFC has been suggested as a potential source of top-down modulation of SC neurons during antisaccades (Munoz and Everling, 2004).
Here, we used the system controlling saccadic eye movements as a model to characterize the signals sent by output neurons of the DLPFC to a target structure. We used antidromic activation to identify DLPFC output neurons sending direct projections to the SC and recorded their activity while monkeys performed a task in which they performed blocks of prosaccades and antisaccades. Many neurons responded selectively to the location of the visual stimulus or the direction of the impending saccade. More interestingly, we discovered the presence of neuronal signals selective for antisaccades that could act as a signal to inhibit reflexive prosaccades. This constitutes the first evidence that DLPFC neurons send task-selective signals directly to a subcortical target in nonhuman primates.
Materials and Methods
Two rhesus monkeys (Macaca mulatta) weighing 6.5 and 8 kg were subjects in the present experiment. Recording chambers were stereotaxically implanted over the right lateral DLPFC of both animals based on coordinates reconstructed from MRI images. Details of the surgical procedures have been described previously (DeSouza and Everling, 2004). All experimental methods described were performed in accordance with the guidelines of the Canadian Council on Animal Care policy on the care and use of experimental animals and an ethics protocol approved by the Animal Users Subcommittee of the University of Western Ontario Council on Animal Care.
Behavioral task.
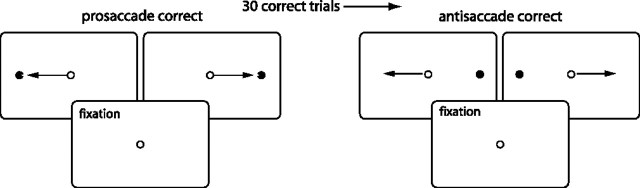
Monkeys performed an experimental task in which they were required to alternate between blocks of prosaccades in which they had to look toward a flashed peripheral stimulus and antisaccade trials that required the suppression of the automatic prosaccade toward the stimulus in favor of a voluntary saccade away from the stimulus to its mirror location (Fig. 1). Each trial began with the presentation of a small fixation point at the center of the display screen. Monkeys were required to fixate this spot within a 0.5 × 0.5° window for a variable period of between 1100 and 1400 ms. After this, a 0.15° visual stimulus was presented pseudorandomly with equal probability either 8° to the left or right of fixation. The central fixation remained illuminated throughout the trial (overlap condition) to increase the animal's performance on antisaccade trials (Everling et al., 1999). To obtain a juice reward, the animals were required to generate a saccade either toward the stimulus (prosaccade trials) or to a location diametrically opposite to the stimulus (antisaccade trials) within 500 ms. Saccade endpoints were required to fall within a 5 × 5° window. Once 30 correct trials had been performed, the task rule switched without any explicit signal to the animals. We used this task in previous studies (Everling and DeSouza, 2005). Eye movements were recorded at 1000 Hz using a magnetic search coil technique (David Northmore Institute, Newark, DE). Both monkeys were able to perform the task with an accuracy of >80%. Saccadic reaction times (SRTs) for prosaccades had a mean ± SD value of 183.7 ± 24.8 ms, and the mean ± SD reaction time for antisaccades was 222.5 ± 39.9 ms.
Figure 1.
Experimental task. Each trial began with the presentation of a fixation point at the center of the screen, which the monkey was required to fixate. A visual stimulus then appeared to the right or left of fixation. Monkeys were required to make either a prosaccade or antisaccade (arrows) depending on the task rule in effect. After 30 correct responses, the task rule switched without explicit signal to the animals.
Recording technique.
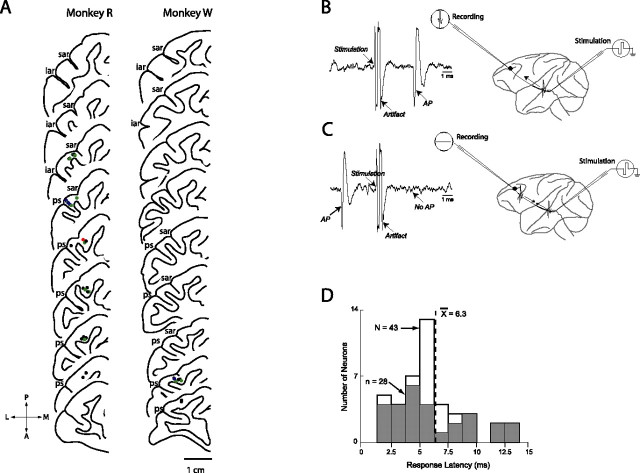
We recorded single corticotectal neurons from the right lateral prefrontal cortex. Arrays of two to six electrodes were driven individually within the DLPFC recording chambers using a computer-controlled multi-microelectrode drive (NAN; Plexon, Dallas TX). Neurons were identified as sending a direct projection to the SC using antidromic activation. Arrays of three to four electrodes were chronically implanted in the intermediate layers of SC ipsilateral to the DLPFC recording site. The intermediate layers were identified using single-neuron recordings and microstimulation (Everling and Munoz, 2000). One electrode was implanted at an eccentricity of <2° (rostral electrode), and the others were implanted at eccentricities of 5–15° (caudal electrodes) on the collicular motor map (Robinson, 1972). Electrodes were implanted for a period of 4–6 weeks. In a given experimental session, we first isolated the activity of a single DLPFC neuron. The activity of the neuron was then monitored while single biphasic current pulses (0.15–0.3 ms per phase) were delivered to the SC through one of the implanted electrodes and an indifferent electrode. Neurons were classified as antidromic if stimulation elicited action potentials meeting several criteria, including fixed threshold, fixed latency, ability to follow high-frequency twin pulses, and collision testing (Lipski, 1981) (see Fig. 2A,B). The threshold for eliciting antidromic responses was defined as the current level that elicited an action potential ∼50% of the time. Threshold varied between 100 and 1400 μA, with a mean value of 591 μA.
Figure 2.
Recording locations and schematic representation of antidromic identification technique. A, Recording locations in monkeys R and W, reconstructed from MRI images. Slices are separated by 1 mm. Blue dots represent locations of neurons showing significant effect of stimulus location, red dots indicate locations of neurons showing significant effect of saccade direction, and green dots indicate locations of neurons showing a significant interaction between stimulus location and saccade direction. iar, Inferior arcuate sulcus; ps, principal sulcus; sar, superior arcuate sulcus. A, Anterior; L, lateral; M, medial; P, posterior. B, Right, schematic representation of experimental method for antidromic activation. Left, Waveforms depicting activity recorded in the DLPFC showing artifact caused by stimulation and stimulation-elicited action potential (AP). C, Right, Schematic representation of collision test. Left, Activation waveforms depicting DLPFC activity during collision test. Spontaneous action potential (AP) triggers stimulation pulse to SC. After collision, no action potential is observed in DLPFC neuron. D, Antidromic latencies. No difference in latency was found between neurons showing significant effects and those showing no effects (ANOVA, p > 0.05).
Data collection commenced once an antidromic neuron had been identified. Waveforms were digitized, stored, and sorted off-line using two- and three-dimensional principal components analysis (Plexon). To verify that none of our recording locations were in the FEFs, we subsequently delivered stimulation pulses at our recording sites. No eye movements were elicited by microstimulation currents of up to 200 μA (100 ms, 300 Hz, 0.3 ms biphasic pulses).
Data analysis.
Analysis of preparatory activity was performed with a t test on the mean activity for each neuron in the prosaccade and antisaccade conditions calculated during the 500 ms immediately preceding presentation of the peripheral stimulus. t tests were evaluated at p < 0.05. To quantify differences in preparatory activity between prosaccade and antisaccade trials in individual neurons, we calculated a contrast ratio (CR) based on the mean activity in the 500 ms preceding stimulus presentation for prosaccade and antisaccade trials for each neuron using the following equation: CR = (anti − pro)/(anti + pro). Values of this ratio could range between −1.0 and 1.0, with positive values indicating greater activity for antisaccades and negative values indicating greater activity for prosaccades.
Analysis of perisaccadic activity was performed using a two × two ANOVA with the factors stimulus location and saccade direction. Interaction between these factors indicated task-related differences in activity. All ANOVAs were evaluated at p < 0.05. Perisaccadic activity was classified as occurring within a statistical window that began 80 ms after presentation of the peripheral stimulus and ended 50 ms after saccade onset. This dynamic window was chosen because it included the responses to the visual stimuli and excluded activity that occurred after the saccade, such as visual feedback. This window was also unaffected by differences in SRT between the task conditions as the end of the window is always 50 ms after saccade onset. We also performed this analysis with static statistical windows varying from 100 to 200 ms in length with similar results.
To quantify stimulus, saccade, and task selectivity, we calculated a set of three indices (Xstim, Xsac, and Xtask) (Zhang et al., 1997) for each neuron showing a significant effect in the ANOVA described above. Each index was calculated using the mean perisaccadic activity for each neuron in each of the four experimental conditions: prosaccades directed toward the side contralateral to the recording site [pro(c)], prosaccades directed toward the side ipsilateral to the recording site [pro(i)], antisaccades directed toward the side contralateral to the recording site [anti(c)], and antisaccades directed toward the side ipsilateral to the recording site [anti(i)]. In all cases, contralateral and ipsilateral refer to the direction of the saccade relative to the recording site. To be able to directly compare the indices for each neuron, we performed a normalization procedure in which we set the maximum activity for each neuron to a value of 50 and calculated the activity of the neuron in the other three conditions relative to this value. Index values could therefore vary between −100 and +100 for each neuron. Xstim, Xsac, and Xtask were calculated in the following manner: Xstim = pro(c) + anti(i) − anti(c) − pro(i); Xsac = pro(c) + anti(c) − anti(i) − pro(i); and Xtask = pro(c) + pro(i) − anti(c) − anti(i). Xstim computes the difference in response between conditions in which the visual stimulus is presented on the contralateral side and those in which the visual stimulus is presented on the ipsilateral side. Negative values indicate greater activity for ipsilateral stimuli, and positive values indicate greater activity for contralateral stimuli. Xsac computes the difference between the conditions in which saccades are directed to the contralateral side and those in which saccades are directed to the ipsilateral side, with negative values indicating greater activity for ipsilateral saccades and positive values greater activity for contralateral saccades. Xtask computes the difference in activity between the prosaccade and antisaccade conditions, with negative values indicating greater activity for antisaccades and positive values indicating greater activity for prosaccades. After computing these indices, we plotted the values for each neuron on a three-dimensional plot. We tested whether significantly more neurons had index values falling within a given quadrant of this plot using a χ2 test evaluated at p < 0.05.
To investigate the relationship between neural activity and SRT, we computed correlations between SRT in each experimental condition described above and mean neural activity in the same condition calculated in an 80 ms window immediately after presentation of the peripheral stimulus. This window was chosen because it excluded any response to the visual stimulus, because visual response latencies of DLPFC neurons are typically 100 ms or longer (Funahashi et al., 1990). The statistical significance of correlations was assessed using t tests evaluated at p < 0.05.
All analyses described above were based on correct trials. Trials associated with incorrect responses, broken, incorrect, or inaccurate fixation, or failure to generate a saccade within 500 ms were excluded. We attempted to perform an analysis on error trials, but because both animals were quite proficient at the experimental task, only a small number of trials were available for analysis. In addition, most errors occurred at the time of the task rule switch. In this case, errors were the result of the monkeys' performing the previously rewarded response or a trial and error strategy used to determine the new task rule. Thus, we did not consider these trials as typical antisaccade task errors because they were not the result of an inability to inhibit reflexive prosaccades. For these reasons, error trials immediately after the task switch should be excluded. Once these trials were excluded from the already small number of error trials, an insufficient number remained for analysis.
Results
Corticotectal neurons
We report the activity of 43 neurons from the right DLPFC of two monkeys (35 for monkey R, eight for monkey W) (Fig. 2A) that were identified as sending a direct projection to the SC. Of these neurons, 29 of 43 (67.4%) showed a statistically significant difference in activity in at least one of the trial epochs we analyzed.
The latencies of the antidromic responses of the population of corticotectal neurons we recorded are presented in Figure 2D. No difference in latency was found between neurons showing significant effects in any of our statistical analyses of neural activity (gray bars) and neurons showing no effects (white bars) (t test, p < 0.05).
All neurons were antidromically activated through microstimulation of an electrode implanted in the rostral SC. Only three neurons (7%) could also be antidromically stimulated through one the caudal SC electrodes. The maximal biphasic stimulation current that we used to test whether a neuron could be antidromically activated was 2000 μA.
DLPFC neurons send preparatory activity to the SC
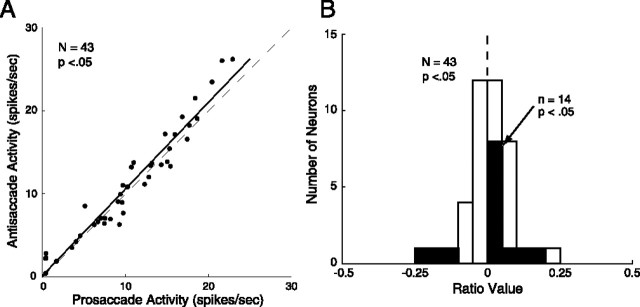
To determine whether corticotectal DLPFC neurons sent task-selective preparatory signals to the SC, we performed t tests comparing the activity for prosaccade and antisaccade trials during the 500 ms immediately preceding presentation of the peripheral stimulus for each neuron. During this period, the monkeys were required to maintain fixation on a central white fixation point. Because the monkeys were fixating during this period and because our behavioral task contained no explicit signal indicating which response would be rewarded, both visual input and eye position were the same for both prosaccade and antisaccade trials. A number of neurons showed task-selective responses during this period (14 of 43, 32.6%). The majority of task-selective neurons had a higher level of activity on antisaccade trials than prosaccade trials (11 of 14, 78.6%; χ2 test, p < 0.05) (Fig. 3A). To quantify this activity, we calculated a pro/anti contrast ratio using the mean activity during the preparatory period. This ratio could range from −1.0 to 1.0, with negative values indicating greater activity on prosaccade trials and positive values indicating greater activity on antisaccade trials. Ratio values are presented in Figure 3B. These ratio values were significantly less than zero for the population of antidromic neurons we recorded (t test, p < 0.05), indicating that, overall, preparatory activity was greater on antisaccade trials than prosaccade trials. Almost all neurons showing a significant task effect in the preparatory period also showed differences in the analyses described below (13 of 14, 93%).
Figure 3.
Neural activity during the preparatory period. A, Mean activity during the 500 ms immediately preceding presentation of the peripheral stimulus for antisaccade trials (ordinate) and prosaccade trials (abscissa). Solid line indicates least-squares fit of data points, and dashed line represents unity. B, Pro/anti contrast ratios for the population of corticotectal neurons recorded. Ratios were calculated using mean activity in the preparatory period described above. Black bars represent neurons with ratio values significantly greater than zero. Three additional neurons not shown have ratio values above 0.5.
DLPFC neurons send stimulus, saccade, and combined signals to the SC
To investigate whether the responses of corticotectal DLPFC neurons encoded the location of the visual stimulus, the direction of the upcoming saccade, or both, we performed ANOVAs on the perisaccadic activity of each neuron (see Materials and Methods). We found that a large number of DLPFC neurons (28 of 43, 65.1%) sent signals selective for one or more of these factors directly to the SC.
Some neurons (6 of 43, 14%) showed only a main effect of stimulus location. One such neuron is presented in Figure 4A. This neuron responded strongly to ipsilateral visual stimuli, regardless of whether the upcoming saccade was directed toward or away from the stimulus location (bottom panel). No such response was observed for stimuli presented on the contralateral side (top panel). We found two neurons (2 of 43, 4.7%) that exhibited only a main effect for saccade direction. The neuron presented in Figure 4B exhibited activity that peaked after saccade onset, with a greater response for contralateral prosaccades than ipsilateral antisaccades (top panel), as well as a greater response for contralateral antisaccades than ipsilateral prosaccades (bottom panel). Thus, the activity of this neuron was highest for saccades directed to the contralateral side, regardless of whether those saccades were directed toward or away from the visual stimulus. Two neurons showed a significant main effect for both stimulus location and saccade direction (2 of 43, 4.7%). The majority of selective neurons showed interaction effects (19 of 43, 44.2%), i.e., their pattern of activity that was dependent on both stimulus location and saccade direction. Figure 4C depicts one such neuron. This neuron showed a greater level of activity for stimuli presented on the contralateral side (top panel) than ipsilateral stimuli (bottom panel). In addition, the responses of this neuron were modulated by the task rule. It responded more strongly to contralateral stimuli when the upcoming saccade was directed away from the stimulus (i.e., an antisaccade) than when the upcoming saccade was directed toward the stimulus. This neuron also showed a greater level of preparatory activity for antisaccades than prosaccades (t test, p < 0.05)
Figure 4.
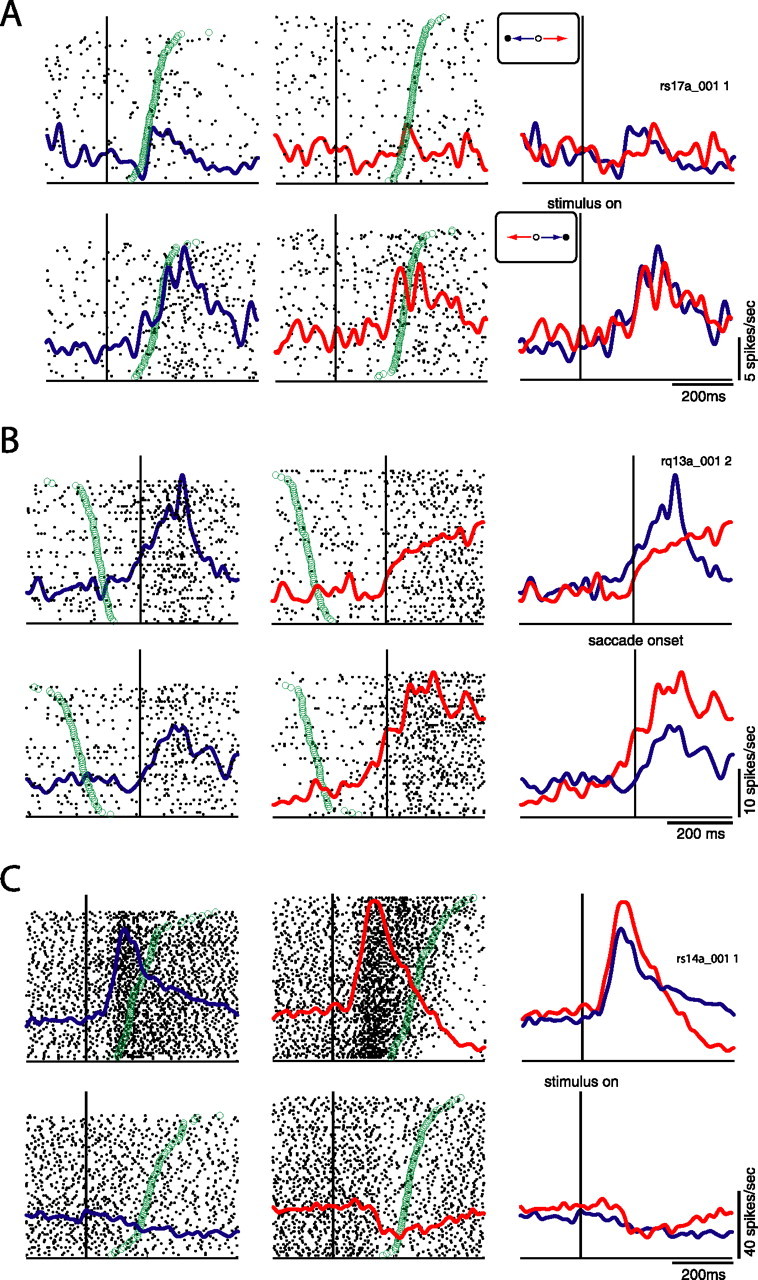
Single neuron examples. Red lines depict activity on antisaccade trials, and blue lines prosaccade trials. A, Neuron showing a significant effect of stimulus location. Functions and rasters are aligned on stimulus onset. Green circles represent SRTs. Trials are sorted by reaction time. Top, Activity for conditions in which stimulus is presented on the contralateral side (contralateral prosaccades, ipsilateral antisaccades). Bottom, Activity for conditions in which stimulus is presented on the ipsilateral side (ipsilateral prosaccades, contralateral antisaccades). Neuron was more active for ipsilateral stimuli regardless of saccade direction or task rule. B, Neuron showing significant effect of saccade direction. Spike density functions and rasters are aligned on saccade onset. Green circles represent time of stimulus presentation. Top, Activity for contralateral prosaccades and ipsilateral antisaccades, as in A. Bottom, Also same as A. Neuron was more active for contralateral saccades regardless of stimulus location or task rule. C, Neuron showing an interaction between stimulus location and task rule. Functions and rasters are aligned on stimulus onset. Green circles represent SRT. Activity depicted in top and bottom as in A and B. Neuron was more active for condition in which stimulus was presented on the contralateral side and more active for antisaccades than prosaccades in this condition. All spike density functions were constructed by convolving spike trains with a 30 ms Gaussian activation function. Statistical significance for all neurons was evaluated by ANOVA at p < 0.05.
The population response of DLPFC output neurons during prosaccades and antisaccades
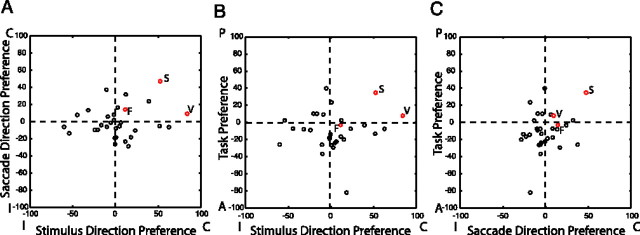
To quantify the perisaccadic responses sent from the DLPFC to the SC, we calculated a set of three selectivity indices (Xstim, Xsac, and Xtask) based on normalized firing rates for each identified DLPFC efferent neuron (see Materials and Methods). The values of each index could range in value from −100 to +100, with greater values of the index indicating stronger selectivity. Xstim represents the stimulus location selectivity of each neuron. Negative values represent a higher level of activity for stimuli presented on the side ipsilateral to the recording site, and positive values represent a higher level of activity for stimuli presented on the contralateral side. Xsac represents saccade direction selectivity, with negative values indicating greater activity for saccades directed toward the side ipsilateral to the recording site and positive values indicating greater activity for saccades directed toward the contralateral side. Xtask represents selectivity of each neuron for the prosaccade/antisaccade task rule. In this case, negative values represent higher levels of activity for antisaccades than prosaccades, whereas positive values represent the opposite pattern of activity. The relative values of these three indices summarize the stimulus, saccade, and task selectivity of each neuron and can be plotted in a three-dimensional space. Figure 5 presents plots of the values of the selectivity indices for each neuron showing a significant perisaccadic effect. We found that every combination of stimulus, saccade, and task selectivity was carried by the population of antidromic neurons. Interestingly, more neurons showed the strongest responses for stimuli presented on the contralateral side, saccades directed to the ipsilateral side, and the antisaccade task than any other combination (χ2 test, p < 0.05). This pattern of activity was also apparent in the population activity of these neurons (Fig. 6A,B). Thus, the predominant signal carried by DLPFC output neurons was a selectively enhanced response to contralateral stimuli in the antisaccade task.
Figure 5.
Plots of stimulus, saccade, and task indices for all neurons showing significant effects on ANOVA. Black dots, DLPFC neurons. Red dots, Neurons previously recorded from the SC (Everling and Munoz, 2000, their Fig. 4). P, Prosaccade; A, antisaccade; C, contralateral; I, ipsilateral; S, Saccade; V, visual; F, fixation. A, Saccade direction index versus stimulus direction index. B, Task index versus stimulus direction index. C, Task index versus saccade direction index.
Figure 6.
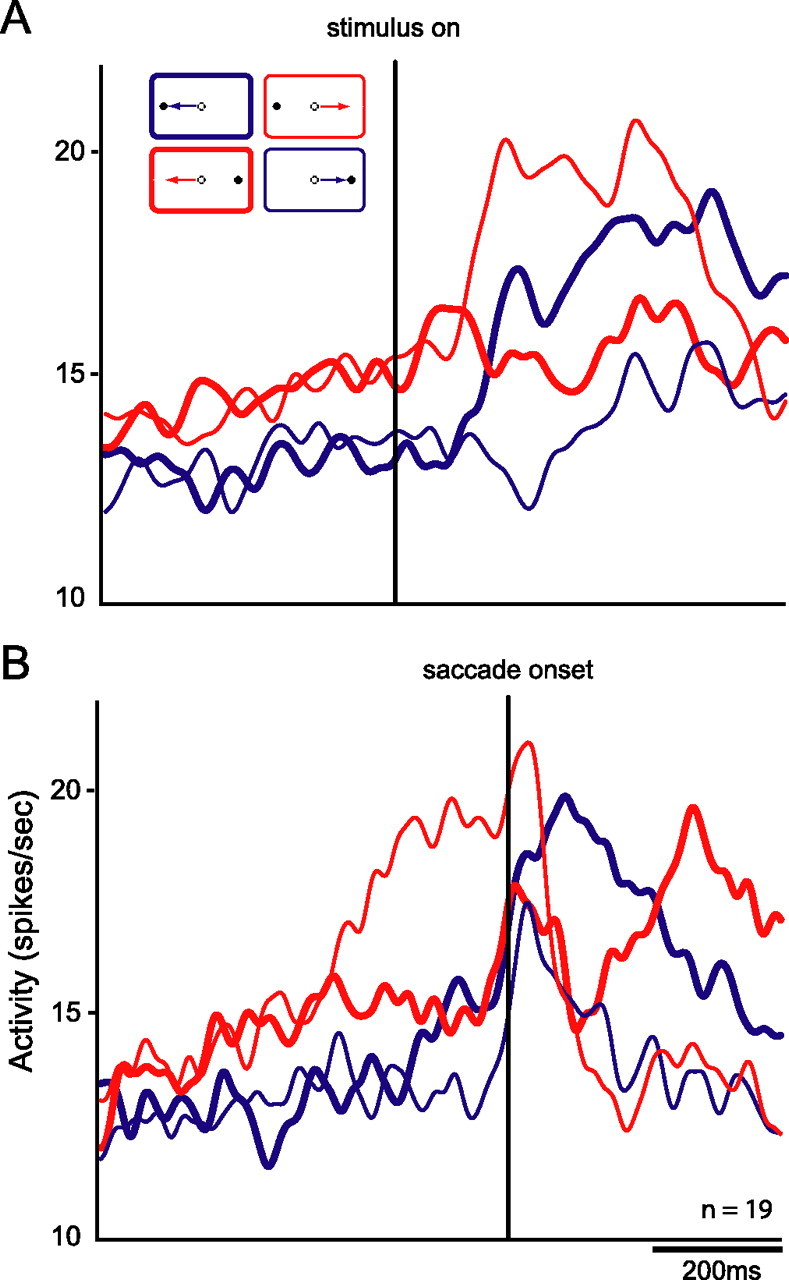
Spike density functions for the population of neurons showing significant interaction on ANOVA. Red lines represent activity on antisaccade trials, and blue lines indicate activity on prosaccade trials. Thick and thin lines represent activity for saccades directed to the side ipsilateral and contralateral to the recorded (right) hemisphere respectively. A, Functions aligned on stimulus onset. B, Functions aligned on saccade onset. More activity is present on antisaccade trials than prosaccade trials. More specifically, an elevated response is observed before the saccade for trials on which the visual stimulus is presented on the contralateral side and saccades are directed toward the ipsilateral side (i.e., contraversive antisaccades).
To compare the selectivity of DLPFC output neurons with the responses of neurons in their target structure, the SC, we also calculated values of these indices for previously recorded SC saccade, visual, and fixation neurons (Fig. 5, S, V, and F) (Everling et al., 1999, their Fig. 4). As can be seen in the figure, the selectivity of these neurons did not closely match that of DLPFC output neurons, suggesting that the signals sent from the DLPFC do not simply mirror those of the SC.
Signals carried by DLPFC output neurons correlate with antisaccade reaction times
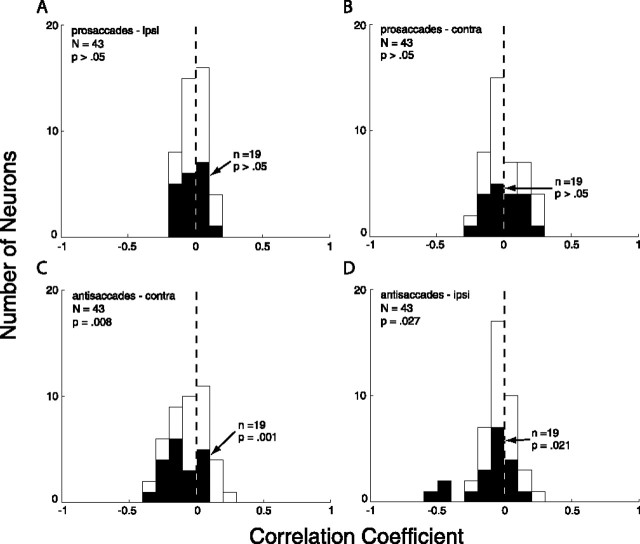
To investigate the behavioral relevance of the signals sent from the DLPFC to the SC, we calculated correlations between the mean prestimulus neural activity and SRT for prosaccades and antisaccades for the entire population of recorded DLPFC output neurons. Prestimulus activity has been shown previously to be negatively correlated with SRT in the SC for both prosaccades (Dorris et al., 1997; Dorris and Munoz, 1998) and antisaccades (Everling et al., 1999). We found significant negative correlations between prestimulus activity and SRT for antisaccades directed to both the contralateral and ipsilateral sides (Fig. 7C,D) but no significant correlation between prestimulus activity and SRT for prosaccades (Fig. 7A,B). This indicates that higher activity in DLPFC neurons is associated with faster SRTs for antisaccades but not prosaccades. These data suggest that the task-selective signals sent to the SC from the DLPFC participate in the preparation of antisaccades.
Figure 7.
Histograms depicting correlations between neural activity and SRT for the population of antidromic neurons for ipsilateral (ipsi) and contralateral (contra) prosaccades and antisaccades. Correlations were computed between mean activity for each condition in an 80 ms window immediately after presentation of the visual stimulus and the SRT for the corresponding condition. Neural activity was correlated with SRT for both ipsilateral and contralateral antisaccades. A, Correlations for ipsilateral prosaccades. B, Correlations for contralateral prosaccades. C, Correlations for contralateral antisaccades. D, Correlations for ipsilateral antisaccades. No significant correlations were found for prosaccades (t tests, evaluated at p < 0.05). Neurons showing a significant interaction effect are highlighted in black.
Discussion
Influential theories of prefrontal function have emphasized the role of the DLPFC in modulating the activity of target structures (Miller and Cohen, 2001). To date, no studies have directly investigated the nature of the signals sent by DLPFC output neurons to a specific cortical or subcortical target in primates. Here, we characterized for the first time the signals carried by identified DLPFC output neurons. We found that the DLPFC neurons send signals selective for stimulus location, saccade direction, and task directly to the SC. Although previous studies demonstrated such activity in DLPFC neurons (Funahashi et al., 1991, 1993; Asaad et al., 2000; Everling and DeSouza, 2005; Johnston and Everling, 2006), this is the first direct evidence that the DLPFC sends such specific signals to another brain area.
To correctly perform the antisaccade task, subjects must override the automatic response to make a saccade toward the location of a visual stimulus and instead generate a saccade in the opposite direction. A number of studies from both the patient (Guitton et al., 1985; Pierrot-Deseilligny et al., 1991; Ploner et al., 2005) and monkey (Condy et al., 2006) literature have suggested that the DLPFC plays a crucial role in antisaccade performance by directly suppressing reflexive saccades. More specifically, it has been suggested that the DLPFC sends an inhibitory signal to oculomotor structures (Pierrot-Deseilligny et al., 1991; Munoz and Everling, 2004). We found that many neurons were more active on antisaccade trials and, specifically, that many DLPFC neurons responded most strongly on antisaccade trials in which the stimulus was presented in the hemifield contralateral to the recorded hemisphere. The top-down inhibitory effect of these neurons on saccade neurons in the SC must be mediated by projections to fixation neurons or inhibitory interneurons in the SC because cortical output is entirely glutamatergic and excitatory in nature (Creutzfeldt, 1983).
It has been shown previously that the activity of SC fixation neurons is enhanced whereas the activity of saccade neurons is reduced on antisaccade trials (Everling et al., 1999). A top-down excitatory signal could facilitate antisaccade performance by enhancing the activity of SC fixation neurons (Fig. 8, blue arrows). In this model, presentation of a visual stimulus to the left of fixation during antisaccade blocks leads to an increased signal in the right DLPFC. This excitatory signal would then be sent directly to fixation neurons in the rostral pole of the ipsilateral and contralateral colliculi (Takahashi et al., 2005), enhancing their activity and suppressing reflexive saccades.
Figure 8.
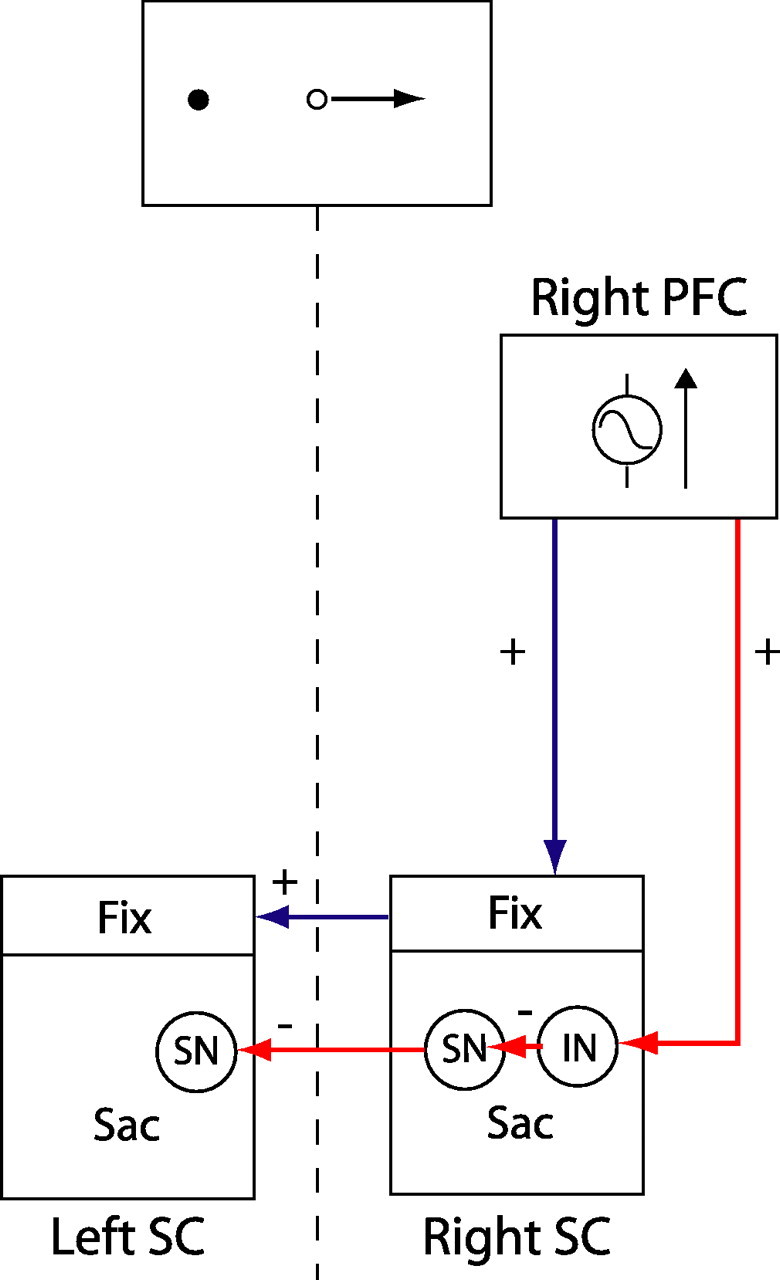
Possible mechanism by which enhanced DLPFC signal for contralateral antisaccade targets could facilitate antisaccade task performance via the SC. Excitatory descending signal could enhance activity of fixation (Fix) neurons in the ipsilateral (right) rostral SC and, in turn, the contralateral (left) SC. Alternatively, excitatory descending signal could be sent to an inhibitory interneuron (IN) in the caudal saccade zone of the SC (Sac), which would in turn inhibit saccade neurons (SN), leading to decreased probability of triggering a reflexive saccade toward the stimulus. This would also lead to decreased inhibition of saccade neurons in the contralateral SC, which would facilitate execution of a rightward saccade.
A second alternative is that an excitatory signal from the DLPFC is sent to inhibitory interneurons in the caudal SC (Fig. 8, red arrows). The SC contains a vast array of inhibitory interneurons that synapse on both fixation and saccade neurons (Munoz and Istvan, 1998). The result of this would be a reduction in the response of saccade neurons to contralateral targets and a concomitant reduction in the likelihood that their activity would exceed the threshold to trigger a saccade toward the visual stimulus (Munoz and Everling, 2004). In addition, because saccade neurons have an inhibitory influence on saccade neurons in the opposite SC (Munoz and Istvan, 1998), this process would result in decreased inhibition of the contralateral SC, which would facilitate the performance of a saccade in the ipsilateral direction. This could account for our finding of a negative correlation between DLPFC activity and reaction time on antisaccade trials. The absence of such a correlation on prosaccade trials suggests that top-down modulation of the SC might be selectively engaged on antisaccade trials.
All neurons that we recorded were activated via stimulation of the rostral SC. Only three neurons were also antidromically activated by microstimulation through a caudal electrode. Sommer and Wurtz (2000) reported that FEF neurons with foveal responses were easier to activate through rostral SC stimulation, whereas FEF saccade-related neurons had lower thresholds for microstimulation through caudal SC electrodes. It is therefore tempting to speculate that the output neurons of the dorsolateral PFC project predominately to the rostral SC. However, the fact that axons enter the colliculus via the rostral pole (Stanton et al., 1988) renders it impossible to determine the exact collicular site to which the DLPFC neurons we recorded projected. Thus, we feel that we cannot confidently distinguish whether DLPFC axons projected to fixation or saccade neurons and therefore suggest that both possibilities described above are equally likely based on our data.
The notion than the DLPFC could participate in inhibition of reflexive saccades by a mechanism such as that described above has been supported by the results of studies in human patients with lesions restricted to the prefrontotectal pathways. These patients commit more antisaccade errors when the visual stimulus is presented in the hemifield contralateral to the side of the lesion (Gaymard et al., 2003; Ploner et al., 2005), suggesting a lateralized failure of top-down control. Results of inactivation studies in the monkey are less clear (Condy et al., 2006). Muscimol inactivation of the area surrounding the principal sulcus lead to an increase in antisaccade errors when the visual stimulus was presented ipsilateral to the inactivated hemisphere, the exact opposite of what our findings would predict. A possible explanation for this is that the inactivated sites corresponded to areas 46 and 9/46 (Petrides and Pandya, 1999), whereas our corticotectal neurons were recorded primarily from area 8a, anterior to FEFs. This area corresponds closely to that identified by Hasegawa et al. (2004), as containing neurons coding saccade suppression.
The FEF has also been shown to send signals related to antisaccade task performance directly to the SC. Corticotectal FEF neurons generally exhibit lower levels of preparatory, stimulus-related, and saccade-related activity on antisaccade trials than prosaccade trials (Everling and Munoz, 2000). This differs substantially from the pattern of activity we observed in DLPFC corticotectal neurons, which overall showed a greater level of preparatory and perisaccadic activity on antisaccade trials than prosaccade trials. This suggests that signals sent from the DLPFC to the SC make a contribution to antisaccade task performance that is distinct from that of the FEF. It has been demonstrated that the FEF sends excitatory output directly to saccade-related neurons in the SC (Helminsky and Segraves, 2003). We propose that the DLPFC performs a more modulatory function, most likely by providing excitatory input to fixation neurons or collicular inhibitory interneurons.
Another distinct difference between corticotectal FEF and DLPFC neurons is their antidromic latency. Mean latencies for FEF neurons are typically ∼2 ms and range from 0.7 to 6.0 ms (Segraves and Goldberg, 1987; Sommer and Wurtz, 1998; Everling and Munoz, 2000; Helminsky and Segraves, 2003), whereas the antidromic latencies we observed in corticotectal DLPFC neurons were much longer, having a mean of 6.3 ms and ranging from 1.3 to 14 ms. This suggests that the cell bodies of DLPFC neurons are smaller than those of FEF neurons or that their axons are thinner, longer, or unmyelinated. Although it is tempting to infer a functional explanation for this latency difference, any such explanation would be speculative at best.
Models of prefrontal function suggested that the DLPFC participates in cognitive control through the active maintenance of patterns of activity representing the requirements of the task at hand and modulation of the activity of other structures to accomplish behavioral goals. This type of control is thought to be most necessary when tasks require the implementation of flexible stimulus–response associations or the ability to override automatic behaviors in favor of weaker, purposive ones (Miller, 1999; Miller and Cohen, 2001). We found that DLPFC neurons sent a task-selective signal to the SC during antisaccades, a task requiring the implementation of cognitive control. We propose that this signal is indicative of the selective engagement of a top-down process that could act via a specific mechanism to modulate the activity of the SC neurons and facilitate antisaccade performance. This supports the general idea that the DLPFC sends bias signals to other brain areas and provides evidence that the DLPFC may indeed influence behavior by orchestrating the activity of target structures.
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research and the EJLB Foundation. We thank T. Admans, S. Ekstrom, and T. DeLangley for outstanding surgical and technical support. We gratefully acknowledge R. Menon and J. Gati for their assistance during MRI scans. We also acknowledge the comments of two anonymous reviewers whose comments greatly improved this manuscript.
References
- Asaad WF, Rainer G, Miller EK. Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol. 2000;84:451–459. doi: 10.1152/jn.2000.84.1.451. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol. 2000;83:1550–1556. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- Condy C, Wattiez N, Rivaud-Pechoux S, Tremblay L, Gaymard B. Antisaccade deficit after inactivation of the principal sulcus in monkeys. Cereb Cortex. 2006 doi: 10.1093/cercor/bhj140. in press. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD. Berlin: Springer; 1983. Cortex cerebri: performance, structural and functional organization of the cortex. [Google Scholar]
- Curtis CE, D'Esposito M. Success and failure suppressing reflexive behaviour. J Cogn Neurosci. 2003;15:409–418. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- DeSouza JFX, Everling S. Focused attention modulates visual responses in the primate prefrontal cortex. J Neurophysiol. 2004;91:855–862. doi: 10.1152/jn.00273.2003. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci. 1998;18:7015–7026. doi: 10.1523/JNEUROSCI.18-17-07015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Pare M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci. 1997;17:8566–8579. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, DeSouza JFX. Rule-dependent activity for prosaccades and antisaccades in the primate prefrontal cortex. J Cogn Neurosci. 2005;17:1483–1496. doi: 10.1162/0898929054985455. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Munoz DP. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. J Neurophysiol. 1998;80:1584–1589. doi: 10.1152/jn.1998.80.3.1584. [DOI] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Klien RM, Munoz DP. Role of primate superior colliculus in preparation of and execution of anti-saccades and pro-saccades. J Neurosci. 1999;19:2740–2754. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Tinsley CJ, Gaffan D, Duncan J. Filtering of neural signals by focused attention in the monkey prefrontal cortex. Nat Neurosci. 2002;5:671–676. doi: 10.1038/nn874. [DOI] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related fMRI. J Neurophysiol. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Visual categorization and the primate prefrontal cortex: neurophysiology and behavior. J Neurophysiol. 2002;88:929–941. doi: 10.1152/jn.2002.88.2.929. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J Neurosci. 2003;23:5235–5246. doi: 10.1523/JNEUROSCI.23-12-05235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Visuospatial coding in primate prefrontal neurons revealed by oculomotor paradigms. J Neurophysiol. 1990;63:814–831. doi: 10.1152/jn.1990.63.4.814. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Neuronal activity related to saccadic eye movements in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1991;65:1464–1483. doi: 10.1152/jn.1991.65.6.1464. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Francois C, Ploner CJ, Condy C, Rivaud-Pechoux S. A direct prefrontotectal tract against distractability in the human brain. Ann Neurol. 2003;53:542–545. doi: 10.1002/ana.10560. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Nauta WJH. Autoradiographic demonstration of a projection from prefrontal association cortex to the superior colliculus in the rhesus monkey. Brain Res. 1976;116:145–149. doi: 10.1016/0006-8993(76)90256-0. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res. 1985;58:455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hasegawa R, Peterson BW, Goldberg ME. Prefrontal neurons coding suppression of specific saccades. Neuron. 2004;43:415–425. doi: 10.1016/j.neuron.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Helminsky JO, Segraves MA. Macaque frontal eye field input to saccade-related neurons in the superior colliculus. J Neurophysiol. 2003;90:1046–1062. doi: 10.1152/jn.00072.2003. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Effects on eye movements of a GABA agonist and antagonist injected into the monkey superior colliculus. Brain Res. 1983;272:368–372. doi: 10.1016/0006-8993(83)90586-3. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S. Neural activity in monkey prefrontal cortex is modulated by task context and behavioral instruction during delayed-match-to-sample and conditional prosaccade-antisaccade tasks. J Cogn Neurosci. 2006;18:749–765. doi: 10.1162/jocn.2006.18.5.749. [DOI] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Spencer RF, Hardy SG, Astruc J. The prefrontal corticotectal projection in the monkey: an anterograde and retrograde horseradish peroxidase study. Neuroscience. 1981;6:1023–1041. doi: 10.1016/0306-4522(81)90068-3. [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurons as an analytic tool in the study of the central nervous system. J Neurosci Methods. 1981;4:1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex: complex neural properties for complex behavior. Neuron. 1999;22:15–17. doi: 10.1016/s0896-6273(00)80673-x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol. 1998;79:1193–1209. doi: 10.1152/jn.1998.79.3.1193. [DOI] [PubMed] [Google Scholar]
- Nieder A, Freedman DJ, Miller EK. Representation of the quantity of visual items in the primate prefrontal cortex. Science. 2002;297:1708–1711. doi: 10.1126/science.1072493. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually guided saccades in man. Brain. 1991;114:1473–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard BM, Rivaud-Pechoux S, Pierrot-Deseilligny C. The prefrontal substrate of reflexive saccade inhibition in humans. Biol Psychiatry. 2005;57:1159–1165. doi: 10.1016/j.biopsych.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Segraves MA, Goldberg ME. Functional properties of corticotectal neurons in the monkey's frontal eye field. J Neurophysiol. 1987;58:1387–1419. doi: 10.1152/jn.1987.58.6.1387. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Composition and topographic organization of signals sent from the frontal eye fields to the superior colliculus. J Neurophysiol. 2000;83:1979–2001. doi: 10.1152/jn.2000.83.4.1979. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey. II. Topography of terminal fields in midbrain and pons. J Comp Neurol. 1988;271:493–506. doi: 10.1002/cne.902710403. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Sugiuchi Y, Izawa Y, Shinoda Y. Synaptic inputs and their pathways from fixation and saccade zones of the superior colliculus to inhibitory burst neurons and pause neurons. Ann NY Acad Sci. 2005;1039:209–219. doi: 10.1196/annals.1325.020. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol. 2003;90:1790–1806. doi: 10.1152/jn.00086.2003. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson C, Miller EK. Single neurons in monkey prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg MA. Activity of superior colliculus in behaving monkeys. IV. Effects of lesions on eye movements. J Neurophysiol. 1972;35:587–596. doi: 10.1152/jn.1972.35.4.587. [DOI] [PubMed] [Google Scholar]
- Zhang J, Riehle A, Requin J, Kornblum S. Dynamics of single neuron activity in monkey primary motor cortex related to sensorimotor transformation. J Neurosci. 1997;17:2227–2246. doi: 10.1523/JNEUROSCI.17-06-02227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]