Abstract
BACKGROUND:
Overweight/obesity (OW) is linked to worse asthma and poorer inhaled corticosteroid (ICS) response in older children and adults.
OBJECTIVES:
Describe the relationships between OW and asthma severity and response to ICS in preschool children.
METHODS:
This post-hoc study of three large multi-center trials involving 2–5 year-old children compared annualized asthma symptom days and exacerbations among normal weight (NW) (body mass index (BMI) 10–84th percentile) versus OW (BMI ≥85th percentile) participants. Participants had been randomized to daily ICS, intermittent ICS or daily placebo. Simple and multivariable linear regression was used to compare BMI-groups.
RESULTS:
Within the group not treated with a daily controller, OW children had more asthma symptom days (AD) (90.7 vs. 53.2, p=0.020) and exacerbations (1.4 vs. 0.8, p=0.009) compared to NW children. Within the ICS-treated groups, OW and NW children had similar AD (daily ICS: 47.2 vs. 44.0 days, p=0.44; short-term ICS: 61.8 vs. 52.9 days, p=0.46; as-needed ICS: 53.3 vs. 47.3 days, p=0.53), and similar exacerbations (daily ICS: 0.6 vs 0.8, p=0.10, short-term ICS: 1.1 vs 0.8 days, p=0.25; as-needed ICS: 1.0 vs 1.1, p=0.72). Compared to placebo, daily ICS in OW led to fewer annualized asthma symptom days (90.7 vs. 41.2, p=0.004) and exacerbations (1.4 vs. 0.6, p=0.006), while similar protective ICS effects were less apparent among NW.
CONCLUSION:
In preschool children off controller therapy, OW is associated with greater asthma impairment and exacerbations. However, unlike older asthmatics, overweight/obese preschool children do not demonstrate reduced responsiveness to ICS therapy.
Keywords: Asthma, Overweight, Obesity, Children, Infants, Exacerbation
Capsule Summary:
Overweight/obesity in preschoolers is associated with greater asthma symptom days and exacerbations when off controller therapy, and an overall good response to inhaled corticosteroids.
INTRODUCTION
Asthma is one of the most common chronic diseases of childhood and adolescence(1, 2). High body mass index (BMI) status has a poorly defined relationship with asthma severity. According to national asthma guidelines, classification of asthma severity in controller-naïve patients depends on (1) impairment of daily functioning by asthma symptoms and (2) risk of exacerbations(3). Studies involving older youths and adults have found that overweight or obesity status (OW) worsens asthma symptoms(4–6), asthma-related healthcare utilization(6–8) and response to inhaled corticosteroids (ICS)(9–11). For example, Quinto studied 32,321 children aged 5–17 years within the Kaiser Permanente health system and found that OW was associated with poor asthma control and exacerbations, measured by rescue inhaler and oral steroid dispensing, respectively(6). However, others have found no association between OW and measures of asthma severity(12–14), or found that high BMI was associated with reduced (not greater) airway hyperresponsiveness, a central component of asthma(15–17). Very little data currently exist exploring the effects of OW status on asthma severity in preschool children. In addition, no studies to our knowledge have investigated OW and ICS-response in preschoolers. The lack of research of OW status in preschoolers is an important gap in children’s health considering that preschool children (< age 5 years) are at a particularly high risk for morbidity stemming from asthma or recurrent wheezing. Half of all children experience wheezing by age 5(18), roughly one-third of preschool children suffer prolonged episodes of recurrent asthma symptoms(19), and among preschoolers, asthma symptoms are a leading cause of hospitalizations and ED visits. Additionally, the current prevalence of OW in the United States for 2–5 year olds is 27%(20). Elucidating the factors in preschool children which affect the treatment efficacy of ICS is of particular public health interest. If early life OW status does worsen asthma symptoms and reduces the effectiveness of inhaled corticosteroids, early life nutrition and obesity prevention efforts could become a critically important intervention.
Currently, ICS are the most effective single anti-asthma controller medication available for the prevention of daily symptoms and exacerbations. Therefore, response to daily ICS is an important phenotypic characteristic of childhood asthma. Only a few studies in adults and one study in older children(11) have evaluated the effect of OW on ICS treatment response. Studies have demonstrated a reduced response to ICS among adults with high BMI(9, 21, 22). In the Childhood Asthma Management Program (CAMP) study, Forno and colleagues found that OW children demonstrated a reduced improvement in lung function and asthma-related urgent care use(11) in response to ICS compared to NW children. Using data from three large prospective trials of preschool children enrolled in the Childhood Asthma Research and Education (CARE) and AsthmaNet networks, we evaluated the effects of early life OW-status on prospectively determined asthma symptom days and exacerbations in children treated with either ICS (daily or intermittent step-up) or placebo. We hypothesized that among both placebo-treated and ICS-treated children, OW status would lead to greater AD and exacerbations.
METHODS
Participant Selection
Details of the main studies (Individualized Therapy for Asthma in Toddlers (INFANT), Prevention of Early Asthma in Kids (PEAK), and Maintenance and Intermittent Inhaled Corticosteroids in Wheezing Toddlers (MIST) have been published (23–26). All caregivers of participants signed written informed consents. The present post-hoc study was approved by the Nemours (928923–2) institutional review board. We included baseline and intervention period data from 736 preschool aged participants (24–59 months) with mild persistent asthma or recurrent wheezing who were randomized into one of three multi-center placebo-controlled trials in which they received either daily ICS, intermittent ICS or placebo. Weights were determined using a calibrated electronic or beam balance scale. Standing height was measured without shoes using a calibrated stadiometer accurate to the nearest millimeter. Age and sex-adjusted body mass index percentiles were calculated using a centralized calculator using CDC growth chart data. Because underweight children have also demonstrated more severe asthma(27), participants with a body mass index (BMI) percentile < 10th percentile were excluded in this analysis.
The INFANT study was a multicenter, randomized, double-blind, double-dummy, clinical trial in children 12–59 months (n=300) with persistent asthma, which was factorially linked to the Acetaminophen versus Ibuprofen in Young Children with Asthma (AVICA) study. Because treatment with ibuprofen compared to acetaminophen did not affect asthma outcomes (28), we included INFANT data in the combined analysis. INFANT participants completed a 2–8 week run-in period followed by three 16-week crossover intervention periods with daily ICS (fluticasone propionate, 88μg twice daily, GlaxoSmithKline), daily LTRA (montelukast, 4 mg by mouth, Merck and Co., Inc.), and intermittent “as-needed” ICS treatment (fluticasone propionate, 88μg given given whenever 2 inhalations of albuterol sulfate are needed, GlaxoSmithKline). The PEAK study was a multicenter double-blind two-arm parallel study that randomly assigned 285 participants 2–3 years of age with a positive modified asthma predictive index to treatment with either fluticasone propionate (GlaxoSmithKline) 88μg twice daily or masked placebo for 24 months. The MIST trial was a multi-center randomized double-blind parallel trial which studied 278 children between the ages of 12 and 53 months who had recurrent wheezing and a positive modified asthma predictive index. Participants were randomly assigned to receive a budesonide inhalation suspension (Pulmicort Respules, Astra-Zeneca) for 12 months as either a daily low-dose ICS (0.5 mg nightly) or an intermittent “short-term” ICS (1 mg twice daily for 7 days, starting early during a predefined respiratory tract illness).
Clinical Data
We analyzed demographics, medical and environmental histories, and asthma-related utilization among 736 participants from the three trials who were older than 24 months at enrollment. Intervention period data were collected ranged over a 14-year period (PEAK 2001–2004; MIST 2008–2010; INFANT 2013–15). Participants were classified as normal weight (10–84th percentile BMI)(NW) or overweight/obese (OW) (≥85th percentile BMI) based on BMI percentiles according to the Centers for Disease Control and Prevention classification(29). Figures show asthma symptom days and exacerbations by quartiles to demonstrate the lack of a BMI-percentile trend and to justify combining percentiles 10–84 into one group. Aeroallergen testing was performed by skin prick testing or blood ImmunoCAP (Phadia AB, Uppsala, Sweden) allergen-specific IgE.
During the intervention period, the impairment domain of asthma severity was measured by annualized asthma symptom days (AD). AD in the three studies were defined as days which included any daytime or nighttime asthma-like symptoms (cough, wheezing, nighttime awakening), unscheduled medical visits for respiratory symptoms, or use of any rescue asthma medications. AD were reported by caregivers using study-specific means including twice monthly interviews (PEAK), daily diary cards (MIST) and daily electronic home diaries (INFANT). Annualized rates of AD were determined for each participant. Risk domain of asthma severity was measured by annualized asthma exacerbations. Exacerbations were defined similarly among the studies as events involving an increase in asthma symptoms requiring treatment with systemic corticosteroids to avoid serious worsening of asthma. Study staff blinded to treatment assignment diagnosed exacerbations of asthma based on conventional criteria including symptoms not responding to SABA, frequent SABA use, prolonged moderate-severe symptoms, and physician discretion (23, 25, 26). If caregivers sought care outside of the study staff which resulted in a diagnosis of an asthma exacerbation requiring systemic steroids, the event was considered an exacerbation.
Statistical Analysis
Baseline data were summarized by study and by BMI group (Table 1, 2). The primary analyses were comparisons between NW (BMI 10–84th percentile) and OW (BMI ≥85th percentile) in asthma symptom days and exacerbations. The Chi-square and student’s t test (or Wilcoxon, as appropriate) were used for comparing categorical and continuous variables between two BMI groups, respectively. Multivariable generalized linear regression under the negative binomial likelihood was used to compare main outcomes between BMI groups and study treatments with race and ethnicity as additional covariates. For analyses combining multiple studies, study was also included as a covariate. The effect of BMI status on response to ICS was determined by including an interaction between BMI group and study treatment. To demonstrate the appropriateness of collapsing all children within the BMI range 10–84th percentile and comparing NW vs. OW, we presented annualized AD and exacerbations across four BMI-percentile groups. SAS, version 9.3 (SAS Institute Inc; Cary, NC) was used. All tests were two-tailed at a level of significance of 0.05.
Table 1.
Baseline characteristics of participants by clinical trial
| PEAK Placebo (N=137) | MIST intermittent short-term ICS (N=104) | INFANT intermittent as-needed ICS / Daily ICS (N=245)* | PEAK daily ICS (N=132) | MIST daily ICS (N=118) | |
|---|---|---|---|---|---|
| Age at enrollment (months)† | 35.9 (6.9) | 37.9 (8.2) | 43.6 (10.5) | 36.0 (7.1) | 38.3 (9.2) |
| Age at diagnosis (months) | 15.6 (10.0) | 19.6 (10.4) | 22.1 (12.8) | 18.0 (10.4) | 17.9 (9.9) |
| Female, n (%) | 51 (37.2) | 27 (26) | 101 (41.2) | 51 (38.6) | 41 (34.7) |
| Race/Ethnicity, n (%) | |||||
| Non-Hispanic White | 73 (53.3) | 48 (46.2) | 77 (31.4) | 73 (55.3) | 41 (34.7) |
| Non-Hispanic Black | 20 (14.6) | 13 (12.5) | 73 (29.8) | 13 (9.8) | 21 (17.8) |
| Hispanic | 25 (18.2) | 31 (29.8) | 64 (26.1) | 28 (21.2) | 41 (34.7) |
| Other Race | 19 (13.9) | 12 (11.5) | 31 (12.7) | 18 (13.6) | 15 (12.7) |
| Weight (kg) | 15.2 (2.1) | 15.9 (2.8) | 17.3 (3.7) | 15.3 (2.6) | 16.1 (2.9) |
| BMI percentile | 66.3 (25.6) | 65.0 (25.0) | 67.8 (25.0) | 66.6 (25.9) | 71.8 (20.9) |
| BMI ≥ 85 percentile, n (%) | 44 (32.1) | 33 (31.7) | 89 (36.3) | 40 (30.3) | 38 (32.2) |
| Tobacco smoke exposure, n (%) | 54 (39.4) | 37 (36.3) | 96 (39.2) | 52 (39.4) | 52 (44.1) |
| Pets in home, n (%) | 60 (43.8) | 45 (43.3) | 113 (46.1) | 63 (47.7) | 53 (44.9) |
| Positive aeroallergen test, n (%) | 79 (57.7) | 62 (60.2) | 110 (46.6) | 82 (62.1) | 71 (60.2) |
| Ever have eczema, n (%) | 66 (48.2) | 53 (51) | 156 (63.7) | 74 (56.1) | 59 (50) |
| IgE (kU/L)** | 40.4 (12.1, 111.0) | 58.0 (21.6, 242.0) | 85.5 (27.0, 244.5) | 43.0 (15.0, 117.0) | 59.7 (25.0, 179.0) |
| Blood eosinophils (%)** | 3.0 (1.6, 5.0) | 4.0 (2.0, 5.7) | 3.5 (2.0, 6.0) | 3.8 (2.0, 6.0) | 3.0 (2.0, 6.2) |
| Average SFDs per week | 5.1 (1.7) | 4.7 (2.2) | 6.1 (1.2) | 5.1 (1.6) | 4.7 (2.1) |
| Average SABA puffs per week** | 0.6 (0.0, 1.5) | 0.0 (0.0, 1.0) | 0.0 (0.0, 2.2) | 0.5 (0.0, 1.4) | 0.0 (0.0, 1.0) |
| Urgent/ED visit in the past year, n (%) | 64 (46.7) | 61 (58.7) | 214 (87.3) | 62 (47) | 70 (59.3) |
| Hospitalized in the past year, n (%) | 10 (7.3) | 18 (17.3) | 48 (19.6) | 10 (7.6) | 19 (16.1) |
Values represent means (SD) unless noted.
represents same participants as INFANT daily ICS due to crossover design.
inclusion age ranges varied slightly in the three studies. Analysis included only children 24–59 months at enrollment.
denotes median and interquartile ranges. ICS – inhaled corticosteroid. BMI – body mass index. SFD – symptoms free days. SABA – short acting beta-agonist. PEAK – Prevention of Early Asthma in Kids trial, MIST - Maintenance versus Intermittent Inhaled Steroids in Wheezing Toddlers trial, INFANT - Individualized Therapy for Asthma in Toddlers
Table 2.
Baseline asthma-related characteristics by BMI status
| BMI-percentile | |||
|---|---|---|---|
| 10–84th (N=492) | ≥ 85th (N=244) | p-value | |
| Age at enrollment in months, mean (SD) | 38.9 (9.1) | 39.6 (9.9) | 0.45 |
| Age at diagnosis in months, mean (SD) | 19.4 (11.4) | 18.4 (11.2) | 0.31 |
| Weight in kg, mean (SD) | 15.0 (2.1) | 18.4 (3.7) | <0.0001 |
| Female | 193 (39.2%) | 78 (32%) | 0.055 |
| Race | |||
| Non-Hispanic White | 223 (45.3) | 89 (36.5) | 0.0223 |
| Non-Hispanic Black | 95 (19.3) | 45 (18.4) | 0.78 |
| Hispanic | 108 (22.0) | 81 (33.2) | 0.0010 |
| Other Race | 66 (13.4) | 29 (11.9) | 0.56 |
| Tobacco smoke exposure | 188 (38.3) | 103 (42.4) | 0.16 |
| Pets in Home | 225 (45.7) | 109 (44.7) | 0.79 |
| Positive Aeroallergen Test | 278 (57.3) | 126 (52.3) | 0.20 |
| Child ever have eczema | 279 (56.7) | 129 (52.9) | 0.98 |
| IgE (kU/L)* | 64.5 (21.6, 202.0) | 49.0 (16.1, 161.5) | 0.060 |
| Blood eosinophils %* | 3.7 (2.0, 6.1) | 3.0 (1.9, 5.0) | 0.0068 |
| Average SFDs per week, mean (SD) | 5.3 (1.8) | 5.3 (1.8) | 0.62 |
| Average SABA puffs per week* | 0.4 (0.0, 1.4) | 0.0 (0.0, 1.3) | 0.095 |
| Urgent/ED visit in the past year | 310 (63) | 161 (66) | 0.43 |
| Hospitalized in the past year | 60 (12.2) | 45 (18.4) | 0.0226 |
Values represent counts (%) unless noted. BMI – body mass index, SFD – symptom free days, SABA – short-acting beta-agonist, ED – emergency department.
Wilcoxon test
RESULTS
Baseline Characteristics
The baseline characteristics at randomization for 736 preschool children with asthma are shown by study and treatment in Table 1. Baseline characteristics were similar across studies, and each individual study had regional and racial/ethnic diversity. Participants generally had mild-moderate asthma symptoms, were more likely to be male (271/736, 63%), and typically were diagnosed with asthma prior to age 2. Overweight/obese status affected 33% (244/736) of participants at baseline. A substantial portion of children were exposed to environmental tobacco smoke (291/736, 40%) and one or more pets in the home (334/736, 45%). More than half (404/736, 55%) displayed sensitization to one or more aeroallergen. On average, participants reported 1–2 asthma symptom days/week, while 64% (471/736) reported urgent care/ED use in the previous year for asthma.
Overweight/Obesity Status and Asthma Characteristics
Table 2 shows asthma characteristics at randomization for the 736 participants by overweight/obesity status. The OW group had a slightly lower prevalence of white and higher prevalence of Hispanic children. Reported home exposures to pets and tobacco smoke, and objectively measured aeroallergen sensitization were similar between BMI groups. OW children had a significantly lower percent of blood eosinophils. BMI status was not related to reported baseline symptom free days, rescue SABA use or recent urgent care. OW children had 63% higher odds of a reported hospitalization in the previous 12 months prior to enrollment (OR=1.63, 95% CI: 1.07–2.48).
Asthma Severity during Daily and Step-up ICS Treatment
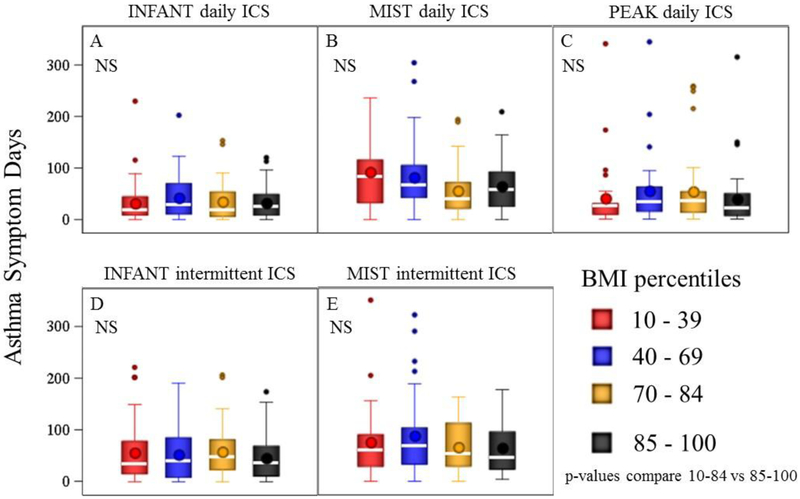
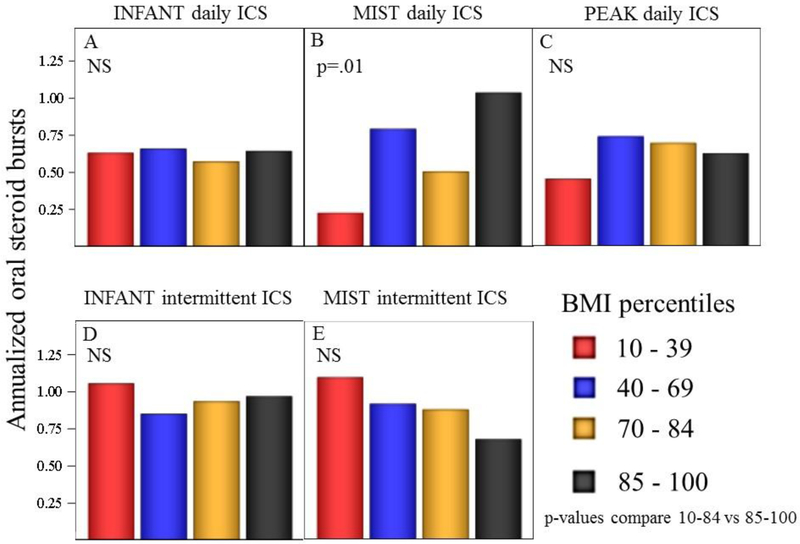
In the three studies, a total of 485 children were randomized to an ICS intervention, either as daily treatment or as one of two strategies of intermittent ICS treatment. We found no evidence of OW status affecting annualized asthma symptom days while on daily ICS or either of the two intermittent ICS treatments (see Table 3; Fig 1, p>0.05 for Panels A-E). OW also did not affect the rate of oral steroid courses while on either of the two intermittent ICS strategies (Fig 2, p>.05 for panels D-E). In the combined analysis of all three studies, OW children given daily ICS did have a significantly higher rate of oral steroid bursts. As association between OW and exacerbations was noted only in the MIST study (see Table 3, Fig 2 Panel B), while in the INFANT and PEAK studies OW participants treated with daily ICS experienced similar rates of exacerbations (Fig Panel A, C).
Table 3.
Asthma severity during daily or step-up inhaled corticosteroid (ICS) intervention by BMI-status
| BMI-percentile | |||
|---|---|---|---|
| Daily ICS* | 10–84th (N=328) | ≥85th (N=157) | p-value |
| Asthma Symptom Days, mean (95% CI)1 | 47.2 (40.8, 54.7) | 43.0 (35.1, 52.6) | 0.443 |
| Prednisone bursts, mean (95% CI)2 | 0.6 (0.5, 0.8) | 0.8 (0.6, 1.1) | 0.103 |
| Intermittent short-term ICS** | |||
| N | 71 | 33 | |
| Total intervention days, median (IQR) | 359 (350, 367) | 362 (343, 369) | |
| Asthma Symptom Days, mean (95% CI)1 | 61.8 (48.1, 79.3) | 52.9 (36.9, 76.0) | 0.464 |
| Prednisone bursts, mean (95% CI)2 | 1.1 (0.8, 1.6) | 0.8 (0.5, 1.3) | 0.254 |
| Intermittent as needed ICS*** | |||
| N | 140 | 75 | |
| Total intervention days, median (IQR) | 113 (111, 117) | 113 (112, 118) | |
| Asthma Symptom Days, mean (95% CI)1 | 53.3 (42.1, 67.4) | 47.3 (35.0, 63.9) | 0.534 |
| Prednisone bursts, mean (95% CI)2 | 1.0 (0.7, 1.4) | 1.1 (0.7, 1.7) | 0.724 |
BMI – body mass index, SFD – symptom free days.
data combined from INFANT, PEAK and MIST trials,
data from MIST trial,
data from INFANT trial.
annualized symptom days.
bursts represent new oral steroid starts.
p-values adjusted for trial and race/ethnicity
p-values adjusted for race/ethnicity
Figure 1.
Annualized Asthma Symptom Days among four BMI-percentile groups. Each panel indicates the study and treatment. Box plots represent medians and intra-quartile ranges. Whiskers represent 95th percentile ranges and points denote outliers. BMI-percentile grouping did not affect asthma symptom days, p-values were non-significant (NS) for all panels A-E comparing BMI-percentiles 10–84 vs. 85–100.
Figure 2.
Annualized exacerbations requiring oral steroid bursts among four BMI-percentile groups. Each panel indicates the study and treatment. Box plots represent medians and interquartile ranges. Whiskers represent 95th percentile ranges and points denote outliers. BMI-percentile grouping did not affect asthma exacerbations with the exception of MIST daily ICS. P-values were non-significant (NS) in panels (A, C, D, E) comparing BMI-percentile groups 10–84 vs. 85–100.
Interaction of Overweight/Obesity Status and Treatment with Daily ICS
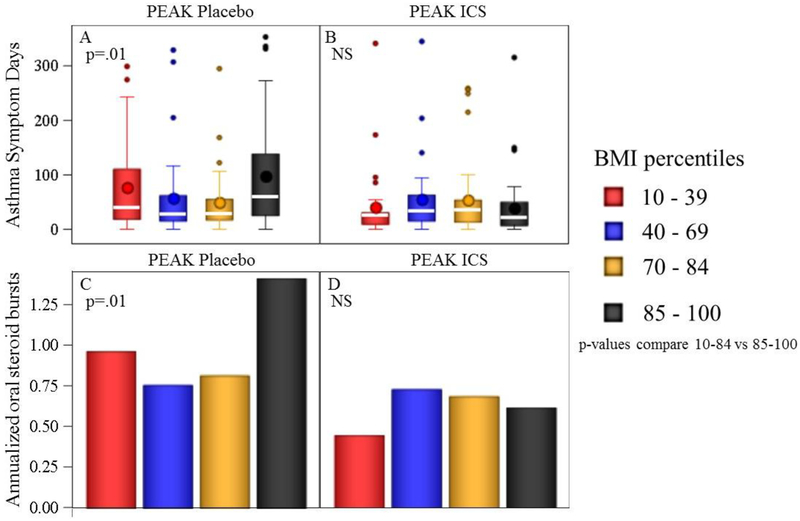
The PEAK trial was the only study which included both ICS-treated and placebo-treated participants in the same trial. Participants in the PEAK trial (n=269) received either daily ICS or placebo for 2 years, and are shown according to OW status (Table 4, Fig 3). Among children given placebo (n=137), OW status was associated with significantly more asthma symptoms days and oral steroid courses compared to NW status (Table 4). Placebo-treated OW children suffered 70% more symptom days (nearly 40 additional symptom days per year), and 75% more exacerbations compared to NW children. However, when similar children were randomized to daily ICS, the deleterious effects of OW status on symptoms days and oral steroid bursts were not observed. Only OW children displayed a significant ICS-related improvement in asthma symptom days (p=0.004) and oral steroid courses (p=0.006). The treatment*OW status interaction p-value approached but did not reach statistical significance for AD (p=0.065) or prednisone courses (p=0.13).
Table 4.
Response to inhaled corticosteroids (ICS) by BMI-status (PEAK study only)
| BMI-percentile | |||
|---|---|---|---|
| Placebo-treated | 10–84th (N=93) | ≥85th (N=44) | p-value3 |
| Total intervention days, median (IQR) | 672 (661, 679) | 672 (667, 685) | |
| Asthma Symptom Days, mean (95% CI)1 | 53.2 (40.1, 70.5) | 90.7 (61.8, 133.2) | 0.021 |
| Exacerbations, mean (95% CI)2 | 0.8 (0.6, 1.1) | 1.4 (1.0, 2.1) | 0.009 |
| Daily ICS | |||
| N | 92 | 40 | |
| Total intervention days, median (IQR) | 672 (663, 679) | 672 (669, 679) | |
| Asthma Symptom Days, mean (95% CI)1 | 44.9 (34.3, 58.8) | 41.2 (27.6, 61.5) | 0.72 |
| Exacerbations, mean (95% CI) | 0.6 (0.4, 0.8) | 0.6 (0.4, 1.0) | 0.78 |
| p-value comparing ASD (Placebo vs ICS) | 0.37 | 0.004 | |
| p-value comparing exacerbations (Placebo vs ICS) | 0.20 | 0.006 | |
| OW status *Treatment interaction on symptom days | 0.065 | ||
| OW status *Treatment interaction on exacerbations | 0.13 | ||
BMI – body mass index,
annualized symptom rate.
bursts represent new oral steroid starts.
p-values adjusted for race/ethnicity, PEAK – Prevention of Early Asthma in Kids trial
Figure 3.
Exacerbations and asthma symptom days among four BMI-percentile groups. Box plots (a, b) represent medians and intra-quartile ranges. Whiskers represent 95th% ranges. BMI-percentile grouping did not affect AD or exacerbations among ICS-treated, p>0.05; OW participants treated with placebo demonstrated significantly greater AD and exacerbations compared to NW (p=0.01 for both comparison). P-values in all panels compare BMI-percentiles 10–84 vs. 85–100.
DISCUSSION
Among preschool children with a past history of asthma symptoms and not on a daily controller, OW status is associated with significantly more asthma symptom days and exacerbations. OW status at baseline was associated with greater likelihood of recent hospitalization for asthma despite similar exposure to tobacco smoke and pets in the home, and reduced blood eosinophils. However, when OW and NW children were treated with ICS (either daily, intermittent step-up or as-needed), their asthma symptom days and exacerbations were similar. The weight effect with daily ICS differed somewhat among the three studies with regard to exacerbations. OW children treated with daily ICS in the MIST trial demonstrated significantly more exacerbations compared to NW children, while this OW-effect on exacerbations while on daily ICS was not seen in the PEAK or INFANT trials. OW preschoolers in the MIST study receiving intermittent ICS did not demonstrate greater exacerbations compared similarly-treated NWs. We conclude that OW status is associated with greater impairment and risk in preschool children who are off controller therapy. However, unlike what has been reported in older children, OW status is not clearly associated with reduced treatment response to ICS. Overall, preschool children in the three trials responded well to ICS, measured by daily asthma symptoms and, to a lesser extent, exacerbations.
This is the first study to our knowledge to examine the effect of high BMI on asthma severity and ICS response in preschool aged children. Strengths of the current study include that it involved a large number of preschoolers from three highly controlled trials with documented drug adherence and extensive phenotyping. The three trials were conducted prospectively by experienced pediatric asthma centers participating in two consecutive NIH-funded research networks and recruited participants from diverse backgrounds from around the US. The three trials had similar inclusion/exclusion criteria which allowed consolidation of data (see table E1).
Monitoring of asthma severity in the preschool age is challenging, and relies mainly on clinical markers of airway disease and in most children does not incorporate measures of airway responsiveness or airway inflammation. The current recommendations for assessing asthma control in this young age group involves daily monitoring for frequency and severity of symptoms and their impact on functioning - which describes the impairment domain of asthma control. Preschool children are most impacted by episodic severe exacerbations of symptoms which often result in systemic steroid treatment and urgent care visits. The risk of asthma exacerbations corresponds to the risk domain of asthma control and is particularly important to the care of preschool children. Past studies which have attempted to evaluate the effect of high BMI in preschool children on asthma severity have been very few in number and have not utilized data from rigorously controlled trials with precise outcomes. For example, Aragona and colleagues conducted a retrospective cohort study using billing system and chart review data of hospitalized patients in the US(34). Among children <5 years of age, overweight/obese children had greater than twice the odds of a repeat emergency department visit for asthma following discharge, while overweight/obesity status exerted no effect in all other outcomes related to asthma including length of intensive care and hospital stay, total health care charges, and repeat admission. This retrospective analysis was limited by the fact that it assessed length of stay resulting from all treatments occurring during hospitalization, and overweight/obesity effects were studied only among a subset of preschoolers (i.e. those requiring hospitalization). Silveira and colleagues(35) conducted a case control study in two Brazilian teaching hospitals involving 3–12 year olds, where cases and controls involved children with persistent asthma and intermittent asthma, respectively. The study did not stratify by age and found that obesity was associated with higher odds of persistent (versus intermittent) asthma. Both of these studies report some association between high BMI and surrogates of asthma severity, however both studies were modest in size and measurement bias and confounding from socioeconomic factors likely had some effect influence. The current study is the first to our knowledge to apply prospectively collected outcomes of both impairment and risk domains to assess the effect of body habitus on asthma severity. OW children in the current study not on daily ICS had nearly double the asthma symptom days (roughly 0.5–1.3 excess symptom days per week, or 22–63 excess symptom days per year) and more than double the average annual exacerbations compared to NW children, which equates to a difference that is clinically meaningful.
Inhaled corticosteroids are clearly efficacious versus placebo in the control and prevention of asthma symptoms for most preschool children(36–39). In fact, ICS appears to be the most efficacious single therapy for the prevention of asthma symptoms in school age children(40–43) and preschoolers(26, 44). However, poor response to ICS among preschoolers and school age children remains a problem as evidenced by the high frequency of breakthrough exacerbations in ICS-treated preschoolers and the high degree of differential response in older children (45, 46). Pooled data suggest that among preschool children the percentage of ICS responders may be as low as 40% (47, 48). Establishing markers in preschool children which predict response to ICS would be a marked advance in clinical care. Current predictors of more favorable ICS response in the PEAK trial included male sex, white race, presence of atopy and recent asthma-related ED use(49). Several studies in adults have reported that ICS is less effective in obese patients as asthma control days (22), rescue use (9), lung function (9, 50) and exacerbations (50). The mechanism(s) underlying these reduced treatment responses in the obese are unclear, but may be related to greater neutrophilic airway inflammation in OW subjects (51, 52), which has been associated with poor ICS response(53). Additionally, OW status may also cause impaired apoptotic airway cell clearance (efferocytosis), which is key to resolving inflammation and airway health (54). Based on our findings, these mechanisms do not appear to be leading in preschool children to reduced ICS efficacy.
Preschoolers in this study were treated with three possible ICS regimens (once daily, intermittent step-up, and intermittent short-term). Each analysis evaluated the effect of OW on both AD and exacerbations. Among the resulting six analyses, OW was associated with worse ICS responses in just one (MIST daily ICS on exacerbations). Among the PEAK analysis, which had the longest observation period, daily ICS significantly improved both exacerbations and AD among OW preschoolers compared to OW preschoolers treated with placebo, while a similar improvement over placebo was not seen in NW preschoolers. We conclude that overall OW preschool children display a robust response to ICS, unlike what has been reported in older OW children and adults. Though more research is needed in refining the optimal ICS strategy in this age-group (daily vs. different intermittent approaches), the mainstay for symptomatic preschool children with asthma or recurrent wheezing at high risk for asthma has been the use of ICS which we propose should remain the case in OW preschoolers.
The current study has several limitations, including its post-hoc nature. Post-hoc analyses are important for the generation of scientific hypotheses but should be regarded cautiously until findings can be replicated. However, the hypothesis and analytic approach of the current study was proposed a priori (before data was released for analysis). Since the study was not specifically powered to analyze OW-related effects, it is possible that a larger analysis could demonstrate different results. We chose the most common convention for defining pediatric overweight/obesity (i.e. BMI>85th percentile), however high BMI percentile is only a marker for adiposity and can become elevated, particularly in shorter or muscular children, though this is probably less of a concern in preschool children compared to older children and adults. Since this analysis did not follow BMI-percentile over several years and did not involve a non-asthmatic comparison group, we are only able to assess phenotypic (impairment, risk) associations with OW among asthmatics. Though we did make statistical adjustments for differences between OW and NW children in race and ethnicity, we did not measure specific markers of socioeconomics (such as income or health literacy) which could be a confounding third factor associated with OW. In addition, our analysis was limited by the fact that we combined data from three studies with slightly different inclusion criteria and outcomes. We were limited to analyzing outcomes which were measured similarly (asthma symptom days, exacerbations) in all three studies and we did not analyze lung function. We chose to analyze only the children who were 12–59 months of age at enrollment to account for the slightly different ages across studies. Lastly, we did not attempt to adjust for possible variations in adherence.
In conclusion, early life high BMI does appear to worsen both impairment and risk domains of asthma severity in preschool children off controller therapy. Interventions which reduce early life weight gain and overweight/obesity status may benefit respiratory health in preschool children and deserves future study of interventions aimed at reducing adiposity. OW status was not clearly associated with reduced response to ICS. Overall, preschool children in the three trials responded well to ICS, measured by daily asthma symptoms and, to a lesser extent, exacerbations, and thus ICS should remain the first-line treatment option for this high morbidity group.
Supplementary Material
Funding source:
Funded by the National Heart, Lung and Blood Institute - AsthmaNet
INFANT https://ClinicalTrials.gov number,
PEAK https://ClinicalTrials.gov number,
MIST https://ClinicalTrials.gov number,
Abbreviations
- ANOVA
analysis of variance
- AD
asthma symptom days
- BMI
body mass index
- CAMP
Childhood Asthma Management Program
- CARE
Childhood Asthma Research and Education Program
- CDC
Centers for Disease Control & Prevention
- CI
confidence intervals
- ED
emergency department
- ICS
inhaled corticosteroids
- INFANT
Individualized Therapy for Asthma in Toddlers
- LTRA
leukotriene receptor antagonist
- MIST
Maintenance versus Intermittent Inhaled Steroids in Wheezing Toddlers trial
- OR
odds ratio
- OW
overweight/obese
- PEAK
Prevention of Early Asthma in Kids trial
- SABA
short-acting Beta-2-agonists
Contributor Information
Jason E. Lang, Division of Allergy/Immunology and Pulmonary Medicine, Duke University School of Medicine, Children’s Hospital and Health Center, Durham, NC
Anne M. Fitzpatrick, Emory University, Department of Pediatrics, Atlanta, GA
David T. Mauger, Penn State University, College of Medicine, Department of Public Health Sciences, Hershey, PA
Theresa W. Guilbert, Cincinnati Children’s Hospital and Medical Center, Cincinnati, OH
Daniel J. Jackson, University of Wisconsin School of Medicine and Public Health, Pediatrics Section of Allergy, Immunology and Rheumatology, Madison, WI
Robert F. Lemanske, Jr., University of Wisconsin School of Medicine and Public Health, Pediatrics, Madison, WI
Fernando D. Martinez, University of Arizona, Arizona Respiratory Center, Tuscon, AZ
Robert C. Strunk, Washington University School of Medicine, St. Louis, MO
Robert S. Zeiger, Kaiser Permanente Medical Center, University of California - San Diego, San Diego, CA
Wanda Phipatanakul, Boston Children’s Hospital, Harvard Medical School, Boston, MA
Leonard B. Bacharier, Washington University School of Medicine, St. Louis, MO
Jacqueline A. Pongracic, Children’s Memorial Hospital, Chicago, IL
Fernando Holguin, University of Pittsburgh, Pittsburgh, PA
Michael D. Cabana, University of California-San Francisco, San Francisco, CA
Ronina A. Covar, National Jewish Health, Denver, CO
Hengameh H. Raissy, University of New Mexico, Albuquerque, NM.
Monica Tang, Division of Allergy/Immunology and Pulmonary Medicine, Duke University School of Medicine, Children’s Hospital and Health Center, Durham, NC
Stanley J. Szefler, Children’s Hospital Colorado, The Breathing Institute, and University of Colorado School of Medicine, Aurora, CO.
References:
- 1.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS data brief. 2012(94):1–8. [PubMed] [Google Scholar]
- 2.Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United States, 2001–2010. Vital & health statistics Series 3, Analytical and epidemiological studies / [US Dept 2012(35):1–67. [PubMed] [Google Scholar]
- 3.NAEPP. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (Full Report 2007). NHLBI/NIH, US Department of Health and Human Services; 2007. August 28, 2007. Contract No.: NIH Publication No. 07–4051. [Google Scholar]
- 4.Michelson PH, Williams LW, Benjamin DK, Barnato AE. Obesity, inflammation, and asthma severity in childhood: data from the National Health and Nutrition Examination Survey 2001–2004. Ann Allergy Asthma Immunol. 2009;103(5):381–5. [DOI] [PubMed] [Google Scholar]
- 5.Luder E, Melnik TA, DiMaio M. Association of being overweight with greater asthma symptoms in inner city black and Hispanic children. J Pediatr. 1998;132(4):699–703. [DOI] [PubMed] [Google Scholar]
- 6.Quinto KB, Zuraw BL, Poon KY, Chen W, Schatz M, Christiansen SC. The association of obesity and asthma severity and control in children. J Allergy Clin Immunol. 2011;128(5):964–9. [DOI] [PubMed] [Google Scholar]
- 7.Carroll CL, Stoltz P, Raykov N, Smith SR, Zucker AR. Childhood overweight increases hospital admission rates for asthma. Pediatrics. 2007;120(4):734–40. [DOI] [PubMed] [Google Scholar]
- 8.Schatz M, Zeiger RS, Zhang F, Chen W, Yang SJ, Camargo CA Jr. Overweight/obesity and risk of seasonal asthma exacerbations. J Allergy Clin Immunol Pract. 2013;1(6):618–22. [DOI] [PubMed] [Google Scholar]
- 9.Camargo CA Jr., Boulet LP, Sutherland ER, Busse WW, Yancey SW, Emmett AH, et al. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J Asthma. 2010;47(1):76–82. [DOI] [PubMed] [Google Scholar]
- 10.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007;101(11):2240–7. [DOI] [PubMed] [Google Scholar]
- 11.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedon JC. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127(3):741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mougey E, Lang JE, Allayee H, Teague WG, Dozor AJ, Wise RA, et al. ALOX5 polymorphism associates with increased leukotriene production and reduced lung function and asthma control in children with poorly controlled asthma. Clin Exp Allergy. 2013;43(5):512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters JI, McKinney JM, Smith B, Wood P, Forkner E, Galbreath AD. Impact of obesity in asthma: evidence from a large prospective disease management study. Ann Allergy Asthma Immunol. 2011;106(1):30–5. [DOI] [PubMed] [Google Scholar]
- 14.Ginde AA, Santillan AA, Clark S, Camargo CA, Jr. Body mass index and acute asthma severity among children presenting to the emergency department. Pediatr Allergy Immunol. 2010;21(3):480–8. [DOI] [PubMed] [Google Scholar]
- 15.Lang JE, Hossain MJ, Lima JJ. Overweight children report qualitatively distinct asthma symptoms: analysis of validated symptom measures. J Allergy Clin Immunol. 2015;135(4):886–93 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP). Thorax. 2003;58(12):1036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Gent R, van der Ent CK, Rovers MM, Kimpen JL, van Essen-Zandvliet LE, de Meer G. Excessive body weight is associated with additional loss of quality of life in children with asthma. J Allergy Clin Immunol. 2007;119(3):591–6. [DOI] [PubMed] [Google Scholar]
- 18.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. The New England journal of medicine. 1995;332(3):133–8. [DOI] [PubMed] [Google Scholar]
- 19.Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatr Pulmonol. 2007;42(8):723–8. [DOI] [PubMed] [Google Scholar]
- 20.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama. 2014;311(8):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutherland ER, Camargo CA Jr., Busse WW, Meltzer EO, Ortega HG, Yancey SW, et al. Comparative effect of body mass index on response to asthma controller therapy. Allergy Asthma Proc. 2010;31(1):20–5. [DOI] [PubMed] [Google Scholar]
- 22.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. The European respiratory journal. 2006;27(3):495–503. [DOI] [PubMed] [Google Scholar]
- 23.Guilbert TW, Morgan WJ, Krawiec M, Lemanske RF Jr., Sorkness C, Szefler SJ, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Controlled clinical trials. 2004;25(3):286–310. [DOI] [PubMed] [Google Scholar]
- 24.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354(19):1985–97. [DOI] [PubMed] [Google Scholar]
- 25.Zeiger RS, Mauger D, Bacharier LB, Guilbert TW, Martinez FD, Lemanske RF Jr., et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med. 2011;365(21):1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzpatrick AM, Jackson DJ, Mauger DT, Boehmer SJ, Phipatanakul W, Sheehan WJ, et al. Individualized Therapy for Persistent Asthma in Young Children. J Allergy Clin Immunol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang JE, Hossain J, Smith K, Lima JJ. Asthma severity, exacerbation risk, and controller treatment burden in underweight and obese children. J Asthma. 2012;49(5):456–63. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan WJ, Mauger DT, Paul IM, Moy JN, Boehmer SJ, Szefler SJ, et al. Acetaminophen versus Ibuprofen in Young Children with Mild Persistent Asthma. N Engl J Med. 2016;375(7):619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 Suppl 4:S164–92. [DOI] [PubMed] [Google Scholar]
- 30.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159(3):785–90. [DOI] [PubMed] [Google Scholar]
- 31.Ater D, Bar BE, Fireman N, Fireman E, Shai H, Tasher D, et al. Asthma-predictive-index, bronchial-challenge, sputum eosinophils in acutely wheezing preschoolers. Pediatr Pulmonol. 2014;49(10):952–9. [DOI] [PubMed] [Google Scholar]
- 32.Larsen JM, Brix S, Thysen AH, Birch S, Rasmussen MA, Bisgaard H. Children with asthma by school age display aberrant immune responses to pathogenic airway bacteria as infants. J Allergy Clin Immunol. 2014;133(4):1008–13. [DOI] [PubMed] [Google Scholar]
- 33.Paul IM, Camera L, Zeiger RS, Guilbert TW, Bacharier LB, Taussig LM, et al. Relationship between infant weight gain and later asthma. Pediatr Allergy Immunol. 2010;21(1 Pt 1):82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aragona E, El-Magbri E, Wang J, Scheckelhoff T, Scheckelhoff T, Hyacinthe A, et al. Impact of Obesity on Clinical Outcomes in Urban Children Hospitalized for Status Asthmaticus. Hosp Pediatr. 2016;6(4):211–8. [DOI] [PubMed] [Google Scholar]
- 35.Silveira DH, Zhang L, Prietsch SO, Vecchi AA, Susin LR. Nutritional status, adiposity and asthma severity and control in children. J Paediatr Child Health. 2015;51(10):1001–6. [DOI] [PubMed] [Google Scholar]
- 36.Bisgaard H, Allen D, Milanowski J, Kalev I, Willits L, Davies P. Twelve-month safety and efficacy of inhaled fluticasone propionate in children aged 1 to 3 years with recurrent wheezing. Pediatrics. 2004;113(2):e87–94. [DOI] [PubMed] [Google Scholar]
- 37.Connett GJ, Warde C, Wooler E, Lenney W. Use of budesonide in severe asthmatics aged 1–3 years. Archives of disease in childhood. 1993;69(3):351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bisgaard H, Munck SL, Nielsen JP, Petersen W, Ohlsson SV. Inhaled budesonide for treatment of recurrent wheezing in early childhood. Lancet. 1990;336(8716):649–51. [DOI] [PubMed] [Google Scholar]
- 39.Gleeson JG, Price JF. Controlled trial of budesonide given by the nebuhaler in preschool children with asthma. BMJ (Clinical research ed 1988;297(6642):163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostrom NK, Decotiis BA, Lincourt WR, Edwards LD, Hanson KM, Carranza Rosenzweig JR, et al. Comparative efficacy and safety of low-dose fluticasone propionate and montelukast in children with persistent asthma. J Pediatr. 2005;147(2):213–20. [DOI] [PubMed] [Google Scholar]
- 41.Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117(1):45–52. [DOI] [PubMed] [Google Scholar]
- 42.Sorkness CA, Lemanske RF Jr., Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119(1):64–72. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Hollenbeak CS, Mauger DT, Zeiger RS, Paul IM, Sorkness CA, et al. Cost-effectiveness analysis of fluticasone versus montelukast in children with mild-to-moderate persistent asthma in the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2011;127(1):161–6, 6 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szefler SJ, Baker JW, Uryniak T, Goldman M, Silkoff PE. Comparative study of budesonide inhalation suspension and montelukast in young children with mild persistent asthma. J Allergy Clin Immunol. 2007;120(5):1043–50. [DOI] [PubMed] [Google Scholar]
- 45.Lemanske RF Jr., Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362(11):975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115(2):233–42. [DOI] [PubMed] [Google Scholar]
- 47.Ducharme FM, Tse SM, Chauhan B. Diagnosis, management, and prognosis of preschool wheeze. Lancet. 2014;383(9928):1593–604. [DOI] [PubMed] [Google Scholar]
- 48.Castro-Rodriguez JA, Rodrigo GJ. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with meta-analysis. Pediatrics. 2009;123(3):e519–25. [DOI] [PubMed] [Google Scholar]
- 49.Bacharier LB, Guilbert TW, Zeiger RS, Strunk RC, Morgan WJ, Lemanske RF Jr., et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J Allergy Clin Immunol. 2009;123(5):1077–82, 82 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camargo CA Jr., Sutherland ER, Bailey W, Castro M, Yancey SW, Emmett AH, et al. Effect of increased body mass index on asthma risk, impairment and response to asthma controller therapy in African Americans. Curr Med Res Opin. 2010;26(7):1629–35. [DOI] [PubMed] [Google Scholar]
- 51.Telenga ED, Tideman SW, Kerstjens HA, Hacken NH, Timens W, Postma DS, et al. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy. 2012;67(8):1060–8. [DOI] [PubMed] [Google Scholar]
- 52.Gibson PG. Obesity and asthma. Ann Am Thorac Soc. 2013;10 Suppl:S138–42. [DOI] [PubMed] [Google Scholar]
- 53.Cox G Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995;154(9):4719–25. [PubMed] [Google Scholar]
- 54.Fernandez-Boyanapalli R, Goleva E, Kolakowski C, Min E, Day B, Leung DY, et al. Obesity impairs apoptotic cell clearance in asthma. J Allergy Clin Immunol. 2013;131(4):1041–7, 7 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.