Abstract
Sexually dimorphic innate immune responses have been observed in several species, but have not been studied in response to a live pathogen challenge in pigs. This study aimed to elucidate sexually dimorphic innate immune responses along with Salmonella translocation patterns in newly weaned pigs orally inoculated with Salmonella. Newly weaned pigs (n = 8 gilts and 12 barrows; 6.2 ± 0.2 kg BW) were obtained from a commercial swine facility and were maintained in an environmentally-controlled facility in individual pens equipped with feeders and nipple waterers. Pigs were allowed ad libitum access to a commercial non-medicated starter ration and water throughout the study. On d 12 post-weaning, pigs were anesthetized to allow placement of a temperature measuring device in the abdominal cavity for measurement of intraperitoneal temperature (TEMP). On d 17, pigs were anesthetized and fitted with indwelling jugular vein catheters. On the following day (d 18), pigs were orally inoculated with 4.7x109Salmonella typhimurium. Blood samples were collected at 0.5-h intervals from -2 to 8 h, and at 8-h intervals from 8 to 72 h post-challenge. Whole blood was analyzed for complete blood cell counts. Serum was isolated for measurement of cortisol. Following collection of the 72 h sample, pigs were humanely euthanized and tissues were collected for Salmonella isolation. There was a sex × time interaction (P < 0.001) for TEMP such that gilts had a greater TEMP response to the Salmonella challenge compared to barrows. There was also a sex × time interaction (P = 0.03) for serum cortisol with gilts having decreased cortisol at 16 h yet greater cortisol at 32 h than barrows. Barrows had greater total white blood cells (17.8 vs. 16.2 ± 0.4 103 cells/μL; P < 0.01; respectively) and neutrophils (7.8 vs. 6.1 ± 0.4 103 cells/μL; P < 0.01; respectively) than gilts. However, gilts had greater lymphocytes (9.6 vs. 9.0 ± 0.2 103 cells/μL; P = 0.05; respectively) than barrows. While immune parameters were influenced by sex, there was no effect of sex (P > 0.05) on Salmonella concentrations from fecal shedding 3 d post-inoculation in the cecum, mesenteric and subiliac lymph nodes, liver, spleen, gallbladder, or kidney tissues. These data demonstrate that weaned gilts appear to produce a stronger acute phase response to a Salmonella challenge compared to barrows, without affecting the tissue translocation or shedding of Salmonella.
Keywords: cortisol, innate immunity, pigs, Salmonella, sexual dimorphism
INTRODUCTION
The responsiveness of the immune system to a perceived challenge can be modulated by various factors, including internal factors such as the hypothalamic-pituitary-adrenal (HPA) axis and energy availability/storage, as well as naturally-occurring factors such as behavior, breed, and sex (Burdick et al., 2009; Carroll et al., 2011; Carroll et al., 2015). Specifically regarding sex, studies conducted by our lab have demonstrated sex-specific innate immune responses to a lipopolysaccharide (LPS), and to a dual corticotropin-releasing hormone (CRH) and vasopressin challenge in cattle (Hulbert et al., 2013; Carroll et al., 2015). Additionally, Williams et al. (2009) observed greater baseline and peak serum TNF-α and IL-6, but decreased IL-1β concentrations in weaned gilts in response to an LPS challenge compared to barrows. Thus, sexual dimorphic effects are found in livestock species, and particularly in pigs utilizing exogenous challenges.
Utilizing substances such as LPS and CRH to exogenously stimulate immune and stress responses in the body have proven to be useful models for elucidating these complex biological systems. However, the use of live pathogen challenge models provides a further means of studying immune and stress responses that are more reflective of real-world scenarios. Live pathogen challenge studies are more indicative and representative of the immunological challenges pigs encounter in the commercial production setting; however increased variation is introduced due to endogenous factors associated with each animal and the specific microorganism. The use of a live Salmonella challenge in swine is of particular relevance due to the health issues associated with Salmonella outbreaks in swine herds, and due to an increasing concern within the livestock industry associated with post-harvest contamination, food safety and human illness (Ojha and Kostrzynska, 2007). Aligned with the observed effects of sex on the innate immune response that have been observed in response to various immune challenges in swine and other species, this study sought to determine whether newly weaned pigs exhibit sexually dimorphic innate immune responses to an oral Salmonella typhimurium challenge.
MATERIALS AND METHODS
All experimental procedures were in compliance with the Guide for the Care and Use of Agricultural Animals in Research and Teaching and approved by the Institutional Animal Care and Use Committee at the Livestock Issues Research Unit (Protocol # 2014–04-JAC18).
Experimental Design
Newly weaned pigs (n = 8 gilts and 12 barrows; 6.2 ± 0.2 kg body weight) were obtained from a commercial swine facility and transported to the Livestock Issues Research Unit's Liberty Farm facility. Pigs were housed in an enclosed, environmentally-controlled facility in individual pens equipped with stainless steel feeders and nipple waterers. Pigs were allowed ad libitum access to a commercial non-medicated starter ration and water throughout the study. On d 12 post-weaning, pigs were anesthetized and a small incision (2 to 2.5 cm) was made in the lower abdominal region for the placement of an indwelling temperature recording device (25.4 mm in length, 8.3 mm in diameter, 3.3 g; Star Oddi DST micro-T; MeterMall USA, Marysville, OH) into the peritoneal cavity. Intraperitoneal temperature was measured at 5-min intervals from the time of the placement of the temperature device until the end of the study. On d 17, pigs were anesthetized and non-surgically fitted with indwelling jugular vein catheters (Carroll et al., 1999). On d 18, pigs were orally inoculated with 4.7 × 109 cfu/pig of a nalidixic acid-resistant Salmonella typhimurium in 10 mL of culture media. One 4.5-mL whole blood sample was collected in Starstedt tubes containing no additive (Sarstedt Inc., Newton, NC) at 0.5 h intervals from -2 to 8 h, and at 8-h intervals from 8 to 72 h post-challenge. Samples were allowed to clot at room temperature for 30 min prior to centrifugation at 1,500 g for 20 min at 4°C. Isolated serum was stored at -80°C until analyzed for cortisol concentrations. A second 4-mL sample was collected in a vacutainer containing EDTA for determination of complete blood counts (CBC) using a ProCyte Dx Hematology Analyzer (IDEXX, Westbrook, ME). Sickness behavior scores were recorded for each pig following the collection of each blood sample. Additionally, individual pig feeders were weighed on d 0, 12, 18, and 22 to measure average daily feed disappearance. Fecal samples were collected on d 19 (0 h), 20 (24 h), 21 (48 h) and 22 (72 h) after the challenge to determine fecal shedding of the inoculated Salmonella. Following collection of the 72 h sample, pigs were humanely euthanized and samples of the cecum, mesenteric lymph node, subiliac lymph node, liver, spleen, gallbladder and kidney were collected to detect any Salmonella translocation into these tissues.
Sickness Behavior Scores
Sickness behavior scores were assigned to each pig by a single observer skilled at assessing sickness behavior in cattle and pigs. Pigs assigned a score of 1 exhibited normal maintenance behaviors. Pigs receiving a score of 2 were calm, but were lying down with increased respiration. A score of 3 was given to pigs exhibiting clinical signs of sickness including increased respiration with drool, scours, and(or) coughing. If a pig was observed to be lying on its side with labored breathing and frothing at the mouth it was given a score of 4, and then was humanely euthanized.
Cortisol Analysis
Serum cortisol concentrations were determined in duplicate using a commercially available, porcine-specific enzyme immunoassay kit (Abnova, Walnut, CA) according to the manufacturer's directions by comparison of unknowns to standard curves generated with known concentrations of cortisol. The minimum detectable cortisol concentration was 0.2 ng/mL, and the intra- and inter-assay coefficients of variation were 10.1% and 8.2%, respectively.
Salmonella Isolation
Prior to experimental infection, fecal samples from all pigs were collected to confirm the absence of Salmonella or the presence of bacteria with similar morphology resistant to nalidixic acid. All fecal and tissue samples were aseptically collected, weighed (10 g), homogenized, and placed in a 1:10 dilution of phosphate buffered saline (PBS). Overnight cultures were serially diluted in PBS and plated on Luria-Bertani (LB) agar with 50 µg/mL nalidixic acid and manually counted the following day after incubation for 18 to 24 h at 37°C. Isolates were confirmed as Salmonella via biochemical reactions on 4 bacterial test media: Triple Sugar Iron Slants, Indole Test, Lysine Iron Agar, and Urease Test. Negative cultures were enriched overnight in tetrathionate broth and re-plated.
Statistical Analysis
Prior to analysis, intraperitoneal temperature (TEMP) was averaged into 1-h intervals. Intraperitoneal temperature, serum cortisol, and CBC data were analyzed using the MIXED procedure of SAS specific for repeated measures (SAS Inst. Inc., Cary, NC). Sex, time and their interaction were included as fixed effects with pig within sex as the experimental unit. Specific treatment comparisons were made using the PDIFF option in SAS, with P ≤ 0.05 considered significant and P ≤ 0.10 considered a tendency. Salmonella tissue and fecal count data were analyzed in SAS using Proc Glimmix of SAS at an α = 0.05 utilizing the Tukey option for mean separation. Positive/negative binomial data to indicate the presence or absence of Salmonella in a tissue were analyzed using logistics regression in Proc Glimmix. All data are presented as the LSM ± SEM.
RESULTS
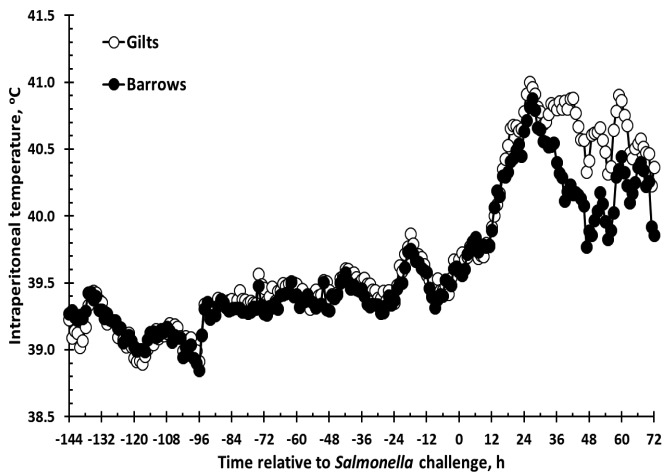
There was a sex × time interaction (P < 0.001) for TEMP. Specifically, gilts had greater TEMP than barrows from 36 to 63 h and at 72 h post-Salmonella challenge (Fig. 1). Additionally, there were also sex (P < 0.001) and time (P < 0.001) effects for TEMP. Intraperitoneal temperature was greater in gilts (39.72 ± 0.01°C) than barrows (39.61 ± 0.01°C). Intraperitoneal temperature generally increased from -144 h (insertion of temperature probes) to 0 h (immediately prior to oral Salmonella challenge; P < 0.001; -144 h vs. 0 h), and subsequently increased in response to the Salmonella challenge beginning at 12 h which persisted through the end of the study (P ≤ 0.02).
Figure 1.
Effect of sex on the intraperitoneal temperature (TEMP) response to an oral Salmonella challenge (4.7 × 109 cfu/pig Salmonella typhimurium) in weaned pigs. Gilts n = 8, Barrows n = 12. Data presented as the LSM. SEM is ± 0.13 for Gilts and ± 0.10 for Barrows. There was a sex × time interaction (P < 0.001) with gilts having greater TEMP response than barrows from 36 to 63 and at 72 h post-Salmonella challenge.
Sickness behavior score was measured on pigs throughout the study. Interestingly, all pigs exhibited normal maintenance behaviors (i.e., a score of 1) throughout the entire study. Therefore, no differences in sickness behaviors were observed. However, differences in feed disappearance were observed during the study. Specifically, average daily feed disappearance increased from d 0 to 12 (0.21 ± 0.02 kg/d) to d 12 to 18 (0.51 ± 0.02 kg/d; P < 0.001), but decreased following Salmonella challenge to the end of the study (d 18 to 22; 0.38 ± 0.02; P < 0.001). There was no effect of sex (P = 0.38) or sex × time (P = 0.75) on average daily feed disappearance.
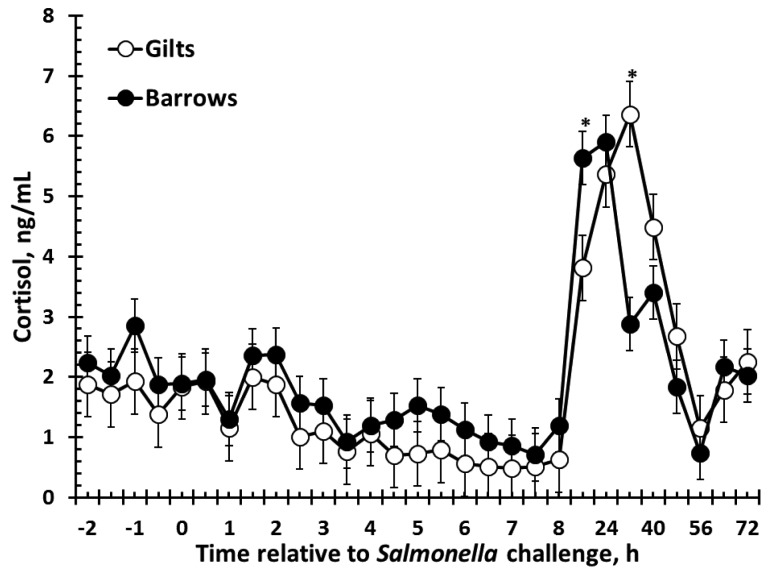
There was a sex × time interaction (P = 0.03) for serum cortisol concentrations. Specifically, cortisol concentrations were greater in barrows than gilts at 16 h (5.6 ± 0.4 vs. 3.8 ± 0.5 ng/mL; P = 0.009), yet cortisol was greater in gilts than barrows 32 h post Salmonella challenge (6.4 ± 0.5 vs. 2.9 ± 0.4 ng/mL; P < 0.001; Fig. 2). Overall, there was no effect of sex (P = 0.16) but there was an effect of time (P < 0.01) such that cortisol concentrations decreased below -2 h values at 3.5 h post-challenge (P = 0.01) and remained there before increasing above -2 h values at 16 h post-challenge (P < 0.001) and returning to baseline values by 64 h post-challenge (P = 0.87; -2 h vs. 64 h).
Figure 2.
Effect of sex on the serum cortisol response to an oral Salmonella challenge (4.7 × 109 cfu/pig Salmonella typhimurium) in weaned pigs. Gilts n = 8, Barrows n = 12. Data presented as the LSM ± SEM. Sex × time interaction (P = 0.03). *Gilts and Barrows differ P ≤ 0.009.
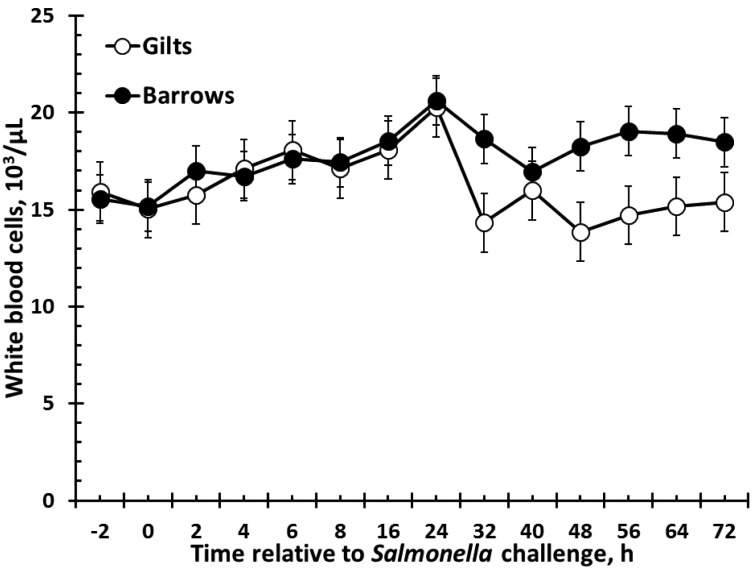
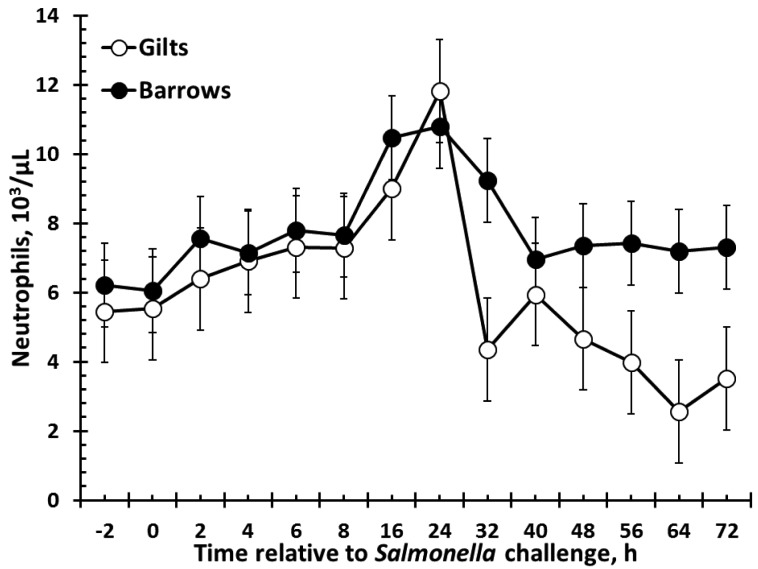
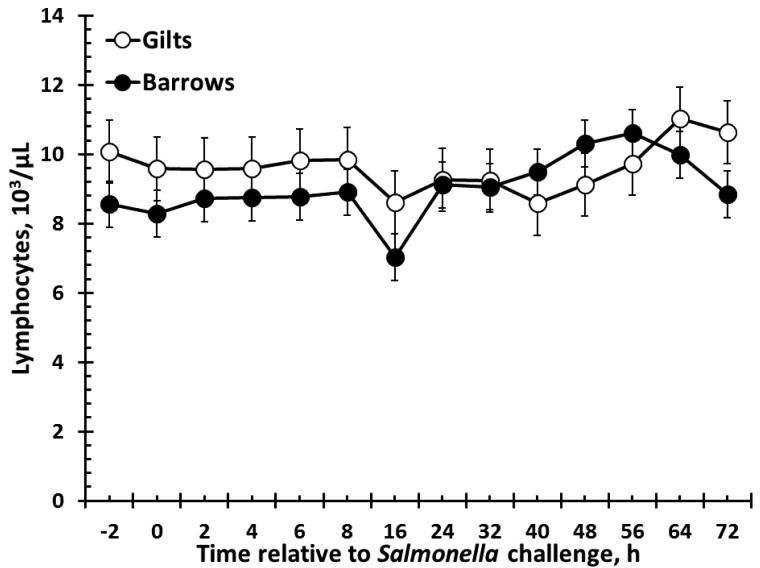
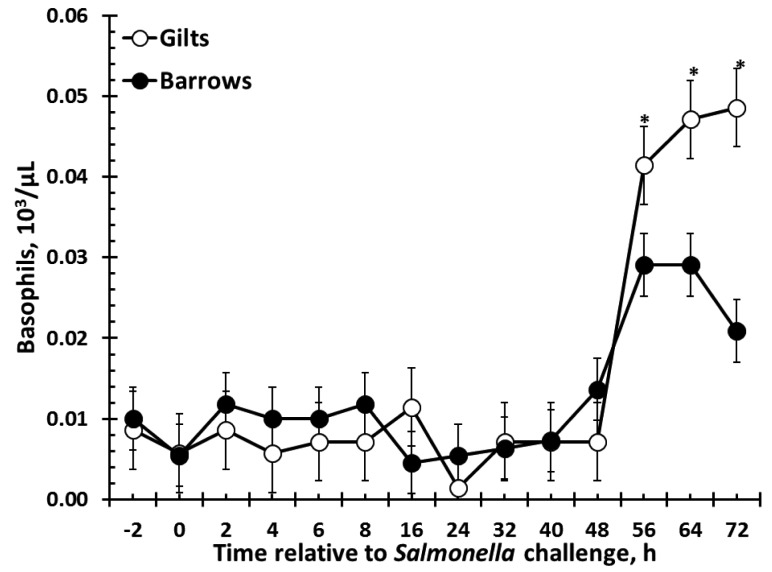
There was no effect of sex on red blood cells, hemoglobin, monocytes, or eosinophils (Table 1). There were sex (P = 0.02) and time (P < 0.001) effects for hematocrit such that barrows had greater hematocrit than gilts, and hematocrit decreased over time (Table 1). Also, similar results were found for platelet counts, such that barrows had greater platelets than gilts (P < 0.001), with counts fluctuating over time (P = 0.003; Table 1). Total white blood cells (WBC) were affected by sex (P = 0.003; Fig. 3). Specifically, barrows had greater total WBC counts than gilts. There was a tendency (P = 0.06) for a decrease in WBC following Salmonella challenge, and there was no sex × time interaction (P = 0.49). There was also a sex effect for neutrophils (P < 0.001) such that barrows had greater neutrophils than gilts (Fig. 4). Neutrophil counts decreased following Salmonella challenge (P < 0.001), but there was no sex × time interaction (P = 0.55). In contrast to total WBC and neutrophils, lymphocytes were greater in gilts than barrows (P = 0.05), yet there was no effect of time (P = 0.27) or sex × time interaction (P = 0.71; Fig. 5). There was a sex × time interaction (P = 0.002) for basophil counts, with gilts having greater basophils from 56 to 72 h (P ≤ 0.05; Fig. 6). There was also an effect of time, with basophil counts increasing from 56 to 72 h post-Salmonella challenge, and there was a tendency (P = 0.10) for gilts to have greater basophil counts than barrows.
Table 1.
Summary of hematology variables measured in gilts and barrows in response to an oral Salmonella typhimurium challenge
| Variable | Sex1 | SEM | P-value | |||
|---|---|---|---|---|---|---|
| Gilts | Barrows | Sex | Time | Sex × time | ||
| Red blood cells, 106/µL | 4.83 | 4.82 | 0.07 | 0.95 | < 0.001 | 1.00 |
| Hemoglobin, g/dL | 8.22 | 8.35 | 0.06 | 0.11 | < 0.001 | 0.99 |
| Hematocrit, % | 26.91 | 27.58 | 0.23 | 0.02 | < 0.001 | 0.99 |
| Platelets, 103/µL | 402.44 | 446.11 | 6.41 | < 0.001 | 0.003 | 0.99 |
| White blood cells, 103/µL | 16.20 | 17.78 | 0.40 | 0.003 | 0.06 | 0.49 |
| Neutrophils, 103/µL | 6.05 | 7.80 | 0.40 | < 0.001 | < 0.001 | 0.55 |
| Lymphocytes, 103/µL | 9.62 | 9.03 | 0.24 | 0.05 | 0.27 | 0.71 |
| Monocytes, 103/µL | 1.10 | 1.09 | 0.05 | 0.80 | < 0.001 | 0.98 |
| Eosinophils, 103/µL | 0.23 | 0.25 | 0.01 | 0.22 | < 0.001 | 0.77 |
| Basophils, 103/µL | 0.02 | 0.01 | 0.00 | 0.10 | < 0.001 | 0.002 |
Values for sex represent average values for each sex pooled over time.
Figure 3.
Effect of sex on the total white blood cell (WBC) response to an oral Salmonella challenge (4.7 × 109 cfu/pig Salmonella typhimurium) in weaned pigs. Gilts n = 8, Barrows n = 12. Data presented as the LSM ± SEM. Barrows had greater WBC counts than gilts (sex: P = 0.003).
Figure 4.
Effect of sex on the neutrophil response to an oral Salmonella challenge (4.7 × 109 cfu/pig Salmonella typhimurium) in weaned pigs. Gilts n = 8, Barrows n = 12. Data presented as the LSM ± SEM. Barrows had greater neutrophils than gilts (sex: P < 0.001).
Figure 5.
Effect of sex on the lymphocyte response to an oral Salmonella challenge (4.7 × 109 cfu/pig Salmonella typhimurium) in weaned pigs. Gilts n = 8, Barrows n = 12. Data presented as the LSM ± SEM. Lymphocytes were greater in gilts than barrows (sex: P = 0.05).
Figure 6.
Effect of sex on the basophil response to an oral Salmonella challenge (4.7 × 109 cfu/pig Salmonella typhimurium) in weaned pigs. Gilts n = 8, Barrows n = 12. Data presented as the LSM ± SEM. Sex × time interaction (P = 0.002). *Gilts and Barrows differ P ≤ 0.05.
While many immune parameters were influenced by sex, there were no effects of sex (P > 0.05) on Salmonella concentrations from fecal shedding 4 d post-inoculation, or within the tissues of the cecum, mesenteric and subiliac lymph nodes, liver, spleen, gallbladder, or kidney tissues (Table 2). The only exception was fecal shedding at 48 h post-inoculation, which was greater in barrows than gilts (P = 0.05).
Table 2.
Summary of fecal shedding and tissue Salmonella counts (log10) in response to an oral Salmonella typhimurium challenge in weaned pigs
| Tissue | Sex1 | SEM | Sex effect | |
|---|---|---|---|---|
| Gilts | Barrows | |||
| Fecal Shedding 24 h | 3.9 | 4.9 | 0.64 | 0.34 |
| Fecal Shedding 48 h | 4.8 | 6.0 | 0.35 | 0.05 |
| Fecal Shedding 72 h | 4.1 | 5.2 | 0.45 | 0.12 |
| Cecum | 4.7 | 4.2 | 0.26 | 0.33 |
| Mesenteric lymph node | 3.5 | 3.5 | 0.11 | 0.45 |
| Subiliac lymph node | 0.5 | 0.4 | 0.29 | 0.88 |
| Liver | 0.5 | 0.2 | 0.22 | 0.49 |
| Spleen | 0.6 | 0.4 | 0.25 | 0.60 |
| Gallbladder | 0.8 | 0.4 | 0.16 | 0.12 |
| Kidney | 0.3 | 0.2 | 0.18 | 0.78 |
Values for sex represent average Salmonella counts (log10; log/g) for each sex.
DISCUSSION
Sexual dimorphism has been reported in many animal species, including humans, cattle, dogs and pigs (Glucksmann, 1974; Williams et al., 2009; Carroll et al., 2015). Understanding the sexually dimorphic responses exhibited in livestock, such as pigs, is important as it may lead to sex-specific treatment or management to address the potential impacts of sexual dimorphism on health and subsequently growth. After thorough review of the literature, we believe this is the first study to demonstrate sexually dimorphic innate immune responses to an oral Salmonella typhimurium challenge in pigs.
Body temperature is an important physiological variable that can be used to detect an inflammatory response, and has been used by industry as an indicator of illness along with behavioral observations. Use of an intraperitoneal temperature device in the current study allowed for the measurement of body temperature at a constant interval throughout the study without having to handle the pigs, which can be stressful and further alter body temperature. Intraperitoneal temperature increased in response to the oral Salmonella challenge, but this increase was not observed until 12 h following the challenge. The temporal response to Salmonella is similar to what has been observed previously in pigs, with an increase in body temperature occurring approximately 12 to 24 h following inoculation (Balaji et al., 2000; Jenkins et al., 2004; Price et al., 2010). In contrast, there was no change in body temperature in finishing barrows nasally inoculated with Salmonella compared to non-challenged controls (Rostagno et al., 2011), which may be a result of route of administration or the sampling timeline. This appears to be the first study to report a sexually dimorphic temperature response to Salmonella challenge in pigs, with gilts producing a greater temperature response compared to barrows. Studies in cattle in response to LPS and CRH challenges have reported differences in rectal temperature responses between heifers and bulls, such that rectal temperature was greater in heifers than bulls (Hulbert et al., 2013; Carroll et al., 2015). Additionally, greater corticosterone responses to LPS have been reported in female mice (Spinedi et al., 1992). Changes in body temperature in response to an infectious agent are regulated by cytokines, including TNF-α, IL-6 and IL-1β. The current study did not measure serum cytokine concentrations in response to Salmonella challenge. Although, a study by Williams et al. (2009) utilizing an LPS challenge in weaned pigs found greater peak TNF-α and IL-6 concentrations in gilts compared to barrows. Perhaps the greater peak concentrations of these 2 thermogenic cytokines observed in response to LPS partially explains the greater intraperitoneal temperature response to Salmonella challenge in the current study. However, further research is warranted to determine if sex alters Salmonella-induced cytokine concentrations in weaned pigs.
It is interesting to note that no differences were observed in sickness behavior. In fact, there were no changes in behavior outside of normal maintenance behaviors observed in any of the pigs throughout the post-challenge period. Typically, animal behavior is used by producers as an objective measurement when selecting animals for treatment of an illness or injury. These behaviors include decreased movement in the pen, decreased feeding and drinking behavior, and lethargy (Ahmed et al., 2015). Ahmed et al. (2015) observed decreases in pig movement and feeding and drinking behavior in response to a Salmonella challenge in growing pigs observed over a 4 wk period using video monitoring. While the current study observed a measureable decrease in average daily feed disappearance following the challenge, the decreased from pre-challenge to post-challenge was minimal (0.13 kg/d), which may not be significant enough to be detected by an observer. Yet, feed disappearance was only measured for a short period following Salmonella challenge, as pigs were euthanized at 72 h post-challenge, and sickness behavior was measured at specific time points throughout the study and did not utilized video surveillance. However, in the current study it could be suggested, based on the lack of sickness behaviors, that these pigs would not be selected for treatment based on periodically-observed behaviors alone, similar to that performed in industry, regardless of the variables that indeed indicate an active infection (i.e., elevated body temperature and changes in blood cell parameters).
Cortisol concentrations varied between barrows and gilts at 2 time points in response to Salmonella, suggesting that barrows had an earlier peak in cortisol compared to gilts. However, overall there was no sex effect on serum cortisol concentrations. This is similar to what was observed by Williams et al. (2009) in response to an LPS challenge in pigs. A similar temporal response was observed in barrows challenged orally with Salmonella (Balaji et al., 2000). Studies in cattle did not find a difference in serum cortisol between heifers and bulls in response to an LPS challenge (Carroll et al., 2015), but a greater cortisol response was observed in response to a CRH challenge (Hulbert et al., 2013). The temporal pattern relative to the increase in cortisol concentration follows that of intraperitoneal temperature such that cortisol concentrations increased at 16 h post-challenge. Larzul et al. (2015) observed differences in cortisol binding globulin (CBG) capacity in pigs, with a correlation between basal cortisol concentrations and CBG in males but not in females. Additionally, female mammals have been noted to have greater adrenal gland weights (Glucksmann, 1974). However, the differences observed in the cortisol response to Salmonella challenge in the current study were minimal.
White blood cells are the cellular component of the immune response, and thus changes in these cell populations within the blood can suggest changes in the response to pathogens. In the current study, various differences in blood cell parameters were observed between gilts and barrows. This is in contrast to Williams et al. (2009) who found no differences in total white blood cell or differential counts in response to LPS between barrows and gilts. Perhaps the nature of the live challenge compared to a more provocative LPS challenge resulted in the differences observed between the 2 studies. Similar results have been observed in cattle, such that Brahman bulls had greater total leukocytes and neutrophils compared to heifers (Hulbert et al., 2013; Carroll et al., 2015). Females have been reported to have a heavier spleen and thymus (Glucksmann, 1974); however, this does not correspond with the decreased total white blood cells and neutrophils observed in gilts compared to barrows. It is possible that the decrease in neutrophils is a result of a greater number of neutrophils leaving the periphery and entering the gut. A study of neutrophil activity and adhesion molecules would help elucidate if this is indeed happening. A study in rats and mice found a greater neutrophil accumulation in tissues following ischemia/reperfusion, and was believed to be regulated by the chemokine Cxcl5, which was able to increase systemic neutrophils in the absence of ischemia/reperfusion (Madalli et al., 2015). Thus, perhaps differences in cytokine and chemokine concentrations are also driving the differences in various immune cell parameters.
Observing almost no differences in fecal shedding and tissue Salmonella content was surprising due to the differences observed in intraperitoneal temperature, serum cortisol, and the complete blood counts. It is possible that although there were differences in blood parameters, this did not influence colonization within the gastrointestinal system and did not influence migration of Salmonella from the gut to peripheral tissues. It is unclear why fecal shedding was influenced by sex 48 h following the challenge, but not at any other time point measured. Further, evidence of sexual dimorphism regarding fecal shedding of bacteria is limited. Reports have indicated a greater incidence of bacterial infections in males compared to females (Green, 1992; Strachan et al., 2008). Yet, when experimentally introduced, it appears there is no difference between weaned gilts and barrows in the tissue Salmonella content.
There have been many studies in several species aimed at elucidating sexually dimorphic responses. From these studies it appears that females are more resilient to infection, yet are more prone to autoimmune diseases (Homodelarche et al., 1991; Spitzer, 1999). For example, female mice of reproductive age appear to have more active polymorphonuclear leukocytes than male counterparts (Spitzer, 1999). Sex hormones, estrogens and androgens, have been implicated in these effects. For example, Kahl and Elsasser (2006) reported greater serum TNF-α and serum amyloid A concentrations in steers administered testosterone 2 d prior to an LPS challenge. Additionally, heifers in estrus (i.e., low progesterone concentrations) had greater mean TNF-α concentrations after LPS administration compared to heifers in diestrus (i.e., high progesterone concentrations). In humans, Dosiou et al. (2004) reported a decrease in the Th1 response during the luteal phase of the menstrual cycle. However it is interesting to note that there are several studies in which sexually dimorphic effects were observed in pre-pubertal animals. Thus, the observed sexually dimorphic effects are likely initiated earlier in life, and likely in utero and/or in the early perinatal period. This is supported by research that reports a change in thymus and T lymphocyte development when female mice were administered testosterone early in life (Leposavic et al., 2009). Based on the findings within our current study, and that of prior reports, it is clear that pigs exhibit a sexually dimorphic immune response which warrants further study.
Conclusion
These data provide further proof that sex can influence the acute phase immune response to a provocative immune challenge. Specifically, gilts had a greater intraperitoneal temperature response, greater lymphocyte and basophil counts, yet decreased hematocrit, platelets, total white blood cell, and neutrophil counts than barrows. These data demonstrate that weaned gilts appear to produce a stronger acute phase response to a Salmonella challenge compared to barrows, without affecting sickness behavior or the tissue translocation or shedding of Salmonella. Based on the differences in cellular immune and serum variables, but the lack of differences in sickness behavior, managing gilts and barrows separately may have significant impacts on herd health management during the weaning/early post-weaning period.
Footnotes
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual's income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA's TARGET Center at (202) 720–2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, DC. 20250–9410, or call (800) 795–3272 (voice) or (202) 720–6382 (TDD).
LITERATURE CITED
- Ahmed S. T., Mun H. S., Yoe H., Yang C. J.. 2015. Monitoring of behavior using a video-recording system for recognition of Salmonella infection in experimentally infected growing pigs. Animal 9:115–121. doi: 10.1017/S1751731114002213 [DOI] [PubMed] [Google Scholar]
- Balaji R., Wright K. J., Hill C. M., Dritz S. S., Knoppel E. L., Minton J. E.. 2000. Acute phase responses of pigs challenged orally with Salmonella typhimurium. J. Anim. Sci. 78:1885–1891. doi: 10.2527/2000.7871885x [DOI] [PubMed] [Google Scholar]
- Burdick N. C., Banta J. P., Neuendorff D. A., White J. C., Vann R. C., Laurenz J. C., Welsh T. H., Randel R. D.. 2009. Interrelationships among growth, endocrine, immune, and temperament variables in neonatal Brahman calves. J. Anim. Sci. 87:3202–3210. doi: 10.2527/jas.2009-1931 [DOI] [PubMed] [Google Scholar]
- Carroll J. A., Burdick N. C., Reuter R. R., Chase C. C. Jr, Spiers D. E., Arthington J. D., Coleman S. W.. 2011. Differential acute phase immune responses by Angus and Romosinuano steers following an endotoxin challenge. Domest. Anim. Endocrinol. 41:163–173. doi: 10.1016/j.domaniend.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Carroll J. A., Burdick Sanchez N. C., Hulbert L. E., Ballou M. A., Dailey J. W., Caldwell L. C., Vann R. C., Welsh T. H. Jr, Randel R. D.. 2015. Sexually dimorphic innate immunological responses of pre-pubertal Brahman cattle following an intravenous lipopolysaccharide challenge. Vet. Immunol. Immunopathol. 166:108–115. doi: 10.1016/j.vetimm.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Carroll J. A., Daniel J. A., Keisler D. H., Matteri R. L.. 1999. Non-surgical catheterization of the jugular vein in young pigs. Lab. Anim. 33:129–134. doi: 10.1258/002367799780578345 [DOI] [PubMed] [Google Scholar]
- Dosiou C., Lathi R. B., Tulac S., Huang S. T. J., Giudice L. C.. 2004. Interferon-related and other immune genes are downregulated in peripheral blood leukocytes in the luteal phase of the menstrual cycle. J. Clin. Endocrinol. Metab. 89:2501–2504. doi: 10.1210/jc.2003-031647 [DOI] [PubMed] [Google Scholar]
- Glucksmann A. 1974. Sexual Dimorphism in Mammals. Biol. Rev. Camb. Philos. Soc. 49:423–475. doi: 10.1111/j.1469-185X.1974.tb01171.x [DOI] [PubMed] [Google Scholar]
- Green N. S. 1992. The Male Predominance in the Incidence of Infectious-Disease in Children: A Postulated Explanation for Disparities in the Literature. Int. J. Epidemiol. 21:381–386. doi: 10.1093/ije/21.2.381 [DOI] [PubMed] [Google Scholar]
- Homo-Delarche F., Fitzpatrick F., Christeff N., Nunez E. A., Bach J. F., Dardenne M.. 1991. Sex Steroids, Glucocorticoids, Stress and Autoimmunity. J. Steroid Biochem. Mol. Biol. 40:619–637. doi: 10.1016/0960-0760(91)90285-D [DOI] [PubMed] [Google Scholar]
- Hulbert L. E., Carroll J. A., Ballou M. A., Burdick N. C., Dailey J. W., Caldwell L. C., Loyd A. N., Vann R. C., Welsh T. H. Jr, Randel R. D.. 2013. Sexually dimorphic stress and pro-inflammatory cytokine responses to an intravenous corticotropin-releasing hormone challenge of Brahman cattle following transportation. Innate Immun. 19:378–387. doi: 10.1177/1753425912462752 [DOI] [PubMed] [Google Scholar]
- Jenkins N. L., Turner J. L., Dritz S. S., Durham S. K., Minton J. E.. 2004. Changes in circulating insulin-like growth factor-I, insulin-like growth factor binding proteins, and leptin in weaned pigs infected with Salmonella enterica serovar Typhimurium. Domest. Anim. Endocrinol. 26:49–60. doi: 10.1016/j.domaniend.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Kahl S., Elsasser T. H.. 2006. Exogenous testosterone modulates tumor necrosis factor-alpha and acute phase protein responses to repeated endotoxin challenge in steers. Domest. Anim. Endocrinol. 31:301–311. doi: 10.1016/j.domaniend.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Larzul C., Terenina E., Foury A., Billon Y., Louveau I., Merlot E., Mormede P.. 2015. The cortisol response to ACTH in pigs, heritability and influence of corticosteroid-binding globulin. Animal 9:1929–1934. doi: 10.1017/S1751731115001767 [DOI] [PubMed] [Google Scholar]
- Leposavić G., Perisic M., Kosec D., Arsenovic-Ranin N., Radojevic K., Stojic-Vukanic Z., Pilipovic I.. 2009. Neonatal testosterone imprinting affects thymus development and leads to phenotypic rejuvenation and masculinization of the peripheral blood T-cell compartment in adult female rats. Brain Behav. Immun. 23:294–304. doi: 10.1016/j.bbi.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Madalli S., Beyrau M., Whiteford J., Duchene J., Nandhra I. S., Patel N. S. A., Motwani M. P., Gilroy D. W., Thiemermann C., Nourshargh S., Scotland R. S.. 2015. Sex-specific regulation of chemokine Cxcl5/6 controls neutrophil recruitment and tissue injury in acute inflammatory states. Biol. Sex Differ. 6:27. doi: 10.1186/s13293-015-0047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha S., Kostrzynska M.. 2007. Approaches for reducing Salmonella in pork production. J. Food Prot. 70:2676–2694. [DOI] [PubMed] [Google Scholar]
- Price K. L., Totty H. R., Lee H. B., Utt M. D., Fitzner G. E., Yoon I., Ponder M. A., Escobar J.. 2010. Use of Saccharomyces cerevisiae fermentation product on growth performance and microbiota of weaned pigs during Salmonella infection. J. Anim. Sci. 88:3896–3908. doi: 10.2527/jas.2009-2728 [DOI] [PubMed] [Google Scholar]
- Rostagno M. H., Eicher S. D., Lay D. C.. 2011. Immunological, Physiological, and Behavioral Effects of Salmonella enterica Carriage and Shedding in Experimentally Infected Finishing Pigs. Food. Pathog. Dis. 8:623–630. doi: 10.1089/fpd.2010.0735 [DOI] [PubMed] [Google Scholar]
- Spinedi E., Suescun M. O., Hadid R., Daneva T., Gaillard R. C.. 1992. Effects of Gonadectomy and Sex-Hormone Therapy on the Endotoxin-Stimulated Hypothalamo-Pituitary-Adrenal Axis- Evidence for a Neuroendocrine-Immunological Sexual Dimorphism. Endocrinology 131:2430–2436. doi: 10.1210/endo.131.5.1330501 [DOI] [PubMed] [Google Scholar]
- Spitzer J. A. 1999. Gender differences in some host defense mechanisms. Lupus 8:380–383. doi: 10.1177/096120339900800510 [DOI] [PubMed] [Google Scholar]
- Strachan N. J. C., Watson R. O., Novik V., Hofreuter D., Ogden I. D., Galan J. E.. 2008. Sexual dimorphism in campylobacteriosis. Epidemiol. Infect. 136:1492–1495. doi: 10.1017/S0950268807009934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. N., Collier C. T., Carroll J. A., Welsh T. H., Laurenz J. C.. 2009. Temporal pattern and effect of sex on lipopolysaccharide-induced stress hormone and cytokine response in pigs. Domest. Anim. Endocrinol. 37:139–147. doi: 10.1016/j.domaniend.2009.04.004 [DOI] [PubMed] [Google Scholar]