Abstract
Dopaminergic neurons are present in both plexuses of the murine bowel and are upregulated after extrinsic denervation but play unknown roles in enteric nervous system (ENS) physiology. Transcripts encoding dopamine (DA) receptors D1–D5 were analyzed by reverse transcription-PCR in stomach ≈ duodenum ≈ ileum ≈ proximal ≫ distal colon. Dissected muscle and myenteric plexus contained transcripts encoding D1–D3 and D5, whereas mucosa contained D1 and D3–D5. D1–D5 expression began in fetal gut [embryonic day 10 (E10)], before the appearance of neurons (E12), and was sustained without developmental regulation through postnatal day 1. In situ hybridization revealed that subsets of submucosal and myenteric neurons contained mRNA encoding D2 or D3. Immunoblots confirmed that D1, D2, and D5 receptor proteins were present from stomach through distal colon. Subsets of submucosal and myenteric neurons were also D1, D2, or D3 immunoreactive. When double labeled by in situ hybridization, these neurons contained mRNA encoding the respective receptors. Total gastrointestinal transit time (TGTT) and colonic transit time (CTT) were measured in mice lacking D2, D3, or D2 plus D3. Both TGTT and CTT were decreased significantly (motility increased) in D2 and D2 plus D3, but not D3, knock-out animals. Mice lacking D2 and D2 plus D3 but not D3 were smaller than wild-type littermates, yet ate significantly more and had greater stool frequency, water content, and mass. Because motility is abnormal when D2 is absent, the net inhibitory DA effect on motility is physiologically significant. The early expression of DA receptors is also consistent with the possibility that DA affects ENS development.
Keywords: dopamine receptor, dopaminergic, colon motility, gastrointestinal transit time, enteric nervous system, development
Introduction
Catecholamines modulate gastrointestinal (GI) motility. Sympathetic nerves release norepinephrine (NE), which inhibits acetylcholine (ACh) release from motor neurons (via α2 adrenoceptors) (Scheibner et al., 2002), evokes IPSPs in submucosal neurons (Hirst and Silinsky, 1975; Frieling et al., 1991; Ren et al., 1999; Wood, 1999), and relaxes smooth muscle (Gershon, 1967). The gut, however, also contains dopamine (DA); moreover, the DA to NE ratio is higher in the bowel than in other sympathetic targets, and the gut contains a high concentration of the specific DA metabolite 3,4-dihydrioxyphenylacetic acid (Eaker et al., 1988). The suggestion from these observations, that DA is an enteric neurotransmitter, has been confirmed recently. Enteric dopaminergic neurons, which express tyrosine hydroxylase (TH) and the dopamine transporter (DAT) but lack dopamine β-hydroxylase, have been identified in mouse, guinea pig (Li et al., 2004), and human (Anlauf et al., 2003).
Although enteric dopaminergic neurons develop perinatally, a subset of the early precursors of enteric neurons (TC cells) transiently contains TH and is catecholaminergic (Cochard et al., 1978; Gershon et al., 1984; Baetge et al., 1990). TC cells depend on the transcription factor Mash1 and give rise to noncatecholaminergic neurons, such as those containing 5-HT (Blaugrund et al., 1996). Whether TC cells secrete norepinephrine or DA is unknown, as is the function of the early catecholamine expression in the developing enteric nervous system (ENS). The potential of catecholamines, such as DA, to influence gut or ENS development remains to be explored.
The function of enteric dopaminergic neurons is not clear. DA relaxes the rat jejunum (Lucchelli et al., 1986, 1990), relaxes smooth muscle isolated from dog colon (Grivegnee et al., 1984), and hyperpolarizes guinea pig submucosal neurons (Hirst and Silinsky, 1975). Electrically induced contractions of mouse colon smooth muscle are small in DAT knock-out mice and restored to normal by the combined inhibition of D1 and D2 DA receptors (Walker et al., 2000). Endogenous DA may thus inhibit colonic motility, an effect that is potentiated in the absence of DAT. Previous studies on enteric DA, however, have been indirect, and enteric DA receptors have not yet been systematically characterized. DA, moreover, can activate β-adrenoceptors (Grivegnee et al., 1984; Lucchelli et al., 1990; Tsai and Cheng, 1992), which complicates analyses of responses to DA, because exogenous DA or endogenous DA in the absence of DAT reaches ectopic sites that are not accessible to endogenously released DA.
There are five subtypes of DA receptor, which can be grouped into two families: D1-like family, including D1 and D5; D2-like family, including D2, D3, and D4 (Hartman and Civelli, 1997; Tan et al., 2003). We therefore studied the expression of all dopamine receptor subtypes in adult and fetal mouse gut. Experiments were undertaken to identify DA receptors, to determine their location and oral–anal distribution, and to ascertain which receptors mediate actions of dopaminergic neurons. We also determined when during enteric ontogeny the expression of each subtype of DA receptor begins. This timing provides insight into when developing enteric cells might become DA responsive. Because the D2 and D3 receptor subtypes were found in the ENS, the relevance of D2- and D3-mediated dopaminergic responses in enteric physiology was investigated by measuring GI motility in transgenic mice lacking one or both of these receptor. These experiments suggest that D2 receptors inhibit intestinal motility and do so physiologically. Because DA receptors were found in the fetal bowel at a time when the gut contains precursors but no neurons, DA receptors may also be developmentally active.
Materials and Methods
Animals and tissue preparation.
Experiments were performed with fetal and adult CD-1 mice (25–30 g; Charles River Laboratories, Wilmington, MA) of either sex and with adult knock-out mice (congenic, C57BL/6) lacking D2, D3, or both D2 and D3 receptors (Jung et al., 1999). CO2 inhalation was used to kill adult animals. This procedure was approved by the Animal Care and Use Committee of Columbia University. The brain, stomach, duodenum, ileum, proximal colon, and distal colon were removed from the animals and processed for molecular and histological studies. Fetal mice were removed from pregnant dams at embryonic day 10 (E10), E12, E14, E16, and E18. Newborn mice at postnatal day 1 (P1) were also investigated. The day at which a vaginal plug was found was designated as day 0 of gestation.
RNA extraction and preparation of cDNA.
The brain, stomach, duodenum, ileum, proximal colon, and distal colon were collected in PBS (0.9% NaCl in 0.01 m sodium phosphate buffer, pH 7.4), which had been treated with 0.1% diethyl pyrocarbonate (depc-PBS). Fetal gut was collected in ice-cold HBSS and stored in RNA Later (Ambion, Austin, TX). After the wall of each piece of gut was opened, the tissue was cleaned with depc-PBS and transferred to Trizol (Invitrogen, Carlsbad, CA) for extraction of total RNA, which was isolated according to the manufacturer’s instructions and stored for further use at −80°C. cDNA was prepared from 3 μg of total RNA by reverse transcription in a 30 μl reaction volume with 0.5 μg of random hexamer primers, 0.5 mm dNTPs, 40 U of Rnasin, and 400 U of Maloney murine leukemia virus reverse transcriptase (Promega, Madison, WI).
PCR.
Pairs of oligonucleotide primers for amplification of cDNA encoding β-actin, β3-tubulin, dopamine receptors (D1, D2, D3, D4, D5), DAT, sucrase-isomaltase, and TH were designed from published mouse cDNA sequences. The programs used for PCR amplification with each primer pair are listed in Table 1. The identities of all PCR products were confirmed by sequence analysis. For this purpose, PCR products were subcloned into pGEM-T Easy vectors (PromegaI) by using the TA-cloning kit (Invitrogen). Inserts in two individual clones were sequenced by the dideoxynucleotide-chain termination method in the DNA facility of Columbia University. The sequences of the PCR products obtained from brain and gut with the indicated primers were found to be identical to those of the appropriate regions of the GenBank sequences of the amplified cDNAs.
Table 1.
Sequences of primers
| Primers | GenBank accession number | Primer sequence | Primer location in the sequence | PCR program |
|---|---|---|---|---|
| β-Actin | X03672 | Forward: 5′-TGT TTG AGA CCT TCA ACA CC-3′ | 448–467 | 94°C 30″+ (94°C 0″ + 57°C 9″ + 72°C 24″) × 40 |
| Reverse: 5′-CAG TAA TCT CCT TCT GCA TCC-3′ | 1035–1015 | |||
| DAT | AF109391 | Forward: 5′-ATC TGC CCT GTC CTG AAA G-3′ | 405–423 | 94°C 30″ + (94°C 0″ + 57°C 5″ + 72°C 5″) × 40 |
| Reverse: 5′-TGG TGA AGG AGG AGA AGA AG-3′ | 522–503 | |||
| D1 | AK044723 | Forward: 5′-GTA GCC ATT ATG ATC GTC AC-3′ | 1138–1157 | D + (94°C 30″ + 55°C 45″ + 72°C 30″) × 38 + E |
| Reverse: 5′-GAT CAC AGA CAG TGT CTT CAG-3′ | 1350–1330 | |||
| D2 | X55674 | Forward: 5′-GCA GCC GAG CTT TCA GAG CC-3′ | 812–821 | D + (94°C 45″ + 64°C 60″ + 72°C 60″) × 38 + E |
| Reverse: 5′-GGG ATG TTG CAG TCA CAG TG-3′ | 1343–1324 | |||
| D3 | X67274 | Forward: 5′-AGG TTT CTG TCA GAT GCC-3′ | 772–789 | D + (94°C 30″ + 55°C 45″ + 72°C 30″) × 38 + E |
| Reverse: 5′-ATT GCT GAG TTT TCG AAC C-3′ | 1047–1029 | |||
| D4 | U19880 | Forward: 5′-CAC CAA CTA CTT CAT CGT GA-3′ | 308–327 | D + (94°C 30″ + 58°C 60″ + 72°C 45″) × 38 + E |
| Reverse: 5′-AAG GAG CAG ACG GAC GAG TA-3′ | 700–681 | |||
| D5 | AK045456 | Forward: 5′-CTA CGA GCG CAA GAT GAC C-3′ | 593–611 | D + (94°C 30″ + 61°C 45″ + 72°C 45″) × 39 + E |
| Reverse: 5′-CTC TGA GCA TGC TCA GCT G-3′ | 952–934 | |||
| β3-Tubulin | BC031357 | Forward: 5′-TGA TGA CGA GGA ATC GGA AG-3′ | 1359–1378 | D + (94°C 30″ + 63°C 1′ + 72°C 45″) × 31 + E |
| Reverse: 5′-CCC GAA TAT AAA CAC AAC CCA G-3′ | 1681–1660 | |||
| Sucrase-isomeltase | XM_143332 | Forward: 5′-GTT CGA AGG AGA AGC ACT GG-3′ | 631–650 | D + (94°C 30″ + 63°C 1′ + 72°C 45″) × 23 + E |
| Reverse: 5′-TGC GGT AGG TTA GAG CAG GT-3′ | 964–945 | |||
| TH | M69200 | Forward: 5′-GCA CAT TTG CCC AGT TCT C-3′ | 1033–1051 | 94°C 30″ + (94°C 0″ + 57°C 5″ + 72°C 5″) × 40 |
| Reverse: 5′-TTT ACA CAG CCC AAA CTC CAC-3′ | 1148–1128 |
D, Denature at 94°C for 2 min; E, extension at 72°C for 5 min; ″, seconds; ′, minutes.
Real-time PCR.
Real-time PCR was used to quantify mRNA encoding DAT and TH in the ileum of transgenic mice lacking D2 and their littermates as described previously (Li et al., 2004). The expression of DAT and TH was normalized to that of β-actin, a housekeeping gene that is not thought to be subject to regulation. Transcripts encoding β-actin in samples of mouse gut were first quantified by real-time PCR with the SYBR Green Jumpstart Taq ReadyMix (Sigma, St. Louis, MO) using a LightCycler instrument (Roche Diagnostics, Indianapolis, IN). Measurements were obtained by referring to standard curves that were prepared by serially diluting plasmid DNA encoding DAT, TH, and β-actin. The dilutions of β-actin and TH plasmid DNA ranged from 1 pg to 10 ng in five series, each of which covered a 10-fold range. Plasmid DNA encoding DAT was serially diluted from 0.01 to 100 pg, again in five series, each of which covered a 10-fold range.
Amplifications were performed in a final volume of 20 μl of a commercial reaction mixture (Sigma) that contained TaqDNA polymerase, reaction buffer, dNTPs in which dTTP is replaced by dUTP, SYBR Green I dye, and MgCl2. The primers for the amplification of cDNA encoding β-actin, DAT, and TH were used at a final concentration of 0.3 μm. The final concentration of MgCl2 was 4.5 mm for the amplification of β-actin, 8 mm for that of DAT, and 7 mm for that of TH. To this mixture was added 2 μl of either the serially diluted plasmid DNA (standards) or the cDNA prepared from tissue. The standards and the cDNA from tissues were simultaneously subjected to real-time PCR analysis in parallel capillary tubes. The PCRs were performed according to the programs in Table 1. The appearance of double-stranded DNA was quantified by measuring the fluorescence of SYBR Green after each step of elongation. A melting point analysis was finally performed to improve the sensitivity and specificity of amplification reactions detected with the SYBR Green I dye; samples were incubated at 95°C for 0 s, at 67°C for 15 s, and then from 67 to 95°C with a transition rate of 0.2°C/s. Data were analyzed with computer assistance using the LightCycler software.
Immunoprecipitation, gel electrophoresis, and immunoblotting.
Tissue was harvested from mouse brain (positive control), stomach, duodenum, ileum, proximal and distal colon, washed with PBS, and homogenized in 300 μl of 50 mm Tris buffer, pH 7.4, containing EDTA (1.0 mm), EGTA (2.0 mm), phenylmethanesulfonyl fluoride (1.0 mm), aprotinin (100 μg/ml), and leupeptin (100 μg/ml) (Li et al., 2004). The homogenate was centrifuged at 10,000 × g for 30 min at 4°C to separate a membrane-containing fraction (pellet) from the cytosol. The membrane-containing fraction was solubilized with Triton X-100 (1%) in the same buffer. An aliquot containing 200 μg of protein was then removed for immunoprecipitation with goat antibodies to the D2 receptor (Santa Cruz Biotechnology, Santa Cruz, CA). Ten microliters of anti-D2 were added to yield a total volume of 50 μl. After incubation at 4°C overnight, 20 μl of washed UltraLink Immobilized Protein A/G (Pierce, Rockford, IL) was added, followed by incubation at 4°C with gentle agitation overnight. The reaction mixture was washed, centrifuged at 2000 × g for 2 min, and the supernatant was removed for analysis of D2 protein by Western blotting. For this purpose, proteins (50 μg) were separated by 8.5% SDS/PAGE. The separated proteins were then electroblotted onto polyvinylidene difluoride membranes and immersed in blocking buffer containing 5% nonfat dry milk in Tris base–sodium chloride buffer (TBS) for 30 min at room temperature (RT). The blot was washed with TBS containing 0.05% Tween 20 (TBST) and finally incubated overnight at 4°C with polyclonal primary antibodies to D1, D2, or D5 (diluted 1:1000 in 3% nonfat dry milk) (Table 2). After washing in TBST, the blot was incubated with goat HRP-labeled secondary antibodies to rabbit IgG (Vector Laboratories, Burlingame, CA) for 1 h at RT. The blot was finally washed with TBST and developed with a chemiluminescent substrate (Pierce).
Table 2.
Primary antibodies
| Antigen | Antibody | Dilution | Source | |
|---|---|---|---|---|
| Immunocytochemistry | Western blot | |||
| D1 | Rat monoclonal | 1:500 | 1:1000 | Sigma |
| D2 | Rabbit polyclonal | 1:800 | 1:1000 | Alpha Diagnostic, San Antonio, TX |
| D2 | Goat polyclonal | 1:800 | 1:1000 | Santa Cruz Biotechnology |
| D3 | Goat polyclonal | 1:300 | N/A | Santa Cruz Biotechnology |
| D5 | Mouse monoclonal | N/A | 1:1000 | Chemicon, Temecula, CA |
N/A, Not applicable.
Immunocytochemistry.
Segments of the ileum were collected in PBS to which the muscle relaxant nicardipine had been added to prevent muscle spasm (10−6m; Sigma) (Li et al., 2004). The contents were flushed out with PBS and the preparations were cut open along the mesenteric border. When whole mounts were to be prepared, the tissue was stretched tautly and pinned flat on balsa wood with the mucosal surface facing down. Specimens were fixed for 2 h at RT with 4% formaldehyde (freshly prepared from paraformaldehyde, pH 7.4) and washed three times with PBS for a total of 30 min. Laminar preparations of longitudinal muscle with adherent myenteric plexus (LMMP) and submucosa [containing the submucosal plexus (SMP)] were obtained by dissection of the gut wall. Tissue to be sectioned was cryoprotected overnight at 4°C with 30% sucrose in PBS containing 0.1% sodium azide. The preparations were embedded in OCT compound (Tissue-Tek; Sakura Finetec, Torrance, CA) and sectioned at 10 μm with a cryostat microtome. Sections were air-dried on slides for 1 h at RT. For immunostaining, sections were permeabilized and blocked by incubation for 30 min at RT with 1% Triton X-100 in PBS containing 10% normal rabbit, goat, or lamb serum, corresponding to the host species used to generate secondary antibodies to prevent nonspecific staining. The whole mounts and sections were incubated with primary antibodies overnight (Table 2), either at RT or at 4°C. For the double labeling of D2 and D3, primary antibodies from different species were used (Table 2). After washing with PBS three times for 10 min, the tissue was incubated with appropriate affinity purified species-specific secondary antibodies for 1–2 h. The working concentrations of the secondary antibodies used for immunofluorescence were: goat anti-rat Alexa 594 (1:200; Invitrogen), biotin-labeled donkey anti-rabbit (1:200; Jackson ImmunoResearch, West Grove, PA), donkey anti-goat Alexa 594 (1:200; Invitrogen), biotin-labeled donkey anti-goat (1:200; Jackson ImmunoResearch), and cyanine 3 (Cy3)-labeled streptavidin (1:3000; Jackson ImmunoResearch).
To increase the detection sensitivity, D2 and D3 receptors were also located by using biotinylated secondary antibodies in conjunction with a preformed avidin–biotin complex (Elite kit; Vector Laboratories). Bound antibodies were visualized by using the Vectastain Elite peroxidase kit. Whole mounts of LMMP and SMP of mouse ileum, prepared as described above, were both used. Briefly, preparations were incubated (30 min) with 10% normal serum (of the species in which the secondary antibodies were raised) to inhibit nonspecific staining. The blocked tissue was then incubated overnight with primary antibodies, washed with PBS, and exposed with intervening washes in PBS to the biotinylated secondary antibodies (2 h), 0.3% H2O2 in 0.3% blocking sera (5 min), Elite ABC reagent (1 h; Vector Laboratories), and 3′,5′-diaminobenzidine solution (2–10 min) until suitable staining developed.
As a control for the specificity of antibodies to the D2 and D3 receptors, attempts were made to immunostain the corresponding receptors in the gut of knock-out animals lacking these receptors.
The immunostained tissue was viewed with a Leica (Nussloch, Germany) DMRXA2 microscope. For epifluorescence, the L5 (Alexa 488, FITC) and M2 (Alexa 594, Texas Red, Cy3) dichroic mirror/filter combinations were used. Fluorescence of the red fluorophores was not visible with the L5 dichroic mirror/filter combination, and fluorescence of the green fluorophores was not visible with the M2 dichroic mirror/filter combination. Images were captured digitally with a cooled CCD camera (QImaging, Burnaby, British Columbia, Canada) operated by a Macintosh computer (Apple Computers, Cupertino, CA) using commercial software (Openlab; Improvision, Coventry, UK). Contrast was adjusted by using the Openlab software, which was also used to analyze double labeling by superimposing images. Bright-field images were captured by using the same microscope, camera, and computer software, except that red–green–blue filters were interposed between the microscope and the camera to permit color imaging. Pictures were sized and assembled using Photoshop 7 (Adobe Systems, San Jose, CA) software for the Macintosh computer.
In situ hybridization. In situ hybridization was performed using methods that have been described previously (Schaeren-Wiemers and Gerfin-Moser, 1993). Briefly, mouse ileum was dissected in ice-cold depc-PBS, stretched, and pinned flat on autoclaved balsa wood. Preparations were fixed on their supports in a freshly made, ice-cold solution containing 4% formaldehyde (freshly prepared from paraformaldehyde) for 1.5 h. After washing with depc-PBST, the preparations were incubated overnight with 30% sucrose/depc-PBS at 4°C, embedded in OCT, and frozen in liquid N2. Sections (12 μm) were cut at −20°C in a cryostat microtome and thaw-mounted onto Colorfrost Plus slides (Fisher Scientific, Pittsburgh, PA). Sections were air-dried for 20–60 min, rinsed in depc-PBST for 5 min, postfixed with 4% formaldehyde at RT for 15 min, and washed again with depc-PBST to eliminate residual fixative. Sections were then permeabilized with 1.0 μg/ml proteinase K in depc-PBS, washed with 2.0 mg/ml glycine in depc-PBST, equilibrated in 0.1 m triethanolamine, pH 8.0, for 2 min, acetylated for 10 min in 0.25% acetic anhydride in 0.1 m triethanolamine, and washed in depc-PBST. Sections were incubated at 68°C for 1 h in prehybridization buffer, containing 50% formamide (Fisher Scientific), 5× SSC, 5× Denhardt’s solution, 0.25 mg/ml yeast tRNA (Roche Diagnostics), 500 μg/ml salmon sperm DNA (Sigma), and 100 μg/ml heparin (Sigma). For hybridization, sections were incubated in the same buffer with 100 ng/ml sense or antisense probes, respectively, for 16 h. The slides were washed in 2× SSC containing 0.1% SDS at RT for 5 min (two times), in 0.1× SSC containing 0.1% SDS at 68°C for 30 min (two times), and in 2× SSC at RT for 5 min. Sections were blocked with 10% goat serum in TBST for 1 h at RT and incubated overnight at 4°C with alkaline phosphatase-labeled Fab fragments of sheep antibodies to digoxigenin (diluted 1:1500; in TBST containing 10% goat serum blocking solution; Roche Diagnostics). After washing in TBST, containing 2 mm levamisole (Sigma) to inhibit endogenous alkaline phosphatase activity, alkaline phosphatase activity was demonstrated with 4-nitroblue tetrazolium according to the directions of the manufacturer (Roche Diagnostics). Sections were coverslipped in a 1:2 mixture of 0.5 m bicarbonate buffer, pH 8.6, and glycerol.
Colon motility.
Colon motility was studied in wild-type mice and in littermates lacking D2, D3, or both D2 and D3 receptors by using previously described methods (Chen et al., 2001). Six mice from each receptor knock-out and from wild-type littermates were used. Briefly, the animals were anesthetized with isoflurane (Baxter Pharmaceutical Products, Deerfield, IL). A glass bead (diameter, 3 mm) was inserted into the colon at a distance of 2 cm from the anal verge. The time required for expulsion of the glass bead was measured and taken as an estimate of colonic motility.
Total GI transit time.
These studies were performed in wild-type mice and in littermates lacking D2, D3, or D2 and D3 receptors by measuring the transit time through the bowel of Bacillus stearothermophilus spores (Mathers et al., 1997). Six mice from each genotype were used. The B. stearothermophilus spores (Merck, Darmstadt, Germany) were suspended in water at a concentration of ∼108 spores/ml. Each mouse received ∼2 × 107 spores in 0.2 ml by gavage. Fecal pellets were collected after 3, 6, 9, 12, 24, 36, 48, 72, and 96 h, vacuum dried overnight, weighed, and ground into a fine powder. The powder was resuspended in water, and 100 μl of the resulting solution was spread on trypticase soy agar plates. Because spores of B. stearothermophilus germinate at 65°C, a temperature that is lethal to normal flora, the plates were incubated at 65°C for 12–16 h. The number of B. stearothermophilus colonies on each plate was counted, and the mean transit time (MTT) was calculated from the following formula: MTT (h) = Σ miti /Σ mi, where mi is the number of B. stearothermophilus spores passed at time ti(h) after gavage.
One hour stool collection.
Ten mice from each genotype were used for this study. Each mouse was placed in a separate clean cage and observed throughout the 60 min collection period. Fecal pellets were collected immediately after expulsion and placed in sealed (to avoid evaporation) 1.5 ml tubes. Tubes were weighed to obtain the wet weight of the stool, which was then dried overnight at 65°C and reweighed to obtain the dry weight. The stool water content was calculated from the difference between the wet and dry stool weights.
Food and water consumption.
Ten mice from each genotype were used for this study. Food and water consumption were studied over the course of a 72 h observation period. Mice were housed separately to permit each animal’s food and water consumption to be calculated from the difference in weights of the food and water supply at the beginning and the end of the observation period.
Statistical analysis.
Differences between animals of each type were compared by one-way ANOVA.
Results
Transcripts encoding five DA receptors are expressed in the mouse gut
Reverse transcription-PCR was used to investigate the expression of mRNA encoding D1, D2, D3, D4, and D5 in the mouse stomach, duodenum, ileum, proximal, and distal colon. Transcripts encoding each of the five DA receptors were found in all of these regions of the bowel and in the brain, which was studied at the same time as a positive control (Fig. 1A). To determine the layer of the gut in which these receptors are expressed, the bowel wall was dissected to isolate the mucosa and the LMMP, total RNA was separately extracted from each, and DA receptor expression was again analyzed by reverse transcription-PCR. To evaluate the possibility that the mucosal preparation was contaminated by neurons or the LMMP by mucosa, the presence of transcripts encoding the neuronal marker β3-tubulin and the mucosal epithelial marker sucrase-isomaltase was also investigated. Transcripts encoding β3-tubulin were detected in RNA extracted from the LMMP but not from the mucosa. In contrast, transcripts encoding sucrase-isomaltase were detected in the mucosa but not in the LMMP (Fig. 1B). These data indicate that there was no mucosal contribution to RNA from the LMMP or neuronal contamination of RNA from the mucosa. Transcripts encoding D1, D2, D3, and D5 were detected in RNA extracted from the LMMP, whereas those encoding D1, D3, D4, and D5 were identified in mucosa (Fig. 1C). The observation that transcripts encoding D2 were only found in the LMMP is consistent with the possibility that the enteric D2 receptor is neuronal. Similarly, the observation that transcripts encoding D4 were only observed in the mucosa is consistent with the possibility that the enteric D4 is non-neuronal. In contrast, both neurons and non-neuronal cells probably express D1, D3, and D5 in the gut.
Figure 1.
DA receptors are expressed in the gut. Expression of transcripts encoding the dopamine receptors D1–D5 was analyzed regionally in the whole gut wall (A) and in dissected layers of the wall of the ileum (B, C). A, Transcripts encoding each of the five DA receptors were detected in the stomach (St), duodenum (Du), ileum (Ile), proximal colon (PC), and distal (DC) colon. The brain (Br) was investigated as a positive control. B, The presence of transcripts encoding the neural marker β3-tubulin and mucosal epithelial marker sucrase-isomaltase was studied to assess the potential contamination of mucosal preparations with RNA from neurons and LMMP preparations with RNA from the mucosal epithelium. As expected, preparations from Br, whole ileum, and LMMP, but not the mucosa (Muc), contained transcripts encoding β3-tubulin. In contrast, transcripts encoding sucrase-isomaltase were detected in preparations from whole ileum and mucosa but not in those from brain or LMMP. Cross-contamination, therefore, was negligible. M, Molecular marker. C, Transcripts encoding D1, D3, and D5 were present both in the mucosa and the LMMP; those encoding D2 were present in LMMP but not in mucosa; those encoding D4 were present in mucosa but not in LMMP.
Transcripts encoding DA receptors are expressed in developing mouse gut
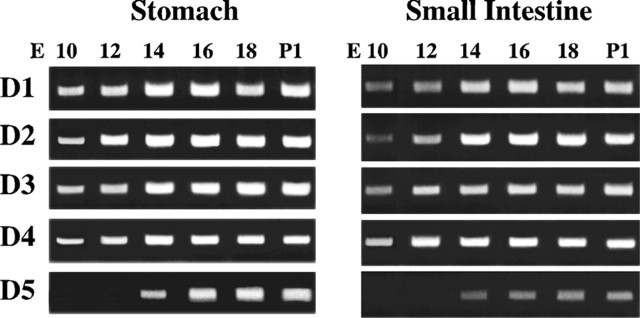
Total RNA was extracted from the small intestine and stomach of fetal mice at E10, E12, E14, E16, and E18 and also from postnatal animals at P1 and analyzed by reverse transcription-PCR. Transcripts encoding D1, D2, D3, and D4 were all detected at each age examined from E10 through P1 in both the small intestine and stomach (Fig. 2A,B). Transcripts encoding D5 were not observed until E14 in either the small intestine or the stomach, but once detected at E14, they too persisted through P1 in both regions of the bowel (Fig. 2A,B). All subtypes except D5 are thus found in the bowel early in ontogeny. Even D5 is present while enteric neurons are developing from precursor cells. Receptor expression, however, is not developmentally regulated; expression of all DA receptor subtypes persists in the mature bowel. This pattern is consistent with actions of DA receptors both in the developing and mature gut.
Figure 2.
Expression of DA receptors begins early in fetal development and persists in the postnatal gut. DA receptor expression was analyzed by reverse transcription-PCR in the stomach and small intestine from fetal day E10 through postnatal day P1. Transcripts encoding DA receptors D1–D4 were detected as early as E10 in both the presumptive stomach and small intestine. Transcripts encoding D5 were not detected in either organ until E14. Expression of each receptor was detected through P1.
DA receptor immunoreactivities were located in the ENS
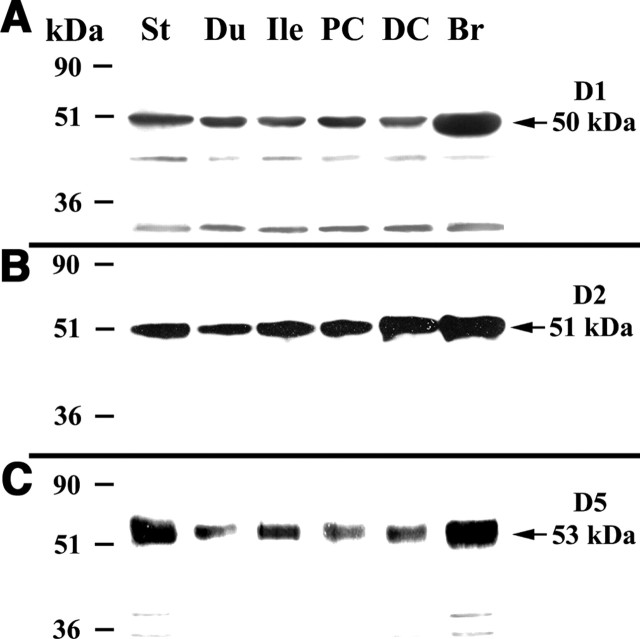
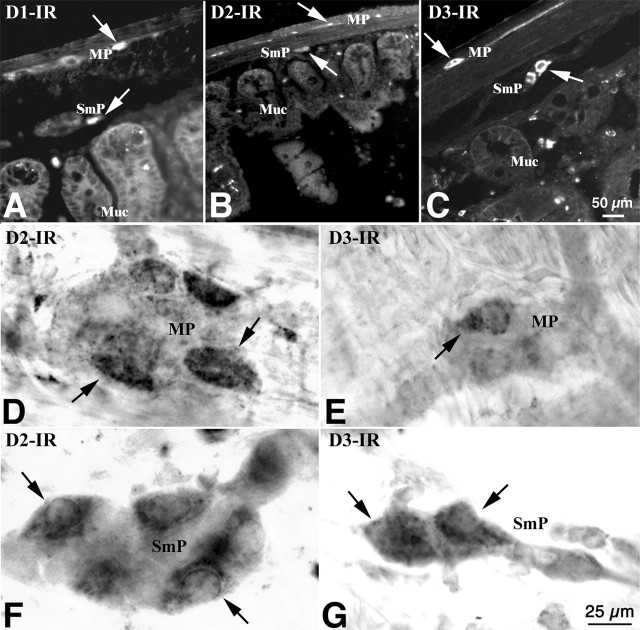
Immunoblots were used to confirm that DA receptor proteins, like the previously detected transcripts, are expressed in the mouse gut. Immunocytochemistry was then used to locate the receptors. The immunoreactivities of D1, D2, and D5 (D3 and D4 were not examined) were detected by immunoblotting in mouse stomach, duodenum, ileum, proximal, and distal colon (Fig. 3A–C, respectively). The electrophoretic mobility of each immunoreactive receptor protein was identical to that of the corresponding protein from brain, which was examined as a positive control. Both frozen sections of gut wall (Fig. 4A–C) and whole mounts of dissected laminar preparations of LMMP and submucosa (Fig. 4D–G) were used for the immunocytochemical localization of DA receptors. D1 (Fig. 4A), D2 (Fig. 4B,D,F), and D3 (Fig. 4C,E,G) immunoreactivities were found in subsets of neurons in both the myenteric and submucosal plexuses.
Figure 3.
DA receptor immunoreactivity was detected in enteric neurons of mouse ileum. The immunoreactivity was visualized with antibodies to D1, D2, and D3 in both frozen section (A–C) and in whole-mount preparations (D–G). A, D1 immunoreactivity is present in the myenteric (MP) and submucosal (SmP) plexuses and in the mucosa (Muc). The arrows indicate the immunoreactive products. B, D, F, D2 immunoreactivity is present in subsets of myenteric and submucosal neurons, but not in the mucosa. C, E, G, D3 immunoreactivity is present in subsets of myenteric and submucosal neurons. Mucosal immunoreactivity is very weak. Scale bars: (in C) A–C, 5μm; (in G) D–G, 25μm.
Figure 4.
DA receptor immunoreactivity was detected in enteric neurons of mouse ileum. The immunoreactivity was visualized with antibodies to D1, D2, and D3 in both frozen sections (A–C) and in whole-mount preparations (D–G). A, D1 immunoreactivity is present in the myenteric (MP) and submucosal (SmP) plexuses and in the mucosa (Muc). The arrows indicate the immunoreactive products. B, D, F, D2 immunoreactivity is present in subsets of myenteric and submucosal neurons, but not in the mucosa. C, E, G, D3 immunoreactivity is present in subsets of myenteric and submucosal neurons. Mucosal immunoreactivity is very weak. Scale bars: (in C) A–C, 50 μm; (in G) D–G, 25 μm.
The locations of transcripts encoding D2 and D3 are coincident with their immunoreactivities in the ENS
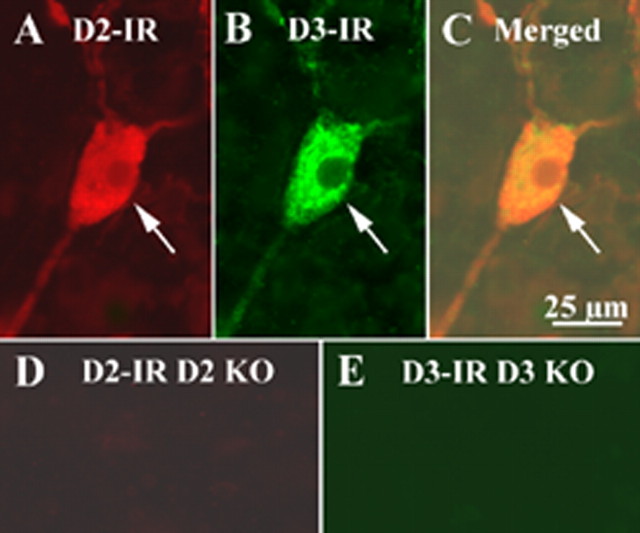
Double labeling by immunocytochemistry and in situ hybridization was used to obtain additional evidence that D2- and D3-immunoreactive cells actually express the corresponding receptors. Transcripts encoding D2 were detected in myenteric neurons of mouse ileum by in situ hybridization. Coincident labeling of each of the same cells was found after the preparations were immunostained with antibodies to D2 (Fig. 5A–D). Similarly, myenteric neurons of the mouse ileum, in which transcripts encoding D3 were detected in by in situ hybridization displayed coincident labeling after the preparations were immunostained with antibodies to D3 (Fig. 5E–H). These observations indicate that subsets of myenteric neurons transcribe and translate D2 and D3 receptor proteins. Because D2 and D3 are both expressed by enteric neurons, double-label immunocytochemistry (using antibodies that were raised in different species and visualized with contrasting fluorophores) was used to determine whether they are expressed in the same or different neurons. The double labeling in the submucosal plexus of the CD-1 mouse ileum revealed an apparently complete coincidence of expression (Fig. 6A–C).
Figure 5.
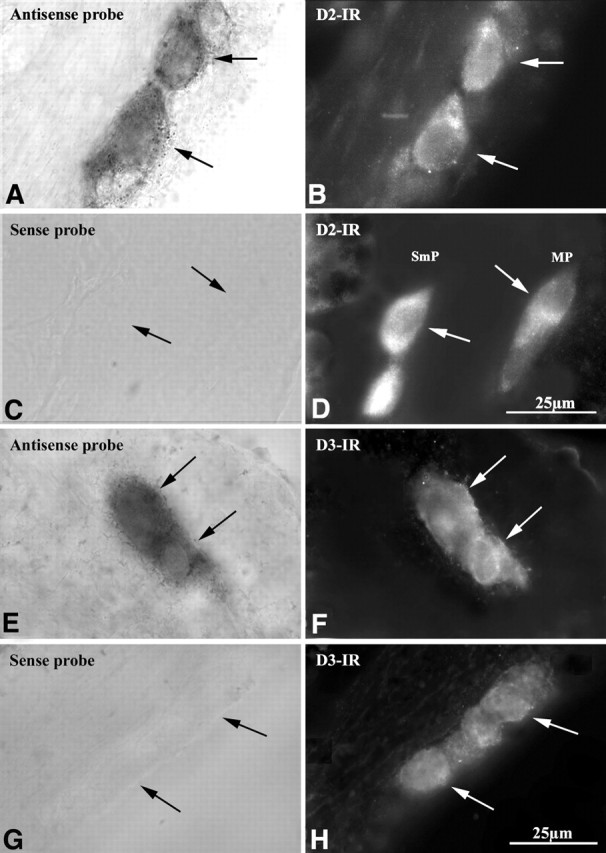
Combined in situ hybridization and immunocytochemistry verifies that D2 and D3 immunoreactivities (IRs) are found in neurons that coexpress transcripts encoding these proteins. In situ hybridization is illustrated in the left panels (A, C, E, G), and immunocytochemistry is illustrated in the right panels (B, D, F, H). Transcripts encoding D2 (A) and D2 immunoreactivity (B) are found in the same neurons. A sense probe (control; C) does not label D2-immunoreactive neurons (D). Transcripts encoding D3 (E) and D3 immunoreactivity (F) are found in the same neurons. A sense probe (control; G) does not label D3-immunoreactive neurons (H). MP, Myenteric plexus.
Figure 6.
D2 and D3 immunocytochemistry were performed on the submucosal plexus of the ileum of CD-1 mice. The same tissue preparation from D2 and D3 KO mice was used as control. D2 receptor immunoreactivity (IR) was revealed by a D2 rabbit antibody and a donkey anti-rabbit Alexa 594 secondary antibody. D3 receptor immunoreactivity was revealed by a D3 goat antibody, a biotinylated donkey anti-goat secondary antibody, and streptavidin FITC. On the tissue of CD-1 mice, D2-immunoreactive products were present in the enteric neurons (A), and D3-immunoreactive products were also present in the enteric neurons (B). D2 and D3 immunoreactivities were colocalized in the same cell (C). However, D2 immunoreactivity was not detected on the tissue of D2 knock-out (KO) mice (D); no D3 immunoreactivity was detected on the tissue of D3 knock-out mouse (E). The arrows indicate the immunoreactive neurons. Scale bar: (in C) 25 μm.
Control immunocytochemical experiments were performed to determine whether antibodies to the D2 and D3 receptors react with gut from transgenic mice that lack these receptors. In contrast to results obtained with wild-type mice, no immunoreactivity was detected when antibodies to D2 were applied to knock-out mice lacking D2 (compare Figs. 6D and 4B,D,F, 6A), and no immunoreactivity was detected when antibodies to D3 were applied to knock-out mice lacking D3 (compare Figs. 6E and 4C,E,G, 6B).
Expression of TH and DAT is increased in the gut of mice that lack D2
Real-time PCR was used to quantify transcripts encoding TH and DAT in the ilea of transgenic mice lacking D2 receptors and in those of their wild-type littermates. Expression of TH and DAT in each animal was normalized to that of β-actin and plotted as a ratio. The abundance of transcripts encoding TH was significantly greater in the animals lacking D2 than in the wild-type mice (p < 0.05; n = 9). Expression of transcripts encoding DAT was also significantly elevated in the D2-deficient animals (p < 0.01; n = 9). Transcripts encoding TH were >10-fold greater than those encoding DAT. These data suggest that dopamine biosynthesis and reuptake both increase when D2 receptors are absent.
Propulsive motility is increased in the gut of mice that lack D2 receptors
The observations that expression of TH and DAT change when D2 receptors are absent suggests that enteric D2 receptors and the dopaminergic neurons that activate them are functionally significant. If so, then an abnormality in gastrointestinal physiology would be expected to be present in mice lacking D2 receptors. We therefore measured total gastrointestinal transit time in animals deficient in D2, D3, or D2 and D3 (double knock-out) receptors (Fig. 7A). D3 was studied as a reference for D2 because both receptors are expressed by enteric neurons. Spores of B. stearothermophilus, administered by gavage, were used to evaluate transit. Because these spores pass through the entire GI tract and appear in the stool without modification, colony counts as a function of time after gavage can be used to quantify GI transit. The mean GI transit time measured in mice lacking D2 receptors (12.7 ± 1.4 h) was significantly faster than that measured in their wild-type littermates (18.3 ± 1.4 h; p < 0.05; n = 6) (Fig. 7A). In contrast, the mean GI transit time measured in mice lacking D3 receptors did not differ significantly from that measured in their wild-type littermates (Fig. 7A). Although the mean GI transit time measured in mice lacking both D2 and D3 receptors (12.4 ± 0.9 h) was significantly faster than that of their wild-type littermates (17.4 ± 0.9 h; p < 0.05; n = 6), it did not differ significantly from the mean GI transit time measured in mice lacking D2 receptors alone (Fig. 7A).
Figure 7.
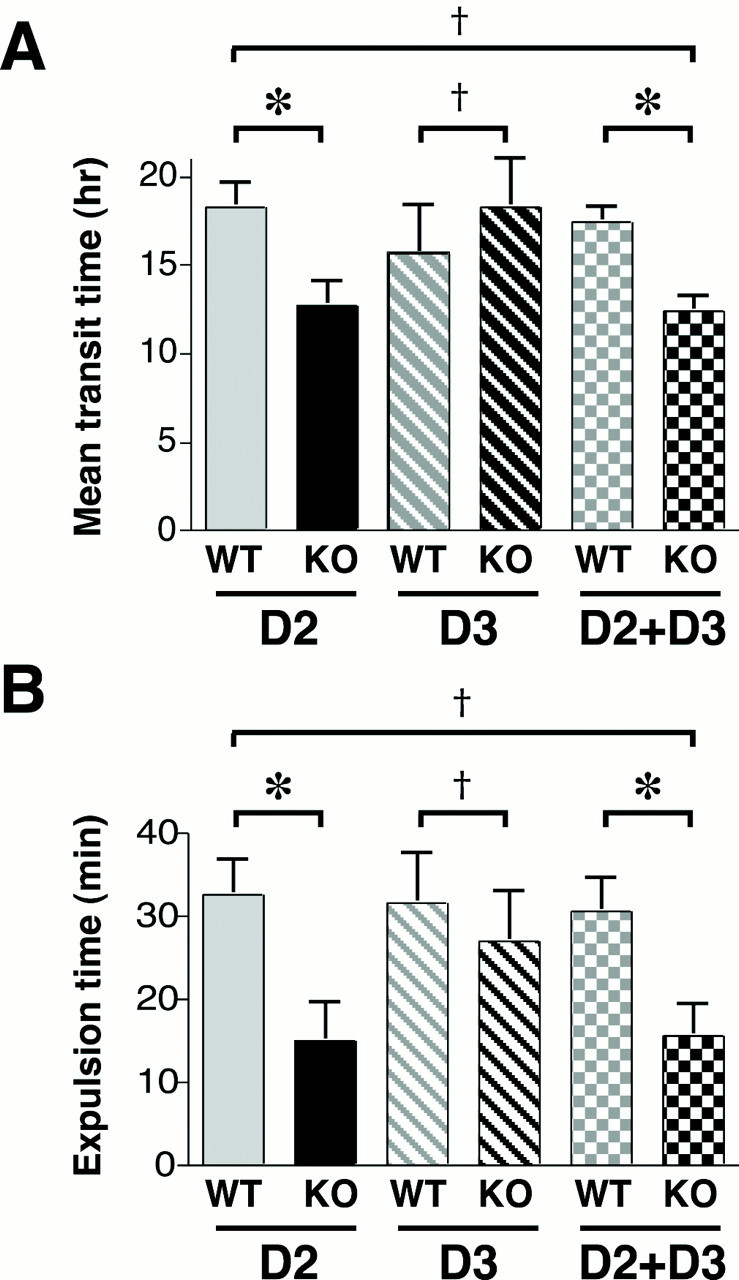
Total gastrointestinal transit time and colonic motility are accelerated in mice that lack D2 receptors. A, Mean gastrointestinal transit time was measured by using spores of B. stearothermophilus. Transit time in mice lacking D2, D3, or D2 and D3 (double knock-out) was compared with that of wild-type littermates. Relative to wild-type littermates, transit time was reduced (faster) in D2 knock-out and double knock-out animals; however, transit time in D3 knock-out mice did not differ from that of their wild-type littermates. Transit time in double knock-out animals was not different from that in mice lacking only D2. B, Colonic motility was estimated by measuring the time require to expel a glass bead inserted into the rectum for a distance of 2 cm. Relative to wild-type littermates, this time was significantly decreased (faster motility) in mice lacking only D2 or D2 plus D3 but was not different in mice lacking only D3. The time required to expel the bead in double knock-out mice was not different from that in mice lacking only D2. *p < 0.05; †, p is not significant. KO, Knock-out; WT, wild type. The error bars indicate SEM.
Because total GI transit time was abnormal in D2 receptor-deficient mice, studies were performed to determine whether propulsion is also faster regionally in the colon where abnormalities have previously been reported in mice lacking DAT (Walker et al., 2000). Colonic propulsion was evaluated by measuring the time required to expel a glass bead inserted into the rectum for a distance of 2 cm. Colonic motility was affected by the knock-out of D2 receptors in a manner that was similar to that of total GI transit (Fig. 7B). The time required to expel glass beads from the colon/rectum of mice lacking D2 receptors (15 ± 5 min) was significantly less than that required by their wild-type littermates (33 ± 4 min; p < 0.05; n = 6) (Fig. 7B). In contrast, expulsion time for the glass beads in mice lacking D3 receptors did not differ significantly from that measured in their wild-type littermates (Fig. 7B). Although the time needed for glass bead expulsion in mice lacking both D2 and D3 receptors (16 ± 4 min; n = 6) was significantly less than that needed by their wild-type littermates (31 ± 4 min; p < 0.05; n = 6), it did not differ significantly from that needed by mice lacking D2 receptors alone (Fig. 7B). These data suggest that D2 plays an important role in the regulation of GI transit and colonic motility.
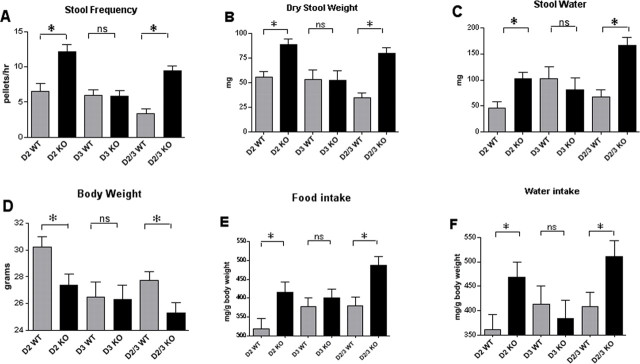
The decrease in gastrointestinal transit time in mice lacking the D2 receptor was reflected in corresponding changes in stool frequency, dry weight, and water content, which were determined from fecal pellets obtained during a 1 h collection period (Fig. 8). Stool frequency (Fig. 8A), dry weight (Fig. 8B), and water content (Fig. 8C) were all significantly greater in D2 knock-out and D2/D3 double knock-out mice than in their wild-type littermates. In contrast, the knock-out of D3 did not cause a significant change in any of these parameters (Fig. 8A–C). The effect of the D2/D3 double knock-out was similar to that seen in animals in which only D2 was deleted. The rapid gastrointestinal and colonic transit in D2 knock-out mice adversely affected the ability of these animals to keep pace with their littermates in growth. Mice lacking D2 and the double knock-out animals lacking both D2 and D3 weighed significantly less than their wild-type littermates (Fig. 8D), yet they consumed significantly more food (Fig. 8E) and water (Fig. 8F). Despite an increased food and water intake, therefore, the D2 and D2/D3 knock-out mice gained weight more slowly than their wild-type littermates. The increased food and water intake of these animals was simply reflected in an increased weight and water content of their stool output, suggesting that the fast rate of GI transit in mice lacking D2 may preclude complete digestion and/or absorption.
Figure 8.
Stool frequency, dry weight, and water content are increased in mice lacking D2, which are smaller than their wild-type littermates, although they consume more food and water. A, Stool frequency. B, Stool dry weight. C, Stool water content. D, Body weight. E, Food intake. F, Water intake. For each of the parameters measured, wild-type (WT) littermates are compared with mice lacking D2 (D2 KO) only, D3 (D3 KO) only, or D2 and D3 (D2/3 KO). *p < 0.05. The error bars indicate SEM.
Discussion
The current study was undertaken to investigate the physiological significance of the enteric dopaminergic neurons that have been found recently to be present in the mammalian ENS (Anlauf et al., 2003; Li et al., 2004). Although DA can activate adrenoceptors (Lucchelli et al., 1990; Tsai and Cheng, 1992), active dopaminergic neurons would probably be more likely to act physiologically through DA receptors than through adrenoceptors, which evolved to respond to norepinephrine and epinephrine. In fact, some previous studies attributed all of the actions of exogenous DA on the bowel to the nonspecific stimulation adrenoceptors and denied the existence of enteric receptors for DA (Grivegnee et al., 1984; Lucchelli et al., 1990). In contrast to these reports, the current observations suggest that the gut is well endowed with specific DA receptors. Transcripts encoding all five classes of DA receptor were expressed throughout the proximodistal axis of the bowel, and the immunoreactivities of all of these receptors, except D4, were also found to be present in layers of the bowel that contain neurons. Transcripts encoding D4 were confined to the mucosal layer, but each of the others potentially mediate dopaminergic neurotransmission. D1, D3, and D5 were expressed both in nerve-containing layers of the gut and in the mucosa; however, both the transcripts encoding D2 and its immunoreactive protein were restricted to neurons. D2 is therefore likely to be an important mediator of neuronal responses to DA. This idea was supported by the observation that transcripts encoding TH and DAT were both increased in transgenic mice that lack D2. Stimulation of D2 thus seems to be important in feedback regulation of neuronal DA biosynthesis (via TH) and reuptake (via DAT). The upregulation of TH, which should increase DA biosynthesis, may be a compensation for the loss of D2-mediated effects. The upregulation of DAT may be a compensation for the increased biosynthesis and consequent release of DA.
The phenotype of mice that lack D2 suggests that D2 is a major mediator of the effects of endogenous DA in the ENS. Consistent with early observations that DA inhibits subsets of enteric neurons that receive an inhibitory innervation (Hirst and Silinsky, 1975), the knock-out of D2 was followed by an increase the rate of total gastrointestinal transit and a regional increase in colonic motility. These effects were D2 specific, in that they were not mimicked by the knock-out of D3; moreover, the alterations in motility induced by the double knock-out of D2 and D3 were indistinguishable from those that followed the knock-out of D2 alone. Essentially, therefore, these experiments suggest that endogenous DA exerts a net inhibitory effect on intestinal motility and does so primarily via enteric neuronal D2 receptors. The abnormally rapid transit that follows the knock-out of D2, moreover, appears to be a severe handicap for mice. The animals ate and drank more than their littermates but still failed to gain weight normally. The excess food and drink lead to increases in the dry weight and water content of stool. One would imagine that animals with this problem would do very poorly in the wild where, in contrast to well regulated animal quarters, access to food and water may be problematic.
Inhibition plays critical roles in the regulation of intestinal motility. For example, the ENS is known to exert a tonic inhibitory influence on myogenic pacemakers of the enteric muculature (Wood, 1980). As a result of this constitutive inhibition, the administration of drugs that suppress the output of enteric motor neurons can lead to intestinal spasm and constipation. Inhibition is also critical in the descending relaxation of the peristaltic reflex (Costa and Furness, 1976). Neither inhibition of the tonic inhibitory output of the ENS nor a direct inhibition of the intestinal musculature is likely to be relevant to the effects of D2 stimulation. The fact that propulsive motility is retained and, in fact, enhanced when D2 is deleted indicates that coordinated peristaltic reflexes must occur in the mutant animals; the gut is not in spasm and the mice are not constipated. It follows that ganglionic microcircuits, as well as the final common excitatory and inhibitory motor neurons used by the ENS to drive the enteric musculature, must also be functioning and subject to regulation. Synaptic transmission within ganglia and release of ACh, nitric oxide, and other transmitters to smooth muscle thus must occur after the deletion of D2.
Rapid transit through the total bowel and the colon of mice that lack D2 suggests that propulsive reflexes occur too often or too powerfully, which implies that the site of D2-mediated inhibition is within ganglia, affecting the microcircuits that govern peristaltic and/or secretory regulation. This suggestion is consistent with the immunocytochemical location of D2, which was prominent on the neuritic processes of both myenteric and submucosal neurons. In this location, endogenous DA acting on axonal D2 receptors is in a position to inhibit the release of ACh and thus to decrease the strength of neurotransmission in prokinetic pathways. In fact, DA has been found to inhibit the evoked release of 3H-ACh from enteric neurons (Kusunoki et al., 1985; Takahashi et al., 1991). This effect, like all of those that are mediated by D2, involves a pertussis toxin-sensitive G-protein; furthermore, the ability of DA to interfere with 3H-ACh release is blocked by the D2 antagonist domperidone. Domperidone, moreover, exerts a gastrokinetic effect that has been attributed to its ability to block D2 receptors (Takahashi et al., 1991). Endogenous DA is released from electrically stimulated neurons of the guinea pig stomach and decreases ACh release (Shichijo et al., 1997). The D2 antagonists, domperidone, and sulpiride both enhance the release of ACh, suggesting that endogenous DA release provides a constitutive level of inhibition that is relieved by the antagonists. The knock-out of D2 would be expected to mimic the effects of the D2 antagonists and increase release of ACh within the ENS.
It is striking that the effects of knocking out D2 are similar to those exerted on the bowel by 5-HT4 agonists. Tegaserod, a partial agonist in vivo at 5-HT4 receptors, accelerates gastric emptying and gastrointestinal transit (Degen et al., 2001; Liu et al., 2005). 5-HT4 receptors, moreover, like D2, are most concentrated on neurites within enteric ganglia (Liu et al., 2005). Domperidone (Barone, 1999; Drolet et al., 2000) and tegaserod are both used therapeutically as prokinetic agents, although domperidone is often thought to be more effective on the proximal gut (Longo and Vernava, 1993; Jost, 1997) and so has been used to relieve the symptoms of dyspepsia and gastroparesis (Horowitz and Fraser, 1995; Tonini et al., 2004), whereas tegaserod is used to treat chronic constipation and constipation-predominant irritable bowel syndrome (Prather et al., 2000; Muller-Lissner et al., 2001; Johanson, 2004; Galligan and Vanner, 2005). Domperidone and DA, however, have both been shown to exert effects on the colon and rectum as well as on the upper bowel (Wiley and Owyang, 1987), whereas tegaserod accelerates gastric emptying (Degen et al., 2001; James et al., 2004; Crowell et al., 2005) and has also been useful in treating dyspepsia and gastroparesis (Banh et al., 2005; Galligan and Vanner, 2005; Zuberi et al., 2005). The acceleration by D2 knock-out of total gastrointestinal and colonic motility suggests that D2 receptors are important at all levels of the gut and are not restricted in their function to the proximal bowel. This suggestion is consistent with the even distribution of transcripts encoding D2 and other DA receptors throughout the proximodistal axis of the gut. Inhibition or deletion of D2 and stimulation of 5-HT4 receptors, therefore, appear to achieve similar prokinetic effects in both the upper and lower bowel. This would occur if the strength of neurotransmission in prokinetic pathways within the ENS were to be regulated by opposing receptors, D2 mediating inhibition and 5-HT4 mediating excitation.
The earliness of the development of enteric DA receptors was unexpected. Except for D5, all were present by E10. Colonization of fetal mouse gut by émigrés from the neural crest begins at E9.5–E10 (Rothman et al., 1984; Baetge and Gershon, 1989; Young et al., 2003), although enteric neurons cannot be detected until ∼E12 (Rothman et al., 1984). Expression of DA receptors, including the neuronally associated D2 subtype, thus precedes the development of terminally differentiated enteric neurons, although it is coincident with the presence in the gut of crest-derived neuronal/glial precursors. The early expression of enteric DA receptors is consistent with the possibility that DA affects the development of the bowel and/or the ENS. The sustained expression of these receptors suggests that their function is not restricted to development but also involves signaling in the mature gut.
Footnotes
This work was supported by National Institutes of Health Grants NS12969 and NS15547.
References
- Anlauf M, Schafer MK, Eiden L, Weihe E (2003). Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol 459:90–111. [DOI] [PubMed] [Google Scholar]
- Baetge G, Gershon MD (1989). Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetal mice: relationship to the development of enteric neurons. Dev Biol 132:189–211. [DOI] [PubMed] [Google Scholar]
- Baetge G, Pintar JE, Gershon MD (1990). Transiently catecholaminergic (TC) cells in the bowel of fetal rats and mice: precursors of non-catecholaminergic enteric neurons. Dev Biol 141:353–380. [DOI] [PubMed] [Google Scholar]
- Banh HL, MacLean C, Topp T, Hall R (2005). The use of tegaserod in critically ill patients with impaired gastric motility. Clin Pharmacol Ther 77:583–586. [DOI] [PubMed] [Google Scholar]
- Barone JA (1999). Domperidone: a peripherally acting dopamine2-receptor antagonist. Ann Pharmacother 33:429–440. [DOI] [PubMed] [Google Scholar]
- Blaugrund E, Pham TD, Tennyson VM, Lo L, Sommer L, Anderson DJ, Gershon MD (1996). Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers, and Mash-1-dependence. Development 122:309–320. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Zhishan L, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD (2001). Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high affinity serotonin transporter (SERT): abnormal intestinal motility and the expression of cation transporters. J Neurosci 21:6348–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard P, Goldstein M, Black IB (1978). Ontogenetic appearance and disappearance of tyrosine hydroxylase and catecholamines. Proc Natl Acad Sci USA 75:2986–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Furness JB (1976). The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol 294:47–60. [DOI] [PubMed] [Google Scholar]
- Crowell MD, Mathis C, Schettler VA, Yunus T, Lacy BE (2005). The effects of tegaserod, a 5-HT receptor agonist, on gastric emptying in a murine model of diabetes mellitus. Neurogastroenterol Motil 17:738–743. [DOI] [PubMed] [Google Scholar]
- Degen L, Matzinger D, Merz M, Appel-Dingemanse S, Osborne S, Luchinger S, Bertold R, Maecke H, Beglinger C (2001). Tegaserod, a 5-HT4 receptor partial agonist, accelerates gastric emptying and gastrointestinal transit in healthy male subjects. Aliment Pharmacol Ther 15:1745–1751. [DOI] [PubMed] [Google Scholar]
- Drolet B, Rousseau G, Daleau P, Cardinal R, Turgeon J (2000). Domperidone should not be considered a no-risk alternative to cisapride in the treatment of gastrointestinal motility disorders. Circulation 102:1883–1885. [DOI] [PubMed] [Google Scholar]
- Eaker EY, Bixler GB, Dunn AJ, Moreshead WV, Mathias JR (1988). Dopamine and norepinephrine in the gastrointestinal tract of mice and the effects of neurotoxins. J Pharmacol Exp Ther 244:438–442. [PubMed] [Google Scholar]
- Frieling T, Cooke HJ, Wood JD (1991). Synaptic transmission in submucosal ganglia of guinea pig distal colon. Am J Physiol 260:G842–G849. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Vanner S (2005). Basic and clinical pharmacology of new motility promoting agents. Neurogastroenterol Motil 17:643–653. [DOI] [PubMed] [Google Scholar]
- Gershon MD (1967). Inhibition of gastrointestinal movement by sympathetic nerve stimulation: the site of action. J Physiol (Lond) 189:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Rothman TP, Joh TH, Teitelman GN (1984). Transient and differential expression of aspects of the catecholaminergic phenotype during development of the fetal bowel of rats and mice. J Neurosci 4:2269–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivegnee AR, Fontaine J, Reuse J (1984). Effect of dopamine on dog distal colon in-vitro. J Pharm Pharmacol 36:454–457. [DOI] [PubMed] [Google Scholar]
- Hartman DS, Civelli O (1997). Dopamine receptor diversity: molecular and pharmacological perspectives. Prog Drug Res 48:173–194. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Silinsky EM (1975). Some effects of 5-hydroxytryptamine, dopamine and noradrenaline on neurones in the submucous plexus of guinea-pig small intestine. J Physiol (Lond) 251:817–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Fraser RJ (1995). Gastroparesis: diagnosis and management. Scand J Gastroenterol Suppl 213:7–16. [PubMed] [Google Scholar]
- James AN, Ryan JP, Crowell MD, Parkman HP (2004). Regional gastric contractility alterations in a diabetic gastroparesis mouse model: effects of cholinergic and serotoninergic stimulation. Am J Physiol Gastrointest Liver Physiol 287:G612–G619. [DOI] [PubMed] [Google Scholar]
- Johanson JF (2004). Review article: tegaserod for chronic constipation. Aliment Pharmacol Ther 7:[Suppl 20], 20–24. [DOI] [PubMed] [Google Scholar]
- Jost WH (1997). Gastrointestinal motility problems in patients with Parkinson’s disease. Effects of antiparkinsonian treatment and guidelines for management. Drugs Aging 10:249–258. [DOI] [PubMed] [Google Scholar]
- Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, Robakis NK, Polites HG, Pintar JE, Schmauss C (1999). Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience 91:911–924. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Taniyama K, Tanaka C (1985). Dopamine regulation of [3H]acetylcholine release from guinea-pig stomach. J Pharmacol Exp Ther 234:713–719. [PubMed] [Google Scholar]
- Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD (2004). Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J Neurosci 24:1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD (2005). Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol 289:G1148–G1163. [DOI] [PubMed] [Google Scholar]
- Longo WE, Vernava AM III (1993). Prokinetic agents for lower gastrointestinal motility disorders. Dis Colon Rectum 36:696–708. [DOI] [PubMed] [Google Scholar]
- Lucchelli A, Boselli C, Chiari MC, Grana E (1986). Analysis of the relaxing effect of dopamine on the isolated rat jejunum. Arch Int Pharmacodyn Ther 279:234–247. [PubMed] [Google Scholar]
- Lucchelli A, Boselli C, Grana E (1990). Dopamine-induced relaxation of the guinea-pig isolated jejunum is not mediated through dopamine receptors. Pharmacol Res 22:433–444. [DOI] [PubMed] [Google Scholar]
- Mathers JC, Smith H, Carter S (1997). Dose-response effects of raw potato starch on small-intestinal escape, large-bowel fermentation and gut transit time in the rat. Br J Nutr 78:1015–1029. [PubMed] [Google Scholar]
- Muller-Lissner SA, Fumagalli I, Bardhan KD, Pace F, Pecher E, Nault B, Ruegg P (2001). Tegaserod, a 5-HT4 receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther 15:1655–1666. [DOI] [PubMed] [Google Scholar]
- Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G (2000). Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology 118:463–468. [DOI] [PubMed] [Google Scholar]
- Ren J, Hu HZ, Liu S, Xia Y, Wood JD (1999). Glutamate modulates neurotransmission in the submucosal plexus of guinea-pig small intestine. NeuroReport 10:3045–3048. [DOI] [PubMed] [Google Scholar]
- Rothman TP, Nilaver G, Gershon MD (1984). Colonization of the developing murine enteric nervous system and subsequent phenotypic expression by the precursors of peptidergic neurons. J Comp Neurol 225:13–23. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A (1993). A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100:431–440. [DOI] [PubMed] [Google Scholar]
- Scheibner J, Trendelenburg AU, Hein L, Starke K, Blandizzi C (2002). Alpha 2-adrenoceptors in the enteric nervous system: a study in alpha 2A-adrenoceptor-deficient mice. Br J Pharmacol 135:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichijo K, Sakurai-Yamashita Y, Sekine I, Taniyama K (1997). Neuronal release of endogenous dopamine from corpus of guinea pig stomach. Am J Physiol 273:G1044–G1050. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kurosawa S, Wiley JW, Owyang C (1991). Mechanism for the gastrokinetic action of domperidone. In vitro studies in guinea pigs. Gastroenterology 101:703–710. [DOI] [PubMed] [Google Scholar]
- Tan S, Hermann B, Borrelli E (2003). Dopaminergic mouse mutants: investigating the roles of the different dopamine receptor subtypes and the dopamine transporter. Int Rev Neurobiol 54:145–197. [DOI] [PubMed] [Google Scholar]
- Tonini M, Cipollina L, Poluzzi E, Crema F, Corazza GR, De Ponti F (2004). Review article: clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther 19:379–390. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Cheng JT (1992). The effect of exogenous dopamine on ileal smooth muscle of guinea-pigs. Chin J Physiol 35:133–141. [PubMed] [Google Scholar]
- Walker JK, Gainetdinov RR, Mangel AW, Caron MG, Shetzline MA (2000). Mice lacking the dopamine transporter display altered regulation of distal colonic motility. Am J Physiol Gastrointest Liver Physiol 279:G311–G318. [DOI] [PubMed] [Google Scholar]
- Wiley J, Owyang C (1987). Dopaminergic modulation of rectosigmoid motility: action of domperidone. J Pharmacol Exp Ther 242:548–551. [PubMed] [Google Scholar]
- Wood JD (1980). Intracellular study of effects of morphine on electrical activity of myenteric neurons in cat small intestine. Gastroenterology 79:1222–1230. [PubMed] [Google Scholar]
- Wood JD (1999). Neurotransmission at the interface of sympathetic and enteric divisions of the autonomic nervous system. Chin J Physiol 42:201–210. [PubMed] [Google Scholar]
- Young HM, Bergner AJ, Muller T (2003). Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J Comp Neurol 456:1–11. [DOI] [PubMed] [Google Scholar]
- Zuberi BF, Quraishy MS, Faisal N, Ahmed S (2005). Idiopathic gastroparesis. J Coll Physicians Surg Pak 15:566–567. [PubMed] [Google Scholar]