Abstract
Extinction of conditioned fear responses is an active learning process resulting from the repeated presentation of a conditioned stimulus in the absence of the unconditioned aversive stimulus. Recent research implicates the medial prefrontal cortex (mPFC) in the mediation of fear extinction in rodents and the pathophysiology of posttraumatic stress disorder. However, there is currently little understanding of precisely how stress can impact fear extinction and the neural circuitry subserving this behavior. The present study examined the effects of brief exposure to an uncontrollable stressor on (1) fear conditioning and fear extinction, and (2) dendritic morphology of pyramidal neurons in the infralimbic (IL) and prelimbic (PL) regions of the mPFC in mice. Exposure to three episodes of stress ending 24 h before fear conditioning significantly attenuated the rate of cued fear extinction relative to nonstressed controls, but did not affect fear conditioning or cue or context recall. Analysis of Golgi-stained neurons showed that one or three exposures to daily swim stress caused significant retraction of terminal branches of apical, but not basilar, dendrites of IL neurons. In contrast, PL neuronal morphology was unaltered by stress. These data demonstrate that IL, but not PL, neurons are highly sensitive to even brief exposure to stress, and that this same form of stress impairs fear extinction. Present findings suggest that trauma may compromise the functional integrity of the mPFC with implications for the pathophysiology of certain neuropsychiatric disorders.
Keywords: stress, infralimbic cortex, fear extinction, mPFC, PTSD, dendritic arborization
Introduction
Extinction is an active form of learning in which the expression of a conditioned fear response is reduced after repeated experience of the conditioned stimulus in the absence of the unconditioned, aversive stimulus (Pavlov, 1927). Impaired fear extinction is a major symptom of anxiety disorders caused by emotional trauma, such as posttraumatic stress disorder (PTSD) [Diagnostic and Statistical Manual of the American Psychiatric Association IV (DSM-IV)]. However, although there is improved understanding of the neural systems subserving fear extinction, little is known about how these systems might be functionally compromised by stress.
Fear conditioning and extinction appear to involve partially distinct molecular and neural circuitry (Maren and Quirk, 2004; Pare et al., 2004; Barad, 2005). A growing literature implicates the medial prefrontal cortex (mPFC), via connections to the amygdala, in fear extinction (Canteras et al., 1992; McDonald et al., 1996; Smith et al., 2000; Berretta et al., 2005). In both healthy humans and rats, mPFC is activated during fear extinction (Barrett et al., 2003; Phelps et al., 2004; Santini et al., 2004) and relatively lesser mPFC volume is associated with poor fear extinction (Cintron and Quirk, 2004; Milad et al., 2005). Furthermore, electrical stimulation of rat mPFC inhibits the neuronal output of the amygdala and mimics the effects of extinction learning on conditioned freezing, whereas, conversely, lesions of the mPFC cause deficits in extinction learning and/or subsequent recall of extinction memory (Morgan et al., 1993; Quirk et al., 2000, 2003; Milad and Quirk, 2002; Lebron et al., 2004).
Together, these converging lines of evidence suggest that stress-induced changes in mPFC function could impair fear extinction. Interestingly in this context, PTSD patients have smaller mPFC volume and exhibit lesser mPFC activation during fear extinction than normal controls (Rauch et al., 2003; Bremner et al., 2005; Shin et al., 2005). Moreover, recent rodent studies have shown that stress causes neuronal remodeling in the mPFC as well as changes in long-term potentiation in the mPFC-amygdala pathway (Maroun and Richter-Levin, 2003). For example, chronic (3–6 weeks) restraint stress or corticosterone treatment causes significant dendritic retraction of mPFC pyramidal neurons (Wellman, 2001; Cook and Wellman, 2004; Radley et al., 2006). Indeed, demonstrating the profound sensitivity of these neurons to stress, similar morphological changes can be induced to some extent by repeated exposure to mild stressors (Seib and Wellman, 2003; Brown et al., 2005).
However, a number of critical questions remain: first, can exposure to a brief but sufficiently intense stressor produce changes in mPFC neuronal morphology; second, are specific subregions of the mPFC [i.e., infralimbic (IL) and prelimbic (PL) cortex] vulnerable to these effects; and third, are these changes associated with alterations in fear extinction. The present study aimed to address these questions.
Materials and Methods
Subjects.
Adult male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were housed five per cage in a temperature- and humidity-controlled vivarium under a 12 h light/dark cycle (lights on 6:00 A.M.). Experimental procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the local Animal Care and Use Committee.
Uncontrollable stressor.
The experimental design is depicted in Figure 1A. Mice were forced to swim in a 20-cm-diameter cylinder filled halfway with 24 ± 1°C water for 10 min. Forced swimming is commonly used to assess stress-related behavior in mice (Cryan and Holmes, 2005) and causes profound activation of the hypothalamic–pituitary–adrenal axis (Anisman et al., 2001) (our unpublished observations). Co-housed mice were either subjected to one swim stress or three swim stresses on consecutive days, or remained in the home cage.
Figure 1.
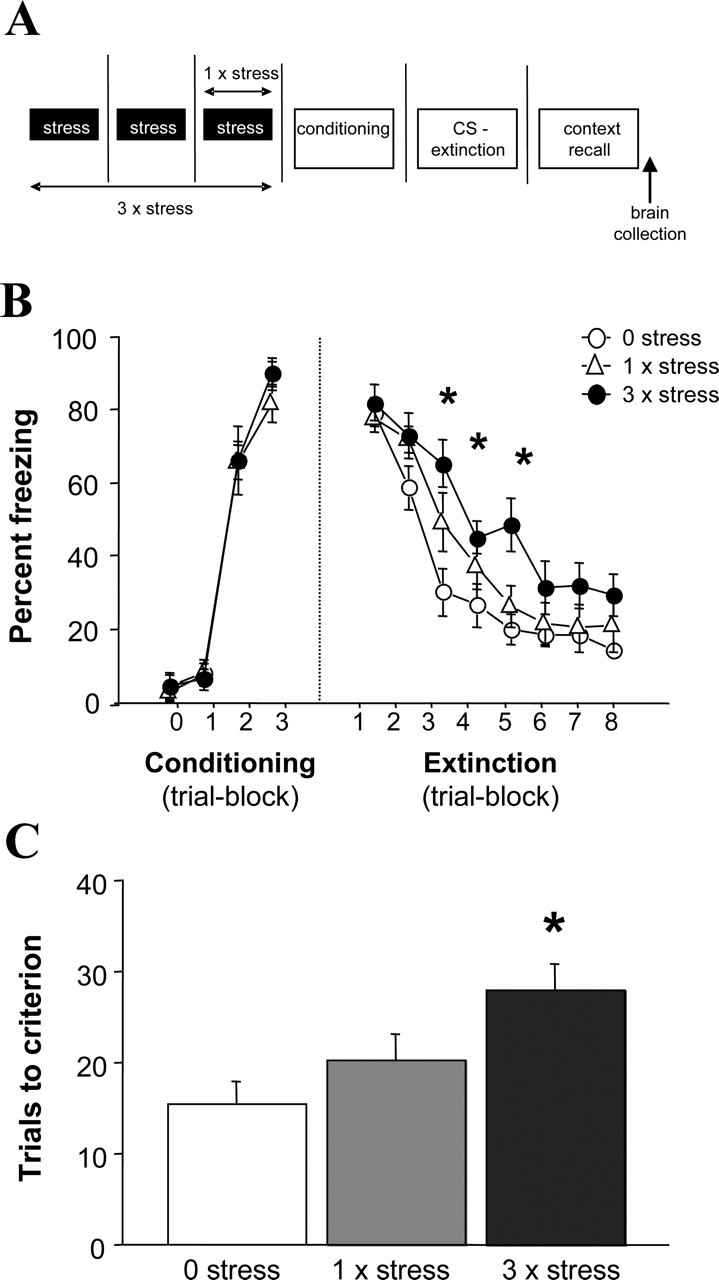
Uncontrollable stress causes deficits in fear extinction. A, Schematic depiction of the behavioral testing procedure. B, Stress significantly increased freezing during extinction training but did not affect fear acquisition or initial recall of the conditioned tone. C, Stress significantly increased the number of trials to extinction criterion (n = 10–11/stress condition). Data in Figures 1–3 are means ± SEM. *p < 0.05 versus zero stress.
Fear conditioning and extinction.
Mice were fear conditioned 24 h after the final stress. Zero stress controls were conditioned concurrently. Conditioning was conducted in a 27 × 27 × 11 cm chamber with transparent walls and a metal-rod floor, cleaned with a 79.5% water/19.5% ethanol/1% vanilla-extract solution. After a 120 s acclimation period, mice received three pairings (60–120 s variable interpairing interval) of the conditioned stimulus (CS; 30 s, 80 dB, 3 kHz tone) and the unconditioned stimulus (US; 2 s, 0.6 mA scrambled footshock), in which the US was presented during the last 2 s of the CS by the San Diego Instruments (San Diego, CA) Freeze Monitor system. After a 120 s no-stimulus consolidation period after the final CS–US pairing, mice were returned to the home cage.
Twenty-four hours later, CS recall and extinction learning were assessed. Mice were placed in a novel context (black/white-checkered walls and a solid Plexiglas opaque floor cleaned with a 50% ethanol/50% water solution) housed in a different room. After an initial 120 s acclimation period, the mouse received 40 presentations of the CS, each lasting 30 s and separated by a 5 s no-stimulus interval. Twenty-four hours later, mice were returned to the original training chamber/testing room for 5 min to assess context recall.
Freezing (no visible movement except respiration) was scored every 5 s by an observer blind to condition and converted to a percentage [(freezing observations/total observations) x 100]. Freezing during extinction was averaged into 5-trial blocks for analysis. The number of extinction trials taken to reach a criterion of two successive trials of one or fewer instances of freezing per CS exposure was also calculated. The effect of stress on freezing was analyzed using ANOVA, with repeated measures for trial block in the case of extinction, and Bonferroni’s post hoc comparisons.
Dendritic analyses.
Within 1 h of context recall, six randomly chosen mice from each stress condition were overdosed with isoflurane and transcardially perfused with 0.9% saline. Brains were removed and processed using Glaser and Van der Loos’ (1981) modified Golgi stain. Briefly, tissue was immersed in Golgi-Cox solution for 10 d. Brains were then dehydrated, infiltrated with a graded series of celloidins, and embedded in 8% celloidin. Coronal sections were cut at 200 μm on a sliding microtome (Histoslide SM2000R; Leica, Nussloch, Germany). Free-floating sections were alkalinized, developed, fixed, dehydrated, mounted, and coverslipped.
Pyramidal neurons in the IL and PL (Paxinos and Franklin, 2001) were identified in Golgi-stained sections based on position relative to major landmarks (e.g., forceps minor and genu of the corpus callosum, nucleus accumbens) and characteristic cytoarchitecture (e.g., the IL is markedly thinner and has fewer, less well defined layers than the PL). Pyramidal neurons were defined by a distinct, single apical dendrite extending from the apex of the soma toward the pial surface of the cortex, two or more basilar dendritic trees extending from the base of the soma, and dendritic spines (see Figs. 2B, 3B). Neurons selected for reconstruction were located in the middle third of the section, did not have truncated branches, and were unobscured by neighboring neurons and glia, with dendrites that were easily discriminable by focusing through the depth of the tissue. IL neurons were sampled from all cortical layers and PL neurons from layers II and III (Cook and Wellman, 2004; Radley et al., 2004). All pyramidal neurons meeting the criteria were reconstructed.
Figure 2.
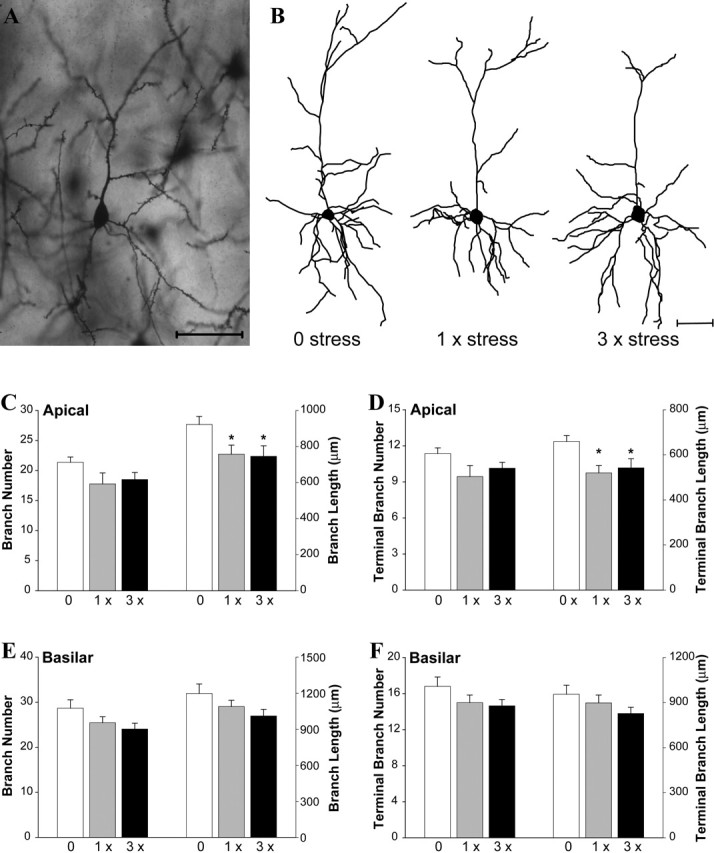
Uncontrollable stress rapidly causes retraction of apical dendrites in IL pyramidal neurons. A, Golgi-stained IL pyramidal neuron from an unstressed mouse. B, Computer-assisted reconstructions of representative IL pyramidal neurons in mice exposed to zero, one, or three episodes of stress. C, D, Overall (C) and terminal (D) branch length, but not number, of apical dendrites were significantly reduced after one or three stress exposures relative to zero stress controls. E, F, Stress did not affect overall (E) or terminal (F) basilar branch number or length (n = 6/stress condition). Scale bar, 50 μm. *p < 0.05 versus zero stress.
Figure 3.
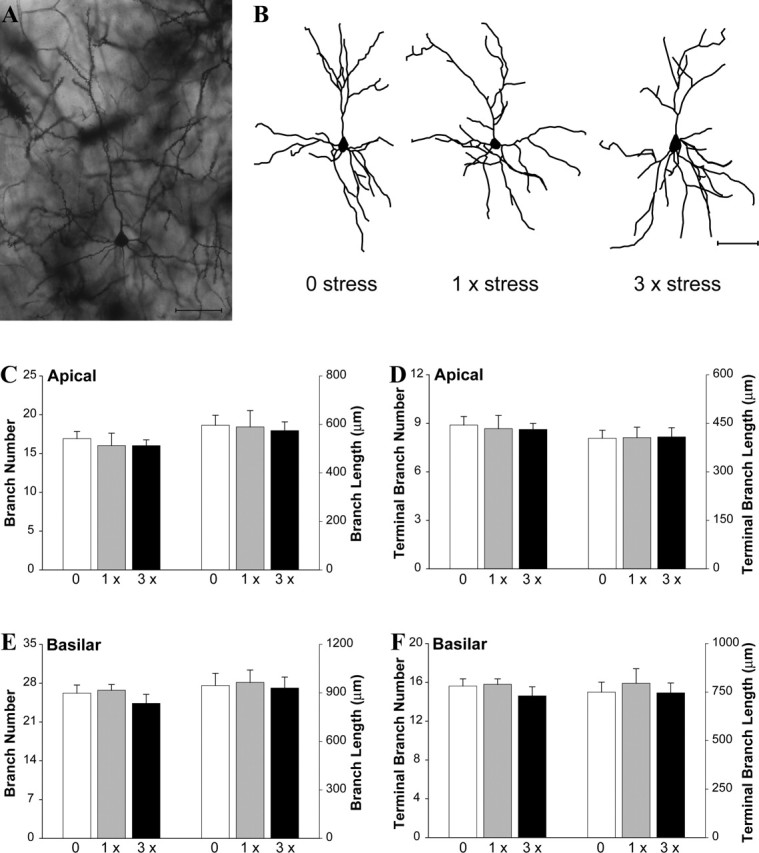
Uncontrollable stress does not alter dendritic morphology of PL pyramidal neurons. A, Golgi-stained PL pyramidal neuron from an unstressed mouse. B, Computer-assisted reconstructions of representative PL pyramidal neurons in mice exposed to zero, one, or three episodes of stress. Scale bars, 50 μm. C–F, Stress did not affect overall or terminal branch number or length of apical (C, D) or basilar (E, F) dendrites (n = 6/stress condition).
Six to 13 neurons per region were reconstructed for each mouse at 600x (average within-animal error, 12.1 ± 0.5%). Morphology of apical and basilar arbors was quantified in three dimensions using a computer-based neuron tracing system (Neurolucida; MicroBrightField, Williston, VT) with the experimenter blind to condition. To rule out artifactual differences in dendritic morphology caused by differential sampling across cortical layers, the soma-to-pial surface distance was measured in each neuron and compared across conditions using ANOVA. The effect of stress on the number and length of apical and basilar dendrites, both overall and specifically at terminals, were analyzed using ANOVA and Bonferroni’s post hoc comparisons.
Results
Stress effects on fear conditioning and extinction
All mice displayed robust fear conditioning, with no differences across stress conditions (Fig. 1B). Stress did not affect freezing before the first CS presentation or immediately after conditioning. Freezing during context testing 24 h after extinction training was also unaffected by stress (0 × stress = 31 ± 4% freezing; 1 × stress = 32 ± 4%; 3 × stress = 36 ± 4%).
Freezing during extinction was significantly affected by stress (F(2,28) = 5.70; p < 0.01) and trial-block (F(7,196) = 67.86; p < 0.01). Although freezing progressively decreased in all groups over extinction trial blocks, 3 × stress mice showed significantly higher freezing than 0 × stress controls on the third, fourth, and fifth extinction trial blocks (p < 0.05), with a nonsignificant trend for higher freezing in the 1 × stress group on the third trial block (p = 0.07) (Fig. 1B). Mice exposed to three episodes of stress also took significantly more extinction trials to reach criterion than 0 × stress controls (main effect of stress, F(2,28) = 4.94, p = 0.01; post hoc, p < 0.05) (Fig. 1C).
Stress effects on mPFC dendritic morphology
Examination of mPFC showed complete impregnation of multiple pyramidal neurons (Fig. 2A). Reconstructed neurons were located at depths of 107–533 μm for the IL and 104–434 μm for the PL. Average soma-to-pial surface distance did not vary across stress conditions for either the IL or PL, indicating sampling across equivalent cortical layers.
In the IL, apical dendritic branch length was significantly affected by stress (F(1,15) = 3.69; p < 0.05). Mice exposed to one or three episodes of stress displayed significantly shorter apical branches than 0 × stress controls (p < 0.05) (Fig. 2B, C) because of a specific reduction in the length of terminal branches (F(1,15) = 4.89, p < 0.05; post hocs, p < 0.05) (Fig. 2D). This effect was not localized to a specific cortical layer, as the magnitude of the reductions was similar in neurons sampled from superficial and deep layers (for superficial neurons, apical branch length was decreased by ∼15% averaged across both stress conditions; for deep neurons, apical branch length was decreased by ∼18%). Neither total nor terminal branch number was affected by stress (Fig. 2C,D). Basilar dendritic morphology was also not different across groups (Fig. 2E,F) (all p values > 0.12).
Stress did not affect any measure of apical or basilar morphology in PL neurons (Fig. 3A–F) (all p values > 0.40). This was confirmed using Sholl analysis that assessed the amount and distribution of dendritic material in both apical and basilar PL arbors (Sholl, 1956) (both p values > 0.60; data not shown).
Discussion
Although there is a strong clinical relationship between exposure to emotional trauma and deficits in fear extinction, a direct link between the two has not been clearly established (DSM-IV) (Orr et al., 2000). The present study demonstrated that exposure to uncontrollable stress before fear conditioning impaired subsequent fear extinction in mice. As compared with nonstressed controls, mice exposed to 3 consecutive days of swim stress showed higher levels of freezing during early extinction training and a greater number of trials to reach extinction criterion. Stressed mice were able to extinguish to rates comparable with nonstressed controls with further training. Moreover, rates of freezing during conditioning, initial CS recall, and context recall were unaffected by stress. Together, this pattern of effects suggests that stress produced a selective deficit in fear extinction rather than a nonspecific enhancement of fear conditioning, or a more general impairment in learning and memory.
Whereas present data concur with previous evidence that mPFC lesions produce deficits in extinction learning in rats (Morgan et al., 1993; Morgan and LeDoux, 1995), other studies point to a specific role for the IL in mediating recall of extinguished fear memories (Quirk et al., 2000, 2003; Herry and Garcia, 2002; Lebron et al., 2004). Because our objective was to complete three phases of behavioral testing (conditioning, CS recall/extinction, and context recall) and obtain mPFC samples within the shortest time frame after the cessation of stress, extinction recall was not tested. Previous studies in rats have also found that various types of stressors, including restraint and footshock as well as forced swimming, can facilitate fear conditioning (Shors et al., 1992; Conrad et al., 1999; Rau et al., 2005). In addition, recent evidence demonstrates that chronic restraint stress can produce selective deficits in extinction recall in rats (Miracle et al., 2006). Thus, together with the present findings, these data show that stress may produce effects on fear conditioning, extinction, or extinction recall, and that the precise nature of these effects may depend on the stressor type, intensity, and chronicity. Notwithstanding, the finding that even brief exposure to a sufficiently stressful event can cause resistance to fear extinction provides a novel model of stress-induced psychopathology in neuropsychiatric conditions characterized by impaired extinction, such as PTSD. Moreover, the observation that these effects occur in mice has implications for further identifying the neural and genetic factors involved, given the utility of this species as a model system for emotional disorders (Cryan and Holmes, 2005; Hariri and Holmes, 2006).
The present study showed that the same stressor that produced extinction deficits also caused alterations in neuronal morphology in the mPFC. Exposure to three episodes of swim stress produced remodeling of dendritic arbors of mPFC pyramidal neurons within 72 h of the final stressor. Specifically, stress caused significant retraction of terminal branches of apical, but not basilar, dendrites. Remarkably, changes of the same specificity and magnitude were found in mice subjected to just one episode of stress, demonstrating the exquisite sensitivity of these neurons to stress. This finding not only provides novel insight into the plasticity of ventromedial PFC neurons, but also has implications for predicting how behaviors mediated by this brain region might be vulnerable to disruption by exposure to a brief traumatic stressor (see below).
Previous studies have shown that various forms of chronic stress in rats produce morphological changes in the mPFC comparable with those presently observed, notably retraction of apical, not basilar, dendritic terminals (Wellman, 2001; Cook and Wellman, 2004; Brown et al., 2005; Radley et al., 2006). Furthermore, although the present study is the first to show that such changes can be produced by an acute stressor, rapid dendritic remodeling in response to behavioral and physiological events is not without precedent. For example, Siberian ground squirrels exhibit a marked increase in the apical branch number and length of CA3 hippocampal neurons within 2 h of emergence from torpor (Popov et al., 1992), whereas the 24 h transition from proestrus to estrus in female rats is characterized by decreased spine density of CA1 hippocampal neurons (Woolley et al., 1990).
The present data also showed that stress-induced changes in neuronal morphology were specific to a subregion of the mPFC: dendritic retraction occurred in the IL, but not PL, neurons. This is the first demonstration of morphological changes in the IL occurring in response to stress (the aforementioned studies, which demonstrated effects of more chronic stress in the PL, did not examine the IL). One interpretation of these findings is that IL neurons are acutely sensitive to stress, whereas more prolonged exposure to stress is necessary to induce changes in the PL. Additional studies could test this hypothesis and assess how such region-specific morphological changes might translate into differential effects on behavior.
As noted in the Introduction, there is compelling evidence that a neural pathway connecting the mPFC and amygdala mediates fear extinction. Within this circuit, the IL appears to be critical. For example, neuronal activity in the IL signals successful retention and expression of an extinguished fear memory (Milad and Quirk, 2002). More generally, inactivation of the IL causes impairments in other behaviors that, like fear extinction, are characterized by inhibition of inappropriate responding to learned cues (Chudasama et al., 2003; Murphy et al., 2005). These findings have clear parallels with the present finding that stress effects on fear extinction are associated with changes in IL neuronal morphology, and raise the possibility that the two effects may be functionally linked.
Precisely how morphological changes in IL neurons might alter their function remains to be determined. Differences in dendritic patterns and distribution are known to determine the functional properties of cortical neurons (Rall et al., 1992; Mainen and Sejnowski, 1996; Koch and Segev, 2000), and alterations in neuronal excitability are associated with changes in dendritic morphology (Muller et al., 2000; Gazzaley et al., 2002; Monfils and Teskey, 2004; Monfils et al., 2004). For instance, repeated high-frequency stimulation of callosal fibers in behaving rats results in both increases in dendritic length and potentiation of excitability of cortical pyramidal cells (Monfils et al., 2004), whereas repeated low-frequency stimulation produces dendritic retraction and concomitant decreases in the excitability of cortical pyramidal cells (Monfils and Teskey, 2004). Furthermore, stimulation of apical tufts of cortical pyramidal neurons is thought to disproportionately excite the cells (Rhodes and Llinás, 2001). Thus, stress-induced retraction of terminal branches of IL apical dendrites could result in decreased excitability of these neurons. Given that activation of mPFC neurons can depress amygdala output (Quirk et al., 2003; Laviolette et al., 2005; Likhtik et al., 2005), the retraction of IL dendrites and concomitant decrease in mPFC activation could impair inhibitory modulation of the amygdala during extinction.
However, the present results do not exclude effects of stress on regions other than the IL, and indeed stress-induced alterations in the IL alone may not be sufficient to impair extinction. Consistent with this notion, although both one and three episodes of stress were sufficient to causes dendritic retraction in the IL, the more prolonged stress regime was necessary to produce statistically significant deficits in fear extinction. Chronic stress is known to alter neuronal morphology in the amygdala, hippocampus, and PL (Conrad et al., 1999; Vyas et al., 2002; Cook and Wellman, 2004; Laviolette et al., 2005). Therefore, one possibility is that the IL is an initial target for stress and that loss of functional integrity in this brain region renders the wider neural circuitry supporting extinction vulnerable to additional stress. Additional studies will help elucidate the precise nature of stress effects on this circuitry and may ultimately provide insights into the pathophysiology of neuropsychiatric disorders ranging from PTSD to drug addiction.
Footnotes
*A.I. and C.L.W. contributed equally to this work.
This work was supported by the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program, a National Alliance for Research on Schizophrenia and Depression grant to A.H., and National Institute of Mental Health Grant MH067607 to C.L.W. We thank Joy E. Garrett for technical assistance.
References
- Anisman H, Hayley S, Kelly O, Borowski T, Merali Z (2001). Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: mouse strain-dependent outcomes. Behav Neurosci 115:443–454. [PubMed] [Google Scholar]
- Barad M (2005). Fear extinction in rodents: basic insight to clinical promise. Curr Opin Neurobiol 15:710–715. [DOI] [PubMed] [Google Scholar]
- Barrett D, Shumake J, Jones D, Gonzalez-Lima F (2003). Metabolic mapping of mouse brain activity after extinction of a conditioned emotional response. J Neurosci 23:5740–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D (2005). Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience 132:943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS (2005). Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med 35:791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL (2005). Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex 15:1714–1722. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW (1992). Connections of the posterior nucleus of the amygdala. J Comp Neurol 324:143–179. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW (2003). Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146:105–119. [DOI] [PubMed] [Google Scholar]
- Cintron B, Quirk GJ (2004). The size of the infralimbic cortex is correlated with recall of fear extinction. Soc Neurosci Abstr 30:328.13. [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS (1999). Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113:902–913. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL (2004). Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 60:236–248. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A (2005). The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4:775–790. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Kay S, Benson DL (2002). Dendritic spine plasticity in hippocampus. Neuroscience 111:853–862. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H (1981). Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods 4:117–125. [DOI] [PubMed] [Google Scholar]
- Hariri A, Holmes A (2006). Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci 10:182–191. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R (2002). Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci 22:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Segev I (2000). The role of single neurons in information processing. Nat Neurosci 3:Suppl, 1171–1177. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA (2005). A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci 25:6066–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ (2004). Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem 11:544–548. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D (2005). Prefrontal control of the amygdala. J Neurosci 25:7429–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ (1996). Influence of dendritic structure on firing pattern in model neocortical neurons. Nature 382:363–366. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ (2004). Neuronal signalling of fear memory. Nat Rev Neurosci 5:844–852. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G (2003). Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci 11:4406–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL (2006). Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem 85:213–218. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L (1996). Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71:55–75. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2002). Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420:70–74. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL (2005). Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA 102:10706–10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Teskey GC (2004). Induction of long-term depression is associated with decreased dendritic length and spine density in layers II and V of sensorimotor neocortex. Synapse 53:114–121. [DOI] [PubMed] [Google Scholar]
- Monfils MH, VandenBerg PM, Kleim JA, Teskey GC (2004). Long-term potentiation induces expanded movement representation and dendritic hypertrophy in layer V of rat sensorimotor neocortex. Cereb Cortex 14:586–593. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE (1995). Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109:681–688. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE (1993). Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett 163:109–113. [DOI] [PubMed] [Google Scholar]
- Muller D, Toni N, Buch PA (2000). Spine changes associated with long-term potentiation. Hippocampus 10:596–604. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Dalley JW, Robbins TW (2005). Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 179:99–107. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK (2000). De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol 109:290–298. [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE (2004). New vistas on amygdala networks in conditioned fear. J Neurophysiol 92:1–9. [DOI] [PubMed] [Google Scholar]
- Pavlov IP (1927). In: Conditioned reflexes London: Oxford UP.
- Paxinos KBJ, Franklin G (2001). In: The mouse brain in stereotaxic coordinates London: Academic.
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43:897–905. [DOI] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS, Bragin AG (1992). Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience 48:45–51. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K (2000). The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20:6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D (2003). Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23:8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH (2004). Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125:1–6. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH (2006). Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 16:313–320. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Holmes WR, Jack JJ, Redman SJ, Segev I (1992). Matching dendritic neuron models to experimental data. Physiol Rev 72:S159–186. [DOI] [PubMed] [Google Scholar]
- Rau V, Decola JP, Fanselow MS (2005). Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev 29:1207–1223. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N (2003). Selectively reduced regional cortical volumes in post-traumatic stress disorder. NeuroReport 14:913–916. [DOI] [PubMed] [Google Scholar]
- Rhodes PA, Llinás RR (2001). Apical tuft input efficacy in layer 5 pyramidal cells from rat visual cortex. J Physiol (Lond) 536:167–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ (2004). Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci 24:5704–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib LM, Wellman CL (2003). Daily injections alter spine density in rat medial prefrontal cortex. Neurosci Lett 337:29–32. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 62:273–281. [DOI] [PubMed] [Google Scholar]
- Sholl DA (1956). The measurable parameters of the cerebral cortex and their significance in its organization. Prog Neurobiol 324–333. [PubMed]
- Shors TJ, Weiss C, Thompson RF (1992). Stress-induced facilitation of classical conditioning. Science 257:537–539. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D (2000). Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol 416:496–508. [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22:6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL (2001). Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol 49:245–253. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS (1990). Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci 10:4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]


