Abstract
Chemical stimulation of a region extending from the most caudal ventrolateral medulla into the upper cervical spinal cord evoked large sympathetically mediated pressor responses. These responses were not dependent on the integrity of the rostral ventrolateral medulla (RVLM) and may be mediated by glutamatergic neurons embedded in the white matter that project to the thoracic spinal cord. We term this new region the medullo-cervical pressor area (MCPA). This region is distinct from the caudal pressor area, because blockade of the RVLM with muscimol inhibited this pressor response but not that evoked from the MCPA. This is the first study to provide functional evidence for a cardiovascular role for neurons in the cervical spinal cord white matter that innervate sympathetic preganglionic neurons (Jansen and Loewy, 1997). Using retrograde tracing, in combination with immunohistochemistry and in situ hybridization, we identified two groups of spinally projecting neurons in the region. Approximately 50% of neurons in one group were excitatory because they contained vesicular glutamate transporter 1 (VGluT1)/VGluT2 mRNA, whereas the other contained a mixed population of neurons, some of which contained either VGluT1/VGluT2 or GAD67 (glutamic acid decarboxylase 67) mRNA. Despite the fact that activation of the MCPA causes potent sympathoexcitation, it does not act to restore arterial pressure after chemical lesion of the RVLM so that a role for this novel descending sympathoexcitatory region remains to be elucidated.
Keywords: blood pressure regulation, caudal pressor area, sympathetic nervous system, baroreceptor reflex, bulbospinal, in situ hybridization
Introduction
The ventral medulla oblongata plays a crucial role in cardiovascular regulation. Several discrete regions have been characterized including the rostral ventrolateral medulla (RVLM), well known for its role in tonic and reflex control of arterial blood pressure, the GABAergic sympathoinhibitory interneurons of the caudal ventrolateral medulla (CVLM) that mediate the sympathetic baroreflex, and the caudal pressor area (CPA) (Pilowsky and Goodchild, 2002).
Some confusion surrounds the location, extent, and function of the CPA. Previous studies described a sympathetically dependent pressor area, caudal to the depressor region of the CVLM, in both the cat and rat (Feldberg and Guertzenstein, 1986; Gordon and McCann, 1988). Some attempts to define the region have been made (Sun and Panneton, 2002, 2005). More recently, pressor responses evoked from the region(s) at various distances caudal to the CVLM were shown to be dependent on relay neurons in the CVLM (Natarajan and Morrison, 2000; Horiuchi and Dampney, 2002) and/or the RVLM (Gordon and McCann, 1988; Possas et al., 1994; Natarajan and Morrison, 2000).
Even more caudally, there are neurons in the white matter of the upper cervical spinal cord that innervate sympathetic preganglionic neurons in the intermediolateral column of the thoracic spinal cord (Jansen and Loewy, 1997) and therefore could potentially influence vascular function.
The objective of this study was to explore the role of the ventrolateral region of the medullo-cervical junction, from the most caudal levels of the medulla into the upper cervical spinal cord, in sympathetic control of the circulation. First, we identified a site in the most caudal ventrolateral medulla from which large pressor responses were evoked by glutamate microinjection. Second, we determined whether or not these pressor responses were dependent on the RVLM and whether they could be distinguished from those evoked from the CPA. Third, we determined whether or not spinally projecting neurons were found in the region and, if so, identified their neurochemical signatures. Finally, we identified the most caudal extent from which glutamate injection evoked pressor and sympathoexcitatory responses.
Materials and Methods
Animals
Experiments were performed on 35 adult male Sprague Dawley rats (350–500 g) from Gore Hill Research Laboratories (Sydney, Australia). All procedures conducted were in accordance with the guidelines of the Royal North Shore Hospital/University of Technology, Sydney Animal Care and Ethics committee.
Physiological/pharmacological experiments
Subjects and surgical procedures.
Rats were anesthetized with urethane (1.3 g/kg, i.p.; Sigma-Aldrich, St. Louis, MO) in all but one group of experiments, in which sodium pentobarbital (60 mg/kg) was used. The right femoral vein and artery were cannulated for intravenous access and arterial blood pressure recording, respectively. The trachea was cannulated, and animals were paralyzed (pancuronium; 0.8 mg initially, then 0.4 mg/h) and ventilated. The left greater splanchnic nerve was isolated, and the distal end was cut to permit recording of efferent sympathetic nerve activity (SNA). In a subset of the urethane-anesthetized animals, the left cervical sympathetic nerve (n = 4) and the phrenic nerves (n = 7) were also isolated to provide an additional sympathetic outflow and central inspiratory information, respectively. In an additional subset of the animals (n = 3), the aortic depressor nerve (ADN) was isolated as described previously (Goodchild et al., 2000). Nerve recordings were made using bipolar silver wire electrodes. Nerve signals were amplified, filtered (30–1000 Hz), and recorded using a CED 1401 data capture system and Spike 2 software (CED, Cambridge, UK). The dorsal medulla was exposed after an occipital craniotomy extending to the C5 cervical vertebra. The level of anesthesia was assessed by checking the withdrawal reflex and/or arterial blood pressure changes after hindpaw pinch. A complete transection of the brain at the level of the CVLM was achieved in two animals (see below). At completion of all experiments, the brainstem was removed and fixed by immersion in 4% formaldehyde in 0.1 m phosphate buffer, pH 7.4, and histology was performed to identify injection sites marked with albumin adsorbed to colloidal gold (Sigma-Aldrich) as described previously (Seyedabadi et al., 2001). Some brain sections were processed for immunohistochemical staining for the neuron-specific nuclear protein NeuN (1:5000; Chemicon, Boronia Victoria, Australia) using procedures as described previously (Li et al., 2005).
Drugs.
The drugs used included glutamate (100 mm, 50 nl; Sigma-Aldrich), muscimol (10 mm, 100 nl; Sigma-Aldrich), vehicle (PBS, pH 7.4), and phenylephrine (10 μg/kg, 0.2 ml, i.v.). All microinjections were made using multibarrel glass pipettes. Injection sites (both the RVLM and most caudal ventral medulla) were all marked, at the conclusion of the experiment, with microinjections of colloidal gold (Seyedabadi et al., 2001).
Physiological studies
Five studies were performed. In study 1, glutamate microinjections were made into the most caudal ventrolateral medulla under urethane anesthesia. Studies 2 and 3 were very similar but used different anesthetics (n = 7 pentobarbital, n = 7 urethane), and their aim was to determine whether the pressor response evoked by glutamate in the most caudal ventrolateral medulla was dependent on the RVLM. Sites in the most caudal ventrolateral medulla and in the RVLM, where a large pressor response (>40 mmHg) was evoked by glutamate microinjection, were selected. Muscimol was then microinjected bilaterally into the RVLM. To determine the extent of RVLM muscimol blockade, two tests were performed. First glutamate was reinjected into the RVLM. Second, in urethane-anesthetized animals only, the baromediated sympathoinhibition evoked by intravenous phenylephrine injection was tested again. After confirmation of complete RVLM inhibition, glutamate was again microinjected into the most caudal ventrolateral medulla. To determine whether the pressor region identified in the most caudal ventrolateral medulla could be distinguished from the CPA, study 3 was repeated (study 4; n = 3) with the additional identification, using glutamate microinjection of the CPA, at a site 1.3 mm from the pressor site in the most caudal ventrolateral medulla, both before and after muscimol blockade of the RVLM. Testing of baroreceptor function before and after RVLM blockade was performed by stimulation of the ADN, using a 1 s train of stimuli at 100 Hz frequency, as well as by phenylephrine-evoked changes in arterial pressure. In study 5, the pressor region in the most caudal ventrolateral medulla was identified using glutamate microinjection, and the brain was transected at the level of the CVLM, freehand, beginning through the caudal part of the cerebellum, using a scalpel (n = 2). After transection, glutamate was again injected into the pressor region.
Peak changes in mean arterial blood pressure (MAP; millimeters mercury), sympathetic (splanchnic and/or cervical) nerve activity (percentage of preinjection baseline), and phrenic nerve activity [duration of apnea (seconds)] were determined and expressed as mean ± SEM. Zero SNA was taken as the level after the animals were killed. Student’s t test was used to analyze drug effects, and p < 0.05 was considered significant. Each injection site within the RVLM, caudal ventral medulla, and cervical spinal cord were identified and reproduced on standard sections. The transected brains were removed, fixed, and sectioned. The transections were complete and were found to be caudal to the facial nucleus at the level of the CVLM, in both cases.
Anatomical experiments
Retrograde labeling of spinally projecting neurons with cholera toxin B subunit.
Rats (n = 9) were anesthetized with sodium pentobarbital (60 mg/kg, i.p.), and thoracic segments T1–T2 were exposed. Cholera toxin B subunit (CTB; 1%, 200 nl; List Biological Laboratories, Campbell, CA) was microinjected bilaterally into the spinal cord (0.5 mm lateral from midline and 0.8 mm ventral to dorsal surface). Rats were allowed to recover for 2–3 d.
Rats were re-anesthetized with pentobarbital (70 mg/kg, i.p.) and perfused transcardially with 250 ml of 0.9% sodium chloride, followed by 250 ml of 4% paraformaldehyde in phosphate buffer (0.1 m, pH 7.4). The brainstem was removed and fixed overnight with the same fixative at 4°C and sectioned coronally at 40 μm using a vibrating microtome (VT1000S; Leica, Wetzlar, Germany).
Digoxigenin-labeled riboprobe preparation.
Both antisense and sense probes targeting mRNAs for rat vesicular glutamate transporter 1 (VGluT1) and VGluT2, preprotachykinin A (PPT-A), and glutamic acid decarboxylase 67 (GAD67) were synthesized as follows: DNA fragments for VGluT1, VGluT2, PPT-A, and GAD67 were first amplified by PCR from rat brain cDNA using forward and reverse primers with SP6 and T7 promoters attached at the 5′ end, respectively (Table 1). The antisense and sense riboprobes were then transcribed in vitro using digoxigenin-11-UTP (Roche Applied Science, Basel, Switzerland) and the T7 or Sp6 RiboMAX large-scale RNA production system (Promega, Madison, WI).
Table 1.
Primers used for PCR
| Primer | Sequence (5′–3′)a | Size of the fragmentb | GenBank accession number |
|---|---|---|---|
| VGluT1-F | GGATCCATTTAGGTGACACTATAGAAGagatcagcaaggtgggactg | 894 bp | U07609 |
| VGluT1-R | GAATTCTAATACGACTCACTATAGGGAGAagaaggagagagggctggtc | ||
| VGluT2-F | GGATCCATTTAGGTGACACTATAGAAGtcaatgaaatccaacgtcca | 886 bp | NM_053427 |
| VGluT2-R | GAATTCTAATACGACTCACTATAGGGAGAcaagagcacaggacaccaaa | ||
| GAD67-F | GGATCCATTTAGGTGACACTATAGAAGttatgtcaatgcaaccgc | 812 bp | NM_017007 |
| GAD67-R | GAATTCTAATACGACTCACTATAGGGAGAcccaacctctctatttcctc | ||
| PPT-F | GGATCCATTTAGGTGACACTATAGAAGtccgacagtgaccaaatcaa | 783 bp | NM_012666 |
| PPT-R | GAATTCTAATACGACTCACTATAGGGAGAcacaacacaggaaacatgctg |
F, Forward; R, reverse.
aCapital letters are attached sequences. T7 (reverse primer) or SP6 (forward primer) promoter sequences are underlined.
bThe size of the fragment includes 56 bp attached sequences.
Combined in situ hybridization and immunohistochemistry.
Free-floating brain sections were processed with a combined method of in situ hybridization and immunocytochemistry, as described in detail previously (Li et al., 2005). In brief, sections were first hybridized with each riboprobe (VGluT1 and VGluT2, or GAD67, or PPT-A), washed in descending concentrations of salt, and reacted with primary antibodies against digoxigenin [alkaline phosphatase-conjugated rabbit anti-digoxigenin (1:1000; Dako, Glostrup, Denmark), tyrosine hydroxylase (TH; 1:2000, from mouse; Sigma-Aldrich), and CTB (1:1000, from sheep; List Biological Laboratories, Campbell, CA)]. TH was revealed by incubation overnight with Texas Red-conjugated donkey anti-mouse IgG (1:500; Jackson ImmunoResearch, West Grove, PA), CTB with FITC-conjugated donkey anti-sheep IgG (1:500; Jackson ImmunoResearch), and digoxigenin-labeled in situ neurons by a histochemical reaction using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate salts.
Imaging and quantitation.
Sections were mounted onto slides using Prolong Antifade (Molecular Probes, Eugene, OR) medium and viewed using a DML fluorescence microscope (Leica). TH- and CTB-positive neurons were visualized using appropriate filter sets to discriminate fluorescent tags, whereas in situ-positive neurons were visualized using bright-field illumination (Li et al., 2005). Neurons were considered to be double or triple labeled only when the images were completely overlapping and were in the same focal plane. Images were acquired and processed using a Spot 2 digital camera and software (Diagnostic Instruments, Livingston, UK).
The section that contained the most caudal part of the inferior olive was used as reference plane (14.6 mm caudal to bregma). Neurons were counted bilaterally in the six sections, each separated by 200 μm, caudal to the reference plane. Results are expressed as mean ± SEM.
Results
Study 1: a pressor region in the most caudal ventrolateral medulla
Unilateral glutamate injection into the ventrolateral region of the medulla immediately caudal to the caudal pole of the inferior olive (Fig. 1A) evoked a large pressor and sympathoexcitatory response, in both cervical (cSNA) and splanchnic (sSNA) nerves in urethane-anesthetized animals (Fig. 1B). Grouped data show that pressor responses of 60 ± 5 mmHg mediated by increases in both sympathetic outflows (sSNA, 184 ± 19%; cSNA, 248 ± 75%) but negligible changes in heart rate were consistently evoked from the area. A cessation of phrenic nerve activity bursts for 6 ± 1 s accompanied this response. We have termed this region the medullo-cervical pressor area (MCPA).
Figure 1.
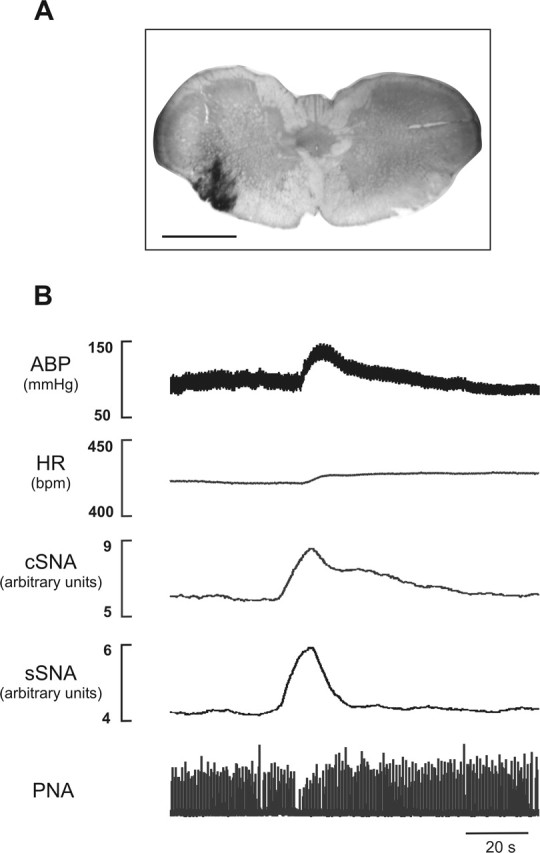
Cardiorespiratory effects of unilateral glutamate microinjection into the MCPA. A, Injection site marked by silver-enhanced colloidal gold at a level −14.6 mm from bregma. Scale bar, 1 mm. B, Glutamate (100 mm, 50 nl) evokes an increase in arterial blood pressure (ABP), SNA (cSNA and sSNA), and cessation of activity (apnea) in phrenic nerve activity (PNA). HR, Heart rate.
Studies 2–4: the pressor response evoked from the MCPA is independent of the RVLM and distinct from that evoked from the CPA
Studies 2–4 each tested whether the MCPA-evoked pressor response was affected by RVLM blockade achieved using bilateral muscimol injection, except that studies 2 and 4 were performed in urethane-anesthetized animals and study 3 was performed in pentobarbital-anesthetized animals. Phenylephrine was used to test baroreceptor function to evaluate the extent of RVLM blockade in study 2. Additionally, in study 4, the extent of RVLM blockade was tested using ADN stimulation, and the effect of glutamate stimulation of the CPA was tested before and after RVLM blockade.
Figure 2 shows a continuous trace from an experiment in study 4. Data from all studies are illustrated in Figure 3.
Figure 2.
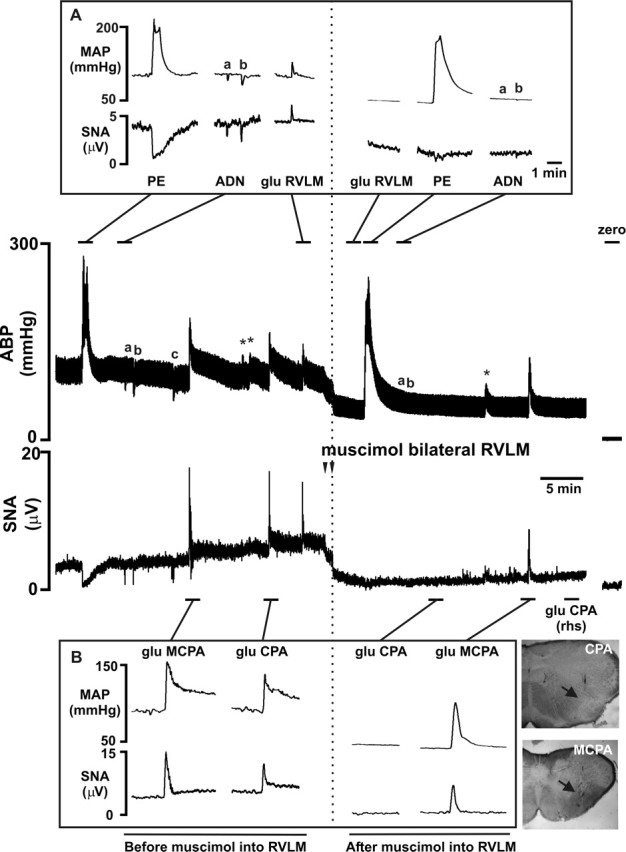
The pressor and sympathoexcitatory response evoked by glutamate microinjection into the MCPA is independent of the RVLM, although the pressor response evoked from the CPA is dependent on the RVLM. A representative recording of arterial blood pressure (ABP) and sSNA in a urethane-anesthetized rat is shown. Distinct sites where glutamate evoked a pressor and sympathoexcitatory response (MCPA, CPA, and RVLM) were identified throughout the ipsilateral extension of the ventrolateral medulla. Blockade of the RVLM with bilateral muscimol injections resulted in spinal levels of ABP and an almost complete inhibition of SNA. Note the zero levels of ABP and SNA after death. A, The responses evoked by tests used to determine the effectiveness of RVLM blockade. Pressor responses could no longer be evoked from the RVLM, and sympathoinhibitory responses to phenylephrine or ADN stimulation were abolished. The asterisks refer to lower-dose glutamate injections or at off-site coordinates. B, After RVLM blockade, the CPA-evoked response [ipsilateral and contralateral (rhs)] was completely abolished, whereas the MCPA-evoked response remained intact. Unstained histological sections showing electrode tracks and injection sites (arrows) in the CPA and MCPA are shown adjacent to B. The ADN stimulation protocol was 100 Hz for 1 s at 2.5 V (a) or 5 V (b), and a longer stimulus was sometimes applied (5 s; c). PE, Phenylephrine; glu, glutamate; rhs, right-hand side.
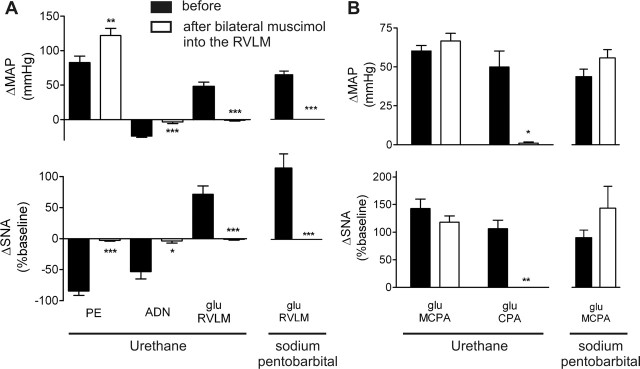
Figure 3.
Grouped data illustrating that pressor and sympathoexcitatory responses evoked from glutamate injection into the MCPA are preserved after RVLM blockade with muscimol, under two different anesthetic agents (n = 7 per group). A, Completeness of RVLM blockade was confirmed by the absence of glutamate-evoked effects in the RVLM and abolition of sympathoinhibitory responses after intravenous phenylephrine (PE) or ADN stimulation. B, The large increase in MAP and SNA evoked from the MCPA was unaffected by RVLM blockade, whereas the pressor and sympathoexcitatory responses evoked from the CPA were completely abolished. Results are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.005 before versus after muscimol. glu, Glutamate.
Initially phenylephrine (intravenously) evoked a potent baroreflex-mediated sympathoinhibition of −84 ± 7% (Figs. 2A, 3A), whereas ADN stimulation evoked a depressor response of −24 ± 2 mmHg and a sympathoinhibition of −53 ± 12%. Glutamate initially microinjected into the MCPA elicited a pressor response of 60 ± 3 mmHg (pentobarbital, 49 ± 5 mmHg) and an increase in SNA (143 ± 17%; pentobarbital, 99 ± 15%) (Figs. 2B, 3B). Glutamate microinjection into the RVLM also elicited a pressor response (48 ± 6 mmHg; pentobarbital, 65 ± 5 mmHg) and a 71 ± 13% (pentobarbital, 127 ± 25%) increase in SNA (Figs. 2A, 3A). The pressor responses elicited by glutamate injections into the MCPA and RVLM were not significantly different (p = 0.12), whereas the increase in SNA evoked from the MCPA was significantly greater than from the RVLM (p = 0.006). Bilateral muscimol microinjections (100 nl, 10 mm) into the RVLM evoked a fall in MAP (−65 ± 3 mmHg; pentobarbital, −72 ± 10 mmHg) and SNA (−96 ± 1%; pentobarbital, −98 ± 1%), indicating successful inhibition of the RVLM. After RVLM blockade, phenylephrine evoked a larger pressor response, but no baroreceptor-evoked sympathoinhibition was evident (Figs. 2A, 3A). Similarly, ADN stimulation failed to evoke any change in MAP or SNA (Figs. 2A, 3A). RVLM blockade was further confirmed by unilateral glutamate microinjection into the RVLM; no response was evoked under either anesthetic (Figs. 2A, 3A). After RVLM blockade, glutamate microinjection into the MCPA still evoked an increase in both MAP (67 ± 5 mmHg; pentobarbital, 62 ± 6 mmHg) and SNA of 118 ± 11% (pentobarbital, 158 ± 43%) (Figs. 2B, 3B). This MCPA-evoked pressor response after RVLM blockade was, if anything, larger than that obtained before RVLM blockade (p = 0.12; pentobarbital, p = 0.14; MAP). No significant differences were seen in urethane- compared with pentobarbital-anesthetized animals. In study 4, the CPA was also identified ipsilaterally to the MCPA, and glutamate microinjection evoked a pressor and sympathoexcitatory response of 50 ± 10 mmHg and 106 ± 16% SNA (Figs. 2B, 3B). After RVLM blockade, the CPA-evoked response was completely abolished, whereas the MCPA-evoked response remained intact (Figs. 2B, 3B).
Study 5: glutamate stimulation evokes a pressor response from the MCPA after brain transection at the level of the CVLM
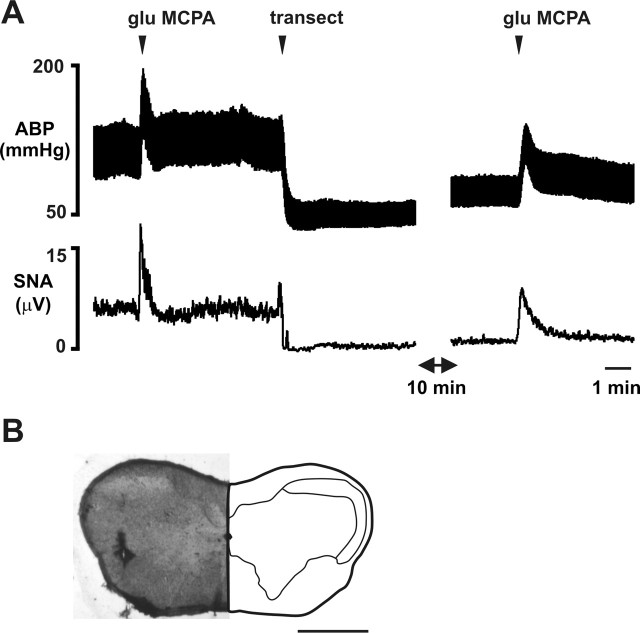
Glutamate microinjections were made into the MCPA before and after medullary transection at the level of the CVLM in two animals. Figure 4 shows the results from one animal. Transection caused arterial pressure to fall to similar levels evoked by RVLM blockade (Fig. 3). Glutamate stimulation still evoked a significant pressor response from the MCPA in each animal.
Figure 4.
The MCPA-evoked sympathoexcitation and pressor response is not abolished by brain transection at the level of the CVLM. A, Glutamate (glu) microinjection into the MCPA evokes a large increase in ABP and sSNA before and after transection (transect) at the level of the CVLM. Transection causes a large decrease in ABP and sSNA. B, Site of glutamate microinjection into the MCPA described in A. Scale bar, 1 mm.
Spinally projecting neurons in the most caudal ventrolateral medulla
The glutamate-evoked MCPA response, unlike the CPA-evoked response, was not relayed through the RVLM, and the MCPA pressor response could be elicited in rats where the brainstem was transected. Thus, the possibility of a direct spinal projection from the MCPA was examined.
Two groups of spinally projecting neurons were identified in the most caudal ventrolateral medulla after CTB injections at theT1–T2 spinal levels (Fig. 5). The lateral group was located immediately ventromedial to the caudal part of the spinal trigeminal nucleus (Sp5C). The ventral aspect of this bulbospinal cell group overlapped with nonspinally projecting TH-immunoreactive (IR) cells (A1; data not shown). The medial group was located ventral to the ventral medullary reticular nucleus and medial to the medial longitudinal fasciculus.
Figure 5.
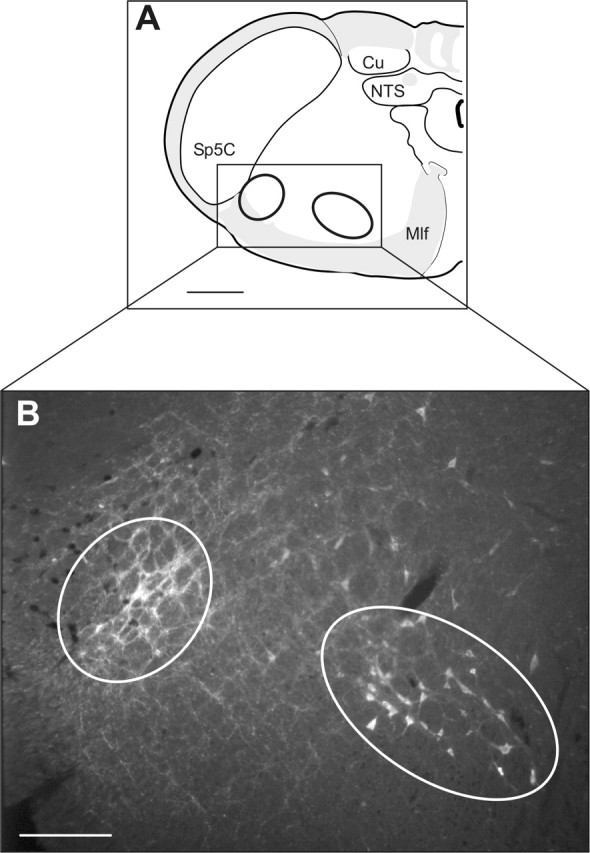
Bulbospinal neurons are present in the MCPA. A, A schematic diagram illustrating the location of the photomicrograph in B. Cu, Cuneate nucleus; Mlf, medial longitudinal fasciculus; NTS, nucleus tractus solitary; Sp5C, caudal part of the spinal trigeminal nucleus. B, Two groups of bulbospinal neurons are found in the MCPA. A lateral and a medial group of neurons showed CTB immunoreactivity after injections of CTB into the upper thoracic spinal cord. Scale bars: A, 500 μm; B, 200 μm.
Neurochemistry of the spinally projecting neurons
To determine the chemical content of these bulbospinal neurons, in situ hybridization was performed using antisense probes targeting the mRNA of VGluT1/VGluT2, GAD67, and PPT-A. The CTB-IR neurons in the ventral medulla were explored from14.6 mm (level of the most caudal pole of the inferior olive) to 16 mm caudal to bregma and examined for VGluT1/VGluT2+ or GAD67+ or PPT-A+ mRNA. Both VGluT1/VGluT2- and GAD67-labeled neurons were found in most regions of the gray matter, with PPT-A-labeled neurons having a restricted distribution that was localized mainly to a densely stained group at the boundary of the ventral medullary reticular nucleus and the caudal spinal trigeminal nucleus (Fig. 6). A1 cells in this region are neither VGluT1/VGluT2+ nor GAD67+ (data not shown)
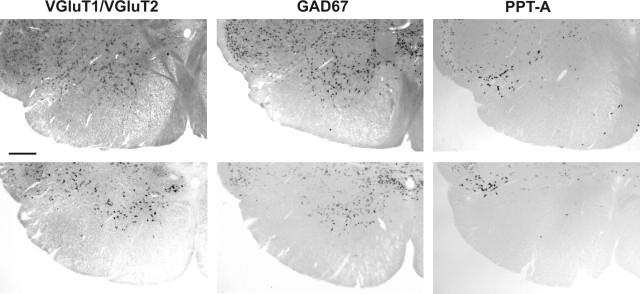
Figure 6.
Distribution of VGluT1/VGluT2+ or GAD67+ or PPT-A+ mRNA in the ventral medulla at two levels within the MCPA, bregma −14.6 mm (top panels) and first cervical segment (bottom panels). VGluT1/VGluT2+ and GAD67+ neurons were found widely distributed throughout the gray matter. PPT-A+ neurons had more a more restricted distribution with a heavily labeled group ventromedial to the caudal spinal trigeminal nucleus. At both levels, the distribution of labeled neurons within the MCPA was similar. Scale bar, 500 μm.
Of the lateral group of spinally projecting neurons, 48 ± 2% contained VGluT1/VGluT2 mRNA (132 of 274; n = 2) (Fig. 7A) and 12.9 ± 3% contained PPT-A mRNA (49 of 367 cell; n = 3) (Fig. 7B); no GAD67 spinally projecting neurons were detected in this group. In the medial group of spinally projecting neurons, there are mixed populations of VGluT1/VGluT2 mRNA- and GAD67 mRNA-containing neurons. Of the spinally projecting neurons, 27.1 ± 0.7% contained VGluT1/VGluT2 mRNA (92 of 338 cells; n = 2) (Fig. 7C) and 13.0 ± 1.9% contained GAD67 mRNA (91 of 710 cells; n = 3) (Fig. 7D), whereas neurons containing PPT-A mRNA were rarely observed.
Figure 7.
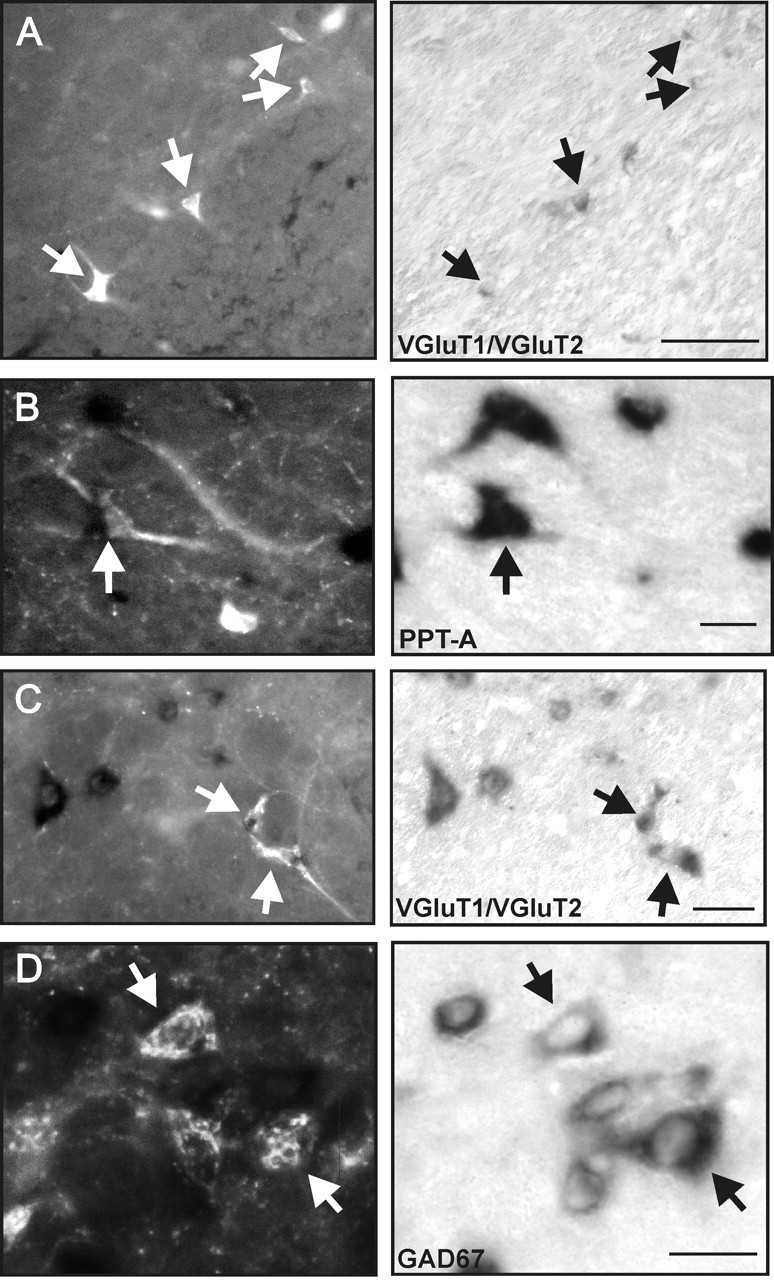
A, B, Neurons of the lateral bulbospinal cell group often contained VGluT1/VGluT2 mRNA (A) and occasionally contained PPT-A mRNA (B), but none contained GAD67 mRNA. C, D, Of the medial bulbospinal cell group, some contained VGluT1/VGluT2 mRNA (C) and occasional neurons contained GAD67 mRNA (D), but none contained PPT-A m RNA. The arrows in the left panels show CTB+ neurons, the in situ labeling of which is shown in the right panels. Scale bars: A, D, 25 μm; B, 100 μm; C, 50 μm.
Caudal extension of the pressor region in the most caudal medulla
Because the spinally projecting neurons extended into the cervical spinal cord, the caudal extent of the pressor region was determined by glutamate microinjection in 10 urethane-anesthetized animals using 127 microinjections (Fig. 8). Large pressor (>20 mmHg) and sympathoexcitatory responses were evoked from 14.6 mm caudal from bregma to the caudal part of the third cervical segment (Fig. 8A–C). Although C4 and C5 spinal levels were quite extensively explored, only very small pressor responses were ever evoked (Fig. 8D). Figure 8 also shows that sites from which pressor responses were evoked were those that contained NeuN-IR neurons scattered among fibers in the lateral and ventral white matter.
Figure 8.
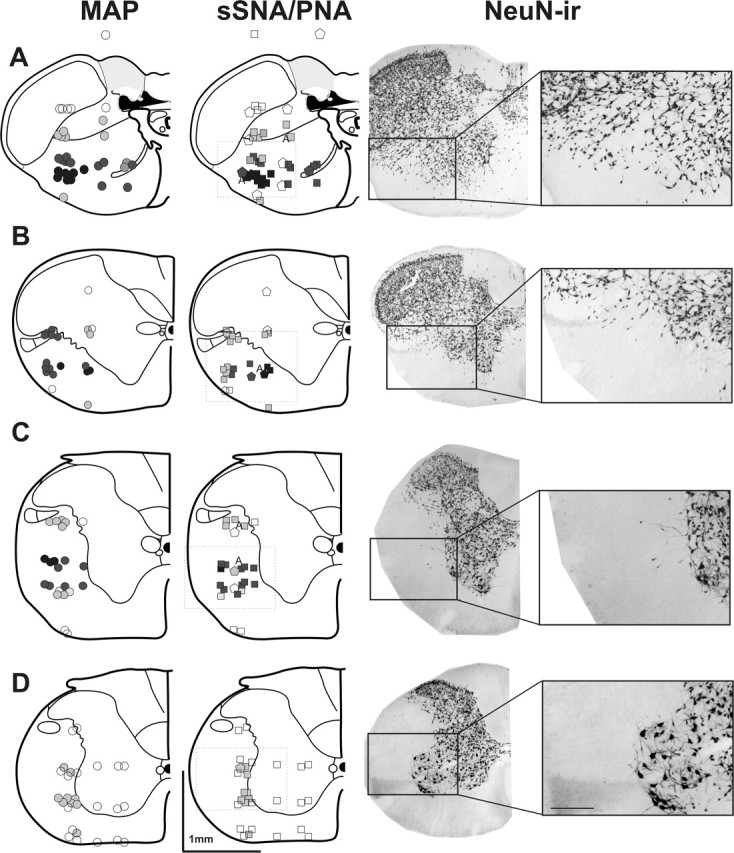
Representative coronal sections at the level of the 14.6 mm caudal to bregma (A), first cervical segment (B), second and third cervical segments (C), and fourth and fifth cervical segments (D) illustrating glutamate injection sites and the evoked changes in MAP (circles), sSNA (squares), and the phrenic nerve (PNA; pentagons). The symbols are graded from dark to light indicating maximal and minimal responses. Black indicates >40 mmHg change in MAP, >100% increase in sSNA, and >60% decrease in the PNA. The letter A indicates sites where the apnea was evoked. Dark gray indicates a response between 20 and 50 mmHg, between 40 and 100% increase in sSNA, and between 30 and 60% decrease in the PNA, whereas light gray indicates a response between 10 and 20 mmHg, between 10 and 20% increase in sSNA, and between 10 and 30% decrease in the PNA. Open symbols indicate no response. The right column shows anatomically matched sections immunohistochemically labeled for neuron-specific nuclear protein NeuN. The largest pressor and sympathoexcitatory responses (100 mm, 50 nl) were found in A, however large responses were also evoked in the region extending into the third cervical segment (B, C). Large responses were not elicited anywhere in the fourth and fifth cervical segments (D). Note that pressor responses were evoked from regions extending into the white matter where scattered neurons were found. ir, Immunoreactive.
Discussion
These data identify a novel pressor and sympathoexcitatory region in the most caudal medulla that is not dependent on the integrity of the RVLM: the MCPA. This pressor region extends caudally from the medulla at the level of the caudal pole of the inferior olive to the fourth cervical segment. This pressor region is distinct from the CPA. Because the pressor area does not appear to mediate its effects via suprabulbar regions and spinally projecting neurons are found in this region the MCPA may be another major presympathetic cell group. We suggest that this is the first study to provide functional evidence for a cardiovascular role for neurons in cervical spinal cord white matter, first reported by Jansen and Loewy (1997), that innervate sympathetic preganglionic neurons. Some previous investigators of the CPA may have, in fact, explored the more caudally located CMPA, which could explain the ambiguous findings described for the CPA.
The most striking finding of this study is the identification of a pressor region in the most caudal ventrolateral medulla from which pressor and sympathoexcitatory responses can be evoked. We have termed this new pressor region the MCPA. In keeping with previous studies, we have demonstrated that bilateral RVLM blockade eliminates the responsiveness of the more rostrally located CPA (Gordon and McCann, 1988; Possas et al., 1994; Natarajan and Morrison, 2000). In contrast, and for the first time, we have shown that responses evoked from the MCPA are unaffected by bilateral RVLM blockade. Thus, the MCPA is distinct in both location and axonal trajectory to the CPA. These findings support our idea that up to three distinct pressor areas exist in the ventral medulla, caudal to the CVLM (Goodchild et al., 2002). Additional evidence for separate areas or at least cell groups currently called the CPA is indicated (1) by the different responses obtained when the CPA is inhibited by GABA or glycine (Gordon and McCann, 1988; Possas et al., 1994; Natarajan and Morrison, 2000; Horiuchi and Dampney, 2002), (2) by the fact that differently signed projections from the CPA to the CVLM to RVLM have been postulated (Campos et al., 1994; Natarajan and Morrison, 2000; Horiuchi and Dampney, 2002), and (3) by the fact that the anatomical sections showing injection sites indicate, in some instances at least, a more rostrally located region [Possas et al. (1994), their Fig. 1].
It could perhaps be argued that the RVLM was not completely silenced in the present study. This is unlikely. First, we went to great lengths to ensure absolute inhibition of the RVLM using well established and widely used drug, dose, and injection volumes of the potent and long-acting GABAA receptor agonist muscimol (Gordon and McCann, 1988; Horiuchi and Dampney, 2002). Second, the functional blockade of the RVLM was tested both pharmacologically by local microinjections of glutamate and physiologically by testing baroreceptor function in two ways: response to intravenous phenylephrine injection and to ADN stimulation. No responses were evoked after RVLM blockade. Third, bilateral muscimol blockade of the RVLM evoked profound falls in blood pressure and abolished SNA. Fourth, the MCPA independence of the RVLM was verified under anesthesia with two agents that have quite different mechanisms of action (Rojas et al., 2006; Chan, 1985). Finally, and most compelling, was that bilateral RVLM blockade eliminated the responsiveness of the CPA, as documented previously (Gordon and McCann, 1988; Possas et al., 1994; Natarajan and Morrison, 2000), while leaving the MCPA unaffected. Because transection in two animals also did not eliminate the response evoked from the MCPA, it is clear that neither the RVLM nor suprabulbar regions are essential for generation of the glutamate evoked sympathetically mediated pressor response from the MCPA.
Present data do not exclude the possibility of a projection from the MCPA to the RVLM. Campos and McAllen (1999) found that chemical stimulation in a region in the very caudal medulla activated bulbospinal barosensitive neurons of the RVLM. The schematic map of CPA injection sites presented by these authors indicates that their glutamate injections could overlap with part of the MCPA. Furthermore, Sun and Panneton (2005), using anterograde tracing from a region that may overlap the MCPA, demonstrated projections to more rostral regions. However, the substantial fiber tracts in the region and the lack of neuronal identity make this study a little difficult to interpret. Curiously, the spinal cord was not investigated in this study.
In the present study, large pressor responses were evoked from a ventrolateral region extending from the caudal pole of the inferior olive to the fourth cervical spinal cord. Although this region appeared continuous, it is possible that unresponsive intercalated zones may exist. Some investigators of the CPA have explored varying ranges of more caudal regions (often using restricted dorsoventral and mediolateral exploration) and have not identified other pressor regions (Possas et al., 1994; Natarajan and Morrison, 2000; Horiuchi and Dampney, 2002; Sun and Panneton, 2002). This may indicate coverage of the area between the CPA and the MCPA or is just the result of poor coverage of the region. Although anesthetics could affect these responses, the results of the present study demonstrate that glutamate sensitivity of MCPA is retained under two anesthetics.
Although the pressor regions of the MCPA appear to overlap the white matter, we and others (Jansen and Loewy, 1997) found that neuronal somata exist here. Furthermore, we demonstrate, using retrograde tracing, that neurons projecting to thoracic levels are present in the region. Evidence in support of neurons within the MCPA region that innervate sympathetic preganglionic neurons of the thoracic cord comes from two sources. First, neurons in the lateral funiculus and lateral spinal nucleus are labeled after pseudorabies viral injection in either the kidney or the stellate ganglion and thus innervate sympathetic preganglionic neurons (Schramm et al., 1993; Jansen and Loewy, 1997). Second, anterograde tracing studies from both the lateral funiculus and the lateral spinal nucleus at the C3 spinal level demonstrate that their main projections are to the intermediolateral cell column of the thoracic spinal cord, although other spinal regions also receive projection (Jansen and Loewy, 1997). Because glutamate-evoked pressor responses of the MCPA do not appear to depend on suprabulbar regions, the simplest interpretation of these data are that MCPA-evoked responses are mediated by bulbospinal sympathetic neurons in the region. Because the sympathoexcitatory effects extended as far as the end of the third cervical spinal segment, it is pertinent that the neurons within the lateral funiculus (the medial spinally projecting cell groups described here) appear to share this caudal extent (Jansen and Loewy, 1997).
The chemical heterogeneity of the two groups of spinally projecting neurons described here may indicate functional heterogeneity within the MCPA. The lateral cell group is predominantly excitatory in nature, whereas the medial cell group contains both excitatory and inhibitory neurons. These cell groups are unrelated to the adjacent A1 cell group that also extends significantly into the first cervical spinal segment (Paxinos et al., 1999). The rostrocaudal extent of these cell groups beyond 1.2 mm caudal to the caudal pole of the inferior olive groups remains to be determined. It is noteworthy in respect of functional heterogeneity that excitation of the dorsolateral surface of the cervical spinal cord in spinally transected or RVLM-inhibited rats evoked inhibition of the renal nerve, whereas in the intact preparation, sympathoexcitation was evoked (Schramm and Livingstone, 1987; Poree and Schramm, 1992). In this respect, effects of MCPA excitation on sympathetic outflows, other than the cervical sympathetic and splanchnic tested here, will be of interest. Furthermore, cell groups in a similar region to the MCPA are activated after pain to deep somatic regions of the body (Clement et al., 2000), indicating that the MCPA may be important in signaling autonomic effects in response to pain.
A glutamate-evoked apnea in the caudal medulla has been described previously, although it was accompanied by a decrease in arterial pressure (Chitravanshi and Sapru, 1999) and therefore may be attributed to a region more closely associated with the CVLM. Inspiratory neurons are located in the upper cervical spinal cord (Lipski and Duffin, 1986; Lipski et al., 1993), and activation of these may have evoked apnea. Any role that these neurons play in cardiovascular control is unknown; however, they appear to project to the thoracic spinal levels presumably innervating motoneurons supplying abdominal and intercostal muscles. It is possible that the spinally projecting neurons seen in the present study may have included these neurons. However, it is not possible that the cardiovascular effects seen here could be secondary to activation of respiratory musculature, because the animals were paralyzed and ventilated.
Thus, the present study describes data supporting our working hypothesis that the MCPA extends caudally as far as the third cervical segment, does not provide an obligatory relay to the RVLM (or more rostrally), and contains spinally projecting neurons (which are neurochemically heterogeneous) that directly innervate the sympathetic preganglionic neurons. The MCPA is distinct from the previously described, more rostrally located CPA and does not rely on projections to suprabulbar regions. Interestingly, the MCPA does not appear to play a role in maintaining vasomotor tone after RVLM blockade. Hence, the physiological role that this cell group plays in circulatory control remains to be determined.
Footnotes
This work was supported by grants from the National Health and Medical Research Council of Australia (211023 and 211196), the Northern Sydney Area Health Service (2005:27), and the Garnett Passe and Rodney Williams Memorial Foundation. J.R.P. was supported by Australian Postgraduate Awards and the Northern Sydney Health Centenary Foundation. A.K.G. is a Fellow of the Foundation for High Blood Pressure Research of Australia.
References
- Campos RR, McAllen RM (1999). Tonic drive to sympathetic premotor neurons of rostral ventrolateral medulla from caudal pressor area neurons. Am J Physiol 276:R1209–R1213. [DOI] [PubMed] [Google Scholar]
- Campos RR Jr, Possas OS, Cravo SL, Lopes OU, Guertzenstein PG (1994). Putative pathways involved in cardiovascular responses evoked from the caudal pressor area. Braz J Med Biol Res 27:2467–2479. [PubMed] [Google Scholar]
- Chan MY (1985). Naloxone interaction with some CNS depressants. Pharmacology 31:294–297. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN (1999). Phrenic nerve responses to chemical stimulation of the subregions of ventral medullary respiratory neuronal group in the rat. Brain Res 821:443–460. [DOI] [PubMed] [Google Scholar]
- Clement CI, Keay KA, Podzebenko K, Gordon BD, Bandler R (2000). Spinal sources of noxious visceral and noxious deep somatic afferent drive onto the ventrolateral periaqueductal gray of the rat. J Comp Neurol 425:323–344. [DOI] [PubMed] [Google Scholar]
- Feldberg W, Guertzenstein PG (1986). Blood pressure effects of leptazol applied to the ventral surface of the brain stem of cats. J Physiol (Lond) 372:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild AK, Van Deurzen BT, Sun QJ, Chalmers J, Pilowsky PM (2000). Spinal GABAA receptors do not mediate the sympathetic baroreceptor reflex in the rat. Am J Physiol Regul Integr Comp Physiol 279:R320–R331. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Moon EA, Pilowsky PM (2002). Cardiovascular and respiratory nuclei in the ventral medulla: a high-resolution anatomical and physiological correlation using glutamate microinjection in rat. Soc Neurosci Abstr 28:862.1. [Google Scholar]
- Gordon FJ, McCann LA (1988). Pressor responses evoked by microinjections of l-glutamate into the caudal ventrolateral medulla of the rat. Brain Res 457:251–258. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Dampney RA (2002). Evidence for tonic disinhibition of RVLM sympathoexcitatory neurons from the caudal pressor area. Auton Neurosci 99:102–110. [DOI] [PubMed] [Google Scholar]
- Jansen AS, Loewy AD (1997). Neurons lying in the white matter of the upper cervical spinal cord project to the intermediolateral cell column. Neuroscience 77:889–898. [DOI] [PubMed] [Google Scholar]
- Li Q, Goodchild AK, Seyedabadi M, Pilowsky PM (2005). Preprotachykinin A mRNA is colocalized with tyrosine hydroxylase-immunoreactivity in bulbospinal neurons. Neuroscience 136:205–216. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J (1986). An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res 61:625–637. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J, Kruszewska B, Zhang X (1993). Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Exp Brain Res 95:477–487. [DOI] [PubMed] [Google Scholar]
- Natarajan M, Morrison SF (2000). Sympathoexcitatory CVLM neurons mediate responses to caudal pressor area stimulation. Am J Physiol Regul Integr Comp Physiol 279:R364–R374. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Carrive P, Wang H, Wang P-Y (1999). In: Chemoarchitectonic atlas of the rat brainstem London: Academic.
- Pilowsky PM, Goodchild AK (2002). Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens 20:1675–1688. [DOI] [PubMed] [Google Scholar]
- Poree LR, Schramm LP (1992). Interaction between medullary and cervical regulation of renal sympathetic activity. Brain Res 599:297–301. [DOI] [PubMed] [Google Scholar]
- Possas OS, Campos RR Jr, Cravo SL, Lopes OU, Guertzenstein PG (1994). A fall in arterial blood pressure produced by inhibition of the caudalmost ventrolateral medulla: the caudal pressor area. J Auton Nerv Syst 49:235–245. [DOI] [PubMed] [Google Scholar]
- Rojas MJ, Navas JA, Rector DM (2006). Evoked response potential markers for anesthetic and behavioral states. Am J Physiol Regul Integr Comp Physiol in press. [DOI] [PubMed]
- Schramm LP, Livingstone RH (1987). Inhibition of renal nerve sympathetic activity by spinal stimulation in rat. Am J Physiol 252:R514–R525. [DOI] [PubMed] [Google Scholar]
- Seyedabadi M, Goodchild AK, Pilowsky PM (2001). Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension 38:1087–1092. [DOI] [PubMed] [Google Scholar]
- Sun W, Panneton WM (2002). The caudal pressor area of the rat: its precise location and projections to the ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 283:R768–R778. [DOI] [PubMed] [Google Scholar]
- Sun W, Panneton WM (2005). Defining projections from the caudal pressor area of the caudal ventrolateral medulla. J Comp Neurol 482:273–293. [DOI] [PubMed] [Google Scholar]