Abstract
P2X4 purinergic receptors are calcium-permeable, ATP-activated ion channels. In the CA1 area of the hippocampus, they are located at the subsynaptic membrane somewhat peripherally to AMPA receptors. The possible role of P2X4 receptors has been difficult to elucidate because of the lack of selective antagonists. Here we report the generation of a P2X4 receptor knock-out mouse and show that long-term potentiation (LTP) at Schaffer collateral synapses is reduced relative to that in wild-type mice. Ivermectin, which selectively potentiates currents at P2X4, was found to increase LTP in wild-type mice but had no effect in P2X4 knock-out mice. We suggest that calcium entry through subsynaptic P2X4 receptors during high-frequency stimulation contributes to synaptic strengthening.
Keywords: LTP, P2X4, knock-out, mice, hippocampus, ivermectin
Introduction
P2X4 purinergic receptors are widely distributed throughout the CNS (Buell et al., 1996; Collo et al., 1996; Séguéla et al., 1996; Soto et al., 1996). Immunogold electron microscopy shows that, in the CA1 region of the hippocampus, they have a perisynaptic location on postsynaptic membranes apposed to terminals of Schaffer collaterals (Rubio and Soto, 2001). P2X4 receptors have a relatively high permeability to calcium (PCa/PNa ≈ 4) (Soto et al., 1996; North, 2002; Egan and Khakh, 2004); therefore, any ATP released at these synapses might contribute to changes in synaptic strength during repetitive activity. It is well known that long-lasting changes in synaptic strength can be induced at these synapses by calcium entering through NMDA receptors (Bliss and Collingridge, 1993; Malenka and Nicoll, 1993).
Involvement of P2X4 receptors in hippocampal synaptic transmission has been difficult to test experimentally because of the lack of effective antagonists (Buell et al., 1996; North and Surprenant, 2000; North, 2002). A role for P2X receptors in general was inferred from one study (Pankratov et al., 2002): in this case, a brief (0.2 s) high-frequency stimulation that did not normally elicit long-term potentiation (LTP) was able to do so after application of the antagonist pyridoxal-phophate-6-axophenyl-2′,4′-disulphonic acid (PPADS) (20 μm). This action of PPADS seems unlikely to result from block of P2X4 receptors because, at least in the rat, they are quite insensitive to this antagonist (Buell et al., 1996; North and Surprenant, 2000; North, 2002); it may act by blocking different P2X receptors (e.g., P2X2 or P2X6) or other receptors.
Ivermectin is an antifilarial drug that is becoming an increasingly useful tool in the study of P2X4 receptors. It potentiates ATP-induced currents at P2X4 receptors but not at other P2X receptors (Khakh et al., 1999; Priel and Silberberg, 2004). However, ivermectin has actions on other ion channels (e.g., mouse GABAA receptors, Krusek and Zemková, 1994; human α7 nicotinic receptors, Krause et al., 1998), and this has limited confidence in the interpretation of its actions. The paucity of tools with which to probe the P2X4 receptor function led us to generate a P2X4 knock-out mouse, so that hippocampal synaptic transmission could be compared between wild-type and knock-out animals. We argued that any effect of ivermectin that is present in the wild-type but lacking in the knock-out mice should indicate P2X4 receptor involvement.
Materials and Methods
Generation of P2X4 receptor-deficient mice.
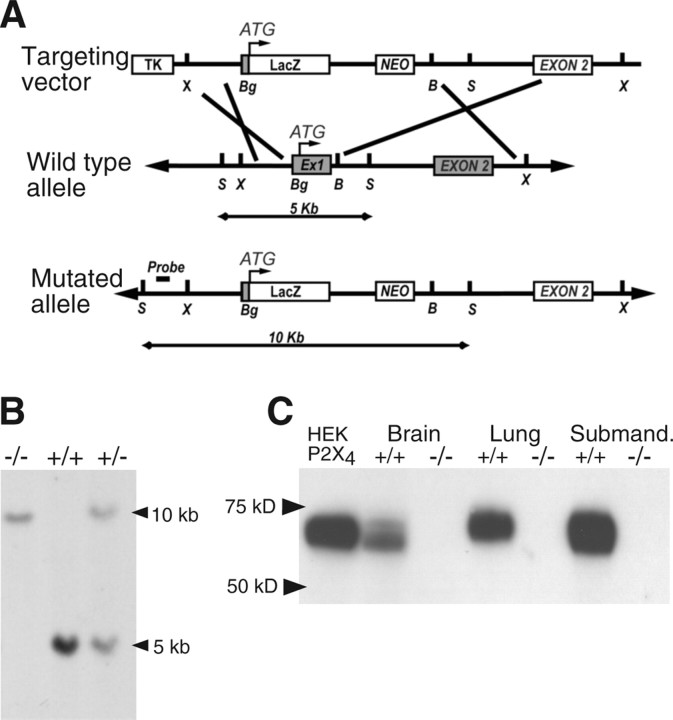
Mice carrying a targeted null mutation of the P2X4 gene were generated according to published protocols (Conquet, 1995). A 10 kb genomic DNA containing the first two exons of the P2X4 subunit was isolated from a genomic library obtained from 129/Sv mice. The targeting vector was constructed by the insertion of a LacZ neomycin cassette, between a BgIII site located 30 bases upstream of P2X4-initiating methionine and a BamHI site 172 bases downstream of the first exon/intron boundary. This also resulted in a 337 bases deletion encompassing the first exon (including P2X4-initiating methionine) and 172 bases of the downstream intron (Fig. 1A). Embryonic stem cells were cultured, transfected, and screened using standard methods. Targeted embryonic stem cells and mice carrying the mutant allele were identified by Southern blotting (Fig. 1B), using a probe specific to a genomic region upstream of the targeted locus as indicated in Figure 1A. Germ-line chimeras were crossed with C57BL/6J to generate heterozygotes and then backcrossed >15 times onto the C57BL/6J strain.
Figure 1.
Targeted disruption of the mouse P2X4 gene. A, Maps of the P2X4 wild-type allele, targeting vector, and mutant allele. Boxes represent open reading frames; LacZ gene insertion upstream of the P2X4-initiating methionine resulted in the deletion of all the coding region of exon 1 and of the first exon–intron splice site. TK, Thymidine kinase; NEO, neomycin; Ex1, exon 1; B, BamH1; Bg, BgIII; S, SacI; X, XbaI. Southern blotting probe was designed outside of the targeting locus between 5′ SacI and XbaI sites. As shown, after digestion with SacI, this probe will hybridize to a 5 or 10 kb DNA fragment for wild-type and mutant allele, respectively. B, Southern blot analysis of genomic DNA digested with SacI from +/+, +/−, and −/− mice using the probe described in A. C, Western blot of membrane protein fraction from brain, lung, and submandibular gland of wild-type P2X4+/+ mice showed a band at 60 kDa, similar to that observed in P2X4-expressing HEK293 cells. No band of equivalent size was observed from knock-out P2X4−/− mice.
Adult (25–60 d) male homozygotes and their corresponding wild-type C57BL/6J mice were used in Western blotting and immunocytochemistry. Young adult mice (21–26 d) were used for electrophysiological studies. All procedures were performed according to United Kingdom Home Office legislation.
Immunocytochemistry and LacZ staining.
Immunocytochemistry using nickel–diaminobenzidine tetrahydrochloride chromagen with glucose oxidase was performed as described previously (Sim et al., 2004). The primary antibody used was polyclonal P2X4 antibody (1:1000 dilution) from Alomone Labs (Jerusalem, Israel) and goat anti-rabbit peroxidase-conjugated secondary antibody from Vector Laboratories (Peterborough, UK). Controls comprised the omission of the primary antibodies from the immunostaining protocol and the use of P2X4 antibodies preadsorbed with the corresponding peptide. β-Galactosidase staining was as described previously (Sim et al., 2004). After mounting, sections were viewed using an Axiovert 25 microscope, and images were acquired using Zeiss (Oberkochen, Germany) Axiocam digital camera with AxioVision software. For immunofluorescence, free-floating sections were incubated in blocking solution (0.01% Triton X-100 and 10% FCS) for 30 min at room temperature and then incubated overnight at 4°C with a home-produced rabbit antibody raised against the mouse P2X4 receptor terminal peptide (1:500 dilution). After three washes in PBS, sections were incubated for 2 h with goat-anti rabbit Alexa488 secondary antibody (Invitrogen, Carlsbad, CA). Sections were mounted and viewed with a Leica (Nussloch, Germany) DMRA2 fluorescent microscope. Images were acquired using a cool-snap HQ (PhotoMetrics, Huntington Beach, CA) digital camera controlled by the Metaview software suite.
Western blotting.
Mice were killed by isoflurane anesthesia. The brain and submandibular gland were removed; membrane fractions were prepared and used for Western blotting as described previously (Sim et al., 2004). The primary antibody was anti-rat P2X4 polyclonal antibody (1:5000 dilution; Alomone Labs) and the goat anti-rabbit HRP-conjugated secondary antibody (1:2000; DakoCytomation, Ely, UK). Positive controls were membrane fractions from HEK293 cells transiently transfected with mouse P2X4 receptors.
Electrophysiology.
The brain was rapidly removed and placed in ice-cold artificial CSF (aCSF) containing the following (in mm): 124 NaCl, 3 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgSO4, 10 d-glucose, and 0.1 picrotoxin (bubbled with 95% O2/5% CO2). Transverse hippocampal slices (400 μm thick) were prepared using a vibroslice (Leica). Hippocampal slices were stored in aCSF (20–25°C) for 1–2 h before transferring to the recording chamber, in which they were submerged in aCSF (∼30°C) flowing at ∼ 2 ml/min. Extracellular field potentials were recorded in the CA1 region using glass electrodes containing NaCl (4 m). A stimulating electrode in CA2 was used to evoke EPSPs (constant voltage, 100 μs duration, repeated at 30 s intervals). Long-term potentiation was evoked by four trains of stimuli (each 100 Hz, 1 s; repeated at 15 s intervals). The amplitude of the evoked field potential responses was measured and expressed relative to the normalized preconditioning baseline. Experiments in which changes in the fiber volley occurred were discarded.
Data were recorded using an Axoclamp 2B amplifier (Molecular Devices, Palo Alto, CA), monitored, analyzed on-line, and reanalyzed off-line (Anderson and Collingridge, 2001). Data were used from only one slice per mouse. Data pooled across slices are expressed as the mean ± SEM, and effects of conditioning stimulation were measured between 45 and 50 min after tetanus stimuli. The significance of difference from baseline was tested using two-tailed t tests.
Results
Western blotting showed that the P2X4 receptor protein was completely absent from the brain, lung, and submandibular gland of the knock-out P2X4−/− mice (Fig. 1C). Immunocytochemistry showed the presence of P2X4 receptors in the hippocampus of wild-type mice (Fig. 2A,C,E) that were not detectable in knock-out animals (Fig. 2B,D,F). The P2X4 immunoreactivity obvious in many other parts of the brain, such as Purkinje neurons (Fig. 2I) and in submandibular glands (Fig. 2L), was completely absent in P2X4−/− mice (Fig. 2J,M). Conversely, the P2X4−/− mouse showed strong staining of β-galactosidase in hippocampus (Fig. 2G), cerebellum (Fig. 2K), and submandibular glands (Fig. 2N). The distribution of β-galactosidase staining in the brain of P2X4−/− mice mapped well onto that reported previously for P2X4 receptor immunoreactivity (Lê et al., 1998; Rubio and Soto, 2001).
Figure 2.
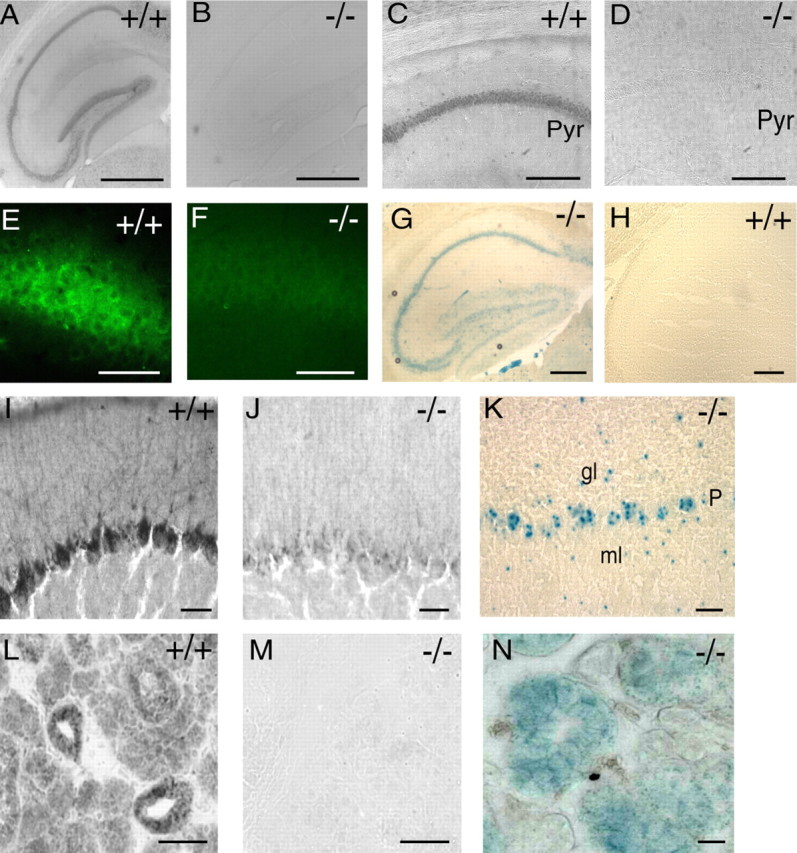
Expression of P2X4 receptors in wild-type P2X4+/+ but not in knock-out P2X4−/− mice. A, C, E, P2X4 subunit immunoreactivity throughout cell body layers of hippocampus is absent in knock-out mice (B, D, F). Pyr, Pyramidal cell. G, H, Detection of β-galactosidase. 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) staining in hippocampus of knock-out P2X4−/− mice but not in wild-type P2X4+/+ mice. Scale bars: A, B, G, H, 500 μm; C, D, 50 μm; E, F, 40 μm. I, P2X4 subunit immunoreactivity in cerebellar Purkinje cells is absent in the knock-out P2X4−/− mice (J). K, X-gal staining shows the expression of β-galactosidase in cerebellar Purkinje cells (P), as well as some cells of the granular layer (gl) and mossy layer (ml) from the knock-out P2X4−/− mouse. L, P2X4 subunit immunoreactivity in acinar and ductal cells of the submandibular gland is completely absent in the knock-out P2X4−/− mouse (M). N, Extensive expression of β-galactosidase in submandibular gland of the knock-out P2X4−/− mouse. Scale bars: I, J, K, 20 μm; L, M, N, 50 μm.
EPSPs were recorded extracellularly from the CA1 area of the hippocampus in slices from knock-out P2X4−/− or wild-type P2X4+/+ mice. Stimulation of the Schaffer collaterals (four trains of 100 Hz for 1 s, at 15 s intervals) resulted in a sustained increase in the amplitude of the field EPSP. The magnitude of LTP was greater in wild-type P2X4+/+ (169 ± 4% of baseline; n = 12) than in knock-out P2X4−/− mice (136 ± 4% of baseline; n = 12) (Fig. 3A); this difference was significant (p < 0.001; n = 12).
Figure 3.
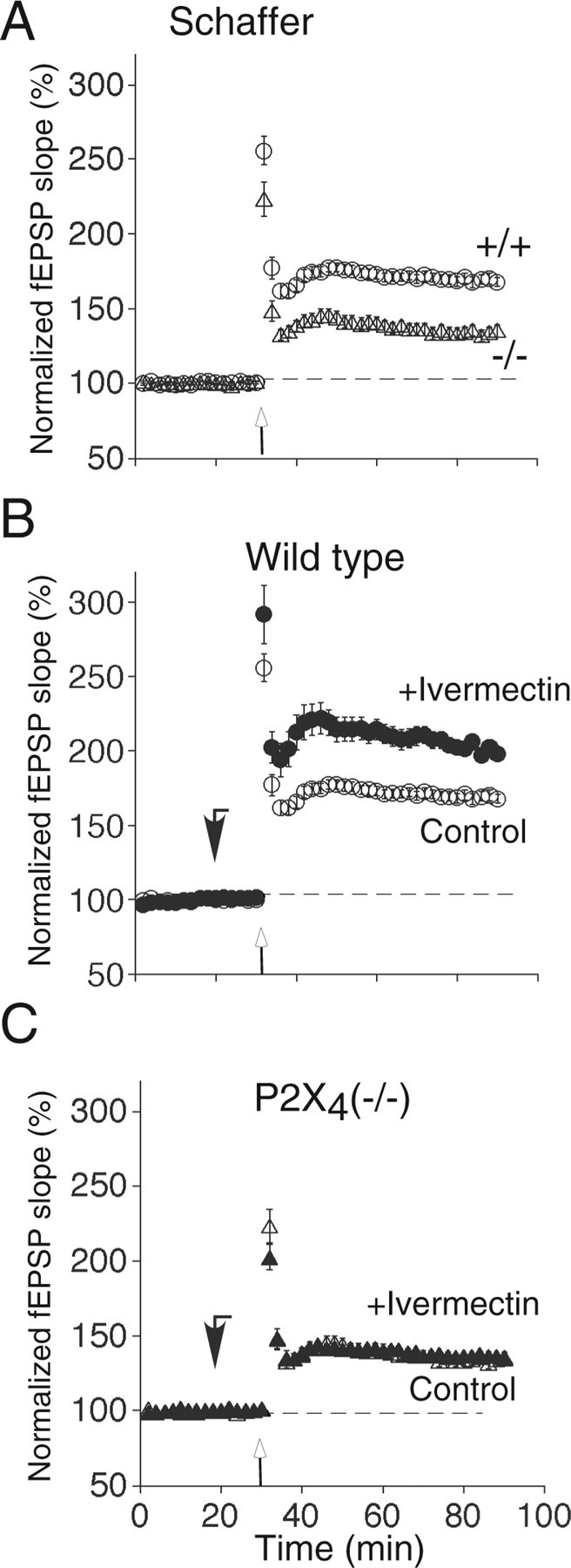
Contribution of P2X4 receptor activation to tetanus-induced LTP in hippocampal CA1 pyramidal cells. A, The magnitude of LTP elicited by Schaffer collateral stimulation was significantly reduced in P2X4−/− mice (▵; n = 12) compared with wild-type P2X4+/+ mice (○; n = 12; p < 0.001). B, LTP is enhanced by ivermectin (3 μm; ·) in wild-type P2X4+/+ mice but not in knock-out P2X4−/− mice (C) (▴; n = 15; p > 0.05), because they were superimposable on control values (▵). Ivermectin was applied 10 min before tetanic stimulation (downward filled arrows); it did not change the baseline of field EPSP. Tetanic stimulation (4 times at 100 Hz for 1 s) was applied at the upward open arrows. All data points are mean ± SEM, for n mice. Control data points in A are the same as in B and C.
LTP was greater in hippocampal slices from wild-type P2X4+/+ mice that had been treated with ivermectin (3 μm) (213 ± 9%; n = 15) (Fig. 3C). However, ivermectin had no effect on the magnitude of LTP recorded from knock-out P2X4−/− mice (138 ± 2%; n = 15) (Fig. 3D). Together, these findings strongly suggest that ivermectin is exerting its effect by potentiating field EPSPs through P2X4 receptors. Ivermectin did not introduce an NMDA receptor-independent form of long-term synaptic plasticity because, in the presence of ivermectin, the LTP in wild-type mice was completely blocked by the NMDA antagonist d-(−)-2-amino-5-phosphono-pentanoic acid (50 μm) (105 ± 8%; n = 4; data not shown). Therefore, ivermectin may act by increasing the activity of P2X4 receptors in the postsynaptic membrane (Toulmé et al., 2006).
Discussion
In certain experimental conditions, it can be shown that a component of the EPSC in hippocampal CA1 neurons is actually mediated by ATP (Pankratov et al., 1998). However, the proportion of the total current is rather small, as judged by the fraction remaining after blockade of different types of glutamate receptor (<10%), and the functional significance is therefore unclear. Our observation that ivermectin did not alter the field EPSP under resting conditions would be consistent with such a minor role. However, P2X4 receptors have very substantial calcium permeability (Soto et al., 1996; North, 2002; Egan and Khakh, 2004), and, unlike NMDA receptors, they are not subject to block by magnesium at resting potentials. The results with ivermectin, most strikingly its lack of effect in the P2X4−/− knock-out mouse, lead us to hypothesize that sufficient calcium, which enters through perisynaptic P2X4 receptors, is able to fine-tune the NMDA-dependent component of long-term synaptic plasticity of hippocampal CA1 neurons. Additional studies will be necessary to elucidate the details of the cellular elements primarily involved, but ivermectin and P2X4−/− knock-out mice will be useful tools in that endeavor.
Footnotes
This work was supported by the Medical Research Council, The Royal Society, The Wellcome Trust, Institut National de la Santé et de la Recherche Médicale, and Centre National de la Recherche Scientifique.
References
- Anderson WW, Collingridge GL. The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conquet F. Inactivation in vivo of metabotropic glutamate receptor 1 by specific chromosomal insertion of reporter gene lacZ. Neuropharmacology. 1995;34:865–870. doi: 10.1016/0028-3908(95)00042-5. [DOI] [PubMed] [Google Scholar]
- Egan TM, Khakh BS. Contribution of calcium ions to P2X channel responses. J Neurosci. 2004;24:3413–3420. doi: 10.1523/JNEUROSCI.5429-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X4 receptor channels. J Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Ivermectin: a positive allosteric effector of the α7 neuronal nicotinic receptor. Mol Pharmacol. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Krusek J, Zemková H. Effect of ivermectin on γ-aminobutyric acid-induced chloride currents in mouse hippocampal embryonic neurons. Eur J Pharmacol. 1994;259:121–128. doi: 10.1016/0014-2999(94)90500-2. [DOI] [PubMed] [Google Scholar]
- Lê KT, Villeneuve P, Ramjaun AR, McPherson PS, Beaudet A, Séguéla P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience. 1998;83:177–190. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur J Neurosci. 1998;10:3898–3902. doi: 10.1046/j.1460-9568.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- Pankratov YV, Lalo UV, Krishtal OA. Role for P2X receptors in long-term potentiation. J Neurosci. 2002;22:8363–8369. doi: 10.1523/JNEUROSCI.22-19-08363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel A, Silberberg SD. Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol. 2004;123:281–293. doi: 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ME, Soto F. Distinct localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci. 2001;21:641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguéla P, Haghighi A, Soghomonian JJ, Cooper E. A novel neuronal P2X ATP receptor ion channel with widespread distribution in the brain. J Neurosci. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24:6307–6314. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé E, Soto F, Garret M, Boué-Grabot E. Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharmacol. 2006;69:576–587. doi: 10.1124/mol.105.018812. [DOI] [PubMed] [Google Scholar]