Abstract
A commonly occurring polymorphic variant of the human 5-hydroxytryptamine (5-HT) transporter (5-HTT) gene that increases 5-HTT expression has been associated with reduced anxiety levels in human volunteer and patient populations. However, it is not known whether this linkage between genotype and anxiety relates to variation in 5-HTT expression and consequent changes in 5-HT transmission. Here we test this hypothesis by measuring the neurochemical and behavioral characteristics of a mouse genetically engineered to overexpress the 5-HTT. Transgenic mice overexpressing the human 5-HTT (h5-HTT) were produced from a 500 kb yeast artificial chromosome construct. These transgenic mice showed the presence of h5-HTT mRNA in the midbrain raphe nuclei, as well as a twofold to threefold increase in 5-HTT binding sites in the raphe nuclei and a range of forebrain regions. The transgenic mice had reduced regional brain whole-tissue levels of 5-HT and, in microdialysis experiments, decreased brain extracellular 5-HT, which reversed on administration of the 5-HTT inhibitor paroxetine. Compared with wild-type mice, the transgenic mice exhibited a low-anxiety phenotype in plus maze and hyponeophagia tests. Furthermore, in the plus maze test, the low-anxiety phenotype of the transgenic mice was reversed by acute administration of paroxetine, suggesting a direct link between the behavior, 5-HTT overexpression, and low extracellular 5-HT. In toto, these findings demonstrate that associations between increased 5-HTT expression and anxiety can be modeled in mice and may be specifically mediated by decreases in 5-HT transmission.
Keywords: serotonin, serotonin transporter, anxiety, polymorphism, microdialysis, serotonergic
Introduction
Brain 5-hydroxytryptamine (5-HT; serotonin) neuronal pathways are strongly implicated in the pathophysiology of a number of common and severe psychiatric disorders, and biological variation in 5-HT neurotransmission is likely to be a major psychiatric disorder risk factor. Reuptake of 5-HT by the 5-HT transporter (5-HTT; SERT) is a key control point in 5-HT neurotransmission because it is the main route by which released 5-HT is cleared from the synapse (Blakely et al., 1994; Lesch and Gutknecht, 2005). The human 5-HTT gene has a number of commonly occurring polymorphisms that may be a source of variation in 5-HTT expression in the human population (Heils et al., 1996; Ogilvie et al., 1996; Heinz et al., 2000; Kilic et al., 2003; Ozaki et al., 2003).
Of special interest are two alleles, the short (s) and long (l), in a repetitive element of varying length (5-HTTLPR) in a promoter region of the 5-HTT gene (44 bp insertion/deletion) that alters 5-HTT gene transcription and expression. Cultured cells expressing two copies of the l allele exhibit an increase in 5-HTT mRNA and a twofold increase in 5-HT uptake compared with cells expressing either one or two copies of the s allele (Lesch et al., 1993). Moreover, it is reported that individuals with the l/l genotype have increased 5-HT uptake, 5-HTT binding sites, and mRNA in brain and platelets compared with individuals with the s/s and s/l genotypes, in postmortem as well as neuroimaging studies (Hanna et al., 1998; Little et al., 1998; Greenberg et al., 1999; Heinz et al., 2000). Also, there is up to a twofold to threefold difference in the number of brain 5-HTT binding sites from one healthy individual to the next (Malison et al., 1998; Mann et al., 2000), and this variation may be genetically driven, at least in part through polymorphisms in the 5-HTT gene promoter region.
Recent gene linkage studies find that l/l allele carriers have decreased anxiety-related traits compared with carriers of the s/l and s/s alleles. Thus, l/l allele carriers exhibited decreased neuroticism scores (Lesch et al., 1996; Greenberg et al., 2000) and showed reduced anxiety-related responses to fearful stimuli compared with carriers of the s allele (Hariri et al., 2002; Pezawas et al., 2005). It is reasonable to assume that these gene associations are linked to altered 5-HTT expression because there are well established and strong connections between 5-HT and anxiety (Griebel, 1995; Handley, 1995), and mice with null mutations of the 5-HTT gene are anxious in anxiety paradigms (Holmes et al., 2003a,b). However, this linkage is not proven because the latter mouse does not model the level of natural variation in 5-HTT expression that likely occurs among individuals with different 5-HTT genotypes.
Recently, we reported preliminary accounts of a novel mouse genetically engineered to overexpress the human 5-HTT (Loder et al., 2000; Jennings et al., 2003). Here we examine the neurochemical and behavioral characteristics of this transgenic mouse and test the hypothesis that increased 5-HTT expression leads to altered anxiety-like behavior.
Materials and Methods
Animals
Experiments were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986. Male mice (CBA × C57BL/6J; 3–6 months of age; heterozygote transgenic mice and wild-type littermates) were generated as described below. Mice were group housed (unless stated otherwise) on a 12 h light/dark cycle (lights off 8:00 P.M. to 8:00 A.M.) in a temperature-controlled environment (21 ± 1°C). Behavioral testing was performed during the first 6 h of the light phase of the light/dark cycle.
Generation of 5-HTT-overexpressing mice
The 500 kb yeast artificial chromosome (YAC) 35D8 consisted of the human 5-HTT (h5-HTT) gene flanked by 150 kb of 5′ and 300 kb of 3′ sequences with the “short” allele of the 5-HTTLPR in the promoter region and the 10-repeat allele of the variable number tandem repeat in intron 2 (Shen et al., 2000a). This YAC was modified by homologous recombination to include a hemagglutinin (HA) epitope tag at the C terminus of the h5-HTT protein and a lacZ reporter gene downstream of an internal ribosomal entry site using placZSERT and pYAM4 (Shen et al., 2000b). The vector placZSERT was constructed by inserting (1) a fragment of h5-HTT genomic DNA extending 5 kb upstream from the stop codon (obtained by PCR using forward primer 5′-ACTGCATAGCGGCCGCATCTTTCATTTGCATCCCC-3′ and reverse primer 5′-TGTGCTCGAGAGCATTCAAGCGGATGT-3′) into the NotI–XhoI sites of pYIV3 and (2) the sequence downstream of the stop codon (obtained by PCR with forward primer 5′-CTCCTCGAGAGGAAAAAGGCTTCT-3′ and reverse primer 5′-TAGGTACCCTGTTCTCTCCTACGCAGTTT-3′) into the SalI–KpnI sites of pYIV3. The unmodified and placZSERT-modified YAC 35D8 were then transformed with NotI-linearized pYAM4 to amplify YAC DNA.
YAC DNA was purified by pulsed field gel electrophoresis as described by Schedl et al. (1996) and injected into the fertilized eggs (CBA × C57BL/6J). Transgenic mice were identified by PCR using primer pairs for h5-HTT (exon 1A, exon 1B, intron 1A, and 3′ untranslated region), sequence tag site markers (D17S2009, D17S2004, D1S1294, and D17S1549), and the YAC vector arms (Shen et al., 2000a). The transgene copy number was determined using densitometric measurement (NIH Image) of the ratios of the 2.6 kb h5-HTT over the 3.8 kb mouse 5-HTT (m5-HTT) genomic DNA, which were amplified by PCR with primers E4F (5′-CTACCTCATCTCCTCCTTCACG-3′) and E7R (5′-TTGTTGTTGAACTTGTTGTAGC-3′) in the exponential phase (15–23 cycles).
Reverse transcription-PCR
RNA was extracted from the cortex and brainstem of the transgenic mice and littermate controls using RNAzol B (Biogenesis, Bournemouth, UK), and cDNA was synthesized with Omniscript kits (Qiagen, Chatsworth, CA). Both m5-HTT and h5-HTT cDNA were amplified with primers E4F and E7R. Digestion of the 513 bp reverse transcription (RT)-PCR products with HpaII produced a 461 bp fragment from m5-HTT and a 295 bp fragment from h5-HTT. Digestion with HinfI resulted in a 513 bp fragment from the h5-HTT and fragments of 177 bp and a 249 bp from the m5-HTT. Digested DNA products were separated on 5% nondenaturing acrylamide or 2% agarose gels. In some experiments, the PCR primers were 32P labeled using T4 polynucleotide kinase, and restriction fragments of PCR products were detected by autoradiography of dried gels using Eastman Kodak (Rochester, NY) Biomax film.
Western blotting
Tissue was examined for the presence of the HA-tagged epitope using Western blotting. Protein extracts were prepared by homogenizing fresh lung tissue in treatment buffer (4 m urea, 3.8% SDS, 20% glycerol, 75 mm Tris, pH 6.8, and 50 mm DTT). Extracted protein (300 μg) and a positive control (HA-tagged ubiquilin) were separated on 10% SDS-polacrylamide gels and transferred to nitrocellulose membranes in transfer buffer (20% methanol, 0.1% SDS, 192 mm glycine, and 25 mm Tris-HCl, pH 8.5) for 60 min at 75 V. Membranes were blocked in 5% milk powder in Tris-buffered saline with Tween (TBST; 150 mm NaCl, 50 mm Tris-HCl, pH 7.5, and 0.1% Tween 20) for 1 h. Primary (chicken anti-HA, AB 3254; Chemicon, Temecula, CA) and secondary (HRP-conjugated anti-chicken; The Jackson Laboratory, Bar Harbor, ME) antibodies were applied in 5% milk powder in TBST at concentrations of 1:1000 and 1:5000, respectively, for 1 h each. The membrane was washed three times for 5 min in 5% milk powder in TBST after application of each antibody. Antibodies were visualized using Pierce (Rockford, IL) ECL Western blotting substrate.
Measurement of 5-HTT mRNA using in situ hybridization
Mice (five to six per group) were killed by cervical dislocation, brains were snap-frozen in isopentane/solid CO2, and coronal sections (12 μm) were cut on a microtome cryostat (−20°C), collected onto gelatin-coated slides, and stored at −70°C.
Oligonucleotide probes included a 40-mer m5-HTT-specific probe (5′-CGTGTCACCAGCTAATGTGGCAGTAAGTCCAAGAGAGTTC-3′; Sigma-Genosys, The Woodlands, TX) and a 42-mer h5-HTT probe (5′-CACCCAGCAGATCCTCCAgAACCAcCCcGGGCTGAAGCCgAG-3′) with four mismatches to the m5-HTT (indicated in lowercase). The probes (1 pmol/μl) were end labeled with [35S]dATP (12.5 mCi/ml; NEN Life Science Products, Boston, MA) by incubation at 37°C for 30 min with terminal deoxynucleotidyl transferase (Promega, Madison, WI), purified through Sephadex G-50, and diluted to 1.2 × 106 cpm/100 μl in hybridization buffer containing 50% formamide, 2× or 4× SSC, and 0.05 mm DTT. Each slide was loaded with a 100 μl probe, covered with a glass coverslip, and placed in lidded incubation trays containing filter paper soaked in 50 ml of 50% formamide in 4× SSC. Sections were hybridized overnight in 50% formamide with 4× SSC at 34°C (m5-HTT probe, high stringency), 50% formamide with 2× SSC at 37°C (h5-HTT probe, high stringency), or 50% formamide with 4× SSC at 33°C (low stringency to detect both m5-HTT and h5-HTT mRNA). Hybridized sections were washed in 1× SSC at 55°C three times for 20 min and at room temperature two times for 1 h. Slides were rinsed in water, dried, and exposed to Betamax film (Amersham Biosciences, Buckinghamshire, UK) for 4 weeks.
The abundance of mRNA in selected areas was determined by densitometric quantification of autoradiograms (MCID, St. Catherines, Ontario, Canada). Optical density values were calibrated to [35S] tissue equivalents using [14C] microscales (Amersham Biosciences) as described previously (Pei et al., 2003). Densitometric values measured from three sections of each animal were averaged and expressed as nanocurie per gram of tissue.
Measurement of 5-HTT binding sites
Membrane binding assay.
5-HTT binding sites in tissue membranes were assayed using standard radioligand binding methodology (Marston et al., 1999). In brief, the cerebral cortex from transgenic mice and littermate controls was homogenized in 50 vol (w/v) of ice-cold 50 mm Tris-HCl buffer, pH 7.4, and centrifuged (30,000 × g, 10 min). The membrane pellet was resuspended in 50 mm Tris-HCl and incubated at 25°C for 10 min, and membranes were washed by centrifugation (30,000 × g, two times for 10 min). The final membrane pellet was resuspended in 20 vol of buffer and stored (−20°C). On the assay day, membranes were suspended in 200 vol of 50 mm Tris-HCl buffer (120 mm NaCl and 5 mm KCl, pH 7.4), and binding of 0.25 nm [3H]citalopram (specific activity, 83 Ci/mmol; NEN Life Science Products) was measured in duplicate in the absence or presence of unlabeled citalopram (0.001–100 nm). Nonspecific binding was defined using 1 μm paroxetine. Samples were incubated for 1 h at 25°C, binding was terminated by filtration, and radioactivity was measured by scintillation counting. Data were analyzed using iterative, nonlinear least-squares curve fitting to provide binding-site affinity (KD) and density (Bmax) values (Prism; GraphPad, San Diego, CA) and tested statistically using Student's unpaired t test.
Receptor autoradiography.
Brains from six heterozygous transgenic males and five wild-type littermates (age, 3–6 months) were removed, frozen in an isopentane bath at −30°C, and stored at −70°C before sectioning. Coronal sections (12 μm) were cut using a microtome cryostat (−20°C), mounted on gelatin-coated slides, and stored at −70°C. Slides were subsequently thawed and preincubated in 50 mm Tris-HCl buffer (120 mm NaCl and 5 mm KCl, pH 7.4) for 15 min, before incubation in 50 mm Tris-HCl buffer containing 2 nm [3H]citalopram for 60 min. Nonspecific binding was defined by 1 μm imipramine. Sections were washed in ice-cold Tris-HCl buffer (two times for 10 min) and rinsed in ice-cold distilled water before drying in a cold air stream.
Slides were exposed to [3H]-sensitive Hyperfilm (Amersham Biosciences) at 4°C for 6 weeks. The relative abundance of 5-HTT binding sites was determined by densitometric quantification of autoradiograms, calibrated to tritiated tissue equivalents (Amersham Biosciences), and corrected for nonspecific signals. Optical densities from three sections per animal were averaged and expressed as femtomoles per milligram of tissue. Data were analyzed statistically on a region-by-region basis using Student's unpaired t test. In addition, regional binding was compared across genotypes using a correlation analysis (Pearson's).
Measurement of whole-tissue 5-HT and other analytes
Mice (10 per group) were either treatment naive or administered the aromatic amino acid decarboxylase inhibitor NSD 1015 (100 mg/kg, i.p.) 30 min before they were killed. Brains were removed, and regions (including the frontal cortex, striatum, and hippocampus) were rapidly dissected out on ice and frozen in isopentane (−20°C) and stored at −70°C.
On the day of analysis, samples were homogenized in 1 ml of 0.1 m perchloric acid and centrifuged (15,000 × g, 15 min). Supernatants were stored on ice before analysis using HPLC with electrochemical detection (see below). Data were converted into picomoles per milligram of wet weight, and comparisons between genotype were made on a region-by-region basis using Student's unpaired t test.
Microdialysis
Microdialysis experiments were performed using anesthetized mice because of technical difficulties perfusing and administering drugs to freely moving mice. Previous studies in rats found similar effects of 5-HTT inhibitors under awake and anesthetized conditions (Gartside et al., 1995; Castro et al., 2003).
Mice (five to six per group) were anesthetized (chloral hydrate; 500 mg/kg, i.p., 50–100 mg/kg/h thereafter), and single cannula microdialysis probes were stereotaxically implanted into either the medial prefrontal cortex [rostrocaudal, lateral, and dorsoventral coordinates +1.9 mm, +0.4 mm, and +2.0 mm, respectively, relative to bregma and dura surface (Franklin and Paxinos, 1997)] or the hippocampus (coordinates: +1.1 mm, +1.5 mm, +3.6 mm). Probes were perfused continuously with artificial CSF (in mm: 140 NaCl, 3 KCl, 1.2 Na2HPO4, 0.27 NaH2PO4, 1 MgCl2, 2.4 CaCl2, and 7.2 glucose) at a rate of 2 μl/min.
After a 1.5 h postimplantation period, baseline dialysates (20 min samples) were collected for 2 h before switching to CSF containing high potassium (56 mm KCl and 87 mm NaCl) for 20 min, followed by perfusion with normal CSF for an additional 1 h. In another set of experiments, after the 2 h baseline period, either the perfusion medium was switched to CSF containing 1 μm paroxetine or 5 mg/kg paroxetine was injected intraperitoneally. At the end of each experiment, brains were removed to confirm correct probe placement by histological analysis. Under the above conditions, 5-HT levels were stable after the 2 h baseline period, and removal of calcium from the perfusion medium reduced 5-HT levels by 70–80% (n = 5).
Microdialysis data were analyzed either as raw data (picomoles per 40 μl dialysate) or percentage of baseline, using the last time point before treatment as 100%. Genotypes were compared using one- or two-way repeated-measures ANOVA as appropriate, and post hoc testing was by a Bonferroni's t test.
HPLC analysis
Dialysates and tissue supernatants were assayed for 5-HT and other analytes (dopamine, noradrenaline, 5-HIAA, 5-HTP, DOPAC, DOPA) using HPLC with electrochemical detection. Analytes were separated on Microsorb C18 reverse-phase columns (4.6 × 100–150 mm) and detected (LC-4B electrochemical detector) using a glassy carbon working electrode (+0.7 V vs silver/silver chloride). The mobile-phase compositions (flow rate, 1 ml/min) for separation of the different analytes were as follows: 5-HT, 5-HIAA, and DOPAC: 12.5% methanol, 0.1 m NaH2PO4, 0.8 mm EDTA, and 0.01 mm sodium octane sulfonate, pH 3.55; 5-HTP and DOPA: 12% methanol, 0.1 m NaH2PO4, 0.8 mm EDTA, and 1.7 mm sodium octane sulfonate, pH 3.8; noradrenaline and dopamine: 14.5% methanol, 0.1 m NaH2PO4, 0.8 mm EDTA, and 3.2 mm sodium octane sulfonate, pH 3.35.
Behavioral testing
Plus maze.
The plus maze apparatus was elevated 50 cm and comprised open (29 × 4 cm, 0.5 cm walls) and closed (27 × 8 cm, 30 cm walls) arms joined by a central open space (8 × 4 cm). Transgenic and wild-type mice (eight per group) were placed at the distal end of a closed arm, and the amount of time spent in the open arms, the number of entries into the open arms, the total number of arm entries, and the latency to first enter the open arm were measured. Testing was videotaped in the absence of the investigator, with the apparatus lit by a 60 W light bulb directed away from the apparatus. In separate experiments, mice (four groups of eight) were administered paroxetine (10 mg/kg, i.p.) or saline vehicle 30 min before testing.
Hyponeophagia.
Food consumption of singly housed mice was monitored over a 16 h period (including overnight) and restricted to one-third of this amount the night before testing. The following day, mice (eight per group) were placed singly in a transparent plastic box (26 × 16 × 17 cm) with the floor covered with an even layer of food pellets (Noyes, Lancaster, NH). This method ensures that the mouse is immediately aware of the food and sensorimotor factors do not confound the anxiety measurement. Latency to begin feeding was defined as the time taken for a mouse to hold a pellet in the forepaws and eat continuously (at least 3 s).
Open-field test.
The open-field test was performed as described previously (Deacon et al., 2002) using an open-topped rectangular box (50 × 30 × 30 cm) constructed from gray plastic with the floor divided into 15 × 10 cm squares, under low-light conditions. Mice (eight per group) were placed individually in a corner square, and the number of squares entered in a 3 min period was measured.
Behavioral data were analyzed statistically using either Student's unpaired t test or two-way ANOVA followed by a post hoc Bonferroni's test, as appropriate. All cases of significance detected using Student's t test were confirmed using the Mann–Whitney U test.
Results
Generation of 5-HTT-overexpressing mice
The successful integration of the YAC DNA into transgenic lines and its integrity were verified by the presence of the PCR products of primer pairs a–j (Fig. 1A and data not shown). Transgene copy numbers were estimated from the ratios of PCR products of h5-HTT (2.6 kb) and m5-HTT (3.8 kb) genomic DNA in the exponential phase of amplification, using primers E4F and E7R (Fig. 1B). The transgenic mouse lines A102.3 (eight copies) and A104 (three copies) made with the modified YAC contained all markers examined, and line A145.1 (five copies) made with unmodified YAC DNA lacked the short vector arm only.
Figure 1.
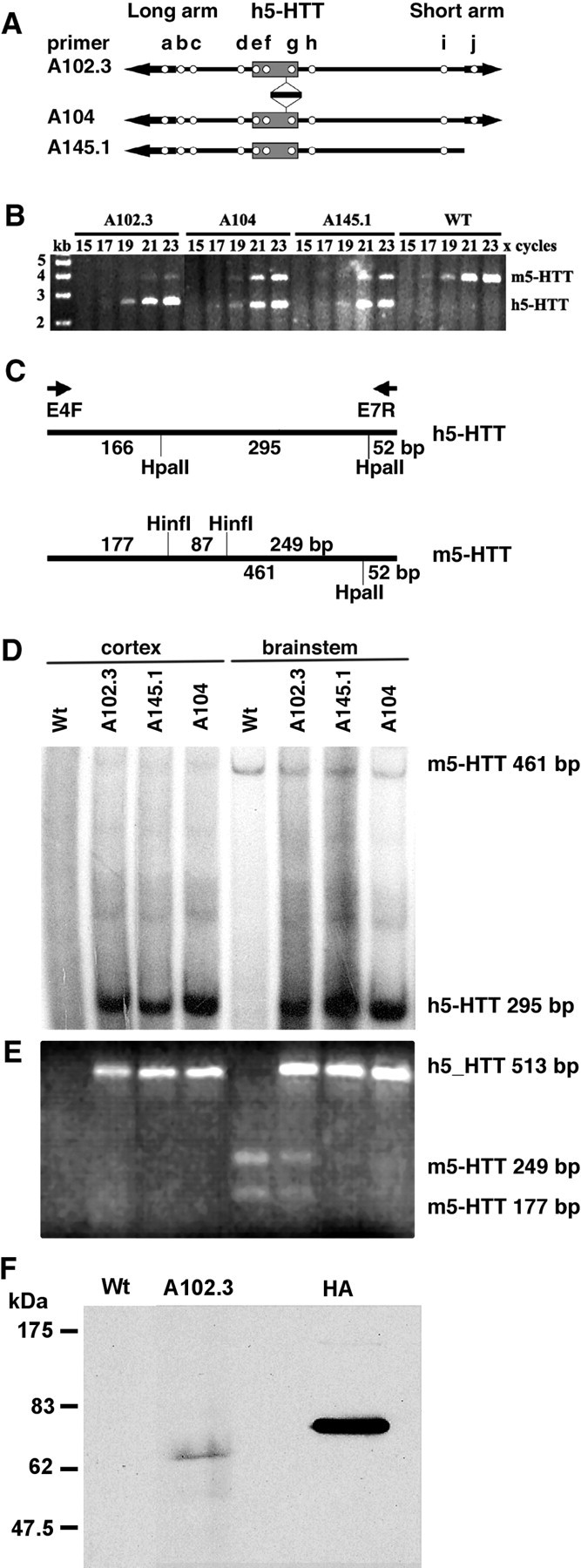
Creation and characterization of transgenic mice expressing the h5-HTT. Three lines of transgenic mice were generated (lines A145.1, A102.3, and A104). A, Open circles indicate the presence of markers in each transgenic line. The primer pairs used (from left to right) were for the long YAC vector arm (a), D17S2004 (b), D17S2009 (c), h5-HTT promoter (d), intron 1A (e), intron 1B (f), 3′ untranslated region (g), D17S1294 (h), D17S1549 (i), and the short arm of the YAC vector (j). B, PCR amplification of a 2.6 kb genomic DNA fragment from the human 5-HTT gene and a 3.8 kb fragment from the m5-HTT gene using primers E4F and E7R. These data were used to estimate the transgene copy number. C, Expression of 5-HTT mRNA was determined by RT-PCR with primer pairs E4F and E7R and by subsequent restriction digestion. D, An autoradiogram of PCR products obtained using 32P-labeled primers and digested with HpaII. E, Restriction fragments of PCR products obtained using unlabeled primers and detected by ethidium bromide staining. RNA templates were from the cortex (lanes 1–4) and brainstem (lanes 5–8) of wild-type (lanes 1, 5), A102.3 (lanes 2, 6), A145.1 (lanes 3, 7), and A104 (lanes 4, 8) transgenic mice. F, Western blot demonstrating the presence a HA-tagged epitope in lung tissue from transgenic (A102.3) but not wild-type mice. The positive control lane (HA) represents HA-tagged ubiquilin. Wt, Wild type.
Expression of the h5-HTT in transgenic mice
To examine h5-HTT expression, total RNA was extracted from the cortex and brainstem (including midbrain raphe nuclei) of wild-type and transgenic mice and analyzed by RT-PCR for both m5-HTT and h5-HTT cDNA (Fig. 1C). m5-HTT was detected in the brainstem (Fig. 1D,E, lane 5) but not in the cortex (Fig. 1D,E, lane 1) of wild-type controls. Expression of the h5-HTT was detected in both the brainstem (Fig. 1D,E, lanes 6–8) and cortex (Fig. 1D,E, lanes 2–4) of all transgenic lines (Fig. 1D,E). The three transgenic lines (A102.3, A104, and A145.1) overexpressed comparable levels of the h5-HTT mRNA. The A102.3 line of transgenic mice was selected for investigation in more detail.
Immunocytochemical studies of brain tissue failed to detect specific staining of β-galactosidase or of the HA tag using a number of commercial antibodies or (in the case of β-galactosidase) by X-Gal staining. This may indicate that the level of expression of the human SERT protein was too low to be detectable by immunocytochemistry. In addition, LacZ transgenes often function poorly in adult animals (Cohen-Tannoudji et al., 2000).
In another attempt to verify expression of the HA epitope in the transgenic animals, additional Western blotting experiments were performed on lung tissue. This tissue has a higher level of 5-HTT expression than brain (Ramamoorthy et al., 1993), which is further enhanced in the transgenic mice (MacLean et al., 2004), thereby making the HA epitope easier to detect. Indeed, lung tissue from the transgenic mice showed a distinct HA-immunolabeled band at the molecular weight predicted for the 5-HTT (∼70 kDa), that was absent in the wild-type controls (Fig. 1F).
Localization of 5-HTT mRNA in transgenic mice
The brain localization of 5-HTT transgene was investigated (n = 6 transgenic and wild-type mice) by in situ hybridization with a m5-HTT-specific probe under high-stringency conditions that detected m5-HTT transcripts only (Fig. 2A,F) and with a h5-HTT probe under high-stringency conditions that detected h5-HTT RNA only (data not shown), or under less stringent conditions that detected both m5-HTT and h5-HTT RNA (Fig. 2B–E,G–J).
Figure 2.
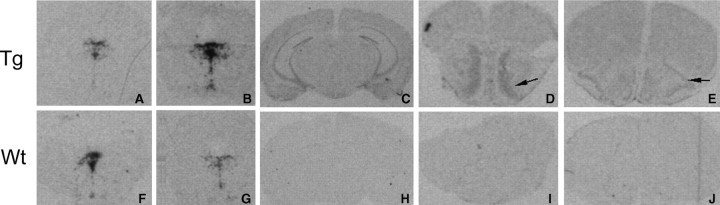
Detection of 5-HTT expression by in situ hybridization in 5-HTT-overexpressing transgenic (Tg) and wild-type (Wt) mice. 5-HTT expression in the raphe (A, B, F, G), hippocampus (C, H), olfactory bulb (D, I), and piriform cortex (E, J) of the transgenic (A–E) and wild-type (F–J) mice using m5-HTT-specific probe (A, F) and h5-HTT probe (B–E, G–J).
In wild-type controls, m5-HTT was found predominantly in the dorsal raphe nucleus (Fig. 2F–G) with lower abundance in the median raphe and ventrolateral dorsal raphe nuclei, consistent with the expression pattern reported previously (Bengel et al., 1997). There was no detectable m5-HTT expression in any other brain region examined (Fig. 2H–J). The transgenic mice expressed the h5-HTT in a pattern similar to the endogenous 5-HTT with predominant hybridization signals observed in the raphe nuclei (Fig. 2B).
Total 5-HTT mRNA was increased 2.9-fold in the dorsal raphe nucleus (2578 ± 266 vs 890 ± 71 nCi/g tissue for transgenic and wild-type mice, respectively), 1.8-fold in the median raphe nucleus (938 ± 164 vs 521 ± 37 nCi/g tissue), and 2-fold in the ventrolateral dorsal raphe nucleus (1118 ± 161 vs 539 ± 60 nCi/g tissue). These differences were statistically significant (p < 0.05). The increase was attributable to expression of the transgene, because the h5-HTT probe displayed weaker hybridization signals in the wild-type mice (Fig. 2G) than in transgenic mice (Fig. 2B) and the m5-HTT-specific probe showed similar levels and pattern of m5-HTT RNA expression in transgenic (Fig. 2A) and wild-type (Fig. 2F) mice.
In addition to overexpression in the raphe nuclei, the transgenic mice also showed low levels (6–8% of that in the raphe) of h5-HTT expression outside the brainstem, including the CA1–CA3 regions and dentate gyrus of the hippocampus (Fig. 2C), the piriform cortex (Fig. 2E, arrow), and the granular cell layer of the olfactory bulb (Fig. 2D, arrow). No endogenous m5-HTT was detected in these regions of the wild-type controls (Fig. 2H–J).
5-HTT binding sites in transgenic mice
5-HTT binding was analyzed using a membrane binding assay and receptor autoradiography, performed at equilibrium conditions with [3H]citalopram. In the membrane binding assay, transgenic mice and wild-type controls showed similar KD values for [3H]citalopram (1.75 ± 0.14 vs 1.29 ± 0.21 nm, respectively) with Hill coefficients close to unity (0.89 ± 0.11 vs 1.03 ± 0.08, respectively). Transgenic mice, however, displayed a significant (3.2-fold) increase (p < 0.01) in the density of [3H]citalopram binding sites in cortical tissue compared with wild-type controls (2800 ± 625 vs 862 ± 312 fmol/mg protein, respectively).
Autoradiograms revealed a distribution of [3H]citalopram binding sites (Fig. 3A,B) in wild-type mice similar to that reported in previous studies (Le Marec et al., 1998). The density of [3H]citalopram binding sites was greater in the transgenic mice compared with the wild-type controls. The increase in [3H]citalopram binding sites ranged from ∼3.3-fold in cortical regions to 1.5- to 1.8-fold in the thalamus and hippocampus (Fig. 3B). The regional pattern of [3H]citalopram binding sites in transgenic mice was similar to wild-type controls (Fig. 3A,B). Thus, in both transgenic and wild-type mice, [3H]citalopram binding sites were most abundant in the dorsal and median raphe nuclei, hypothalamus, thalamus, and hippocampus and least abundant in striatum and anterior cortical regions (Fig. 3B). There was a strong correlation (r = 0.943; p < 0001) between 5-HTT binding in brain regions of the wild types versus transgenic mice (Fig. 3C), indicative of a similar 5-HTT expression pattern across the genotypes.
Figure 3.
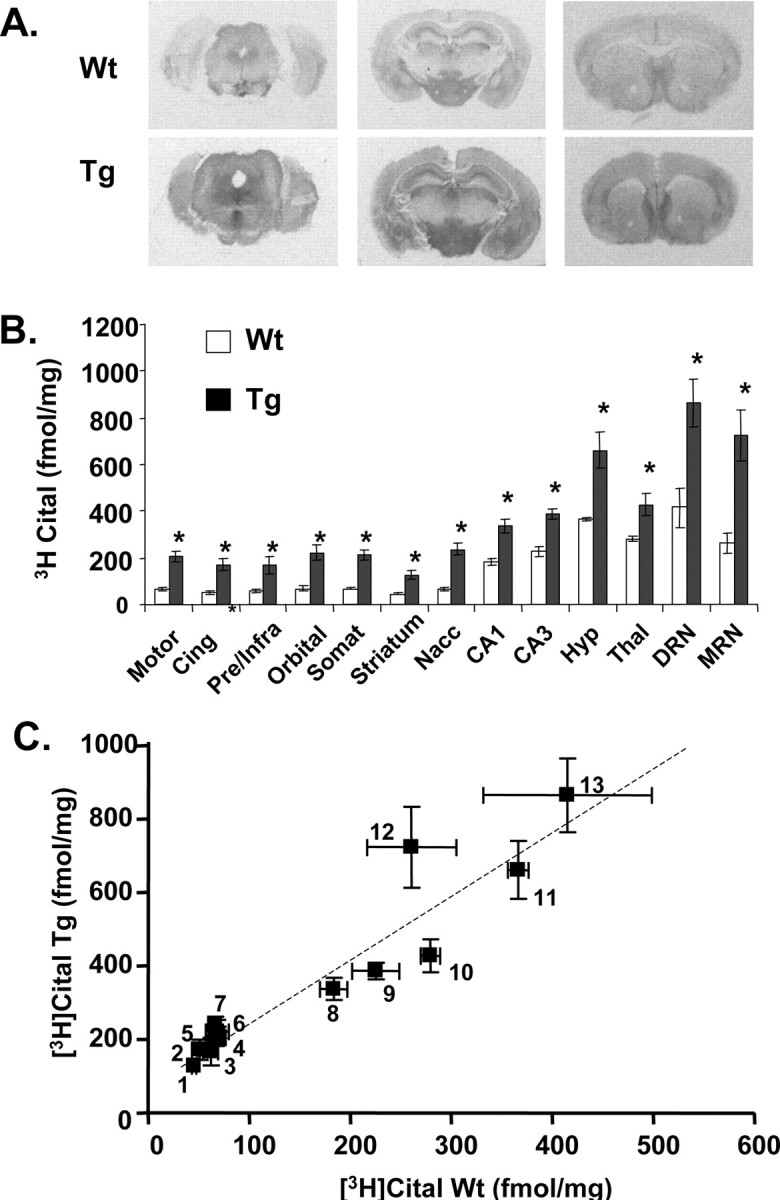
Autoradiograms showing the distribution (A), quantification (B), and regional correlation (C) of [3H]citalopram (Cital) binding sites in brain sections of 5-HTT-overexpressing (Tg) and wild-type (Wt) mice. Sections were cut at approximately levels 21–23 (bottom), 46–48 (middle), and 67–69 (top) according to the mouse stereotaxic atlas of Franklin and Paxinos (1997). The abbreviations for B with corresponding numbers for C are as follows: motor, motor cortex (4); Cing, cingulate cortex (3); Pre/Infra, prelimbic/infralimbic cortex (2); Orbital, orbital cortex (6); Somat, somatosensory cortex (5); Striatum, striatum (1); Nacc, nucleus accumbens (7); CA1, CA1 (8); CA3, CA3 (9); Hyp, hypothalamus (11); Thal, thalamus (10); DRN, dorsal raphe nucleus (13); MRN, medial raphe nucleus (12). All determinations in the transgenic mice were statistically significant (*p < 0.05, Student's unpaired t test) compared with wild-type controls. Error bars indicate SEM.
Brain tissue levels of 5-HT and 5-HT synthesis and metabolism
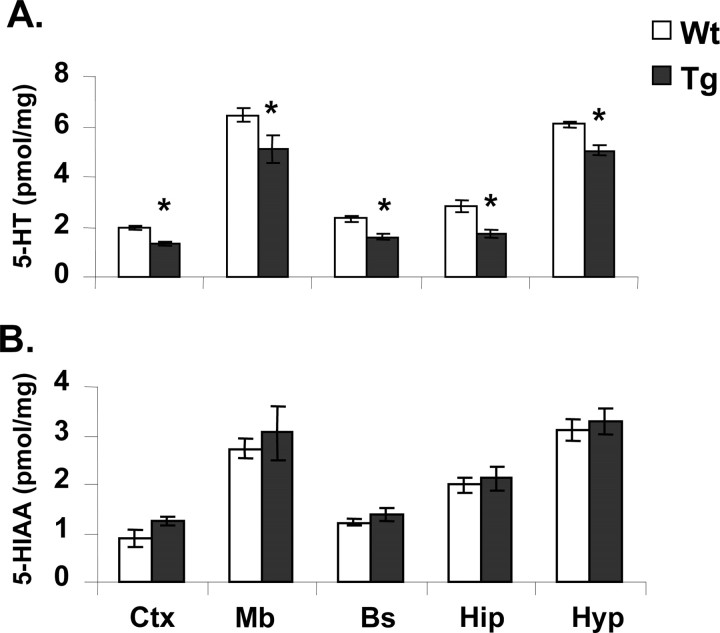
Transgenic mice showed reduced tissue levels (−15 to 35%) of 5-HT in the hippocampus, frontal cortex, hypothalamus, brainstem, and midbrain compared with wild-type animals (Fig. 4). In comparison, levels of the 5-HT metabolite, 5-HIAA, were not significantly different (Fig. 4).
Figure 4.
Tissue levels of 5-HT (A) and 5-HIAA (B) in brain regions of 5-HTT-overexpressing (Tg) and wild-type (Wt) mice. Ctx, Cortex; Bs, brainstem; Mb, midbrain; Hip, hippocampus; Hyp, hypothalamus. Mean ± SEM (n = 8) values are shown. *p < 0.05, wild-type versus transgenic mice (Student's unpaired t test).
In separate experiments, accumulation of the 5-HT precursor 5-HTP (after aromatic amino acid decarboxylase inhibition) in the cortex, striatum, and hippocampus was not different between wild-type and transgenic mice (Table 1). These experiments also confirmed that tissue levels of 5-HT were significantly lower in transgenic mice than wild types and that 5-HIAA levels were unchanged (data not shown). Regional levels of the dopamine metabolite DOPAC and accumulation of the catecholamine precursor DOPA were not different between transgenic and wild-type mice (Table 1 and data not shown).
Table 1.
5-HTP and l-DOPA accumulation in brain regions of 5-HTT-overexpressing transgenic (Tg) and wild-type (Wt) mice
| Frontal cortex |
Striatum |
Hippocampus |
||||
|---|---|---|---|---|---|---|
| Wt | Tg | Wt | Tg | Wt | Tg | |
| 5-HTP (pmol/mg) | 0.83 ± 0.038 | 0.79 ± 0.053 | 1.1 ± 0.051 | 1.1 ± 0.093 | 2.5 ± 0.37 | 2.1 ± 0.30 |
| l-DOPA (pmol/mg) | 0.84 ± 0.074 | 0.94 ± 0.085 | 3.8 ± 0.17 | 3.7 ± 0.34 | 0.79 ± 0.14 | 0.58 ± 0.092 |
Mice (n = 10 per group) were administered the aromatic amino acid decarboxylase inhibitor NSD 1015 (100 mg/kg) 30 min before tissue removal. The data shown are mean ± SEM values.
Brain extracellular 5-HT levels
In microdialysis experiments, 5-HTT-overexpressing mice demonstrated constantly lower (−50 to 60%) basal extracellular levels of 5-HT in both the prefrontal cortex (effect of genotype; F(1,9) = 5.4; p < 0.05) and hippocampus (F(1,8) = 13.0; p < 0.05) compared with wild-type mice (Fig. 5A,B). This difference was stable and maintained over 3–4 h of baseline sampling. Also, in both regions, the increase in 5-HT evoked by local application of high potassium (56 mm) was significantly (p < 0.05) less (40–80%) in the transgenic mice compared with wild types (Fig. 5A,B).
Figure 5.
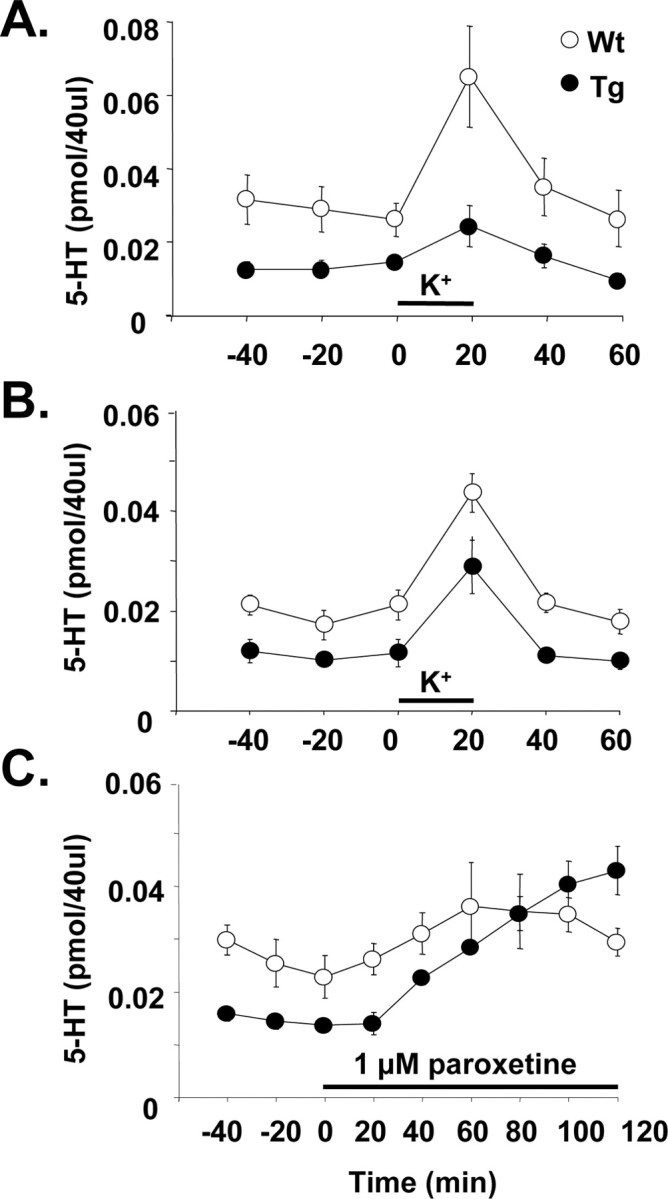
Levels of 5-HT in microdialysates from anesthetized 5-HTT-overexpressing (Tg) and wild-type (Wt) mice. A, Basal and potassium-evoked 5-HT in the medial prefrontal cortex. B, Basal and potassium-evoked 5-HT in the hippocampus. C, Basal 5-HT in the medial prefrontal cortex and effect of locally applied paroxetine. High potassium (56 mm) and paroxetine (1 μm) were added to the perfusion medium as indicated by the bars. Mean ± SEM values are shown (n = 5–6 per group).
Separate experiments showed that the low levels of extracellular 5-HT in the prefrontal cortex of 5-HTT-overexpressing mice could be reversed by local application of 1 μm paroxetine. Thus, whereas the transgenic mice had 50% lower 5-HT levels than the wild types (F(1,8) = 8.5; p < 0.02), after paroxetine 5-HT levels were not significantly different (Fig. 5C). Also, systemically administered paroxetine (5 mg/kg, s.c.) caused a rise in cortical extracellular 5-HT in transgenic mice that was similar in magnitude to that observed in wild-type mice [+203.4 ± 65.9% (n = 5) vs +235.3 ± 70.5% (n = 5) of predrug values at t = 60 min after paroxetine].
Transgenic and wild-type mice did not differ in basal extracellular levels of either dopamine in the prefrontal cortex [0.040 ± 0.006 pmol/40 μl (n = 5) vs 0.054 ± 0.008 pmol/40 μl (n = 5)] and striatum [0.214 ± 0.089 pmol/40 μl (n = 5) vs 0.133 ± 0.046 pmol/40 μl (n = 5)] or noradrenaline in the prefrontal cortex [0.108 ± 0.020 pmol/40 μl (n = 5) vs 0.090 ± 0.012 pmol/40 μl (n = 5)] and hippocampus [0.041 ± 0.009 pmol/40 μl (n = 5) vs 0.029 ± 0.005 pmol/40 μl (n = 5)].
Anxiety tests
Elevated plus maze
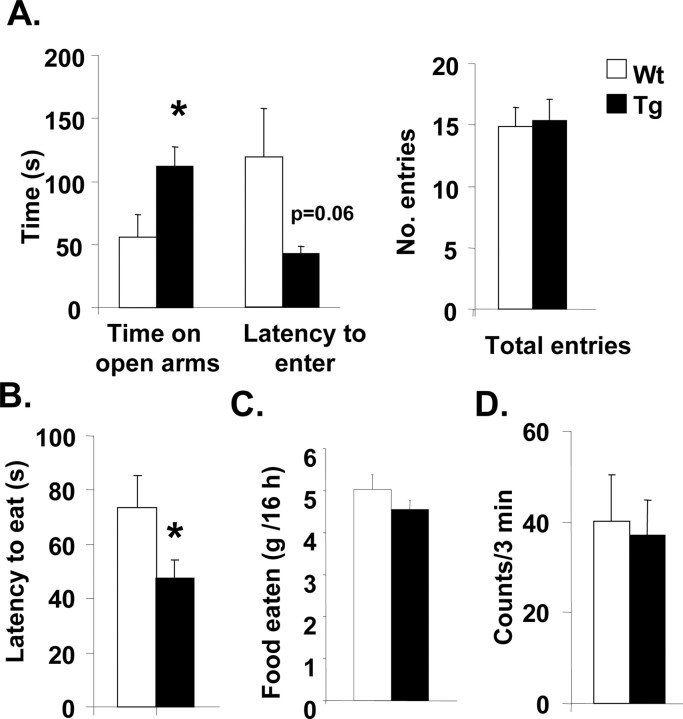
Drug naive 5-HTT-overexpressing mice spent significantly more time on the open arms of the plus maze (p < 0.05) and showed a shorter latency (p = 0.06) to first enter the open arm than drug naive wild-type mice, whereas total arm entries were not different between genotypes (Fig. 6A).
Figure 6.
Behavioral measures of anxiety in 5-HTT-overexpressing (Tg) and wild-type (Wt) mice. A, Latency to enter open arms, time on open arms, and total number of arm entries in the elevated plus maze (left to right). B, Latency to eat in hyponeophagia test. C, Overnight food consumption. D, Activity in the open-field test. The data shown are mean ± SEM values. *p < 0.05 transgenic versus wild-type mice (Student's unpaired t test).
Open and closed arm entries by the transgenic mice (6 ± 1 and 10 ± 1, respectively) were not significantly different from wild-type mice (4 ± 1 and 11 ± 1). Similarly, ratios for open/closed and open/total entries by the transgenic mice (0.65 ± 0.11 and 0.37 ± 0.04, respectively) were not significantly different from wild-type mice (0.40 ± 0.14 and 0.24 ± 0.06), suggesting that the decreased latency to enter the open arms and increased time spent on the open arms is specifically linked to reduced anxiety and not a function of changes in locomotor activity.
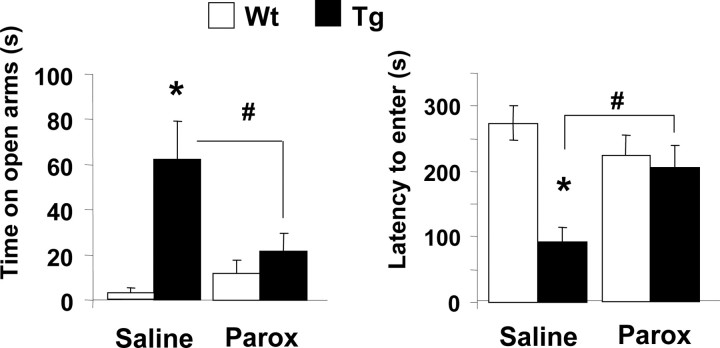
Additional plus maze experiments on a separate cohort of mice confirmed that the transgenic mice spent significantly more time on the open arms of the plus maze (p < 0.05) and showed a shorter latency (p < 0.05) to first enter the open arm than wild-type mice (Fig. 7). These experiments also demonstrated that the low-anxiety phenotype of the transgenic mice could be reversed by inhibition of the 5-HTT (Fig. 7). Thus, transgenic mice administered 10 mg/kg paroxetine intraperitoneally spent significantly less time on the open arm, and showed a significantly greater latency to enter the open arms, compared with transgenic mice administered saline (p < 0.02) (Fig. 7). In wild-type controls, paroxetine (10 mg/kg, i.p.) had no effect on either the time on the open arm or latency to enter the open arms (Fig. 7).
Figure 7.
Behavioral measures of anxiety in 5-HTT-overexpressing (Tg) and wild-type (Wt) mice treated with the 5-HTT inhibitor paroxetine (Parox) or saline vehicle. Paroxetine (10 mg/kg, i.p.) was administered 30 min before testing. The data shown are mean ± SEM values. *p < 0.05 transgenic versus wild-type mice; #p < 0.05, paroxetine versus saline (Student's unpaired t test).
Hyponeophagia
In the hyponeophagia test, 5-HTT-overexpressing mice displayed a significantly decreased latency to eat compared with wild-type mice (Fig. 6B) (p < 0.05). 5-HTT-overexpressing mice showed no significant difference in the amount of food consumed overnight compared with wild types (Fig. 6C).
Open field
5-HTT-overexpressing mice did not show a difference in the number of squares entered in the open field compared with wild types (Fig. 6D).
Discussion
A common, naturally occurring polymorphism in the 5-HTT gene promoter region of the human 5-HTT gene (l/l genotype) that increases 5-HTT expression has been associated with reduced anxiety levels in human volunteers and in patient populations (see Introduction). The present study investigated the neurochemical and behavioral effects of increased 5-HTT expression by generating a novel transgenic mouse model that overexpresses the h5-HTT from a YAC construct. These data suggest that increased 5-HTT expression produces a low-anxiety phenotype and that this is mediated by decreased 5-HT transmission.
General features of the 5-HTT-overexpressing mice
A selected transgenic mouse line (A102.3) showed the presence of h5-HTT mRNA in brainstem extracts analyzed by RT-PCR, and in situ hybridization studies demonstrated the increased abundance of 5-HTT mRNA in the midbrain raphe nuclei. The transgenic mice also had increased 5-HTT protein expression across a range of forebrain and midbrain regions, as determined by autoradiographic measurement of 5-HTT binding sites using the selective 5-HTT radioligand [3H]citalopram. Although the number of 5-HTT binding sites increased in transgenic animals, the pattern of expression showed a close correlation with that seen in wild-type mice and corresponded with that seen in other studies using [3H]citalopram and similarly selective 5-HTT radioligands such as 3H-paroxetine (De Souza and Kuyatt, 1987; Le Marec et al., 1998). It is possible that differences in distribution of 5-HTT between these studies and other reports (Bengel et al., 1997) relates to differences in selectivity of the radioligands used.
An interesting feature of the transgenic mouse is that the level of 5-HTT expression (mRNA and binding sites) was twofold to threefold greater than wild-type mice. A similar degree of variation in 5-HTT binding sites is reported in healthy individuals in postmortem and neuroimaging studies (Malison et al., 1998; Mann et al., 2000). Naturally occurring variation in 5-HTT expression may, at least in part, be genetically driven because individuals with the l/l genotype show about a twofold increase in 5-HT uptake, 5-HTT binding sites, and mRNA in studies of brain and platelets compared with individuals with the s/s and s/l genotypes (Hanna et al., 1998; Little et al., 1998; Greenberg et al., 1999; Heinz et al., 2000).
In addition to showing increased abundance of 5-HTT mRNA in the midbrain raphe nuclei, the transgenic mice also had trace amounts of 5-HTT mRNA in the forebrain. Although the present study did not detect extra raphe 5-HTT mRNA in wild-type mice, this finding was not entirely unexpected, because low levels of 5-HTT mRNA have been reported previously in rat cortex and hippocampus (Lesch et al., 1993) and in human cortex (Hernandez and Sokolov, 1997; Sun et al., 2001) using sensitive PCR-based methods. It has been speculated previously that the low levels of 5-HTT mRNA in the forebrain might arise through trafficking to the 5-HT nerve terminals (Lesch et al., 1993). Also, expression of 5-HTT protein in non-5-HT neurons has been detected in the limbic brain regions (Pickel and Chan, 1999). Nevertheless, the presence of 5-HTT mRNA in the forebrain of the transgenic mice may be evidence of a low level of 5-HTT expression in atypical sites. Although the present study did not measure 5-HTT expression in peripheral tissues of the 5-HTT-overexpressing mice, a recent paper reported increased 5-HTT immunoreactivity and binding sites in the lungs of these animals (MacLean et al., 2004).
Neurochemical changes in the 5-HTT-overexpressing mice
The 5-HTT-overexpressing mice demonstrated a robust and specific neurochemical phenotype in terms of decreased extracellular 5-HT as well as decreased whole tissue levels of 5-HT in different brain regions. In in vivo microdialysis experiments, basal extracellular levels of 5-HT in mPFC and hippocampus in the transgenic mice were lower (50%) than wild types, and the 5-HT response to a depolarizing stimulus of high potassium was significantly reduced. Importantly, extracellular 5-HT in the transgenic mice increased toward wild-type levels in response to administration of the 5-HTT inhibitor paroxetine. This observation suggests that an increased 5-HTT activity contributes to the low extracellular levels of 5-HT observed in the transgenic mice.
Interestingly, the transgenic mice had low whole-tissue levels of 5-HT (−35 to 60% of wild types) across a range of brain regions examined. Therefore, it is likely that less 5-HT is available for release, and this may also contribute to the low extracellular levels that these animals demonstrate. The origin of the decreased 5-HT tissue content is uncertain because levels of both metabolism (5-HIAA) and 5-HT synthesis (5-HTP accumulation) were unchanged. However, because the major contributor to whole-tissue 5-HT levels is likely to be the vesicular storage compartment, the transgenic mice may have a 5-HT storage deficit. This notion would be consistent with studies showing that 5-HT storage depletion by inhibition of vesicular monoamine uptake (VMAT2) with reserpine results in reduction in both tissue and extracellular 5-HT levels in the brain, without changed 5-HT metabolism (Heslop and Curzon, 1994; Hatip-Al-Khatib et al., 2001). The 5-HTT-overexpressing mice do not show evidence of altered VMAT2 mRNA expression (our unpublished observation), but another possible source of a 5-HT storage deficit could be disrupted interactions between the 5-HTT and proteins involved in vesicle formation and utilization (Rothman, 1996; Bajjalieh, 2001; Kavalali, 2002; Quick, 2002, 2003).
Behavioral changes in the 5-HTT-overexpressing mice
Compared with wild-type mice, the 5-HTT-overexpressing mice showed evidence of decreased anxiety-like behavior in two animal models of anxiety. On the elevated plus maze test, the transgenic mice were quicker to enter the open arms and spent more time on these arms, and in the hyponeophagia test, the mice showed a decreased latency to eat. The findings in both models have been confirmed using a separate colony of animals (Figs. 6, 7, and our unpublished data). It is unlikely that reduced anxiety-like behavior has come about as a result of altered locomotion or feeding activity as the two tests place distinct sensorimotor and motivational demands on the animal. Moreover, in a battery of behavioral tests of exploratory function and motor coordination, as well as daily food and water consumption, the transgenic mice were not different from wild-type controls (see Results and our unpublished data).
In the plus maze test, administration of paroxetine increased anxiety levels of the transgenic mice such that the mice were not distinguishable from wild-type controls. Paroxetine was without significant effect on the plus maze performance of the wild-type mice. These results have two implications. First, the results suggest that the low-anxiety phenotype of the transgenic mice is driven by an ongoing overexpression of the 5-HTT. Although recent findings suggest that the changes in anxiety observed in 5-HT1A receptor null mutant mice are developmental in origin (Gross and Hen, 2004), this appears not to be the case for the changes in anxiety observed in the 5-HTT-overexpressing mice.
Second, the results with paroxetine suggest that the low-anxiety phenotype of the 5-HTT-overexpressing mice is linked to the decrease in presynaptic 5-HT function observed in the neurochemical experiments. This idea is strengthened by evidence from microdialysis experiments that paroxetine caused a rise in extracellular 5-HT in the transgenic mice. Moreover, there is much previous evidence associating drug-induced reductions in 5-HT function with decreased anxiety in a range of animal models (Griebel, 1995; Handley, 1995). In particular, 5-HT depletion by 5-HT synthesis inhibition or 5-HT lesions is reported to lower anxiety in the plus maze test (Briley et al., 1990; Soderpalm and Engel, 1990; Critchley et al., 1992).
Comparison of 5-HTT-overexpressing and 5-HTT knock-out mice
The neurochemical and behavioral phenotypes of the 5-HTT-overexpressing mice reported here are essentially opposite to those reported in 5-HTT null mutant mice. Thus, in microdialysis experiments, homozygous 5-HTT knock-out mice demonstrate raised extracellular 5-HT in various brain regions (Fabre et al., 2000; Shen et al., 2004), and in the elevated plus maze and hyponeophagia models, these animals exhibit high levels of anxiety (Holmes et al., 2003a,b; Lira et al., 2003).
These observations reinforce the argument that there is a causal relationship between altered 5-HT function and behavioral changes in both models. Moreover, these results suggest that variation in 5-HTT expression over a wide range may lead to proportionate changes in 5-HT function and anxiety-related behaviors. Thus, despite the many mechanisms that might compensate for the effects of 5-HTT gene variation, this single gene appears to be a strong determinant of anxiety-related behavior.
Curiously, as with 5-HTT knock-out mice (Kim et al., 2005), 5-HTT-overexpressing mice have reduced whole-tissue levels of 5-HT. It is plausible that this reflects a common underlying mechanism such as disruption in 5-HT storage mechanisms, possibly through altered interactions between the 5-HTT and proteins involved in vesicular formation and trafficking (Bengel et al., 1998; Kim et al., 2005).
In summary, 5-HTT-overexpressing mice demonstrate reduced presynaptic 5-HT function and low-anxiety-like behavior, and our data indicate that these neurochemical and behavioral phenotypes are linked. We propose that associations between increased 5-HTT expression and anxiety can be modeled in mice and may be specifically mediated by decreases in 5-HT transmission.
Footnotes
This work was supported by a European Community Integrated Network (NEWMOOD; LSHM-CT-2004-503474), an MRC Priority Area Research Studentship (K.A.J.), and the Oxford Ion Channel Group (R.M.J.D.).
References
- Bajjalieh S. SNAREs take the stage: a prime time to trigger neurotransmitter secretion. Trends Neurosci. 2001;24:678–680. doi: 10.1016/s0166-2236(00)01965-2. [DOI] [PubMed] [Google Scholar]
- Bengel D, Johren O, Andrews AM, Heils A, Mossner R, Sanvitto GL, Saavedra JM, Lesch KP, Murphy DL. Cellular localization and expression of the serotonin transporter in mouse brain. Brain Res. 1997;778:338–345. doi: 10.1016/s0006-8993(97)01080-9. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Briley M, Chopin P, Moret C. Effect of serotonergic lesion on “anxious” behaviour measured in the elevated plus-maze test in the rat. Psychopharmacology (Berl) 1990;101:187–189. doi: 10.1007/BF02244124. [DOI] [PubMed] [Google Scholar]
- Castro E, Tordera RM, Hughes ZA, Pei Q, Sharp T. Use of Arc expression as a molecular marker of increased postsynaptic 5-HT function after SSRI/5-HT1A receptor antagonist co-administration. J Neurochem. 2003;85:1480–1487. doi: 10.1046/j.1471-4159.2003.01782.x. [DOI] [PubMed] [Google Scholar]
- Cohen-Tannoudji M, Babinet C, Morello D. lacZ and ubiquitously expressed genes: should divorce be pronounced? Transgenic Res. 2000;9:233–235. doi: 10.1023/a:1008916910392. [DOI] [PubMed] [Google Scholar]
- Critchley MA, Njung'e K, Handley SL. Actions and some interactions of 5-HT1A ligands in the elevated X-maze and effects of dorsal raphe lesions. Psychopharmacology (Berl) 1992;106:484–490. doi: 10.1007/BF02244819. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Croucher A, Rawlins JN. Hippocampal cytotoxic lesion effects on species-typical behaviours in mice. Behav Brain Res. 2002;132:203–213. doi: 10.1016/s0166-4328(01)00401-6. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Kuyatt BL. Autoradiographic localization of 3H-paroxetine-labeled serotonin uptake sites in rat brain. Synapse. 1987;1:488–496. doi: 10.1002/syn.890010513. [DOI] [PubMed] [Google Scholar]
- Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, Murphy DL, Lanfumey L, Hamon M, Martres MP. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic; 1997. [Google Scholar]
- Gartside SE, Umbers V, Hajos M, Sharp T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet. 1999;88:83–87. [PubMed] [Google Scholar]
- Greenberg BD, Li Q, Lucas FR, Hu S, Sirota LA, Benjamin J, Lesch KP, Hamer D, Murphy DL. Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population sample. Am J Med Genet. 2000;96:202–216. doi: 10.1002/(sici)1096-8628(20000403)96:2<202::aid-ajmg16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Griebel G. 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol Ther. 1995;65:319–395. doi: 10.1016/0163-7258(95)98597-j. [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- Handley SL. 5-Hydroxytryptamine pathways in anxiety and its treatment. Pharmacol Ther. 1995;66:103–148. doi: 10.1016/0163-7258(95)00004-z. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Himle JA, Curtis GC, Koram DQ, Veenstra-VanderWeele J, Leventhal BL, Cook EH., Jr Serotonin transporter and seasonal variation in blood serotonin in families with obsessive-compulsive disorder. Neuropsychopharmacology. 1998;18:102–111. doi: 10.1016/S0893-133X(97)00097-3. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hatip-Al-Khatib I, Mishima K, Iwasaki K, Fujiwara M. Microdialysates of amines and metabolites from core nucleus accumbens of freely moving rats are altered by dizocilpine. Brain Res. 2001;902:108–118. doi: 10.1016/s0006-8993(01)02382-4. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Hernandez I, Sokolov BP. Abnormal expression of serotonin transporter mRNA in the frontal and temporal cortex of schizophrenics. Mol Psychiatry. 1997;2:57–64. doi: 10.1038/sj.mp.4000215. [DOI] [PubMed] [Google Scholar]
- Heslop KE, Curzon G. Depletion and repletion of cortical tissue and dialysate 5-HT after reserpine. Neuropharmacology. 1994;33:567–573. doi: 10.1016/0028-3908(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003a;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003b;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Jennings KA, Sheward WJ, Harmar AJ, Sharp T. In vivo evidence that mice overexpressing the 5-HT transporter have decreased cortical 5-HT release. Proceedings of the 10th International Conference in In Vivo Methods; Stockholm. 2003. Jun, [Google Scholar]
- Kavalali ET. SNARE interactions in membrane trafficking: a perspective from mammalian central synapses. BioEssays. 2002;24:926–936. doi: 10.1002/bies.10165. [DOI] [PubMed] [Google Scholar]
- Kilic F, Murphy DL, Rudnick G. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol Pharmacol. 2003;64:440–446. doi: 10.1124/mol.64.2.440. [DOI] [PubMed] [Google Scholar]
- Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Le Marec N, Hebert C, Amdiss F, Botez MI, Reader TA. Regional distribution of 5-HT transporters in the brain of wild type and “Purkinje cell degeneration” mutant mice: a quantitative autoradiographic study with [3H]citalopram. J Chem Neuroanat. 1998;15:155–171. doi: 10.1016/s0891-0618(98)00041-6. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gutknecht L. Pharmacogenetics of the serotonin transporter. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1062–1073. doi: 10.1016/j.pnpbp.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Aulakh CS, Wolozin BL, Tolliver TJ, Hill JL, Murphy DL. Regional brain expression of serotonin transporter mRNA and its regulation by reuptake inhibiting antidepressants. Brain Res Mol Brain Res. 1993;17:31–35. doi: 10.1016/0169-328x(93)90069-2. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- Loder MK, Shen S, Wren PB, Pei Q, Olverman HJ, Sharp T, Harmar AJ. The production and analysis of transgenic mice over-expressing the human serotonin transporter. Soc Neurosci Abstr. 2000;26:146.6. [Google Scholar]
- MacLean MR, Deuchar GA, Hicks MN, Morecroft I, Shen S, Sheward J, Colston J, Loughlin L, Nilsen M, Dempsie Y, Harmar A. Overexpression of the 5-hydroxytryptamine transporter gene: effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation. 2004;109:2150–2155. doi: 10.1161/01.CIR.0000127375.56172.92. [DOI] [PubMed] [Google Scholar]
- Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–1098. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- Marston HM, Reid ME, Lawrence JA, Olverman HJ, Butcher SP. Behavioural analysis of the acute and chronic effects of MDMA treatment in the rat. Psychopharmacology (Berl) 1999;144:67–76. doi: 10.1007/s002130050978. [DOI] [PubMed] [Google Scholar]
- Ogilvie AD, Battersby S, Bubb VJ, Fink G, Harmar AJ, Goodwim GM, Smith CA. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet. 1996;347:731–733. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:895:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Pei Q, Zetterstrom TS, Sprakes M, Tordera R, Sharp T. Antidepressant drug treatment induces Arc gene expression in the rat brain. Neuroscience. 2003;121:975–982. doi: 10.1016/s0306-4522(03)00504-9. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J. Ultrastructural localization of the serotonin transporter in limbic and motor compartments of the nucleus accumbens. J Neurosci. 1999;19:7356–7366. doi: 10.1523/JNEUROSCI.19-17-07356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW. Role of syntaxin 1A on serotonin transporter expression in developing thalamocortical neurons. Int J Dev Neurosci. 2002;20:219–224. doi: 10.1016/s0736-5748(02)00021-7. [DOI] [PubMed] [Google Scholar]
- Quick MW. Regulating the conducting states of a mammalian serotonin transporter. Neuron. 2003;40:537–549. doi: 10.1016/s0896-6273(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci USA. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. The protein machinery of vesicle budding and fusion. Protein Sci. 1996;5:185–194. doi: 10.1002/pro.5560050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl A, Grimes B, Montoliu L. YAC transfer by microinjection. Methods Mol Biol. 1996;54:293–306. doi: 10.1385/0-89603-313-9:293. [DOI] [PubMed] [Google Scholar]
- Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Shen S, Battersby S, Weaver M, Clark E, Stephens K, Harmar AJ. Refined mapping of the human serotonin transporter (SLC6A4) gene within 17q11 adjacent to the CPD and NF1 genes. Eur J Hum Genet. 2000a;8:75–78. doi: 10.1038/sj.ejhg.5200400. [DOI] [PubMed] [Google Scholar]
- Shen S, Spratt C, Sheward WJ, Kallo I, West K, Morrison CF, Coen CW, Marston HM, Harmar AJ. Overexpression of the human VPAC2 receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc Natl Acad Sci USA. 2000b;97:11575–11580. doi: 10.1073/pnas.97.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm B, Engel JA. Serotonergic involvement in conflict behaviour. Eur Neuropsychopharmacol. 1990;1:7–13. doi: 10.1016/0924-977x(90)90004-t. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang L, Johnston NL, Torrey EF, Yolken RH. Serial analysis of gene expression in the frontal cortex of patients with bipolar disorder. Br J Psychiatry Suppl. 2001;41:s137–s141. doi: 10.1192/bjp.178.41.s137. [DOI] [PubMed] [Google Scholar]