Abstract
The hippocampus is necessary for declarative memory in humans and episodic memory in rodents. Considerable current research is focused on the role of plasticity within specific subfields of the hippocampus. Here, using a viral vector to temporally control a focal deletion of the NR1 gene, we show that learning novel paired associations between specific cues and their context is dependent on CA3 NMDA receptors. Deletion of CA3 NR1 genes in <30% of the dorsal hippocampus was sufficient to disrupt new learning, whereas the same treatment does not prevent expression of previously acquired paired associates and does not affect the ability to discriminate contexts or paired associate learning when either the cues or the context is familiar. The findings suggest that CA3 NMDA receptors specifically support the encoding of new experiences to involve incidental and contingent associations.
Keywords: learning, memory, NR1, adeno-associated virus, conditional, knock-out, declarative memory disorder model
Introduction
Recent studies have indicated that CA3 neuronal ensembles demonstrate pattern separation and pattern completion of complex cue combinations (Lee et al., 2004; Leutgeb et al., 2004) and are required for pattern separation of spatial cues (Gilbert and Kesner, 2003). Correspondingly, NMDA receptors (NMDARs) in CA3 are requisite for pattern completion in a situation in which sensory cues are degraded (Nakazawa et al., 2002; Gold and Kesner, 2005). A central question remains open as to whether hippocampal CA3 NMDARs also play a special role in pattern separation when learning requires distinguishing complex environmental contingencies.
We used a viral vector technique (Scammell et al., 2003) to induce a temporally and anatomically controlled targeted gene deletion of the NR1 gene, essential to NMDAR function. This technique allowed an examination of learned response after a targeted gene deletion that was acquired under control conditions. As with other knock-out (KO) techniques, acquisition may also be examined after the gene deletion; however, we were able to additionally compare acquisition before and after targeted gene deletion.
Our study used a paired associate learning task in combination with the focal gene deletion method to examine the role of CA3 NR1 genes in the acquisition of contingent associations. The elements of these associations consisted of a pair of environmental contexts and a pair of proximal olfactory cues. The same pair of olfactory cues was used in both contexts; however, the reward assignment of each cue was contingent on the context. We examined learning under conditions of varying previous experience with the cues and contextual elements. We observed that CA3 NR1 genes were essential for acquisition of the paired associate task only when both elements (the contexts and the olfactory cues) were unfamiliar.
Materials and Methods
CA3 NMDA receptor knock-out
Transgenic “knock-in” mice with two loxP sites flanking exons 11–22 of the NR1 gene were made as described by Tsien et al. (1996), and a breeding pair was given to us from the Tonegawa laboratory. We have developed PCR-based genotyping using primer sequences kindly provided by T. Iwasato (RIKEN, Saitama, Japan), who also gave us the clone used to make the probe used for in situ hybridization (ISH) of NR1 message needed to localize the extent of the knock-out.
We used a reliable method of adeno-associated virus (AAV)–Cre delivery, using stereotaxic localization of a glass micropipette containing the AAV–Cre (Scammell et al., 2003). The AAV–Cre and AAV–β-galactosidase gene (LacZ) were obtained from the Gene Therapy Initiative, Harvard Institute of Human Genetics (Boston, MA; Director, Dr. Richard Mulligan; Associate Director, Dr. Jeng-Shin Lee). Timed pulses of high pressure, applied to the pipette, delivered a well controlled volume (0.8 μl) over ∼20 min, and then the tip was left in place for another 20 min. Decreasing the time of injection from 1 h (Scammell et al., 2003) to 20 min has resulted in less spread of the AAV–Cre to ∼0.5–1 mm rostrodorsally and to ∼20% laterally in the dorsal hippocampus. This has limited AAV–Cre expression to 10–30% of total CA3 in dorsal hippocampus and avoided the spread to either the CA1 field or the dentate, as is shown in Figure 2.
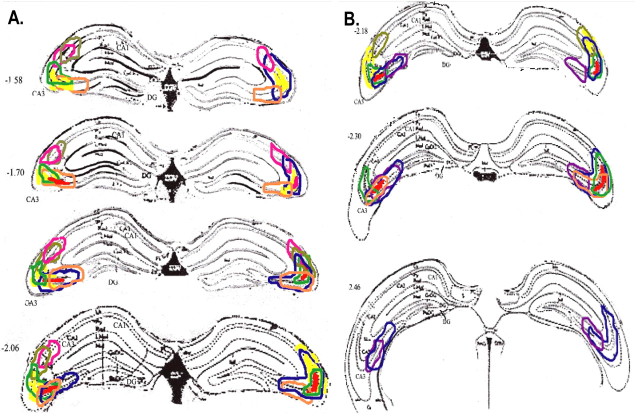
Figure 2.
The extent of the NR1 gene deletions are shown for each animal (1 color per animal with bilateral injections of AAV–Cre) with line drawings of horizontal sections through the hippocampus from anteroposterior (AP) –1.58 to AP –2.06 (A) and from AP –2.18 to 2.06 (B). The color-coded lines indicate the well demarcated extent of the loss of NR1 message using an in situ radiolabeled NR1-specific RNA probe. DG, Dentate gyrus.
For every injected animal, the limit of the region that sustained loss of the NR1 gene was verified by in situ autoradiography for NR1 message. The extent of the molecular lesion was estimated using the NR1 signal in CA3 by examination of every third 14-μm-thick section for loss of message, throughout the hippocampus (and elsewhere, but it was diminished only within the CA3 field in every case; neither CA1 nor the dentate showed any loss of signal). The loss of signal was clearly demarcated (densitometry measures showed >85% loss of message in all cases compared with AAV–lacZ-injected mice). In all cases, the knock-out was restricted to the dorsal hippocampus. The length of the CA3 cell layer missing NR1 signal divided by the total length of the pyramidal cell layer of the CA3 region (from the same number of sections comprising the dorsal hippocampus) was used to estimate the percentage of the area lesioned.
Previous studies using astroglial and microglial markers indicate that that there is no inflammatory response induced by AAV or expression of AAV constructs (Scammell et al., 2003). Nevertheless, we additionally used a positive control of AAV–LacZ in genetically identical animals.
Immunocytochemistry for Cre and β-galactosidase
After the last phase of behavioral manipulation, mice were anesthetized deeply with Avertin (0.0165–0.0175 ml/g, i.p.) and perfused with saline followed by 4% paraformaldehyde. Brains were postfixed for 16 h at 4°C, placed in 20% glycerol/1× PBS until they sank, and then sectioned at 30 μm into 1× PBS plus 0.01% NaAzide and stored at 4°C, alternating with 14 μm sections for in situ hybridization. Immunohistochemistry (IH) was performed on slide-mounted sections. Primary antibodies included rabbit anti-Cre (1:1000; Novagen, Madison, WI) and rabbit anti-β-galactosidase (1:10,000; 3′5′; Novagen). After blocking for 60–90 min in 3% normal goat serum (NGS), 0.3% Triton X-100 in 1× PBS, slides were incubated with primary antibodies in 3% NGS and 0.3% Triton X-100 in 1× PBS for 12–20 h at room temperature (room temperature). Sections were then washed in 1× PBS and incubated in secondary antibody (1: 200) in 1× PBS for 2–4 h at room temperature. Slides were then washed and dehydrated in rapid ethanol series (50, 75, 90, 100, 100%; 10 s each; twice for 3 min in Citrosolve). Then, they were mounted in a mixture of distyrene, tricresyl phosphate, and xylene.
In situ hybridization for NR1
To determine the loss of NMDA-NR1 message at the site of AAV–Cre injection, we performed NR1 in situ hybridization on 14 μm brain sections. The in situ hybridization protocol is based on that described by Iwasato et al. (1997). Antisense and sense probes were made from pSP72–ASp clone using SP6 and T7 RNA polymerases and EcoRI and HindIII restriction endonucleases, respectively. pSP72–ASp was generated by inserting AvrII–SphI 0.4 kb (probe-R) of NR1 cDNA into XbaI–SphI sites of pSP72 (Promega, Madison, WI). Probe-R detected all splice variants of the NR1 gene. Hybridization was done at 60°C overnight in hybridization buffer with 1.0 × 106 cpm/μl of 35S-labeled RNA probe. Sections were washed in a series of SSC/DTT buffers after RNase A treatment. The final wash was done in 0.1× SSC, 1 mm DTT at 60°C for 60 min. After dehydration in ethanol, we exposed the sections to an x-ray film for 4–7 d.
For quantification, we used NIH Image 1.6 comparing the mean optical densities of a knock-out region to those of a noninjected region (e.g., a comparable nontransfected part of CA3).
Animals behavioral manipulations
Mice (12–18 weeks of age) were housed in a pathogen-free barrier facility maintained at 21.5–22.5°C with lights on at 7:00 A.M. and off at 7:00 P.M. Mice had food and water available ad libitum except on days of behavioral manipulation. On these days, mice were food deprived for 8 h before testing, from 12:00 A.M. to 8:00 A.M. The Institutional Animal Care and Use Committees approved the protocols.
Behavioral apparatus. All tests were conducted in a standard (30 × 19 cm) clear Plexiglas cages, modified to be distinct contexts. A context refers to the total representation of environmental elements such as a specific combination of different shaded pieces of paper cut in different geometric forms and attached on the walls of the plastic cage from the outside, specific flooring texture, specific location in the behavioral room, and specific amount of light. During all the behavioral manipulations, two opaque plastic cups were located at one end of the cage. These cups were filled with sterilized playground sand, and one of them has a small piece of chocolate (∼15 mg of Hershey's Huggs; The Hershey CompanyHershey, PA) hidden at the bottom of the cup. Depending on the context, the cups were scented with two different odors. The odor stimuli were chosen from the following common spices and mixed with the sand in a 1% concentration by weight: cinnamon, paprika, cumin, sage, apple pie, and ginger. The position of each scented cup was randomly assigned to right or left in counterbalanced design.
Shaping phase. All mice underwent a shaping phase before behavioral manipulation. On the first day of shaping, each fasting mouse was exposed in a cage to two nonscented sand-filled cups. One of the cups was baited with chocolate. On first day of shaping, chocolate pieces were dispersed throughout the sand and on the surface of the baited cup. Mice were allowed a maximum of 1 h to dig in the sand and retrieve all the treats. This step was repeated three times on the first day. On the second day, the same procedure was repeated three times, except that this time, only hidden multiple treats were presented (i.e., none on the surface). On day 3, each mouse underwent the same procedures as day 2, except that there was only one hidden treat in the sand of one cup instead of multiple treats. Days 4 and 5 were similar to day 3, except that each mouse was given only 8 min to retrieve the treat. After 8 min, if the mouse did not dig, a treat was presented on the surface of the unscented sand. If on day 5 the mouse was not digging consistently on all three trials within 8 min, the animal was excluded from the study.
Acquisition of a paired associate learning task before injections. Each mouse was exposed to one set of odor/context reward assignments, and subjects and controls were counterbalanced with respect to the sets. Each set consisted of two contexts (CX1 and CX2) and two cups filled with two different scents: “A” and “B.” In CX1, the cup with A-scented sand was baited with chocolate, whereas in CX2, the B-scented cup was baited. The placement of the cups with respect to each other was pseudorandom so that in each environment, a cup with a particular odor had a 50% chance of being to the left or right. Importantly, both cups were presented simultaneously in each context. Each trial started by placing the mouse in CX1 or CX2 and ended when the mouse retrieved the treat. The contexts consisted of two distinct environments composed of modified Plexiglas cages placed in different locations in the experimental room. Accordingly, the animals could have four different experiences for CX1 and CX2 and for scents A and B as follows: (1) if CX1, then A is rewarded; (2) if CX1, then B is not rewarded; (3) if CX2, then A is not rewarded; and (4) if CX2, then B is rewarded (Preston et al., 1986).
For the acquisition phase, each mouse underwent eight trials per day for 5 (or 7) days, four in each context, following a pseudorandom order constrained by not having more than two trials of the same type, consecutively, and counterbalanced for right–left position of the baited cup. The intertrial interval was 15–20 min. Note that if the mouse started digging first in the nonbaited cup, it was allowed to correct itself. The response was considered correct or incorrect if the mouse started digging in the baited cup or the nonbaited cup, respectively.
Responses were scored by observers blind to subject assignments as correct when the mouse dug in the rewarded cup first. After acquisition of the task, we determined that choices were unaffected by the absence of the reward on probe trials, showing that the mouse was not directly detecting the presence of the reward buried in the cup.
Retention of paired associate performance after injections. Ten days after the injections, all mice underwent testing for 5 d in the same set of contexts and scents on which they originally trained. Performance was assessed following the same protocol as in acquisition.
Acquisition of a novel paired associates problem after injections. This phase was similar to acquisition before injections, except that another set of contexts and scents was used for each mouse.
Acquisition of a context discrimination task. A pair of contexts (CX1, CX2) and one odor for each context pair was used in a counterbalanced design for subjects and controls. Injected mice were trained on one pair of dissimilar nonoverlapping contexts (e.g., CX1, CX2) and one odor. Each trial started by placing the mouse in CX1 or CX2 for 2 min and then presented with the scented cup. The trial ended when the mouse retrieved the treat. Each mouse underwent eight trials per day for 5 d, four in each context, following a randomly interleaved order. The intertrial interval was 15–20 min. The latencies to approach and start digging in the single cup in either context were measured. Then, for each mouse, a ratio of the mean latency to dig in the rewarded context over the mean latency to dig in the nonrewarded context was generated. Thus, as a subject learned to discriminate between the two contexts, the latency to dig in the rewarded context decreased, whereas the latency to dig in the nonrewarded context increased, resulting in a decrease in the ratio from chance value of 1.0. Each mouse was given a maximum of 5 min in each context to dig, and the trial was terminated if the mouse did not dig within these 5 min.
Electrophysiological recordings
Slice electrophysiology. Coronal hippocampal slices (∼350 μm thick) were prepared from 12- to 18-week-old male homozygous floxed-NR1 (fNR1) uninjected (control) mice or injected (CA3 KO) mice. Animals were anesthetized with isoflurane and decapitated. The brains were rapidly removed and placed into an ice-cold, oxygenated (95% O2/5% CO2) sucrose Ringer's solution, pH 7.4, containing the following (in mm): 125 sucrose, 3 KCl, 1.2 KH2PO4, 2 MgSO4, 26 NaHCO3, 10 glucose, and 2 CaCl2. The brain was divided along the midline for preparation of hippocampal brain slices. Individual slices were then placed in a holding chamber at room temperature containing oxygenated Ringer's solution with 126 mm NaCl in place of the sucrose, pH 7.37–7.41. The osmolality of the Ringer's solution was 295–305 mOsm. Sections remained in the holding chamber for at least 1 h before recording.
Patch-clamp recordings. Slices were transferred into the recording chamber, which was perfused with fresh, oxygenated, Ringer's solution at room temperature via a gravity-feed system at ∼4 ml/min. The Ringer's solution for all electrophysiological experiments contained 50 μm picrotoxin to block GABAA receptors. The whole-cell patch-clamp technique was used to record from single pyramidal neurons using a MultiClamp Commander 700A (Molecular Devices, Union City, CA). Microelectrodes with a resistance of 5–7 MΩ were pulled using a P-87 micropipette puller (Sutter Instruments, Novato, CA). The internal solution in the borosilicate glass microelectrodes consisted of the following (in mm): 130 K gluconate, 10 KCl, 10 HEPES, 3 MgCl2,5 N-ethyl bromide quaternary salt, 2 ATP magnesium salt, and 1 GTP sodium salt, osmolality 280–290 mOsm, pH 7.3 with KOH. Synaptic responses were evoked in either CA3 or CA1 with a 0.1 ms current pulse to generate a reliable EPSC at low frequency (0.05 Hz) delivered to the CA3–collateral association (CA3–C/A) pathway (stimulating electrode placed in the CA3 stratum radiatum) or the CA1–Schaffer collateral (CA1–SC) pathway (stimulating electrode placed in the stratum radiatum). For long-term potentiation (LTP) experiments, induction occurred by administration of three, 1 s trains (100 pulses at 100 Hz paired with a 1 s depolarizing pulse of 200–500 pA; one train per 20 s) administered to CA3–C/A or CA1–SC synaptic pathways. For pharmacological isolation of NMDAR EPSCs, cells were voltage-clamped to –60 mV, and the slices were superfused with Ringer's containing 0 mm Mg2+ and 10 μm 2,3-dihydroxy-6-nitro-7-sulfonyl-benzo[f]quinoxaline (NBQX). NMDAR EPSCs were characterized by their slow time course, insensitivity to NBQX, and sensitivity to APV.
Results
Transfection-induced focal NR1 gene knock-out within CA3 field
To assess the role of hippocampal CA3 NMDARs in learning paired association discrimination, we used a Cre–loxP system together with an adeno-associated viral vector for Cre recombinase (AAV–Cre) to temporally and anatomically limit the deletion of the NR1 gene in homozygous fNR1 mice. AAV–Cre was stereotaxically microinjected (∼0.8 μl of AAV; titer of 108–10), bilaterally, into the dorsal CA3 of adult, male fNR1 mice (Cre mice) using previously described and verified procedures (Scammell et al., 2003). Controls were microinjected with AAV–lacZ (LacZ mice). A set of IH and ISH studies verified the localization of injections to within 10–30% of the total dorsal hippocampal CA3. Quantitative densitometry showed a 85–100% loss of message (average, ∼92% loss for all AAV–Cre injections) in the well demarcated transfected region of the CA3 (Figs. 1, 2). To evaluate the functional phenotype for the knock-out, we analyzed wholecell patch-clamp recordings from pyramidal neurons in Cre and noninjected fNR1 mice. There was a loss of NMDAR-dependent currents and LTP at the CA3–commissural/associational synapse in CA3-injected areas but robust currents and LTP at the CA3–C/A synapse in noninjected area as well as at CA1–SC synapses in CA3-injected and noninjected slices (Fig. 3).
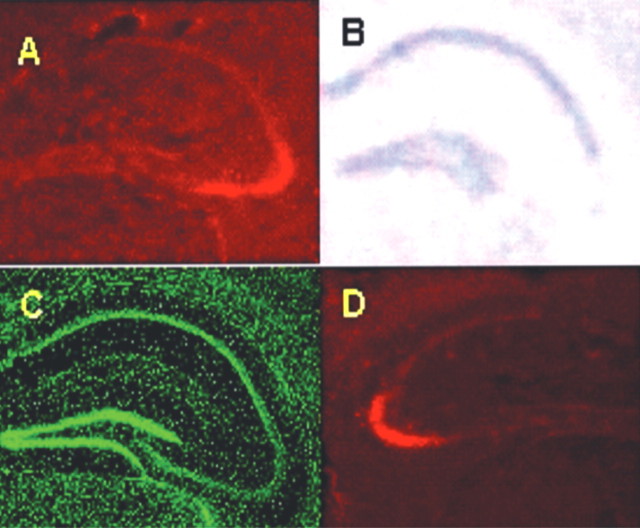
Figure 1.
AAV–Cre and AAV–LacZ microinjections localized to CA3. A, Cre IH shows the expression of Cre in the hippocampus limited to the CA3 region in Cre mice. B, On an adjacent section, ISH for NR1 shows an absent signal in the CA3 region corresponding to the Cre expression in A. C, The section in B is counterstained with PicoGreen stain, which reveals no evidence of tissue damage secondary to the injection. D, β-Galactosidase IH is also localized within CA3 regions in LacZ mice.
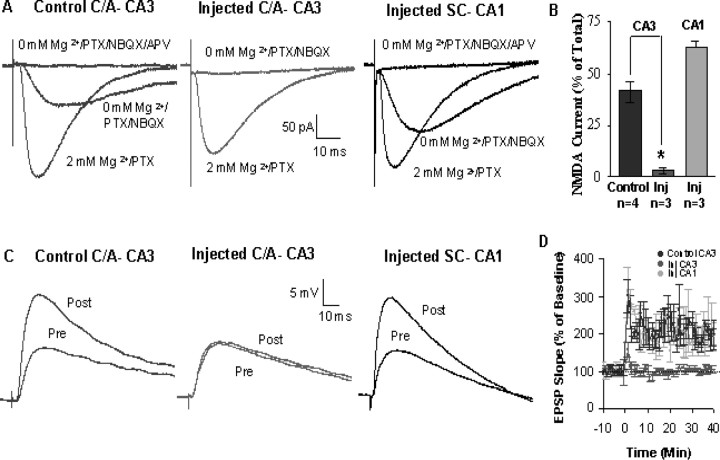
Figure 3.
Functionally absent NMDARs at CA3–C/A synapses in CA3 AAV–Cre-injected slices. A, Pharmacologically isolated NMDAR EPSCs (isolated with NBQX and blocked with APV) were evoked by stimulation. Representative averaged (15 consecutive responses recorded at 0.05 Hz) current traces are shown as follows: left, collateral fiber-evoked NMDAR EPSC from a control mouse (noninjected fNR1); middle, absence of C/A-evoked NMDAR EPSC from an AAV–Cre (injected into CA3) fNR1 mouse; right, a Schaffer collateral-evoked NMDAR EPSC recorded from the same mouse as shown in the middle trace. PTX, Picrotoxin. B, Summary bar graphs show synaptic plasticity induced by high-frequency stimulation (3, 1 s trains of 100 pulses at 100 Hz paired with a 1 s depolarizing pulse every 20 s). Inj, Injected. C, Representative voltage traces show that CA3–collateral fiber LTP (indicated by the post-tetanic increased amplitude of the EPSP) is selectively absent from AAV–Cre-injected mice. Pre, Before injection; Post, after injection. D, Summary data for LTP of EPSPs in noninjected fNR1 (CA3) and Cre mice (CA3 and CA1). *p < 0.01.
Expression of learned paired associations
To examine the effect of the NR1 gene deletion, localized to the dorsal hippocampal CA3 subfield as described above, we used a paired associate learning task (see Materials and Methods). Each day, the mice experienced four trials with one scent rewarded and the other scent unrewarded when the odors were presented in one context and four other trials (eight in total) with opposite reward assignments when the odors were presented in the other context. With an intact hippocampus, we think that each context–cue–reward association is encoded as a distinct experience. If this kind of encoding is disrupted, then the mouse is faced with the more difficult task of dealing with variable reward predictability of the cues and contexts.
Before the AAV injections, mice (AAV–Cre group, n = 7; AAV–LacZ group, n = 6) were trained for 5 d on the paired associates problem; all mice reached a level of >75% correct of eight trials by day 4, and this level was maintained on retesting with eight trials on day 5 [Cre: F(4,34) = 4.31, p < 0.008, Bonferroni's post-tests (day 1 vs day 5), p < 0.05; LacZ: F(4,29) = 11.46, p < 0.0001, Bonferroni's post-tests (day 1 vs day 5), p < 0.001]. The two groups significantly differed in overall performance (4.44% of the variance; F(1,55) = 5.12; p < 0.03), but this was most likely because of the low performance of LacZ group on day 1, because the Bonferroni's post-tests for day-to-day comparisons showed no significant differences.
After initial training, mice were injected with either AAV–Cre or AAV–LacZ bilaterally in the CA3 field of the hippocampus. After 10 d, all animals were retested for expression of the learned responses (Fig. 4B). There was no difference in performance between the two groups (F(1,55) = 0.26; p = 0.614), and both groups showed no additional learning after injections (Cre: F(4,34) = 0.64, p = 0.638; LacZ: F(4,29) = 0.41, p = 0.797). These findings show that intact CA3 NMDARs are not required for perceptual, motor, motivational, or other nonmemory aspects of the task or to express learning previously acquired paired associates, consistent with other studies showing intact working memory in spatial delayed nonmatch to place (Steele and Morris, 1999; Lee and Kesner, 2002) and intact spatial navigation performance (Nakazawa et al., 2002).
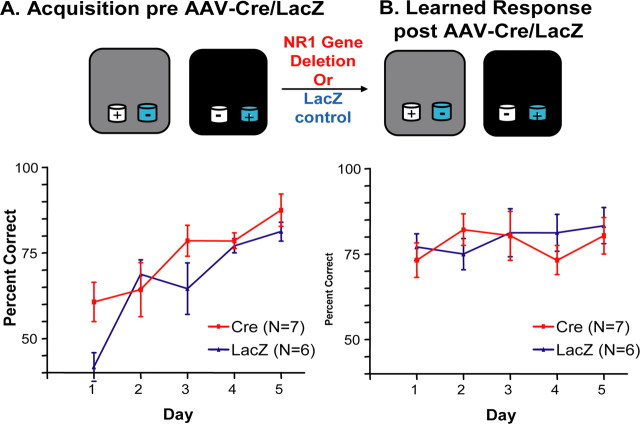
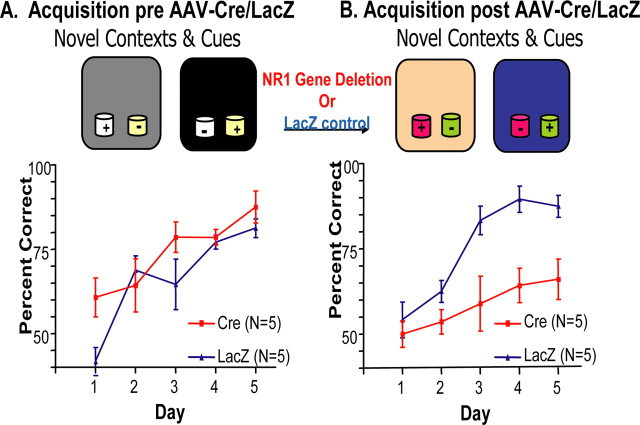
Figure 4.
Intact CA3 NMDA receptors are not required for expression of a previously learned paired associate learning problem. A, Training on the problem involved two different contexts (represented by gray and black) and two different odors (represented by white and blue). As expected, before (pre) application of AAV–Cre (red squares) or AAV–LacZ (blue triangles), the two groups showed significant learning but were not different from one another. B, Ten days after the AAV–Cre/LacZ application (post), both groups showed complete learning retention.
Paired associate learning of novel elements
We next tested the need for CA3 NMDARs in learning new associations between novel cues and contexts using the same two groups of mice.
The mice with reduced NR1 CA3 genes could not acquire the new problem in 5 d in contrast to the mice treated with AAV–LacZ [Cre: F(4,34) = 1.53, p = 0.218; LacZ: F(4,29) = 15.91, p < 0.0001; Bonferroni's post-tests (day 1 vs day 5), p < 0.001]. The AAV–LacZ mice could not be distinguished from acquisition before injection and were superior to CA3 NR1–KO mice (21.92% of the variance; F(1,55) = 28.49; p < 0.0001). Bonferroni's post hoc comparisons revealed significant differences in performance on day 3 (p < 0.01), day 4 (p < 0.01), and day 5 (p < 0.05) (Fig. 5A,B). Thus, learning paired associations between novel contexts and cues required at least 70–90% of CA3 NMDA receptors.
Figure 5.
Intact NMDARs are needed for acquisition of paired associations composed of novel contexts and odor cues. Mice were trained for 5 d before AAV–Cre/LacZ was applied (pre; A), and then they were retrained for 5 d on a new problem using novel contexts and odors (post; B). Mice were trained with eight trials per day (4 trials with each context), and the average percentage of correct choices (x-axis) for each day (y-axis) is plotted before (left plot) and after (right plot) focal, dorsal, CA3 hippocampal deletion of the NR1 gene.
Context discrimination
Although the previous experiment shows hippocampal involvement in the formation of contingent associations, a number of studies indicate that destruction of the majority of hippocampal neurons does not prevent acquisition of some nonlinear conditional tasks or of tasks that are likely to involve conjunctive representations (for review, see O'Reilly and Rudy, 2001). Nevertheless, NMDARs in the hippocampus are necessary for learning that is likely to involve conjunctive representations such as spatial learning (Steele and Morris, 1999; Lee and Kesner, 2002) and contextually conditioned fear responses (Young et al., 1994).
The focal NR1 CA3 gene deletion may have prevented a sufficiently useful contextual representation to allow for a paired association of the olfactory cue and context needed for acquisition of our paired associate task. This possibility was tested in two ways. First, we examined the ability of these mice to discriminate two contexts without a contingently paired cue (Fig. 6A).
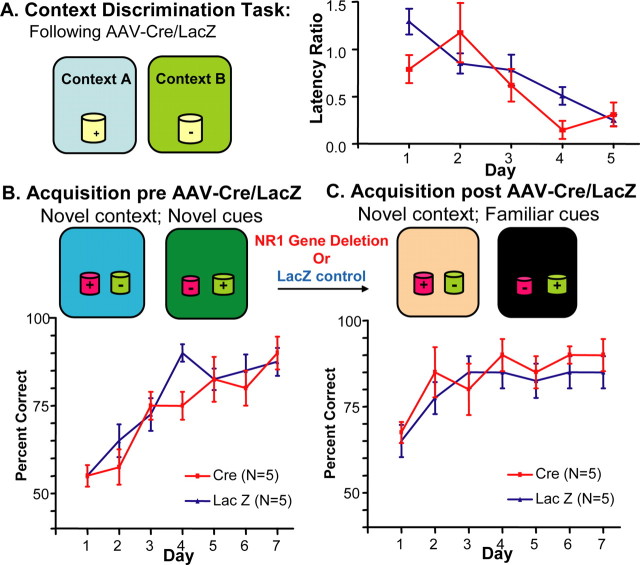
Figure 6.
Neither the discrimination of contexts nor the contingent use of novel contexts to discriminate familiar olfactory cues requires intact CA3 NMDARs. A, On the left, the context discrimination task is illustrated, and on the right, grouped data comparing acquisition (assessed by the ratio of the time to approach the rewarded to the time to approach the unrewarded cup) over 5 d of training after application of AAV–Cre (red squares) or AAV–LacZ (blue triangles) bilaterally to the dorsal CA3 subfield. B, Before application of AAV (pre), mice were randomly assigned to two groups and trained on the paired associate learning task. Pooled data for learning, as assessed by percentage correct choices, are shown for 7 d of training. C, Ten days after either AAV–Cre or AAV–LacZ application (post), pooled data for learning the task, using novel contexts but the same two olfactory cues as before application, are shown for 7 d of training. The group receiving the AAV–Cre does not differ from the group receiving AAV–LacZ with learning either task.
In one environment, a single scented cup was rewarded and, in the other, the same cup was not was not rewarded. The latency to approach the cup in each of the two environments was measured, and the ratio of the latency to dig in the rewarded cup, to the latency to dig in the unrewarded cup, was obtained. The same interleaved training schedule was used.
Both the AAV–Cre-(n = 6) and AAV–LacZ-(n = 6) injected groups showed a significant decrease in the latency ratios [Cre: F(4,25) = 4.657, p < 0.006, Bonferroni's post-tests (day 2 vs day 4 or 5), p < 0.05; LacZ: F(4,25) = 11.00, p < 0.0001, Bonferroni's post-tests (day 1 vs day 4 or 5), p < 0.0001]. Performance between the two groups did not differ overall (F(1,50) = 1.625; p = 0.208) or on day-to-day comparisons (Bonferroni's post-tests, p > 0.05). Thus, the disruption of NR1 CA3 genes did not disrupt the ability to distinguish contexts or to assign them differential reward values.
Paired associate learning with familiar and novel elements
We also examined whether previous familiarity with the olfactory cues influenced the extent to which CA3 NMDARs are needed to learn a paired associate problem. We first trained mice, randomly assigned to two groups, on a novel paired associate task (n = 5 for each group). Then, 10 d after application of AAV–Cre to one group and AAV–LacZ to the other, both groups were trained on a new paired associate problem, composed of novel contexts and the same olfactory cues that they trained with before injection. The AAV–Cre group and the AAV–LacZ groups performed similarly (F(1,56) = 1.46; p = 0.23) (Fig. 6B,C), such that both groups scored >75% correct starting on day 2. Both groups showed no additional significant learning attributable to the high level of performance shown on the first day (LacZ: F(6,34) = 2.49, p = 0.05; Cre: F(6,34) = 2.43, p = 0.05) (Fig. 6C). A direct comparison of the performance on first 2 d on the problem learned before versus after treatment indicated a significant improvement in learning with familiar olfactory cues for the AAV–Cre group (p < 0.004 for two-way ANOVA group effect). A similar comparison in the AAV–LacZ group showed a trend toward improved learning (p = 0.052). Thus, the AAV–Cre-induced disruption of NR1 CA3 genes did not have a negative impact on the paired associate learning of novel contexts and familiar olfactory cues (Fig. 6C).
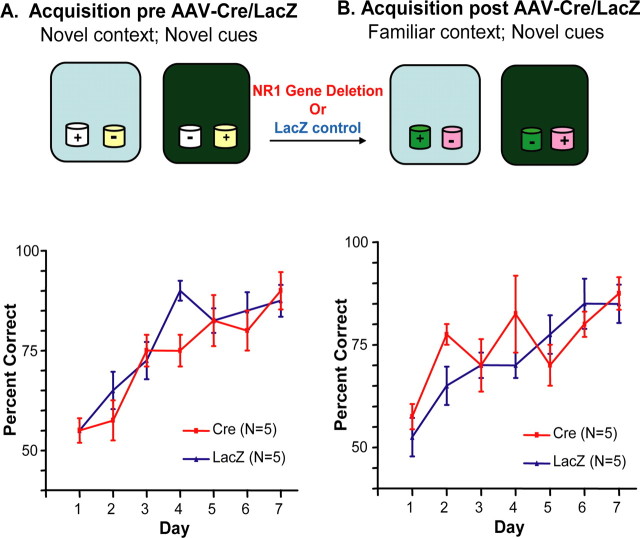
We also examined whether NMDARs are needed to learn paired associates that involve familiar contexts and novel olfactory cues. This was tested with a protocol similar to that used for novel contexts and familiar cues, except that after the injections of AAV–Cre or AAV–lacZ, the contexts that had been experienced previously were used together with novel olfactory cues.
Both the AAV–Cre group and the AAV–LacZ group showed significant learning of a paired associates problem involving familiar contexts and novel olfactory cues [LacZ: F(6,34) = 6.54, p = 0.0002, Bonferroni's post-tests (day 1 vs days 5, 6, and 7), p < 0.05, p < 0.001, and p < 0.001, respectively; Cre: F(6,34) = 3.61, p < 0.01, Bonferroni's post-tests (day 1 vs day 4 and 7), p < 0.01, p < 0.05, and p < 0.01] (Fig. 7), and the two groups did not differ on the problem learned after injections (F(1,56) = 1.185; p = 0.28) or on learning before injections, suggesting that intact NR1 CA3 genes are not required for new paired associations involving familiar contexts and novel olfactory cues.
Figure 7.
Intact CA3 NMDARs are not required to form new paired associations between novel olfactory cues and familiar contexts. Mice were first trained on the paired associate task for 7 d (A). Then, AAV–Cre or AAV–LacZ was applied bilaterally to the dorsal CA3 subfield, and the mice were retrained using the same context as in the previous training but with new olfactory cues(B). Acomparison of the curves (percentage of choice of the rewarded cup on each training day) from before (pre; A) and after (post; B) NR1 focal gene deletion shows no change in acquisition, nor is any change apparent by comparison of the control LacZ group (blue triangles) to the Cre group (red squares) either before or after application of the AAV.
The same two groups of mice that were tested on the paired associate problem using familiar olfactory cues (Fig. 6) or familiar contexts (Fig. 7) were also tested on an additional problem using novel contexts and cues (Fig. 8).
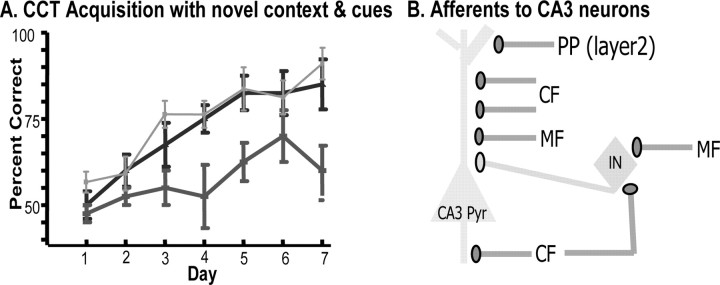
Figure 8.
A, The same mice that normally acquired the paired associate task with familiar contexts or familiar cues cannot acquire the same task using novel contexts and novel cues. The acquisition curve for mice before AAV injection (light squares) is similar to after injection with AAV–LacZ (dark triangles). However, the group injected with AAV–Cre (dark rectangles) is significantly slower. B, Pyramidal neurons (Pyr) and interneurons (IN) receive mossy fiber (MF) inputs from the dentate and collateral fibers (CF) from other CA3 pyramidal cells. The pyramidal cells also receive a perforant path (PP) input from layer 2 of the entorhinal cortex. A focal CA3 deletion of the NR1 gene prevents synaptic plasticity of the PP–Pyr and CF–Pyr synapses but not at the MF–Pyr synapse.
Those treated with the AAV–Cre were unable to reach a level of 75% correct on either day 4 or 5 of training in contrast to the AAV–LacZ treated mice, which showed significant learning by the fifth day of training [LacZ: F(6,34) = 5.75, p = 0.0005, Bonferroni's post-tests (day 1 vs day 5, 6, or 7), p < 0.01] (Fig. 8A). AAV–Cre mice group did eventually show significant learning, but only after 10 d of training [F(12,64) = 4.61, p < 0.0001, Bonferroni's post-tests (day 1 vs day 11, 12, and 13), p < 0.01, p < 0.01, and p < 0.05; data not shown]. The two groups differed in performance during the first 5 d of training (18.59% of the variance; F(1,40) = 15.54; p = 0.0003), and Bonferroni's post hoc tests revealed significant differences on days 4 and 5 (p < 0.05), confirming the original observation of impaired learning when both the context and olfactory cues are novel (Figs. 5, 8A).
Discussion
These findings indicate that intact CA3 NMDA receptors are critical for learning a novel paired associates problem but not for expression of a previously acquired problem. Because only 10–30% of the dorsal hippocampal CA3 NMDARs were affected, this kind of learning requires >70% of CA3 neurons and their NMDARs.
Many studies have indicated a role for the hippocampus in episodic memory (Eichenbaum, 2004) that is essential for fundamental aspects of declarative memory. Episodic memory may involve hippocampal-dependent encoding of a conjunctive entity (or entities) derived from multiple cues that assume a different meaning or salience from that of the individual cues. However, nonlinear discrimination tasks requiring conjunctive representations may be acquired in rodents with extensive hippocampal lesions (O'Reilly and Rudy, 2001), including tasks that are formally indistinguishable from the paired associate task used in the present study (Winocur and Olds, 1978; McDonald et al., 1997; Good et al., 1998). Thus, a requirement for conjunctive encoding does not by itself incur hippocampal dependence for learning.
The present findings help to clarify the specific role of CA3 NMDARs in conjunctive learning. Mice with reduced CA3 NMDARs might have been impaired because of the requirement to use spatial cues that distinguished the environmental contexts (O'Keefe, 1979; Steele and Morris, 1999). However, AAV–Cre mice were normal in their ability to discriminate and assign differential reward values to different contexts, and they normally acquired a paired associate problem that involved novel contexts if the olfactory cues were familiar. The impairment in learning a novel paired associate problem might have been caused by impairment in rapid learning about novel odors or novel associations with odors. However, AAV–Cre mice normally acquired a paired associate problem that involved new odors if the environmental contexts were familiar. Only in the situation in which both the context and olfactory cues were novel were AAV–Cre mice impaired, and this impairment was observed although the animals were experienced with success in learning a different paired associate problem before, or even after, treatment that eliminated NMDARs. Also, AAV–Cre mice could eventually learn a paired associate problem with substantially extended training. Thus, it appears CA3 NMDARs are specifically required for the relatively rapid concurrent acquisition of multiple, novel stimuli, together with their contingent reward assignments, such that each combination of a particular context and a particular odor cue has a unique associated outcome.
In our paradigms, the number of new associations remains the same, although some elements are new and others old. It cannot be ruled out that the reason for the deficit observed in lesioned mice with new contexts and cues was that this task was simply more demanding than the tasks with either familiar olfactory cues or familiar contexts. Assuming that a context is more complex than a single cue, the least complex task to learn is with familiar contexts and new cues, a more complex task to learn is with new contexts with old cues, and the most complex task is with new contexts and new cues. The quantitative complexity argument suggests that the learning deficits might be the least with new cues and familiar contexts, more severe with new contexts and old cues, and greatest with new contexts and new cues. In fact, learning is more rapid with familiar cues and new contexts compared with old contexts and new cues (compare Figs. 6, 7). These observations are most consistent with the use of a different neuronal mechanism when both context and olfactory cues are unfamiliar.
The present observations add to growing evidence that CA3 networks play a critical and selective role in pattern completion and pattern separation. CA3 cells more rapidly change their spatial firing patterns in response to an alteration in the arrangement of spatial cues than CA1 cells (Lee et al., 2004), are more strongly influenced by specific combinations of local and distal cues (Leutgeb et al., 2004), and are more likely to complete or separate stimulus patterns (Lee et al., 2004; Leutgeb et al., 2004; Vazdarjanova and Guzowski, 2004). Mice lacking CA3 NMDA receptors are impaired in rapid learning of new spatial patterns (Nakazawa et al., 2003) and in pattern completion from partial cues (Gold and Kesner, 2005), resulting in reduced activity by CA1 cells with the presentation of partial cues (Nakazawa et al., 2002). Correspondingly, CA3, and not CA1, is critical in composing a contextual representation (Lee and Kesner, 2004), and CA3, not CA1, is critical for odor–place and object–place association (Gilbert et al., 2001; Gilbert and Kesner, 2003). Pharmacological blockade of NMDA receptors in CA3, and not CA1, impaired learning a novel spatial pattern (Lee and Kesner, 2002). The necessary role of CA3 NMDARs does not rule out a necessary role for upstream pattern separation mechanisms as, for example, has been suggested for the dentate (Treves and Rolls, 1992; Kesner et al., 2004) and as is indicated by the presence of place cells in the entorhinal cortex (Fyhn et al., 2004). The present results extend these observations, showing that CA3 NMDARs are specifically critical for pattern separation that involves the composition of environments and cues into coherent and distinct experiences. Our observations suggest this would include contingent and incidental associations and that this is especially important when exposed to unfamiliar cues (not previously experienced as either incidental or contingent).
The lesion used in this study was restricted to a single gene, necessary for NMDAR function, and localized to a minority (10–30%) of the CA3 field of the dorsal hippocampus, bilaterally. NMDAR-dependent synaptic plasticity was compromised, affecting synapses from the entorhinal layer 3 (van Groen et al., 2003) input to CA3 neurons and from recurrent excitatory synapses from neighboring CA3 neurons (Fig. 8). This loss of plasticity might affect the observed deficit in two ways (not mutually exclusive). At least part of the orthogonalization process (making similar patterns of input distinct) might depend on plastic changes in the entorhinal to CA3 pyramidal cell synapses. Of note in this regard, presynaptic plasticity of the dentate granule cell to CA3 pyramidal cell, mossy fiber input, is not affected by postsynaptic NMDAR disruption (Nicoll and Malenka, 1995), suggesting that it was not sufficient to allow acquisition of the pattern separation task. The other affected synapse, mediating CA3 pyramidal cell to cell, recurrent input, is important for pattern completion tasks (Nakazawa et al., 2002). The paired associate learning task used in the present study did not involve a degradation or loss of cues that has been used to demonstrate pattern completion. Nevertheless, this same process may contribute to the stabilization of separated patterns of input and in this manner contribute to the acquisition of pattern separation tasks. Finally, rapid encoding of orthogonalized encoded patterns needed for trial-to-trial incremental learning in our paradigm would also be affected.
The focal gene deletion in CA3 also affected the kinetics of glutamatergic synapses with NMDARs because the slower, NMDAR-dependent, excitatory activation of projection cells and interneurons (slower compared with AMPA receptor activation) was lost. The effects on interneurons are of particular importance because of their widely divergent projections throughout CA3, so that disruption of interneuron activity restricted to 10–30% of the CA3 region, as occurred in the present study, affects inhibition and, accordingly, the timing of pyramidal cell firing throughout the CA3 region (Freund and Buzsaki, 1996). Although this did not affect learned responses, the coordinated timing of pyramidal cell firing may be necessary for effective synaptic plasticity and paired associate learning.
Declarative memory may be disrupted by an inability to encode a particular experience as a configuration that distinguishes it from others. Thus, severe hippocampal damage does not result in loss of language but rather the ability to recollect the context of the sentence needed to provide its meaning. For example, that a sentence was uttered by a particular person, under particular circumstances, is critical to knowing its meaning (Fortin et al., 2004; Squire et al., 2004). The present study suggests that >70–90% of intact NMDARs in the CA3 may be necessary. Furthermore, thought disorder symptoms of patients suffering from schizophrenia may provide an example of dysfunction, rather than loss of function, of hippocampal-dependent systems. These patients show deficits in paradigms requiring contextual information processing (Servan-Schreiber et al., 1996; Bazin et al., 2000; Stratta et al., 2000; Titone et al., 2000; Martins et al., 2001; Liu et al., 2002), which may be resolved by the kind of pattern separation needed in situations involving context–cue–reward (or perhaps saliency) discriminations, as in the present study. Similar deficits were reported in healthy subjects exposed to NMDAR antagonist (Umbricht et al., 2000) as part of an acute clinical psychosis that is similar in most respects to that of schizophrenia (Krystal et al., 1994). Moreover, such deficits have been correlated with clinically defined thought disorder of schizophrenia (Bazin et al., 2000; Stratta et al., 2000). Our findings show, for the first time, a dependence of this kind of pattern separation processing on NMDAR function in the hippocampus. This suggests that NMDAR antagonism can cause a deficit in contextual information processing by disruption of CA3-dependent acquisition of contextually meaningful cues that are not otherwise acquired.
Footnotes
This work was supported by National Institute of Mental Health Conte Center for Neuroscience Research Grant MH60450 and the Department of Veteran Affairs. We thank X. Liao, L. Nugyen, and T. Thai for technical assistance.
Correspondence should be addressed to Dr. Robert W. Greene, Dallas Veterans Affairs Medical Center, 116A, 4500 South Lancaster Road, Dallas, TX 75216. E-mail: robertw.greene@utsouthwestern.edu.
DOI:10.1523/JNEUROSCI.4194-05.2006
Copyright © 2006 Society for Neuroscience 0270-6474/06/260908-08$15.00/0
References
- Bazin N, Perruchet P, Hardy-Bayle MC, Feline A (2000) Context-dependent information processing in patients with schizophrenia. Schizophr Res 45: 93–101. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2004) Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron 44: 109–120. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H (2004) Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature 431: 188–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G (1996) Interneurons of the hippocampus. Hippocampus 6: 347–470. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB (2004) Spatial representation in the entorhinal cortex. Science 305: 1258–1264. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP (2003) Localization of function within the dorsal hippocampus: the role of the CA3 subregion in paired-associate learning. Behav Neurosci 117: 1385–1394. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I (2001) Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus 11: 626–636. [DOI] [PubMed] [Google Scholar]
- Gold AE, Kesner RP (2005) The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus 15: 808–814. [DOI] [PubMed] [Google Scholar]
- Good M, de Hoz L, Morris RG (1998) Contingent versus incidental context processing during conditioning: dissociation after excitotoxic hippocampal plus dentate gyrus lesions. Hippocampus 8: 147–159. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Erzurumlu RS, Huerta PT, Chen DF, Sasaoka T, Ulupinar E, Tonegawa S (1997) NMDA receptor-dependent refinement of somatotopic maps. Neuron 19: 1201–1210. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P (2004) A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci 15: 333–351. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers Jr MB, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP (2002) Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nat Neurosci 5: 162–168. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP (2004) Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus 14: 301–310. [DOI] [PubMed] [Google Scholar]
- Lee I, Rao G, Knierim JJ (2004) A double dissociation between hippocampal subfields: differential time course of CA3 and CA1 place cells for processing changed environments. Neuron 42: 803–815. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI (2004) Distinct ensemble codes in hippocampal areas CA3 and CA1. Science 305: 1295–1298. [DOI] [PubMed] [Google Scholar]
- Liu SK, Chiu CH, Chang CJ, Hwang TJ, Hwu HG, Chen WJ (2002) Deficits in sustained attention in schizophrenia and affective disorders: stable versus state-dependent markers. Am J Psychiatry 159: 975–982. [DOI] [PubMed] [Google Scholar]
- Martins SA, Jones SH, Toone B, Gray JA (2001) Impaired associative learning in chronic schizophrenics and their first-degree relatives: a study of latent inhibition and the Kamin blocking effect. Schizophr Res 48: 273–289. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Murphy RA, Guarraci FA, Gortler JR, White NM, Baker AG (1997) Systematic comparison of the effects of hippocampal and fornixfimbria lesions on acquisition of three configural discriminations. Hippocampus 7: 371–388. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S (2002) Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S (2003) Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 38: 305–315. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC (1995) Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature 377: 115–118. [DOI] [PubMed] [Google Scholar]
- O'Keefe J (1979) A review of the hippocampal place cells. Prog Neurobiol 13: 419–439. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW (2001) Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev 108: 311–345. [DOI] [PubMed] [Google Scholar]
- Preston GC, Dickinson A, Mackintosh NJ (1986) Contextual conditional discriminations. Q J Exp Psychol 38B: 217–237. [Google Scholar]
- Scammell TE, Arrigoni E, Thompson MA, Ronan PJ, Saper CB, Greene RW (2003) Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J Neurosci 23: 5762–5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S (1996) Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry 53: 1105–1112. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RG (1999) Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist d-AP5. Hippocampus 9: 118–136. [DOI] [PubMed] [Google Scholar]
- Stratta P, Daneluzzo E, Bustini M, Prosperini P, Rossi A (2000) Processing of context information in schizophrenia: relation to clinical symptoms and WCST performance. Schizophr Res 44: 57–67. [DOI] [PubMed] [Google Scholar]
- Titone D, Prentice KJ, Wingfield A (2000) Resource allocation during spoken discourse processing: effects of age and passage difficulty as revealed by self-paced listening. Mem Cognit 28: 1029–1040. [DOI] [PubMed] [Google Scholar]
- Treves, A, Rolls ET (1992) Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 2: 189–199. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S (1996) The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87: 1327–1338. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC (2000) Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry 57: 1139–1147. [DOI] [PubMed] [Google Scholar]
- van Groen T, Miettinen P, Kadish I (2003) The entorhinal cortex of the mouse: organization of the projection to the hippocampal formation. Hippocampus 13: 133–149. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF (2004) Differences in hippocampal neuronal population responses to modifications of an environment context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci 24: 6489–6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Olds J (1978) Effects of context manipulation on memory and reversal learning in rats with hippocampal lesions. J Comp Physiol Psychol 92: 312–321. [DOI] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS (1994) NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav Neurosci 108: 19–29. [DOI] [PubMed] [Google Scholar]