Abstract
Deep brain stimulation (DBS) of the ventrolateral thalamus stops several forms of tremor. Microelectrode recordings in the human thalamus have revealed tremor cells that fire synchronous with electromyographic tremor. The efficacy of DBS likely depends on its ability to modify the activity of these tremor cells either synaptically by stopping afferent tremor signals or by directly altering the intrinsic membrane currents of the neurons. To test these possibilities, whole-cell patch-clamp recordings of ventral thalamic neurons were obtained from rat brain slices. DBS was simulated (sDBS) using extracellular constant current pulse trains (125 Hz, 60–80 μs, 0.25–5 mA, 1–30 s) applied through a bipolar electrode. Using a paired-pulse protocol, we first established that thalamocortical relay neurons receive converging input from multiple independent afferent fibers. Second, although sDBS induced homosynaptic depression of EPSPs along its own pathway, it did not alter the response from a second independent pathway. Third, in contrast to the subthalamic nucleus, sDBS in the thalamus failed to inhibit the rebound potential and the persistent Na+ current but did activate the Ih current. Finally, in eight patients undergoing thalamic DBS surgery for essential tremor, microstimulation was most effective in alleviating tremor when applied in close proximity to recorded tremor cells. However, stimulation could still suppress tremor at distances incapable of directly spreading to recorded tremor cells. These complementary data indicate that DBS may induce a “functional deafferentation” of afferent axons to thalamic tremor cells, thereby preventing tremor signal propagation in humans.
Keywords: deep brain stimulation, high-frequency stimulation, thalamus, essential tremor, paired pulse, synaptic depression
Introduction
Thalamic surgery has been used to successfully relieve different forms of tremor since the 1950s (Tasker and Kiss, 1995). Intraoperative microelectrode recordings in the thalamus have revealed neurons that fire in bursts synchronous with electromyographic (EMG) tremor (Lenz et al., 1988). Thalamic lesions are most effective when made in the ventrolateral (VL) nucleus in regions containing these “tremor cells” (Lenz et al., 1995). More recently, deep brain stimulation (DBS) of VL-thalamus has replaced lesions for tremor (Schuurman et al., 2000) and currently is primarily used for essential tremor. The most effective site for electrical stimulation is similar to that used for thalamotomy (Kiss et al., 2003a), suggesting that interventions that use stimulation or lesioning may share similar mechanisms.
Despite its increasing clinical use, how DBS works remains poorly understood. Although several mechanisms have been suggested (for review, see Vitek, 2002; Breit et al., 2004), two specific mechanisms of DBS-mediated tremor stoppage in the thalamus may include inhibition of incoming tremor signals from afferent axons and/or altering the excitability and membrane currents intrinsic to thalamic neurons (Beurrier et al., 2001; Do and Bean, 2003; Anderson et al., 2004a). Because essential tremor is thought to arise from dysfunction of the glutamatergic olivocerebellar pathway, which terminates in the VL thalamus (for review, see DeLong, 1978; Deuschl and Bergman, 2002), we studied this region in rat thalamic brain slices. Simulated DBS (sDBS) primarily induced a synaptically mediated, glutamatergic membrane depolarization (Anderson et al., 2004a). In most neurons, this depolarization was transient, occurring only at the onset of stimulation, and was followed by a period of quiescence with no detectable activity. Therefore, we proposed that DBS could introduce a functional deafferentation of synaptic input, thereby stopping tremor propagation beyond the thalamus.
An important feature of functional deafferentation is the role of synapses and their adaptation to continuous high-frequency stimulation that is not present under physiological conditions. Such influence of DBS on synaptic transmission is generally overlooked in current models of DBS, which focus on the properties of axonal conduction and inhibition of intrinsic membrane currents (McIntyre and Grill, 2002; McIntyre et al., 2004; Rubin and Terman, 2004). Here, we tested key predictions derived from the concept of functional deafferentation, including: (1) sDBS must be able to depress afferent synaptic transmission; (2) functional deafferentation can be achieved independently from inhibition of intrinsic membrane currents; and (3) in humans, tremor stoppage can be obtained remotely by applying DBS at distances beyond the range of current expected to affect tremor cell somata directly. Preliminary data have been reported in abstract form (Anderson et al., 2005).
Materials and Methods
Slice preparation. All experiments were performed in accordance with the Canadian Council on Animal Care guidelines and approved by the University of Calgary Animal Care Committee. Male Sprague Dawley rats [postnatal day 14 (P14) to P21] were decapitated under halothane anesthesia, and the brain was quickly removed. Coronal thalamic slices (400 μm thick) were cut on a vibratome (VT 1000S; Leica, Nussloch, Germany) in 0°C modified artificial CSF (aCSF) containing the following (in mm): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 2.6 MgCl2, 1.0 CaCl2, 18 NaHCO3, and 11 glucose. Slices were then hemisected and incubated in a holding chamber with this medium kept at 30°C for 1–2 h. The slices were then transferred to a recording chamber and superfused with carboxygenated (95% O2, 5% CO2) aCSF consisting of the following (in mm): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 18 NaHCO3, and 11 glucose.
Electrophysiological recording. Thalamic slices submerged with aCSF were initially visualized under brightfield for identification of the ventral thalamus (Paxinos and Watson, 1998). Whole-cell recordings were obtained from thalamic neurons visually identified using an upright microscope (Olympus Optical BX51W; Olympus Optical, Tokyo, Japan) fitted with infrared differential interference contrast optics. All recordings were obtained at 32°C using borosilicate glass microelectrodes (tip resistance, 6–8 MΩ) filled with intracellular solution containing the following (in mm): 116 K-gluconate, 2 MgCl2, 8 Na-gluconate, 8 KCl, 1 EGTA, 4 K2ATP, and 0.3 Na2GTP, buffered with 10 HEPES. The pH of the internal solution was adjusted to 7.3 using KOH or HCl as required and filtered before use. The electrode capacitance and bridge circuit were appropriately adjusted, and series/access resistance (10–40 MΩ) was always <10% of membrane resistance. The liquid junction potential was measured to be ∼10 mV and was not subtracted.
An Axopatch-200A (Molecular Devices, Sunnyvale, CA) was used in either current- or voltage-clamp mode for all experiments. For voltage-clamp recordings, the membrane potential was clamped at –60 mV unless otherwise stated. Transmembrane current pulses were driven and captured (1–10 kHz filter, 10 kHz digitization) using an A-D interface (Digidata 1200B; Molecular Devices) operated by pClamp software (Molecular Devices). Input and series/access resistance were monitored during the course of each experiment between protocols and did not vary >15%. Our previous study (Anderson et al., 2004a) showed that the sDBS response was not sensitive to GABAA receptor blockade with picrotoxin (50 μm). Therefore, in most experiments, it was included in the aCSF to pharmacologically eliminate any fast IPSCs.
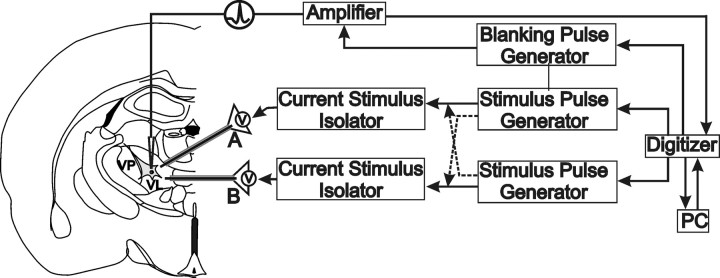
Stimulation protocol. Two concentric bipolar stimulating electrodes (NEX-100; Rhodes Medical Instruments, Woodland, CA) separated by 0.5 mm were placed within the ventral thalamus for delivery of extracellular evoked current pulses. The stimulating electrodes were designated “A” or “B” for experimental clarity. Current pulses (0.25–5 mA) were delivered through individual constant-current stimulators (A360 or A365; World Precision Instruments, Sarasota, FL) activated by a pulse generator (A-M Systems 2100; A-M Systems, Carlsborg, WA) or from the pClamp interface (Fig. 1). The positions of the stimulating electrodes and recording sites were varied within the ventral thalamus. Recorded neurons were located between 0.1 and 1.2 mm from both stimulating electrodes. The responses seen in juvenile rats did not differ from that reported previously for adult rats when stimulation parameters were appropriately controlled (Anderson et al., 2004b).
Figure 1.
Experimental methods. Schematic representation of whole-cell patch-clamp recording setup in thalamic rat brain slices. Left, Recordings were made from thalamic relay neurons in the VL thalamus. Extracellular stimulation (A or B) was selectively delivered through one (or combination) of two stimulating electrodes (NEX-100) placed within the VL. Right, Flow chart detailing experimental design enabling independent stimulus pulse generation along an individual or across electrodes.
During high-frequency stimulation (125 Hz), a “blanking” operation was necessary to eliminate the stimulus artifacts (Fig. 1). The sDBS blanking pulse did not interfere with the detection of action potentials or evoked potentials (Kiss et al., 2002; Anderson et al., 2004a). Artifacts from stimulus pulses delivered at 5 Hz to simulate tremor were not blanked.
Experimental solutions. Picrotoxin (50 μm), tetrodotoxin (TTX; 0.3 μm), and kynurenate (2 mm) were purchased from Sigma-Aldrich (St. Louis, MO). Cyclothiazide (CTZ; 100 μm), 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288; 20 μm), (R, S)-α-2-methyl-4-sulfonophenylglycine (MSPG; 100 μm), and dl-threo-β-benzyloxyaspartate (TBOA; 100 μm) were purchased from Tocris Cookson (Bristol, UK). All drugs were bath applied and prepared daily from a stock solution. All vehicle concentrations (DMSO, HCl) were <0.1% of final and had no effect on recordings.
Human experiments. Patients undergoing thalamic surgery for relief of essential tremor had recording and stimulation with microelectrodes performed as part of the routine brain mapping surgery (Tasker and Kiss, 1995). Recordings were performed continuously with a microelectrode (exposed tip, 15–40 μm; 0.2–0.5 MΩ at 1 kHz) advanced by a hydraulic microdrive (data recorded at 0.1 mm resolution). Recording and stimulation were performed through the same microelectrode. Data regarding types of cells encountered were recorded every 0.1 mm, and stimulation was applied at standard 1 mm intervals. Characteristic cell types such as voluntary, kinaesthetic, or tactile were identified by their response properties (Kiss et al., 2003b). Tremor cells were recognized by their burst firing pattern and their relation to observed tremor (Lenz et al., 1985, 1988, 1990, 1994). Microstimulation was applied with a monophasic constant-current stimulus isolator (A360 and A310; World Precision Instruments) in combination with a stimulus generator (these are the components that make up the ARS Neurosystem-2, Microelectrode guided neurosurgical system; Atlantic Research Systems, Atlanta, GA). Cathodal pulses were applied at the electrode tip, referenced to the guide tube containing the microelectrode. Microstimulation (333 Hz, 0.2 ms duration, 1–2 s trains) was performed every 1 mm along each trajectory (Dostrovsky et al., 1993a). The amplitude of microstimulation was gradually increased (5–100 μA) until threshold for patient perception or a tremor effect was observed. Tremor reduction/arrest was determined by observation and/or continuous surface EMG or accelerometry. The effect of current intensity on tremor was measured for neurons recorded at a fixed distance from the stimulation site (<1 mm), whereas the effect of distance on tremor was determined for a fixed current intensity (50 μA). Tremor effects were then correlated with both stimulation current and distance from identified tremor cells.
All data are presented as mean ± SE. Statistical significance was tested with Student's paired t test or one-way ANOVA. Nonparametric data were analyzed using the Kruskal–Wallis one-way ANOVA on repeated measures. An α value of <0.05 was interpreted as significant.
Results
Animal data
Results were obtained from 85 neurons in the ventral thalamus. All cells exhibited the characteristics of a thalamic relay neuron, including low-threshold Ca2+ spike (LTS) and a resting membrane potential of less than –55 mV (Jahnsen and Llinas, 1984a). Only neurons displaying a transient membrane depolarization [referred to as type 1 in the study by Anderson et al. (2004a)] in response to sDBS were studied.
sDBS evokes homosynaptic depression in rat thalamic slices
In the first set of experiments, we examined whether presynaptic afferents to a single thalamic neuron can be selectively depressed during sDBS. We first recorded EPSCs from the same neuron evoked by stimulation of two independent fiber pathways (Fig. 1A,B). Independence of these pathways was determined using a paired-pulse protocol (Fig. 2). A conditioning pulse (80 μs, 0.25–5 mA) was first applied to pathway A (A), which was followed 20 ms later by a test pulse (aA) delivered to the same pathway through the same electrode using the same stimulation parameters (homosynaptic pairing). We then repeated this experiment cross-synaptically by delivering the conditioning pulse to pathway B (B) followed by the test pulse to pathway A (aB) using the same stimulation pulse parameters (Fig. 2i). The same homosynaptic and cross-synaptic test was then repeated for pathway B (Fig. 2ii). The data are summarized in Figure 2 iii/iv. Homosynaptic pairings (aA/A or bB/B) lead to a paired-pulse facilitation (PPF) with a ratio of 1.39 ± 0.05 for pathway A (n = 18) and 1.36 ± 0.07 for pathway B (n = 15). Cross-synaptic pairings (aB/A or bA/B), however, produced no PPF and had an EPSC ratio of 1.06 ± 0.04 for pathway A and 0.99 ± 0.02 for pathway B. Similar results were obtained when paired pulses were delivered 40 ms apart (n = 15).
Figure 2.
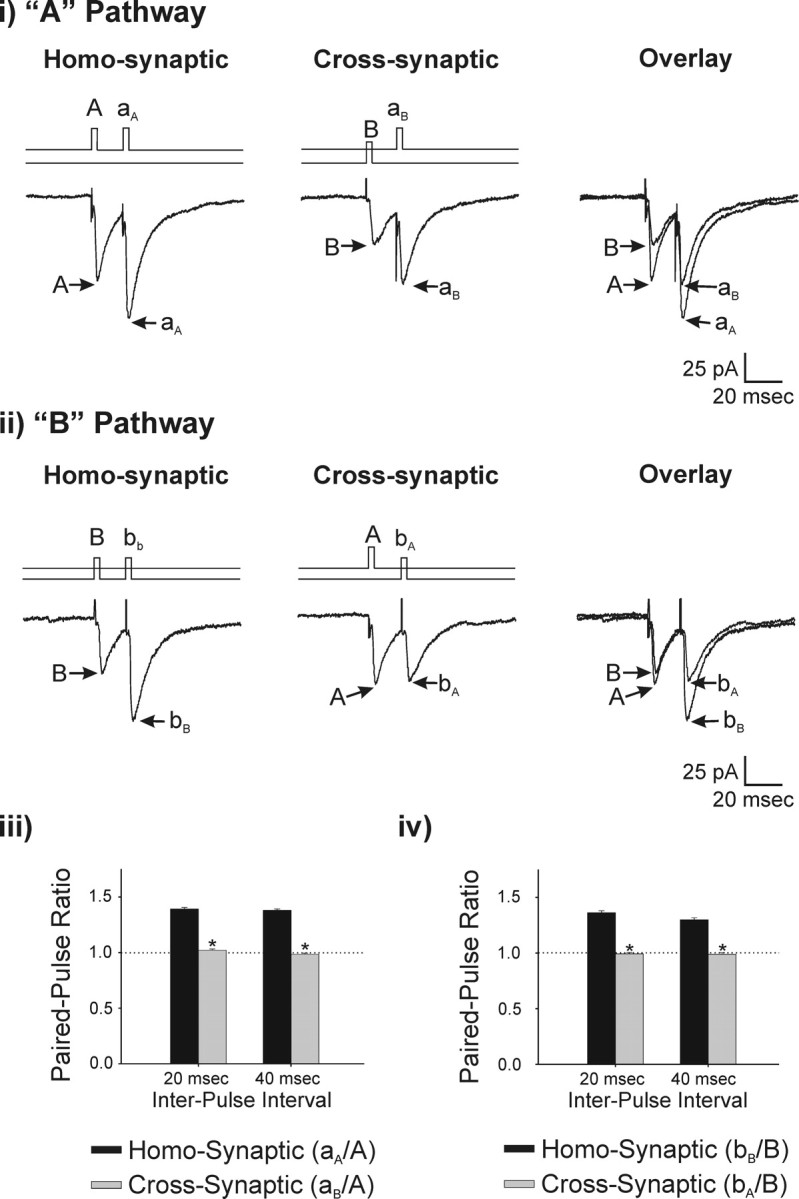
Selective activation of independent thalamic afferents. A test for independence was conducted by comparing the paired-pulse ratio obtained from homosynaptic or cross-synaptic stimulation. Cells were held at –60 mV. i, The stimulation protocol is illustrated above each voltage-clamp recording. The effect of a homosynaptic prepulse (A) on a second homosynaptic pulse (aA) is shown. Similarly, the effect of cross-synaptic prepulse (B) on the A pathway (aB) is shown (n = 18). ii, A similar experimental protocol was used to test pathway B (n = 15). Summary data of the resulting paired-pulse ratio is seen for pulses delivered at 20 and 40 ms apart for pathway A (iii) and pathway B (iv).
Once pathway independence was established, we tested whether sDBS applied to each pathway could inhibit low-frequency synaptic potentials that mimic tremor. We evoked uninterrupted EPSPs at tremor frequency (∼5 Hz) before, during, and after sDBS (10 s, 125 Hz). Both EPSPs and sDBS were generated with the same pulse width (80 μs) and stimulation intensity (0.25–5 mA). As seen in Figure 3i, before the onset of sDBS, EPSPs were reliably produced with each stimulus pulse. However, when sDBS was applied homosynaptically through the same electrode, the low-frequency tremor-mimicking EPSPs were completely eliminated during the sDBS train, however, recovered fully by 2.1 ± 0.6 s after the end of sDBS. We next tested whether this strong synaptic depression was mediated homosynaptically in a pathway-specific manner or if it involved nonspecific depression of all synaptic afferents to a cell. To this end, we continued to deliver sDBS down one pathway (A) but delivered tremor-mimicking EPSPs in the second independent pathway (B). The results of this set of experiments are shown in Figure 3ii, which clearly demonstrate the lack of cross-pathway inhibition of sDBS on EPSPs evoked by another pathway. Summarized data of normalized EPSP amplitude for each pathway during sDBS applied to pathway A is presented in Figure 3iii. These results were repeatable when the stimulation protocol was retested for sDBS of pathway B. Hence, sDBS evoked synaptic depression is homosynaptic and likely occurred presynaptically without affecting the overall neuronal excitability and postsynaptic responsiveness. This conclusion was further supported by the observation that cyclothiazide (100 μm), which blocks AMPA receptor desensitization (Trussell et al., 1993; Arai and Lynch, 1998) did not have any effect on the response to sDBS (n = 5). Furthermore, MSPG (100 μm; n = 3), which blocks presynaptic metabotropic glutamate receptors also failed to prevent sDBS-induced homosynaptic depression. Similarly, application of glutamate uptake inhibitor TBOA (100 μm; n = 5) had no effect on sDBS (data not shown). Therefore, given that these results indicate a rapid onset and recovery from homosynaptic depression, a lack of cross talk and a lack of effect from blockade of postsynaptic AMPA receptor desensitization suggest the involvement of a presynaptic mechanism in mediating sDBS-induced synaptic depression.
Figure 3.
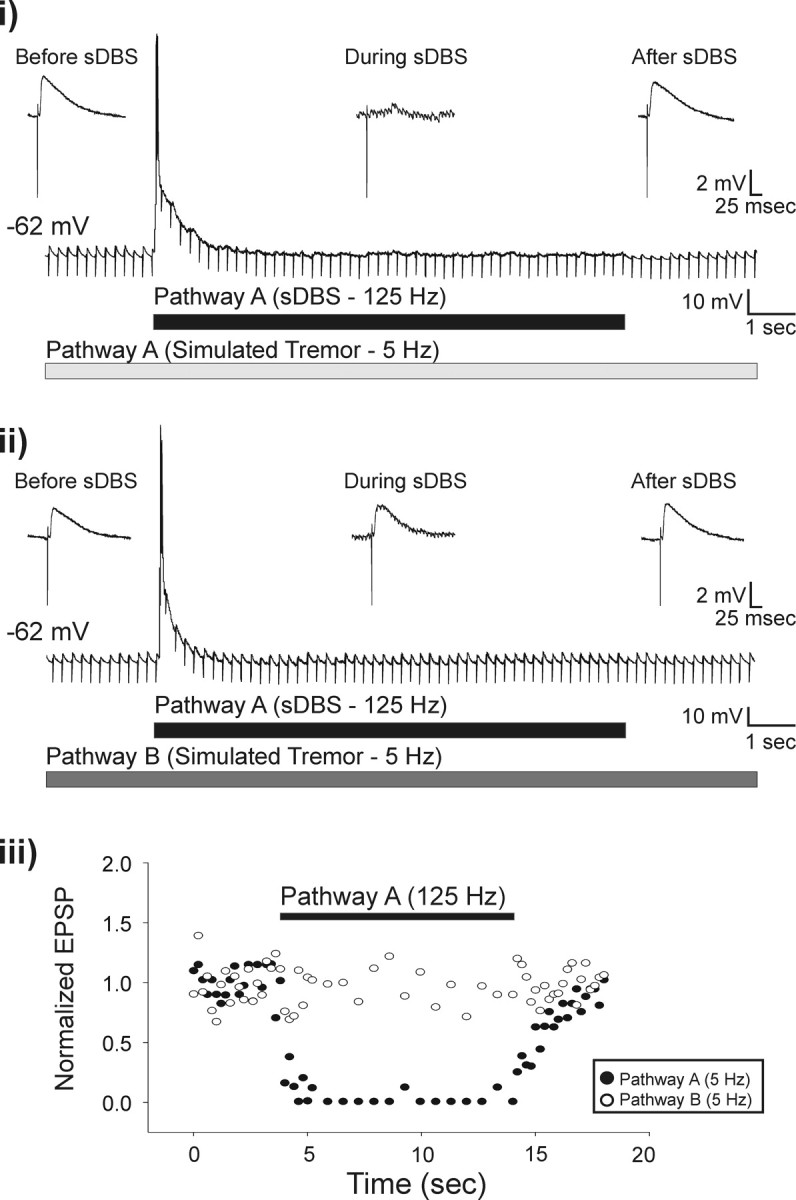
sDBS selectively inhibits extracellular simulated tremor. Tremor was mimicked in the slice by extracellularly evoking EPSPs at 5 Hz. sDBS applied homosynaptically during simulated tremor resulted in complete inhibition of the tremor signal (i) but had no effect on tremor generated along the cross-synaptic pathway (ii; n = 5). Insets show expanded views of single EPSPs before, during, and after sDBS. The effects of sDBS on the amplitude of the homosynaptic (closed circles) and cross-synaptic (open circles) tremor EPSPs as a function of their respective controls are plotted over time (iii). Note that the tremor stimuli were delivered at a subharmonic frequency of the sDBS train to ensure noninterference when delivered homosynaptically down the same electrode. To confirm successful delivery, the tremor stimulation artifacts were not blanked.
Although recording during the high-frequency sDBS train required the use of a blanking pulse, the tremor shock artifacts were not blanked. Furthermore, the tremor pulses were generated between the sDBS pulses. Therefore, the continued presence of the tremor shock artifacts during the sDBS train ensured that the observed inhibition of EPSPs was not attributable to interference between the two stimulus pulses. We also tested sDBS applied at different frequencies. Frequencies <50 Hz were incapable of inducing homosynaptic depression (n = 5). We repeated these experiments without picrotoxin in the aCSF and observed the same results (n = 9).
The effect of sDBS on intrinsic membrane currents
Depression of intrinsic membrane currents during high-frequency electrical stimulation has been reported in subthalamic neurons (Beurrier et al., 2001; Do and Bean, 2003). In the thalamus, how different membrane currents respond to sDBS has not been specifically examined. In our previous study, sDBS induced a small direct current-mediated increase in neuronal excitability without a change in the steady-state input resistance (Anderson et al., 2004a). However, this did not rule out the possibility of changes in active conductances. In this set of experiments, we characterized the effects of sDBS on several membrane currents prominently expressed in thalamic cells and theoretically important to bursting activity (Jahnsen and Llinas, 1984b; Steriade and Deschênes, 1984; Huguenard and McCormick, 1992; McCormick and Huguenard, 1992; Parri and Crunelli, 1998).
Hyperpolarization activated mixed cation current (Ih)
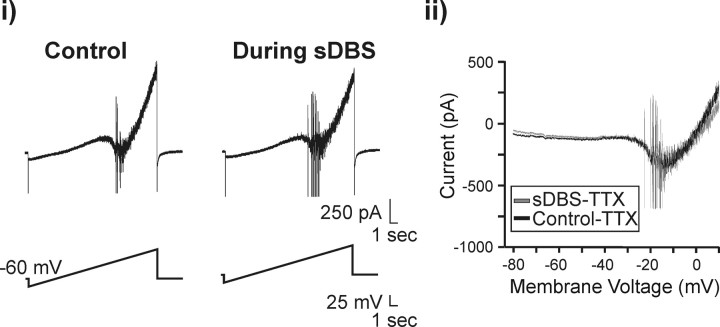
To examine the effect of sDBS on Ih, we conducted both current- and voltage-clamp experiments. Under current clamp, a series of transmembrane current pulses were delivered in control conditions or during sDBS. In control, hyperpolarizing pulses induced a slow onset rectification that developed as a depolarizing sag in the membrane potential (Fig. 4Ai). During sDBS, this depolarizing sag was absent (n = 5). This sDBS effect on Ih was further studied under voltage clamp. The membrane voltage was adjusted to –70 mV for these experiments to decrease the contribution of low-threshold-activated Ca2+ currents to the Ih rebound. All data were derived by subtracting ZD7288 (20 μm)-resistant currents from the controls. As seen in Figure 4Bi, hyperpolarizing steps evoked a time-dependent inward rectifying current Ih (n = 6). During sDBS, there was an apparent loss of the time-dependent activation of Ih (Fig. 4Bii). This lack of time-dependent activation during sDBS can be explained by an increased inward current (–187.5 ± 33.1 pA) activated by sDBS throughout its application over control levels (–86.3 ± 27.0) (Fig. 4Biii). Hence, most Ih channels were already open when the voltage step commands were applied, leading to an apparent lack of activation of Ih macroscopic current. Consistent with this observation, sDBS-mediated modulation of Ih was not observed in the presence of ZD7288. However, it persisted in the presence of 2 mm kynurenate (n = 3), suggesting that sDBS alters the Ih current through a non-synaptic mechanism. I–V curves of instantaneous (Ii) and steady state (Imax) for control and during sDBS are presented in Figure 4Bv. Although significantly more current developed during sDBS (both instantaneously and at steady state) compared with control (p < 0.001), there was no statistical difference between instantaneous and steady-state current during sDBS. Finally, a comparison of the normalized instantaneous current to maximum steady-state current in control and during sDBS reveals a rightward and upward shift in the activation curve of the Ih current (Fig. 4Biv). Similar results were observed when the tail current was analyzed in control or during sDBS (data not shown).
Figure 4.
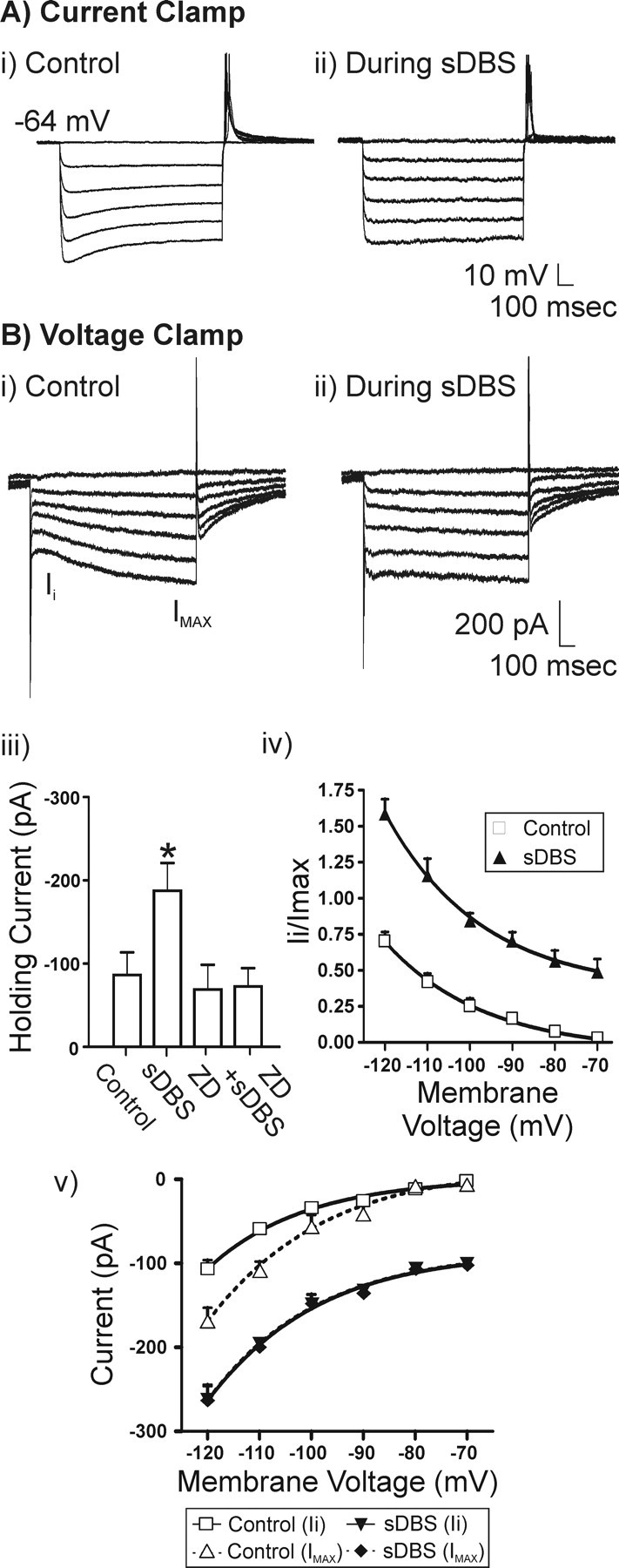
sDBS-mediated modulation of Ih. A, Representative current-clamp recording and V–I relationship (20 pA for each step) obtained during control (i) and sDBS (ii)(n = 5). B, Representative voltage-clamp recording and I–V relationship (10 mV for each step) obtained from another neuron. i, In control, Ih is seen as a slowly activating inward current on the hyperpolarizing steps (n = 6). ii, During extracellular sDBS, the time dependency of Ih activation is nearly absent as the current is activated tonically with the onset of sDBS and consequently has reached steady state before application of the hyperpolarizing test pulse. All data are derived from ZD7288 (20 μm)-sensitive current and obtained after subtracting ZD7288-resistant currents from the controls (n = 6). iii, A comparison of holding current before or during sDBS. Cells were held at –70 mV. Note that sDBS increased the holding current from control levels (p < 0.001), but this sustained inward current was blocked by ZD7288. iv, Ih activation curve obtained based on the ratio of Ii versus Imax (control step to –120 mV). v, I–V relationship for instantaneous (Ii) and steady-state (Imax) current. Imax was obtained at the end of each 1 s voltage step. During sDBS, a significant increase in instantaneous and steady-state current was observed over control (p < 0.001). However, there was no statistical difference between instantaneous and steady-state current that developed during sDBS. This indicates a tonic activation of Ih induced by sDBS.
Rebound current (low-threshold Ca2+ spike)
When the holding potential was adjusted to –60 mV, a rebound current (predominantly mediated by IT) (McCormick and Huguenard, 1992) was observed following a hyperpolarizing command. When intracellular hyperpolarizing current steps (–50 mV, 500 ms) were repeatedly applied at 1 Hz in control (Fig. 5) or during sDBS, no alteration in the amplitude, latency to onset, or duration of the rebound current occurred. However, there was a small decrease in the latency of Na+ spike onset triggered by the rebound current (i.e., low-threshold spike) (n = 7). As reported previously (Anderson et al., 2004a), similar results were observed under current clamp. An expanded overlay of control and sDBS rebound current is presented in Figure 5.
Figure 5.
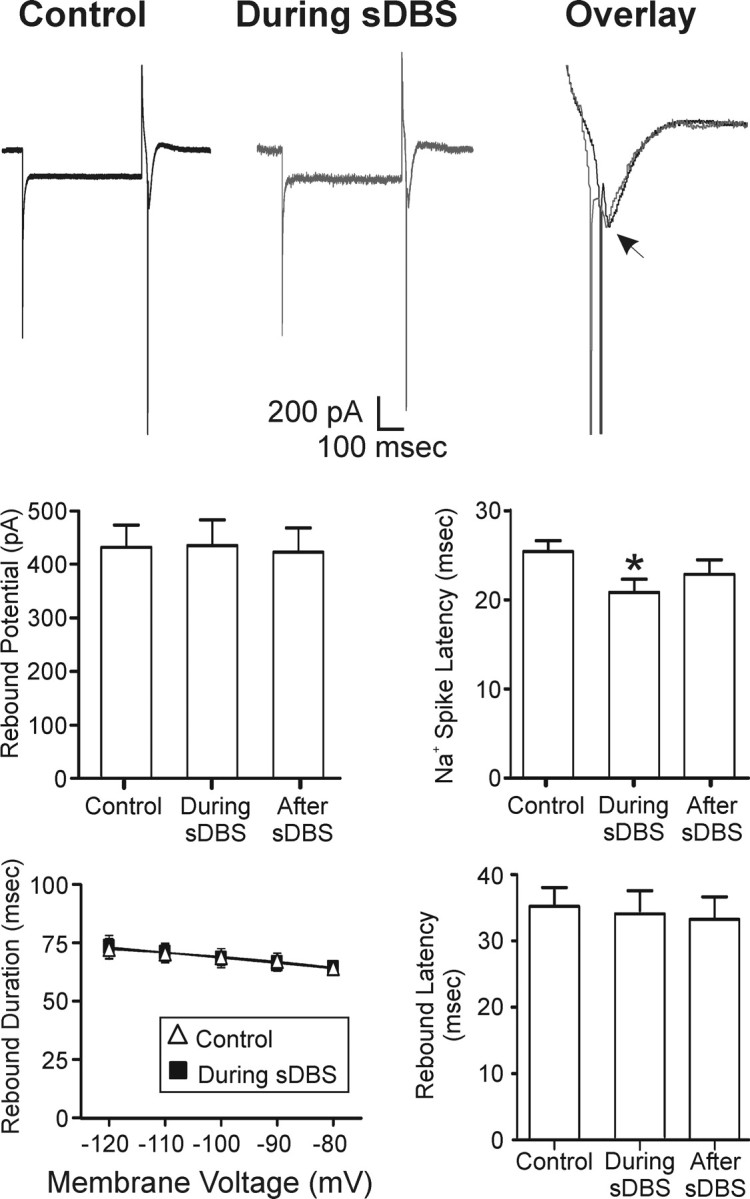
Lack of modulatory effects of sDBS on IT. Intracellular voltage steps (–50 mV, 500 ms) were delivered repeatedly once per second in control or during extracellular sDBS (n = 7). There was no statistical difference in the peak amplitude, onset latency, or duration of the rebound potential. However, there was a decrease in the latency of Na+ spike onset (p < 0.05). The arrow indicates peak of rebound potential. Action potentials were truncated in overlay for clarity.
Persistent Na+ current (INap)
A slow inward current was recorded during a slow ramp (5 mV/s) voltage command from –80 to 10 mV that had the characteristics of an INap current (Parri and Crunelli, 1998; Beurrier et al., 2001). This current remained unaltered during sDBS (Fig. 6i). I–V relationships of the TTX (0.3 μm)-sensitive INap current were not statistically different between control or during sDBS (p = 0.52; n = 4) (Fig. 6ii).
Figure 6.
Effect of sDBS on the persistent Na+ current. i, A slow voltage ramp command (5 mV/s) was applied from –80 to +10 mV resulting in a slow inward current (INap). This current was unaltered during sDBS (n = 4). ii, Overlay of TTX (0.3 μm)-sensitive INap current in control or during sDBS. The INap current was obtained by subtracting the current evoked in the presence of TTX from that obtained under normal aCSF. Action potentials were truncated for clarity.
Together, the above data strongly suggest that sDBS in the thalamus does not inhibit the rebound current or INap intrinsic membrane currents as it does in subthalamic neurons (Beurrier et al., 2001; Do and Bean, 2003).
Human data
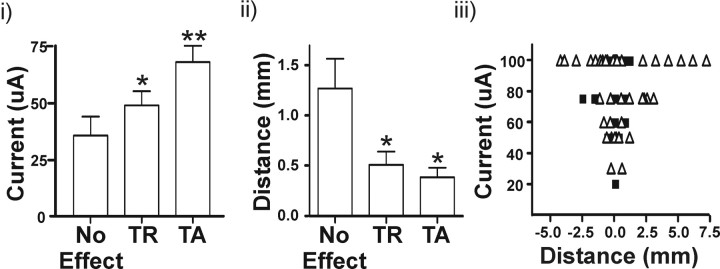
In this last set of experiments, we performed extracellular recording in eight patients undergoing implantation of thalamic DBS electrodes for essential tremor. We tested the prediction, based on the functional deafferentation hypothesis, that tremor suppression does not require direct current spread to tremor cell soma. The effect of microstimulation at various current intensities and distances from 36 thalamic tremor cells was determined. When stimulation was performed within 1 mm of an identified tremor cell(s), the current intensity and degree of tremor suppression were compared. As shown in Figure 7i, no effect on tremor was observed <37.3 ± 6.9 μA. Gradually increasing the current initially produced tremor reduction (TR; 54.3 ± 6.4 μA) followed by tremor arrest (TA; 67.9 ± 7.1 μA). Similarly, at a constant stimulation intensity of 50 μA, the effectiveness of stimulation increased as the electrode approached the tremor cell(s) (no effect, 1.29 ± 0.29 mm; TR, 0.51 ± 0.13 mm; TA, 0.39 ± 0.10) (Fig. 7ii). Hence, the closer the electrical stimulation was applied to tremor cells, the lower the current required to suppress tremor and the more likely tremor arrest would occur (Fig. 7iii). However, tremor suppression was frequently (16 of 61 successful stimulation sites) observed when the stimulating electrode was >2 mm from a tremor cell, a distance well beyond the maximum spread of the current from the stimulating microelectrode (Bagshaw and Evans, 1976).
Figure 7.
Alleviation of tremor is related with stimulation proximity to tremor cells. Thalamic microelectrode stimulation was performed in eight patients before implantation of the DBS macroelectrode. We identified 36 tremor cells and applied microstimulation at multiple sites at various current intensities. i, At stimulation sites <1 mm away from a tremor cell, increasing the stimulation current intensity increased the effectiveness of tremor stoppage. ii, At a constant stimulation intensity of 50 μA, better tremor suppression was achieved at stimulation sites closer to recorded tremor cells. iii, The relationship between current and distance for tremor reduction (open triangles) and tremor arrest (closed squares) are plotted. Note that tremor suppression was observed at distances >2 mm from recorded tremor cells. However, at these distances, low-current intensity-induced tremor reduction was not observed, suggesting the absence of other nearby tremor cells. TR, Tremor reduction; TA, tremor arrest. *p < 0.05 from control (no effect); **p < 0.05 from control (no effect) and TR.
Discussion
High-frequency stimulation of thalamic motor nuclei is effective in treating various forms of tremor. Although the underlying therapeutic mechanisms are unknown, it is thought that DBS disrupts local neural network activities essential for tremor genesis or propagation (Benabid et al., 1996; Kiss et al., 2002; Anderson et al., 2003). Here, we found that the primary effect of sDBS on thalamic neurons is a homosynaptic depression, without an inhibition of the persistent sodium (INap) or the predominantly IT-mediated rebound current, in contrast with findings from STN (Beurrier et al., 2001; Do and Bean, 2003). Furthermore, whereas sDBS tonically activated the Ih current and induced a small increase in membrane excitability, it did not result in a significant membrane depolarization under current clamp (Anderson et al., 2004a). sDBS-induced homosynaptic depression was restricted to the stimulated pathway, leaving the postsynaptic cell responsive to synaptic input from another converging independent pathway. In humans, microstimulation was most effective in suppressing tremor when applied close to tremor cells. However, tremor arrest was also induced with microstimulation at a location up to 2.5 mm away from a recorded tremor cell. These data all point to a synaptically mediated mechanism for DBS-based tremor therapy in the thalamus.
sDBS selectively disrupts afferent transmission
The ventral motor thalamus receives afferent input from multiple areas (cortical, cerebellar, pallidal, intrathalamic) while providing major output to the frontal premotor and motor cortex (Steriade et al., 1997). The ability of DBS to reduce tremor without disrupting normal motor control function suggests that its effect may be targeting selective sets of synaptic afferents. In this regard, the predominant cellular effect of sDBS in the thalamus appears surprisingly consistent: a homosynaptic depression of signal transmission that is quickly reversible and does not cross over to other nonstimulated pathway(s). Low-frequency stimulation (<50 Hz) is ineffective. High-frequency stimulation is required to depress synaptic input, including afferent tremor signals, in a pathway-restricted manner. These characteristics revealed at the cellular level are consistent with the clinical picture, in which thalamic DBS tremor therapy displays robust high-frequency dependency (Benabid et al., 1993, 1996), functional reversibility, and can be continuously applied for years without causing overt morphological damage (Boockvar et al., 2000) or motor disability (Rehncrona et al., 2003). Whereas the slice preparation does not allow us to identify the exact source of synaptic input (i.e., corticothalamic vs cerebellothalamic), the predominance and consistency of this form of homosynaptic depression suggest functional deafferentation being a general mechanism by which thalamic DBS limits tremor genesis and/or propagation.
Previous in vivo intracellular recordings in cat VL thalamus have shown that EPSPs from corticothalamic and cerebellothalamic pathways exhibit no evident potentiation rather than simple summation (Deschenes and Hu, 1990). Anatomically synaptic afferents from extrathalamic sources ending on relay neuron dendrites are highly compartmentalized in mammals (Steriade et al., 1997), and stimulation of the internal capsule in slices can evoke noninteracting synaptic responses in the same thalamic cell (Castro-Alamancos and Calcagnotto, 1999). Our findings based on intrathalamic cross-pathway stimulation are consistent with these features in that synaptic facilitation and depression occurred homosynaptically and did not “spill over” to nonstimulated pathways.
An important factor contributing to the pathway selectivity of the sDBS response derives from the fact that the homosynaptic depression is essentially a presynaptic phenomenon. The lack of effects of CTZ on sDBS-induced synaptic depression suggests that desensitization of postsynaptic AMPA receptors does not play a significant role. Similarly, the absence of cross talk does not favor a postsynaptic mechanism such as that mediated by diffusion of extrasynaptic glutamate or activation of intracellular processes. A more likely cause for homosynaptic depression is transmitter depletion. Indeed, the relatively fast depression of synaptic currents induced by sDBS (i.e., the onset and recovery time) are mostly compatible with that described for glutamatergic terminals during transmitter depletion and recovery (Stevens and Tsujimoto, 1995; Rosenmund and Stevens, 1996; Stevens and Wesseling, 1998).
Compared with humans, the rat thalamus has few GABAergic interneurons (Williams and Faull, 1987), and therefore the effect of inhibitory synaptic potentials within the ventral thalamus in response to DBS is difficult to compare between humans and rat slices. However, the presence of GABAergic projection fibers and innervations appears to be insufficient to alter the main features of the thalamic sDBS response in vitro (Lee et al., 2005).
sDBS-induced alteration of intrinsic thalamic membrane currents
DBS delivers high-frequency current pulses directly toward neuronal somata and dendrites. In theory, membrane channels that are sensitive to changes in membrane voltage, local neurotransmitter, or metabolite levels could be inhibited or modulated by DBS to reduce neuronal excitability. This issue can be addressed in brain slices by examining whether sDBS can directly modulate different membrane currents. Studies of STN neurons have reported that high-frequency stimulation induces a direct inhibition of membrane currents, specifically the persistent sodium, and the T- and L-type calcium currents (Beurrier et al., 2001). Do and Bean (2003) also observed that when STN neurons are deprived of synaptic input, the persistent, transient, and resurgent sodium currents are inhibited. In contrast to these studies, we found that high-frequency stimulation (i.e., sDBS) in the thalamus induced no obvious inhibition in the rebound and the INap currents. The most consistent effect we found was a tonic activation of Ih and a slight increase in membrane excitability (Anderson et al., 2004a). Indeed, in current-clamp mode and in the presence of glutamate receptor blockade, sDBS induces a residual membrane depolarization of no more than 2 mV (Anderson et al., 2004a), which may be related to the observed Ih activation seen in this study. There are several reasons that may explain the discrepancy between thalamic and STN neurons. These include the presence of a resurgent sodium current in STN but not in thalamic neurons (Do and Bean, 2003), as well as potential differences in tissue preparations used in the various studies (Anderson et al., 2004b). The sensitivity of the Ih current to extracellular potassium (McCormick and Pape, 1990a; Maccaferri et al., 1993) and/or the evoked release of nonglutamatergic neurotransmitters (Pape and McCormick, 1989; McCormick and Pape, 1990b) may also play a role. Our observation that there was no inhibition of the rebound current (low-threshold spike) is, however, consistent with in vivo recordings obtained in tremor patients, which have shown that thalamic neurons preserve their burst firing capacity immediately after a DBS train (Dostrovsky et al., 1999). It is possible that the extracellular stimulus pulses of sDBS are too brief (<200 μs) to alter the kinetics of activation and/or inactivation of most Na+ and K+ channels expressed in the somata of thalamic neurons but may effect those currents expressed predominantly on thin dendrites, such as Ih (Magee, 1998). Regardless, such activation of Ih did not seem to be responsible for the synaptic depression induced by sDBS, nor did it alter the synaptic responsiveness to other input.
Location dependence of the DBS effect in human thalamus
In eight essential tremor patients, we investigated the relationship between tremor suppression, stimulation intensity, and electrode location. We found: (1) the effect of stimulation on tremor is not an “all-or-none” phenomenon but is graded, varying with both location and stimulation parameters; (2) using a fixed stimulation intensity (100 μA), the therapeutic effect of microstimulation can be observed at distances >2 mm from a recorded tremor cell, far greater than the estimated current spread (∼0.5 mm) from the microelectrode (Bagshaw and Evans, 1976; Dostrovsky et al., 1993a); and (3) that whereas stimulation at a distance was effective, stimulation closer to tremor cells was better at producing tremor arrest.
The mechanism of DBS action has been ascribed to synaptic activation and/or a direct current effect that alters cellular membrane properties (Anderson et al., 2004a) (for review, see Breit et al., 2004). Our current human studies revealed that DBS was effective in mediating tremor arrest and reduction, at distances beyond the spread of direct current from the stimulating microelectrode to identified tremor cells. As a result, the human data suggest that direct modulation of intrinsic membrane current is not required to achieve the beneficial effects of thalamic DBS on essential tremor and instead suggest a presynaptic mechanism mediated by axonal activation. The same conclusion is also reached by our slice work in which axonal activation (and synaptic transmission failure), but not direct current modulation, of intrinsic membrane currents dominate sDBS responses.
Our human recordings are derived from essential tremor patients. In Parkinson's disease, tremor cell activity in the thalamus has been thought to occur as a result of membrane hyperpolarization-triggered LTS rebound burst (Pare et al., 1990). However, this hypothesis has not been supported by recordings from the human thalamus (Zirh et al., 1998). As for patients with essential tremor, very little is known about the mechanism of tremor cell burst. Although in the awake state the majority of thalamic relay cells are in a tonic firing mode, a small percentage of cells remain in burst mode (Gaze et al., 1964; Lenz et al., 1989). Bursting is particularly prominent in nonlemniscal thalamic neurons (He and Hu, 2002; Ramcharan et al., 2005), in which a more hyperpolarized membrane potential may allow bursts to be triggered through EPSP-LTS coupling (Hu, 2003; Mooney et al., 2004). Therefore, interactions between excitatory synaptic afferents (Pinto et al., 2003; Molnar et al., 2004) and intrinsic membrane currents may play an important role in essential tremor activities. If this conjecture is correct, both mechanisms of synaptic depression and direct facilitation of Ih may contribute to tremor stoppage.
Because our human data were obtained intraoperatively using microelectrodes with exposed tips of 15–40 μm and the stimulation parameters and configuration were similar to that used in our in vitro study, the human and rat slice data may be comparable. For instance, the time course of the onset and recovery of sDBS-induced synaptic depression in slices mirrors the time course for human microstimulation-induced tremor stoppage and tremor return after cessation of stimulation (both of which are on the order of seconds). Consequently, our synaptic deafferentation hypothesis based on brain slice data provides the most parsimonious explanation for the distance phenomenon of DBS in the human thalamus.
A mechanism of presynaptic activation initially appears at odds with our finding that in humans, stimulation closer to tremor cells was more effective in alleviating tremor. However, tremor cells in human thalamus cluster (Lenz et al., 1995) are likely driven by multiple tremor signal carrying afferents. Therefore, the efficacy of DBS in reducing tremor at a particular location should be influenced by the numbers of tremor afferents affected by the stimulation. This conjecture is supported by our slice work, which shows that sDBS-induced synaptic depletion is pathway specific and restricted only to the stimulated pathway. Therefore, if the microelectrode is moved closer to where the afferent tremor fibers converge onto a single and/or a cluster of tremor cells, it will increase the probability that more tremor-related synaptic afferents will be affected by local DBS current, thereby producing a more pronounced effect on tremor. Indeed, our data show that DBS closer to tremor cells produced more tremor arrest compared with more distant stimulation.
Finally, it has been suggested that the effects of DBS may include direct modulation of thalamocortical efferent outflow, decoupled from somatic activity (McIntryre et al., 2004) or antidromic activation of thalamic neurons. Our animal and human data suggest that axons are indeed a key target of DBS (Kiss et al., 2003c; Anderson et al., 2004a), but that elimination of afferent tremor signals to thalamic tremor cells is sufficient to stop tremor propagation. This conjecture is consistent with the fact that local application of muscimol (Pahapill et al., 1999), lidocaine (Dostrovsky et al., 1993b), or lesions close to thalamic tremor cells (Lenz et al., 1995) are equally effective as thalamic DBS in stopping tremor. Therefore, we believe that thalamic DBS may stop tremor by activating afferent axons, inducing synaptic depression, and preventing continued propagation of the tremor signal.
Footnotes
This work was supported by the Alberta Heritage Foundation for Medical Research (AHFMR) and the Canadian Institutes of Health Research (CIHR). T.R.A. holds a postdoctoral fellowship from the Parkinson Society of Canada. Z.H.T.K. is Clinician-Scientist at the CIHR and Clinical Investigator for AHFMR.
Correspondence should be addressed to Dr. Zelma H. T. Kiss, Hotchkiss Brain Institute, Department of Clinical Neurosciences, University of Calgary, Heritage Medical Research Building, Room 182A, 3330 Hospital Drive Northwest, Calgary, Alberta, Canada T2N 4N1. E-mail: zkiss@ucalgary.ca.
DOI:10.1523/JNEUROSCI.3523-05.2006
Copyright © 2006 Society for Neuroscience 0270-6474/06/260841-10$15.00/0
References
- Anderson ME, Postupna N, Ruffo M (2003) Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol 89: 1150–1160. [DOI] [PubMed] [Google Scholar]
- Anderson T, Hu B, Pittman Q, Kiss ZH (2004a) Mechanisms of deep brain stimulation: an intracellular study in rat thalamus. J Physiol (Lond) 559: 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T, Hu B, Pittman Q, Kiss ZH (2004b) The effects of deep brain stimulation of rat ventral thalamus are age and stimulation parameter dependent. Soc Neurosci Abstr 30: 753.13. [Google Scholar]
- Anderson T, Hu B, Kiss ZH (2005) The “roadblock” to tremor signal propagation: a selective action of deep brain stimulation on afferent synaptic transmission. Soc Neurosci Abstr 31: 988.12. [Google Scholar]
- Arai A, Lynch G (1998) AMPA receptor desensitization modulates synaptic responses induced by repetitive afferent stimulation in hippocampal slices. Brain Res 799: 235–242. [DOI] [PubMed] [Google Scholar]
- Bagshaw EV, Evans MH (1976) Measurement of current spread from microelectrodes when stimulating within the nervous system. Exp Brain Res 25: 391–400. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Seigneuret E, Hoffmann D, Gay E, Perret J (1993) Chronic VIM thalamic stimulation in Parkinson's disease, essential tremor and extra-pyramidal dyskinesias. Acta Neurochir Suppl (Wien) 58: 39–44. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, Payen I, Benazzouz A (1996) Chronic electrical stimulation of the ventralis inter-medius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg 84: 203–214. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C (2001) High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 85: 1351–1356. [DOI] [PubMed] [Google Scholar]
- Boockvar JA, Telfeian A, Baltuch GH, Skolnick B, Simuni T, Stern M, Schmidt ML, Trojanowski JQ (2000) Long-term deep brain stimulation in a patient with essential tremor: clinical response and postmortem correlation with stimulator termination sites in ventral thalamus. Case report. J Neurosurg 93: 140–144. [DOI] [PubMed] [Google Scholar]
- Breit S, Schulz JB, Benabid AL (2004) Deep brain stimulation. Cell Tissue Res 318: 275–288. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Calcagnotto ME (1999) Presynaptic long-term potentiation in corticothalamic synapses. J Neurosci 19: 9090–9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR (1978) Possible involvement of central pacemakers in clinical disorders of movement. Fed Proc 37: 2171–2175. [PubMed] [Google Scholar]
- Deschenes M, Hu B (1990) Electrophysiology and pharmacology of corticothalamic input in neurons of the lateral thalamic nuclei: an intracellular study in the cat. Eur J Neurosci 2: 140–152. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Bergman H (2002) Pathophysiology of nonparkinsonian tremors. Mov Disord 17 [Suppl 3]: S41–S48. [DOI] [PubMed] [Google Scholar]
- Do MT, Bean BP (2003) Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron 39: 109–120. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Davis KD, Lee L, Sher GD, Tasker RR (1993a) Electrical stimulation-induced effects in the human thalamus. Adv Neurol 63: 219–229. [PubMed] [Google Scholar]
- Dostrovsky JO, Sher GD, Davis KD, Parrent AG, Hutchison WD, Tasker RR (1993b) Microinjection of lidocaine into human thalamus: a useful tool in stereotactic surgery. Stereotact Funct Neurosurg 60: 168–174. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Wu JP, Levy R, Hutchison WD, Davis KD, Tasker RR, Lozano AM (1999) Microstimulation-induced effects on neurons in human globus pallidus and motor thalamus. Soc Neurosci Abstr 25: 150.14. [Google Scholar]
- Gaze RM, Gillingham FJ, Kalyanaraman S, Porter RW, Donaldson AA, Donaldson IM (1964) Microelectrode recordings from the human thalamus. Brain 87: 691–706. [DOI] [PubMed] [Google Scholar]
- He J, Hu B (2002) Differential distribution of burst and single-spike responses in auditory thalamus. J Neurophysiol 88: 2152–2156. [DOI] [PubMed] [Google Scholar]
- Hu B (2003) Functional organization of lemniscal and nonlemniscal auditory thalamus. Exp Brain Res 153: 543–549. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA (1992) Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol 68: 1373–1383. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R (1984a) Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol (Lond) 349: 205–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R (1984b) Voltage-dependent burst-to-tonic switching of thalamic cell activity: an in vitro study. Arch Ital Biol 122: 73–82. [PubMed] [Google Scholar]
- Kiss ZH, Mooney DM, Renaud L, Hu B (2002) Neuronal response to local electrical stimulation in rat thalamus: physiological implications for mechanisms of deep brain stimulation. Neuroscience 113: 137–143. [DOI] [PubMed] [Google Scholar]
- Kiss ZH, Wilkinson M, Krcek J, Suchowersky O, Hu B, Murphy WF, Hobson D, Tasker RR (2003a) Is the target for thalamic deep brain stimulation the same as for thalamotomy? Mov Disord 18: 1169–1175. [DOI] [PubMed] [Google Scholar]
- Kiss ZHT, Davis KD, Tasker RR, Lozano AM, Hu B, Dostrovsky JO (2003b) Kinaesthetic neurons in thalamus of humans with and without tremor. Exp Brain Res 150: 85–94. [DOI] [PubMed] [Google Scholar]
- Kiss ZH, Anderson T, Hansen T, Kirstein D, Suchowersky O, Hu B (2003c) Neural substrates of microstimulation-evoked tingling: a chronaxie study in human somatosensory thalamus. Eur J Neurosci 18: 728–732. [DOI] [PubMed] [Google Scholar]
- Lee KH, Hitti FL, Shalinsky MH, Kim U, Leiter JC, Roberts DW (2005) Abolition of spindle oscillations and 3-Hz absence seizure like activity in the thalamus by using high-frequency stimulation: potential mechanism of action. J Neurosurg 103: 538–545. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Tasker RR, Kwan HC, Schnider S, Kwong R, Murphy JT (1985) Cross-correlation analysis of thalamic neurons and EMG activity in parkinsonian tremor. Appl Neurophysiol 48: 305–308. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Tasker RR, Kwan HC, Schnider S, Kwong R, Murayama Y, Dostrovsky JO, Murphy JT (1988) Single unit analysis of the human ventral thalamic nuclear group: correlation of thalamic “tremor cells” with the 3–6 Hz component of parkinsonian tremor. J Neurosci 8: 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR (1989) Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Res 496: 357–360. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR, Murphy JT, Lenz YE (1990) Single unit analysis of the human ventral thalamic nuclear group. Activity correlated with movement. Brain 113: 1795–1821. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Martin RL, Tasker RR, Dostrovsky JO, Lenz YE (1994) Single unit analysis of the human ventral thalamic nuclear group. Tremor-related activity in functionally identified cells. Brain 117: 531–543. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Normand SL, Kwan HC, Andrews D, Rowland LH, Jones MW, Seike M, Lin YC, Tasker RR, Dostrovsky JO (1995) Statistical prediction of the optimal site for thalamotomy in parkinsonian tremor. Mov Disord 10: 318–328. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Mangoni M, Lazzari A, DiFrancesco D (1993) Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J Neurophysiol 69: 2129–2136. [DOI] [PubMed] [Google Scholar]
- Magee JC (1998) Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR (1992) A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol 68: 1384–1400. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC (1990a) Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol (Lond) 431: 291–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC (1990b) Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol (Lond) 431: 319–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM (2002) Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J Neurophysiol 88: 1592–1604. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM, Sherman DL, Thakor NV (2004) Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol 91: 1457–1469. [DOI] [PubMed] [Google Scholar]
- Molnar GF, Sailer A, Gunraj CA, Lang AE, Lozano AM, Chen R (2004) Thalamic deep brain stimulation activates the cerebellothalamocortical pathway. Neurology 63: 907–909. [DOI] [PubMed] [Google Scholar]
- Mooney DM, Zhang L, Basile C, Senatorov VV, Ngsee J, Omar A, Hu B (2004) Distinct forms of cholinergic modulation in parallel thalamic sensory pathways. Proc Natl Acad Sci USA 101: 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahapill PA, Levy R, Dostrovsky JO, Davis KD, Rezai AR, Tasker RR, Lozano AM (1999) Tremor arrest with thalamic microinjections of muscimol in patients with essential tremor. Ann Neurol 46: 249–252. [DOI] [PubMed] [Google Scholar]
- Pape HC, McCormick DA (1989) Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature 340: 715–718. [DOI] [PubMed] [Google Scholar]
- Pare D, Curro'Dossi R, Steriade M (1990) Neuronal basis of the parkinsonian resting tremor: a hypothesis and its implications for treatment. Neuroscience 35: 217–226. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V (1998) Sodium current in rat and cat thalamocortical neurons: role of a non-inactivating component in tonic and burst firing. J Neurosci 18: 854–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The rat brain in stereotactic coordinates. San Diego: Academic.
- Pinto AD, Lang AE, Chen R (2003) The cerebellothalamocortical pathway in essential tremor. Neurology 60: 1985–1987. [DOI] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM (2005) Higher-order thalamic relays burst more than first-order relays. Proc Natl Acad Sci USA 102: 12236–12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehncrona S, Johnels B, Widner H, Tornqvist AL, Hariz M, Sydow O (2003) Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord 18: 163–170. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF (1996) Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16: 1197–1207. [DOI] [PubMed] [Google Scholar]
- Rubin JE, Terman D (2004) High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J Comp Neurosci 16: 211–235. [DOI] [PubMed] [Google Scholar]
- Schuurman PR, Bosch DA, Bossuyt PM, Bonsel GJ, van Someren EJ, de Bie RM, Merkus MP, Speelman JD (2000) A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med 342: 461–468. [DOI] [PubMed] [Google Scholar]
- Steriade M, Deschênes M (1984) The thalamus as a neuronal oscillator. Brain Res Brain Res Rev 8: 1–63. [DOI] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick D (1997) Thalamus, Ed 2. New York: Elsevier.
- Stevens CF, Tsujimoto T (1995) Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc Natl Acad Sci USA 92: 846–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF, Wesseling JF (1998) Activity-dependent modulation of the rate at which synaptic vesicles become available to undergo exocytosis. Neuron 21: 415–424. [DOI] [PubMed] [Google Scholar]
- Tasker RR, Kiss ZH (1995) The role of the thalamus in functional neurosurgery. Neurosurg Clin N Am 6: 73–104. [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM (1993) Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron 10: 1185–1196. [DOI] [PubMed] [Google Scholar]
- Vitek JL (2002) Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord 17 [Suppl 3]: S69–S72. [DOI] [PubMed] [Google Scholar]
- Williams MN, Faull RL (1987) The distribution and morphology of identified thalamocortical projection neurons and glial cells with reference to the question of interneurons in the ventrolateral nucleus of the rat thalamus. Neuroscience 21: 767–780. [DOI] [PubMed] [Google Scholar]
- Zirh TA, Lenz FA, Reich SG, Dougherty PM (1998) Patterns of bursting occurring in thalamic cells during parkinsonian tremor. Neuroscience 83: 107–121. [DOI] [PubMed] [Google Scholar]