Abstract
The brains of fetal alcohol syndrome patients exhibit impaired neuronal migration, but little is known about the mechanisms underlying this abnormality. Here we show that Ca2+ signaling and cyclic nucleotide signaling are the central targets of alcohol action in neuronal cell migration. Acute administration of ethanol reduced the frequency of transient Ca2+ elevations in migrating neurons and cGMP levels and increased cAMP levels. Experimental manipulations of these second-messenger pathways, through stimulating Ca2+ and cGMP signaling or inhibiting cAMP signaling, completely reversed the action of ethanol on neuronal migration in vitro as well as in vivo. Each second messenger has multiple but distinct downstream targets, including Ca2+/calmodulin-dependent protein kinase II, calcineurin, protein phosphatase 1, Rho GTPase, mitogen-activated protein kinase, and phosphoinositide 3-kinase. These results demonstrate that the aberrant migration of immature neurons in the fetal brain caused by maternal alcohol consumption may be corrected by controlling the activity of these second-messenger pathways.
Keywords: cerebellar development, granule cell, neuronal cell migration, cAMP, cGMP, rate of cell movement
Introduction
Prolonged exposure to alcohol during gestation and lactation correlates with a pattern of abnormal development in newborns (Chiriboga, 2003; Lemoine et al., 2003; Sokol et al., 2003; Goodlett et al., 2005). Jones and Smith (1973) called this developmental disturbance “fetal alcohol syndrome” (FAS). The disturbance of the CNS is the most serious feature of FAS (Marcus, 1987; Riley and McGee, 2005; Welch-Carre, 2005). For example, microencephaly is common among patients with FAS (Wisniewski et al., 1983). Several aspects of development are involved in the alcohol-induced malformation of the brain (Jones, 1988; Coulter et al., 1993; Guerri, 2002). Among them, the most striking abnormalities appear to involve the impairment of neuronal and glial migration (Miller, 1986, 1993).
The fundamental mechanisms by which alcohol administration leads to the disturbance of brain development have not been delineated definitively. Previous studies suggest that alcohol or a metabolic product is responsible for the teratogenic effects expressed in FAS (Randall et al., 1990; West et al., 1994), and may adversely affect the developing brain through multiple mechanisms (Kennedy, 1984). These include direct effects such as transient impairment of uterine vessels and reduced fetal cerebral metabolic rate (Kennedy, 1984). Alcohol is also capable of affecting voltage-gated ion channels, neurotransmitter receptors, signal transduction, transcription of multiple genes, trophic support of neurons, and membrane fluidization (West et al., 1994; Costa et al., 2004; Olney, 2004).
In this study, we focused on how alcohol affects the migration of immature neurons. To this end, we used cerebellar granule cells from the early postnatal mouse as a model system (Cudd, 2005). Children with FAS show neurological signs associated with cerebellar damage, such as delayed motor development, problems with fine tasks, and ataxia (Little et al., 1989; Green et al., 2002; Hauser et al., 2003; Coffin et al., 2005; Manzardo et al., 2005). The most vulnerable period of cerebellar development in humans is during the third trimester (Clarren, 1986). The equivalent time of development in mice is during the early postnatal period (Kornguth et al., 1979), and alcohol exposure results in abnormal development of the postnatal cerebellum (Kornguth et al., 1979; Sakata-Haga et al., 2001; Dikranian et al., 2005). In particular, the numbers of granule cells in the internal granular layer (IGL), where postmigratory granule cells reside and make synaptic connections with mossy fiber terminals, were significantly reduced in the alcohol-treated animals (Borges and Lewis, 1983), suggesting that alcohol affects the migration of immature granule cells from their birthplace to their final destination.
We first determined the quantitative relationship between the amount of ethanol exposure and the effects on cerebellar granule cell migration. Second, we examined the intracellular mechanisms by which ethanol affects the migration of granule cells. In particular, we focused on the roles of Ca2+ signaling and cyclic nucleotide signaling in the ethanol-induced impairment of granule cell migration. Finally, we examined whether the impaired migration of granule cells caused by ethanol can be reversed by controlling the second-messenger pathways.
Materials and Methods
All animal procedures were approved by the Internal Animal Care and Use Committee of the Cleveland Clinic Foundation.
Granule cell migration in acute slice preparations. Cerebella of postnatal 7-, 10-, and 13-d-old mice (CD-1) were sectioned transversely or sagittally into 150- to 200-μm-thick slices on a vibrating blade microtome (Komuro and Rakic, 1995, 1998a; Komuro et al., 2001). To label granule cells, cerebellar slices were incubated for 3 min in a fluorescent lipophilic carbocyanine dye [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)] (7 μg/ml) (Invitrogen, Eugene, OR), which was added to the culture medium. The culture medium consisted of a minimum essential medium (Invitrogen) supplemented with (in mm)30 glucose, 1.8 glutamine, and 24 NaHCO3. One or 2 h after staining with DiI, cerebellar slices were transferred into the chamber of a microincubator (Medical Systems Corp., Greenvale, NY) attached to the stage of a confocal microscope (Leica, Nussloch, Germany). Chamber temperature was kept at 37°C, and the cells were provided with a constant gas flow (95% O2, 5% CO2). The tissue was illuminated with a 488-nm-wavelength light from an argon laser through a 40× oil-immersion objective (numerical aperture, 1.25; Leica), and fluorescence emission was detected at 530 ±15 nm. To determine the effects of ethanol on neuronal migration, images of granule cells labeled with DiI were collected every minute for up to 5 h before and after application of ethanol. Ethanol is volatile, and to maintain ethanol concentrations in the medium, we continuously perfused the culture medium with ethanol ranging from 2.5 to 100 mm. The inhibition of migration was evaluated by dividing the distance traveled during the first 60 min in the absence of ethanol by the distance traveled during the next 60 min after its application.
Migration of isolated granule cells in microexplant culture. Cerebella of postnatal day 0–3 (P0–P3) mice (CD-1) were placed in ice-chilled HBSS and freed from meninges and choroid plexus (Komuro and Rakic, 1996, 1999; Yacubova and Komuro, 2002a; Kumada and Komuro, 2004). Cerebellar slices were then made with a surgical blade, from which white matter and deep cerebellar nuclei were removed. Rectangular pieces (50–100 μm) were dissected out from the remaining tissue, which consisted mainly of cerebellar gray matter, under a dissecting microscope. Small pieces of cerebellum were placed on 35 mm glass-bottom microwell dishes (MatTec, Ashland, MA) that had been coated with poly-l-lysine (100 μg/ml)/laminin (20 μg/ml). Each dish was put in a CO2 incubator (37°C, 95% air, 5% CO2). The incubation medium consisted of DMEM/F-12 (Invitrogen) with N2 supplement, 90 U/ml penicillin, and 90 μg/ml streptomycin. In these cultures, >95% of the migrating neurons were granule cells, which were easily distinguished from other neurons by the small size of their cell bodies (Komuro and Rakic, 1996; Yacubova and Komuro, 2002a). Although granule cells were prepared from the external granule layer (EGL) and the IGL of all lobules of the cerebellum, most of the granule cells were derived from the EGL, because at the age of P0–P3, the IGL contains only a small number of postmigratory granule cells (Yacubova and Komuro, 2002a); therefore, most of the granule cells were at the same developmental stage. One day after plating, dishes were transferred into the chamber of a micro-incubator (Medical Systems Corp.) attached to the stage of a confocal microscope (Leica). Chamber temperature was kept at 37°C, and the cells were provided with a constant gas flow (95% air, 5% CO2). The transmitted images of migrating granule cells at 488 nm were collected every 60 s for up to 5 h. To examine the effects of ethanol on granule cell migration, 25–200 mm ethanol was added to the culture medium.
Measurement of granule cell migration in vivo. P9 (CD-1) mice were injected intraperitoneally with 5-bromo-2′-deoxyuridine (BrdU; 50 mg/kg body weight) (Komuro et al., 2001). One day after BrdU injection, one group of mice was injected with 100 μl of saline, caffeine (15 mg/kg body weight), NMDA (2 mg/kg body weight), Rp-cAMPS (2 mg/kg body weight), or Br-cGMP (2 mg/kg body weight) into the peritoneal cavity with or without an ethanol injection (5 g/kg body weight; 25%, v/v mixed in saline). The other group of mice was injected with 5 μl of saline, caffeine (2 mg/kg body weight), NMDA (0.01 mg/kg body weight), Rp-cAMPS (0.4 mg/kg body weight), or Br-cGMP (0.4 mg/kg body weight) into the subarachnoid space between the skull and the surface of the cerebellum and injected with ethanol (5 g/kg body weight; 25%, v/v mixed in saline) or saline into the peritoneal cavity. Two days after BrdU injection, all animals were transcardially perfused with 4% paraformaldehyde. Brains were postfixed in 4% paraformaldehyde for 24 h, stored in a 30% sucrose solution, and sectioned sagittally into 30-μm-thick slices. In each section, cells that had incorporated BrdU into DNA were detected by an anti-BrdU monoclonal antibody (BrdU labeling and Detection Kit I; Boehringer Mannheim, Indianapolis, IN) and fluorescein-conjugated secondary antibody (Komuro et al., 2001). Fluorescent signals were detected and processed with a confocal microscope.
Ca2+ measurements in isolated granule cells. Small pieces of P0–P3 mouse (CD-1) cerebellum were placed on 35 mm glass-bottom microwell dishes that had been coated with poly-l-lysine (100 μg/ml)/laminin (20 μg/ml) (Komuro and Rakic, 1996; Yacubova and Komuro, 2002b). Each dish was put in a CO2 incubator (37°C, 95% air, 5% CO2). The incubation medium consisted of DMEM/F-12 (Invitrogen) with N2 supplement, 90 U/ml penicillin, and 90 μg/ml streptomycin. One day after plating, the granule cells in the microexplant cultures were incubated for 30 min with the cell-permeant, acetoxymethyl ester form of 1 μm Oregon Green 488 BAPTA-1 (Invitrogen) diluted in the culture medium. The cells were subsequently washed three times with the culture medium, and the dye was allowed to de-esterify for an additional 30–60 min in the CO2 incubator. A confocal microscope was used to examine the changes in Ca2+ levels and cell movement. The granule cells loaded with Oregon Green 488 BAPTA-1 were illuminated with 488-nm-wavelength light, and fluorescence images for Ca2+ measurements (at 530 ± 15 nm) and transmitted images for monitoring cell movement were collected simultaneously every 1–10 s for up to 2 h. The changes in fluorescence intensity of each granule cell were normalized to its baseline fluorescent intensity. To examine the effects of ethanol on the frequency of Ca2+ transients in the granule cell somata and the rate of cell migration, 25–200 mm ethanol was added to the culture medium.
Determination of cAMP and cGMP levels. Forty-six P10 (CD-1) mice were injected intraperitoneally with ethanol (5 g/kg body weight; 25%, v/v mixed in saline). At 1 h after ethanol injection, the mice were killed, and whole cerebella were isolated. Then, the cerebella were weighed and frozen in liquid nitrogen to inactivate the endogenous enzymes. Thereafter, the frozen cerebella were homogenized in 10 vol of 0.1 m HCl. The homogenates were centrifuged at 10,000 rpm for 5 min, and the supernatants were used for the assay. cAMP levels and cGMP levels in the cerebella were determined by use of the Direct cAMP Enzyme Immunoassay kit (Sigma, St. Louis, MO) and the Direct cGMP Enzyme Immunoassay kit (Sigma), according to the manufacturer's instructions. Furthermore, the total amount of proteins was estimated with the BCA Protein Assay kit (Pierce, Rockford, IL).
Detection of apoptotic cell death of granule cells. P10 (CD-1) mice were injected intraperitoneally with 100 μl of saline, caffeine (15 mg/kg body weight), NMDA (2 mg/kg body weight), Rp-cAMPS (2 mg/kg body weight), or Br-cGMP (2 mg/kg body weight) with or without ethanol (5 g/kg body weight; 25%, v/v mixed in saline). One day after injection, all animals were transcardially perfused with 4% paraformaldehyde. Brains were postfixed in 4% paraformaldehyde for 24 h, stored in a 30% sucrose solution, and sectioned into 30-μm-thick slices. In each section, apoptotic cell death of granule cells and their precursors was determined by the terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) assay kit (Roche Diagnostics, Roswell, GA), according to the manufacturer's instructions. After staining with TUNEL, the sections were incubated with 400 μl of 0.33 mm TO-PRO3 (Invitrogen) for 5 min at room temperature for nuclear staining.
Determination of blood alcohol levels. Thirty P10 (CD-1) mice were injected intraperitoneally with ethanol (5 g/kg body weight; 25%, v/v mixed in saline). At 0.5, 1, 2, 3, 4, 6, 8, 10, 12, and 24 h after ethanol injection, blood samples were collected from the mice, and ethanol concentrations in blood were determined by the use of the NAD-ADH Reagent Multiple Test Vial (Sigma), according to the manufacturer's instructions.
Statistical analysis. Statistical differences were determined with a Student's t test. Statistical significance was defined at p < 0.05 or p < 0.01.
Results
Dose-dependent reduction of the rate of granule cell migration in three cortical layers of early postnatal mouse cerebellum by ethanol
We first examined the effects of ethanol on cerebellar granule cell migration in vitro. In the developing cerebellum, postmitotic granule cells migrate from their birthplace at the top of the EGL, where their precursor cells actively proliferate every 19–22 h, to their final destination in the IGL (Rakic, 1971; Hatten and Heintz, 1995; Rakic and Komuro, 1995; Komuro and Yacubova, 2003). During this trip, the cells exhibit three distinct modes of migration: (1) tangential migration in the EGL; (2) Bergmann glia-guided radial migration in the molecular layer (ML); and (3) glia-independent radial migration in the IGL (Komuro and Rakic, 1998b; Yacubova and Komuro, 2003). Real-time observation of cell movement in cerebellar slices revealed that administration of ethanol (100 mm) immediately slows the tangential migration of granule cells in the EGL of a P10 mouse (Fig. 1A,B), and induces morphological change in the soma as indexed by the length/width ratio (Fig. 1C). Pharmacologically relevant concentrations of ethanol were based on blood ethanol concentrations attained by alcohol consumption in humans (Jones, 1988). Because ethanol readily crosses the placental and blood–brain barriers and diffuses rapidly into all aqueous compartments of the body (West et al., 1994), these levels would readily be found in cases of alcohol consumption during pregnancy. The effects of ethanol on the rate of granule cell migration in the cerebellar slices obtained from P10 mice were dose dependent (Fig. 1D). For example, although 2.5 mm ethanol failed to alter the rate of cell movement, 10 mm ethanol (equivalent to blood ethanol level <50 mg/dl) significantly decreased the rate of cell movement to 62% (EGL), 76% (ML), and 82% (IGL) of the control rate. In the presence of 50 mm ethanol, the rate of cell movement was reduced to 40% (EGL), 55% (ML), and 62% (IGL) of the controls. Finally, when 100 mm ethanol was added, the movement declined to 35% (EGL), 50% (ML), and 56% (IGL) of the control. These results demonstrate that ethanol slows granule cell migration in a dose-dependent manner. It is noteworthy that the vulnerability of granule cells to ethanol exposure decreased as the cells migrated from the EGL to the IGL (Fig. 1E). The differential vulnerability of granule cells among the three cortical layers suggests that the stage of differentiation (or maturation) is critical in producing the harmful effects of ethanol on granule cell migration.
Figure 1.
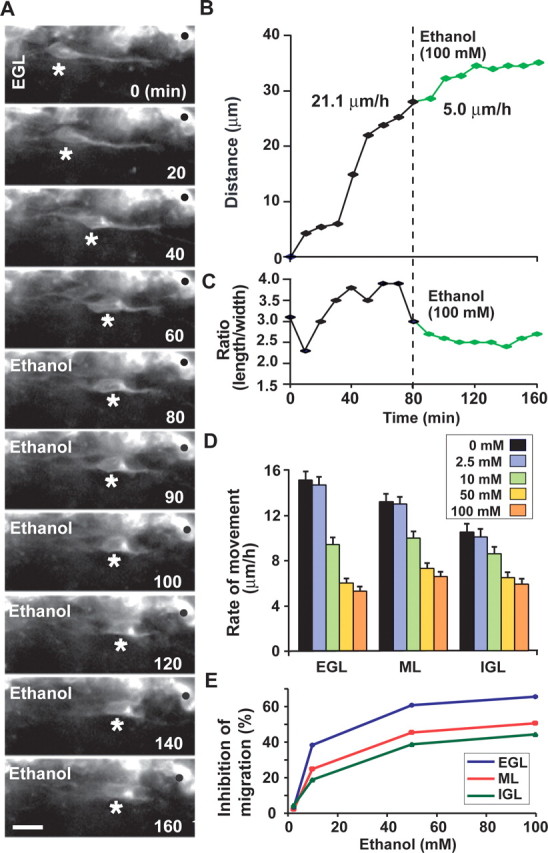
Ethanol slows the migration of cerebellar granule cells in a dose-dependent manner. A, Time-lapse images showing that acute administration of ethanol (100 mm) slows the tangential migration of granule cells in the EGL of a cerebellar slice obtained from a P10 mouse. Asterisks and dots mark the granule cell soma and reference points for cell movement, respectively. Elapsed time is indicated in the bottom right of each photograph. Scale bars, 12 μm. B, C, Effects of 100 mm ethanol on the total distance traversed by the granule cell soma (B) shown in A and the length/width ratio of the soma (C). D, Effects of different doses (2.5–100 mm) of ethanol on the migration rate of granule cells among three cerebellar cortical layers. Each column represents the average rate of cell movement obtained from at least 60 migrating cells. Error bars indicate SD. E, Inhibition rates of granule cell migration in the three cerebellar cortical layers by acute administration of ethanol (2.5–100 mm).
Next, we determined whether ethanol differentially affects granule cell migration at different ages. To address this question, we used cerebellar slices obtained from P7 and P13 mice, because in the mouse cerebellum, most of the granule cells migrate from the EGL to the IGL during a period ranging from P5 to P15 (Miale and Sidman, 1961; Fujita, 1967). We found that the granule cells in the cerebellum of younger mice are more susceptible to ethanol exposure than those of older mice. For example, in cerebellar slices obtained from P7 mice, an acute administration of 10 mm ethanol decreased the rate of granule cell migration to 53% (EGL), 69% (ML), and 74% (IGL) of the control rate, 50 mm ethanol decreased the rate to 36% (EGL), 49% (ML), and 55% (IGL) of the control rate, and 100 mm ethanol decreased the rate to 31% (EGL), 44% (ML), and 48% (IGL) of the control rate. In contrast, in cerebellar slices obtained from P13 mice, 10 mm ethanol decreased the rate of granule cell migration to 68% (EGL), 81% (ML), and 85% (IGL) of the control rate, 50 mm ethanol decreased the rate to 43% (EGL), 58% (ML), and 64% (IGL) of the control rate, and 100 mm ethanol decreased the rate to 38% (EGL), 55% (ML), and 59% (IGL) of the control rate. The different susceptibility of granule cells between different ages of animals suggests that microenvironments surrounding the granule cells modulate the effects of ethanol on cell migration.
Direct action of ethanol on the intrinsic migratory behavior of isolated granule cells in microexplant cultures
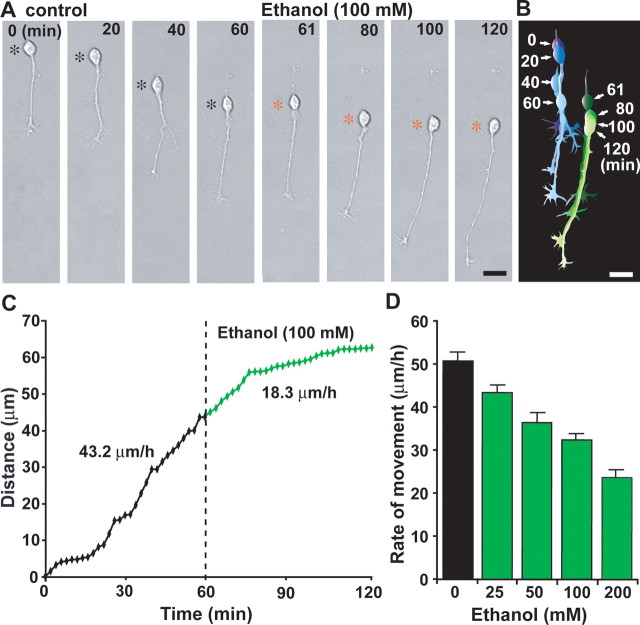
Alcohol may alter the motility of granule cells directly or indirectly by modifying the surrounding environment. For example, ethanol exposure affects the development and functions of glia (Miller and Robertson, 1993; Gonzalez-Burgos and Alejandre-Gomez, 2005), which in turn may alter granule cell motility. To determine whether alcohol directly affects the migration of granule cells, we used microexplant cultures of P0–P3 mouse cerebella (Komuro and Rakic, 1996; Yacubova and Komuro, 2002a,b). In these cultures, isolated granule cells actively migrate in the absence of cell–cell contact (Yacubova, and Komuro, 2002a). The addition of 100 mm ethanol slowed the migration of isolated granule cells from 43.2 to 18.3 μm/h, although ethanol did not affect the extension of the leading process or the morphological features of the process (Fig. 2A–C). It has been shown that isolated granule cells exhibit systematic fluctuations in their migration rate every 2–3 h (Yacubova, and Komuro, 2002a); therefore, to confirm the effects of ethanol on the migration of isolated granule cells, we averaged the data obtained from at least 100 isolated granule cells at each dose level of ethanol. We found that ethanol at concentrations ranging from 25 to 200 mm appreciably slowed the movement of isolated granule cells (Fig. 2D). For example, the average rate of cell movement was reduced to 85% (25 mm ethanol), 71% (50 mm ethanol), 63% (100 mm ethanol), and 46% (200 mm ethanol) of the control (Fig. 2D). These results indicated that alcohol acts directly on the migration of granule cells in a dose-dependent manner.
Figure 2.
Ethanol directly inhibits the migration of isolated granule cells in the microexplant cultures. A, Time-lapse images showing that an application of ethanol (100 mm) directly slows the migration of isolated granule cells in the microexplant cultures of P1 mouse cerebellum. Black and red asterisks mark the granule cell soma. Elapsed time is indicated at the top of each photograph. Scale bars, 12μm. B, Two sets of superimposed images of the granule cells shown in A represent the cell movement before and after an application of 100 mm ethanol. Scale bars, 12 μm. C, Sequential changes in the distance traveled by the granule cell soma shown in A before and after an application of 100 mm ethanol. D, Dose-dependent reduction of the rate of granule cell migration in the microexplant cultures by an acute exposure of ethanol (25–200 mm). Each column represents the average rates obtained from at least 100 migrating cells. Error bars indicate SD.
Reduction of frequency of spontaneous Ca2+ transients in somata of migrating granule cells by ethanol
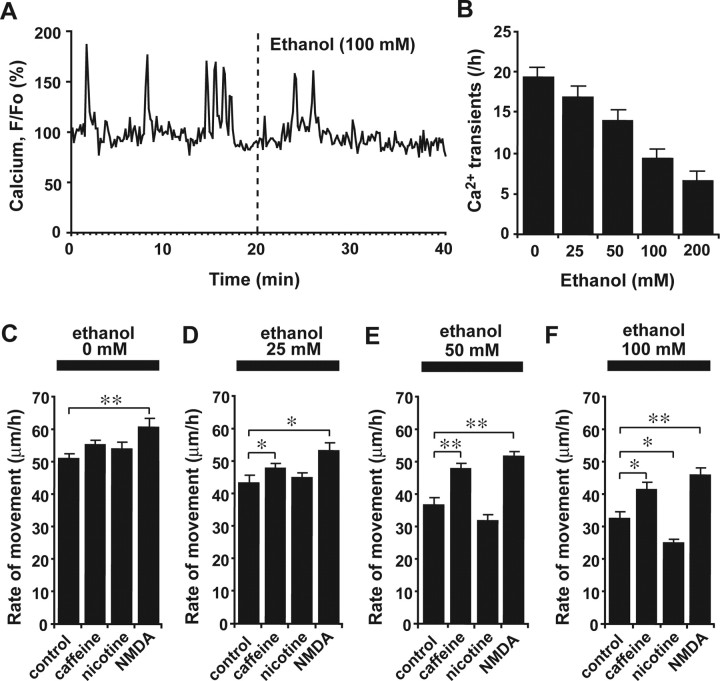
Through what molecular mechanisms does ethanol slow the migration of granule cells? Although the effects of ethanol were initially believed to arise from alcohol-induced perturbations in the order of membrane lipids, the effects on membrane lipids are actually quite small at clinically relevant concentrations (Peoples et al., 1996). It has been reported that even low levels of ethanol can modulate the functions of voltage-gated and ligand-gated Ca2+ channels by binding to a hydrophobic pocket on the proteins (Walter and Messing, 1999), suggesting that administration of ethanol may affect the intracellular Ca2+ levels of migrating granule cells. This possibility is intriguing, because it is known that the migration of granule cells is highly sensitive to changes in intracellular Ca2+ levels and Ca2+ signaling (Komuro and Rakic, 1992, 1993; Kumada and Komuro, 2004; Komuro and Kumada, 2005). On the basis of these previous studies, we hypothesized that ethanol affects the migration of granule cells by altering intracellular Ca2+ levels and Ca2+ signaling. To test this hypothesis, we examined whether the application of ethanol changes the intracellular Ca2+ levels of migrating granule cells. With the use of a Ca2+ indicator dye (Oregon Green 488 BAPTA-1), we found that the administration of ethanol significantly lowers the frequency of spontaneous Ca2+ transients in the granule cell somata in a dose-dependent manner (Fig. 3A,B). For example, the average number of spontaneous Ca2+ transients in the granule cells was reduced to 88% (25 mm ethanol), 73% (50 mm ethanol), 48% (100 mm ethanol), and 35% (200 mm ethanol) of the control after administration of ethanol (Fig. 3B). These results suggest that one of the cellular mechanisms underlying ethanol action on neuronal migration is precisely the alteration of Ca2+ signaling.
Figure 3.
Ethanol action on granule cell migration depends on intracellular Ca2+ signaling. A, Reduction of spontaneous intracellular Ca2+ transients in the granule cell soma by ethanol (100 mm). F/F0 represents the fluorescent intensity of Oregon Green 488 BAPTA-1 divided by its baseline fluorescent intensity. B, Histograms showing the dose-dependent reduction of the frequency of Ca2+ transients in the granule cell somata by ethanol (25–200 mm). Each column represents the average frequency of spontaneous Ca2+ transients obtained from at least 50 migrating cells. Error bars indicate SD. C–F, Changes in the effects of various concentrations (0 mm in C, 25 mm in D, 50 mm in E, and 100 mm in F) of ethanol on the rate of granule cell migration by an application of caffeine (1 mm), nicotine (1 μm), and NMDA (30 μm). Each column represents the average rate of cell movement obtained from at least 40 migrating cells. Error bars indicate SD. *p < 0.05; **p < 0.01.
Reversal of ethanol action on granule cell migration by enhancing Ca2+ signaling
If ethanol slows granule cell migration by inhibiting Ca2+ signaling, enhancing Ca2+ release from internal Ca2+ stores or Ca2+ influxes across the plasma membrane may reduce the effect of ethanol on granule cell migration. To test this possibility, we applied caffeine, which increases internal Ca2+ release through the ryanodine receptors, NMDA and nicotine, both of which induce Ca2+ influxes through NMDA-type glutamate receptors and nicotinic acetylcholine receptors. Intoxicating levels of ethanol have been reported to alter the activity of these receptors (Lovinger et al., 1989; Mezna et al., 1996; Narahashi et al., 1999). We added caffeine, NMDA, and nicotine to the culture medium at dose levels that are able to activate their receptors and have fewer toxic side effects (Rossi and Slater, 1993; Narahashi et al., 2001; El Yacoubi et al., 2003; Connole et al., 2004; Dash et al., 2004; Chen and Harle, 2005; Riddoch et al., 2005). In the absence of ethanol, the addition of nicotine (1 μm) or caffeine (1 mm) to the culture medium did not appreciably change the migration rate of isolated granule cells, whereas the addition of NMDA (30 μm) significantly accelerated the rate of migration (Fig. 3C). Moreover, it is interesting that when caffeine (1 mm) or NMDA (30 μm) was added to the culture medium with ethanol, the effects of ethanol on the rate of granule cell movement were noticeably reduced at all dose levels (25–100 mm) (Fig. 3D–F). In contrast, when nicotine (1 μm) was added to the culture medium with low doses (25 and 50 mm) of ethanol, nicotine had little effect on the rate of migration (Fig. 3D,E). In the presence of a high dose (100 mm) of ethanol, the application of nicotine significantly amplified the effects of ethanol on granule cell migration (Fig. 3F). These results suggest that ethanol affects the migration of granule cells by altering multiple and distinct components of Ca2+ signaling. Furthermore, these results demonstrate that the action of ethanol may be ameliorated by controlling Ca2+ signaling.
Reciprocal control of ethanol action on granule cell migration by cAMP signaling
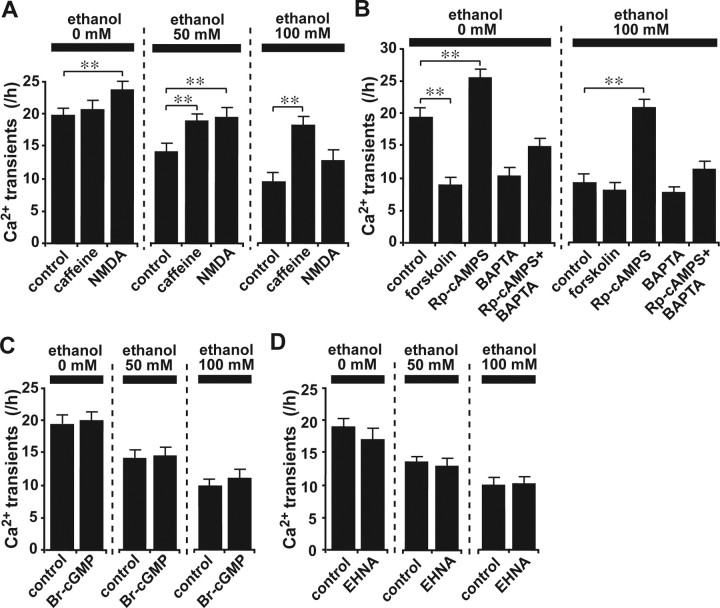
Ca2+ signaling pathways interact with numerous other signaling systems (Berridge et al., 2003). Among them, cyclic nucleotide pathways such as those involving cAMP or cGMP are of particular interest, because alterations of these systems affect the motility of various types of cells (Gorbunova and Spitzer, 2002; Xiang et al., 2002; Howe et al., 2005; Veltman et al., 2005). Accordingly, with the use of the cAMP enzyme immunoassay, we first investigated whether ethanol alters cAMP levels in the cerebellum. We found that an intraperitoneal injection of ethanol (5 g/kg body weight) into P10 mice markedly increases cAMP levels in the cerebellum 1 h after injection (Fig. 4A), which suggests that cAMP signaling also plays a role in the effects of ethanol on granule cell migration. We then examined whether altering the cAMP signaling pathways likewise alters the effects of ethanol on the rate of granule cell migration. In the absence of ethanol, stimulating adenylyl cyclase (AC) with forskolin (30 μm), which is upstream of cAMP signaling, markedly reduced the rate of granule cell movement; however, inhibiting protein kinase A (PKA) with PKA inhibitor fragment 14-22, myristoylated trifluoroacetate salt (PKI) (5 μm), which is downstream of cAMP signaling, markedly increased the migration rate of isolated granule cells (Fig. 4C). Similarly, without ethanol, Sp-cAMPS (20 μm), a competitive cAMP agonist, reduced the rate of granule cell migration, whereas Rp-cAMPS (100 μm), a competitive cAMP antagonist, increased the rate, although these changes were not statistically significant (Fig. 4C). These results demonstrate that in the absence of ethanol, stimulating cAMP signaling reduces the rate of granule cell migration, whereas inhibiting cAMP signaling increases the rate of cell migration.
Figure 4.
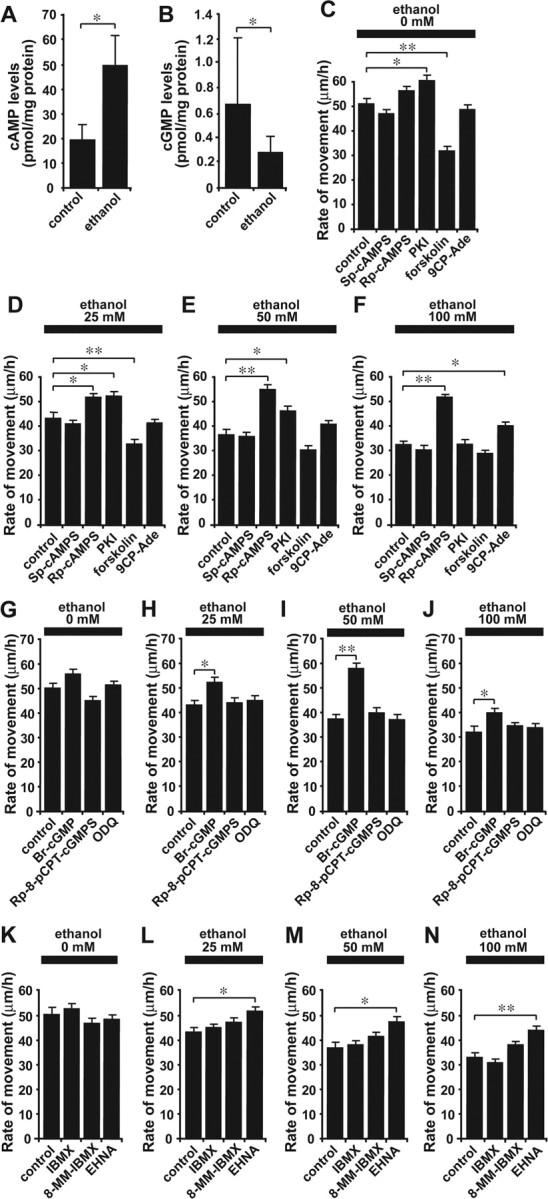
Cyclic nucleotide signaling plays a key role in the ethanol-induced inhibition of granule cell migration. A, B, Changes in cAMP levels (A) and cGMP levels (B) in the P10 mouse cerebellum induced by an intraperitoneal injection of ethanol (5 g/kg body weight). Each column represents the average values obtained from 11 mouse cerebella for cAMP measurements and from 12 mouse cerebella for cGMP measurements. C–F, Changes in the effects of various concentrations (0 mm in C, 25 mm in D, 50 mm in E, and 100 mm in F) of ethanol on the rate of granule cell migration by altering cAMP signaling with Sp-cAMPS (20 μm), Rp-cAMPS (100 μm), PKI (5μm), forskolin (30μm), or 9CP-Ade (30μm). G–J, Changes in the effects of various concentrations (0 mm in G, 25 mm in H, 50 mm in I, and 100 mm in J) of ethanol on the rate of granule cell migration by altering cGMP signaling with Br-cGMP (100 μm), Rp-8-pCPT-cGMPS (5 μm), or ODQ (1.5 μm). K–N, Changes in the effects of various concentrations (0 mm in K,25 mm in L,50mm in M, and 100 mm in N) of ethanol on the rate of granule cell migration by altering the activity of cyclic nucleotide PDEs with IBMX (30μm), 8-MM-IBMX (20μm), or EHNA (10 μm). In C–N, each column represents the average rate of cell movement obtained from at least 40 migrating cells. Error bars indicate SD. *p < 0.05; **p < 0.01.
Interestingly, when the activity of PKA was inhibited with PKI (5 μm), the effects of low doses (25 and 50 mm) of ethanol on the rate of granule cell movement were reduced significantly (Fig. 4D,E), although the inhibition of PKA activity failed to change the action of a high dose of ethanol (100 mm) on cell migration (Fig. 4F). Furthermore, application of a competitive cAMP antagonist, Rp-cAMPS (100 μm), completely reversed the effects of ethanol on the rate of granule cell migration at all dose levels (25–100 mm) (Fig. 4D–F). Moreover, when the activity of AC was inhibited with 9-cyclopentyladenine (30 μm), the effects of high-dose ethanol (100 mm) were reduced significantly (Fig. 4F). In contrast, when AC was stimulated with forskolin (30 μm), the effects of low-dose ethanol (25 mm) on the rate of migration were enhanced significantly (Fig. 4D). Together, these results demonstrate that the effects of ethanol on granule cell migration are highly sensitive to changes in the activity of cAMP signaling pathways: stimulating cAMP signaling amplifies the effects of ethanol in granule cell migration, whereas inhibiting cAMP signaling reduces the effects. Because the application of ethanol increases cAMP levels, ethanol may slow the migration of granule cells by stimulating cAMP signaling pathways.
Reduction of ethanol action on granule cell migration through stimulation of the cGMP signaling pathway
To determine the involvement of the cGMP signaling pathway in the action of ethanol on granule cell migration, we first examined whether application of ethanol alters cGMP levels. Use of the cGMP enzyme immunoassay revealed that an intraperitoneal injection of ethanol (5 g/kg body weight) into P10 mice significantly decreases cGMP levels in the cerebellum 1 h after injection (Fig. 4B), suggesting that cGMP signaling also is a target for ethanol action in cell migration. Next we examined the role of cGMP signaling in the ethanol-induced inhibition of granule cell migration. In the absence of ethanol, stimulating cGMP signaling with the cGMP analog Br-cGMP (100 μm) slightly increased the rate of granule cell movement, whereas inhibiting cGMP signaling with the cGMP antagonist Rp-8-pCPT-cGMPS (5 μm) slightly decreased the rate (Fig. 4G); however, these changes were not statistically significant. Furthermore, inhibiting the guanylyl cyclase with 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (1.5 μm) did not affect the rate of granule cell migration (Fig. 4G). It is significant that when the cGMP signaling pathway was stimulated with Br-cGMP (100 μm), the effects of ethanol on granule cell migration were markedly reduced at all dose levels (25–100 mm) (Fig. 4H–J). In contrast, inhibiting the cGMP signaling pathway with Rp-8-pCPT-cGMPS (5 μm) or ODQ (1.5 μm) did not change the action of ethanol on the rate of granule cell migration (Fig. 4H–J). Cumulatively, these results demonstrate that cGMP signaling is also targeted by ethanol action in granule cell migration. Application of ethanol may slow granule cell migration by reducing the activity of cGMP signaling pathways.
Modification of ethanol action on granule cell migration through alteration of the activity of cyclic nucleotide phosphodiesterase
If alcohol affects granule cell migration by altering cAMP and cGMP signaling pathways, one mechanism controlling the action of ethanol might be the degradation of these cyclic nucleotides. To test this possibility, we inhibited the activity of cyclic nucleotide phosphodiesterases (PDEs), which catalyze the hydrolysis of cAMP and/or cGMP. In the absence of ethanol, the application of 3-isobutyl-1-methylxanthine (IBMX) (30 μm), a broad-spectrum PDE inhibitor, did not affect the rate of granule cell migration (Fig. 4K). Both 8-methoxymethyl-IBMX (8-MM-IBMX) (20 μm), a PDE1 inhibitor that blocks the Ca2+/calmodulin-dependent cleavage of cAMP and cGMP (Juilfs et al., 1999), and erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA) (10 μm), a PDE2 inhibitor that blocks the cGMP-dependent cleavage of cAMP and cGMP (Juilfs et al., 1999), slightly reduced the rate of granule cell migration, although these changes were not statistically significant (Fig. 4K). Interestingly, when EHNA (10 μm) was added to the culture medium with ethanol, the action of ethanol on the rate of granule cell migration was significantly reduced at all dose levels (25–100 mm) (Fig. 4L–N). Moreover, application of 8-MM-IBMX (20 μm) slightly reduced the effects of a high dose (100 mm) of ethanol on granule cell migration, although the change was not statistically significant (Fig. 4 N); however, IBMX (30 μm) failed to alter ethanol action on granule cell migration (Fig. 4 L–N). These results indicate that ethanol may affect granule cell migration by altering the amplitude and duration of cyclic nucleotide signals by modifying the activity of a specific PDE family. In particular, the activity of cGMP-activated cyclic nucleotide phosphodiesterases (PDE2) may be a key target for the action of ethanol on cell migration.
Ca2+ transient-dependent and Ca2+ transient-independent mechanisms underlying the reversal of ethanol-induced inhibition of granule cell migration
It has been reported that changes in the frequency of Ca2+ transients positively correlate with changes in the rate of granule cell migration (Komuro and Rakic, 1996; Kumada and Komuro, 2004). Do caffeine, NMDA, cAMP antagonists, and cGMP agonists reduce the effects of ethanol on granule cell migration by increasing the frequency of Ca2+ transients? To test this, we examined whether application of caffeine, NMDA, cAMP antagonists, and cGMP agonists induces changes in the frequency of Ca2+ transients in granule cell somata. In the absence of ethanol, NMDA (30 μm) treatment significantly increased the frequency of Ca2+ transients in the somata of migrating granule cells, whereas caffeine (1 mm) did not affect the frequency (Fig. 5A). When NMDA (30 μm) was added to the culture medium with ethanol, there was a marked decrease in the effects of 50 mm ethanol on the frequency of Ca2+ transients in the granule cell somata (Fig. 5A), yet interestingly, the effects of high-dose ethanol (100 mm) on the frequency of Ca2+ transients were reduced significantly by caffeine (1 mm) (Fig. 5A).
Figure 5.
Effects of caffeine, NMDA, forskolin, Rp-cAMPS, Br-cGMP, and EHNA on the ethanol-induced reduction of transient Ca2+ elevations in the granule cell somata. A, Effects of caffeine (1 mm) and NMDA (30μm) on the frequency of Ca2+ transients in the granule cell somata in the presence and absence of ethanol (50 and 100 mm). B, Effects of forskolin (30μm), Rp-cAMPS (100 μm), and BAPTA-AM (10 μm) on the frequency of Ca2+ transients in the granule cell somata in the presence and absence of ethanol (100 mm). C, Effects of Br-cGMP (100μm) on the frequency of Ca2+ transients in the granule cell somata in the presence and absence of ethanol (50 and 100 mm). D, Effects of EHNA (10 μm) on the frequency of Ca2+ transients in the granule cell somata in the presence and absence of ethanol (50 and 100 mm). In A–D, each column represents the average values obtained from atleast 50 migrating cells. Error bars indicate SD. *p < 0.05; **p < 0.01.
The modulation of cAMP signaling pathways also altered the frequency of Ca2+ transients in the granule cell somata. For example, in the absence of ethanol, stimulating the activity of adenylyl cyclase with forskolin (30 μm) significantly reduced the frequency of Ca2+ transients (Fig. 5B). In contrast, without ethanol, inhibiting cAMP signaling with a competitive cAMP antagonist, Rp-cAMPS (100 μm), significantly increased the frequency of Ca2+ transients (Fig. 5B). Noteworthy too is that when Rp-cAMPS (100 μm) was added to the culture medium with ethanol, the effects of ethanol (100 mm) on the frequency of Ca2+ transients were eliminated completely (Fig. 5B). Such effects of Rp-cAMPS on the frequency of Ca2+ transients were significantly reduced by a coadministration of intracellular Ca2+ chelator, BAPTA-AM (10 μm) (Fig. 5B).
In contrast to altering Ca2+ signaling and cAMP signaling, stimulating cGMP signaling with a cGMP analog, Br-cGMP (100 μm), did not change the frequency of Ca2+ transients in the granule cell somata in the presence or absence of 50–100 mm ethanol (Fig. 5C). Likewise, inhibiting the activity of PDE2 with EHNA (10 μm) did not significantly alter the frequency of Ca2+ transients in the presence or absence of 50–100 mm ethanol (Fig. 5D).
Together, these results indicate that Ca2+ signaling and cAMP signaling may reverse the action of ethanol on the migration of granule cells by increasing the frequency of Ca2+ transients in their somata, whereas cGMP signaling and cyclic nucleotide PDEs may reduce the action of ethanol on migration without altering Ca2+ transients.
Ca2+ signaling, cAMP signaling, and cGMP signaling require the activities of multiple but distinct downstream targets for reversing the action of ethanol on granule cell migration
What downstream effectors are essential for the reversal of ethanol action on granule cell migration by controlling Ca2+, cAMP or cGMP signaling? Although it has been known that these signalings interact with large varieties of downstream targets, we chose PKC, Ca2+/calmodulin-dependent protein kinase II (CaMKII), calcineurin, protein phosphatase 1 (PP1), Rho GTPase, mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase (PI3K) as potential targets that are known to be involved in controlling the motility of various types of cells (Bandyopadhyay et al., 2002; Parameswaran et al., 2004; Pfleiderer et al., 2004; Affolter and Weijer, 2005; Cai et al., 2005; Domin et al., 2005; Rajalingam et al., 2005; Titus et al., 2005; Yang and Huang, 2005).
First, we investigated whether the activity of PKC is necessary for the reversal of ethanol action on granule cell migration by Ca2+, cAMP, or cGMP signaling. In the absence of ethanol, inhibiting the activity of PKC with calphostin-C (100 nm) markedly reduced the rate of granule cell migration, implying that the activity of PKC is necessary for the migration of granule cells (Fig. 6A). When calphostin-C (100 nm) was added to the culture medium, the effects of ethanol (100 mm) on granule cell migration were amplified significantly (Fig. 6A). Interestingly, with a coapplication of calphostin-C (100 nm), NMDA (30 μm) and Rp-cAMPS (100 μm) failed to reverse the action of ethanol (100 mm) on granule cell migration (Fig. 6A). In contrast, the effects of caffeine (1 mm) and Br-cGMP (100 μm) on the ethanol-induced inhibition of granule cell migration were not affected by the application of calphostin-C (100 nm) (Fig. 6A).
Figure 6.
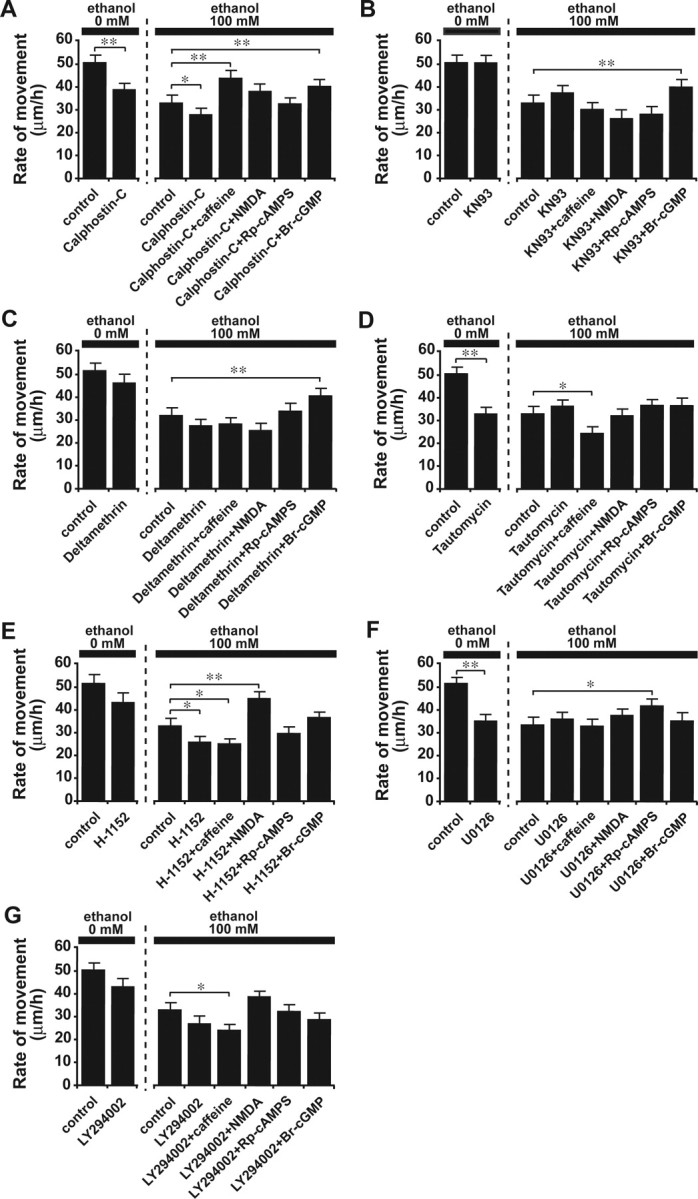
The reversal of ethanol-induced aberrant neuronal migration by Ca2+ and cyclic nucleotide signaling is mediated by multiple and distinctive downstream effectors. A–G, Changes in the effects of caffeine (1 mm), NMDA (30 μm), Rp-cAMPS (100 μm), and Br-cGMP (100 μm) on the ethanol (100 mm)-induced inhibition of granule cell migration by inhibiting protein kinase C with calphostin C (100 nm)(A), inhibiting CaMKII with KN93 (5 μm)(B), inhibiting calcineurin with deltamethrin (10 nm)(C), inhibiting PP1 with tautomycine (4 nm)(D), inhibiting Rho GTPase with H-1152 (1μm)(E), inhibiting MAPK with U0126 (10μm) (F), and inhibiting PI3K with LY294002 (10μm)(G). In A–G, each column represents the average rate of cell movement obtained from at least 50 migrating cells. Error bars indicate SD. *p < 0.05; **p < 0.01.
Second, we examined the role of CaMKII in the reversal of ethanol action on granule cell migration by Ca2+, cAMP, or cGMP signaling. Without ethanol, the inhibition of the activity of CaMKII with 2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)] amino-N-(4-cholrocinnamyl)-N-methylbenzylamine (KN93) (5 μm) did not affect the rate of granule cell migration (Fig. 6 B). Interestingly, when the activity of CaMKII was inhibited by KN93 (5 μm), caffeine (1 mm), NMDA (30 μm), and Rp-cAMPS (100 μm) failed to reduce the effects of ethanol (100 mm) on granule cell migration (Fig. 6 B). In contrast, the effects of Br-cGMP (100 μm) on the ethanol-induced retardation of granule cell migration did not depend on CaMKII activity (Fig. 6B).
Third, we tested whether the activity of calcineurin is required for the reversal of ethanol action on granule cell migration by Ca2+, cAMP, or cGMP signaling. In the absence of ethanol, the inhibition of activity of calcineurin with deltamethrin (10 nm) slightly decreased the rate of granule cell migration, although the change was not statistically significant (Fig. 6C). Interestingly, application of deltamethrin (10 nm) eliminated the effects of caffeine (1 mm), NMDA (30 μm), and Rp-cAMPS (100 μm) on the ethanol-induced inhibition of granule cell migration (Fig. 6C); however, the effects of Br-cGMP (100 μm) on ethanol action in granule cell migration were not affected by deltamethrin (10 nm) (Fig. 6C).
Fourth, we determined the role of PP1 in the reversal of ethanol action on granule cell migration by Ca2+, cAMP, or cGMP signaling. Inhibiting the activity of PP1 with tautomycine (4 nm) markedly reduced the rate of granule cell migration in the absence of ethanol (Fig. 6D), suggesting that the activity of PP1 is crucial for maintaining an active movement of granule cells. When tautomycine (4 nm) was added to the culture medium with ethanol, application of NMDA (30 μm), Rp-cAMPS (100 μm), or Br-cGMP (100 μm) did not change the action of ethanol (100 mm) on granule cell migration (Fig. 6 D). Coapplication of tautomycine (4 nm) and caffeine (1 mm) significantly increased the effects of ethanol (100 mm) on granule cell migration (Fig. 6 D).
Fifth, we examined whether the activity of Rho GTPase is required for the reversal of ethanol action on granule cell migration by Ca2+, cAMP, or cGMP signaling. In the absence of ethanol, inhibiting Rho GTPase with (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinolinyl)sulfonyl]homopiperazine (H-1152) (1 μm) decreased the rate of granule cell migration, although the change was not statistically significant (Fig. 6E). Application of H-1152 (1 μm) significantly increased the effects of ethanol (100 mm) on granule cell migration (Fig. 6E). Furthermore, when H-1152 (1 μm) was added to the culture medium with ethanol (100 mm), the effects of Rp-cAMPS (100 μm) and Br-cGMP (100 μm) on the ethanol-induced inhibition of granule cell migration were eliminated (Fig. 6E). Coapplication of H-1152 (1 μm) and caffeine (1 mm) resulted in a significant reduction of the rate of granule cell migration in the presence of ethanol (100 mm). In contrast, the effects of NMDA (30 μm) on the ethanol-induced inhibition of granule cell migration were not altered by an application of H-1152 (1 μm) (Fig. 6E).
Sixth, we investigated the role of MAPK in the reversal of ethanol action on granule cell migration by Ca2+, cAMP, or cGMP signaling. In the absence of ethanol, the inhibition of the activity of MAPK with 1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene (U0126) (10 μm) significantly slowed the migration of granule cells (Fig. 6F), suggesting that the activity of MAPK is essential for an active migration of granule cells. When U0126 (10 μm) was applied to the culture medium with ethanol, application of caffeine (1 mm), NMDA (30 μm), and Br-cGMP (100 μm) failed to reduce the effects of ethanol (100 mm) on the granule cell migration (Fig. 6F); however, the effects of Rp-cAMPS (100 μm) on the ethanol-induced impairment of granule cell migration did not depend on the activity of MAPK (Fig. 6F).
Finally, we tested whether the activity of PI3K is required for the reversal of ethanol action on granule cell migration by Ca2+, cAMP, or cGMP signaling. In the absence of ethanol, inhibiting the activity of PI3K with 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) (10 μm) reduced the rate of granule cell migration, although the change was not statistically significant (Fig. 6G). When LY294002 (10 μm) was added to the culture medium with ethanol, an application of NMDA (30 μm), Rp-cAMPS (100 μm), and Br-cGMP (100 μm) failed to reduce the action of ethanol (100 mm) on granule cell migration (Fig. 6G). Coapplication of LY294002 (10 μm) and caffeine (1 mm) significantly increased the effects of ethanol (100 mm) on granule cell migration (Fig. 6G).
Cumulatively, these results demonstrate that the reversal of ethanol action on granule cell migration by controlling Ca2+, cAMP, and cGMP signaling pathways requires the activities of multiple but distinctive downstream effectors: (1) caffeine needs the activities of CaMKII, calcineurin, PP1, Rho GTPase, MAPK, and PI3K; (2) NMDA needs the activities of PKC, CaMKII, calcineurin, PP1, MAPK, and PI3K; (3) Rp-cAMP needs the activities of PKC, CaMKII, calcineurin, PP1, Rho GTPase, and PI3K; and (4) Br-cGMP needs the activities of PP1, Rho GTPase, MAPK, and PI3K.
Reversal of ethanol action on granule cell migration in vivo by administration of caffeine, NMDA, Rp-cAMPS, and Br-cGMP
Our in vitro studies demonstrate that the effects of an acute administration of ethanol on granule cells migration are significantly reduced by controlling Ca2+, cAMP, and cGMP signaling pathways. The next question was whether controlling Ca2+, cAMP, and cGMP signaling pathways could also reduce the effects of ethanol on granule cell migration in vivo. To address this question, we first determined the sequential changes in blood ethanol levels after a single injection of ethanol into the early postnatal mouse. An intraperitoneal injection of ethanol (5 g/kg body weight) into P10 mice resulted in an increase in blood ethanol levels to ∼80 mm (equivalent to blood ethanol level <400 mg/dl) within 1 h after injection (Fig. 7A). Thereafter, blood ethanol levels decreased gradually, ending in 14 mm at 10 h after injection (Fig. 7A). The injection of this amount of ethanol made the pups intoxicated and decreased the activity of spontaneous movement for several hours; however, this dose level of ethanol did not cause the death of the animals. After establishing the sequential changes in blood ethanol levels, we then investigated the chronology of migration of cerebellar granule cells in vivo in the absence of ethanol. To this end, we injected P9 mice intraperitoneally with BrdU (50 mg/kg body weight), which is incorporated only into proliferating cells such as granule cell precursors. One day after BrdU injection (at P10), the BrdU-labeled granule cells were detected in the entire EGL of the cerebellum (Fig. 7B). Two days after BrdU injection (at P11), ∼50% of BrdU-labeled granule cells left the EGL and translocated their soma into the ML, Purkinje cell layer (PCL), or IGL (Fig. 7B), indicating that within 48 h after final mitosis, many postmitotic granule cells begin to migrate radially toward the IGL, and some of them have already reached their final destinations in the IGL.
Figure 7.
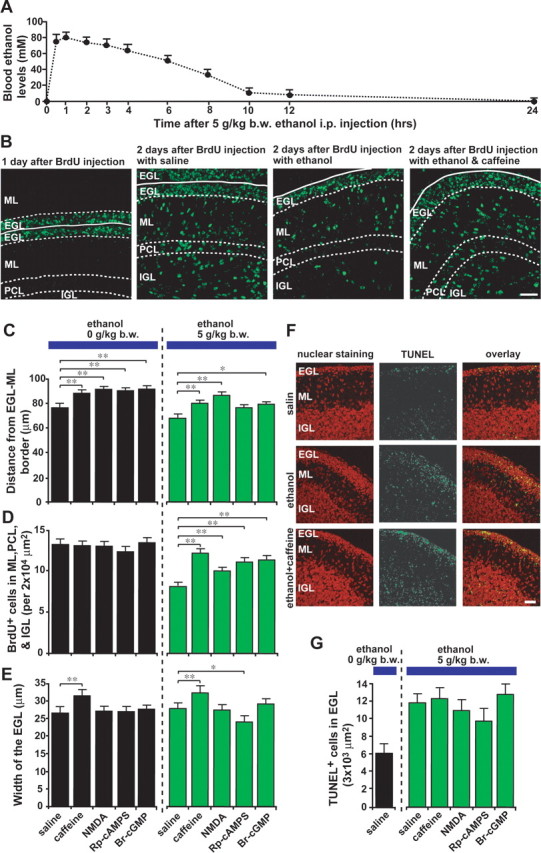
Ethanol action on granule cell migration in vivo is reversed by administration of caffeine, NMDA, Rp-cAMPS, and Br-cGMP. A, Sequential changes in blood ethanol levels after intraperitoneal injection of ethanol (5 g/kg body weight; 25%, v/v mixed in saline) into P10 mice. B, Effects of ethanol and caffeine on the translocation of BrdU-labeled granule cells in the postnatal mouse cerebellum. P9 mice were injected intraperitoneally with BrdU (50 mg/kg body weight). One day after BrdU injection (at P10), mice were injected with 100 μl of saline or caffeine (15 mg/kg body weight) into the peritoneal cavity with or without ethanol injection (5 g/kg body weight; 25%, v/v mixed in saline). C–E, Effects of intraperitoneal injection of 100 μl of caffeine (15 mg/kg body weight), NMDA (2 mg/kg body weight), Rp-cAMPS (2 mg/kg body weight), or Br-cGMP (2 mg/kg body weight) on ethanol (5 g/kg body weight)-induced changes in the distance of the BrdU-labeled granule cells from the EGL–ML borders (C), in the number of BrdU-labeled cells in the ML, PCL, and IGL (D), and in the width of the EGL (E). Each column represents the average values obtained from >1200 migrating cells in C and D and 38 external granular layers in E. F, Apoptotic cell death of granule cells and their precursors by an intraperitoneal injection of ethanol (5 g/kg body weight) alone or ethanol (5 g/kg body weight) and caffeine (15 mg/kg body weight). G, Effects of intraperitoneal injection of caffeine (15 mg/kg body weight), NMDA (2 mg/kg body weight), Rp-cAMPS (2 mg/kg body weight), and Br-cGMP (2 mg/kg body weight) on the ethanol (5 g/kg body weight)-induced apoptotic cell death of granule cells and their precursors in the EGL. In G, each column represents the average value obtained from at least 400 cells. Error bars indicate SD. *p < 0.05; **p < 0.01. b.w., Body weight; i.p., intraperitoneal.
After establishing the chronology of granule cell migration in vivo, we ascertained the effects of an acute administration of ethanol on granule cell migration in vivo. We injected ethanol intraperitoneally (5 g/kg body weight) into P10 mice, which 1 d earlier (at P9) had been injected intraperitoneally with BrdU (50 mg/kg body weight). One day after ethanol injection (at P11), the mice were killed, and the distribution of BrdU-labeled granule cells was analyzed. We found that a single injection of ethanol into P10 mice results in (1) a reduction of the average distance of BrdU-labeled granule cells from the EGL–ML border and (2) a decrease in the number of BrdU-labeled granule cells in the ML, PCL, and IGL (Fig. 7B–D). These results demonstrate that a single injection of ethanol prevents granule cells from entering the ML and slows radial migration in the ML, PCL, and IGL.
After establishing the effects of an acute administration of ethanol on granule cell migration in vivo, we then examined whether controlling Ca2+, cAMP, and cGMP signaling pathways can also reduce the effects of ethanol on granule cell migration in vivo. To this end, we intraperitoneally injected P10 mice with caffeine (15 mg/kg body weight), NMDA (2 mg/kg body weight), Rp-cAMPS (2 mg/kg body weight), and Br-cGMP (2 mg/kg body weight) in separate experiments, when a single intraperitoneal injection of ethanol (5 g/kg body weight) was applied. One day after an intraperitoneal injection, we found that injection of caffeine, NMDA, or Br-cGMP significantly reduces the effect of ethanol on the average distance of BrdU-labeled granule cells from the EG-L–ML border and the number of BrdU-labeled cells in the ML, PCL, and IGL (Fig. 7B–D). Furthermore, injection of Rp-cAMPS also significantly reduced the effect of ethanol on the number of BrdU-labeled cells in the ML, PCL, and IGL but did not significantly affect the action of ethanol on the average distance of BrdU-labeled cells from the EGL–ML border (Fig. 7C,D). These results suggest that the action of ethanol on granule cell migration in vivo can be reduced by an intraperitoneal injection of caffeine, NMDA, Rp-cAMPS, or Br-cGMP.
Despite a marked decrease in the number of granule cells that migrated out of the EGL after a single injection of ethanol, the width of the EGL did not increase (Fig. 7E), which suggests that ethanol may induce cell death in granule cell precursors and/or postmitotic granule cells in the EGL. Accordingly, we examined whether a single injection of ethanol induces the apoptotic death of granule cell precursors and postmitotic granule cells in the EGL. With the use of the TUNEL assay, we found that an intraperitoneal injection of ethanol (5 g/kg body weight) into P10 mice increases the apoptotic cell death of granule cell precursors and postmitotic granule cells in the EGL within 1 d after injection (Fig. 7F,G). Furthermore, intraperitoneal injection of caffeine, NMDA, Rp-cAMPS, or Br-cGMP failed to rescue granule cell precursors and postmitotic granule cells in the EGL from cell death caused by ethanol (Fig. 7F,G), although intraperitoneal injections of caffeine and Rp-cAMPS with ethanol significantly altered the width of the EGL (Fig. 7E).
Although an intraperitoneal injection of caffeine, NMDA, Rp-cAMPS, and Br-cGMP significantly reduced the effects of ethanol on granule cell migration in vivo, this may be caused by the outcome of multiple and complex indirect effects, because in fact, these chemicals might not easily reach the cerebellum. Therefore, to examine the direct effects of these reagents on ethanol-induced inhibition of granule cell migration in vivo, we injected reduced amounts of caffeine (2 mg/kg body weight), NMDA (0.01 mg/kg body weight), Rp-cAMPS (0.4 mg/kg body weight), and Br-cGMP (0.4 mg/kg body weight) into the subarachnoid space between the skull and the surface of the P10 mouse cerebellum in separate experiments, when a single intraperitoneal injection of ethanol (5 g/kg body weight) was applied. We found that 1 d after an injection (at P11), the application of caffeine, Rp-cAMPS, and Br-cGMP completely reversed the effect of ethanol on the average distance of BrdU-labeled cells from the EGL–ML border and the number of BrdU-labeled cells in the ML, PCL, and IGL (Fig. 8A,B). In contrast, the application of NMDA failed to reduce the action of ethanol on granule cell migration in vivo (Fig. 8A,B). The direct administration of NMDA (>0.01 mg/kg body weight) into the subarachnoid space between the skull and the surface of the cerebellum often caused the death of injected pups, possibly from the neurotoxic effects of high doses of NMDA. In contrast to the case of an intraperitoneal injection, the width of the EGL was decreased significantly by coadministration of caffeine, Rp-cAMPS, or Br-cGMP with ethanol into the subarachnoid space between the skull and the surface of the cerebellum (Fig. 8C). This reduction of the width of the EGL suggests that injection of caffeine, Rp-cAMPS, or Br-cGMP fails to rescue the granule cell precursors and postmitotic granule cells from apoptotic cell death induced by ethanol, because the numbers of BrdU-labeled granule cells in the ML, PCL, and IGL in the pups that had been injected with caffeine, Rp-cAMPS, or Br-cGMP with ethanol were similar to those in the pups that had been injected with saline, but without ethanol as a control. Together, these results demonstrate that controlling Ca2+, cAMP, and cGMP signaling pathways reduces the effects of ethanol on granule cell migration in vivo, although these modifications of the second-messenger signaling pathways do not affect the action of ethanol on cell survival in vivo.
Figure 8.
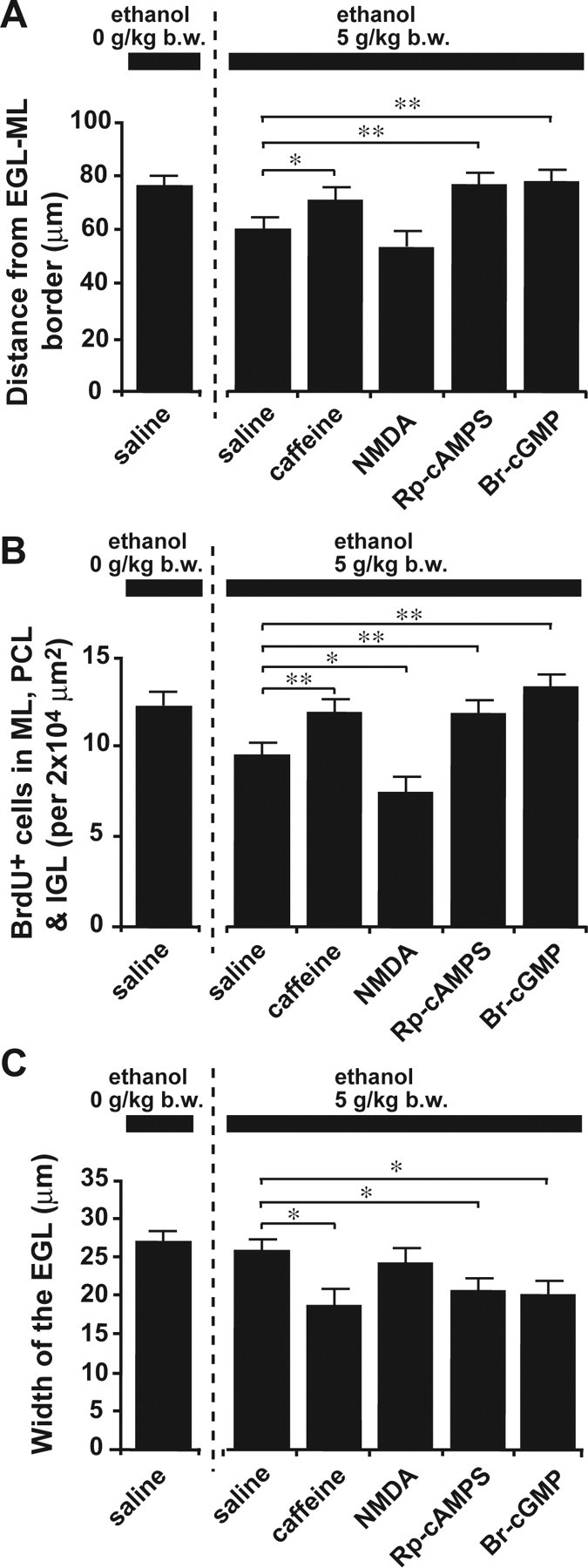
Reversal of ethanol action on granule cell migration in vivo by injection of caffeine, Rp-cAMPS, and Br-cGMP into the subarachnoid space between the skull and the surface of the cerebellum. A–C, Effects of injection of 5 μl of caffeine (2 mg/kg body weight), NMDA (0.01 mg/kg body weight), Rp-cAMPS (0.4 mg/kg body weight), and Br-cGMP (0.4 mg/kg body weight) into the subarachnoid space between the skull and the surface of the cerebellum on the ethanol (5 g/kg body weight)-induced changes in the distance of the BrdU-labeled granule cells from the EGL-ML borders (A), the number of BrdU-labeled cells in the ML, PCL, and IGL (B), and the width of the EGL (C). In A–C, each column represents the average value obtained from >1000 migrating cells in A and B and 40 external granular layers in C. Error bars indicate SD. *p < 0.05; **p < 0.01.
Discussion
Cellular mechanisms by which alcohol affects the migration of immature neurons in the developing brain have not been resolved, despite more than two decades of research. In this series of experiments, we demonstrate that Ca2+ and cyclic nucleotide signalings are central targets for alcohol action in neuronal cell migration. Acute administration of ethanol reduced the frequency of Ca2+ transients and cGMP levels but increased cAMP levels. Stimulating Ca2+ signaling with caffeine and NMDA increased the frequency of Ca2+ transients in the somata of migrating granule cells in the presence of ethanol and significantly reduced the effects of ethanol exposure on granule cell migration in vitro. In the case of cyclic nucleotide signalings, without ethanol, cAMP signaling acts as a “brake” on granule cell movement, whereas cGMP signaling acts as an “accelerator.” In the presence of ethanol, inhibiting the cAMP signaling pathways significantly reduced the effects of ethanol exposure on granule cell migration in vitro as well as in vivo. Likewise, stimulating the cGMP signaling pathways reduced ethanol action on cell migration in vitro as well as in vivo. Measurement of the changes in intracellular Ca2+ levels suggests that inhibiting cAMP signaling pathways reverses the action of ethanol on granule cell migration by increasing the frequency of Ca2+ transients, whereas stimulating cGMP signaling pathways reduces the action of ethanol on cell migration independent of Ca2+ signaling. Results obtained from the PDE2 inhibitors support the conclusions that cAMP and cGMP signaling pathways reciprocally regulate the action of ethanol on granule cell migration, because PDE2 is known to play a role in cells with physiological functions that are regulated in an opposite manner by cAMP and cGMP (Juilfs et al., 1999). To reduce the effects of ethanol exposure on granule cell migration, the Ca2+ and cyclic nucleotide signaling pathways require the activities of multiple but distinct downstream targets, including PKC, CaMKII, calcineurin, PP1, Rho GTPase, MAPK, and PI3K.
The present results demonstrate that acute administration of ethanol directly and immediately slows the migration of granule cells in a dose-dependent manner; however, we cannot rule out the possibility that ethanol may also indirectly affect the migration of granule cells by altering the function of Bergmann glial cells. In the developing ML, granule cells migrate toward the PCL along the processes of Bergmann glial cells (Rakic, 1971). This interaction between the granule cells and the processes of Bergmann glial cells is essential for migration of granule cells in the ML and is mediated by several cell adhesion molecules expressed on the plasma membrane of granule cells and Bergmann glial cells (Hatten, 1990; Cameron and Rakic, 1994). It has been reported that ethanol exposure alters the expression and function of cell adhesion molecules (Bearer et al., 1999; Minana et al., 2000; Ozer et al., 2000; Guerri, 2002; Miller and Luo, 2002), suggesting that these alterations of cell adhesion molecules by ethanol may affect the migration of granule cells in the ML. Furthermore, it has been shown that radial glial cells, which support the radial migration of cortical neurons in the developing cerebrum, exhibit spontaneous elevations of intracellular Ca2+ levels (Weissman et al., 2004), and the disruption of Ca2+ elevations affects the proliferative activity of neuronal precursors in the ventricular zone of the cerebrum (Weissman et al., 2004). Similarly, we observed spontaneous elevations of intracellular Ca2+ levels in Bergmann glial cells during a period of glial-guided neuronal migration (H. Komuro and T. Kumada, unpublished results); however, the role of Ca2+ transients in Bergmann glial cells in controlling granule cell migration remains to be determined.
To date, at least 11 different families of PDE are recognized (Soderling and Beavo, 2000; Beavo and Brunton, 2002). Most cells contain representatives of more than one PDE gene family but in different amounts, proportions, and subcellular locations. Different PDEs integrate the amplitude and duration, termination, and specificity of cyclic nucleotide signals and actions (Houslay and Milligan, 1997; Rich et al., 2001). Some PDE families are rather specific for cAMP hydrolysis (PDE4, -7, and -8); others are cGMP-specific (PDE5, -6, and -9); some hydrolyze both cGMP and cAMP (PDE1, -2, -3, -10, and -11). Present results demonstrate that an application of IBMX, a broad-spectrum PDE inhibitor, does not alter ethanol action on granule cell migration, whereas EHNA, a PDE2 inhibitor, significantly reduces the effects of ethanol on cell migration, suggesting that the action of ethanol on neuronal cell migration is involved in the activity of a specific type of PDE.
In this study, we used NMDA to increase Ca2+ influxes across the plasma membrane; however, it has been reported that NMDA also increases intracellular cGMP levels through the sequential stimulation of the following systems: nitric oxide synthase–nitric oxide-soluble guanylyl cyclase–cGMP (Pantazis et al., 1998). Likewise, caffeine also affects intracellular levels of cAMP and cGMP by altering the activity of PDE (Shafer et al., 1998). Furthermore, most of the intracellular signaling systems are interconnected with each other like a spider web. For example, PKA may alter NMDA receptor activity through its phosphorylation (Woodward, 1999), and cGMP may alter Ca2+ signaling by the activation of cGMP-gated ion channels (Lucas et al., 2000). Therefore, to completely understand the role of signaling pathways in the ethanol-induced inhibition of granule cell migration, a systematic approach with the combined use of gene knockout animals and pharmacological manipulations will be required.
The changes in Ca2+, cAMP, and cGMP signalings induced by ethanol exposure affect the migratory behavior of granule cells. How do the alterations in Ca2+, cAMP, and cGMP signaling reverse ethanol action on granule cell migration? Although the precise mechanisms remain to be examined, there are several possible scenarios. It has been reported that the Ca2+ signaling plays a critical role in organizing the assembly and disassembly of cytoskeletal components (Henley and Poo, 2004), which is essential for the migration of immature neurons (Rakic et al., 1996). Therefore, the frequency of the Ca2+ transients may affect the rate of granule cell migration by controlling the frequency of the assembly and disassembly of cytoskeletal components (Komuro and Rakic, 1996; Kumada and Komuro, 2004). The Ca2+ transients may also control the formation of focal adhesion by regulating the activity of phosphorylated focal adhesion kinase (Conklin et al., 2005). The vulnerability of granule cells to ethanol exposure decreases as the cells migrated from the EGL to the IGL. These changes in vulnerability may be caused by changes in Ca2+ signaling, because it has been shown that the frequency of Ca2+ transients in granule cells sequentially and systematically changes during migration from the EGL to the IGL (Kumada and Komuro, 2004). Although previous studies show a discrepancy in the role of cAMP and cGMP signalings in controlling cell motility (Bolsover et al., 1992; Haase and Bicker, 2003), the activity of cAMP and cGMP signalings may also play crucial roles in controlling the cytoskeletal components. For example, the activation of cAMP and cGMP signalings changes the distribution of F-actin in the somata of migrating neurons in insect embryo (Haase and Bicker, 2003). Phosphorylation by PKA can switch off the activity of oncoprotein 18, a regulator of microtubule dynamics (Gradin et al., 1998). Furthermore, PKA-dependent phosphorylation of RhoA, which is involved in regulating the cytoskeleton, leads to termination of RhoA signaling (Lang et al., 1996).
In this study, a single injection of ethanol into P10 mice prevented granule cells from entering the ML and slowed migration in the ML, PCL, and IGL. These results indicate that even a single administration of a high level of ethanol during a period of pregnancy can cause serious effects in the migration of immature neurons. Importantly, these effects of ethanol on granule cell migration in vivo were reduced significantly by an intraperitoneal injection of caffeine, NMDA, or Br-cGMP or by the injection of caffeine, Rp-cAMPS, and Br-cGMP into the subarachnoid space between the skull and the surface of the cerebellum. These results suggest that Ca2+ and cyclic nucleotide signaling pathways are new targets for preventing the brain malformation that is associated with alcohol consumption during pregnancy. Whether controlling Ca2+ and cyclic nucleotide signaling can rescue immature neurons in other parts of the developing brain, especially the cerebral cortex, from alcohol-induced aberrant migration remains to be determined. Moreover, it should be determined whether the Ca2+ and cyclic nucleotide signaling are also targets for the action of chronic ethanol exposure on neuronal cell migration and brain development.
This study demonstrates that even a single administration of ethanol increases apoptotic cell death in granule cell precursors and postmitotic granule cells in vivo. The cell death was not reversed by application of caffeine, NMDA, Rp-cAMPS, or Br-cGMP. It has been reported that administration of pituitary adenylate cyclase-activating polypeptide reduces the ethanol-induced apoptotic cell death of granule cells and their precursors through the activation of pituitary adenylate cyclase-1 receptors (Vaudry et al., 2002). Therefore, it may be interesting to examine whether coadministration of caffeine, Rp-cAMPS, and Br-cGMP with pituitary adenylate cyclase-activating polypeptide can reduce the effects of ethanol exposure on the migration as well as the survival of granule cells in the early postnatal cerebellum.
Footnotes
H. K. was supported by National Institutes of Health Grant AA 13613 and Whitehall Foundation Grant 2001-12-35. We thank Grahame Kidd, Jacqueline Morris, Donald Cameron, and Carol Haney for critically reading this manuscript.
Correspondence should be addressed to Dr. Hitoshi Komuro, Department of Neurosciences/NC30, Lerner Research Institute, The Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195. E-mail: komuroh@ccf.org.
DOI:10.1523/JNEUROSCI.4478-05.2006
Copyright © 2006 Society for Neuroscience 0270-6474/06/260742-15$15.00/0
References
- Affolter M, Weijer CJ (2005) Signaling to cytoskeleton dynamics during chemotaxis. Dev Cell 9: 19–34. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay J, Lee J, Lee J, Lee JI, Yu JR, Jee C, Cho JH, Jung S, Lee MH, Zannoni S, Singson A, Kim do H, Koo HS, Ahnn J (2002) Calcineurin, a calcium/calmodulin-dependent protein phosphatase, is involved in movement, fertility, egg laying, and growth in Caenorhabditis elegans Mol Biol Cell 13: 3281–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer CF, Swick AR, O'Riordan MA, Cheng G (1999) Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J Biol Chem 274: 13264–13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL (2002) Cyclic nucleotide research: still expanding after half a century. Nat Rev Mol Cell Biol 3: 710–718. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signaling: dynamics, homeostasis and remodeling. Nat Rev Mol Cell Biol 4: 517–529. [DOI] [PubMed] [Google Scholar]
- Bolsover SR, Gilbert SH, Spector I (1992) Intracellular cyclic AMP produces effects opposite to those of cyclic GMP and calcium on shape and motility of neuroblastoma cells. Cell Motil Cytoskeleton 22: 99–116. [DOI] [PubMed] [Google Scholar]
- Borges S, Lewis PD (1983) Effects of ethanol on postnatal cell acquisition in the rat cerebellum. Brain Res 271: 388–391. [DOI] [PubMed] [Google Scholar]
- Cai L, Holoweckyj N, Schaller MD, Bear JE (2005) Phosphorylation of coronin 1B by protein kinase C regulates interaction with Arp2/3 and cell motility. J Biol Chem 280: 31913–31923. [DOI] [PubMed] [Google Scholar]
- Cameron RS, Rakic P (1994) Polypeptides that comprise the plasmalemmal microdomain between migrating neuronal and glial cells. J Neurosci 14: 3139–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Harle LK (2005) Interactive effect of alcohol and nicotine on developing cerebellum: an investigation of the temporal pattern of alcohol and nicotine administration. Alcohol Clin Exp Res 29: 427–442. [DOI] [PubMed] [Google Scholar]
- Chiriboga CA (2003) Fetal alcohol and drug effects. Neurologist 9: 267–279. [DOI] [PubMed] [Google Scholar]
- Clarren SK (1986) Neuropathology in fetal alcohol syndrome. In: Alcohol and brain development (West JR, ed), p 158. New York: Oxford UP.
- Coffin JM, Baroody S, Schneider K, O'Neill J (2005) Impaired cerebellar learning in children with prenatal alcohol exposure: a comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex 41: 389–398. [DOI] [PubMed] [Google Scholar]
- Conklin MW, Lin MS, Spitzer NC (2005) Local calcium transients contribute to disappearance of pFAK, focal complex removal and deadhesion of neuronal growth cones and fibroblasts. Dev Biol 287: 201–212. [DOI] [PubMed] [Google Scholar]
- Connole L, Harkin A, Maginn M (2004) Adenosine A1 receptor blockade mimics caffeine's attenuation of ethanol-induced motor incoordination. Basic Clin Pharmacol Toxicol 95: 299–304. [DOI] [PubMed] [Google Scholar]
- Costa LG, Vitalone A, Guizzetti M (2004) Signal transduction mechanisms involved in the antiproliferative effects of ethanol in glial cells. Toxicol Lett 149: 67–73. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Leech RW, Schaefer GB, Scheithauer BW, Brumback RA (1993) Midline cerebral dysgenesis, dysfunction of the hypothalamic-pituitary axis, and fetal alcohol effects. Arch Neurol 50: 771–775. [DOI] [PubMed] [Google Scholar]
- Cudd TA (2005) Animal model systems for the study of alcohol teratology. Exp Biol Med 230: 389–393. [DOI] [PubMed] [Google Scholar]
- Dash PK, Moore AN, Moody MR, Treadwell R, Felix JL, Clifton GL (2004) Post-trauma administration of caffeine plus ethanol reduces contusion volume and improves working memory in rats. J Neurotrauma 21: 1573–1583. [DOI] [PubMed] [Google Scholar]
- Dikranian K, Qin YQ, Labruyere J, Nemmers B, Olney JW (2005) Ethanol-induced neuroapoptosis in the developing rodent cerebellum and related brain stem structures. Dev Brain Res 155: 1–13. [DOI] [PubMed] [Google Scholar]
- Domin J, Harper L, Aubyn D, Wheeler M, Florey M, Haskard D, Yuan M, Zicha D (2005) The class II phosphoinositide 3-kinase PI3K-C2beta regulates cell migration by a PtdIns(3)P dependent mechanism. J Cell Physiol 205: 452–462. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM (2003) Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology 45: 977–985. [DOI] [PubMed] [Google Scholar]
- Fujita S (1967) Quantitative analysis of cell proliferation and differentiation in the cortex of the postnatal mouse cerebellum. J Cell Biol 32: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Alejandre-Gomez M (2005) Cerebellar granule cell and Bergmann glial cell maturation in the rat is disrupted by pre- and postnatal exposure to moderate levels of ethanol. Int J Dev Neurosci 23: 383–388. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC (2005) Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med 230: 394–406. [DOI] [PubMed] [Google Scholar]
- Gorbunova Y, Spitzer NC (2002) Dynamic interactions of cyclic AMP transients and spontaneous Ca2+ spikes. Nature 418: 93–96. [DOI] [PubMed] [Google Scholar]
- Gradin HM, Larsson N, Marklund U, Gullberg M (1998) Regulation of microtubule dynamics by extracellular signals: cAMP-dependent protein kinase switches off the activity of oncoprotein 18 in intact cells. J Cell Biol 140: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Tran T, Steinmetz JE, Goodlett CR (2002) Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eye-blink conditioning in adult rats. Brain Res 956: 302–311. [DOI] [PubMed] [Google Scholar]
- Guerri C (2002) Mechanisms involved in central nervous system dysfunctions induced by prenatal ethanol exposure. Neurotox Res 4: 327–335. [DOI] [PubMed] [Google Scholar]
- Haase A, Bicker G (2003) Nitric oxide and cyclic nucleotides are regulators of neuronal migration in an insect embryo. Development 130: 3977–3987. [DOI] [PubMed] [Google Scholar]
- Hatten ME (1990) Riding the glial monorail: a common mechanism for glial-guided migration in different regions of the developing brain. Trends Neurosci 13: 179–184. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Heintz N (1995) Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci 18: 385–408. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Khurdayan VK, Goody RJ, Nath A, Saria A, Pauly J (2003) Selective vulnerability of cerebellar granule neuroblasts and their progeny to drugs with abuse liability. Cerebellum 2: 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley J, Poo MM (2004) Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol 14: 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Milligan G (1997) Tailoring cAMP-signaling responses through isoform multiplicity. Trends Biochem Sci 22: 217–224. [DOI] [PubMed] [Google Scholar]
- Howe AK, Baldor LC, Hogan BP (2005) Spatial regulation of the cAMP-dependent protein kinase during chemotactic cell migration. Proc Natl Acad Sci USA 102: 14320–14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DG (1988) Influence of ethanol on neuronal and synaptic maturation in the central nervous system-morphological investigations. Prog Neurobiol 31: 171–197. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW (1973) Recognition of the fetal alcohol syndrome in early infancy. Lancet 1: 999–1001. [DOI] [PubMed] [Google Scholar]
- Juilfs DM, Soderling S, Burns F, Beavo JA (1999) Cyclic GMP as substrate and regulator of cyclic nucleotide phosphodiesterases (PDEs). Rev Physiol Biochem Pharmacol 135: 67–104. [DOI] [PubMed] [Google Scholar]
- Kennedy LA (1984) The pathogenesis of brain abnormalities in the fetal alcohol syndrome: an integrating hypothesis. Teratology 29: 363–368. [DOI] [PubMed] [Google Scholar]
- Komuro H, Kumada T (2005) Ca2+ transients control CNS neuronal migration. Cell Calcium 37: 387–393. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P (1992) Selective role of N-type calcium channels in neuronal migration. Science 257: 806–809. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P (1993) Modulation of neuronal migration by NMDA receptors. Science 260: 95–97. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P (1995) Dynamics of granule cell migration: a confocal microscopic study in acute cerebellar slices preparations. J Neurosci 15: 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P (1996) Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron 17: 275–285. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P (1998a) Distinct modes of neuronal migration in different domains of developing cerebellar cortex. J Neurosci 18: 1478–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P (1998b) Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J Neurobiol 37: 110–130. [PubMed] [Google Scholar]
- Komuro H, Rakic P (1999) In vitro analysis of signal mechanisms involved in neuronal migration. In: The neuron in tissue culture (Haynes LW, ed), pp 57–69. New York: Wiley.
- Komuro H, Yacubova E (2003) Recent advances in cerebellar granule cell migration. Cell Mol Life Sci 60: 1084–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Yacubova E, Yacubova E, Rakic P (2001) Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci 21: 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornguth SE, Rutledge JJ, Sunderland E, Siegal F, Carlson I, Smollens J, Juhl U, Young B (1979) Impeded cerebellar development and reduced serum thyroxine levels associated with fetal alcohol intoxication. Brain Res 177: 347–360. [DOI] [PubMed] [Google Scholar]
- Kumada T, Komuro H (2004) Completion of neuronal migration regulated by loss of Ca2+ transients. Proc Natl Acad Sci USA 101: 8479–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J (1996) Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J 15: 510–519. [PMC free article] [PubMed] [Google Scholar]
- Lemoine P, Harousseau H, Borteyru JP, Menuet JC (2003) Children of alcoholic parents–observed anomalies: discussion of 127 cases. Ther Drug Monit 25: 132–136. [DOI] [PubMed] [Google Scholar]
- Little RE, Anderson KW, Ervin CH, Worthington-Roberts B, Clarren SK (1989) Maternal alcohol use during breast-feeding and infant mental and motor development at one year. N Engl J Med 321: 425–430. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF (1989) Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243: 1721–1724. [DOI] [PubMed] [Google Scholar]
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA (2000) Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52: 375–413. [PubMed] [Google Scholar]
- Manzardo AM, Penick EC, Knop J, Nickel EJ, Hall S, Jensen P, Gabrielli Jr WF (2005) Developmental differences in childhood motor coordination predict adult alcohol dependence: proposed role for the cerebellum in alcoholism. Alcohol Clin Exp Res 29: 353–357. [DOI] [PubMed] [Google Scholar]
- Marcus JC (1987) Neurological findings in the fetal alcohol syndrome. Neuropediatrics 18: 158–160. [DOI] [PubMed] [Google Scholar]
- Mezna M, Patchick T, Tovey S, Michelangeli F (1996) Inhibition of the cerebellar inositol 1,4,5-trisphosphate-sensitive Ca2+ channel by ethanol and other aliphatic alcohols. Biochem J 314: 175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miale LL, Sidman RL (1961) An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol 4: 277–296. [DOI] [PubMed] [Google Scholar]
- Miller MW (1986) Effects of alcohol on the generation and migration of cerebral cortical neurons. Science 233: 1308–1311. [DOI] [PubMed] [Google Scholar]
- Miller MW (1993) Migration of cortical neurons is altered by gestational exposure to ethanol. Alcohol Clin Exp Res 17: 304–314. [DOI] [PubMed] [Google Scholar]
- Miller MW, Luo J (2002) Effects of ethanol and transforming growth factor beta (TGF beta) on neuronal proliferation and nCAM expression. Alcohol Clin Exp Res 26: 1281–1285. [DOI] [PubMed] [Google Scholar]
- Miller MW, Robertson S (1993) Prenatal exposure to ethanol alters the postnatal development and transformation of radial glia to astrocytes in the cortex. J Comp Neurol 337: 253–266. [DOI] [PubMed] [Google Scholar]
- Minana R, Climent E, Barettino S, Segui JM, Renau-Piqueras J, Guerri C (2000) Alcohol exposure alters the expression pattern of neural cell adhesion molecules during brain development. J Neurochem 75: 954–964. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Aistrup GL, Marszalec, Nagata K (1999) Neuronal nicotinic acetylcholine receptors: a new target site of ethanol. Neurochem Int 35: 131–141. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Soderpalm B, Ericson M, Olausson P, Engel JA, Zhang X, Nordberg A, Marszalec W, Aistrup GL, Schmidt LG, Kalouti U, Smolka M, Hedlund L (2001) Mechanisms of alcohol-nicotine interactions: alcoholics versus smokers. Alcohol Clin Exp Res 25: 152S–156S. [DOI] [PubMed] [Google Scholar]
- Olney JW (2004) Fetal alcohol syndrome at the cellular level. Addict Biol 9: 137–149. [DOI] [PubMed] [Google Scholar]
- Ozer E, Sarioglu S, Gure A (2000) Effects of prenatal ethanol exposure on neuronal migration, neurogenesis and brain myelination in the mice brain. Clin Neuropathol 19: 21–25. [PubMed] [Google Scholar]
- Pantazis NJ, West JR, Dai D (1998) The nitric oxide-cyclic GMP pathway plays an essential role in both promoting cell survival of cerebellar granule cells in culture and protecting the cells against ethanol neurotoxicity. J Neurochem 70: 1826–1838. [DOI] [PubMed] [Google Scholar]
- Parameswaran K, Radford K, Zuo J, Janssen LJ, O'Byrne PM, Cox PG (2004) Extracellular matrix regulates human airway smooth muscle cell migration. Eur Respir J 24: 545–551. [DOI] [PubMed] [Google Scholar]
- Peoples RW, Li C, Weight FF (1996) Lipid vs protein theories of alcohol action in the nervous system. Annu Rev Pharmacol Toxicol 36: 185–201. [DOI] [PubMed] [Google Scholar]
- Pfleiderer PJ, Lu KK, Crow MT, Keller RS, Singer HA (2004) Modulation of vascular smooth muscle cell migration by calcium/calmodulin-dependent protein kinases II-delta 2. Am J Physiol 286: C1238–C1245. [DOI] [PubMed] [Google Scholar]
- Rajalingam K, Wunder C, Brinkmann V, Churin Y, Hekman M, Sievers C, Rapp UR, Rudel T (2005) Prohibition is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat Cell Biol 7: 837–843. [DOI] [PubMed] [Google Scholar]
- Rakic P (1971) Neuron-glia relationship during granule cell migration in developing cerebellar cortex: a Golgi and electron microscopic study in Macacus rhesus. J Comp Neurol 141: 283–312. [DOI] [PubMed] [Google Scholar]
- Rakic P, Komuro H (1995) The role of receptor/channel activity in neuronal cell migration. J Neurobiol 26: 299–315. [DOI] [PubMed] [Google Scholar]
- Rakic P, Knyihar-Csillik E, Csillik B (1996) Polarity of microtubule assemblies during neuronal cell migration. Proc Natl Acad Sci USA 93: 9218–9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Ekbald U, Anton RF (1990) Perspectives on the pathophysiology of fetal alcohol syndrome. Alcohol Clin Exp Res 14: 807–812. [DOI] [PubMed] [Google Scholar]
- Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW (2001) A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci USA 98: 13482–13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddoch FC, Rowbotham SE, Brown AM, Redfern CP, Cheek TR (2005) Release and sequestration of Ca2+ by a caffeine- and ryanodine-sensitive store in a sub-population of human SH-SY5Y neuroblastoma cells. Cell Calcium 38: 111–120. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL (2005) Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med 230: 357–365. [DOI] [PubMed] [Google Scholar]
- Rossi D, Slater TN (1993) The developmental onset of NMDA receptor channel activity during neuronal migration. Neuropharmacology 32: 1239–1248. [DOI] [PubMed] [Google Scholar]
- Sakata-Haga H, Sawada K, Hisano S, Fukui Y (2001) Abnormalities of cerebellar foliation in rats prenatally exposed to ethanol. Acta Neuropathol 102: 36–40. [DOI] [PubMed] [Google Scholar]
- Shafer SH, Phelps SH, Williams CL (1998) Reduced DNA synthesis and cell viability in small cell lung carcinoma by treatment with cyclic AMP phosphodiesterase inhibitors. Biochem Pharmacol 56: 1229–1236. [DOI] [PubMed] [Google Scholar]
- Soderling SH, Beavo JA (2000) Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol 12: 174–179. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B (2003) Fetal alcohol spectrum disorder. JAMA 290: 2996–2999. [DOI] [PubMed] [Google Scholar]
- Titus B, Schwartz MA, Theodorescu D (2005) Rho proteins in cell migration and metastasis. Crit Rev Eukaryot Gene Expr 15: 103–114. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Rousselle C, Basille M, Falluel-Morel A, Pamantung TF, Fontaine M, Fournier A, Vaudry H, Gonzalez BJ (2002) Pituitary adenylate cyclase-activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proc Natl Acad Sci USA 99: 6398–6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman DM, Roelofs J, Engel R, Visser AJ, Van Haastert PJ (2005) Activation of soluble guanylyl cyclase at the leading edge during Dictyostelium chemotaxis. Mol Biol Cell 16: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter HJ, Messing RO (1999) Regulation of neuronal voltage-gated calcium channels by ethanol. Neurochem Int 35: 95–101. [DOI] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR (2004) Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 43: 647–661. [DOI] [PubMed] [Google Scholar]
- Welch-Carre E (2005) The neurodevelopmental consequences of prenatal alcohol exposure. Adv Neonatal Care 5: 217–229. [DOI] [PubMed] [Google Scholar]
- West JR, Chen WJ, Pantazis NJ (1994) Fetal alcohol syndrome: the vulnerability of the developing brain and possible mechanisms of damage. Metab Brain Dis 9: 291–322. [DOI] [PubMed] [Google Scholar]
- Wisniewski K, Damabska M, Sher JH, Qazi Q (1983) A clinical neuropathological study of the fetal alcohol syndrome. Neuropediatrics 14: 197–205. [DOI] [PubMed] [Google Scholar]
- Woodward JJ (1999) Overview of the effects of alcohol on the cerebral nervous system. Neurochem Int 35: 107–113. [PubMed] [Google Scholar]
- Xiang Y, Li Y, Zhang Z, Cui K, Wang S, Yuan XB, Wu CP, Poo MM, Duan S (2002) Nerve growth cone guidance mediated by G protein-coupled receptors. Nat Neurosci 5: 843–848. [DOI] [PubMed] [Google Scholar]
- Yacubova E, Komuro H (2002a) Intrinsic program for migration of cerebellar granule cells in vitro. J Neurosci 22: 5966–5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubova E, Komuro H (2002b) Stage-specific control of neuronal migration by somatostatin. Nature 415: 77–81. [DOI] [PubMed] [Google Scholar]
- Yacubova E, Komuro H (2003) Cellular and molecular mechanisms of cerebellar granule cell migration. Cell Biochem Biophys 37: 213–234. [DOI] [PubMed] [Google Scholar]
- Yang S, Huang XY (2005) Ca2+ influx through L-type Ca2+ channels controls the trailing tail contraction in growth factor-induced fibroblast cell migration. J Biol Chem 280: 27130–27137. [DOI] [PubMed] [Google Scholar]