Abstract
Physical activity (PA) is a known behavior to reduce cancer risk and improve cancer survivorship, yet adherence to PA guidelines is poor among the general population and cancer survivors. The purpose of this study was to determine the extent to which patients referred for exercise consultation within a clinical cancer prevention setting were meeting aerobic and resistance physical activity (PA) guidelines and to identify factors associated with guideline adherence. Between 2013 and 2015, cancer prevention patients and cancer survivors were interviewed by an exercise physiologist within an Integrative Health Program at The University of Texas MD Anderson Cancer Prevention Center. PA adherence was defined as at least 150-minutes of moderate-intensity or 75-minutes of vigorous-intensity PA per week, along with resistance training at least 2 days per week. Logistic regression was used to determine factors associated with meeting or not meeting PA guidelines for aerobic exercise, resistance exercise, and aerobic and resistance exercise combined. Among 1,024 cancer prevention patients and survivors, 9% of patients adhered to guideline-based PA. Adherence to aerobic and resistance guidelines were 20% and 12%, respectively. Overweight or obesity was associated with not meeting guideline-based PA in both cancer prevention patients and cancer survivors. Among breast cancer survivors, combination treatment with surgery, radiation, and chemotherapy (‘multimodal therapy’) was robustly associated with not meeting aerobic guidelines (OR 2.20, 95% CI: 1.17 to 4.16). BMI and breast cancer treatment history are key determinants of PA behavior among cancer prevention patients and survivors. Poor adherence to PA guidelines is a key issue for cancer prevention patients and survivors, particularly obese patients and women who receive multimodal therapy for breast cancer. Identifying and connecting patients at highest risk of poor PA adherence with exercise programs is needed to improve PA, a key modifiable cancer risk factor.
Introduction
Physical activity is a known behavior to reduce cancer risk [1–8]. Moreover, among cancer survivors, engaging in physical activity is associated not only with improvements in survival [9–12], but also with improvements in quality of life [13, 14], body image, emotional well-being, sleep, sexuality [14], muscular strength and cardiorespiratory fitness [13], and reductions in fatigue [13, 14], depression [13], and pain [14]. Among the lifestyle guidelines linked with cancer prevention (i.e. physical activity, diet, weight management), adherence to physical activity guidelines has exhibited the strongest association with lower mortality rates in cancer survivors [10, 15]. As such, national guidelines are in place and promote 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity physical activity per week, along with resistance training 2–3 days per week [16].
Significant reductions in cancer risk and improved survival has been demonstrated among those who adhere to the physical activity guidelines [17–19]. For example, a prospective study conducted in nearly 500,000 men and women in the United States observed a significant inverse association (p<0.05) between adherence to aerobic physical activity guidelines and both cancer incidence and cancer-specific mortality [18]. In addition, in a retrospective cohort based on the National Institute of Health AARP Diet and Health Study, conducted among 215,122 men and women, participation in resistance training was significantly associated with a lower risk of colon cancer (p-trend = 0.003) and trend towards lower risk of kidney cancer (p-trend = 0.06) [20]. Further, a retrospective study based on the National Health Interview Survey, conducted among nearly 14,000 cancer survivors at least three-years post-treatment, observed that adherence to both aerobic and resistance physical activity guidelines was associated with a 48%, 46%, and 40% reduction in cancer-specific, cardiovascular disease-specific, and all-cause mortality, respectively [19]. This work supports the importance of promoting physical activity in both cancer prevention and survivorship.
Unfortunately, population-based studies have shown that adherence to the physical activity guidelines is generally poor in both the general population and cancer survivors. According to surveillance data from the Centers for Disease Control and Prevention, approximately 20% of US adults are meeting both aerobic and resistance physical activity guidelines [4]. Among cancer survivors, 9–20% meet both aerobic and resistance physical activity guidelines [19, 21–26], with 22–44% only meeting aerobic guidelines [24, 25] and about 10–34% only meeting resistance guidelines [24, 25, 27, 28]. Evidence suggests that physical activity counseling from a health care provider improves physical activity behaviors, both among cancer survivors [29–31] and the general population [31]. However, initial factors that associate poor adherence to guideline-based physical activity before counseling is not well understood, though of key importance when developing and promoting initiatives to help patients reach recommendations.
At the University of Texas MD Anderson Cancer Prevention Center, patients with and without a history of cancer are seen for preventive services. The primary aim of the present investigation was to determine the extent to which cancer prevention patients and survivors seen in this setting were meeting aerobic physical activity guidelines, resistance physical activity guidelines, and the combination of aerobic and resistance physical activity guidelines. Our secondary aim was to identify clinical factors independently of physical activity guideline adherence among cancer survivors.
Methods
Design, patient population, and exercise counseling
A retrospective chart review was conducted from patients that were referred for consultation from an exercise physiologist between 2013 and 2015 in the Cancer Prevention Center at MD Anderson Cancer Center. The protocol and waiver of informed consent was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board.
The patient population seen at MD Anderson’s Cancer Prevention Center (CPC) consists of patients of average to high risk for cancer (cancer prevention patients) and cancer survivors who have completed treatment. Cancer prevention patients of average risk may be representative of the general population, whereas higher risk prevention patients are unique to this setting.
The CPC offers a range of services to help patients (prevention patients and survivors) learn how to reduce cancer risk or to detect cancer early. The CPC implemented an integrative health program that assists patients in adopting healthy lifestyle behaviors to prevent cancer occurrence or recurrence. Included in this program is the opportunity for in-depth one-on-one exercise counseling with a clinical exercise physiologist. When patients arrive for their clinic appointment, they are asked if they would like the opportunity to meet with a clinical exercise physiologist for exercise counseling. Additionally, patients can be referred to the exercise physiologist for exercise counseling by their provider if the need or interest for exercise counseling is identified by the patient or provider. Providers in the CPC consist of internists/family physicians, medical oncologists, and advance nurse practitioners.
Within the exercise counseling sessions, our clinical exercise physiologists work with our patients to identify their personal goals related to increasing physical activity behavior and strategize a personalized exercise plan to help them achieve their goals. The degree of follow-up and mode of follow-up (e.g.- phone call or face-to-face appointment) is individualized based on patient preference. Data regarding adherence to both aerobic and resistance guidelines are collected during these counseling sessions (please see assessment of adherence to physical activity guidelines section for details).
Between 2013 and 2015, a total of 10,000 patients were seen by the integrative health program. During this time exercise physiologist interviewed a total of 1,126 patients. We limited the analysis to patient charts including information on aerobic activity and resistance training (n = 1,035). We then eliminated patients with a BMI < 18.5 kg/m2 (n = 4) and patients with an undetermined race/ethnicity (n = 7) due to inadequate sample size in these cells for analyses. Therefore, a total of 1,024 cancer prevention patients and cancer survivors were included in this investigation.
Patient demographics and clinical factors
Information regarding the following demographic and clinical factors was collected from the medical record: age, sex, race/ethnicity, body mass index (BMI), smoking history, cancer history, diabetes status and menopause status. For the sample of cancer survivors, time since diagnosis and cancer therapy history (i.e. history of chemotherapy, anthracycline therapy, radiation therapy, radiation side, surgery, surgery type) were also collected. Multimodal therapy was defined as patients undergoing all three types of therapy (chemotherapy, radiation, surgery) as part of their treatment plan.
Assessment of adherence to physical activity guidelines
Current self-reported physical activity levels and adherence to the physical activity guidelines were determined during the exercise physiologist consultation. Patients were asked the following questions within a conversation pertaining to their exercise behavior: how many days per week do you perform moderate exercise; on average, how long do you perform these exercises; how many days per week do you perform vigorous exercises; on average, how long do you perform these exercises; how many days per week do you practice muscle strengthening exercises; on average, how long do you perform these exercises; what is the mode of activity you typically engage in; what are the exercise resources available to you (i.e. gym, fitness classes, exercise videos). Patients were also provided information defining what is considered moderate intensity and vigorous intensity aerobic activity, and resistance training. Patients were considered adherent to the aerobic physical activity guidelines if they reported participating in at least 150 minutes of moderate intensity aerobic activity per week, or at least 75 minutes of vigorous intensity aerobic activity per week, or a combination of both. Patients were considered adherent to the resistance training guidelines if they reported participating in at least two days of resistance training per week that worked all major muscle groups. Patients were considered adherent to both aerobic and resistance training guidelines if they reported participating in the aforementioned amount of weekly aerobic and resistance training activity.
Statistical methods
The variables pertaining to meeting aerobic, resistance, and the combination of aerobic and resistance guidelines were dichotomized as “meeting” or “not meeting” the guidelines. Frequencies and descriptive statistics were used to determine patient characteristics and the extent of which patients were adherent to aerobic, resistance, and the combination of aerobic and resistance guidelines. Chi-square tests were used to examine if there was a significant association between adherence to aerobic guidelines, resistance guidelines, and the combination of aerobic and resistance guidelines, by demographic and clinical factors among the total sample (n = 1,024) and by treatment exposure among female breast cancer survivors (n = 346). For the cancer survivor analyses, female breast cancer was the cancer type used in the analysis due to the small sample size and heterogeneity of cancer types other than female breast cancer. The other most common types of cancer (>1%) seen in the Cancer Prevention Center included: skin cancers other than melanoma (7%, n = 36), thyroid cancer (6%, n = 30), melanoma (3%, n = 15), colon cancer (2%, n = 12), and endometrial cancer (2%, n = 9).
Univariate logistic regression modeling was performed to determine the relative odds of not meeting aerobic physical activity guidelines (yes/no) based on individual baseline demographics and clinical factors for the overall CPC population (n = 1,024) and breast cancer survivors (n = 346). Similar modeling was performed for not meeting resistance guidelines only (yes/no) and not meeting both aerobic and resistance physical activity guidelines (yes/no). Multivariable models adjusted for age, BMI, race/ethnicity, insurance status, smoking history, and diabetes status. Additional adjustments were made for menopause status and multimodal breast cancer therapy for breast cancer specific models. All data were analyzed with SPSS statistical software package, version 23 (Chicago, IL).
Results
Cancer prevention patients and survivors referred to an exercise physiologist
Demographic and clinical factors
Of the 1,024 patients included in the present study, 97% (n = 989) were women and 3% (n = 35) were men. The mean age of participants was 57±10 years old. Sixty-two percent (63%) were Caucasian (n = 642), 21% were African-American (n = 217), 12% were Hispanic (n = 125), and 4% were Asian (n = 40). The mean BMI was 33.4±7.1 kg/m2. A total of 9% of patients were normal weight (BMI 18.5–24.9 kg/m2, n = 94), 24% were overweight (BMI 25–29.9 kg/m2, n = 245), and 67% were obese (BMI ≥30 kg/m2, n = 685). A total of 76% (n = 778) of patients were never smokers, and 21% and 3% were former (n = 215) or current (n = 31) smokers respectively. A total of 83% did not have a history of diabetes (n = 852), while 3% had pre-diabetes (n = 29), and 14% had diabetes (n = 143). Finally, among women, 18% were pre-menopausal (n = 179), 2% were peri-menopausal (n = 22), and 80% were post-menopausal (n = 788). Fifty percent of patients (n = 513) were cancer prevention patients and 50% were cancer survivors (n = 511).
Among breast cancer survivors, mean age and BMI of participants was 61±10 years old and 32±7 kg/m2. A total of 15% of patients were normal weight (BMI 18.5–24.9 kg/m2, n = 53), 25% were overweight (BMI 25–29.9 kg/m2, n = 85), and 60% were obese (BMI ≥30 kg/m2, n = 208). A total of 10% were pre-menopausal (n = 35), 2% were peri-menopausal (n = 7), and 88% were post-menopausal (n = 304). Sixty percent (60%) were Caucasian (n = 207), 20% were African-American (n = 70), 15% were Hispanic (n = 51), and 5% were Asian (n = 18). A total of 75% (n = 260) of patients were never smokers, 23% (m = 78) were former smokers and 2% (n = 8) were current smokers. A total of 82% did not have a history of diabetes (n = 285), while 3% had pre-diabetes (n = 9), and 15% had diabetes (n = 52).
Adherence to physical activity guidelines by demographic and clinical factors
Among our cohort 20% met aerobic physical activity guidelines. Men were more likely to meet aerobic guidelines compared to women (34% compared to 20%, p = 0.03) (Table 1). Individuals with a weight classification of obesity were less likely to meet aerobic physical activity guidelines compared to overweight or normal weight individuals (16% compared to 26% and 35%, respectively, p<0.001). Individuals with a cancer history were more likely to meet aerobic physical activity guidelines compared to patients with no cancer history (23% compared to 17%, p = 0.02). Univariate associations were not observed for age (p = 0.07), race/ethnicity (p = 0.10), smoking history (p = 0.82), history of diabetes (p = 0.06), or menopausal status (p = 0.61). Regarding resistance guidelines, 12% of cancer prevention patients and survivors met recommended guidelines. Both BMI (p<0.001) and cancer history (p = 0.04) were associated with adherence to resistance guidelines, where individuals with normal BMI or cancer survivors were more likely to meet guidelines. Moreover, a significant association was also observed for age, such that middle-aged (45–64 years old) individuals were more likely to meet resistance physical activity guidelines (p = 0.01). No other univariate associations were observed regarding adherence to resistance guidelines (p>0.05).
Table 1. Adherence to physical activity guidelines by demographic and clinical factors.
| Variable | Aerobic Guidelines | Resistance Guidelines | Aerobic & Resistance Guidelines | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Met | Not Met | p-level | Met | Not Met | p-level | Met | Not Met | p-level | |||
| Age (years) |
< 45 | Count | 14 | 102 | 0.07 | 6 | 110 | 0.01 | 4 | 112 | 0.07 |
| (n = 116) | % | 12.1% | 87.9% | 5.2% | 94.8% | 3.4% | 96.6% | ||||
| 45–54 | Count | 56 | 204 | 41 | 219 | 29 | 231 | ||||
| (n = 260) | % | 21.5% | 78.5% | 15.8% | 84.2% | 11.2% | 88.8% | ||||
| 55–64 | Count | 92 | 308 | 57 | 343 | 40 | 360 | ||||
| (n = 400) | % | 23.0% | 77.0% | 14.3% | 85.8% | 10.0% | 90.0% | ||||
| 65–74 | Count | 39 | 169 | 21 | 187 | 14 | 194 | ||||
| (n = 208) | % | 18.8% | 81.3% | 10% | 90% | 6.7% | 93.3% | ||||
| ≥ 75 | Count | 5 | 35 | 2 | 38 | 2 | 38 | ||||
| (n = 40) | % | 12.5% | 87.5% | 5.0% | 95.0% | 5.0% | 95.0% | ||||
| Sex | Female | Count | 194 | 795 | 0.03 | 119 | 870 | 0.06 | 83 | 906 | 0.07 |
| (n = 989) | % | 19.6% | 80.4% | 12.0% | 88.0% | 8.4% | 91.6% | ||||
| Male | Count | 12 | 23 | 8 | 27 | 6 | 29 | ||||
| (n = 35) | % | 34.3% | 65.7% | 22.9% | 77.1% | 17.1% | 82.9% | ||||
| Race/ Ethnicity |
White (non-Hispanic) | Count | 142 | 500 | 0.10 | 89 | 553 | 0.12 | 64 | 578 | 0.29 |
| (n = 642) | % | 22.1% | 77.9% | 13.9% | 86.1% | 10.0% | 90.0% | ||||
| Asian | Count | 10 | 30 | 7 | 33 | 3 | 37 | ||||
| (n = 40) | % | 25.0% | 75.0% | 17.5% | 82.5% | 7.5% | 92.5% | ||||
| African American | Count | 35 | 182 | 19 | 198 | 13 | 204 | ||||
| (n = 217) | % | 16.1% | 83.9% | 8.8% | 91.2% | 6.0% | 94.0% | ||||
| Hispanic or Latino |
Count | 19 | 106 | 12 | 113 | 9 | 116 | ||||
| (n = 125) | % | 15.2% | 84.8% | 9.6% | 90.4% | 7.2% | 92.8% | ||||
| BMI (kg/m2) |
18.5–24.99 | Count | 33 | 61 | <0.001 | 21 | 73 | <0.001 | 16 | 78 | <0.001 |
| (n = 94) | % | 35.1% | 64.9% | 22.3% | 77.7% | 17.0% | 83.0% | ||||
| 25–29.99 | Count | 63 | 182 | 41 | 204 | 30 | 215 | ||||
| (n = 245) | % | 25.7% | 74.3% | 16.7% | 83.3% | 12.2% | 87.8% | ||||
| ≥ 30 | Count | 110 | 575 | 65 | 620 | 43 | 642 | ||||
| (n = 685) | % | 16.1% | 83.9% | 9.5% | 90.5% | 6.3% | 93.7% | ||||
| Smoking History | Never | Count | 159 | 619 | 0.82 | 89 | 689 | 0.23 | 65 | 713 | 0.79 |
| (n = 778) | % | 20.4% | 79.6% | 11.4% | 88.6% | 8.4% | 91.6% | ||||
| Former | Count | 42 | 173 | 34 | 181 | 21 | 194 | ||||
| (n = 215) | % | 19.5% | 80.5% | 15.8% | 84.2% | 9.8% | 90.2% | ||||
| Current | Count | 5 | 26 | 4 | 27 | 3 | 28 | ||||
| (n = 31) | % | 16.1% | 83.9% | 12.9% | 87.1% | 9.7% | 90.3% | ||||
| Cancer History | No | Count | 88 | 425 | 0.02 | 53 | 460 | 0.04 | 36 | 477 | 0.06 |
| (n = 513) | % | 17.2% | 82.8% | 10.3% | 89.7% | 7.0% | 93.0% | ||||
| Yes | Count | 118 | 393 | 74 | 437 | 53 | 458 | ||||
| (n = 511) | % | 23.1% | 76.9% | 14.5% | 85.5% | 10.4% | 89.6% | ||||
| Diabetes | No | Count | 183 | 671 | 0.06 | 113 | 741 | 0.19 | 82 | 772 | 0.07 |
| (n = 854) | % | 21.4% | 78.6% | 13.2% | 86.8% | 9.6% | 90.4% | ||||
| Pre-diabetes | Count | 4 | 23 | 2 | 25 | 1 | 26 | ||||
| (n = 27) | % | 14.8% | 85.2% | 7.4% | 92.6% | 3.7% | 96.3% | ||||
| Yes | Count | 19 | 124 | 12 | 131 | 6 | 137 | ||||
| (n = 143) | % | 13.3% | 86.7% | 8.4% | 91.6% | 4.2% | 95.8% | ||||
| Menopause (n = 989) | Pre-menopausal | Count | 33 | 146 | 0.61 | 21 | 158 | 0.08 | 17 | 162 | 0.04 |
| (n = 179) | % | 18.4% | 81.6% | 11.7% | 88.3% | 9.5% | 90.5% | ||||
| Peri-menopausal | Count | 6 | 16 | 6 | 16 | 5 | 17 | ||||
| (n = 22) | % | 27.3% | 72.7% | 27.3% | 72.7% | 22.7% | 77.3% | ||||
| Post-menopausal | Count | 155 | 633 | 92 | 696 | 61 | 727 | ||||
| (n = 788) | % | 19.7% | 80.3% | 11.7% | 88.3% | 7.7% | 92.3% | ||||
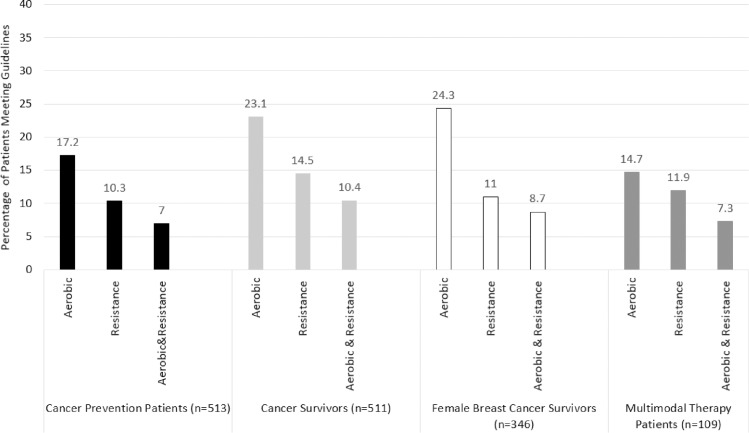
Overall, 9% of cancer prevention patients and survivors met both aerobic and resistance physical activity guidelines. Only 6% of obese individuals met both guidelines, whereas 12% of overweight and 17% of normal weight met both guidelines, respectively (p<0.001). Among women, a significant association was observed for menopausal status, where peri-menopausal women were most-likely to meet both guidelines (22.7%) followed by pre-menopausal (9.5%) then post-menopausal women (7.7%) (p = 0.04). No other significant associations were observed (p>0.05 for all) (Fig 1).
Fig 1. Percentage of patients meeting physical activity guidelines.
Female breast cancer survivors
The mean time from diagnosis to physical activity assessment was 9.1 years (95% confidence interval [CI] 8.5–9.7). Among female breast cancer survivors, there was no association between meeting aerobic physical activity guidelines and time since cancer diagnosis (p = 0.53), history of chemotherapy (p = 0.19), anthracycline therapy (p = 0.61), or surgery (p = 0.13). Univariable associations were observed for radiation therapy, such that women who did not undergo radiation therapy were more likely to meet aerobic activity guidelines (31%) compared to women who did have radiation therapy (20%, p = 0.02). Additionally, women who had a mastectomy were more likely to meet aerobic activity guidelines compared to women who have had lumpectomy, 32% compared to 20% respectively (p = 0.01). Associations between all other variables in relation to resistance training guidelines alone and both aerobic and resistance guidelines are seen in Table 2.
Table 2. Adherence to physical activity guidelines by treatment exposure among female breast cancer survivors.
| Variable | Aerobic Guidelines | Resistance Guidelines | Aerobic & Resistance Guidelines | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meeting | Not Meeting | p-level | Meeting | Not Meeting | p-level | Meeting | Not Meeting | p-level | |||
| Time Since Cancer Diagnosis (n = 346) | ≤ 3 years | Count | 12 | 45 | 0.53 | 8 | 49 | 0.98 | 6 | 51 | 0.90 |
| (n = 57) | % | 21.1% | 78.9% | 14.0% | 86.0% | 10.5% | 89.5% | ||||
| > 3 years | Count | 72 | 217 | 41 | 248 | 32 | 257 | ||||
| (n = 289) | % | 24.9% | 75.1% | 14.2% | 85.8% | 11.1% | 88.9% | ||||
| Chemotherapy (n = 346) | No | Count | 52 | 141 | 0.19 | 29 | 164 | 0.60 | 24 | 169 | 0.33 |
| (n = 193) | % | 26.9% | 73.1% | 15.0% | 85.0% | 12.4% | 87.6% | ||||
| Yes | Count | 32 | 121 | 20 | 133 | 14 | 139 | ||||
| (n = 153) | % | 20.9% | 79.1% | 13.1% | 86.9% | 9.2% | 90.8% | ||||
| Anthracycline Therapy (n = 153) | No | Count | 2 | 5 | 0.61 | 2 | 5 | 0.21 | 1 | 6 | 0.63 |
| (n = 7) | % | 28.6% | 71.4% | 28.6% | 71.4% | 14.3% | 85.7% | ||||
| Yes | Count | 30 | 116 | 18 | 128 | 13 | 133 | ||||
| (n = 146) | % | 20.5% | 79.5% | 12.3% | 87.7% | 8.9% | 91.1% | ||||
| Radiation Therapy (n = 346) | No | Count | 42 | 94 | 0.02 | 20 | 116 | 0.82 | 17 | 119 | 0.47 |
| (n = 136) | % | 30.9% | 69.1% | 14.7% | 85.3% | 12.5% | 87.5% | ||||
| Yes | Count | 42 | 168 | 29 | 181 | 21 | 189 | ||||
| (n = 210) | % | 20.0% | 80.0% | 13.8% | 86.2% | 10.0% | 90.0% | ||||
| Radiation Side (n = 210) | Left | Count | 22 | 88 | 1.00 | 14 | 96 | 0.63 | 8 | 102 | 0.17 |
| (n = 110) | % | 20.0% | 80.0% | 12.7% | 87.3% | 7.3% | 92.7% | ||||
| Right | Count | 20 | 80 | 15 | 85 | 13 | 87 | ||||
| (n = 100) | % | 20.0% | 80.0% | 15.0% | 85.0% | 13.0% | 87.0% | ||||
| Surgery (n = 346) | No | Count | 0 | 7 | 0.13 | 0 | 7 | 0.28 | 0 | 7 | 0.35 |
| (n = 7) | % | 0.0% | 100.0% | 0.0% | 100.0% | 0.0% | 100.0% | ||||
| Yes | Count | 84 | 255 | 49 | 290 | 38 | 301 | ||||
| (n = 339) | % | 24.8% | 75.2% | 14.5% | 85.5% | 11.2% | 88.8% | ||||
| Surgery Type (n = 339) | Mastectomy | Count | 46 | 100 | 0.01 | 27 | 119 | 0.07 | 23 | 123 | 0.02 |
| (n = 146) | % | 31.5% | 68.5% | 18.5% | 81.5% | 15.8% | 84.2% | ||||
| Lumpectomy | Count | 38 | 155 | 22 | 171 | 15 | 178 | ||||
| (n = 193) | % | 19.7% | 80.3% | 11.4% | 88.6% | 7.8% | 92.2% | ||||
In multivariable models, obese breast cancer survivors were at 2-fold higher odds (Odds Ratio [OR] 2.07, 95% CI: 1.0, 4.33) of not meeting aerobic physical activity guidelines compared to overweight or normal weight survivors. Moreover, breast cancer survivors who underwent multimodal therapy (surgery, chemotherapy, and radiation) were also at 2-fold higher odds of not meeting aerobic physical activity guidelines (OR 2.20, 95%CI: 1.17, 4.16). No other independent associations were demonstrated in models for aerobic physical activity guidelines. Regarding resistance training guidelines, breast cancer survivors with diabetes exhibited nearly 8-fold higher odds of not meeting resistance guidelines (OR 7.95, 95%CI: 1.05, 60.46); the wide confidence interval reflects a low number of women representing this category. No other independent associations were observed in models related to meeting the combination of aerobic and resistance training guidelines (Table 3).
Table 3. Factors associated with non-adherence to activity guideline in female BC survivors (n = 346).
| Variable | Cases | Aerobic Guidelines | Resistance Guidelines | Aerobic & Resistance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p-level | OR | 95%CI | p-level | OR | 95%CI | p-level | ||||||
| BMI | 18.5–24.99 kg/m2 | 53 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||||
| 25–29.99 kg/m2 | 85 | 1.656 | .755 | 3.633 | .094 | 1.521 | .615 | 3.763 | .364 | 1.741 | .648 | 4.676 | .271 | |
| ≥ 30 kg/m2 | 208 | 2.077 | .996 | 4.334 | .019 | 1.889 | .802 | 4.451 | .146 | 2.389 | .927 | 6.156 | .071 | |
| Race/ Ethnicity |
White (non-Hispanic) | 207 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||||
| Asian | 18 | .919 | .300 | 2.817 | .883 | .723 | .216 | 2.418 | .598 | 1.321 | .305 | 5.726 | .710 | |
| African American | 70 | 1.769 | .807 | 3.880 | .155 | 1.824 | .630 | 5.283 | .268 | 1.739 | .526 | 5.754 | .365 | |
| Hispanic or Latino | 51 | 1.501 | .647 | 3.484 | .344 | 1.373 | .472 | 3.995 | .561 | 1.305 | .399 | 4.273 | .660 | |
| Insurance Status | Self-Pay | 11 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||||
| Medicare or Medicaid | 114 | .650 | .110 | 3.829 | .634 | .761 | .075 | 7.755 | .817 | .910 | .083 | 9.912 | .938 | |
| Private Insurance | 185 | .662 | .123 | 3.560 | .630 | .524 | .058 | 4.747 | .565 | .738 | .078 | 6.992 | .791 | |
| Not Reported | 36 | .523 | .084 | 3.238 | .486 | .601 | .055 | 6.518 | .675 | .577 | .051 | 6.544 | .657 | |
| Smoking History | Never Use | 260 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||||
| Ever Use | 86 | 1.240 | .662 | 2.325 | .502 | 1.166 | .545 | 2.492 | .692 | 2.081 | .805 | 5.379 | .131 | |
| Diabetes | No | 285 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||||
| Pre-diabetes | 9 | 3.218 | .367 | 28.196 | .291 | * | * | * | * | * | * | * | * | |
| Yes | 52 | 1.796 | .741 | 4.352 | .195 | 7.951 | 1.046 | 60.458 | .045 | 6.381 | 0.824 | 49.403 | .076 | |
| Menopause | Pre-menopausal | 35 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||||
| Peri-menopausal | 7 | .873 | .109 | 6.978 | .898 | 1.701 | .133 | 21.727 | .683 | 1.222 | .091 | 16.310 | .880 | |
| Post-menopausal | 304 | 1.004 | .368 | 2.738 | .994 | 1.490 | .444 | 4.998 | .519 | 2.017 | .537 | 7.581 | .299 | |
| Multimodal Therapy | No | 237 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||||
| Yes | 109 | 2.204 | 1.167 | 4.161 | .015 | 1.169 | .562 | 2.430 | .676 | 1.554 | .648 | 3.723 | .323 | |
Adjusted for age, BMI, race/ethnicity, insurance status, cancer history, smoking history, diabetes, heart condition, chemotherapy, radiation, and surgery history* Not enough cases meeting guidelines to determine OR.
Discussion
In the current study, we found that only 9% of cancer prevention patients and survivors referred to an exercise physiologist for consultation adhered to both aerobic and resistance physical activity guidelines. We observed that being overweight or obese was associated with whether patients were meeting guideline-based physical activity among both prevention patients and breast cancer survivors. Moreover, we observed that adjuvant therapy consisting of a combination of surgery, radiotherapy, and chemotherapy (i.e. multimodal therapy) was independently associated with poor adherence to physical activity guidelines among breast cancer survivors.
Our finding of low adherence to physical activity guidelines among cancer prevention patients and survivors is consistent with surveillance data from the Behavioral Risk Factor Surveillance System (BRFSS), National Health Interview Survey (NHIS), National Health and Nutrition Survey (NHANES), and the Surveillance, Epidemiology, and End Results Program (SEER) [4, 32, 33]. In aggregate, these data speak to the overwhelming need to provide systematic screening and interventions for patients with the goal of increasing physical activity, a key risk factor of cancer occurrence and recurrence. Refinement of health care delivery and reimbursement models are needed to encourage providers and health care systems across all clinical care settings to develop programs centered around physical activity screening and engagement.
Our role in the integrative health program within the Cancer Prevention Center at MD Anderson is to provide these types of services to patients in order to increase physical activity. We have done this by developing an algorithm that is integrated within our clinical workflow. Access to our algorithm is publicly available here: https://www.mdanderson.org/for-physicians/clinical-tools-resources/clinical-practice-algorithms.html.
Essentially, qualified clinical personnel assess the patient’s current level of activity by asking the patient about his/her exercise frequency, intensity (assessed via rating of perceived exertion of self-evaluation of exercise tolerance), duration, ad type of exercise engaged in. If the patient’s self-reported physical activity behavior meets the ACSM physical activity guidelines, then the patient is asked whether he/she is interested in increasing activity. If the patient is not, then positive reinforcement and encouragement to continue meeting guidelines is provided. If the patient is interested in increasing activity, then the patient is assessed for pre-defined (per developed algorithm) conditions that may require clearance from a medical provider. If the patient does not require medical clearance, then he/she may be referred to a clinical exercise physiologist for exercise counseling. If the patient does require medical clearance, then the patient is provided with a physical activity clearance form to be completed by her provider.
Alternatively, if the patient’s self-reported physical activity behavior is not meeting ACSM physical activity guidelines, then the patient is asked whether he/she is interested in starting or increasing activity. If the patient is not, then review of activity guidelines and motivational interviewing to encourage physical activity and limit sedentary time is provided. Additionally, an explanation of physical activity benefits for cancer prevention and a handout related to tips to getting fit is provided. Reassessment of physical activity will then occur at the next clinic visit. However, if the patient is interested in starting or increasing physical activity, then the he/she will be assessed for pre-defined conditions that may require medical clearance as described above, and this pathway in the algorithm will be followed.
Our secondary aim was to identify clinical factors associated with poor adherence to physical activity guidelines. We observed significant inverse associations between BMI and adherence to physical activity guidelines for both cancer prevention patients and breast cancer survivors. This result was largely expected, given obesity and physical inactivity are interrelated (e.g. physical inactivity may lead to weight gain, weight gain may lead to further physical inactivity, etc.). Unfortunately, obesity is an epidemic in the US population and is considered a key risk factor for 13 different types of cancer, in addition to physical inactivity [12, 34]. To this end, lifestyle programs aimed at increasing physical activity as part of weight loss efforts should be considered for both cancer prevention patients and breast cancer survivors, when appropriate.
In the current study, we found that attention to prior treatment exposures may be a way to identify breast cancer survivors who are least likely to adhere to physical activity guidelines. While breast cancer survivors who received particular adjuvant therapies (e.g. radiation, lumpectomy) were less likely to adhere to guideline-based activity compared to those not receiving such therapies, only multimodal therapy (surgery, radiation, and chemotherapy) was independently associated with poor adherence to physical activity guidelines in the current study. Our results support other work by our group demonstrating that cardiorespiratory fitness, measured via exercise treadmill testing, is significantly lower in breast cancer survivors receiving multimodal therapy compared to those receiving individual therapies alone [35, 36]. In both studies, physical activity and cardiorespiratory fitness were measured years (> 5 years on average) after the initial treatment exposure. These data reveal that low levels of physical activity or fitness are not short-term issues for breast cancer survivors receiving multimodal therapy [35, 36].
Additionally, it may also be postulated that breast cancer survivors with a history of multimodal therapy may exhibit lower adherence to physical activity guidelines and lower levels of cardiorespiratory fitness due to increased cancer-related fatigue secondary to their treatment history. A recent meta-analysis conducted by van Vulpen and colleagues [37] observed statistically significant improvements in general fatigue and physical fatigue when physical activity was conducted during adjuvant therapy among breast cancer survivors. Furthermore, a recent Cochrane systematic review conducted by Lahart and colleagues [38] observed statistically significant but smaller improvements in fatigue when physical activity was conducted post-adjuvant therapy among breast cancer survivors. Taken together, creating an infrastructure to promote and deliver exercise programs for breast cancer patients during and after multimodal therapy is needed.
Strengths of the current study include a large sample size, consideration of adherence to aerobic and resistance physical activity guidelines alone and in combination, and assessment of multimodal therapy in relation to physical activity guideline adherence. Limitations of this study include self-reported physical activity behavior, inability to examine factors associated with physical activity adherence in other cancer populations due to small sample sizes, cross-sectional design of the study, and referral to see an exercise physiologist based on provider recommendation or patient interest/inquiry. The latter may have selected for more motivated patients since some patients inquired for a referral, though our physical activity adherence percentages were in line with population-based studies. We consider the fact that our study included one-on-one counseling with a clinical exercise physiologist as both a strength and a limitation of this study. While the expertise of an exercise physiologist is certainly a benefit to our patients and to the assessment of guideline adherence, this is also a limitation as not many cancer centers provide this service and so the generalizability of our model at this time may be limited. Future research should consider targeting men and normal weight cancer prevention patients and survivors in similar analyses, assessment of objectively measured aerobic and resistance activity, assessment of other cancer types in relation to physical activity guideline adherence, and dissemination and implementation research aimed at utilizing the identified factors associated with non-adherence to physical activity guidelines to identify the most appropriate patients in need of physical activity promotion interventions.
Conclusions
Poor adherence to physical activity guidelines among cancer prevention patients and survivors seen in a clinical cancer prevention center were similar to findings observed from surveillance and population-based cohort studies. Based on our results, a review of BMI and cancer treatment history, specifically history of multimodal therapy, should be considered in order to identify individuals at greatest risk for non-adherence to physical activity guidelines. Moreover, a greater focus on integrating physical activity as part of the conversation providers have with patients, and including activity counseling, promotion programs and resources by trained professionals into the clinical care setting will be critical in order to improve adherence to physical activity guidelines at the population level. This change will require engagement with 3rd party payers regarding reimbursement of preventive services in clinical setting as well as require innovative approaches (i.e. mobile health technology) to systematically reach and promote physical activity in prevention patients and cancer survivors.
Supporting information
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by a gift from the Duncan Family to the University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment. This study was also supported by the Center for Energy Balance in Cancer Prevention and Survivorship, Duncan Family Institute. We would like to thank the National Cancer Institute of the National Institutes of Health under Award Number R25CA057730 (PI: Shine Chang, PhD) for supporting AMC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All funders had not role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Abioye AI, Odesanya MO, Abioye AI, and Ibrahim NA. Physical activity and risk of gastric cancer: a meta-analysis of observational studies. Br J Sports Med. 2015;49:224–29. 10.1136/bjsports-2013-092778 [DOI] [PubMed] [Google Scholar]
- 2.Albanes D, Blair A, and Taylor PR. Physical activity and risk of cancer in the NHANES I population. Am J Public Health. 1989;79(6):744–50. 10.2105/ajph.79.6.744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCullough ML, Patel AV, Kushi LH, Patel R, Willett WC, Doyle C, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1089–97. 10.1158/1055-9965.EPI-10-1173 [DOI] [PubMed] [Google Scholar]
- 4.McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women's Health Initiative Cohort Study. JAMA. 2003;290(10):1331–6. 10.1001/jama.290.10.1331 [DOI] [PubMed] [Google Scholar]
- 5.Sanchis-Gomar F, Lucia A, Yvert T, Ruiz-Casado A, Pareja-Galeano H, Santos-Lozano A, et al. Physical inactivity and low fitness deserve more attention to alter cancer risk and prognosis. Cancer Prev Res (Phila). 2015;8(2):105–10. 10.1158/1940-6207.CAPR-14-0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thune I, Brenn T, Lund E, and Gaard M. Physical activity and the risk of breast cancer. N Engl J Med. 1997;336(18):1269–75. 10.1056/NEJM199705013361801 [DOI] [PubMed] [Google Scholar]
- 7.Thune I and Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc. 2001;33(6 Suppl):S530–50. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Zhang D, and Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Cancer Res Treat. 2013;137:869–82. [DOI] [PubMed] [Google Scholar]
- 9.Farrell SW, Cortese GM, LaMonte MJ, and Blair SN. Cardiorespiratory fitness, different measures of adiposity, and cancer mortality in men. Obesity (Silver Spring). 2007;15(12):3140–9. 10.1038/oby.2007.374 [DOI] [PubMed] [Google Scholar]
- 10.Inoue-Choi M, Robien K, and Lazovich D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22(5):792–802. 10.1158/1055-9965.EPI-13-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irwin M, Smith A, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland F, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–64. 10.1200/JCO.2007.15.9822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakoski S, Willis B, Barlow C, Leonard D, Gao A, Radford N, et al. Midlife Cardiorespiratory Fitness, Incident Cancer, and Survival After Cancer in Men: The Cooper Center Longitudinal Study. JAMA Oncol. 2015;1(2):231–7. 10.1001/jamaoncol.2015.0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leun SS, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70 10.1136/bmj.e70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;CD007566 10.1002/14651858.CD007566.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, and Courneya KS. Physical activity and survival after prostate cancer. Eur Urol. 2016;70(4):576–85. 10.1016/j.eururo.2015.12.032 [DOI] [PubMed] [Google Scholar]
- 16.Pescatello LS, Arena R, Riebe D, and Thompson PD, eds. ACSM's Guidelines for Exercise Testing and Prescription. Ninth ed 2013, Lippincott, Williams & Wilkins: Baltimore, MD. [DOI] [PubMed] [Google Scholar]
- 17.2018 Physical Activity Guidelines Advisory Committee Scientific Report. 2018. U.S. Department of Health and Human Services: Washington, DC.
- 18.Kabat GC, Matthews CE, Kamensky V, Hollenbeck AR, and Rohan TE. Adherence to cancer prevention guidelines and cancer incidence, cancer mortality, and total mortality: a prospective cohort study. Am J Clin Nutr. 2015;101:558–69. 10.3945/ajcn.114.094854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarasenko YN, Linder DF, and Miller EA. Muscle-strengthening and aerobic activities and mortality among 3+ year cancer survivors in the U.S. Cancer Causes Control. 2018;29(4–5):475–84. 10.1007/s10552-018-1017-0 [DOI] [PubMed] [Google Scholar]
- 20.Mazzilli KM, Matthews CE, Salerno EA, and Moore SC. Weight Training and Risk of 10 Common Types of Cancer. Med Sci Sports Exerc. 2019;Epub ahead of print. 10.1249/MSS.0000000000001987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loprinzi P, Smit E, Lee H, Crespo C, Andersen R, and Blair SN. The "fit but fat" paradigm addressed using accelerometer-determined physical activity data. N Am J Med Sci. 2014;6(7):295–301. 10.4103/1947-2714.136901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SG and Chagpar AB. Adherence to physical activity guidelines in breast cancer survivors. Am Surg. 2010;76(9):962–5. [PubMed] [Google Scholar]
- 23.Crawford JJ, Holt NL, Vallance JK, and Courneya KS. A new paradigm for examining the correlates of aerobic, strength, and combined exercise: an application to gynecologic cancer survivors. Support Care Cancer. 2016;24(8):3533–41. 10.1007/s00520-016-3173-7 [DOI] [PubMed] [Google Scholar]
- 24.Ottenbacher A, Yu M, Moser RP, Phillips SM, Alfano C, and Perna FM. Population Estimates of Meeting Strength Training and Aerobic Guidelines, by Gender and Cancer Survivorship Status: Findings From the Health Information National Trends Survey (HINTS). J Phys Act Health. 2015;12(5):675–9. 10.1123/jpah.2014-0003 [DOI] [PubMed] [Google Scholar]
- 25.Vallerand JR, Rhodes RE, Walker GJ, and Courneya KS. Correlates of meeting the combined and independent aerobic and strength exercise guidelines in hematologic cancer survivors. Int J Behav Nutr Phys Act. 2017;14(1):44 10.1186/s12966-017-0498-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trinh L, Strom DA, Wong JN, and Courneya KS. Modality-specific exercise guidelines and quality of life in kidney cancer survivors: A cross-sectional study. Psychooncology. 2018;27(10):2419–26. 10.1002/pon.4844 [DOI] [PubMed] [Google Scholar]
- 27.Forbes C, Blanchard C, Mummery W, and Courneya K. Prevalence and correlates of strength exercise among breast, prostate, and colorectal cancer survivors. Oncol Nurs Forum. 2015;42(2):118–27. 10.1188/15.ONF.42-02AP [DOI] [PubMed] [Google Scholar]
- 28.Short C, James E, Vandelanotte C, Courneya K, Duncan M, Rebar A, et al. Correlates of resistance training in post-treatment breast cancer survivors. Support Care Cancer. 2014;22(10):2757–66. 10.1007/s00520-014-2273-5 [DOI] [PubMed] [Google Scholar]
- 29.Fisher A, Williams K, Beeken R, and Wardle J. Recall of physical activity advice was associated with higher levels of physical activity in colorectal cancer patients. BMJ Open. 2015;5(4):e006853 10.1136/bmjopen-2014-006853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones LW, Courneya KS, Fairey AS, and Mackey JR. Effects of an oncologist's recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: a single-blind, randomized controlled trial. Ann Behav Med. 2004;28(2):105–13. 10.1207/s15324796abm2802_5 [DOI] [PubMed] [Google Scholar]
- 31.Tarasenko Y, Miller E, Chen C, and Schoenberg N. Physical activity levels and counseling by health care providers in cancer survivors. Prev Med. 2017;99:211–17. 10.1016/j.ypmed.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 32.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484–91. [PMC free article] [PubMed] [Google Scholar]
- 33.Nayak P, Holmes HM, Nguyen HT, and Elting LS. Self-reported physical activity among middle-aged cancer survivors in the United States: Behavioral Risk Factor Surveillance System Survey, 2009. Prev Chronic Dis. 2014;11:E156 10.5888/pcd11.140067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, and Straif K. Body fatness and cancer- viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–8. 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakoski SG, Barlow CE, Koelwyn GJ, Hornsby WE, Hernandez J, Defina LF, et al. The influence of adjuvant therapy on cardiorespiratory fitness in early-stage breast cancer seven years after diagnosis: the Cooper Center Longitudinal Study. Breast Cancer Res Treat. 2013;138(3):909–16. 10.1007/s10549-013-2478-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peel AB, Thomas SM, Dittus K, Jones LW, and Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc. 2014;3(1):e000432 10.1161/JAHA.113.000432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Vulpen JK, Peeters PH, Velthuis MJ, van der Wall E, and May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: A meta-analysis. Maturitas. 2016;85:104–11. 10.1016/j.maturitas.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 38.Lahart IM, Metsios GS, Nevill AM, and Carmichael AR. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018;CD011292 10.1002/14651858.CD011292.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.