Abstract
Objective:
To estimate the incidence of failed pregnancy and menstrual irregularities among Liberian women who had survived Ebola virus disease (EVD) and to identify host-specific and disease-specific factors associated with these outcomes.
Methods:
A cross-sectional questionnaire-based study was conducted between August 10, 2016, and February 7, 2017. The study population comprised 111 women aged 18–45 years who had survived EVD and were enrolled in the Longitudinal Liberian Ebola Survivor study based at the Eternal Love Winning Africa Hospital, Monrovia, Liberia. Self-reported data on outcomes related to pregnancy and menstrual changes since recovery from EVD were collected.
Results:
In all, 29 (26.1%) of the participants had become pregnant since surviving EVD. Of the 23 women whose pregnancies continued to term, 10 (43.4%) reported live birth, 11 (47.8%) reported spontaneous abortion, and two (8.7%) reported stillbirth. Of the 105 women who reported having regular menstruation before EVD, 27 (29.0%) reported experiencing irregular menstruation for unknown reasons after EVD. In bivariate logistic models, no associations were found between failed pregnancy or irregular menstruation and any of the factors of interest.
Conclusions:
Adverse pregnancy outcomes and irregular menstruation were frequently reported among EVD survivors in Liberia.
Keywords: Ebola virus disease, Liberia, Menstruation, Post-Ebola sequelae, Pregnancy, Spontaneous abortion, Stillbirth, Survivor
1. INTRODUCTION
The 2014–2016 West African Ebola virus disease (EVD) outbreak led to the infection of more than 28 600 individuals, a figure that was several-fold higher than the total number of all previous known cases of EVD combined [1]. The case fatality rate of EVD is high (50%–70%); nonetheless, affected individuals can survive. Indeed, it is thought that there are more than 10 000 survivors of the 2014–2016 EVD outbreak living in Guinea, Liberia, and Sierra Leone [2].
Cohorts of survivors have reported persistent clinical and psychological manifestations of EVD. Such post-EVD sequelae were first documented among survivors of the 1995 outbreak in the Democratic Republic of Congo [3, 4]. Presentation of post-EVD sequelae vary, both by type and severity, but can include arthralgia, ocular complications, depression, abdominal pain, and insomnia [2, 5, 6, 7]. Although the mechanisms underlying these clinical complications of EVD remain unknown, some studies found an association between the severity of the acute illness, as measured by viral load, with sequelae after recovery [8].
Ebola virus can persist in certain immunologically protected parts of the body, including semen, the interior compartments of the eye, and central nervous system [9–11]. Ebola virus RNA has been detected in the semen of EVD survivors at more than 2 years after recovery [11]. In addition, sexual transmission of the virus from male survivors to their female sexual partners has been identified as the cause of at least three cases of EVD recrudescence in West Africa [12].
Most studies on the impact of Ebola virus on female reproduction have been limited to women who were pregnant at the time of infection. Fetal or early neonatal death is almost certain to occur among women who were pregnant when they developed EVD [13, 14]. Some evidence suggests that Ebola virus could persist in the uteruses of survivors who were pregnant when they became infected [5]. Women who experienced spontaneous abortion after discharge from the Ebola treatment unit (ETU) had negative blood test results for Ebola virus; however, products of conception (i.e. the fetus, placenta, and amniotic sac) have tested positive for Ebola virus RNA [5]. For women who were not pregnant at the time of infection, there is evidence that Ebola virus RNA might remain in the vaginal fluid for up to 1 month after the onset of symptoms [9].
Guidance on the clinical care of EVD survivors released by WHO in April 2016 [2] provided anecdotal evidence of stillbirths among women who had conceived after EVD, and warned that such pregnancies should be treated as high-risk for fetal complications. The same guidelines indicated that menorrhagia, metrorrhagia, and amenorrhea had been reported by some EVD survivors. However, little research has examined the ongoing reproductive health of female EVD survivors after recovery from acute infection [6, 15, 16].
The aims of present study were to estimate the frequency of adverse reproductive health outcomes (failed pregnancy and irregular menstruation) and to identify factors associated with these outcomes among Liberian EVD survivors.
2. MATERIALS AND METHODS
A cross-sectional questionnaire-based study was conducted between August 10, 2016, and February 7, 2017. The population comprised women who had survived EVD and were enrolled in the Longitudinal Liberian Ebola Survivor study, which was based at the Eternal Love Winning Africa (ELWA) Hospital, Monrovia, Liberia. The research protocol, data collection tools, and consent forms were approved by the institutional review boards of the University of North Carolina (Chapel Hill, USA) and the University of Liberia Pacific Institute for Research and Evaluation (Monrovia, Liberia). All participants provided written informed consent before enrolling in the Longitudinal Liberian Ebola Survivor study.
Two ETUs were based at ELWA Hospital during the 2014–2016 EVD outbreak. This hospital has since established a clinic for EVD survivors in the Monrovia area, where they can undergo evaluation and receive treatment for issues related to post-EVD sequelae.
All study participants were recruited from the ELWA Hospital Ebola Survivor Clinic and local communities through word of mouth, as survivors often told other survivors about the Longitudinal Liberian Ebola Survivor study and encouraged them to enroll. Inclusion criteria for the present study were history of EVD (evidenced by a discharge certificate from an ETU); female sex; and age 18–45 years. All women who met these criteria also had to be willing and able to consent to participation. There were no specific exclusion criteria for the current analysis.
Although participants in the Longitudinal Liberian Ebola Survivor study attended ELWA Hospital every 3 months, the data used for the present study were cross-sectional. These data were collected from an interviewer-administered questionnaire (Box S1). All interviews were conducted in Liberian English in a private room at ELWA Hospital.
In the present study, the outcomes ‘spontaneous abortion’ and ‘stillbirth’ were self-defined by the participants, rather than using the WHO definition of fetal death occurring before or after 28 weeks of gestation[17]. The term ‘failed pregnancy’ was used to include both stillbirths and spontaneous abortions.
Participants were categorized as experiencing ‘regular menstruation post-EVD’ if they reported having regular menstruation (cycles lasting approximately 30 days) both before and after infection with Ebola virus. Women were categorized as experiencing ‘irregular menstruation post-EVD for unknown reason’ if they reported having regular menstruation before infection but irregular or no menstruation at any point between ETU discharge and data collection, and did not identify a reason for this change. Women who reported irregular menstruation for unknown reason were further categorized based on their qualitative description of menstrual change. The definitions used for menstrual irregularities are outlined in Table 1.
Table 1.
Terms used to describe menstrual irregularities [27].
| Term | Definition |
|---|---|
| Irregular menstruation, known reason | Absent or irregular menstruation owing to use of hormonal contraception, pregnancy, breastfeeding, medical procedure, etc. |
| Irregular menstruation, unknown reason | Absent or irregular menstruation without a plausible explanation or reason. |
| Amenorrhea | Absence of menstruation for ≥3 mo. |
| Oligomenorrhea | Infrequent menstruation, reduced duration of flow, light or scanty flow. |
| Dysmenorrhea | Experience of abdominal pain, cramping, or backache during menstruation. |
| Menorrhagia | Frequent menstruation, increased duration of flow, and/or heavy flow. |
All data were entered into Access version 16.0 (Microsoft, Redmond, WA, USA) and then imported into SAS version 9.4 (SAS Institute, Cary, NC, USA) for the analysis. The percentage of women reporting the outcomes of interest (failed pregnancy and irregular menstruation for unknown reason) was calculated. Bivariate and multivariable logistic regression models were used to identify potential factors associated with failed pregnancy and irregular menstruation. Odds ratios and 95% confidence intervals were estimated to compare variables by pregnancy and menstruation outcomes. Adjusted ORs that controlled for the variables of maternal age or duration of illness owing to EVD were also used. Variables were considered for inclusion in the current analysis based on the existing literature regarding factors related to clinical sequelae among EVD survivors.[8, 15, 18–20] They included duration of illness owing to EVD; the number of post-EVD sequelae reported (e.g. vision issues, auditory issues, joint pain, etc.); maternal age at the time of enrolling in the Longitudinal Liberian Ebola Survivor study; and time between ETU discharge and conception.
3. RESULTS
A total of 111 women were included in the present study. Their characteristics are outlined in Table 2. The mean age at the time of EVD infection was 29.4 years. On average, interviews were conducted at 709 days after ETU discharge; the mean age at interview was 31.6 years.
Table 2.
Characteristics of the participants (n=111). a
| Characteristic | Distribution |
|---|---|
| Age at time of EVD, y | 29.4 ± 7.2 |
| Age at time of the present study, y | 31.6 ± 7.3 |
| Duration of EVD, d | 28.2 ± 13.9 |
| Time between ETU release and enrollment in the present study, d | 709 ± 45 |
| Education level | |
| None | 18 (16.2) |
| Primary | 26 (23.4) |
| Secondary | 56 (50.5) |
| Post-secondary | 11 (9.9) |
| Relationship status | |
| Not in a relationship | 11 (9.9) |
| In a relationship, not married | 78 (70.3) |
| In a relationship, married | 22 (19.8) |
| Habitation status | |
| Living alone | 24 (21.6) |
| Living with partner or family | 87 (78.4) |
Abbreviations: ETU, Ebola treatment unit; EVD, Ebola virus disease.
Values are given as mean ± SD or number (percentage).
A total of 29 (26.1%) participants reported becoming pregnant at least once after EVD (Figure 1 and Table 3). Four (13.8%) of these women had become pregnant twice since ETU discharge; however, the current analysis was limited to the outcomes of the first pregnancy following EVD for each woman. As shown in Figure 1, of the 23 pregnancies continued to terminus for which outcome data were available, 13 (56.5%) resulted in either spontaneous abortion (n=11, 47.8%) or stillbirth (n=2, 8.7%). The mean reported gestational age at the time of spontaneous abortion was 11 weeks. Both stillbirths had a reported gestational age of 40 weeks, suggesting that fetal death had occurred at the time of labor and delivery.
Figure 1.
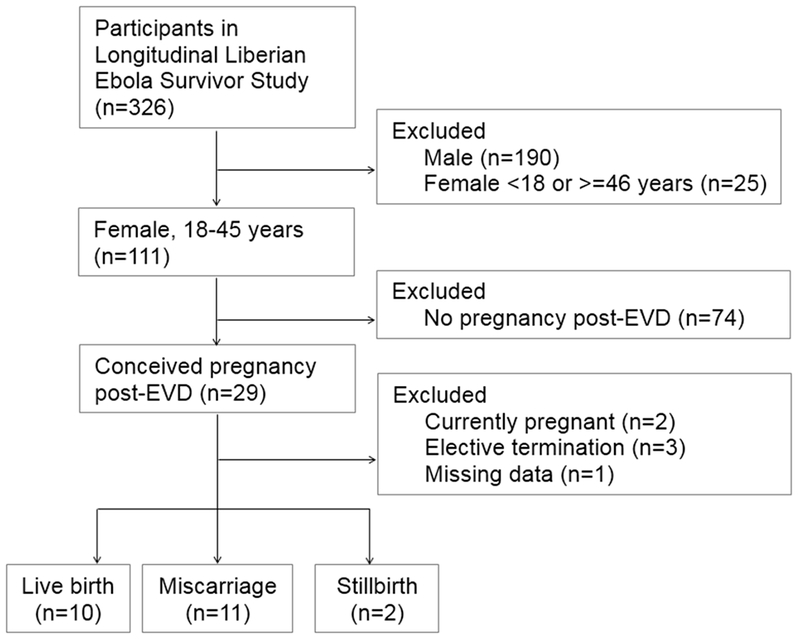
Study population for analyzing pregnancy outcomes. Abbreviation: EVD, Ebola virus disease.
Table 3.
Reproductive health outcomes among the participants (n=111) after Ebola virus disease. a
| Outcome | Distribution |
|---|---|
| Pregnant | 29 (26.1) |
| Pregnancy outcomes | |
| Live birth | 10 (34.5) |
| Stillbirth | 2 (6.9) |
| Spontaneous abortion | 11 (37.9) |
| Elective induced abortion | 3 (10.3) |
| Currently pregnant | 2 (6.9) |
| Unknown | 1 (3.5) |
| Regular menstruation before EVD | 105 (94.6) |
| Menstruation status after EVD | |
| Regular menstruation | 66 (62.9) |
| Irregular menstruation, b known reason c | 12 (11.4) |
| Irregular menstruation, b unknown reason | 27 (25.7) |
Abbreviation: EVD, Ebola virus disease.
Values are given as number (percentage).
Irregular menstruation included no menstruation; light or infrequent menstruation; heavy menstruation; frequent menstruation; and/or abdomen or back pain during menstruation.
Known reasons for irregular menstruation included pregnancy; breastfeeding; have reached menopause at the age of 45 years or older; or using hormonal contraceptives.
Of the 93 women who reported having regular menstruation before EVD, 27 (29.0%) reported that their menstruation became irregular after EVD due to unknown reasons (Figure 2 and Table 3). A total of 22 (81.5%) menstrual irregularities were characterized as amenorrhea or oligomenorrhea (Figure 3). Dysmenorrhea and/or menorrhagia were reported by four (14.8%) women.
Figure 2.
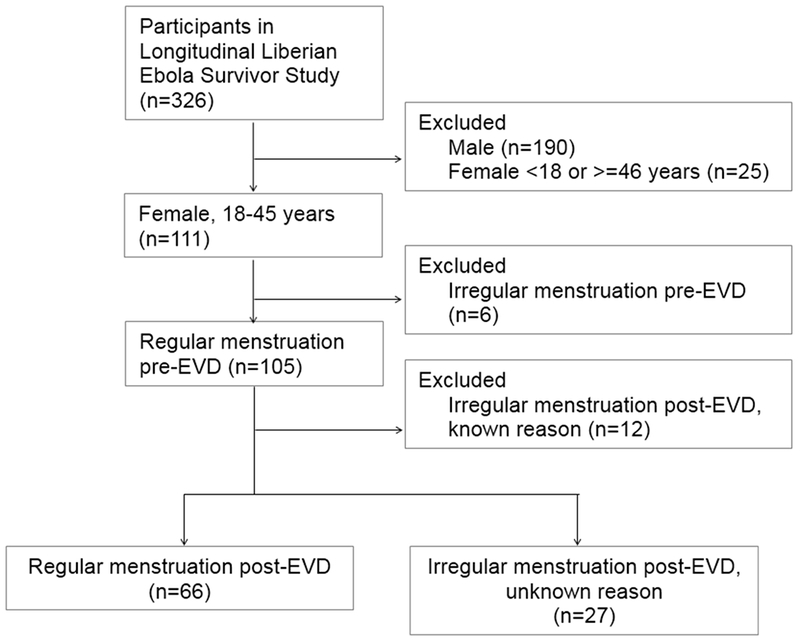
Study population for analyzing menstrual outcomes. Abbreviation: EVD, Ebola virus disease.
Figure 3.
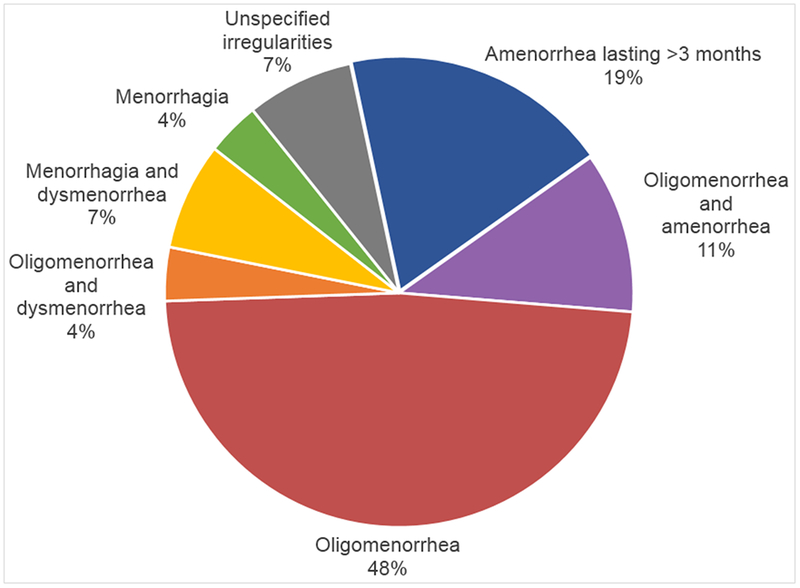
Menstrual irregularities among women who reported having regular menstruation before developing Ebola virus disease (n=27).
Table 4 shows the association of demographic and EVD-related factors with pregnancy outcomes. In bivariate logistic models, no associations were found between failed pregnancy and any of the factors of interest. In addition, no associations were found between pregnancy outcome and potential factors after adjusting for age of the mother or duration of EVD.
Table 4.
| Characteristic | Pregnancy with live birth (n=10) | Failed pregnancy c (n=13) | Model 1, OR (95% CI) | Model 2, OR (95% CI) | Model 3, OR (95% CI) |
|---|---|---|---|---|---|
| Maternal age at time of EVD, y | 27.3 ± 6.9 | 28.5 ± 7.9 | 1.02 (0.91–1.15) | NA | 1.02 (0.91–1.15) |
| Duration of EVD, d | 27.5 ± 16.2 | 31.4 ± 12.6 | 1.02 (0.96–1.09) | 1.02 (0.96–1.09) | NA |
| Time between ETU discharge and conception, d | 220 ± 130 | 287 ± 177 | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) |
| No. of reported post-EVD sequelae | 6.0 ± 3.4 | 4.7 ± 2.2 | 0.83 (0.60–1.16) | 0.84 (0.60–1.17) | 0.82 (0.58–1.15) |
Abbreviations: CI, confidence interval; EVD, Ebola virus disease; ETU, Ebola treatment unit; NA, not applicable; OR, odds ratio.
Values are given as mean ± SD, unless indicated otherwise.
Model 1 was unadjusted; model 2 was adjusted by maternal age; and model 3 was adjusted by the duration of EVD.
Included spontaneous abortion and stillbirth.
Table 5 shows the association of demographic and EVD-related factors with menstruation outcomes. No bivariate associations were found between menstrual irregularities due to unknown reasons and any of the factors of interest. These associations remained null even after adjusting for maternal age.
Table 5.
Association of demographic and Ebola-related factors with menstrual changes among women who reported having regular menstruation before Ebola virus disease. a, b
| Characteristic | Regular menstruation (n=66) | Irregular menstruation, c unknown reason (n=27) | Model 1, OR (95% CI) | Model 2, OR (95% CI) |
|---|---|---|---|---|
| Maternal age at time of EVD, y | 28.8 ± 7.4 | 31.2 ± 7.2 | 1.05 (0.98–1.12) | NA |
| Duration of EVD, d | 27.8 ± 11.6 | 29.0 ± 11.2 | 1.01 (0.97–1.05) | 1.02 (0.98–1.06) |
| No. of reported post-EVD sequelae | 4.9 ± 2.5 | 5.0 ± 2.7 | 1.02 (0.86–1.22) | 1.02 (0.83–1.25) |
Abbreviations: CI, confidence interval; EVD, Ebola virus disease; ETU, Ebola treatment unit; NA, not applicable; OR, odds ratio.
Values are given as mean ± SD, unless indicated otherwise.
Model 1 was unadjusted and model 2 was adjusted by maternal age.
Irregular menstruation included no menstruation; light or infrequent menstruation; heavy menstruation; frequent menstruation; and/or abdomen or back pain during menstruation.
4. DISCUSSION
The present study found high rates of pregnancy failure and irregular menstruation among Liberian survivors of EVD. These findings highlighted the substantial long-term impact of EVD among female survivors after acute infection. As such, they represent an important contribution to the previous literature regarding reproductive health outcomes in this population.
The observed rate of failure of first pregnancy after EVD owing to spontaneous abortion (47.8%) was higher than the reported rate of 15%–20% for spontaneous abortion in general populations [27]; however, no substantiated data are available for general populations in West Africa. In addition, the current finding that 8.7% of first pregnancies after EVD resulted in stillbirth is also greater than the 36 stillbirths per 1000 live births (3.6%) estimated for pregnancies in Sub-Saharan Africa [28]. A small qualitative study that conducted in-depth interviews among 13 female survivors, who had conceived after EVD and continued to terminus found no cases of spontaneous abortion but three (23%) cases of stillbirth, suggesting that pregnancy failure was increased in this cohort [16]. The present findings were also consistent with another study of female survivors (n=68) in Liberia, which found increased rates of post-EVD pregnancy failure (22.1% for spontaneous abortion and 5.8% for stillbirth) [15]. However, the present study included pregnancies that were conceived up to 4 months later in the post-EVD recovery period. Importantly, the results of the present study potentially indicated a greater frequency of failed pregnancies (>50%) among female EVD survivors than was previously suggested.
The present study found that more than one-quarter of women who reported regular menstruation before EVD had experienced irregular menstruation after EVD. Oligomenorrhea was the most frequently reported type of menstrual irregularity, followed by amenorrhea. The frequency of irregular menstruation found in the present study was higher than the 5%–17% rate estimated for women in low-income settings [26], and the 16% rate estimated for women in rural Gambia [27]. A previous Liberian study found that approximately half of all EVD survivors assessed had experienced menstrual changes after infection [16]; however, that study did not estimate the frequency of amenorrhea, dysmenorrhea, and oligomenorrhea separately. In another study of post-EVD menstrual sequelae, 10% of survivors seen at 1–3 months after discharge from an ETU reported experiencing amenorrhea [6]; however, this short follow-up period would not have captured cases of menstrual irregularity that developed after that time. By contrast, the present study had a mean follow-up of approximately 2 years. Thus, the current findings indicated an excess of menstrual irregularity among EVD survivors after acute infection when compared with the general population in West Africa. Furthermore, the present study extended the previous literature by providing specificity about the types of menstrual irregularity that might develop at some time after EVD.
The underlying mechanisms by which Ebola virus infection affects pregnancy outcomes and menstruation after EVD remain unclear. Sustained immune activation and/or dysregulation might to contribute to the high incidence of failed pregnancies recorded after EVD [5]. The strong association between immune dysregulation and spontaneous abortion in the general population lends credence to this hypothesis [28]. Inflammation of the placenta has been associated with stillbirth [26]. Furthermore, ongoing inflammation after infection could provide an etiology for some post-EVD clinical manifestations such as arthritis and uveitis [5]. Irregular menstruation among EVD survivors in the present study could be attributable to multiple underlying factors, including weight loss and stress [27]. Given that survivors would have lost large amounts of weight during the period of acute EVD [3], and are likely to have experienced high levels of stress after Ebola, it is plausible that these factors (in isolation or in combination) could explain the menstrual changes observed in the present study. Persistent inflammation following EVD might also contribute to irregular menstruation, as menstruation is driven by interactions between ovarian hormones and the immune system [28].
The current analysis had some limitations. The study population comprised only EVD survivors; therefore, the lack of a control group (i.e. reproductive-aged women who had not experienced EVD) limited the findings to descriptive statistics. The present study was susceptible to selection bias in that individuals who access services at the ELWA Hospital Ebola Survivor Clinic might experience more severe post-EVD sequelae than EVD survivors who do not access services at that hospital. Conversely, the sickest EVD survivors might not have been able to travel to ELWA Hospital and so were excluded from the present study. Also, the use of self-reported and self-defined data for the failed pregnancy outcomes could have resulted in some early stillbirths being reported as spontaneous abortions, as the women might not have known how to differentiate between these two outcomes. One of the greatest limitations was the lack of data on pre-EVD pregnancy history. It was unclear how many of the participants had experienced a failed pregnancy before EVD, thereby placing them at increased risk of failed pregnancy after EVD [27]. Finally, the sample size (n=111) resulted in small numbers of women reporting on the various outcomes of interest. Consequently, the analysis of these outcomes for potential associations with other factors, while descriptive, suffered from low statistical power.
In the present study, a substantial proportion of female EVD survivors in Liberia experienced adverse reproductive health outcomes. Additional research is needed to identify the mechanisms by which EVD causes these clinical sequelae among survivors and the unique ways in which EVD affects women. Future studies on post-EVD sequelae should include specific questions regarding menstruation and pregnancy, as well as the collection of biomarkers of reproductive health. In addition, programs offering psychosocial or clinical support to female EVD survivors should include counseling on potential reproductive health complications.
Supplementary Material
Synopsis:
Adverse pregnancy outcomes and irregular menstruation were frequently reported among female survivors of Ebola virus disease in Liberia.
Acknowledgments
This work was supported by the Bill & Melinda Gates Foundation; the National Institutes of Health R01 AI123535 (DAW and WAF) and K24DA037101 (DAW); and the University of North Carolina at Chapel Hill.
Footnotes
Conflicts of interest
EED has received consulting fees from Epi Excellence and Bohn Epidemiology. The other authors declare no conflicts of interest
Supporting information
Box S1 The reproductive health questionnaire.
References
- [1].World Health Organization; Ebola situation report. Geneva: WHO; 2016. https://apps.who.int/iris/bitstream/handle/10665/208883/ebolasitrep_10Jun2016_eng.pdf;jsessionid=A6CAD8F42BD8C17AC7D678D1D803CF38?sequence=1. Accessed April 15, 2019. [Google Scholar]
- [2].World Health Organization; Clinical care for survivors of Ebola virus disease. Geneva, Switzerland: World Health Organization; 2016. https://www.who.int/csr/resources/publications/ebola/guidance-survivors/en. Accessed April 15, 2019. [Google Scholar]
- [3].Bwaka MA, Bonnet M-J, Calain P, Colebunders R, De Roo A, Guimard Y, et al. : Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis 1999;179(Supplement 1): S1–S7. [DOI] [PubMed] [Google Scholar]
- [4].Rowe AK, Bertolli J, Khan AS, Mukunu R, Muyembe-Tamfum J, Bressler D, et al. : Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. J Infect Dis 1999;179(Supplement 1): S28–S35. [DOI] [PubMed] [Google Scholar]
- [5].Vetter P, Kaiser L, Schibler M, Ciglenecki I, Bausch DG: Sequelae of Ebola virus disease: the emergency within the emergency. Lancet Infect Dis 2016;16(6): e82–e91. [DOI] [PubMed] [Google Scholar]
- [6].Tiffany A, Vetter P, Mattia J, Dayer J-A, Bartsch M, Kasztura M, et al. : Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin Infect Dis 2016;62(11): 1360–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clark DV, Kibuuka H, Millard M, Wakabi S, Lukwago L, Taylor A, et al. : Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis 2015;15(8): 905–912. [DOI] [PubMed] [Google Scholar]
- [8].Mattia JG, Vandy MJ, Chang JC, Platt DE, Dierberg K, Bausch DG, et al. : Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis 2016;16(3): 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bausch DG, Towner JS, Dowell SF, Kaducu F, Lukwiya M, Sanchez A, et al. : Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007;196(Supplement 2): S142–S147. [DOI] [PubMed] [Google Scholar]
- [10].Howlett P, Brown C, Helderman T, Brooks T, Lisk D, Deen G, et al. : Ebola virus disease complicated by late-onset encephalitis and polyarthritis, Sierra Leone. Emerg Infect Dis 2016;22(1): 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fischer WA, Brown J, Wohl DA, Loftis AJ, Tozay S, Reeves E, et al. Ebola virus ribonucleic acid detection in semen more than two years after resolution of acute Ebola virus infection. Open Forum Infect Dis 2017;4:ofx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee H, Nishiura H: Recrudescence of Ebola virus disease outbreak in West Africa, 2014–2016. Int J Infect Dis 2017;64: 90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mupapa K, Mukundu W, Bwaka MA, Kipasa M, De Roo A, Kuvula K, et al. : Ebola hemorrhagic fever and pregnancy. J Infect Dis 1999;179(Supplement 1): S11–S12. [DOI] [PubMed] [Google Scholar]
- [14].Nelson J, Griese S, Goodman A, Peacock G: Live neonates born to mothers with Ebola virus disease: a review of the literature. J Perinatol 2016;36:411. [DOI] [PubMed] [Google Scholar]
- [15].Fallah MP, Skrip LA, Dahn BT, Nyenswah TG, Flumo H, Glayweon M, et al. : Pregnancy outcomes in Liberian women who conceived after recovery from Ebola virus disease. Lancet Glob Health 2016;4(10): e678–e679. [DOI] [PubMed] [Google Scholar]
- [16].Godwin CL, Buller A., Bentley M, Singh K Understanding the personal relationships and reproductive health changes of female survivors of Ebola infection in Liberia In: Schwartz JAA DA, Abramowitz S, eds. Pregnant in the Time of Ebola Women and Their Children in the 2013–2015 West African Epidemic. New York: Springer, 2019:103–120. [Google Scholar]
- [17].Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. : Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 2016;387(10018): 587–603. [DOI] [PubMed] [Google Scholar]
- [18].Etard JF, Sow MS, Leroy S, et al. : Multidisciplinary assessment of post-Ebola sequelae in Guinea (Postebogui): an observational cohort study. Lancet Infect Dis. 2017;17(5): 545–552. [DOI] [PubMed] [Google Scholar]
- [19].Hansen JP. Older maternal age and pregnancy outcome: a review of the literature. Obstet Gynecol Surv. 1986:41(11): 726–742. [DOI] [PubMed] [Google Scholar]
- [20].Li J, Duan HJ, Chen HY, et al. : Age and Ebola viral load correlate with mortality and survival time in 288 Ebola virus disease patients. Int J Infect Dis. 2016:42: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brown S Miscarriage and its associations. Semin Reprod Med. 2008;26:391–400. [DOI] [PubMed] [Google Scholar]
- [22].Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. : National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 2016;4(2): e98–e108. [DOI] [PubMed] [Google Scholar]
- [23].Harlow SD, Schuman P, Cohen M, Ohmit SE, Cu-Uvin S, Lin X, et al. : Effect of HIV infection on menstrual cycle length. J Acquir Immune Defic Syndr 2000;24(1): 68–75. [DOI] [PubMed] [Google Scholar]
- [24].Walraven G, Ekpo G, Coleman R, Scherf C, Morison L, Harlow SD: Menstrual disorders in rural Gambia. Stud Fam Plann 2002;33(3): 261–268. [DOI] [PubMed] [Google Scholar]
- [25].Christiansen OB, Nielsen HS, Kolte AM. Inflammation and miscarriage. Semin Fetal Neonatal Med. 2006;11(5):302–8. [DOI] [PubMed] [Google Scholar]
- [26].Kim CJ, Romero R, Chaemsaithong P, Kim J-S: Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 2015;213(4): S53–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Harlow SD, Ephross SA: Epidemiology of menstruation and its relevance to womens health. Epidemiol Rev 1995;17(2): 265–286. [DOI] [PubMed] [Google Scholar]
- [28].Critchley HO, Kelly RW, Brenner RM, Baird DT: The endocrinology of menstruation–a role for the immune system. Clin Endocrinol 2001;55(6): 701–710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


