Abstract
Background:
Little is known about event-level patterns of marijuana co- or tri-use with alcohol and tobacco. Thus, the study goal was to examine patterns of same-day alcohol, cigarette, and marijuana co- and tri-use at the individual level in non-treatment-seeking alcohol users.
Methods:
Participants (N = 551) completed an in-person interview for alcohol, cigarette, and marijuana use over the previous 30 days, and the event-level substance use patterns of n = 179 participants who reported using each of these substances at least once per month were analyzed.
Results:
The use of alcohol, marijuana, or cigarettes independently increased the probability of subsequent, simultaneous co-use of one of the two remaining substances. The co-use of alcohol with cigarettes and marijuana with cigarettes produced generally additive effects on the odds of same day tri-use of marijuana and alcohol, respectively. Conversely, the co-use of alcohol and marijuana produced sub-additive effects on likelihood of cigarette use. Sex moderated several of the observed patterns of co- and tri-use: the relationship between alcohol or cigarette use predicting marijuana co-use was stronger in men, whereas the observed additive relationships between drug co-use leading to tri-use was stronger in women.
Conclusions:
The presented results may aid in the understanding of how simultaneous co-use of marijuana with alcohol and/or tobacco relates to the etiology, maintenance, and treatment of comorbid and trimorbid substance use disorder. Replication and extension of the results in treatment seeking populations using more fine-grained analysis approaches, e.g. ecological momentary assessment, is needed.
Keywords: Alcohol, Tobacco, Marijuana, Comorbidity, Co-use, Polydrug abuse, Sex differences
1. Introduction
Alcohol, tobacco, and marijuana are the three most commonly used drugs of abuse in the US (Substance Abuse and Mental Health Services Administration, 2017), and cross-sectional, epidemiological findings suggest that it is common for individuals to report concurrently using these substances (Kessler et al., 1997; Prince van Leeuwen et al., 2014). The prevalence of, problems arising from, and motives underlying the co-use of alcohol and tobacco have been well documented (for review, see McKee and Weinberger, 2013; Roche et al., 2016a). Approximately 20% of regular tobacco smokers are also heavy-drinkers (Dawson, 2000), and those who use both substances tend to regularly do so simultaneously (i.e., at the same time or within close temporal proximity; (Piasecki et al., 2008; Shiffman et al., 2012). The chronic, simultaneous use of cigarettes and alcohol yields adverse consequences. First, heavy-drinking tobacco smokers experience more frequent and severe negative health consequences as compared to those who use either drug alone (Durazzo et al., 2007; Ebbert et al., 2005). Second, this simultaneous co-use creates substantial impediments to smoking cessation among this sub group. Alcohol use is associated with substantially poorer smoking cessation rates and, at a more fine-grained level of analysis, a smoking lapse is four times more likely to occur in the context of a drinking episode as compared to a non-drinking episode (Kahler et al., 2010; Shiffman et al., 1996). The understanding of the daily, event-level patterns of simultaneous cigarette and alcohol co-use, for example how use of one drug can acutely increase craving for and drive use of the other (Perkins, 1997; Shiffman et al., 1996), contributed to line of research focused on developing pharmacological and behavioral treatments that are specifically tailored for individuals who are dependent on both substances (Falk et al., 2015; Fucito et al., 2011; McKee et al., 2009; McKee and Weinberger, 2013; Mitchell et al., 2012). Thus, characterizing patterns of drug co-use at the individual rather than population level may be beneficial in identifying the behavioral mechanisms that drive problematic, simultaneous substance use in order to leverage that knowledge into targeted treatments for co-abusing populations. While marijuana is the most commonly used illicit drug in the world and is becoming increasingly legal in the USA, relatively little is known about event-level patterns of marijuana co-use with alcohol and/or tobacco.
Marijuana is the most used illicit drug in the world and third most commonly used drug of abuse in the nation. In the US, past year marijuana use more than doubled between 2001–2002 and 2012–2013 (~4% to > 9%) with a near parallel magnitude of increase in the prevalence of cannabis use disorder (~1.5% – 3%; CUD; (Hasin et al., 2015a). While there is still some debate on this topic (Kerr et al., 2017; Pacula and Smart, 2017), the national rise in the prevalence of marijuana use, particularly in adults, appears to be related to the increasing number of states that fully legalized or legalized medicinal use over this same time (Cerdá et al., 2012; Hasin et al., 2015b; Legislatures, 2016; Mauro et al., 2017; Wen et al., 2014). As more states legalize or decriminalize marijuana use and its use becomes more tacitly accepted across the country, it is expected that prevalence of marijuana use and CUD will continue to rise (Compton et al., 2016; Pacula and Smart, 2017; Pew Research Center, 2014). Although marijuana is considered less harmful to self and others compared with alcohol and tobacco (Nutt et al., 2010), acute and chronic marijuana use is indeed associated with a wide variety of health risks (Hall, 2016; Meier et al., 2012), and treatment outcomes for CUD are generally poor across various intervention types (Budney et al., 2006; Copeland et al., 2001; Kadden et al., 2007; Levin et al., 2011; Marijuana Treatment Project Research Group, 2004; Stephens et al., 2000). These adverse consequences from marijuana use and poor treatment outcomes are thought to be exacerbated by the commonality of marijuana being used concurrently with other substances (Cohn et al., 2015, 2016; Conway et al., 2013; Olthuis et al., 2013; Tzilos et al., 2014). Given the rising prevalence of marijuana use, CUD, and their related health and treatment problems, it is critical to characterize situations and patterns in which marijuana is concurrently(i.e., individuals report use of both but not necessarily simultaneous use) and simultaneously used with other drugs of abuse.
At the population level, concurrent alcohol and marijuana use is quite common, with over 75% of marijuana users reporting alcohol use (Agrawal et al., 2007; Butterworth et al., 2014; Haas et al., 2015; Hyggen and Hammer, 2014; Midanik et al., 2007). Large scale, long-itudinal survey data suggest that most who report concurrently using alcohol and marijuana also use both drugs simultaneously, and simultaneous use is associated with heightened heavy-drinking behavior, drunk driving, adverse social consequences, and harm to self and others (Midanik et al., 2007; Subbaraman and Kerr, 2015; Terry-McElrath et al., 2014). As alcohol consumption across youth to adulthood is substantially higher in marijuana users than non-users (Hyggen and Hammer, 2014), it is not surprising that marijuana use and CUD are each associated with the development and maintenance of AUD (Agrawal et al., 2007; Weinberger et al., 2016). Simultaneous marijuana and alcohol use is increasing in younger populations, and in states that have recently legalized marijuana use, there have been early indications of increases in impaired driving stemming from simultaneous co-use (Terry-McElrath and Patrick, 2018; Rocky Mountain High Intensity Drug Trafficking Area, 2015; Washington Traffic Safety Commission, 2016). Lastly, concurrent alcohol and marijuana use has consequences for treatment as well: using marijuana during alcohol treatment is associated with poorer alcohol treatment outcomes (Mojarrad et al., 2014; Subbaraman et al., 2017), and when attempting to reduce their marijuana use, drinkers with and without AUD have reported increased alcohol craving and consumption (Allsop et al., 2014; Copersino et al., 2006; Peters and Hughes, 2010; Stephens et al., 1994). As observed with the co-use of alcohol and tobacco, alcohol and marijuana appear to regularly be co-administered in a pattern that escalates severity of use of each drug and creates impediments in reduction of drug use.
Similar to findings with alcohol, epidemiological studies suggest concurrent marijuana and cigarette use is highly prevalent and problematic. Recent findings indicate that more than two-thirds of current marijuana users concurrently use tobacco (Caulkins et al., 2015; Richter et al., 2008; Schauer et al., 2017, 2015), and up to 53% of current tobacco users also use marijuana (Leatherdale et al., 2007, 2006; Ramo et al., 2012; Substance Abuse and Mental Health Services Administration, 2017). The co-use of these substances is increasing, particularly in individuals who were initially tobacco only users (Schauer et al., 2015) and/or live in states where marijuana use is legal (Wang and Cataldo, 2016). Relatedly, there is bi-directional evidence that tobacco or marijuana use precedes and increases the likelihood of future use of the other substance (Humfleet and Haas., 2004; Patton et al., 2005; Tarter et al., 2006; Timberlake et al., 2007; Agrawal et al., 2008; Panlilio et al., 2013; Kandel and Kandel, 2015). Concurrent marijuana and tobacco use, vs. use of either substance alone, is associated with increased risk of CUD, more psychosocial and mental health problems, more severe nicotine dependence, heavier alcohol consumption, and poorer treatment outcomes for both substances (Agrawal et al., 2012; de Dios et al., 2009; Haney et al., 2013; Moore and Budney, 2001; Peters et al., 2012; Ramo et al., 2012; Schauer and Peters, 2018; Wang et al., 2016). As with alcohol co-use, simultaneous use of marijuana and tobacco is common among youths and adults and associated with more severe drug use and worse health outcomes than concurrent use (Akre et al., 2009; Amos et al., 2004; Barrett et al., 2006; Golub, 2012; Kelly, 2012; Soldz et al., 2003). For example, individuals who simultaneously use marijuana and tobacco are at heightened risk for escalating consumption to hazardous levels, development of dependence, and poor cessation outcomes for each substance (Agrawal and Lynskey, 2009; Baggio et al., 2014; Fairman, 2015; Ford et al., 2002; Ream et al., 2008).
In summary, epidemiological studies indicate that marijuana and tobacco or alcohol are commonly co-used in a concurrent and simultaneous fashion, and the co-use of these substances, particularly when used simultaneously, is related to greater quantity and frequency of use, development of dependence, and health problems above and beyond the use of each substance alone. While these studies provide compelling data about the scope of problems stemming from the co-use of these substances, a fundamental limitation of such population-level, cross-sectional studies is that they are unable to answer questions about event-level patterns of use. That is, the cross-sectional nature of these studies cannot provide information about the pattern and predictive relationship of simultaneous co-use within a given day or drug-use event, which may be especially critical to understanding the co-use of marijuana with other substances. For example, individuals report both using marijuana as a substitute for tobacco or alcohol (i.e., either using marijuana completely in place of either drug or reducing tobacco/alcohol consumption due to the use of marijuana) and in a sequential/simultaneous manner to produce additive or subtractive subjective effects (Berg et al., 2018; Reiman, 2009; Schauer et al., 2016). It is difficult to differentiate such patterns of use unless examining event-level data. The few, recent studies that have used a fine-grained approach to study simultaneous use, while important, have limitations that may affect generalizability. A study examining event-level alcohol and marijuana co-use in adolescents did not report patterns of co-use, only the context of and consequences from simultaneous co-use (Lipperman-Kreda et al., 2017). Another study that examined daily patterns of marijuana and alcohol co-use, but not cigarette use, in a predominantly male (94%), veteran population found that moderate and heavy-drinking were more likely to occur on days which marijuana was used. Further, while individuals with AUD or comorbid AUD + CUD were more likely to drink heavily on such days, individuals with CUD were less likely to drink heavily, which the authors interpreted as supporting marijuana substitution (Metrik et al., 2018). Lastly, Gunn et al., (2018) found that daily marijuana use was associated with greater alcohol consumption in college students, and this predictive relationship strengthened over a two-year period. However, they did not report on tobacco use nor the influence of sex on the relationship between daily marijuana and alcohol use.
In light of the high rates of marijuana co-use with alcohol and tobacco in epidemiological studies but relatively absent data on event-level patterns of use, the goal of the present study was to examine daily patterns of alcohol, tobacco, and marijuana co- and tri-use (i.e., using all three substances at once) in non-treatment seeking drinkers who report regularly using tobacco and marijuana. To our knowledge, no studies have examined the daily co-use or tri-use of all three substances at the individual, event-level in the same sample. Because of the strong evidence for the co-use of all three substances, we hypothesized that use of one substance would indiscriminately increase the odds of same-day use of a second substance, and the use of two substances would subsequently increase the odds of using the third. Finally, as an exploratory aim, we examined whether sex moderated any observed daily patterns of co- or tri-use. While men tend to use marijuana, alcohol, and cigarettes earlier, heavier, more frequently, and have greater dependence rates than women (Carliner et al., 2017; Higgins et al., 2015; Johnston et al., 2018; White et al., 2015), women may have more severe consequences from substance abuse and enter treatment earlier than men (Diehl et al., 2007; Hernandez-Avila et al., 2004; Mann et al., 2005; Randall et al., 1999). Given the general dearth of event-level substance use studies, sex differences in patterns of simultaneous co-use have obviously not been well characterized. However, at the population level, men have higher rates of marijuana co-use with each alcohol and tobacco and display a more rapid escalation in the frequency of this co-administration than women (Crane et al., 2015; Guxens et al., 2007; Penetar et al., 2005; Schauer et al., 2015; Victoir et al., 2006). The characterization of sex differences in patterns of event-level co-use also may have important implications for understating the etiology and treatment of addiction.
2. Materials and methods
2.1. Participants and parent studies
All study procedures were approved by the University of California, Los Angeles Institutional Review Board and conducted in accordance with the Declaration of Helsinki. The reported sample draws from baseline data collected as a part of four human laboratory studies. Three studies examined pharmacotherapies for alcohol use: naltrexone in an Asian American sample (n = 199; (Ray et al., 2018), ibudilast (n = 138; (Ray et al., 2017), and ivermectin (n = 74; (Roche et al., 2016b). The fourth study was an alcohol self-administration study (n = 140; (Bujarski et al., 2018), resulting in a total sample of 551 participants. Each study recruited a sample of non-treatment seeking, regular drinkers from the Los Angeles area using identical recruitment methods of print and online advertisements.
Interested participants completed an initial telephone screening to determine eligibility. During the telephone screening, all participants were asked to report their drinking over the past three months prior to enrollment. The drinking requirement for each study had the following inclusion criteria: naltrexone in Asian Americans – female requirement of > 4 drinks per week and male requirement of > 6 drinks per week, as well as have an Alcohol Use Disorder Identification Test (AUDIT; (Saunders et al., 1993) score greater than 8; ibudilast and ivermectin – requirement of > 48 drinks per month and score > 1 on the CAGE questionnaire (Ewing, 1984) assessing for alcohol problems; self-administration – female requirement of > 7 drinks per week and male requirement of > 14 drinks per week. Age restrictions for the naltrexone and ibudilast study were between 21–55, whereas participants had to be between 21–65 for the ivermectin study and 21–45 for the self-administration study. Only two studies had ethnicity requirements. Participants in the naltrexone in Asian American study were of East Asian ethnicity (i.e. Chinese, Korean, Japanese, or Taiwanese) and participants in the self-administration study were Caucasian.
All studies shared the following exclusion criteria: 1) current involvement in treatment programs for alcohol use or having received treatment in the past 30 days; 2) use of non-prescription drugs (e.g. methamphetamine, cocaine) or prescription medications for recreational purposes; 3) self-reported history of exclusionary psychiatric disorders (e.g. bipolar disorder, manic-depression, psychotic disorders) assessed during telephone interview; 4) currently using antidepressants, mood stabilizers, sedatives, anti-anxiety medications, seizure medications, or prescription pain killers ; 5) self-reported history of contra-indicated medical conditions (e.g. chronic liver disease, ulcer disease, cardiac disease,) or any other medical condition that may interfere with study participation; 6) intense fear of needles or adverse reactions to needle puncture; and 7) if female, pregnancy, nursing, planning to get pregnant in the next 6 months or refusal to use reliable method of birth control. Specific to the ivermectin study, participants were excluded if they had a Body Mass Index (BMI) less than 18.5 or greater than 30. For the self-administration study, participants were excluded if they weighed over 265 pounds.
If participants were eligible following the telephone screening, they completed an in-person screening visit where written informed consent was obtained. During the screening visit, participants were required to produce a breath alcohol concentration (BrAC) of 0.000 g/dl, and test negative for pregnancy and drug use, except for marijuana, on a urine toxicology screening.
2.2. Measures
Participants completed a battery of measures at the screening visit. A demographics questionnaire assessed age, sex, and ethnicity. The Timeline Follow-Back (TLFB; (Sobell and Sobell, 1992) queried daily alcohol consumption in standard drinks, number of cigarettes smoked per day, and marijuana use during the previous thirty days. Marijuana use was assessed in a dichotomous fashion (i.e., Q: “Did you use marijuana on this day? A: “Yes or No”); route of marijuana administration was not recorded. Alcohol and cigarette use was assessed as a continuous variable. Use of other tobacco products, e.g. snus or chewing tobacco, was not assessed. The Fagerström Test of Nicotine Dependence (FTND; (Heatherton et al., 1991) queried extent of nicotine dependence. The AUDIT was administered to evaluate severity of drinking. The Cannabis Use Disorders Identification Test (CUDIT-R; (Adamson et al., 2010), a reliable and valid adaptation of the AUDIT, was given to assess marijuana use severity.
2.3. Data analysis
Of the 551 total subjects who were screened for the four studies from which we culled data, 541 (98.2%) reported using alcohol on the AUDIT and/or TLFB, 296 (53.7%) reported using marijuana on the CUDIT and/or TLFB, and 260 (47.2%) reported using cigarettes on the FTND. As this study aimed to understand patterns of co- and tri-use among all three substances, we included only participants who reported using alcohol, cigarettes, and marijuana on a monthly basis. This selection resulted in a final sample of N = 179 participants. While this represents a significant decrease in the number of subjects, statistical power is still quite high for these analyses. The proposed analyses test the association between drug use on a per-day basis (i.e., Level 1 effects). Thus, the sample size for this study is properly conceptualized in terms of both the number of subjects, 179, but also the number of Level 1 observations which is 5390 total days. To confirm that this study is well powered, GPower 3.1.9.2. was used to conduct a power analysis. Based on a simplified repeated measures approach, a small effect size (i.e., Cohen’s d = 0.2) and a nominal α = 0.05 threshold, power for this study was exceptionally high (i.e., > 99.99%).
To explore patterns of marijuana, alcohol, and/or cigarette co-use a series of multilevel logistic models were run on 30-day timeline follow-back drug use data. Owing to the one-on-one clinical interview nature of data collection for the key variables, there was no missing data in this study. Only individuals who reported using all three substances at least once per month were analyzed. Multilevel logistic modeling was chosen because (1) the data structure is nested with days (Level 1) nested within subjects (Level 2) which is appropriately modeled with a multilevel modeling approach and (2) the outcome variable of whether a given drug was used on a given day is binary necessitating the logistic modeling approach. Multilevel logistic models were run via PROC GLIMMIX in SAS version 9.4 (SAS Institute, 2015) with a binomial dependent variable distribution and a logit link function. Models were run with cigarette use and marijuana use as the dependent variable and the other drug classes treated as predictor variables with main effects and interactions to test for potentially synergistic effects of combined use on the likelihood on the third drug use (i.e., a positive interaction), or a suppressive effect where use of both drugs is associated with the same risk as singular use (i.e., a negative interaction). Variables tested were (1) Drink, a binary Level 1 variable coding whether alcohol was consumed on a given day, (2) Smoke, a binary Level 1 variable coding whether cigarettes were smoked on a given day, and (3) Marijuana, a binary Level 1 variable coding whether marijuana was used on a given day. To disentangle within-person effects (i.e., effects of use on a given day) from between-person effects (i.e. tendency for heavier users of one drug to be heavier users of all drugs), person-means for each predictor variable (e.g., the average drinking frequency across the 30 days assessed) were entered into models as Level 2 variables. To further ensure that the effects reported are within-subject effects, all Level 1 variables were treated as random at Level 2, meaning the effects were allowed to vary between subjects.
Statistical results are presented with an accompanying odds ratio effect size and 95% confidence intervals. Where interactions were observed, analysis of simple slopes were conducted through a recentering scheme to test the lower-order effects at specific levels of the interacting variables. In accordance with the NIH policy on considering sex as a biological variable (Health, 2015) and given the sex differences in the prevalence of marijuana, tobacco, and alcohol use in the US (Carliner et al., 2017), we also tested for sex differences in the propensity for drug co-use. To test the robustness of these results several covariates were explored including: age, ethnicity, and source study, all of which were included as Level 2 variables. Ethnicity was examined as a covariate because one of the source studies was completely composed of individuals of East Asian descent (Ray et al., 2018), and age was included due to findings that patterns of co-use may differ by age (Schauer et al., 2015; Terry-McElrath and Patrick, 2018). In line with the recommendations of (Simmons et al., 2011), where discrepancies between models which included vs. omitted covariates were observed, we report the results of both models.
3. Results
Sample demographics for the final sample of 179 participants are displayed in Table 1. Overall the sample was in early-mid adulthood (mean age = 29.02, SD = 8.19), majority male (72.47%), ethnically diverse (36.16% Asian American, 31.07% Caucasian, 12.43% African American, 9.04% Hispanic, and 7.34% Native American), and pulled from all four studies (Self-Administration Study: 25.70%; Naltrexone in Asian Americans Study: 32.40%; Ibudilast Study: 26.82%; Ivermectin Study: 15.08%). The sample on average reported very hazardous drinking as indicated by the AUDIT (mean = 17.20, SD = 7.66), a high prevalence of hazardous marijuana use on the CUDIT-R (mean = 7.83, SD = 5.98), and low to moderate nicotine dependence on the FTND (mean = 2.85, SD = 1.06). In total, these 179 participants reported drinking alcohol on 3073 days, smoking cigarettes on 2750 days, and consuming marijuana on 1598 days. 4.47% of subjects drank alcohol daily, 10.0% used marijuana daily and 22.9% smoked cigarettes daily. On average, participants smoked cigarettes on 51% of days (SD = 41%), drank alcohol on 57% of days (SD = 25%), and used marijuana on 30% of days (SD = 35%). Further, participants reported co-administering cigarettes and alcohol on 35% of days (SD = 31.51%), cigarettes and marijuana on 19.55% of days (SD = 30.21%), marijuana and alcohol on 20.08% of days (SD = 26.58%), and using all three substances on 14.81% of days (SD = 24.61%).
Table 1.
Sample demographics for study completers (N = 179) who reported using alcohol, marijuana, and cigarettes on self-report measures and/or timeline follow-back interviews.
| Mean (SD) or % | |
|---|---|
| Age | 29.02 (8.19) |
| Sex (% women) | 27.53% |
| Ethnicity | |
| American Indian | 7.34% |
| Asian | 36.16% |
| African American | 12.43% |
| Hispanic/Latino | 9.04% |
| Caucasian | 31.07% |
| Multiple Ethnicities reported | 1.69% |
| Other | 2.26% |
| Source Study | |
| Self-Administration Study | 25.70% |
| Naltrexone in Asian Americans Study | 32.40% |
| Ibudilast Study | 26.82% |
| Ivermectin Study | 15.08% |
| Cannabis Use Disorder IdentificationTest - Revised (CUDIT-R) | 7.83 (5.98) |
| Alcohol Use Disorder Identification Test (AUDIT) | 17.20 (7.66) |
| Fagerstrom Test for Nicotine Dependence (FTND) | 2.85 (1.06) |
Subjects recruited from different source studies differed in terms of their average marijuana use frequency (F(1, 3) = 12.32, p < 0.001; Self-Administration Study 13.9% of days (SD = 19.1%), Naltrexone in Asian Americans Study: 22.5% (SD = 29.5%), Ibudilast Study: 39.0% (SD = 38.8%), Ivermectin Study: 56.5% (SD = 40.8%)). Studies also differed in their average drinking frequency (F(1, 3) = 13.97, p < 0.001; Self-Administration Study 61.1% of days (SD = 21.5%), Naltrexone in Asian Americans Study: 42.0% (SD = 23.5%); Ibudilast Study: 67.8% (SD = 20.4%); Ivermectin Study: 64.8% (SD = 24.5%)). No difference in cigarette smoking frequency was found between studies (p = 0.36).
3.1. Correlations between average drug use frequency
Please see supplemental information for summary tables of all results. To first test whether average drug use frequency across these three drugs of abuse were correlated at the subject level, a series of linear regressions were conducted analyzing the correlation between proportion of days using each drug from the TLFB. Both drinking frequency and marijuana use frequency were found to independently predict cigarette smoking frequency (B = 0.35, SE = 0.12, t = 2.87, p < 0.01 and B = 0.24, SE = 0.09, t = 2.89, p < 0.01, respectively), but drinking frequency did not predict marijuana use frequency (p = 0.17). While these results suggest that subjects who use cigarettes and drink alcohol more often also use marijuana more frequently, these analyses do not address the central question posed in this paper of whether use of one substance on a particular day increases the likelihood of co-use or tri-use on that same day.
3.2. Predicting same-day cigarette use
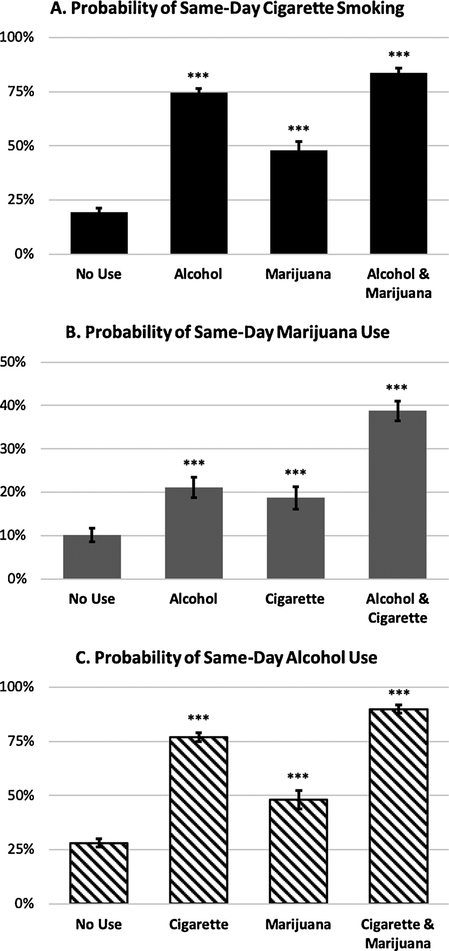
To test whether use of one drug increases the likelihood of same-day co-use, a series of multilevel models were run with daily use of each drug included as Level 1 variables and drug use frequency person means also included as covariates to disentangle the between-subject effects summarized in 3.1 from same-day effects. Over and above the effect of more frequent drug use generally, drinking alcohol on a given day was associated with a dramatic increase in the likelihood of same-day cigarette smoking (B = 2.27, SE = 0.15, t (5172) = 15.63, p < 0.001, OR = 9.71, 95% CI [7.29, 12.91]). Likewise, marijuana use was also associated with an increase in the likelihood of same-day cigarette smoking, though to a smaller degree than alcohol (B = 0.82, SE = 0.18, t (5172) = 4.61, p < 0.001, OR = 2.27, 95% CI [1.60, 3.21]). A significant and negative Drink × Marijuana interaction term was observed (B = −0.78, SE = 0.27, t (5171) = −2.92, p < 0.01) such that the effect of combined use of alcohol and marijuana on a given day was sub-additive (Fig. 1A). This interaction was such that on non-drinking days, marijuana use was associated with relatively large increases in the likelihood to smoke cigarettes (OR = 3.82, 95% CI [2.32, 6.28], but on drinking days, the effect of marijuana use on cigarette co-use was substantially smaller (OR = 1.74, 95% CI [1.17, 2.58]). As expected in these models, average cigarette use, marijuana use, and alcohol use frequency were all significantly associated with likelihood to smoke cigarettes on a given day (p < 0.01). Neither sex, age, ethnicity, nor source study were significant covariates, and their inclusion in the model had no effect on these reported outcomes.
Fig. 1.
Probability of (A) same-day cigarette smoking on days of no drug use, alcohol use, marijuana use, or both, (B) same-day marijuana use on days of no drug use, alcohol use, cigarette use, or both, and (C) same day alcohol use on days of no drug use, cigarette use, marijuana use, or both. Plotted probabilities are computed based on the final multilevel logistic model including main effects and interaction and covarying for person-mean use frequencies for each drug. Error bars represent 95% confidence intervals. *** p < 0.001 as compared to “No Use” days.
3.3. Predicting same-day marijuana use
Drinking alcohol on a given day was associated with an increase in the likelihood of same-day marijuana use over and above the effect of cigarette smoking (B = 0.94, SE = 0.12, t (5172) = 7.63, p < 0.001, OR = 2.56, 95% CI [2.01, 3.27]). Likewise, smoking cigarettes on a given day was also associated with an increase in the likelihood of same-day marijuana use (B = 0.80, SE = 0.17, t (5172) = 4.76, p < 0.001, OR = 2.24, 95% CI [1.60, 3.11]). The effect of drinking and cigarette smoking on marijuana use was generally additive as evidenced by a non-significant interaction term (p = 0.46). As a result of this additivity, the odds of marijuana use on days where both alcohol and cigarettes were used are 5.59 times greater than on days of no alcohol or cigarette use (Fig. 1B). No covariates were significant, and their inclusion did not affect any of the effects reported.
3.4. Predicting same-day alcohol use
Smoking cigarettes on a given day was associated with an increase in the odds of same-day alcohol use over and above the effects of same-day marijuana use (B = 2.17, SE = 0.13, t (5172) = 16.98, p < 0.001, OR = 8.80, 95% CI [6.84, 11.30]). Marijuana use was also associated with an increase in alcohol drinking likelihood (B = 0.92, SE = 0.12, t (5172) = 7.52, p < 0.001, OR = 2.52, 95% CI [1.98, 3.21]). The interaction between cigarette and marijuana use was not significant (p = 0.52) suggesting a generally additive relationship (Fig. 1C). As with the results predicting cigarette smoking and marijuana use, the effects predicting alcohol use were not affected by the inclusion of covariates, and no covariates predicted alcohol use likelihood.
3.5. Sex differences
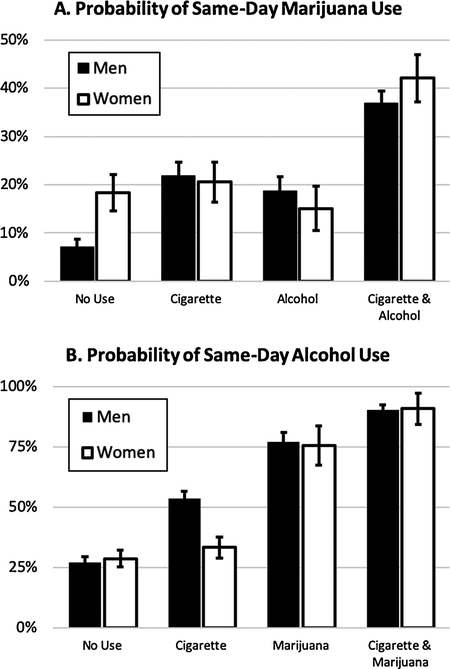
The effects of cigarette and alcohol use on the probability of same-day marijuana use were moderated by sex. Specifically, a significant sex × alcohol use interaction was observed (B = −0.48, SE = 0.23, t (5142) = −2.09, p < 0.05) such that the effect of drinking on same-day marijuana use was greater for men (OR = 2.99, 95% CI [2.24, 3.99]) than for women (OR = 1.85, 95% CI [1.26, 2.72]). Furthermore, a sex × alcohol use × cigarette use interaction was also significant (B = 1.63, SE = 0.48, t (5139) = 3.42, p < 0.001, Fig. 2A). This three-way interaction was such that the two-way alcohol use × cigarette use interaction was significant and positive representing a synergistic effect for women (B = 1.27, SE = 0.40, t (5139) = 3.20, p < 0.01), but nonsignificant for men (B = −0.36, SE = 0.26, t (5139) = −1.38, p = 0.17). Further breaking down this complex sex difference, the effects of singular alcohol and cigarette use on marijuana use were both significant for men (ORAlcohol = 3.65, 95% CI [2.39, 5.56]; ORCigarette = 2.99, 95% CI [1.88, 4.78]), but small and non-significant for women (ORAlcohol = 1.15, 95% CI [0.70, 1.87]; ORCigarette = 0.79, 95% CI [0.40, 1.56]).
Fig. 2.
Sex differences in the probability of (A) same day marijuana use associated with alcohol and/or cigarette use and (B) same day alcohol use associated with cigarette and marijuana use. Plotted probabilities are computed based on the final multilevel logistic model including main effects and interaction and covarying for person-mean use frequencies for each drug. Error bars represent 95% confidence intervals.
A significant sex × cigarette use × marijuana use interaction predicting alcohol use likelihood was also observed (B = 1.06, SE = 0.39, t (5139) = 2.74, p < 0.01). This effect was such that the synergistic effect of combined cigarette and marijuana use was greater among women (B = 0.95, SE = 0.34, t (5139) = 2.82, p < 0.01) than among men (B = −0.11, SE = 0.18, t (5139) = −0.60, p = 0.55, see Fig. 2B). No other sex differences were observed (p ≥ 0.26).
All reported sex differences were robust to controlling for ethnicity, source study, and age.
4. Discussion
The present study was the first to examine event-level, daily patterns of co-use of marijuana, alcohol, and cigarettes in a sample of non-treatment seeking individuals. Alcohol consumption was associated with increased odds of same-day cigarette or marijuana co-use. Similarly, any cigarette smoking increased the probability of same-day alcohol or marijuana co-use, and marijuana use also increased the odds of same-day alcohol or cigarette co-use. Additionally, we found generally additive effects of simultaneous co-use on the likelihood of using a third substance (i.e., tri-use); the co-use of alcohol with cigarettes and marijuana with cigarettes increased the odds of same day marijuana and alcohol use by over five times, respectively. When taken together, these results indicate that the use of either marijuana, alcohol, or tobacco substantially increases the probably of the co-use one of the two other substances, and if two of these substances are co-used, the likelihood of a using the third is further amplified. Our results may aid in the understanding of how simultaneous co-use of marijuana with alcohol and/or tobacco relates to the etiology, maintenance, and treatment of AUD, CUD, and tobacco use disorder (TUD).
Our event-level findings that marijuana, alcohol, and/or cigarette use substantially increased odds of simultaneous co- and tri-use in non-treatment seeking, regular substance users support epidemiological data that describe highly prevalent concurrent and simultaneous co-use of these three substances. The behavioral mechanisms underlying the relationship between alcohol and tobacco co-use have been well characterized and may be applicable to understanding co-use of each substance with marijuana. As reviewed in detail elsewhere (Roche et al., 2016a; Verplaetse and McKee, 2017), the underlying motivation for simultaneous co-administration of alcohol and tobacco appears to be predominantly driven by cue-conditioned cross-reactivity, in which each substance elicits cue-induced craving for the other via Pavlovian conditioning, and the additive or synergistic reinforcing effects of the drugs when used in combination. The findings of the present study may suggest that individuals are simultaneously using marijuana with alcohol and/or tobacco due to similar mechanisms. Such motives for co-or tri-use would be consistent with the majority of preclinical and clinical studies examining the combined effects or patterns of co-use of marijuana with alcohol or tobacco. For example, tobacco and marijuana co-users have reported simultaneously using both substances because each drug increases craving for the other, tobacco enhances the subjective effects of marijuana, and simultaneous co-use produces additive subjective effects (Amos et al., 2004; Berg et al., 2018; Ramo et al., 2013, 2012; Schauer et al., 2016). Furthermore, the majority of molecular and behavioral pharmacology studies in rodents and humans suggest additive, or even synergistic, reinforcing as well as impairing effects of combined marijuana and alcohol (Bramness et al., 2010; Downey et al., 2013; Liguori et al., 2002; Lukas and Orozco, 2001; Perez-Reyes et al., 1988; Ramaekers et al., 2004). Interestingly, a recent study found that alcohol consumption was positively associated with being open to experiment with tobacco or marijuana co-use in different places and with different people, suggesting a contextual or social influence on co-use in addition to the pharmacological factors discussed above (Berg et al., 2018).
If the pattern of simultaneous co- and tri-use observed in this study is representative of a chronic behavior, we speculate that additive co-reinforcement and cue-cross-reactivity, as well as the likely development of cross-tolerance due to overlapping neurobiological effects (Le Foll et al., 2008; Maldonado et al., 2006; Roche et al., 2016a), could lead to escalation of substance use to hazardous levels and underlie the development of comorbid or even trimorbid CUD, AUD, and/or TUD. This proposed progression would be consistent with epidemiological literature indicating that the simultaneous use of marijuana with tobacco or alcohol is associated with psychological and physiological harm, negative social consequences, high risk substance use, development of dependence, more severe dependence levels, and poorer treatment outcomes above and beyond both concurrent and single drug use (Baggio et al., 2014; Mojarrad et al., 2014; Subbaraman et al., 2017; Subbaraman and Kerr, 2015; Weinberger et al., 2016). Yet, there is a sizeable literature suggesting marijuana is sometimes used as a substitution for alcohol or cigarettes. Individuals who use marijuana concurrently with alcohol or tobacco report using marijuana in place of both drugs (Berg et al., 2018; Lau et al., 2015; Reiman, 2009; Schauer et al., 2016). Furthermore, cessation studies have also shown that as marijuana use declines, craving and use of alcohol or tobacco may rise, which indirectly supports a substitution pattern of use (Choi et al., 2018; Copersino et al., 2006; Peters and Hughes, 2010; Schaub et al., 2010). Indeed, some have argued for marijuana to be positioned as a substitute for alcohol and other illicit drug abuse as a harm reduction strategy (Charlton, 2005; Reiman, 2009). Marijuana may have a superior safety profile to alcohol or tobacco (Nutt et al., 2010), but the concept of drug substitution as a harm reduction strategy is predicated on the idea that use of the substituted drug decreases rather than increases the likelihood of target drug use. Although marijuana use strongly augmented the odds of same-day drug co-use in our sample, we also observed that the co-use of alcohol and marijuana was associated with a decrease in the odds of cigarette consumption compared with non-drinking days. One possible interpretation of this result is that individuals were substituting marijuana for cigarettes in this particular co-use event. Despite this single sub-additive result, our findings when taken as a whole suggest additive co-use effects and indicate further research of event-level, simultaneous co-use in both treatment-seeking and non-treatment-seeking populations is needed before considering marijuana as a harm reduction strategy for AUD or TUD.
Sex was a significant moderator of several of the observed patterns of co- and tri-use between marijuana, alcohol, and tobacco. The effect of alcohol and cigarette use independently increasing the odds of same-day marijuana co-use was stronger in men than women. This finding is broadly consistent with epidemiological data showing that men, vs. women, have higher rates of marijuana, alcohol, and cigarette use, start using these substances at a younger age, use them in greater quantities, and have greater prevalence of dependence (Carliner et al., 2017; Higgins et al., 2015; Johnston et al., 2018; White et al., 2015). More specifically, men have higher rates of marijuana co-use with each alcohol and tobacco and display a more rapid escalation in the frequency of this co-administration than women, both of which directly support the patterns of co-use observed in the present study (Crane et al., 2015; Guxens et al., 2007; Penetar et al., 2005; Schauer et al., 2015; Victoir et al., 2006).
Interestingly, while men had stronger relationships of single drug use predicting simultaneous marijuana co-use, women were more likely to have drug co-use turn into tri-use. We observed that the odds of alcohol use after simultaneous cigarette and marijuana co-use and marijuana use after cigarette and alcohol co-use were greater in women than men. An event-level pattern of tri-use such as this, i.e., with greater odds of progressing from simultaneously using two substances to co-using three substances in an event, could plausibly be related to more severe consequences from substance use in women even if they consumed less overall quantity than men. While men use marijuana, tobacco, and alcohol more heavily and have higher rates of dependence than women, women often experience more severe consequences from use. Some, but not all (Keyes et al., 2010), studies have demonstrated that women display “telescoping” in the development of AUD and CUD. That is, while men have higher rates of the disorders, women tend to enter treatment for CUD and AUD after fewer years and quantity of use than men (Diehl et al., 2007; Hernandez-Avila et al., 2004; Mann et al., 2005; Randall et al., 1999). Additionally, women are at greater risk for lost productivity, alcohol-induced blackouts, more severe neurocognitive impairment, brain atrophy, and a variety of physiological problems due to alcohol abuse despite drinking less and for a shorter amount of time than men (Diehl et al., 2007; Hommer, 2003; Nixon et al., 2014; Nolen-Hoeksema, 2004; White et al., 2002). Sex differences in patterns of co- and tri-use could inform sex specific treatment and intervention of comorbid substance use disorders. However, given the exploratory nature of our comparison of sex differences and the paucity of studies that have included sex as a variable when examining event-level patterns of co-use, our sex-related results should be viewed as preliminary and are in need of replication in independent samples.
As the original purpose for collecting the data that was analyzed in this manuscript was participant screening, and the analysis presented in this manuscript was ad hoc, there are several important limitations that should be considered when interpreting our results. The primary study limitation is the potential for low external validity due to the very specific composition of our sample. The recruitment goals of the four parent studies were in part to screen individuals who were regular-to-heavy-drinkers but who did not have other serious psychiatric disorders or medical conditions. Additionally, one of the parent studies only enrolled individuals of East Asian descent (Ray et al., 2018). The resultant sample in the present study is reflective of these parameters; that is, one with a higher than expected percentage of Asian Americans who are very hazardous drinkers, have low nicotine dependence, have borderline hazardous marijuana use, and report having no serious medical or psychiatric conditions. However, as outlined in the introduction, individuals from the general population who simultaneously co-administer alcohol, marijuana, and/or cigarettes on a regular basis would likely present with comorbid psychiatric disorders and serious health problems, and this may be especially true for treatment-seeking populations. Further, individuals of East Asian descent generally report lower alcohol consumption and have reduced risk of AUD development than other ethnicities (Eng et al., 2007; Frank et al., 2012), so it is potentially unlikely that this ethnic background would be responsible for 36% of the individuals who use alcohol, marijuana, and cigarettes in the real world. Although controlling for ethnicity in all analyses increases external validity and confidence in the presented results, it is still unclear how the presented results may generalize to both the general population of substance using adults as well as those seeking treatment for AUD, CUD, and/or TUD.
Additional limitations may be related to the use of the TLFB to retrospectively assess patterns of drug co-use. When compared to same-day assessment, the use of the TLFB interview to retrospectively record drug use introduces a risk of recency bias (Gmel and Daeppen, 2007; Vinson et al., 2003). However, this recency effect appears to be mostly related to underreporting measurements of consumption levels (e.g., number of drinks) rather than accuracy in dichotomously assessing whether any drug was consumed on a given day (Searles et al., 2002), which would mitigate any negative influence of recall bias on the present results. Also, because our standard procedures for TLFB administration was to assess marijuana use as a dichotomous “Yes/No” variable, no information was collected on the route, formulation, or quantity of marijuana that was consumed on a given day. For example, in our data we have no ability to distinguish whether a single “hit” from a vaporizer, 30 mg of marijuana extract taken orally, or three entire blunts was consumed in a day; all could feasibly be coded identically in our dataset. Furthermore, while we do interpret the self-report of co-use within a day as simultaneous rather than concurrent use, we do not have data directly indicating that all substances were consumed during a single drug-use event. It is conceivable, albeit unlikely, that an individual would regularly use one drug in the morning and a second in the evening, for example. Yet, we believe we are warranted to interpret same-day co-use as simultaneous given prior findings indicating that polydrug users simultaneously co-administer drugs the far majority of the time and that marijuana is commonly self-reported as being used simultaneously with alcohol or tobacco (Barrett et al., 2006; Martin et al., 1992; Midanik et al., 2007; Subbaraman and Kerr, 2015). Lastly, although overall a clear strength of our study, our data only allows us to examine co-use within a given day. Thus, we are unable to determine causal pathways underlying specific sequences of co-use, and future studies, for example with ecological momentary assessment (EMA) methods, should examine the temporal relationship between marijuana, alcohol, and tobacco use within a given drug-use episode.
4.1. Conclusions
In conclusion, the present study suggests that, as observed with the co-use of alcohol and tobacco, marijuana is simultaneously co-used with tobacco and alcohol in a predictable and incremental pattern at the individual and event-level. The individual use of marijuana, alcohol, or tobacco significantly increased the odds of using a second substance, and the use of a second substance generally produced additive effects in increasing the likelihood of using a third. We did observe one exception to this pattern in that the co-use of alcohol and marijuana produced sub-additive effects on likelihood of also using cigarettes. This finding was surprising, considering the otherwise generally additive effects on co- and tri-use, and suggests that alcohol or marijuana may at times be used a substitute for cigarettes in certain co-use situations.
Lastly, in our exploratory analysis, we found that sex moderated several of the observed patterns of co- and tri-use. The relationship between alcohol or cigarette use increasing the odds of marijuana couse was stronger in men, whereas the observed additive relationships between drug co-use leading to tri-use was stronger in women. As individuals who co-use marijuana with tobacco and alcohol have more severe health and social consequences and worse treatment outcomes, additional studies using more fine-grained analysis approaches, e.g. EMA, are needed to replicate these findings and elucidate their role in the etiology and treatment of CUD, AUD, and TUD.
Supplementary Material
Acknowledgements
This work was supported by the following grants: R01 AA021744 (LAR), R21 AA022214 (LAR), R21 AA022752 (LAR), and 1K01AA026005 (D.J.O.R.).
Footnotes
Conflict of interest
Lara Ray is a paid consultant for GSK and has received medication from Pfizer and Medicinova. No other authors have conflicts to disclose.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2019.02.035.
References
- Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, Sellman JD, 2010. An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. 110, 137–143. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, 2009. Tobacco and cannabis co-occurrence: does route of administration matter? Drug Alcohol Depend. 99, 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Madden PAF, Bucholz KK, Heath AC, Lynskey MT, 2008. Transitions to regular smoking and to nicotine dependence in women using cannabis. Drug Alcohol Depend. 95 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, Madden PA, Bucholz KK, Heath AC, 2007. A latent class analysis of illicit drug abuse/dependence: results from the national epidemiological survey on alcohol and related conditions. Addiction 102, 94–104. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Budney AJ, Lynskey MT, 2012. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction 107, 1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akre C, Michaud P-A, Berchtold A, Suris J-C, 2009. Cannabis and tobacco use: where are the boundaries? A qualitative study on cannabis consumption modes among adolescents. Health Educ. Res 25, 74–82. [DOI] [PubMed] [Google Scholar]
- Allsop DJ, Dunlop AJ, Sadler C, Rivas GR, McGregor IS, Copeland J, 2014. Changes in cigarette and alcohol use during cannabis abstinence. Drug Alcohol Depend. 138, 54–60. 10.1016/j.drugalcdep.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Amos A, Wiltshire S, Bostock Y, Haw S, McNeill A, 2004. ‘You can’t go without a fag… you need it for your hash’—a qualitative exploration of smoking, cannabis and young people. Addiction 99, 77–81. [DOI] [PubMed] [Google Scholar]
- Baggio S, Studer J, Mohler-Kuo M, Daeppen J-B, Gmel G, 2014. Concurrent and simultaneous polydrug use among young swiss males: use patterns and associations of number of substances used with health issues. Int. J. Adolesc. Med. Health 26, 217–224. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Darredeau C, Pihl RO, 2006. Patterns of simultaneous polysubstance use in drug using university students. Hum. Psychopharmacol. Clin. Exp 21, 255–263. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Payne J, Henriksen L, Cavazos-Rehg P, Getachew B, Schauer GL, Haardörfer R, 2018. Reasons for marijuana and tobacco co-use among young adults: a mixed methods scale development study. Subst. Use Misuse 53, 357–369. 10.1080/10826084.2017.1327978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramness JG, Khiabani HZ, Mørland J, 2010. Impairment due to cannabis and ethanol: clinical signs and additive effects. Addiction 105, 1080–1087. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST, 2006. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J. Consult. Clin. Psychol 74, 307. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Jentsch JD, Roche DJO, Ramchandani VA, Miotto K, Ray LA, 2018. Differences in the subjective and motivational properties of alcohol across alcohol use severity: application of a novel translational human laboratory paradigm. Neuropsychopharmacology 43, 1891–1899. 10.1038/s41386-018-0086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth P, Slade T, Degenhardt L, 2014. Factors associated with the timing and onset of cannabis use and cannabis use disorder: results from the 2007 Australian national survey of mental health and well-being. Drug Alcohol Rev. 33, 555–564. 10.1111/dar.12183. [DOI] [PubMed] [Google Scholar]
- Carliner H, Mauro PM, Brown QL, Shmulewitz D, Rahim-Juwel R, Sarvet AL, Wall MM, Martins SS, Carliner G, Hasin DS, 2017. The widening gender gap in marijuana use prevalence in the U.S. during a period of economic change, 2002–2014. Drug Alcohol Depend. 170, 51–58. 10.1016/j.drugalcdep.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulkins JP, Kilmer B, Kleiman MA, MacCoun RJ, Midgette G, Oglesby P, Pacula RL, Reuter PH, 2015. Considering Marijuana Legalization: Insights for Vermont and Other Jurisdictions. Rand Corporation, California. [Google Scholar]
- Cerdá M, Wall M, Keyes KM, Galea S, Hasin D, 2012. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend. 120, 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton BG, 2005. Diazepam with your dinner, Sir? The lifestyle drug-substitution strategy: a radical alcohol policy. QJM 98, 457–459. [DOI] [PubMed] [Google Scholar]
- Choi NG, DiNitto DM, Marti CN, 2018. Marijuana use among adults: initiation, return to use, and continued use versus quitting over a one-year follow-up period. Drug Alcohol Depend. 182, 19–26. 10.1016/j.drugalcdep.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Cohn A, Villanti A, Richardson A, Rath JM, Williams V, Stanton C, Mermelstein R, 2015. The association between alcohol, marijuana use, and new and emerging tobacco products in a young adult population. Addict. Behav 48, 79–88. [DOI] [PubMed] [Google Scholar]
- Cohn AM, Johnson AL, Rath JM, Villanti AC, 2016. Patterns of the co-use of alcohol, marijuana, and emerging tobacco products in a national sample of young adults: substance co-use in young adults. Am. J. Addict 25, 634–640. 10.1111/ajad.12456. [DOI] [PubMed] [Google Scholar]
- Compton WM, Han B, Jones CM, Blanco C, Hughes A, 2016. Marijuana use and use disorders in adults in the USA, 2002–14: analysis of annual cross-sectional surveys. Lancet Psychiatry 3, 954–964. [DOI] [PubMed] [Google Scholar]
- Conway KP, Vullo GC, Nichter B, Wang J, Compton WM, Iannotti RJ, Simons-Morton B, 2013. Prevalence and patterns of polysubstance use in a nationally representative sample of 10th graders in the United States. J. Adolesc. Health 52, 716–723. 10.1016/j.jadohealth.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Swift W, Roffman R, Stephens R, 2001. A randomized controlled trial of brief cognitive–behavioral interventions for cannabis use disorder. J. Subst. Abuse Treat. 21, 55–64. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA, 2006. Quitting among non-treatment-seeking marijuana users: reasons and changes in other substance use. Am. J. Addict 15, 297–302. [DOI] [PubMed] [Google Scholar]
- Crane NA, Langenecker SA, Mermelstein RJ, 2015. Gender differences in the associations among marijuana use, cigarette use, and symptoms of depression during adolescence and young adulthood. Addict. Behav 49, 33–39. 10.1016/j.addbeh.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, 2000. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 59, 235–249. 10.1016/S0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- de Dios MA, Vaughan EL, Stanton CA, Niaura R, 2009. Adolescent tobacco use and substance abuse treatment outcomes. J. Subst. Abuse Treat. 37, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl A, Croissant B, Batra A, Mundle G, Nakovics H, Mann K, 2007. Alcoholism in women: is it different in onset and outcome compared to men? Eur. Arch. Psychiatry Clin. Neurosci 257, 344–351. [DOI] [PubMed] [Google Scholar]
- Downey LA, King R, Papafotiou K, Swann P, Ogden E, Boorman M, Stough C, 2013. The effects of cannabis and alcohol on simulated driving: influences of dose and experience. Accid. Anal. Prev 50, 879–886. 10.1016/j.aap.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Meyerhoff DJ, 2007. The neurobiological and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol Alcohol. 42, 174–185. 10.1093/alcalc/agm020. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Janney CA, Sellers TA, Folsom AR, Cerhan JR, 2005. The association of alcohol consumption with coronary heart disease mortality and cancer incidence varies by smoking history. J. Gen. Intern. Med 20, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng MY, Luczak SE, Wall TL, 2007. ALDH2, ADH1B, and ADH1C genotypes in Asians: a literature review. Alcohol Res. Health 30, 22–27. [PMC free article] [PubMed] [Google Scholar]
- Ewing J, 1984. Detecting interventions for alcohol problems: a review. Addiction 252, 1905–1907. [Google Scholar]
- Fairman BJ, 2015. Cannabis problem experiences among users of the tobacco–cannabis combination known as blunts. Drug Alcohol Depend. 150, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Castle I-JP, Ryan M, Fertig J, Litten RZ, 2015. Moderators of varenicline treatment effects in a double-blind, placebo-controlled trial for alcohol dependence: an exploratory analysis. J. Addict. Med 9, 296–303. 10.1097/ADM.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DE, Vu HT, Anthony JC, 2002. Marijuana use and cessation of tobacco smoking in adults from a community sample. Drug Alcohol Depend. 67, 243–248. [DOI] [PubMed] [Google Scholar]
- Frank J, Cichon S, Treutlein J, Ridinger M, Mattheisen M, Hoffmann P, Herms S, Wodarz N, Soyka M, Zill P, Maier W, Mössner R, Gaebel W, Dahmen N, Scherbaum N, Schmäl C, Steffens M, Lucae S, Ising M, Müller-Myhsok B, Nöthen MM, Mann K, Kiefer F, Rietschel M, 2012. Genome-wide significant association between alcohol dependence and a variant in the ADH gene cluster. Addict. Biol 17, 171–180. 10.1111/j.1369-1600.2011.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS, 2011. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl.) 215, 655–663. 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmel G, Daeppen J-B, 2007. Recall bias for seven-day recall measurement of alcohol consumption among emergency department patients: implications for case-crossover designs*. J. Stud. Alcohol Drugs 68, 303–310. 10.15288/jsad.2007.68.303. [DOI] [PubMed] [Google Scholar]
- Golub A, 2012. The growth in marijuana use among American youths during the 1990s and the extent of blunt smoking The Cultural/Subcultural Contexts of Marijuana Use at the Turn of the Twenty-First Century. Routledge, pp. 9–30. [Google Scholar]
- Gunn RL, et al. , 2018. Marijuana use is associated with alcohol use and consequences across the first 2 years of college. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav 32 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, Nebot M, Ariza C, 2007. Age and sex differences in factors associated with the onset of cannabis use: a cohort study. Drug Alcohol Depend. 88, 234–243. [DOI] [PubMed] [Google Scholar]
- Haas AL, Wickham R, Macia K, Shields M, Macher R, Schulte T, 2015. Identifying classes of conjoint alcohol and marijuana use in entering freshmen. Psychol. Addict. Behav 29, 620. [DOI] [PubMed] [Google Scholar]
- Hall W, 2016. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction 110, 19–35. 10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, Foltin RW, 2013. Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biol. Psychiatry 73, 242–248. 10.1016/j.biopsych.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, 2015a. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 72, 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Wall M, Keyes KM, Cerdá M, Schulenberg J, O’Malley PM, Galea S, Pacula R, Feng T, 2015b. Medical marijuana laws and adolescent marijuana use in the USA from 1991 to 2014: results from annual, repeated cross-sectional surveys. Lancet Psychiatry 2, 601–608. 10.1016/S2215-0366(15)00217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, FAGERSTROM K-O, 1991. The fagerström test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br. J. Addict 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR, 2004. Opioid-, cannabis-and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 74, 265–272. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Kurti AN, Redner R, White TJ, Gaalema DE, Roberts ME, Doogan NJ, Tidey JW, Miller ME, Stanton CA, 2015. A literature review on prevalence of gender differences and intersections with other vulnerabilities to tobacco use in the United States, 2004–2014. Prev. Med 80, 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW, 2003. Male and female sensitivity to alcohol-induced brain damage. Alcohol Res. Health 27, 181–185. [PMC free article] [PubMed] [Google Scholar]
- Humfleet GL, Haas AL, 2004. Is marijuana use becoming a ‘gateway’ to nicotine dependence? Addiction 99 2004, 5–6. [DOI] [PubMed] [Google Scholar]
- Hyggen C, Hammer T, 2014. From cannabis to problem drinking? Use and abuse from youth to adulthood. Nord. Stud. Alcohol Drugs. 10.2478/nsad-2014-0034. [Epub ahead of print]. [DOI] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME, 2018. Monitoring the Future National Survey Results on Drug Use, 1975–2017: Overview, Key Findings on Adolescent Drug Use. [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM, 2007. Abstinence rates following behavioral treatments for marijuana dependence. Addict. Behav 32, 1220–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J, 2010. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine Tob. Res 10.1093/ntr/ntq083.ntq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D, Kandel E, 2015. The Gateway Hypothesis of substance abuse: developmental, biological and societal perspectives. Acta Paediatr. 104, 130–137. [DOI] [PubMed] [Google Scholar]
- Kelly BC, 2012. Bongs and blunts: notes from a suburban marijuana subculture The Cultural/Subcultural Contexts of Marijuana use at the Turn of the Twenty-First Century. Routledge, pp. 89–106. [Google Scholar]
- Kerr DC, Bae H, Phibbs S, Kern AC, 2017. Changes in undergraduates’ marijuana, heavy alcohol and cigarette use following legalization of recreational marijuana use in Oregon. Addiction 112, 1992–2001. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC, 1997. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch. Gen. Psychiatry 54, 313–321. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Martins SS, Blanco C, Hasin DS, 2010. Telescoping and gender differences in alcohol dependence: new evidence from two national surveys. Am. J. Psychiatry 167, 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau N, Sales P, Averill S, Murphy F, Sato S-O, Murphy S, 2015. A safer alternative: Cannabis substitution as harm reduction. Drug Alcohol Rev. 34, 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Forget B, Aubin H-J, Goldberg SR, 2008. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict. Biol 13, 239–252. 10.1111/j.1369-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherdale ST, Ahmed R, Kaiserman M, 2006. Marijuana use by tobacco smokers and nonsmokers: who is smoking what? Can. Med. Assoc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherdale ST, Hammond DG, Kaiserman M, Ahmed R, 2007. Marijuana and tobacco use among young adults in Canada: are they smoking what we think they are smoking? Cancer Causes Control 18, 391–397. [DOI] [PubMed] [Google Scholar]
- Legislatures, 2016. State Medical Marijuana Laws. N.C. of S.. Author, Wasnington, DC. [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV, 2011. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 116, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori A, Gatto CP, Jarrett DB, 2002. Separate and combined effects of marijuana and alcohol on mood, equilibrium and simulated driving. Psychopharmacology (Berl.) 163, 399–405. [DOI] [PubMed] [Google Scholar]
- Lipperman-Kreda S, Gruenewald PJ, Grube JW, Bersamin M, 2017. Adolescents, alcohol, and marijuana: context characteristics and problems associated with simultaneous use. Drug Alcohol Depend. 179, 55–60. 10.1016/j.drugalcdep.2017.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Orozco S, 2001. Ethanol increases plasma Δ9-tetrahydrocannabinol (THC) levels and subjective effects after marihuana smoking in human volunteers. Drug Alcohol Depend. 64, 143–149. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F, 2006. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 29, 225–232. [DOI] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A, 2005. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol. Clin. Exp. Res 29, 896–901. [DOI] [PubMed] [Google Scholar]
- Marijuana Treatment Project Research Group, 2004. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J. Consult. Clin. Psychol 72, 455–466. 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- Martin CS, Clifford PR, Clapper RL, 1992. Patterns and predictors of simultaneous and concurrent use of alcohol, tobacco, marijuana, and hallucinogens in first-year college students. J. Subst. Abuse 4, 319–326. [DOI] [PubMed] [Google Scholar]
- Mauro CM, Newswanger P, Santaella-Tenorio J, Mauro PM, Carliner H, Martins SS, 2017. Impact of medical marijuana laws on State-level marijuana use by age and gender, 2004–2013. Prev. Sci 10.1007/s11121-017-0848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, 2013. How can We use our knowledge of alcohol-tobacco interactions to reduce alcohol use? Annu. Rev. Clin. Psychol 9, 649–674. 10.1146/annurev-clinpsy-050212-185549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E, 2009. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol. Psychiatry, Neural Mech. Linking Stress and Subst Abuse 66, 185–190. 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE, 2012. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci 201206820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrik J, Gunn RL, Jackson KM, Sokolovsky AW, Borsari B, 2018. Daily patterns of marijuana and alcohol Co-use among individuals with alcohol and Cannabis use disorders. Alcohol. Clin. Exp. Res 42, 1096–1104. 10.1111/acer.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midanik LT, Tam TW, Weisner C, 2007. Concurrent and simultaneous drug and alcohol use: results of the 2000 national alcohol survey. Drug Alcohol Depend. 90, 72–80. 10.1016/j.drugalcdep.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL, 2012. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl.) 223, 299–306. 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojarrad M, Samet JH, Cheng DM, Winter MR, Saitz R, 2014. Marijuana use and achievement of abstinence from alcohol and other drugs among people with substance dependence: a prospective cohort study. Drug Alcohol Depend. 142, 91–97. 10.1016/j.drugalcdep.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Budney AJ, 2001. Tobacco smoking in marijuana-dependent outpatients. J. Subst. Abuse 13, 583–596. [DOI] [PubMed] [Google Scholar]
- National Institute of Health (NIH), 2015. Consideration of Sex as a Biological variable in NIH-Funded Research. Available at https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html (Accessed 8 April 2019).. [Google Scholar]
- Nixon SJ, Prather R, Lewis B, 2014. Sex differences in alcohol-related neurobehavioral consequences Handbook of Clinical Neurology. Elsevier, pp. 253–272. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, 2004. Gender differences in risk factors and consequences for alcohol use and problems. Clin. Psychol. Rev 24, 981–1010. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD, 2010. Drug harms in the UK: a multicriteria decision analysis. Lancet 376, 1558–1565. [DOI] [PubMed] [Google Scholar]
- Olthuis JV, Darredeau C, Barrett SP, 2013. Substance use initiation: the role of simultaneous polysubstance use. Drug Alcohol Rev. 32, 67–71. [DOI] [PubMed] [Google Scholar]
- Pacula RL, Smart R, 2017. Medical marijuana and marijuana legalization. Annu. Rev. Clin. Psychol 13, 397–419. 10.1146/annurev-clinpsy-032816-045128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Zanettini C, Barnes C, Solinas M, Goldberg SR, 2013. PriorExposure to THC Increases the Addictive Effects of Nicotine in Rats. Neuropsychopharmacology 38 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M, 2005. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction 100 1518–1525. [DOI] [PubMed] [Google Scholar]
- Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, Lukas SE, 2005. Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug Alcohol Depend. 79, 211–223. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Hicks RE, Bumberry J, Robert Jeffcoat A, Cook CE, 1988Interaction between marihuana and ethanol: effects on psychomotor performance. Alcohol. Clin. Exp. Res 12, 268–276. [DOI] [PubMed] [Google Scholar]
- Perkins KA, 1997. Combined effects of nicotine and alcohol on subjective, behavioral and physiological responses in humans. Addict. Biol 2, 255–268. 10.1080/13556219772552. [DOI] [PubMed] [Google Scholar]
- Peters EN, Hughes JR, 2010. Daily marijuana users with past alcohol problems increase alcohol consumption during marijuana abstinence. Drug Alcohol Depend. 106, 111–118. 10.1016/j.drugalcdep.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Peters EN, Budney AJ, Carroll KM, 2012. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review: Cannabis-tobacco clinical correlates. Addiction 107, 1404–1417. 10.1111/j.1360-0443.2012.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center, 2014. America’s New Drug Policy Landscape. U.S. politics & policy. [Google Scholar]
- Piasecki TM, McCarthy DE, Fiore MC, Baker TB, 2008. Alcohol consumption, smoking urge, and the reinforcing effects of cigarettes: an ecological study. Psychol. Addict. Behav 22, 230–239. 10.1037/0893-164X.22.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince van Leeuwen A, Creemers HE, Verhulst FC, Vollebergh WAM, Ormel J, van Oort F, Huizink AC, 2014. Legal substance use and the development of a DSM-IV cannabis use disorder during adolescence: the TRAILS study. Addict. Abingdon Engl. 109, 303–311. 10.1111/add.12346. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Berghaus G, van Laar M, Drummer OH, 2004. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 73, 109–119. [DOI] [PubMed] [Google Scholar]
- Ramo DE, Liu H, Prochaska JJ, 2012. Tobacco and marijuana use among adolescents and young adults: a systematic review of their co-use. Clin. Psychol. Rev 32, 105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramo DE, Delucchi KL, Hall SM, Liu H, Prochaska JJ, 2013. Marijuana and tobacco co-use in young adults: patterns and thoughts about use. J. Stud. Alcohol Drugs 74, 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME, 1999. Telescoping of landmark events associated with drinking: a gender comparison. J. Stud. Alcohol 60, 252–260. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Shoptaw S, Roche DJ, Heinzerling K, Miotto K, 2017. Development of the neuroimmune modulator ibudilast for the treatment of alcoholism: a randomized, placebo-controlled, human laboratory trial. Neuropsychopharmacology 42, 1776–1788. 10.1038/npp.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Green R, Roche DJO, Bujarski S, Hartwell EE, Lim AC, Rohrbaugh T, Ghahremani D, Hutchison K, Miotto K, 2018. Pharmacogenetic effects of naltrexone in individuals of East Asian descent: human laboratory findings from a randomized trial. Alcohol. Clin. Exp. Res 42, 613–623. 10.1111/acer.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream GL, Benoit E, Johnson BD, Dunlap E, 2008. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend. 95, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman A, 2009. Cannabis as a substitute for alcohol and other drugs. Harm. Reduct. J 6, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P, Caraballo R, Pederson LL, Gupta N, 2008. Exploring use of nontraditional tobacco products through focus groups with young adult smokers, 2002. Prev. Chronic. Dis 5. [PMC free article] [PubMed] [Google Scholar]
- Roche DJO, Ray LA, Yardley MM, King AC, 2016a. Current insights into the mechanisms and development of treatments for heavy-drinking cigarette smokers. Curr. Addict. Rep 3, 125–137. 10.1007/s40429-016-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJO, Yardley MM, Lunny KF, Louie SG, Davies DL, Miotto K, Ray LA, 2016b. A pilot study of the safety and initial efficacy of ivermectin for the treatment of alcohol use disorder. Alcohol. Clin. Exp. Res 40, 1312–1320. 10.1111/acer.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RockyMountain High Intensity Drug Trafficking Area, 2015. The Legalization of Marijuana in Colorado: The Impact. (Rocky Mountain High Intensity Drug Trafficking Area, Investigative Support Center,). [Google Scholar]
- SAS Institute, 2015. Base SAS 9.4 Procedures Guide. https://www.sas.com.
- Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M, 1993. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Schaub M, Gmel G, Annaheim B, Mueller M, Schwappach D, 2010. Leisure time activities that predict initiation, progression and reduction of cannabis use: a prospective, population-based panel survey. Drug Alcohol Rev. 29, 378–384. 10.1111/j.1465-3362.2009.00156.x. [DOI] [PubMed] [Google Scholar]
- Schauer GL, Peters EN, 2018. Correlates and trends in youth co-use of marijuana and tobacco in the United States, 2005–2014. Drug Alcohol Depend. 185, 238–244. 10.1016/j.drugalcdep.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M, 2015. Assessing the overlap between tobacco and marijuana: trends in patterns of co-use of tobacco and marijuana in adults from 2003–2012. Addict. Behav 49, 26–32. 10.1016/j.addbeh.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Schauer GL, Hall CD, Berg CJ, Donovan DM, Windle M, Kegler MC, 2016. Differences in the relationship of marijuana and tobacco by frequency of use: a qualitative study with adults aged 18–34 years. Psychol. Addict. Behav 30, 406–414. 10.1037/adb0000172. [DOI] [PubMed] [Google Scholar]
- Schauer GL, King BA, McAfee TA, 2017. Prevalence, correlates, and trends in tobacco use and cessation among current, former, and never adult marijuana users with a history of tobacco use, 2005–2014. Addict. Behav 73, 165–171. 10.1016/j.addbeh.2017.04.023. [DOI] [PubMed] [Google Scholar]
- Searles JS, Helzer JE, Rose GL, Badger GJ, 2002. Concurrent and retrospective reports of alcohol consumption across 30, 90 and 366 days: interactive voice response compared with the timeline follow back. J. Stud. Alcohol 63, 352–362. 10.15288/jsa.2002.63.352. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M, 1996. First lapses to smoking: within-subjects analysis of real-time reports. J. Consult. Clin. Psychol 64, 366–379. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C, 2012. Characteristics and smoking patterns of intermittent smokers. Exp. Clin. Psychopharmacol 20, 264–277. 10.1037/a0027546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U, 2011. False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol. Sci 22, 1359–1366. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline follow-back. Measuring Alcohol Consumption. Springer, pp. 41–72. [Google Scholar]
- Soldz S, Huyser DJ, Dorsey E, 2003. The cigar as a drug delivery device: youth use of blunts. Addiction 98, 1379–1386. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE, 1994. Treating adult marijuana dependence: a test of the relapse prevention model. J. Consult. Clin. Psychol 62, 92. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L, 2000. Comparison of extended versus brief treatments for marijuana use. J. Consult. Clin. Psychol 68, 898. [PubMed] [Google Scholar]
- Subbaraman MS, Kerr WC, 2015. Simultaneous versus concurrent use of alcohol and cannabis in the national alcohol survey. Alcohol. Clin. Exp. Res 39, 872–879. 10.1111/acer.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaraman MS, Metrik J, Patterson D, Swift R, 2017. Cannabis use during treatment for alcohol use disorders predicts alcohol treatment outcomes: Cannabis and AUD treatment outcomes. Addiction 112, 685–694. 10.1111/add.13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), 2017. Results from the 2015 National Survey on Drug Use and Health: Summary of National Findings. http://www.samhsa.gov/. [PubMed]
- Tarter RE, Vanyukov M, Kirisci L, Reynolds M, Clark DB, 2006. Predictors of Marijuana Use in Adolescents Before and After Licit Drug Use: Examination of the Gateway Hypothesis. Am. J. Psychiatry 2134–2140. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, Patrick ME, 2018. Simultaneous alcohol and marijuana use among young adult drinkers: age-specific changes in prevalence from 1977 to 2016. Alcohol. Clin. Exp. Res 42, 2224–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, O’Malley PM, Johnston LD, 2014. Alcohol and marijuana use patterns associated with unsafe driving among US high school seniors: High use frequency, concurrent use, and simultaneous use. J. Stud. Alcohol Drugs 75, 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake DS, et al. , 2007. Progression from marijuana use to daily smoking and nicotine dependence in a national sample of U. S. adolescents. Drug Alcohol Depend 88, 272–281. [DOI] [PubMed] [Google Scholar]
- Tzilos GK, Reddy MK, Caviness CM, Anderson BJ, Stein MD, 2014. Getting higher: Co-occurring drug use among marijuana-using emerging adults. J. Addict. Dis 33, 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, McKee SA, 2017. An overview of alcohol and tobacco/nicotine interactions in the human laboratory. Am. J. Drug Alcohol Abuse 43, 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoir A, Eertmans A, Van den Bergh O, Van den Broucke S, 2006. Association of substance-use behaviours and their social-cognitive determinants in secondary school students. Health Educ. Res 22, 81–94. [DOI] [PubMed] [Google Scholar]
- Vinson DC, Reidinger C, Wilcosky T, 2003. Factors affecting the validity of a Timeline Follow-Back interview. J. Stud. Alcohol 64, 733–740. 10.15288/jsa.2003.64.733. [DOI] [PubMed] [Google Scholar]
- Wang JB, Cataldo JK, 2016. Medical marijuana legalization and co-use in adult cigarette smokers. Am. J. Health Behav. 40, 205–214. 10.5993/AJHB.40.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, Ramo DE, Lisha NE, Cataldo JK, 2016. Medical marijuana legalization and cigarette and marijuana co-use in adolescents and adults. Drug Alcohol Depend. 166, 32–38. 10.1016/j.drugalcdep.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington Traffic Safety Commission. Driver Toxicology Testing and the Involvement of Marijuana in Fatal Crashes.
- Weinberger AH, Platt J, Goodwin RD, 2016. Is cannabis use associated with an increased risk of onset and persistence of alcohol use disorders? A three-year prospective study among adults in the United States. Drug Alcohol Depend. 161, 363–367. 10.1016/j.drugalcdep.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Hockenberry J, Cummings JR, 2014. The Effect of Medical Marijuana Laws on Marijuana, Alcohol, and Hard Drug Use. NBER Work. Pap. Ser. http://www.nber.org/papers/w20085. [Google Scholar]
- White IR, Altmann DR, Nanchahal K, 2002. Alcohol consumption and mortality: modelling risks for men and women at different ages. BMJ 325, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Castle I-JP, Chen CM, Shirley M, Roach D, Hingson R, 2015Converging patterns of alcohol use and related outcomes among females and males in the United States, 2002 to 2012. Alcohol. Clin. Exp. Res 39, 1712–1726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.