Abstract
The elucidation of distinct protein conformers or states by fluorine (19F) NMR requires fluorinated moieties whose chemical shifts are most sensitive to subtle changes in the local dielectric and magnetic shielding environment. In this study we evaluate the effective chemical shift dispersion of a number of thiol-reactive trifluoromethyl probes [i.e. 2-bromo-N-(4-(trifluoromethyl)phenyl)acetamide (BTFMA), N-(4-bromo-3-(trifluoromethyl)phenyl)acetamide (3-BTFMA), 3-bromo-1,1,1-trifluoropropan-2-ol (BTFP), 1-bromo-3,3,4,4,4-pentafluorobutan-2-one (BPFB), 3-bromo-1,1,1-trifluoropropan-2-one (BTFA), and 2,2,2-trifluoroethyl-1-thiol (TFET)] under conditions of varying polarity. In considering the sensitivity of the 19F NMR chemical shift to the local environment, a series of methanol/water mixtures were prepared, ranging from relatively non-polar (MeOH:H2O = 4) to polar (MeOH:H2O = 0.25). 19F NMR spectra of the tripeptide, glutathione ((2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)carbamoyl]-2-sulfanylethyl]carbamoyl}butanoic acid), conjugated to each of the above trifluoromethyl probes, revealed that the BTFMA tag exhibited a significantly greater range of chemical shift as a function of solvent polarity than did either BTFA or TFET. DFT calculations using the B3LYP hybrid functional and the 6-31G(d,p) basis set, confirmed the observed trend in chemical shift dispersion with solvent polarity.
Keywords: 19F NMR, Chemical tagging, Chemical shift dispersion, BTFMA, TFET, BTFA
Introduction
While more than one hundred elements are NMR active, the fluorine nucleus has the distinction of possessing one of the highest gyromagnetic ratios, and thus, greatest sensitivity for NMR, next to tritium and 1H nuclei. 19F NMR also exhibits a remarkable range of chemical shift; common fluorinated organics span a chemical shift range of ˜ 1100 ppm (Dolbier 2009). For this reason fluorine NMR has had a significant impact in fragment based drug discovery and protein structure function studies (Gakh et al. 2000; Gerig 1994; Dalvit 2009; Dalvit and Vulpetti 2011; Didenko et al. 2013; Kitevski-LeBlanc and Prosser 2012; Yu et al. 2013). In 19F NMR studies of proteins, fluorinated amino acid derivatives (i.e. tryptophan, phenylalanine, tyrosine, and methionine) are commonly incorporated by biosynthetic means, in which case, the chemical shift dispersion arises not from variations of chemical structure but rather from differences in local secondary and tertiary structure, solvent exposure, and conformational dynamics (Gerig 1994). There are examples of protein studies where 19F NMR resonances arising from fluorophenylalanine (Gerig et al. 1983) and fluorotryptophan (Lian et al. 1994) span as much as 10–20 ppm, reflecting the sensitivity of the chemical shift to environment. It is this exquisite sensitivity to environment that we generally wish to exploit in many protein NMR studies, for purposes of delineating distinct conformers or states, and, through relaxation experiments, their interconversion rates and lifetimes.
In this study we compare the chemical shift sensitivity of a number of common cysteine-specific trifluoromethyl (–CF3) probes whose purpose is to monitor conformers and states of proteins of interest. In each case, these probes are incorporated into the protein through 1- or 2-step reactions under non-denaturing aqueous conditions, as described elsewhere and in the “Materials and methods” section. Trifluoromethyl probes are a preferred form of NMR tag for 19F NMR studies of proteins, given their three-fold (or more) amplification in the signal to noise ratio resulting from three equivalent nuclei and the fact that fast methyl rotation further averages the chemical shift anisotropy (CSA). Since 19F NMR relaxation is often dominated by CSA at high magnetic fields, the use of probes with relatively small CSA is key to the study of protein states, particularly in cases where the protein of interest is on the order of 100 residues or more. 19F NMR studies of large proteins, such as G protein coupled receptors (GPCRs), are further complicated by both slow tumbling of the protein of interest, intermediate timescale exchange, and low sample concentrations which necessitates the use of CF3 tags rather than potential mono- or difluorinated species. It is also possible, for purposes of further enhancing the signal to noise ratio, to resort to sulfhydryl specific probes, consisting of higher numbers of equivalent fluorine nuclei, such as perfluoro tert-butyl moieties (Kalbitzer et al. 1992) and even larger structures (Janjic and Ahrens 2009). In this case, the challenge becomes avoiding perturbation to the states or conformations of interest in the protein. In many cases, this can be assessed either through functional assays or by 15N,1H spectroscopy or circular dichroism, which provides a global perspective of the state of the protein before and after labeling.
In situations employing a cysteine-specific trifluoromethyl 19F NMR probe, we consider whether some CF3 probes might be better than others. In the context of defining states, this question amounts to an issue of chemical shift sensitivity, which may loosely be defined as the maximum possible range of chemical shifts accessible to the tag, as a function of environment, divided by the line width. Thus, a relatively small CSA term, a long transverse relaxation time (T2), and a sensitivity of chemical shift to environment, are required.
Fluorine chemical shifts are known to be strongly influenced by long-range electrostatic effects (Pearson et al. 1993) where solvent accessibility, van der Waals interactions, and local magnetic effects from aromatics (ring currents) and electronically anisotropic species all contribute to difference in shielding at the fluorine nucleus. In particular, electrostatic effects and van der Waals interactions couple with the fluorine lone pair electrons resulting in significant modulation of the paramagnetic shielding term (Lau and Gerig 2000). For example, an adjacent carbonyl moiety typically exerts strong shielding effects on the fluorine nuclei of the trifluoromethyl group (Sloop 2013). Other moieties may contribute to the effective partial charge of the CF3 group, thereby altering shielding of the fluorine nuclei. One might further speculate that the polarizability and molecular geometry of species adjacent to the CF3 group could in turn influence the range of shielding effects and thus the sensitivity of the CF3 moiety to environment. A second possibility is that the nearby atoms, constituting the trifluoromethyl probe physically shield the CF3 group from solvents or the local environment, thereby reducing the overall sensitivity of the probe to environment. These arguments are also relevant to the design of unnatural fluorinated amino acids. In particular, trifluoromethyl-L-phenylalanine has been touted as a sensitive site-specific probe, which can be site-specifically incorporated in proteins expressed in E. coli via a TAG nonsense codon (Jackson et al. 2007; Li et al. 2010).
In the current study, we compare the sensitivity of the observed 19F NMR chemical shift to polarity for a variety of CF3 probes. Polarity is controlled by varying the ratio of methanol to water in mixtures. Significant differences in the chemical shift dispersion are observed. To ascertain the possible origin of these differences we employed Density Functional Theory (DFT) calculations to corroborate the dependence of the chemical shift on polarity while estimating the partial charge on the fluorine atoms and the carbon atom of the CF3 tag, in the hopes of identifying a correlation.
Results and discussion
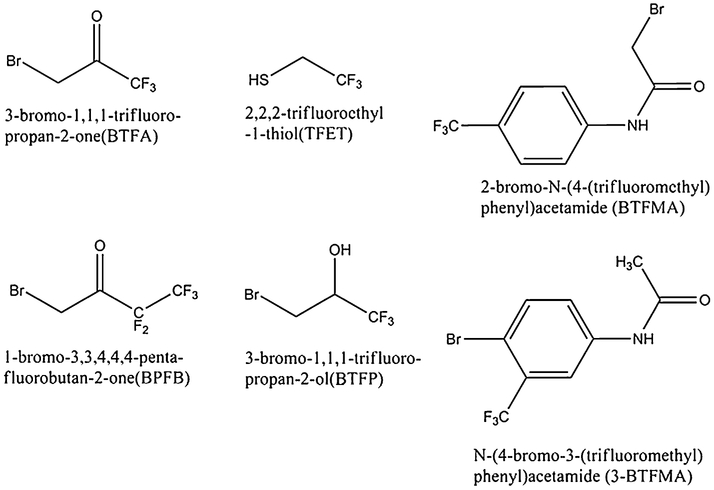
To systematically evaluate spectroscopic features, T2 relaxation and 19F NMR chemical shift sensitivity to environment, we consider a variety of thiol reactive trifluoromethyl probes, shown in Fig. 1. BTFA and TFET represent two commonly used 19F NMR probes that are expected to minimally perturb target proteins due to their relatively small size. With the exception of TFET, all of the probes may be conjugated to free thiols in a single reaction step. Conjugation of TFET to proteins generally involves an additional step where the free thiol(s) are first reacted with dithiopyridine, a relatively bulky moiety which would be expected to preferentially react only with the most exposed cysteine residue(s), possibly avoiding attack of largely-buried cysteines or disulfide linkages (Klein-Seetharaman et al. 1999). One disadvantage of TFET is that the resulting disulfide linkage is somewhat vulnerable to reduction in the presence of reducing agents. BTFA is an equally popular trifluoromethyl tag and may have the advantage that the S-C bond established following reaction with thiols and thiolates is stable (Luchette et al. 2002). Note that the trifluoromethyl moiety in BTFA possesses no scalar coupled protons, thus reducing possible line broadening through dipolar relaxation and obviating the need for 1H decoupling.
Fig. 1.
Structures of cysteine-specific trifluoromethyl probes used in this study
A variety of other commercially available trifluoromethyl moities, depicted in Fig. 1, are expected to provide a range of effects on the fluorine valence electron density, and thus sensitivity to chemical shift. In particular, note that in the case of BTFMA and 3-BTFMA, the trifluoromethyl substituent is directly conjugated to the phenyl group. The delocalized electron density of the aromatic system is highly polarizable and thus, sensitive to environment. The polarizability of the phenyl moiety adjacent to the CF3 group would therefore be expected to contribute to a greater range of shielding effects on the fluorine nuclei, depending on environment. Furthermore, ring current effects are expected to contribute additional shielding/deshielding effects to the 19F nuclei of the trifluoromethyl group. BTFMA and 3-BTFMA are comparable in size to current nitroxide spin-labels used in ESR applications. This might be advantageous in a study involving both NMR and ESR since possible steric perturbations would at least be similar in both cases.
To examine the potential chemical shift sensitivity of the probes, shown in Fig. 1, all were first conjugated to a small test peptide, glutathione, or ((2S)-2-amino-4-{[(1R)-1-[(car-boxymethyl)carbamoyl]-2-sulfanylethyl]carbamoyl}butanoic acid) via the cysteine sulfhydryl, using previously published protocols. 19F NMR spectra of the 19F-labeled peptides were then separately recorded in solvents of varying polarity. Since our interest is in studying proteins by 19F NMR under aqueous conditions, we elected to make use of water combined with methanol over a variety of concentrations such that the water to MeOH ratio ranged from 0.25 to 4.0. MeOH has a dielectric constant of 32.7 and that of water is 80.1, while both solvents are completely miscible. Thus, mixtures of the two solvents provide a range of polar environments while still solubilizing the peptide. While there are many definitions of polarity, we make use of the well-known Py scale for polarity, which is based on the vibronic fine structure of pyrene fluorescence (Dong and Winnik 1982, 1984). Pure water exhibits a Py polarity index of 1.87, while the corresponding value for MeOH is 1.35. Moreover, the Py polarity values for MeOH/water mixtures have been previously determined and were therefore used in the current study (Street and Acree 1986). 19F NMR spectra for the lowest and highest MeOH fractions for each of the probes considered are shown in Supplementary Figure S1 and corresponding chemical shifts as a function of methanol concentration are reported in the Supplementary Table 2.
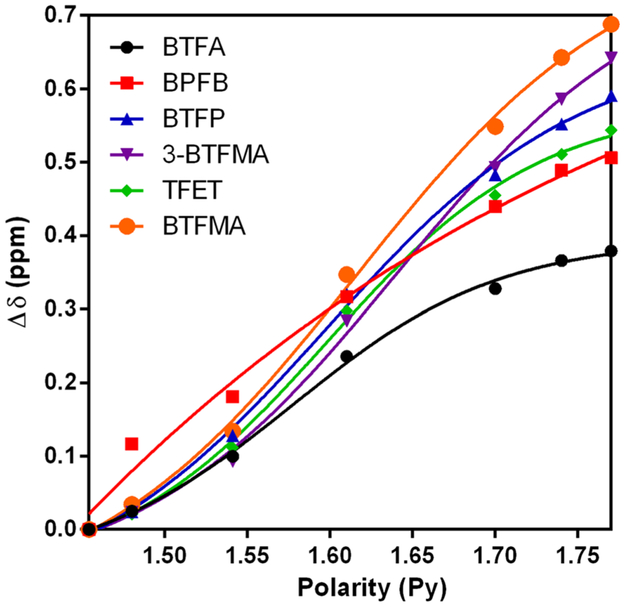
Figure 2 reveals the change in the observed 19F NMR chemical shifts as a function of polarity. Note that the chemical shift changes are defined with respect to the observed shifts for the least polar solvent mixture (i.e. MeOH/ H2O = 4, Py = 1.454). While all of the trifluoromethy1 probes reveal a sensitivity of chemical shift to polarity, both probes possessing a CF3 group directly conjugated to an aromatic, exhibit the greatest chemical shift range by a significant margin over BTFA. Observed changes in chemical shift were fit to the formula Δδ = A/(1 + exp(—(Py — x0)/w)), where A, x0, and w represent fitting parameters, and are presented in Supplemental Table 1, for each probe. In employing this fitting function, A represents the magnitude of the chemical shift change, while 1/w is a measure of steepness of the observed profile. BTFMA clearly exhibits the greatest extent of change in chemical shift (A) over the range of polarities investigated.
Fig. 2.
Changes in 19F chemical shift (Δδ) of various CF3 tags as a function of solvent polarity. Note that all changes are referenced to the most hydrophobic solution investigated (i.e. MeOH/H2O = 4.0 or Py = 1.454). Observed changes in chemical shift were fit to the formula Δδ = A/(1 + exp(—(Py — x0)/w)), where A, x0, and w represent fitting parameters, for each probe
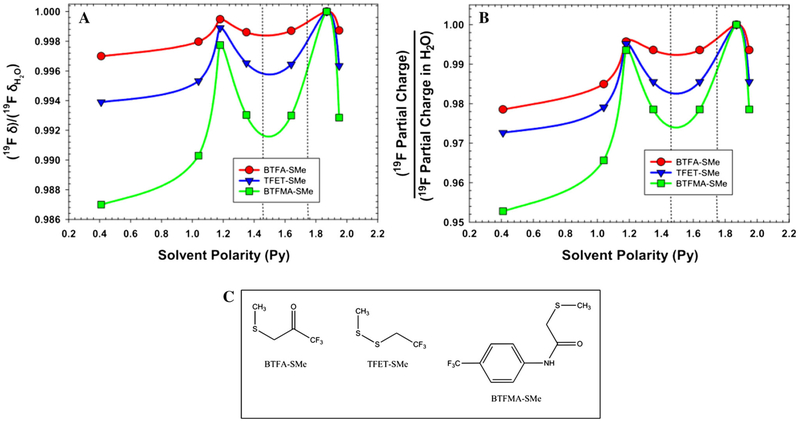
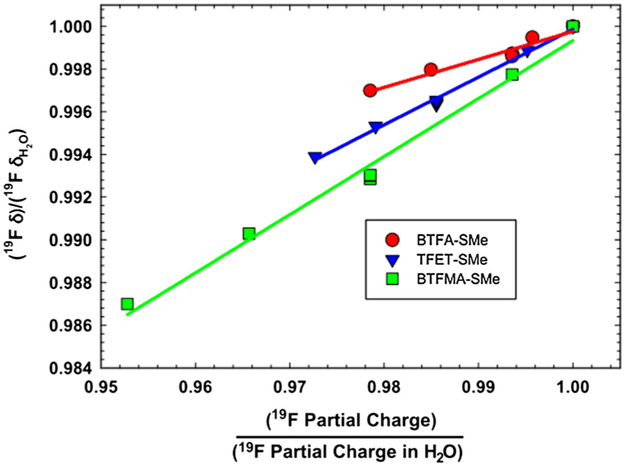
To understand the origin of our experimental results, Density Functional Theory (DFT) calculations of the average 19F NMR chemical shift were performed on three trifluoromethy1 tags, anticipated to be most useful for labeling cysteine residues in NMR studies—namely, BTFA, TFET and BTFMA. To facilitate the DFT calculations, the above probes were each assumed to be conjugated to methanethiol, rather than glutathione, as described in the “Materials and methods” section. Using the B3LYP hybrid functional and the 6-31G(d,p) basis set, isotropic 19F NMR chemical shifts were estimated for each of the three tags for the vapor phase (Py = 0.4) and for solvents of varying polarity. These chemical shifts were then normalized to the predicted shifts in water, as shown in Fig. 3a. There are two clear trends that are apparent from the simulations in Fig. 3 and are most evident in the case of BTFMA. Firstly, larger relative chemical shift differences seem to occur under conditions of lower solvent polarity. Secondly, protic solvents (namely ethanol, for which Py = 1.18, and water, for which Py = 1.87) exhibit very pronounced maxima. As is clear from Fig. 3b, there also appears to be a direct correspondence between the partial charge on the 19F atom and the observed shift. This correlation is further illustrated in Fig. 4. Partial charge is expected to be a consequence of solvent polarity and the chemical structure of the probe itself. As evidenced by the DFT calculations, the 19F atoms on the CF3 moiety associated with BTFMA, exhibit the greatest range of partial charges as a function of polarity (solvent) and correspondingly, the greatest range of 19F NMR chemical shifts.
Fig. 3.
a DFT-based normalized chemical shifts for three CF3 tags conjugated to methanethiol (shown in c). Isotropic NMR shifts for each of the tags were calculated using the B3LYP hybrid functional and the 6-31G(d,p) basis set, under six different solvent conditions and one vapor phase [left to right: vapor phase (Py = 0.4), toluene (Py = 1.04), ethanol (Py = 1.18), methylene chloride (Py = 1.35), acetone (Py = 1.64), water (Py = 1.87), and dimethyl sulfoxide (Py = 1.95)]. b DFT-based normalized average partial charges for 19F atoms for each of the above three CF3 tags, as a function of solvent polarity. The partial charges for each of the 19F atoms in the CF3 group were averaged assuming isotropic conditions and fast methyl rotations and then normalized to the average 19F partial charge for the conjugate in H2O. Dotted lines indicate the polarity region assessed experimentally by NMR via MeOH/H2O solvent mixtures (Fig. 2). c Structure of the three methanethiol conjugates for which DFT calculations were performed
Fig. 4.
Correlation between normalized 19F chemical shifts and normalized 19F electrostatic partial charges. The slope of each plot reflects the extent of 19F shift changes for a given change in partial charge on the fluorine. BTFMA exhibits the greatest chemical shift change for a given change in partial charge. Each point corresponds to a chemical shift and partial electrostatic charge for a given solvent (or solvent polarity)
We emphasize that there are many choices of polarity and the Py scale, used in this paper is one of many. Supplementary Figure S2 shows normalized shifts for the same solvents introduced in Fig. 3, using a different polarity scale. In this case, the trend in shift with polarity appears more monotonic. Our goal in this paper was simply to determine differences or sensitivities in shift to changes in polarity or solvent and the exact shape of the curve is outside the scope of this paper. It is also important to recognize that the calculated chemical shift and partial charge profiles for BTFMA, BTFA, and TFET, shown in Fig. 3, explore a wide range of solvents and a correspondingly wide range of polarities. In contrast, the experimental results shown in Fig. 2, which depict chemical shift sensitivity to polarity for a series of probes, were determined only in methanol/water mixtures over a more limited range of polarities. Moreover, between Py values of 1.454 and 1.78, the experimental and DFT-based results are consistent. The main point is that the computational studies corroborate the observation that BTFMA exhibits a greater sensitivity of 19F NMR chemical shift to polarity, suggesting that the DFT-approach to predicting chemical shift sensitivity could be used as a general method for screening chemical tags for favorable NMR properties.
Another potentially important criteria in the selection of appropriate fluorinated tags for protein structure studies involves T2 relaxation. To evaluate the relative effectiveness of the probes of interest for studies of larger proteins, T2 was measured via a Hahn Echo sequence, after first dissolving the glutathione-fluorinated tag conjugate in 80 % aqueous glycerol, which effectively simulates an environment in which the conjugate exhibits a rotational correlation time similar to a protein of 12 kDa in size. T2 was found to be between 30 and 50 ms for all probes shown in Fig. 1. Thus the trifluoromethy1 probes appear to exhibit similar T2 relaxation times.
Conclusions and final remarks
The above studies were in part motivated by observations from prior and current 19F NMR studies of detergent-stabilized membrane receptor, β2AR, in which a specific cysteine moiety (Cys 265) was tagged by BTFA (Kim et al. 2013), TFET (Liu et al. 2012), or BTFMA (Manglik et al. 2015). In our hands we observed dramatically improved chemical shift dispersion upon substituting BTFA with BTFMA (Manglik et al. 2015). Model studies and DFT studies in this paper confirm these observations and suggest that the CF3 group conjugated to the aromatic should give rise to substantially improved chemical shift sensitivity over more conventional thiol-specific trifluoromethyl tags. Thus, the protein studies do seem to reflect what we observe in the model studies and explain through DFT calculations. Clearly, other factors are involved in determining the chemical shift. Nonetheless, solvent polarity is a relatively easy way of affecting changes in the model system and thus, assessing chemical shift sensitivity to electrostatics, which we know play a role in 19F NMR. Moreover, the correspondence between observed chemical shift measurements in MEOH/water and the DFT simulations suggest that much insight into the potential of next generation fluorinated tags could be obtained by computational and experimental studies such as those outlined above.
Note that other classes of thiol-specific fluorine tags would be expected to exhibit greater chemical shift sensitivity than the trifluoromethyl tags discussed herein. DFT simulations of monofluoro-phenylalanine and monofluoro-tryptophan predict that the chemical shift sensitivity to solvent polarity should be 2–3 times greater than that observed with even the best CF3 tag (BTFMA), although T2 of flouroaromatics is significantly shorter due to the CSA. One might therefore predict that a thiol-specific monofluoroaromatic probe would be very useful in terms of chemical shift sensitivity. DFT calculations confirm that the solvent induced change in partial charge is greatest in fluoroaromatics. However, spectroscopic studies of GPCRs, such as those discussed above, are typically on the threshold of detection using current instruments. High concentrations of purified receptor were difficult to obtain and reported protein concentrations ranged between 20 and 150 μM. At these concentrations, monofluorinated tags are currently impractical for the study of large proteins, particularly where intermediate timescale exchange processes are known to take place.
Materials and methods
Reduced Glutathione (GSH) was conjugated with the following tags as described below. Note that no purification steps were performed upon coupling the tag of interest to GSH. Reactions were therefore performed under conditions of ten-fold molar excess peptide to assure product, which was generally confirmed by mass spectroscopy and NMR.
BTFA. GSH was dissolved in 5 mL phosphate buffered saline (PBS) buffer, pH 7.4, to a final concentration of 10 mM, followed by the addition of BTFA to a final concentration of 1 mM. The mixture was heated to 65 °C for 5 min and the reaction was allowed to continue overnight at room temperature.
BPFB, BTFP, 3-BTFMA and BTFMA. GSH was dissolved in 5 mL PBS, pH 7.4, to a final concentration of 10 mM followed by the addition of the tag to a final concentration of 1 mM. The reaction mixture was then incubated at room temperature overnight without heating.
TFET. The reaction was completed in two steps. 2,2′-dipyridyldisulfide (DPS) was added to 10 mM GSH (pH 7.4 in PBS buffer) in a final concentration of 5 mM, and the mixture was left at room temperature for 6 h in the dark. Subsequently, the TFET was added into solution with a final concentration of 1 mM, and the reaction was allowed to continue overnight at room temperature, thereby replacing the DPS moiety by TFET.
NMR
A series of MeOH/water mixtures were prepared, ranging from MeOH/water (v/v) 4:1 through 1:4. In all cases the labeled peptide was observed to be soluble. NMR experiments were performed on a 600 MHz (1H Larmor frequency) Varian Inova spectrometer equipped with a cryogenic probe capable of 19F NMR spectroscopy, and the chemical shift sensitivity measurements were performed at 35 °C. T2 relaxation times were measured using a standard Hahn echo experiment, using a repetition time of 2 s, and 12–15 echo delay times. Spectra were processed with MestReNova 9.0.1 software, and 19F NMR peaks were referenced to NaF (−122.25 ppm).
LC-MS
LC-MS was used to monitor the extent of conjugation. The sample was diluted into 50 % methanol solution with final concentration of 100 μM supplemented with 0.1 % TFA, and centrifuged at 5000g for 10 min to remove possible precipitated solute/contaminate prior to LC–MS. The flow rate was set to 40 μL/min, and the spectra were acquired with 30 scans with a range of M/Z between 100 and 900 to confirm the conjugation between 19F tag and GSH.
Quantum-mechanical (QM) methods
All calculations were performed using Spartan’10 (Hehre 2003; Shao et al. 2006). Structures of methanethiol (–SMe) conjugates of BTFA, TFET, and BTFMA were first energy minimized using Spartan’s built-in molecular mechanics minimization method. Equilibrium conformers were then predicted via ab initio calculations using the B3LYP hybrid functional with the 6-31G(d) basis set, which has been shown to yield reasonably accurate molecular geometries (Baus-chlicher and Partridge 1995). DFT calculations using B3LYP and 6-31G(d,p) were used to obtain isotropic NMR chemical shits and atomic partial charges (electrostatic) for each of the structures studied, under six different solvent conditions (water, ethanol, methylene chloride, toluene, acetone, and dimethyl sulfoxide). For the trifluoromethyl groups, isotropic chemical shifts for each fluorine nucleus were averaged and normalized to the average isotropic shift determined using water as the solvent. The same was done for 19F electrostatic partial charges. Normalized values were plotted along a solvent coordinate (Py-scale) according to increasing polarity (Dong and Winnik 1982, 1984).
Supplementary Material
Acknowledgments
We would like to thank the Natural Sciences and Engineering Research Council of Canada for support of this research. Supported by NSERC research discovery award (Grant No. 261980).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10858-015-9922-y) contains supplementary material, which is available to authorized users.
References
- Bauschlicher CW, Partridge H (1995) The sensitivity of B3LYP atomization energies to the basis-set and a comparison of basis-set requirements for CCSD(T) and B3LYP. Chem Phys Lett 240:533–540 [Google Scholar]
- Dalvit C (2009) NMR methods in fragment screening: theory and a comparison with other biophysical techniques. Drug Discov Today 14:1051–1057 [DOI] [PubMed] [Google Scholar]
- Dalvit C, Vulpetti A (2011) Fluorine-protein interactions and 19F NMR isotropic chemical shifts: an empirical correlation with implications for drug design. ChemMedChem 6:104–114 [DOI] [PubMed] [Google Scholar]
- Didenko T, Liu JJ, Horst R, Stevens RC, Wührich K (2013) Fluorine-19 NMR of integral membrane proteins illustrated with studies of GPCRs. Curr Opin Struct Biol 23:740–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolbier WR (2009) Guide to fluorine NMR for organic chemists. Wiley, Hoboken [Google Scholar]
- Dong DC, Winnik MA (1982) The Py scale of solvent polarities. Solvent effects on the vibronic fine structure of pyrene fluorescence and empirical correlations with ET and Y values. Photochem Photobiol 35:17–21 [Google Scholar]
- Dong DC, Winnik MA (1984) The Py scale of solvent polarities. Can J Chem Rev Can De Chim 62:2560–2565 [Google Scholar]
- Gakh YG, Gakh AA, Gronenborn AM (2000) Fluorine as an NMR probe for structural studies of chemical and biological systems. Magn Reson Chem 38:551–558 [Google Scholar]
- Gerig JT (1994) Fluorine NMR of proteins. Prog Nucl Magn Reson Spectrosc 26:293–370 [Google Scholar]
- Gerig JT, Klinkenborg JC, Nieman RA (1983) Assignment of fluorine nuclear magnetic-resonance signals from rabbit cyanomethe-moglobin. Biochemistry 22:2076–2087 [DOI] [PubMed] [Google Scholar]
- Hehre WJ (2003) A guide to molecular mechanics and quantum chemical calculations. Wavefunction Inc, Irvine [Google Scholar]
- Jackson JC, Hammill JT, Mehl RA (2007) Site-specific incorporation of a 19F-amino acid into proteins as an NMR probe for characterizing protein structure and reactivity. J Am Chem Soc 129:1160–1166 [DOI] [PubMed] [Google Scholar]
- Janjic JM, Ahrens ET (2009) Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip Rev Nanomed Nanobiotech-nol 1:492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbitzer HR, Rohr G, Nowak E, Goody RS, Kuhn W, Zimmermann H (1992) A new high-sensitivity 19F probe for labeling cysteine groups of proteins. NMR Biomed 5:347–350 [DOI] [PubMed] [Google Scholar]
- Kim TH, Chung KY, Manglik A, Hansen AL, Dror RO, Mildorf TJ, Shaw DE, Kobilka BK, Prosser RS (2013) The role of ligands on the equilibria between functional states of a G protein-coupled receptor. J Am Chem Soc 135:9465–9474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitevski-LeBlanc JL, Prosser RS (2012) Current applications of 19F NMR to studies of protein structure and dynamics. Prog Nucl Magn Reson Spectrosc 62:1–33 [DOI] [PubMed] [Google Scholar]
- Klein-Seetharaman J, Getmanova EV, Loewen MC, Reeves PJ, Khorana HG (1999) NMR spectroscopy in studies of light-induced structural changes in mammalian rhodopsin: applicability of solution 19F NMR. Proc Natl Acad Sci USA 96:13744–13749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau E, Gerig J (2000) Origins of fluorine NMR chemical shifts in fluorine-containing proteins. J Am Chem Soc 122:4408–4417 [Google Scholar]
- Li C, Wang G, Wang Y, Creager-Allen R, Lutz EA, Scronce H, Slade KM, Ruf RAS, Mehl RA, Pielak GJ (2010) Protein 19F NMR in Escherichia coli. J Am Chem Soc 132:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian CY, Lian CY, Le HB, Montez B, Patterson J, Harrell S, Laws D, Matsumura I, Pearson J, Oldfield E (1994) 19F nuclear-magnetic-resonance spectroscopic study of fluorophenylalanine-labeled and fluorotryptophan-labeled avian egg-white lysozymes. Bio-chemistry 33:5238–5245 [DOI] [PubMed] [Google Scholar]
- Liu JJ, Horst R, Katritch V, Stevens RC, Wüthrich K (2012) Biased signaling pathways inβ2-adrenergic receptor characterized by 19F-NMR. Science 335:1106–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchette P, Prosser R, Sanders C (2002) Oxygen as a paramagnetic probe of membrane protein structure by cysteine mutagenesis and 19F NMR spectroscopy. J Am Chem Soc 124:1778–1781 [DOI] [PubMed] [Google Scholar]
- Manglik A, Kim TH, Masureel M, Altenbach C, Yang Z, Hilger D, Lerch MT, Kobilka TS, Thian FS, Hubbell WL, Prosser RS, Kobilka BK (2015) Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JG, Oldfield E, Lee FS, Warshel A (1993) Chemical shifts in proteins—a shielding trajectory analysis of the fluorine Nuclear-Magnetic Resonance spectrum of the Escherichia coli galactose binding protein using a multipole shielding polarizability local reaction field molecular-dynamics approach. J Am Chem Soc 115:6851–6862 [Google Scholar]
- Shao Y et al. (2006) Advances in methods and algorithms in a modern quantum chemistry program package. Phys Chem Chem Phys 8:3172–3191 [DOI] [PubMed] [Google Scholar]
- Sloop JC (2013) 19-Fluorine nuclear magnetic resonance chemical shift variability in trifluoroacetyl species. Rep Org Chem 3:1–12 [Google Scholar]
- Street KW, Acree WE (1986) The Py solvent polarity scale—binary solvent mixtures used in reversed-phase liquid-chromatography. J Liq Chromatogr 9:2799–2808 [Google Scholar]
- Yu JX, Hallac RR, Chiguru S, Mason RP (2013) New frontiers and developing applications in 19F NMR. Prog Nucl Magn Reson Spectrosc 70:25–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.