Abstract
Despite the increasing epidemic of diabetes mellitus affecting populations at different life stages, the global burden of gestational diabetes mellitus (GDM) is not well assessed. Systematically synthesized data on global prevalence estimates of GDM are lacking, particularly among developing countries. The hyperglycemic intrauterine environment as exemplified in pregnancies complicated by GDM might not only reflect but also fuel the epidemic of type 2 diabetes mellitus (T2DM). We comprehensively reviewed available data in the past decade in an attempt to estimate the contemporary global prevalence of GDM by country and region. We reviewed the risk of progression from GDM to T2DM as well. Synthesized data demonstrate wide variations in both prevalence estimates of GDM and the risk of progression from GDM to T2DM. Direct comparisons of GDM burden across countries or regions are challenging given the great heterogeneity in screening approaches, diagnostic criteria, and underlying population characteristics. In this regard, collaborative efforts to estimate global GDM prevalence would be a large but important leap forward. Such efforts may have substantial public health implications in terms of informing health policy makers and healthcare providers for disease burden and for developing more targeted and effective diabetes prevention and management strategies globally.
Keywords: Gestational diabetes, Prevalence, Screening, Diagnosis, Type 2 diabetes, Pregnancy
Introduction
Due to the epidemiologic transition of the population towards aging and more sedentary lifestyle related to urbanization during the past few decades, the prevalence of type 2 diabetes mellitus (T2DM) has been rapidly increasing and the age of onset becomes younger globally [1]. Many developing countries are currently suffering from the increasing burden of T2DM and comorbidities which used to predominantly burden developed countries. Concurrently, the prevalence of gestational diabetes mellitus (GDM), one of the most common pregnant complications, has increased by more than 30 % within one or two decades in a number of countries including developing countries [2, 3], forming an emerging worldwide epidemic [4•].
GDM has been related to substantial short- and long-term adverse health outcomes, such as increased risk of developing cardio-metabolic disorders later in life among both women and their offspring [5, 6••]. Therefore, it is of great public health significance to understand the burden of GDM in a global scope. However, easily accessible and systematically organized data on estimates of global prevalence of GDM are lacking. In particular, data on estimates of GDM prevalence in developing countries are relatively scant. Furthermore, lack of consensus and uniformity in the screening standards, definition, and diagnosis criteria of GDM challenges the comparative assessment of the GDM prevalence across countries and regions.
GDM, defined as any degree of glucose intolerance with onset of first recognition during pregnancy [7], may inevitably include undiagnosed pre-existing T2DM. Therefore, the increasing prevalence of GDM is considered to reflect the underlying T2DM epidemic in the population. On the other hand, women with a history of GDM may be imposed to a sevenfold increased risk of T2DM in later life [6••]. Therefore, the progression of GDM to T2DM may be in turn fueling the T2DM epidemic. Despite the recognition of racial/ethnic disparities in prevalence and disease burden of GDM and T2DM, the geographic and racial/ethnic variation in progression from GDM to T2DM has not been well assessed.
In the present review, we provide an overview of the global prevalence of GDM based on data published in the past decade and discuss the methodological challenges in estimating the global burden. In addition, we discuss contributing factors related to the geographic variation in GDM prevalence and assess the adverse health implications of GDM with respect to its progression to T2DM from a global perspective.
Global Prevalence of GDM by Geographic Regions and Countries
Literature Search
A literature search was conducted in PubMed supplemented by cross-checking relevant references of eligible studies on the prevalence of GDM in the past decade from January 1, 2005 to August 1, 2015, to reflect the contemporary disease prevalence, without language restrictions. Search term combinations were “diabetes, gestational” as a MeSH term or text word, “prevalence” as a MeSH term, and “epidemiology,” “prevalence,” “trend,” or “screening” as a text word. These search criteria yielded 3357 articles, of which titles and abstracts were reviewed to determine the relevance to the research objective (phase 1). In phase 2, full texts of 214 articles were reviewed to extract data on study design, sample size, location, year, sampling strategy, screening approach, diagnostic criteria, prevalence of GDM, and subject characteristics. If duplicate studies on the same study population were identified, the most recently published one was selected. A scoring system developed by the International Diabetes Federation with four domains [sampling (single-hospital, local or multi-site, regional, national; score ranged 0–3), data sources (observational/records, extrapolated/predicted; score ranged 0–1), ascertainment of GDM status (criteria-based, self-reported/record-based; score ranged 0–1), and study year (<2000, 2000–2005, >2005; score ranged 0–2)] was used to assess the quality of each eligible study [8]. In phase 3, we applied the following exclusion criteria for further synthesis: (1) reviews, editorials, comments, letters, and news articles; (2) no specified criteria for diagnosis of GDM; (3) exclusive focus on a particular population (e.g., adolescent pregnancies); (4) studies included women with pre-existing diabetes before the index pregnancy; and (5) studies with a quality score of three or less. Finally, 77 studies with a mean quality score of 4.6 (SD 0.8) were selected. A complete list of the included studies and extracted data is available upon request.
Results of the Literature Search
Among the eligible 77 studies meeting the search criteria, data from a total of 36 countries were included to derive country-specific estimates for GDM prevalence. Some countries such as USA (n=11), Canada (n=4), Australia (n=4), China (n=4), and India (n=3) have multiple published prevalence studies based on various study populations and diagnostic criteria, whereas others (mostly developing countries in Africa and South/Central America) have few published estimates. If more than one estimate of GDM prevalence was available for one country, the country-specific prevalence of GDM was estimated by using the median of all available source data. Likewise, the region-specific prevalence of GDM was estimated by calculating the median prevalence of country-specific estimates within each World Health Organization (WHO) region.
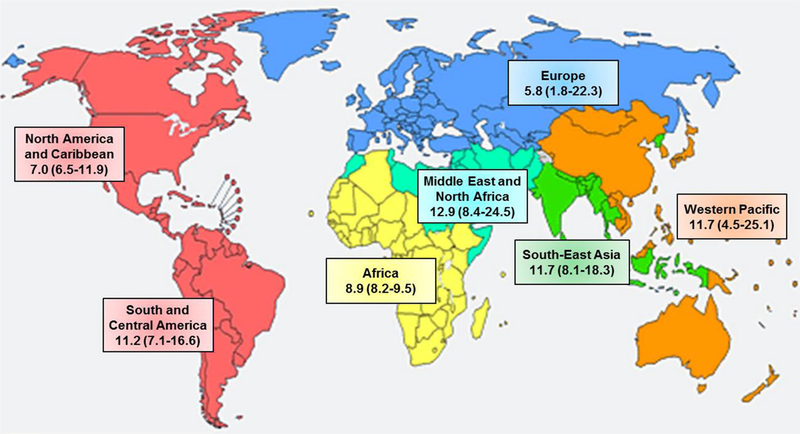
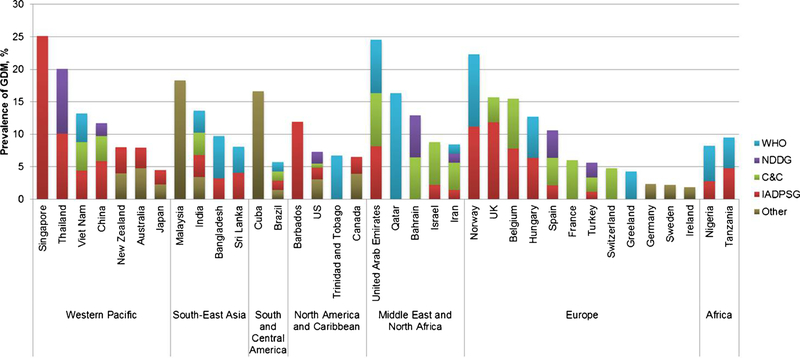
Overall, Middle East and North Africa had the highest prevalence of GDM with a median estimate of 12.9 % (range 8.4–24.5 %), followed by Southeast Asia, Western Pacific, South and Central America, Africa, and North America and Caribbean (median prevalence 11.7, 11.7, 11.2, 8.9, and 7.0 %, respectively), whereas Europe had the lowest prevalence (median 5.8 %; range 1.8–22.3 %) (Fig. 1). Considering the fact that prevalence estimates are subject to the diagnosis criteria applied, we specified diagnosis criteria when describing country-specific estimates of GDM prevalence (Fig. 2). Considerable variations were observed both within and between countries. Within each country, the proportion of which the prevalence estimates were attributable to each major diagnostic criterion was indicated by different colors. Within the Western Pacific region, the prevalence estimates had a wide range from 4.5 % in Japan to 25.1 % in Singapore, to which different GDM diagnosis criteria were applied. The former was based on both the Japan Society of Gynecology and Obstetrics 1984 criteria [10] and the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria [11], whereas the latter was solely based on the IADPSG criteria. Similarly, countries in Europe also had large variations in estimates of GDM prevalence, with Norway leading the prevalence (median 22.3 %; range 13.0–31.5 % using the WHO 1999 [12] and modified IADPSG criteria, respectively) and Ireland having the lowest prevalence of 1.8 % (using the National Institute for Health and Care Excellence criteria [13]). In contrast, countries in the North America and Caribbean region (i.e., Barbados, USA, Trinidad and Tobago, and Canada) had relatively the least variability in estimates of GDM prevalence, ranging from 6.5 % in Canada (mostly diagnosed by the Canadian Diabetes Association criteria [14]) to 11.9 % in Barbados (by the IADPSG criteria). In the Middle East and North Africa region which had on average the highest prevalence of GDM, the prevalence estimates ranged from 8.4 % in Iran to 24.5 % in United Arab Emirates, whereas Qatar, Bahrain, and Israel had intermediate estimates (i.e., 16.3, 12.9, and 8.8 %, respectively). In Southeast Asia, Malaysia had the highest prevalence of 18.3 %, followed by India (13.6 %), Bangladesh (9.7%), and Sri Lanka(8.1%). In South and Central America, data on GDM prevalence were only available in two countries (16.6 and 5.7 % in Cuba and Brazil, respectively).Likewise, only two countries in Africa had qualified and available data on prevalence of GDM (i.e., 8.2 % in Nigeria and 9.5 % in Tanzania), which used both the WHO and IADPSG criteria, respectively.
Fig. 1.
Median (interquartile range) prevalence (%) of GDM by WHO region, 2005–2015. (Map generated from WHO website at http://www.who.int/about/regions/en/ [9])
Fig. 2.
Country-specific prevalence of GDM according to different diagnostic criteria. C&C Carpenter and Coustan criteria, IADPSG International Association of Diabetes and Pregnancy Study Groups, NDDG National Diabetes Data Group, WHO World Health Organization, other included International Classification of Diseases codes and local guidelines or criteria
Of particular note, caution is needed in interpreting the country- and region-specific estimates of GDM prevalence, due to several methodological issues as discussed below. IADPSG criteria emerged just recently. As such, recent studies are more likely to apply the IADPSG criteria while estimating or reevaluating the prevalence of GDM. Thus, the synthesized prevalence estimates based on studies published during the past decade might reflect more about the prevalence defined by the new criteria than the previous ones, especially among countries which did not report their prevalence estimates until recently. In addition, when the prevalence by WHO regions was synthesized, no adjustment was made based on sample sizes and sampling methods.
Methodological Issues in Assessing Global Prevalence of GDM
Screening Approaches
The common screening approaches for GDM include universal or routine screening for all pregnant women, selective screening among high-risk women based on certain risk factors, or a combination of both. Ideally, universal screening should be applied without restriction to high-risk pregnant women in order to identify all potential GDM cases. Selective screening for GDM might lead to missing over 40% of GDM cases [15]. On the other hand, selective screening may be more cost-effective based on the recognition that screening with glucose measurements may be less beneficial to low-risk women [16].
The screening approaches for GDM vary by country, region, and year. For instance, the overall screening rate in the USA has been high in the past two decades, ranging from 87.5 to 96.5 % depending on practices adopted by different health providers [17, 18]. In contrast, although there are no unified national guidelines regarding screening for GDM in Sweden, most regions apply risk factor-based screening [19]. However, surprisingly low rate of compliance with local clinical guidelines was observed, with only 30.7 % of women meeting the criteria for GDM screening were actually screened [20]. Moreover, practices with respect to the timing of screening may also vary by local health practitioners. In Nigeria, regardless of the diagnostic criteria of GDM, the time window for GDM screening might vary from 4–40 weeks of gestation [21], 24–28 weeks [22], 24 weeks onwards [23], to the third trimester [24].
Diagnostic Criteria
There has been continuing debates as to the optimal approach for the diagnosis of GDM, which has resulted in a variety of diagnostic criteria for GDM endorsed by different stakeholders (Table 1).Moreover, diagnostic criteria for GDM have rapidly evolved during the past few decades.
Table 1.
Major diagnostic criteria for gestational diabetes mellitus
| Criteria | Effective years | OGTT type (g) | Abnormal values (n) | Fasting |
1 h |
2 h |
3 h |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mmol/l | mg/dl | mmol/l | mg/dl | mmol/l | mg/dl | mmol/l | mg/dl | ||||
| O’Sullivan and Mahan | Since 1964 | 100 | ≥2 | 5.0 | 90 | 9.2 | 165 | 8.1 | 145 | 7.0 | 125 |
| ACOG | |||||||||||
| Endorsed NDDG 1979 | 2001–present | 100 | ≥2 | 5.8 | 105 | 10.6 | 190 | 9.2 | 165 | 8.0 | 145 |
| Endorsed C&C 1982 | 2001–present | 100 | ≥2 | 5.3 | 95 | 10.0 | 180 | 8.6 | 155 | 7.8 | 140 |
| 75 | ≥2 | 5.3 | 95 | 10.0 | 180 | 8.6 | 155 | – | – | ||
| ADA | |||||||||||
| Endorsed NDDG 1979 | Before 2000 and 2014–present | 100 | ≥2 | 5.8 | 105 | 10.6 | 190 | 9.2 | 165 | 8.0 | 145 |
| Endorsed C&C 1982 | 2000–2010 and 2014–present | 100 | ≥2 | 5.3 | 95 | 10.0 | 180 | 8.6 | 155 | 7.8 | 140 |
| 75 | ≥2 | 5.3 | 95 | 10.0 | 180 | 8.6 | 155 | – | – | ||
| Endorsed IADPSG 2010 | 2011–present | 75 | ≥1 | 5.1 | 92 | 10.0 | 180 | 8.5 | 153 | – | – |
| ADIPS | |||||||||||
| 1991–2011 | 1991–2010 | 75 | ≥1 | 5.5 | 99 | – | – | 8.0 | 180 | – | – |
| Endorsed IADPSG 2010 | 2011–2013 | 75 | ≥1 | 5.1 | 92 | 10.0 | 180 | 8.5 | 153 | – | – |
| Endorsed WHO 2013 | 2014–present | 75 | ≥1 | 5.1–6.9 | 92–125 | 10.0 | 180 | 8.5–11.0 | 153–199 | – | – |
| CDA | |||||||||||
| Before 2013 | 2003–2012 | 75 | ≥2 | 5.3 | 95 | 10.6 | 190 | 8.9 | 160 | – | – |
| Since 2013 | 2013–present | 75 | ≥1 | 5.3 | 95 | 10.6 | 190 | 9.0 | 162 | – | – |
| DIPSI (endorsed WHO 1985) | 2006–present | 75 | ≥1 | – | – | – | – | 7.8 | 140 | – | – |
| EASD | 1996–present | 75 | ≥1 | 6.0 | 108 | – | – | 9.0 | 162 | – | – |
| GDA | 2001–present | 75 | ≥2 | 5.3 | 95 | 10.0 | 180 | 8.6 | 155 | – | – |
| IADPSG | |||||||||||
| IADPSG | 2010–present | 75 | ≥1 | 5.1 | 92 | 10.0 | 180 | 8.5 | 153 | – | – |
| Modified IADPSG | 2010–present | 75 | ≥1 | 5.1 | 92 | – | – | 8.5 | 153 | – | – |
| JSOG | 1984–present | 75 | ≥2 | 5.6 | 100 | 10.0 | 180 | 8.3 | 150 | – | – |
| NICE | 2015–present | 75 | ≥1 | 5.6 | 101 | – | – | 7.8 | 140 | – | – |
| NZSSD | 2004–present | 75 | ≥1 | 5.5 | 99 | – | – | 9.0 | 162 | – | – |
| WHO | |||||||||||
| WHO 1985 | 1985–1998 | 75 | ≥1 | 7.8 | 140 | – | – | 7.8 | 140 | – | – |
| WHO 1999 | 1999–2012 | 75 | ≥1 | 7.0 | 126 | – | – | 7.8 | 140 | – | – |
| WHO 2013 | 2013–present | 75 | ≥1 | 5.1–6.9 | 92–125 | 10.0 | 180 | 8.5–11.0 | 153–199 | – | – |
ACOG American College of Obstetrics and Gynecologists, ADA, American Diabetes Association, ADIPS Australasian Diabetes in Pregnancy Society, CDA Canadian Diabetes Association, C&C Carpenter and Coustan criteria, DIPSI Diabetes in Pregnancy Study Group India, EASD European Association for the Study of Diabetes, GDA German Diabetes Association, IADPSG International Association of Diabetes and Pregnancy Study Groups, JSOG Japan Society of Obstetrics and Gynecology, NDDG National Diabetes Data Group, NZSSD New Zealand Society for the Study of Diabetes, NICE National Institute for Health and Care Excellence, WHO World Health Organization
In 1664, O’Sullivan and Mahan suggested a set of threshold values obtained during a 100-g 3-h oral glucose tolerance test (OGTT) using the Somogyi-Nelson technique for GDM diagnosis [25]. The O’Sullivan criteria were set based on the two standard deviations of the respective mean of each venous whole blood glucose measurements, in order to optimize the ability in predicting development of subsequent diabetes in later life. In 1979, the National Diabetes Data Group (NDDG) modified these thresholds by adding ~15 % to each glucose value to reflect the change of using plasma or serum samples instead of venous whole blood [26]. The American Diabetes Association (ADA) recommended the NDDG criteria for diagnosis of GDM until 1999 and switched to the Carpenter and Coustan (C&C) criteria [27] since 2000 [28]. The C&C criteria were initially published in 1982 based on a new glucose oxidase method for plasma glucose measurement to replace the conventional Somogyi-Nelson technique, which is sensitive to a number of non-glucose substances. Of note, both the NDDG and C&C criteria were based on a 100-g 3-h OGTT test, following a 50-g glucose challenge test with cut-off values ranged from 130 to 140 mg/dl. This combination forms a two-step diagnostic approach for GDM as recommended by the ADA until 2010. Since 2011, the ADA endorsed the new criteria by the IADPSG, which recommended a one-step approach using a 75-g 2-h OGTT with one or more abnormal values [29]; however, in 2014, the ADA modified their recommendations to endorse either the IADPSG or the two-step approach (i.e., C&C or NDDG criteria) [30]. The new criteria were based on the Hyperglycemia and Adverse Pregnancy Outcome Study (HAPO) study which identified a 1.75-fold increase in specific adverse pregnancy outcomes (i.e., birth weight >90th percentile, primary cesarean section delivery, neonatal hypoglycemia, and cord C-peptide >90th percentile) compared to the mean glucose values [11].
In contrast to the ADA diagnostic criteria for GDM exclusive to pregnant women, the WHO suggested that GDM be diagnosed according to the same criteria for diabetes mellitus or impaired glucose tolerance outside pregnancy using a 75-g 2-h OGTT with one or more abnormal values [12, 31]. In 2013, the WHO proposed its new criteria and definition for hyperglycemia first detected during pregnancy (HFDP) including both GDM and diabetes in pregnancy (DIP; previously undiagnosed diabetes based on the 2006 WHO criteria for diabetes [32]) instead of the conventional term GDM [33]. This new definition intends to differentiate between the varied severities of HFDP, which distinguishes GDM as a milder degree of HFDP from the more serious form DIP. To align with the new WHO definition of HFDP, the International Diabetes Federation published prevalence estimates of HFDP instead of GDM largely based on extrapolated data [4•]. The consequent impact on the diagnosis and prevalence estimates of GDM following the new WHO 2013 definition and criteria is yet to be determined.
Overall, the adoption of new criteria at different stages resulted in an increase in prevalence of GDM in the population, to varying extents. Compared to the NDDG criteria, the use of the C&C criteria resulted in higher prevalence of GDM by 33–70 % among members of the Northern California Kaiser Permanente Medical Care Program, varied by women’s age and race/ ethnicity [34]. The lower threshold values of IADPSG further increased the prevalence of GDM by two- to threefolds or even up to sevenfold compared to the previous criteria [35–39]. Similarly, one population-based study in Brazil showed that the prevalence of GDM significantly differed by diagnostic criteria [i.e., 18.0/7.1/2.3 % using the IADPSG/WHO 1999/ADA 2010 criteria, respectively] [35]. One hospital-based study in United Arab Emirates comparing multiple diagnostic criteria also illustrated major discrepancies in the prevalence estimates of GDM (i.e., 45.3/24.5/13.3 % using the IADPSG/WHO 1999/ADA 2004 criteria, respectively) [38]. The revised upward prevalence of GDM by adopting the IADPSG criteria was also seen in a hospital-based cohort in Spain, whose prevalence estimates were 35.5 and 10.6 % according to the IADPSG and C&C criteria, respectively [39].
Even when the same diagnostic criteria were applied, considerable variability in prevalence estimates of GDM was observed both within and between countries. Data from the HAPO study across 15 study centers in nine countries demonstrated great heterogeneity in prevalence of GDM by study center and country [40•] due to variations in the distributions of risk factors of GDM (i.e., age, parity, race/ethnicity, family history of GDM, body adiposity, etc.) in different populations. Among the four HAPO study centers in the USA, the prevalence of GDM ranged from 15.5 % in Providence, RI to 25.5 % in Bellflower, CA. Similarly, the two UK centers also varied in the prevalence estimates with 17.1 % in the Belfast center and 24.3 % in Manchester. Indeed, the considerable variations in estimates of GDM prevalence due to varied diagnostic criteria need to be considered while synthesizing the global prevalence.
Underlying Population Characteristics
The prevalence of GDM is not only influenced by screening approaches and diagnostic criteria but also by inherent characteristics of the study population, which add to the difficulty in comparing prevalence estimates across populations.
Advancing maternal age at childbirth has been recognized as one major risk factor for GDM. For instance, compared with women aged ≤24 years, the prevalence of GDM was more than four times higher among women aged >30 years (14.1 versus 3.0 %) in Turkey [41]. In addition, the increasing trend of GDM prevalence over time in a population could be partially attributable to the increased maternal age. Therefore, age-standardized prevalence estimates would be helpful in terms of facilitating the comparison of prevalence estimates of GDM across populations.
Another strong risk factor for GDM is race/ethnicity. In countries with multi-ethnic populations such as USA, Canada, and Australia, notable differences in racial/ethnic-specific prevalence of GDM have been documented. For instance, one US study in northern California reported that the prevalence was highest among Filipinas (10.9 %) and Asians (10.2 %), intermediate among Hispanics (6.8 %), and lowest among Non-Hispanic White (4.5 %) and African-American (4.4 %) [42]. In Australia, women of South Asia origins were 4.22-fold more likely to develop GDM compared to their Australia/New Zealand-origin counterparts [3], which is consistent with the higher prevalence of GDM among the general Southeast Asian population. The reasons underlying the racial/ethnic differences are still to be further elucidated, but accumulating evidence suggests the mechanisms could be multi-faceted, including differences in body composition, lifestyle (diet and physical activity), acculturation, genetic susceptibility, and healthcare systems and reporting practices [43]. In particular, although Asians generally have a lower body mass index (BMI), they are more prone to accumulate visceral fat and develop abdominal obesity at a given BMI [44], which in turn are positively associated with insulin resistance and impaired β cell function [45, 46]. Genetic predisposition may also contribute to the excessively high prevalence of GDM among certain high-risk racial/ethnic groups [47, 48]. Moreover, the risk of GDM may vary by nativity even within the same racial/ethnic group. For instance, among 133,552 live singleton deliveries from 2004 through 2007 in Florida, foreign-born Asian Indians were twofold more likely to develop GDM compared to their US-born counterparts, independent of BMI, age, parity, and height [49].
Other population characteristics of GDM risk factors including pre-pregnancy adiposity, family history of diabetes, parity, unhealthful diet, sedentary lifestyle, and socioeconomic determinants may also be at play in driving the variations in GDM prevalence across populations [50]. Compared to normal weight women, Caucasian Hungarian women who were overweight or obese prior to pregnancy had approximately twofold increased risk of GDM, regardless of the diagnostic criteria (i.e., WHO 1999 and IADPSG) [51]. In addition, urbanization as an indicator of economic development may also contribute to the varied prevalence of GDM, especially in developing countries. One study in Tanzania showed that the prevalence of GDM in urban communities was approximately five times that of rural counterparts using the IADPSG criteria (17.7 versus 3.7 %), whereas the discrepancy was even greater with an eightfold increase using the WHO 1999 criteria (8.4 versus 1.0 %) [52]. Collectively, it is imperative to take into account the underlying population characteristics while comparing prevalence estimates of GDM across populations.
Progression from GDM to T2DM
Literature Search
GDM was related to substantially increased risk for T2DM after the index pregnancy. The variations in the risk of progression from GDM to T2DM by country and race/ethnicity are not well understood. In this regard, we conducted a literature search on the risk of GDM progression to T2DM by expanding a previous well-conducted systematic review in 2009 [6••]. An electronic search of PubMed was performed on publications from February 1, 2009 to August 1, 2015, without language restrictions. We applied the same search terms as used by the previous review [6••], which included combinations of “gestational diabetes,” “diabetic pregnancy,” “diabetes mellitus,” “type 2 diabetes mellitus,” “NIDDM,” and “non-insulin dependent diabetes mellitus.” Overall, 471 studies were identified. After reviewing the titles and abstracts, 82 studies were included for further synthesis by examining the full texts. Briefly, cohort studies assessing the risk of developing T2DM at least 6 weeks after the index pregnancy complicated by GDM were included, whereas studies were excluded if they included women with known pre-gestational diabetes or did not contain a control group of non-GDM women. A total of seven eligible cohort studies meeting the search criteria were identified [53–59].
Results of the Literature Search
We combined data extracted from the 20 studies included in the previous review and an additional of seven qualified ones published afterwards as source data. The relative risk (RR) of developing T2DM after the index pregnancy complicated by GDM was calculated and grouped by country and WHO region (Fig. 3). The majority of the studies selected were from high-income countries (22 studies in 12 countries), whereas there were data from only five studies in three low-to-middle income countries (i.e., Brazil, China, and India). Despite the relatively high prevalence of GDM in the Southeast Asian region, only one study from India was included [60]. No eligible studies from Africa or Middle East and North Africa were identified. Due to the absence of sufficient study data, we were unable to stratify the results by specific racial/ethnic subgroup. However, most countries included were composed of mixed racial/ethnic groups or Caucasian populations, if not exclusively Caucasian.
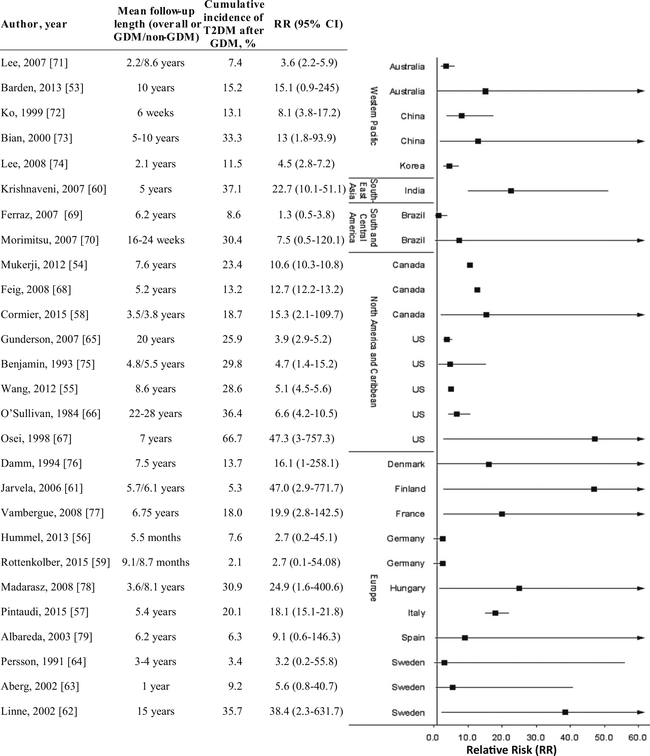
Fig. 3.
Relative risk (95 % confidence interval), length of follow-up, and cumulative incidence of developing type 2 diabetes mellitus (T2DM) after the index pregnancy complicated by gestational diabetes mellitus (GDM) by country and region
Overall, great heterogeneity was observed in the risk of progression from GDM to T2DM across countries and studies, which may be at least partly due to variations in the diagnostic criteria of GDM and/or T2DM, years of follow-up, retention rate, and characteristics of the underlying populations. Among the eligible studies, European countries overall exhibited the greatest heterogeneity with risk estimates ranging from 2.7 in Germany [56, 59] to 47.0 in Finland [61], cumulative incidence of T2DM after GDM ranging from 2.1 % in Germany [59] to 35.7 % in Sweden [62], and mean length of follow-up ranging from 5.5 months [56] to 15 years [62]. The effect sizes for relative risks of developing T2DM seem to vary by length of follow-up after the index pregnancy complicated by GDM. For instance, among the three studies in Sweden, two had increased but non-significant risks for developing T2DM 1 year (RR=5.6; 95 % confidence interval (CI) 0.8–40.7; cumulative risk of T2DM 9.2 %) [63] and 3–4 years (RR=3.2; 95 % CI 0.2–55.8; cumulative risk of T2DM 3.4 %) [64] after the GDM-complicated index pregnancy, respectively [63, 64], whereas the third one had a 38.4-fold increased risk of T2DM after 15 years of follow-up (cumulative risk 35.7 %) [62]. Among the five studies in the USA, four showed similar effect sizes of relative risks ranging from 3.9 [65] to 6.6 [66] with cumulative incidence of T2DM ranging from 25.9 to 36.4 %, although the length of follow-up had a wide range from 4.8 to 28 years. In contrast, the other US study had an exceptionally high risk of developing T2DM after 7 years of follow-up (RR=47.3; 95 % CI 3.0–757.3; cumulative risk of T2DM 66.7 %) comparing African-American women with GDM and a family history of diabetes versus their counterparts who were non-GDM, non-obese, and free of a family history of diabetes [67]. Similarly, two studies in Canada showed similar magnitudes of risk estimates [RR (95 % CI)=10.6 (10.3–10.8) and 12.7 (12.2–13.2), respectively] after 5.2–7.6 years of follow-up [54, 68], whereas a third one showed less precision in risk estimates [RR=15.3; 95 % CI 2.1–109.7] after on average 3.5/3.8 years of follow-up among GDM/non-GDM women [58]. In the only eligible study in Southeast Asia, the risk of developing T2DM was approximately 23-fold greater among South Indian women with previous GDM (cumulative risk of T2DM 37.1 %) compared to their non-GDM counterparts (cumulative risk of T2DM 1.6 %) after 5 years of follow-up [60]. In South and Central America, two eligible studies from Brazil reported 1.3 [69] and 7.5-fold [70] increased risks of T2DM with cumulative incidence ranging from 8.6 to 30.4 % after 6.2 years and 16–24 weeks of follow-up, respectively. Taken together, given the considerable variations in the risk estimates and underrepresentation of low-to-middle income countries, direct comparisons across countries and WHO regions are yet to be evaluated until more qualified data are available.
Conclusions
The epidemic of diabetes poses an enormous public health challenge globally. Given the adverse impacts of GDM on pregnancy outcomes, perinatal morbidity, and development of chronic diseases including T2DM later in life, increasing attention has been drawn to the increasing prevalence of this common pregnancy complication. Overall, our review based on publications during the past decade demonstrates large variations of the prevalence of GDM worldwide, with it being higher among Middle East and North Africa, Southeast Asia, and Western Pacific regions, whereas it is lowest in Europe. However, direct comparisons across countries are challenging at least partly due to varied screening approaches, diagnostic criteria, and underlying population characteristics. In particular, recent studies are more likely to estimate or reevaluate the prevalence of GDM adopting the new IADPSG criteria, which tend to result in a higher prevalence estimate as compared to other criteria used in earlier days. Moreover, the divergence of local or regional practices of GDM screening and diagnosis from the national guidelines, if available, further complexes the reliable estimation of country-specific prevalence. Women whose pregnancies are complicated by GDM have an exceptionally high risk for developing T2DM after the index pregnancy. Data on the risk of progression from GDM to T2DM are still limited and little is known about modifiable factors that may lower the risk, especially among developing and low-to-middle income countries, which have been shown to be suffering from escalating burden of both GDM and T2DM. Taken together, collaborative and continuing efforts to acquire global prevalence data within and across countries may be needed. Such efforts may have substantial public health implications in terms of informing health policy makers and healthcare providers for disease burden and for developing more targeted and effective diabetes prevention and management strategies globally.
Footnotes
Conflict of Interest Yeyi Zhu and Cuilin Zhang declare that they have no conflict of interest.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2012;8(4):228–36. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara A Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30 Suppl 2:S141–6. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 3.Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. 2008;31(12):2288–93. doi: 10.2337/dc08-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.•.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103(2):176–85. doi: 10.1016/j.diabres.2013.11.003.This article estimates the global prevalence of hyperglycemia in pregnancy, including total diabetes in pregnancy (known and previously undiagnosed diabetes) and gestational diabetes, conforming to the new WHO 2013 criteria.
- 5.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 6.••.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and metaanalysis. Lancet. 2009;373(9677):1773–9. doi: 10.1016/S0140-6736(09)60731-5.This systematic review and meta-analysis synthesizes the relative risk of developing type 2 diabetes after gestational diabetes among 675,455 women from 20 studies.
- 7.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21 Suppl 2:B161–7. [PubMed] [Google Scholar]
- 8.Linnenkamp U, Guariguata L, Beagley J, Whiting DR, Cho NH. The IDF Diabetes Atlas methodology for estimating global prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103(2):186–96. doi: 10.1016/j.diabres.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 9.WHO website. http://www.who.int/about/regions/en/. Accessed 10 Sept 2015.
- 10.Obstetrics JSoGa. Committee report on nutrition and metabolism in gyneco-obstetrics: abnormal glucose metabolism in pregnancy, with special reference to the diagnosis of diabetes mellitus in pregnancy. Nihon Sanka Fujinka Gakkai Zasshi. 1984;36(10):2055–8. [PubMed] [Google Scholar]
- 11.International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Organization WH. Definition, diagnosis and classification of diabetes mellitus and its complications Part 1: diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999. [Google Scholar]
- 13.National Institute for Health and Care Excellence (NICE). Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. NICE guidelines [NG3]. National Institute for Health and Care Excellence; 2015. [PubMed] [Google Scholar]
- 14.Canadian Diabetes Association. Canadian diabetes association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32 Suppl 1:S1–201. [DOI] [PubMed] [Google Scholar]
- 15.Ostlund I, Hanson U. Occurrence of gestational diabetes mellitus and the value of different screening indicators for the oral glucose tolerance test. Acta Obstet Gynecol Scand. 2003;82(2):103–8. doi: 10.1034/j.1600-0412.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- 16.Berger H, Sermer M. Counterpoint: selective screening for gestational diabetes mellitus. Diabetes Care. 2009;32(7):1352–4. doi: 10.2337/dc09-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang AL, Soon R, Kaneshiro B. The prevalence of gestational diabetes among Micronesians in Honolulu. Hawaii Med J. 2010;69(5 Suppl 2):4–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM screening program. Diabetes Care. 2005;28(3):579–84. [DOI] [PubMed] [Google Scholar]
- 19.Ignell C, Claesson R, Anderberg E, Berntorp K. Trends in the prevalence of gestational diabetes mellitus in southern Sweden, 2003–2012. Acta Obstet Gynecol Scand. 2014;93(4):420–4. doi: 10.1111/aogs.12340. [DOI] [PubMed] [Google Scholar]
- 20.Persson M, Winkvist A, Mogren I. Surprisingly low compliance to local guidelines for risk factor based screening for gestational diabetes mellitus—a population-based study. BMC Pregnancy Childbirth. 2009;9:53. doi: 10.1186/1471-2393-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuti MA, Abbiyesuku FM, Akinlade KS, Akinosun OM, Adedapo KS, Adeleye JO, et al. Oral glucose tolerance testing outcomes among women at high risk for gestational diabetes mellitus. J Clin Pathol. 2011;64(8):718–21. doi: 10.1136/jcp.2010.087098. [DOI] [PubMed] [Google Scholar]
- 22.Anzaku AS, Musa J. Prevalence and associated risk factors for gestational diabetes in Jos, north-central, Nigeria. Arch Gynecol Obstet. 2013;287(5):859–63. doi: 10.1007/s00404-012-2649-z. [DOI] [PubMed] [Google Scholar]
- 23.Olagbuji BN, Atiba AS, Olofinbiyi BA, Akintayo AA, Awoleke JO, Ade-Ojo IP, et al. Prevalence of and risk factors for gestational diabetes using 1999, 2013 WHO and IADPSG criteria upon implementation of a universal one-step screening and diagnostic strategy in a sub-Saharan African population. Eur J Obstet Gynecol Reprod Biol. 2015;189:27–32. doi: 10.1016/j.ejogrb.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 24.Olarinoye JK, Ohwovoriole AE, Ajayi GO. Diagnosis of gestational diabetes mellitus in Nigerian pregnant women—comparison between 75G and 100G oral glucose tolerance tests. West Afr J Med. 2004;23(3):198–201. [DOI] [PubMed] [Google Scholar]
- 25.O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–85. [PubMed] [Google Scholar]
- 26.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National diabetes data group. Diabetes. 1979;28(12):1039–57. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–73. [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association. Gestational diabetes mellitus. Diabetes care. 2000;23 Suppl 1:S77–9. [PubMed] [Google Scholar]
- 29.American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34:S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37 Suppl 1:S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 31.World Health organization. Diabetes mellitus Report of a WHO study group. Geneva: WHO; 1985. [PubMed] [Google Scholar]
- 32.World Health Organization/International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva: WHO; 2006. [Google Scholar]
- 33.Organization WH. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 34.Ferrara A, Hedderson MM, Quesenberry CP, Selby JV. Prevalence of gestational diabetes mellitus detected by the national diabetes data group or the carpenter and coustan plasma glucose thresholds. Diabetes Care. 2002;25(9):1625–30. [DOI] [PubMed] [Google Scholar]
- 35.Trujillo J, Vigo A, Duncan BB, Falavigna M, Wendland EM, Campos MA, et al. Impact of the international association of diabetes and pregnancy study groups criteria for gestational diabetes. Diabetes Res Clin Pract. 2015;108(2):288–95. doi: 10.1016/j.diabres.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Bodmer-Roy S, Morin L, Cousineau J, Rey E. Pregnancy outcomes in women with and without gestational diabetes mellitus according to the International Association of the Diabetes and Pregnancy Study Groups criteria. Obstet Gynecol. 2012;120(4):746–52. doi: 10.1097/AOG.0b013e31826994ec. [DOI] [PubMed] [Google Scholar]
- 37.Moradi S, Shafieepour MR, Mortazavi M, Pishgar F. Prevalence of gestational diabetes mellitus in Rafsanjan: a comparison of different criteria. Med J Islam Repub Iran. 2015;29:209. [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal MM, Dhatt GS, Othman Y. Gestational diabetes: differences between the current international diagnostic criteria and implications of switching to IADPSG. J Diabetes Complicat. 2015;29(4):544–9. doi: 10.1016/j.jdiacomp.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Duran A, Saenz S, Torrejon MJ, Bordiu E, Del Valle L, Galindo M, et al. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos Gestational Diabetes Study. Diabetes Care. 2014;37(9):2442–50. doi: 10.2337/dc14-0179. [DOI] [PubMed] [Google Scholar]
- 40.•.Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panelrecommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Diabetes Care. 2012;35(3):526–8. doi: 10.2337/dc11-1641.This article is a good example showing the heterogeneity in prevalence estimates of GDM within and across countries even if using the same diagnostic criteria.
- 41.Shirazian N, Emdadi R, Mahboubi M, Motevallian A, Fazel-Sarjuei Z, Sedighpour N, et al. Screening for gestational diabetes: usefulness of clinical risk factors. Arch Gynecol Obstet. 2009;280(6):933–7. doi: 10.1007/s00404-009-1027-y. [DOI] [PubMed] [Google Scholar]
- 42.Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35(7):1492–8. doi: 10.2337/dc11-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SY, Saraiva C, Curtis M, Wilson HG, Troyan J, Sharma AJ. Fraction of gestational diabetes mellitus attributable to overweight and obesity by race/ethnicity, California, 2007–2009. Am J Public Health. 2013;103(10):e65–72. doi: 10.2105/ajph.2013.301469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr. 2007;86(2):353–9. [DOI] [PubMed] [Google Scholar]
- 45.Khoo CM, Sairazi S, Taslim S, Gardner D, Wu Y, Lee J, et al. Ethnicity modifies the relationships of insulin resistance, inflammation, and adiponectin with obesity in a multiethnic Asian population. Diabetes Care. 2011;34(5):1120–6. doi: 10.2337/dc10-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lekva T, Bollerslev J, Godang K, Roland MC, Friis CM, Voldner N, et al. β-cell dysfunction in women with previous gestational diabetes is associated with visceral adipose tissue distribution. Eur J Endocrinol Eur Fed Endocr Soc. 2015;173(1):63–70. doi: 10.1530/eje-15-0153. [DOI] [PubMed] [Google Scholar]
- 47.Junior JP, Frigeri HR, Dos Santos-Weiss IC, de Souza EM, Rego FG, Picheth G, et al. The MTNR1B gene polymorphism rs10830963 is associated with gestational diabetes in a Brazilian population. Gene. 2015;568(1):114–5. doi: 10.1016/j.gene.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 48.Tarquini F, Picchiassi E, Centra M, Pennacchi L, Bini V, Cappuccini B, et al. Body mass index associated to rs2021966 ENPP1 polymorphism increases the risk for gestational diabetes mellitus. Gynecol Endocrinol. 2015;31(1):83–6. doi: 10.3109/09513590.2014.958994. [DOI] [PubMed] [Google Scholar]
- 49.Kim SY, Sappenfield W, Sharma AJ, Wilson HG, Bish CL, Salihu HM, et al. Racial/ethnic differences in the prevalence of gestational diabetes mellitus and maternal overweight and obesity, by nativity, Florida, 2004–2007. Obesity. 2013;21(1):E33–40. doi: 10.1002/oby.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 Suppl):1975S–9. doi: 10.3945/ajcn.110.001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kun A, Tornoczky J, Tabak AG. The prevalence and predictors of gestational diabetes mellitus in Hungary. Horm Metab Res. 2011;43(11):788–93. doi: 10.1055/s-0031-1287795. [DOI] [PubMed] [Google Scholar]
- 52.Mwanri AW, Kinabo J, Ramaiya K, Feskens EJ. Prevalence of gestational diabetes mellitus in urban and rural Tanzania. Diabetes Res Clin Pract. 2014;103(1):71–8. doi: 10.1016/j.diabres.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Barden A, Singh R, Walters B, Phillips M, Beilin LJ. A simple scoring method using cardiometabolic risk measurements in pregnancy to determine 10-year risk of type 2 diabetes in women with gestational diabetes. Nutr Diabetes. 2013;3:e72. doi: 10.1038/nutd.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukerji G, Chiu M, Shah BR. Impact of gestational diabetes on the risk of diabetes following pregnancy among Chinese and South Asian women. Diabetologia. 2012;55(8):2148–53. doi: 10.1007/s00125-012-2549-6. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Chen L, Horswell R, Xiao K, Besse J, Johnson J, et al. Racial differences in the association between gestational diabetes mellitus and risk of type 2 diabetes. J Women’s Health. 2012;21(6):628–33. doi: 10.1089/jwh.2011.3318. [DOI] [PubMed] [Google Scholar]
- 56.Hummel S, Much D, Rossbauer M, Ziegler AG, Beyerlein A. Postpartum outcomes in women with gestational diabetes and their offspring: POGO study design and first-year results. Rev Diabet Stud. 2013;10(1):49–57. doi: 10.1900/rds.2013.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pintaudi B, Lucisano G, Pellegrini F, D’Ettorre A, Lepore V, De Berardis G, et al. The long-term effects of stillbirth on women with and without gestational diabetes: a population-based cohort study. Diabetologia. 2015;58(1):67–74. doi: 10.1007/s00125-014-3403-9. [DOI] [PubMed] [Google Scholar]
- 58.Cormier H, Vigneault J, Garneau V, Tchernof A, Vohl MC, Weisnagel SJ, et al. An explained variance-based genetic risk score associated with gestational diabetes antecedent and with progression to pre-diabetes and type 2 diabetes: a cohort study. BJOG Int J Obstet Gynaecol. 2015;122(3):411–9. doi: 10.1111/1471-0528.12937. [DOI] [PubMed] [Google Scholar]
- 59.Rottenkolber M, Ferrari U, Holland L, Aertsen S, Kammer NN, Hetterich H, et al. The diabetes risk phenotype of young women with recent gestational diabetes. J Clin Endocrinol Metab. 2015;100(6):E910–8. doi: 10.1210/jc.2014-3898. [DOI] [PubMed] [Google Scholar]
- 60.Krishnaveni GV, Hill JC, Veena SR, Geetha S, Jayakurnar MN, Karat CLS, et al. Gestational diabetes and the incidence of diabetes in the 5 years following the index pregnancy in South Indian women. Diabetes Res Clin Pract. 2007;78(3):398–404. doi: 10.1016/j.diabres.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jarvela IY, Juutinen J, Koskela P, Hartikainen AL, Kulmala P, Knip M, et al. Gestational diabetes identifies women at risk for permanent type 1 and type 2 diabetes in fertile age—predictive role of autoantibodies. Diabetes Care. 2006;29(3):607–12. doi: 10.2337/diacare.29.03.06.dc05-1118. [DOI] [PubMed] [Google Scholar]
- 62.Linne Y, Barkeling B, Rossner S. Natural course of gestational diabetes mellitus: long term follow up of women in the SPAWN study. BJOG. 2002;109(11):1227–31. [DOI] [PubMed] [Google Scholar]
- 63.Aberg AEB, Jonsson EK, Eskilsson I, Landin-Olsson M, Frid AH. Predictive factors of developing diabetes mellitus in women with gestational diabetes. Acta Obstet Gynecol Scand. 2002;81(1):11–6. doi: 10.1046/j.0001-6349.2001.00000.x. [DOI] [PubMed] [Google Scholar]
- 64.Persson B, Hanson U, Hartling SG, Binder C. Follow-up of women with previous GDM. Insulin, C-peptide, and proinsulin responses to oral glucose load. Diabetes. 1991;40 Suppl 2:136–41. [DOI] [PubMed] [Google Scholar]
- 65.Gunderson EP, Lewis CE, Tsai AL, Chiang V, Carnethon M, Quesenberry CP, et al. A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception—The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes. 2007;56(12):2990–6. doi: 10.2337/db07-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Sullivan JB. The Boston gestational diabetes studies: review and perspectives. London: Springer-Verlag, Berlin Heidelberg; 1984. [Google Scholar]
- 67.Osei K, Gaillard TR, Schuster DP. History of gestational diabetes leads to distinct metabolic alterations in nondiabetic African-American women with a parental history of type 2 diabetes. Diabetes Care. 1998;21(8):1250–7. doi: 10.2337/diacare.21.8.1250. [DOI] [PubMed] [Google Scholar]
- 68.Feig DS, Zinman B, Wang X, Hux JE. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ. 2008;179(3):229–34. doi: 10.1503/cmaj.080012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferraz TB, Motta RS, Ferraz CL, Capibaribe DM, Forti AC, Chacra AR. C-reactive protein and features of metabolic syndrome in Brazilian women with previous gestational diabetes. Diabetes Res Clin Pract. 2007;78(1):23–9. doi: 10.1016/j.diabres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 70.Morimitsu LK, Fusaro AS, Sanchez VH, Hagemann CCF, Bertini AM, Dib SA. Fibrinolytic dysfunction after gestation is associated to components of insulin resistance and early type 2 diabetes in Latino women with previous gestational diabetes. Diabetes Res Clin Pract. 2007;78(3):340–8. doi: 10.1016/j.diabres.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 71.Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes—a retrospective cohort study using survival analysis. Diabetes Care. 2007;30(4):878–83. doi: 10.2337/dc06-1816. [DOI] [PubMed] [Google Scholar]
- 72.Ko GTC, Chan JCN, Tsang LWW, Li CY, Cockram CS. Glucose intolerance and other cardiovascular risk factors in Chinese women with a history of gestational diabetes mellitus. Aust N J Obstet Gynaecol. 1999;39(4):478–83. [DOI] [PubMed] [Google Scholar]
- 73.Bian XM, Gao P, Xiong XY, Xu H, Qian ML, Liu SY. Risk factors for development of diabetes mellitus in women with a history of gestational diabetes mellitus. Chin Med J Peking. 2000;113(8): 759–62. [PubMed] [Google Scholar]
- 74.Lee H, Jang HC, Park HK, Metzger BE, Cho NH. Prevalence of type 2 diabetes among women with a previous history of gestational diabetes mellitus. Diabetes Res Clin Pract. 2008;81(1):124–9. doi: 10.1016/j.diabres.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 75.Benjamin E, Winters D, Mayfield J, Gohdes D. Diabetes in pregnancy in Zuni Indian women—prevalence and subsequent development of clinical diabetes after gestational diabetes. Diabetes Care. 1993;16(9):1231–5. doi: 10.2337/diacare.16.9.1231. [DOI] [PubMed] [Google Scholar]
- 76.Damm P, Kuhl C, Buschard K, Jakobsen BK, Svejgaard A, Sodoyez-Goffaux F, et al. Prevalence and predictive value of islet cell antibodies and insulin autoantibodies in women with gestational diabetes. Diabet Med J Br Diabet Assoc. 1994;11(6):558–63. [DOI] [PubMed] [Google Scholar]
- 77.Vambergue A, Dognin C, Boulogne A, Rejou MC, Biausque S, Fontaine P. Increasing incidence of abnormal glucose tolerance in women with prior abnormal glucose tolerance during pregnancy: DIAGEST 2 study. Diabet Med. 2008;25(1):58–64. doi: 10.1111/j.1464-5491.2007.02306.x. [DOI] [PubMed] [Google Scholar]
- 78.Madarasz E, Tamas G, Tabak GA, Szalay J, Kerenyi Z. Metabolic syndrome after pregnancy complicated with gestational diabetes: four-year follow-up. Orv Hetil. 2008;149(18):831–8. doi: 10.1556/OH.2008.28242. [DOI] [PubMed] [Google Scholar]
- 79.Albareda M, Caballero A, Badell G, Piquer S, Ortiz A, de Leiva A, et al. Diabetes and abnormal glucose tolerance in women with previous gestational diabetes. Diabetes Care. 2003;26(4):1199–205. doi: 10.2337/diacare.26.4.1199. [DOI] [PubMed] [Google Scholar]