Abstract
Background and aims
While the association between platelet activation and hepatic fibrosis has been previously demonstrated in animal studies; the utility of anti-platelet agents in reversing the progression of hepatic fibrosis requires further review. Utilizing systematic review methods, we provide to our knowledge the first meta-analysis combining evidence from all studies aimed to establish the effect of anti-platelet agents in the prevention of hepatic fibrosis.
Methods
We searched Medline, EMBASE and PubMed databases from inception to October 2018 to identify all studies aimed at evaluating the role of anti-platelet agents in the prevention of hepatic fibrosis. The primary outcome was hepatic fibrosis. The initial title, abstract, and full-text screening were performed in duplicate. Risk of bias was evaluated using the Newcastle–Ottawa Scale. A fixed-effect generic inverse variance method was used to create a pooled estimate of the odds of hepatic fibrosis in patients with anti-platelet agents versus without anti-platelet agents.
Results
Among the 2310 unique articles identified during the title screening, 4 studies with a combined population of 3141 patients were deemed eligible for inclusion into the meta-analysis establishing the effect of anti-platelet agents on hepatic fibrosis. One study failed to report their findings in the entire cohort, electing to instead summarize the effects of anti-platelets within subgroups categorized by fibrotic risk factors. Use of anti-platelets was associated with 32% decreased odds of hepatic fibrosis, (adjusted pooled OR 0.68; CI 0.56–0.82, p 𢙍 0.0001). The statistical heterogeneity among the studies was insignificant.
Conclusion
Use of anti-platelet agents is associated with the decreased odds of hepatic fibrosis. Due to limited evidence, future high-quality randomized controlled trials with larger comparative samples are required to further delineate the potential beneficial effects of these drugs in preventing hepatic fibrosis.
Keywords: Hepatic fibrosis, Aspirin, Anti-platelets, Systematic review
Introduction
Chronic hepatic insult, whether infectious, inflammatory, or autoimmune etiology, leads to fibrosis of the liver which ultimately can progress to irreversible damage in the form of cirrhosis. End-stage liver disease resulting from cirrhosis is a leading cause of mortality both within the United States (US) and worldwide, rising 45% since 1990 [1]. Despite its high case fatality, there are limited treatment modalities for irreversible hepatic injury. As approved anti-fibrotic therapy is currently unavailable, the focus is on medications which can help to prevent the development and progression of hepatic fibrosis. Recent evidence has shifted the focus on the potential role of platelets in hepatic fibrosis [2, 3]. Multiple animal studies have shown that platelets activation increases hepatic fibrosis, while anti-platelet agents demonstrate opposing effects and they have a potential of preventing the onset or progression of hepatic fibrosis [4–9]. In a chronic hepatitis B mouse model, anti-platelet agents (aspirin and clopidogrel) have been shown to decrease the severity of hepatic fibrosis [4]. Similarly, another mouse model of biliary cirrhosis revealed that platelets-derived growth factor-β released by platelets promotes hepatic fibrosis by activation of hepatic stellate cells [5]. While the association between platelet activation and hepatic fibrosis has been previously demonstrated in animal studies, the utility of anti-platelet agents in reversing the progression of hepatic fibrosis requires further review. Utilizing systematic review methods, we provide to our knowledge the first meta-analysis combining evidence from all studies aimed to establish the effect of anti-platelet agents in the prevention of hepatic fibrosis.
Materials and methods
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [10].
Eligibility criteria
All the studies evaluating the association of anti-platelets and hepatic fibrosis were included in the systematic review. We also included the studies which were presented as abstract and not yet fully published. Study size did not restrict the inclusion of the study. The bibliographies of relevant articles were hand-searched to provide additional references. Our primary outcome, hepatic fibrosis, was presented in studies as adjusted odds ratio (OR). Animal studies were excluded. We also excluded the studies which did not report our primary outcome or if anti-platelet agents were not used. In an effort to be inclusive and examine all evidence available, we accepted all definitions and measurements of hepatic fibrosis. This includes hepatic fibrosis as confirmed on imaging, biopsy and histopathology, or commonly accepted serum markers (composite INR, platelet, transaminase, albumin levels).
Information sources and search strategy
A systematic literature search was conducted using the PubMed and Embase databases from inception to October 20, 2018 to identify all original studies that investigated the role of anti-platelets agents in hepatic fibrosis. The systematic literature review was independently conducted by two investigators (U.I and A.A.) using a search strategy. The search terms for PubMed was: “Platelet Aggregation Inhibitors”[Mesh] OR platelet aggregation inhibitors[tiab] OR Aspirin[tiab] or clopidogrel[tiab] AND (“Liver Cirrhosis”[Mesh] OR cirrhosis[tiab] OR cirrhotic[tiab] OR cirrhotics[tiab]) and search terms for Embase were ‘liver cirrhosis’/exp OR cirrhosis:ti,ab OR cirrhotic:ti,ab OR cirrhotics:ti,ab AND ‘antithrombocytic agent’/exp OR ‘antithrombocytic’:ti,ab.
Data collection process and list of items
We screened the title, abstract, and full-text articles in duplicate. Data from eligible studies were independently extracted by 2 reviewers (U.I and M.A.K), then cross-checked by the third author (A.A). Discrepancies were resolved by mutual consensus. We report the kappa statistic to demonstrate the level of agreement between reviewers. The following data were extracted from each primary study: year of publication and country of origin, age, sex, design of the study, sample size, study population methods used to detect hepatic fibrosis, type of anti-platelet agent used, outcome of the study, odds of hepatic fibrosis with aspirin use and adverse events if reported. Data extraction forms were generated by all members of the team.
Quality assessment
Newcastle–Ottawa quality assessment scale was used to evaluate the quality of observational studies in three areas, including the recruitment of cases and controls, the comparability between the two groups and the ascertainment of the outcome of interest. Results of methodological quality assessment did not influence the eligibility of the studies.
Statistical analysis
Data analysis was performed using Review Manager (Rev-Man) 5.3 software from the Cochrane Collaboration (London, UK). A fixed-effects model weighted by the inverse variance method was used to create a pooled estimate of the odds of hepatic fibrosis with anti-platelet agents versus no anti-platelet agent. The pooled odds ratio (OR) was obtained using the risk estimates from the included studies and their corresponding standard errors. We present our pooled findings in separate forest plots. Due to the small number of studies in our analysis, we decided not to assess for publication bias using Egger’s plot. Heterogeneity between the studies was calculated by calculating the inconsistency index (I2). A value of I2 of 0–25% represented insignificant heterogeneity, 26–50% represented low heterogeneity, 51–75% represented moderate heterogeneity, and more than 75% represented high heterogeneity, as set forth by the Cochrane Collaboration [11].
Results
Initial search strategy revealed 2310 relevant articles, 2130 in Embase and 180 in Pubmed. After excluding duplicate articles, 1576 articles underwent title review and abstract review. At this time, 8 studies were included which underwent full-text review. Kappa coefficient for study selection process between reviewers was 0.86. Only four studies including 3141 patients met the inclusion criteria and were included in the systematic review and analyzed in the meta-analysis [12–15]. Literature review process is shown in Fig. 1.
Fig. 1.

Literature review process
Jiang et al. did not report the odds of hepatic fibrosis of the whole cohort but rather reported in 3 groups which included patient population with nonalcoholic steatohepatitis (NASH), alcoholic liver disease and viral hepatitis [12]. There was an overlap of 168 patients as these patients had more than one risk factors for chronic liver disease. To avoid the bias, each of the patient group was included individually in a final meta-analysis along with other 3 included studies. There were significant differences in study population included in the studies. Jiang et al. identified patients by NHANES database with risk factors for chronic liver disease including NASH, viral hepatitis and alcoholic liver disease [12]. Poujol-Robert et al. included hepatitis C virus (HCV)- positive patients following liver transplantation, Singh et al. included patients with type 2 diabetes mellitus with liver biopsy-proven nonalcoholic fatty liver disease (NAFLD), while Schwarzkopf et al. included patients who underwent coronary angiography [13–15]. Patients were noted to have a comparable age range in all the studies. Poujol-Robert et al. showed male predominant population with 83% male, while Singh et al. had 62.7% female. Other baseline characteristics are tabulated in Table 1. Tables 2 and 3 summarize the quality assessment of studies. Majority of studies were noted to have a low risk of bias. Methodological shortcomings are focused in establishing temporality of hepatic fibrosis prior to initiation of anti-platelet therapy as well as ensuring adequate follow-up to support the study conclusions. The use of anti-platelet agents was associated with decreased odds of hepatic fibrosis with the seperate inclusion of all three patient populations from Jiang et al. study, adjusted pooled OR 0.67; CI 0.56–0.81, p ≤ <0.0001 with inclusion of NASH group of Jiang et al. (Fig. 2), and adjusted pooled OR 0.68; CI 0.56–0.82 with inclusion of viral hepatitis and alcoholic liver disease patient populations of Jiang et al. study (Figs. 3, 4) [12]. The statistical heterogeneity as calculated by I2 was insignificant as assessed by I2 = 0.
Table 1.
Baseline characteristics of included studies
| Authors name | Jiang et al. | Poujol-Robert et al. | Schwarzkopf et al. | Singh et al. |
| Country and year of publication | USA 2016 | France 2014 | Germany 2018 | USA 2018 |
| Type of study | Cross-sectional | RCS | PCS | RCS |
| Sample size | N = 1856 | N = 188 | N = 505 | N = 592 |
| Quality assessment | NOQ = 9 | NOQ = 9 | NOQ = 5 | NOQ = 8 |
| Study population | NHANES database was used to identify patients at risk of liver disease such as alcohol abuse, viral hepatitis and NASH. | HCV positive patient without HIV or HBV with transplanted liver between 2000 and 2010 | Consecutive patients undergoing elective coronary angiography. | Patients with type 2 DM with biopsy-proven NAFLD |
| Baseline characteristics | Aspirin users | Recipient age = 52 ± 8 | Anti-platelets users | Age = 52.2 ± 11.7 |
| Age = 46.6 ± 15.4 | Recipient gender = 83% male | Age = 66 (58–75) | Sex = 62.7% female | |
| Sex = 55.4% male | Donor age = 48 ± 17 | Sex = 82% male | Caucasians = 84.8% | |
| BMI = 29.6 ± 6.5 | Donor gender = 70% male | BMI = 27(24–30) | BMI = 92% were overweight or obese | |
| Caucasians = 40% Diabetes = 11.5% HTN = 34.4% Non-aspirin users Age = 43.2 ± 14.7 Sex = 52% male BMI = 29.4 ± 6.5 Caucasians = 23.1 % Diabetes = 11.4% HTN = 27.8% |
Diabetes = 75% HCV genotype 1=63% |
Mean HbA1c = 6.1 Non-aspirin users Age = 66 (58–75) Sex = 82% male BMI = 27(24–30) Mean HbA1c = 5.9 |
Mean HbA1c = 6.4% | |
| Methods to detect liver fibrosis | 4 Non-invasive methods (FIB4, APRI, Forns and NFS) were used | Liver histology using METAVIR system | Fibroscan transient elastography | Histological evaluation of liver fibrosis |
| Anti-platelet agent used | Aspirin | Aspirin | Aspirin and/or P2Y12 receptor antagonists. | Aspirin |
| Outcome | Lower liver fibrosis scores in aspirin group | Lower risk of progression of fibrosis in aspirin group | Lower risk of progression of fibrosis in anti-platelets group | No beneficial effect of aspirin noted in preventing liver fibrosis |
Table 2.
Quality assessment of cohort studies
| Studies | Jiang et al. | Singh et al. | Schwarzkopf et al. |
| Representativeness of the exposed cohort | Low risk | Low risk | Low risk |
| Selection of the non-exposed cohort | Low risk | Low risk | Low risk |
| Ascertainment of exposure | Low risk | Low risk | Low risk |
| Outcome not present at start | Low risk | Unclear bias | Unclear bias |
| Adjustment for primary and secondary factors | Low risk | Low risk | Low risk |
| Assessment of outcome | Low risk | Low risk | Low risk |
| Was follow-up long enough for outcomes to occur | Low risk | Low risk | Unclear bias |
| Adequacy of follow-up of cohorts | Low risk | Low risk | Unclear bias |
| Quality | High quality | Moderate quality | Moderate quality |
Table 3.
Quality assessment of Poujol-Robert study [13] (cross-sectional study)
| Representativeness of the sample | Low risk |
| Sample size | Low risk |
| Non-respondents | Low risk |
| Ascertainment of the exposure | Low risk |
| Adjustment for primary and secondary factors | Low risk |
| Assessment of the outcome | Low risk |
| Statistical test | Low risk |
| Quality | High quality |
Fig. 2.
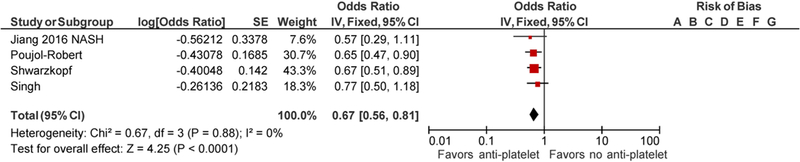
Forest Plot for all studies with inclusion of NASH population of Jiang et al. study
Fig. 3.
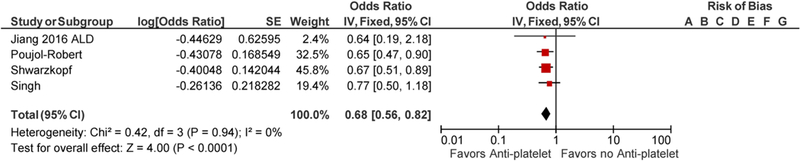
Forest Plot for all studies with inclusion of viral hepatitis (HBV/HCV) population of Jiang et al. study
Fig. 4.
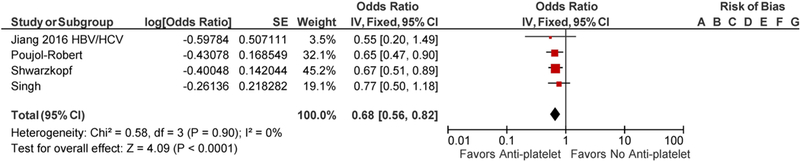
Forest Plot for all studies with inclusion of alcoholic liver disease (ALD) population of Jiang et al. Study
Discussion
As per our knowledge and literature search, this is the first systematic review and meta-analysis evaluating the utility of anti-platelet agents in preventing hepatic fibrosis in humans. Multiple animal models have shown that anti-platelet agents may prevent hepatic fibrosis [4–9]. A study in animal NAFLD model showed that aspirin inhibited the development of hepatic fibrosis [6]. Similar benefits of aspirin and other anti-platelet agents (ticlopidine, dipyridamole and cilostazol) were noted in other animal models where inhibition of platelet aggregation provided protection against hepatic fibrosis [7–9]. There are several plausible mechanisms to explain these effects of anti-platelet agents. First, anti-inflammatory action of some anti-platelet agents like aspirin inhibits transcription of NF-κB in the endothelial cells and prevents adhesion of macrophages and T-lymphocyte. They also reduce the levels of inflammatory cytokines including interleukin-6, tumor necrosis factor-β and PDGF [16]. Inhibition of these key mediators has been shown to be instrumental in the prevention of hepatic fibrosis in various animal models [7–9]. Secondly, these anti-platelet agents have anti-thrombotic effects that might explain and promote anti-fibrotic mechanism. Anti-platelet agents can prevent injury to sinusoidal endothelial cells by inhibiting platelet aggregation and have been shown to reduce chemotherapy induced sinusoidal lesions [17].
A few studies have evaluated the effects of anti-platelet agents on hepatic fibrosis [12–15]. We included 4 studies in our systematic review, 3 of which only used aspirin and one used both either aspirin or P2Y12 inhibitors as anti-platelet therapy. Jiang el al. identified subjects through NHANES database and included 1856 with risk factors for chronic liver disease including viral hepatitis, alcohol abuse and NASH [12]. The study revealed that the use of aspirin was associated with a decline in the risk of hepatic fibrosis calculated by 4 different non-invasive methods (FIB4, APRI, Forns and NFS). Similar beneficial effects of anti-platelet agents were noted in a study performed by Schwarzkopf et al. which included consecutive patients undergoing cardiac catheterization [15]. The hepatic fibrosis was detected by Fibroscan transient elastography and shown that the use of these agents was associated with lower risk of hepatic fibrosis. Poujol-Robert et al. in a study on hepatitis C virus-positive patients in the post-liver transplant setting noted that the use of aspirin was associated with lower risk of progression of hepatic fibrosis [13]. Although the majority of studies showed the beneficial effects of anti-platelet agents in the prevention of hepatic fibrosis, Singh et al. in a retrospective study performed on diabetic population with biopsy-proven NAFLD showed no significant beneficial effects with aspirin use [14]. These results were contradicting to Jiang et al. study which showed that patients with NASH on aspirin were noted to have decreased odds of hepatic fibrosis predicted by FIB4, although no statistical difference was noted when APRI was used to predict hepatic fibrosis. These conflicting results suggest the need for additional larger studies to confirm the beneficial effects of anti-platelet agents in a prospective fashion.
Safety of anti-platelet agents in patients with cirrhosis is controversial. Aspirin is associated with increased risk of renal failure, anemia and gastrointestinal hemorrhage; these complications can be more pronounced because of aspirin-induced thrombocytopenia [18, 19]. A recent study conducted in patients with coronary artery disease and cirrhosis, who were undergoing evaluation for liver transplantation, revealed that aspirin was safe and not associated with increased risk of gastrointestinal bleeding, variceal bleeding and anemia despite significantly thrombocytopenia [20]. Hence, the use of aspirin was not associated with increased mortality and hepatic decompensation in this study [20]. There were no adverse effects reported by Poujol-Robert et al. in patients who were on aspirin [13]. Safety data were missing from the other studies included in our analysis.
The methodology for systematic literature review of our study was comprehensive, and included all the available studies, although one of the included studies was in abstract form and not yet fully published. Moreover, the statistical heterogeneity of this meta-analysis was insignificant. We recognize, however, that this meta-analysis had several limitations and, thus, the results should be interpreted with caution. First, studies included in our analysis are observational in nature as there are no randomized controlled trials available. Although patients’ demographics and comorbidities in both groups were comparable and effect estimates were reported after adjusting for several confounding factors, there remains a risk of residual confounding bias in such data. Secondly, although the statistical heterogeneity was insignificant, and studies included patients with risk factors for chronic liver disease, there is substantial methodological heterogeneity among the studies as population cohorts are different in these studies and methods used to detect hepatic fibrosis are not the same with some utilizing liver biopsy to detect hepatic fibrosis while other relying on non-invasive methods that may underestimate or overestimate the results. Further, data regarding viral hepatitis treatment were not uniformly reported. Poujol-Robert et al. [13] collected data before starting antiviral therapy to avoid confounding effect on hepatic fibrosis, while Jiang et al. [12] did not report data on antiviral therapy. Also, patients who are on aspirin are more likely to be on statins which may confound our results [21]. Lastly, the exact duration of anti-platelets use was unclear, and a larger randomized controlled trial is needed not only to further delineate the treatment duration of anti-platelet agents and its impact on hepatic fibrosis, but also to evaluate safety of these agents in patients with chronic liver disease and hepatic fibrosis.
Conclusion
This systematic review and meta-analysis support the potential beneficial effects of anti-platelet agents in preventing hepatic fibrosis which is associated with significant morbidity and mortality. Given the limited number of studies currently available on efficacy and safety of these drugs in preventing hepatic fibrosis, future high-quality randomized controlled trials are necessary to further delineate the potential beneficial effects of these drugs in the prevention of hepatic fibrosis.
Acknowledgments
Funding There were no funding sources for this study.
Footnotes
Compliance with ethical standards
Conflict of interest Umair Iqbal, Brittany B. Dennis, Andrew A. Li, George Cholankeril, Donghee Kim, Muhammad Ali Khan and Aijaz Ahmed declare no conflict of interest, including financial and/or material support for the preparation of this manuscript.
References
- 1.Ge PS, Runyon BA. Treatment of patients with cirrhosis. N Engl J Med 2016;25(375):767–777 [DOI] [PubMed] [Google Scholar]
- 2.Cohen-Naftaly M, Friedman SL. Current status of novel antifibrotic therapies in patients with chronic liver disease. Ther Adv Gastroenterol 2011;4(6):391–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman SL, Sheppard D, Duffield JS, et al. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 2013;5(167):167sr1 [DOI] [PubMed] [Google Scholar]
- 4.Sitia G, Aiolfi R, Di Lucia P, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci USA 2012;109(32):E2165–E2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida S, Ikenaga N, Liu SB, et al. Extrahepatic platelet-derived growth factor-β, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology 2014;147(6):1378–1392 [DOI] [PubMed] [Google Scholar]
- 6.Denda A, Tang Q, Endoh T, et al. Prevention by acetylsalicylic acid of liver cirrhosis and carcinogenesis as well as generations of 8-hydroxydeoxyguanosine and thiobarbituric acid-reactive substances caused by a choline-deficient, L-Amino acid-defined diet in rats. Carcinogenesis 1994;15(6):1279–1283 [DOI] [PubMed] [Google Scholar]
- 7.Fujita K, Nozaki Y, Wada K, et al. Effectiveness of antiplatelet drugs against experimental non-alcoholic fatty liver disease. Gut 2008;57(11):1583–1591 [DOI] [PubMed] [Google Scholar]
- 8.Chavez E, Castro-Sanchez L, Shibayama M, et al. Effects of acetyl salycilic acid and ibuprofen in chronic liver damage induced by CCl4. J Appl Toxicol 2012;32(1):51–59 [DOI] [PubMed] [Google Scholar]
- 9.Wanless IR, Belgiorno J, Huet PM. Hepatic sinusoidal fibrosis induced by cholesterol and stilbestrol in the rabbit: 1. Morphology and inhibition of fibrogenesis by dipyridamole. Hepatology 1996;24(4):855–864 [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151(4):264–269, W64 [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang ZG, Feldbrugge L, Tapper EB, et al. Aspirin use is associated with lower indices of liver fibrosis among adults in the United States. Aliment Pharmacol Ther 2016;43(6):734–743 [DOI] [PubMed] [Google Scholar]
- 13.Poujol-Robert A, Boelle P-Y, Conti F, et al. Aspirin may reduce liver fibrosis progression: evidence from a multicenter retrospective study of recurrent hepatitis C after liver transplantation. Clin Res Hepatol Gastroenterol 2014;38(5):570–576 [DOI] [PubMed] [Google Scholar]
- 14.Singh A, Gosai F, Lopez R, et al. Regular aspirin use is not protective against advanced fibrosis in type-2 diabetics with biopsy-proven nonalcoholic fatty liver disease. J Hepatol 2018;1(68):S582–S583 [Google Scholar]
- 15.Schwarzkopf K, Bojunga J, Ruschenbaum S. Use of antiplatelet agents is inversely associated with liver fibrosis in patients with cardiovascular disease. Hepatol Commun 2018. 10.1002/hep4.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muhlestein JB. Effect of antiplatelet therapy on inflammatory markers in atherothrombotic patients. Thromb Haemost 2010;103(1):71–82 [DOI] [PubMed] [Google Scholar]
- 17.Brouquet A, Benoist S, Julie C, et al. Risk factors for chemotherapy-associated liver injuries: a multivariate analysis of a group of 146 patients with colorectal metastases. Surgery 2009;145(4):362–371 [DOI] [PubMed] [Google Scholar]
- 18.Segal R, Lubart E, Leibovitz A, Berkovitch M, Habot B, Yaron M, et al. Early and late effects of low-dose aspirin on renal function in elderly patients. Am J Med 2003;115(6):462–466 [DOI] [PubMed] [Google Scholar]
- 19.Lanas A, Wu P, Medin J, Mills EJ. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clin Gastroenterol Hepatol 2011;9(9):762.e6–768.e6 [DOI] [PubMed] [Google Scholar]
- 20.Patel SS, Guzman LA, Lin F-P, Pence T, Reichman T, John B, et al. Utilization of aspirin and statin in management of coronary artery disease in patients with cirrhosis undergoing liver transplant evaluation. Liver Transpl 2018;24(7):872–880 [DOI] [PubMed] [Google Scholar]
- 21.Kamal S, Khan MA, Seth A, Cholankeril G, Gupta D, Singh U, et al. Beneficial effects of statins on the rates of hepatic fibrosis, hepatic decompensation, and mortality in chronic liver disease: a systematic review and meta-analysis. Am J Gastroenterol 2017;112(10):1495–505 [DOI] [PubMed] [Google Scholar]


