Abstract
Background.
Some studies have found a higher frequency of fever with trivalent live attenuated influenza vaccine (LAIV) than with inactivated influenza vaccine (IIV), but quadrivalent LAIV has not been assessed. Understanding fever is important for safety reviews and for parents and providers. In addition, there have been only a limited number of studies in which text messaging was used for vaccine adverse-event (AE) surveillance.
Methods.
We conducted a prospective observational study in 3 community clinics in New York City to assess post-influenza vaccination fever in 24- to 59-month-olds during the 2013–2014 season. Enrolled families of children who received quadrivalent LAIV (LAIV4) or IIV (trivalent IIV3 or quadrivalent IIV4) replied to text messages that assessed their temperature on vaccination night and the next 10 nights (days 0 to 10); missing data were collected via telephone and a diary. We compared frequencies of fever (temperature ≥ 100.4°F) according to vaccine group on days 0 to 2 and 3 to 10 by using χ2 and multivariate log-binomial regression adjusted for age, previous influenza vaccination, and vaccine coadministration. We also assessed outcomes using all sources versus only text messages.
Results.
Most (84.1 % [n = 540]) eligible parents enrolled. Fever frequencies on days 0 to 2 did not differ between LAIV4 and any IIV (3.8% vs 5.7%, respectively; adjusted relative risk [aRR] [95% confidence interval], 0.60 [0.25–1.46]), between LAIV4 and IIV4 (4.2% vs 7.1%, respectively; aRR, 0.58 [0.19–1.72]), or between IIV4 and IIV3 (7.1% vs 6.0%, respectively; aRR, 1.02 [0.30–3.46]). The findings were similar when all data sources versus textmessage data alone were used. There were no significant differences on days 3 to 10.
Conclusions.
Postvaccination fever frequencies were low overall and did not differ according to influenza vaccine type during the 2013–2014 influenza season. The similarity of results when data were limited to text messages lends support to its use for surveillance of vaccine adverse events.
Keywords: fever, influenza, live attenuated, text message, vaccine safety, vaccination
BACKGROUND
Since 2010, influenza vaccination has been recommended for all individuals aged 6 months or older [1]. Several studies had suggested that live attenuated influenza vaccine (LAIV) was more effective than inactivated influenza vaccine (IIV) in children [2–4], which prompted the Advisory Committee on Immunization Practices (ACIP) to review effectiveness and safety data, leading to a recommendation that healthy children aged 2 to 8 years receive a LAIV preferentially over IIV during the 2014–2015 influenza season [5]. Although this preference was removed during the February 2015 ACIP meeting because of new effectiveness data [6], understanding the safety profile of the quadrivalent LAIV (LAIV4) remains important. There is also a need for additional safety data for quadrivalent formulations that were introduced more recently [7].
The ACIP considered fever after receipt of LAIV or IIV to be an important safety review component [5]. Fever after vaccination is relatively common among children [8, 9]. Although most febrile episodes are short lived and uncomplicated, they can be associated with complications such as febrile seizures, especially in young children [8, 10]. Even uncomplicated fever can lead to increased health care utilization [11]. Fevers can also affect parental perception of the vaccine [12, 13], and concerns regarding vaccine adverse effects play an important role in vaccine decision making [14, 15]. Data regarding fever after receipt of LAIV or IIV are limited [2, 16]. In a study in 6- to 59-month-olds, the frequency of fever was higher after trivalent LAIV (LAIV3) (5.4%) than after IIV3 (2.0%) on day 2 (d2) after vaccination [2]. In another study, fever was more frequent after receipt of LAIV4 (5.1%) than after LAIV3 (3.1%) [17]. Neither study assessed coadministration of LAIV with other vaccines.
The primary aim of this study was to assess, through text messaging, the frequency of fever in 24- to 59-month-old children who received LAIV or IIV. We hypothesized that the frequency of fever on the vaccination day and in the first 2 days after vaccination (d0 to d2) would be higher in those who received LAIV than in those who received IIV. The d0 to d2 window was selected because of the increased fever risk observed on d2 after vaccination with LAIV in a previous study [2] and the known d0 to d1 fever-risk window after IIV [18]. The main secondary study aims included determining (1) the clinical importance of reported fevers, (2) frequency of fever on d0 to d2 in children who received IIV4 versus those who received IIV3, and (3) fever on d3 to d10. We also assessed the validity of using text messaging for vaccine adverse-event (AE) surveillance. Although we had successfully used text messaging to assess fever frequency after simultaneous vaccination with IIV3 and 13-valent pneumococcal conjugate vaccine (PCV13) [9], the number of studies using text messaging for vaccine AE surveillance is limited.
METHODS
This prospective observational study (ClinicalTrials.gov identifier NCT01764269) was conducted in 3 community-based clinics affiliated with NewYork-Presbyterian Hospital/Columbia University Medical Center (CUMC) in New York City in collaboration with the US Centers for Disease Control and Prevention. These sites serve a primarily Latino and publicly insured population and share a common electronic health record system. All vaccination decisions were made by the patients’ health care provider and the caregiver. Beginning in the 2013–2014 season, all LAIV products available nationally were quadrivalent (LAIV4). At the study sites, both IIV3 and IIV4 were available, but IIV4 was available only in the dose for ≥3-year-olds. The CUMC institutional review board (IRB) approved the study; the Centers for Disease Control and Prevention IRB relied on the determination of the CUMC IRB.
Study Population and Enrollment
A pilot study was conducted between January and April 2013, and the full-season study was performed between September 2013 and April 2014. The influenza vaccine strain composition differed in the 2 seasons [19, 20]. Families were eligible to enroll if they (1) had a 24- to 59-month-old who was receiving his or her first influenza vaccine (LAIV or IIV) dose of that season at the time of enrollment, (2) had a cell phone with text-messaging capabilities, and (3) spoke English or Spanish. Exclusion criteria included (1) any chronic medical condition considered a contraindication or precaution for LAIV (with the exception of asthma/wheezing history) [5], (2) oral or other systemic steroid use in the previous month, (3) inhaled steroid use in the previous 2 weeks, (4) a temperature of ≥100.4°F at vaccination, (5) administration of any antipyretic within 6 hours before vaccination, (6) a stated intent to use prophylactic antipyretics, or (7) a parent’s inability to read text messages. Receipt of other vaccines was not an exclusion criterion.
After consent was obtained, each family completed an intake form including self-reported demographic information, reviewed the text-message procedures, and enrolled via text message. The families received and were trained to use a temporal artery thermometer [21]. Each family was also given a paper diary in a preaddressed/prestamped envelope to return after the 10-day observation period to receive a round-trip New York City Transit Authority MetroCard.
Study Procedures
The families were asked to take their child’s temperature once per day if they thought he or she was afebrile or as indicated if febrile. Families were sent an interactive text message series on the night of vaccination and over the next 10 nights and were asked to report the highest temperature taken, the time that temperature was taken, name and time of any antipyretics given, and care sought. Study staff reviewed the messages daily and initiated contact with nonresponders to collect missing data and assess whether there was any trouble responding to messages. Using an electronic health record abstraction tool, vaccinations given at enrollment and at all healthcare visits (ambulatory care, pediatric emergency department, and hospital) to NewYork-Presbyterian Hospital/CUMC between d0 and d10 after vaccination were recorded.
Statistical Analysis
The primary outcome assessed was fever (temperature ≥ 100.4°F [38°C]) on d0 to d2 after vaccination. Secondarily, we assessed moderate fever (temperature ≥ 102.2°F [39°C]) on d0 to d2.
For both the 2012–2013 pilot and the 2013–2014 full study, we used χ2 tests to compare the presence of a temperature of ≥100.4°F on d0 to d2 in children who received LAIV versus that in children who received IIV. Children were included in this analysis if they (1) had a temperature of ≥100.4°F reported on d0, d1, or d2, even if a response was invalid or missing on other days, or (2) had valid temperature measurement (defined as a temperature of ≥95°F) reported on all days (d0–d2). Children with or without antipyretic use were classified as having a fever on the basis of the same cutoff values.
On the basis of postvaccination fever frequency in clinical studies, with a sample size of at least 359, we were powered to detect a 2-fold increase in fever frequency when comparing LAIV4 versus IIV, assuming an 80% power and 5% type I error (2-sided). A 2-fold increase was selected on the basis of a previous study that compared fever after LAIV versus fever after IIV [2]. In addition, given the overall fever estimates (5.4%–7.6%) after nonadjuvanted IIV in an analysis of randomized controlled trials, a less than 2-fold increase would likely have little clinical significance [22].
For the full 2013–2014 study, by using multivariate log-binomial regression we determined the association of LAIV versus IIV receipt and fever during d0 to d2 after vaccination. Variables that were included in the model based on a priori clinical considerations included age group (24–35, 36–47, and 48–59 months), history of previous influenza vaccination, concurrent PCV13 vaccination (based on historical association) [9], and the most commonly coadministered inactivated vaccines (diphtheria, tetanus, acellular pertussis, and inactivated poliovirus [DTaP-IPV] [Kinrix] and hepatitis A vaccine) (Supplementary Table 1). In addition, demographic factors (child sex and race/ethnicity [selfreported]), medical problems associated with high risk for influenza complications [5], and enrollment month were planned to be included if the P value was <.10 in univariate analyses. Pairwise correlation was tested via Pearson correlation coefficients, and multicollinearity was assessed on a linear scale.
Five sensitivity analyses were also conducted: (1) including only children with temperature information reported on all days (d0 to d2); (2) including only children who received influenza vaccine alone without other vaccinations; (3) excluding children with reported antipyretic use preceding a reported temperature on d0, d1, or d2; (4) including only children ≥3 years old (in the indicated age range to receive either IIV type available at the sites); and (5) using only data collected via text message.
To verify the d0 to d2 risk window, we assessed the frequency of fever (temperature ≥ 100.4°F) on d3 to d10. Children were included if they had a valid temperature measurement reported on all 8 days or reported fever on any day in that period. Analyses were adjusted for a priori–selected covariates that could affect fever on d3 to d10: age group, previous influenza vaccination, and coadministration of any of the most common live vaccines (measles, mumps, and rubella [MMR], varicella, or MMR–varicella [ProQuad]) (Supplementary Table 1).
In secondary bivariate analyses, we compared differences in the frequencies of fever after IIV3 versus IIV4 and after IIV4 versus LAIV4. Only children ≥3 years of age were included because those who were <3 years could have received only the IIV3.
RESULTS
In the 2012–2013 pilot study, we enrolled 116 children (89.9% of those eligible); 33.6% received IIV3. On d0 to d2, few had a temperature of ≥100.4°F (0.0% [LAIV3] vs 6.0% [IIV3]; P = .55, Fisher’s exact test). There were no temperatures of ≥102.2°F.
In the 2013–2014 season, 540 children (84.1% of those eligible) were enrolled; 41.9% received LAIV4 (Figure 1). Five enrollees were excluded from the IIV3 versus IIV4 analyses because it was unclear which IIV formulation they received. Of the remaining participants, 68.3% received an IIV3. A higher proportion of 24- to 35-month-olds received IIV (Table 1). Daily text-message response rates varied, but on all days the majority of the data were collected via text messaging (Figure 2). The proportion of children for whom all d0 to d2 data were reported via text messaging did not differ according to vaccine type (81.5% [LAIV4] vs 82.9% [IIV3] vs 82.4% [IIV4]; P = .95). Only 39.1% of the participants returned the paper diary; the average time elapsed from d0 to diary receipt was 27 days (range, 8–139 days). The percentage of participants who returned the diary (41.6%–52.4% per response day) was higher for those who responded to text messages than for those who did not respond (15.2%–26.1%).
Figure 1.
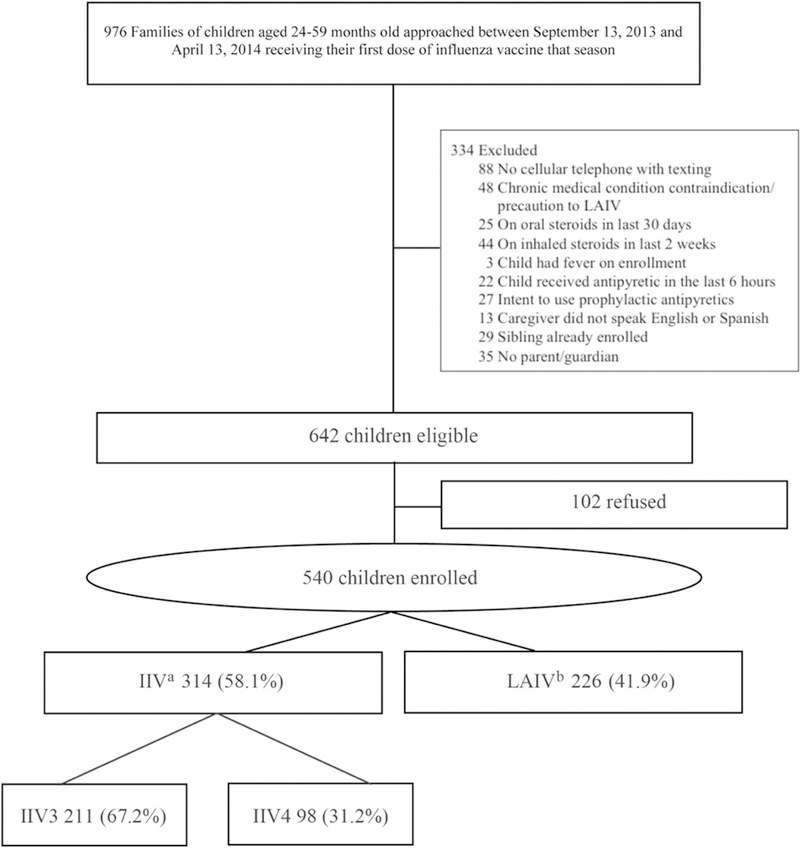
Study flow for enrollment during the 2013–2014 influenza season. aFor 5 children who received inactivated influenza vaccine (IIV), inadequate information regarding subtype was available; bAll live attenuated influenza vaccines (LAIVs) were LAIV4.
Table 1.
Characteristics of Study Population (2013–2014 Season)
| Characteristic | IIV (N = 314) (n [%]) |
LAIV4 (N = 226) (n [%]) |
P (χ2) | IIV3 (N = 211) (n [%]) |
IIV4 N = 98) (n [%]) |
p (χ2) |
|---|---|---|---|---|---|---|
| Child age | <.001 | <.001 | ||||
| 24–35 mo | 135 (43.0) | 54 (23.9) | 130 (61.6) | 0 (0.0) | ||
| 36–47 mo | 85 (27.1) | 85 (37.6) | 45 (21.3) | 40 (40.8) | ||
| 48–59 mo | 94 (29.9) | 87 (38.5) | 36 (17.1) | 58 (59.2) | ||
| Child sex | .007 | .79 | ||||
| Female | 141 (44.9) | 128 (56.6) | 96 (45.5) | 43 (43.9) | ||
| Male | 173 (55.1) | 98 (43.4) | 115 (54.5) | 55 (56.1) | ||
| Child race/ethnicity | .06a | .002a | ||||
| Latino | 256 (81.5) | 203 (89.8) | 176 (83.4) | 75 (76.5) | ||
| Black, non-Latino | 47 (15.0) | 18 (8.0) | 33 (15.6) | 14 (14.3) | ||
| White, non-Latino | 2 (0.6) | 1 (0.4) | 0 (0.0) | 2 (2.0) | ||
| Other, non-Latino | 9 (2.9) | 4 (1.8) | 2 (1.0) | 7 (7.1) | ||
| Child insurance | <.001a | .86a | ||||
| Private | 23 (7.3) | 2 (0.9) | 16 (7.6) | 7 (7.1) | ||
| Medicaid/SCHIP | 282 (89.8) | 220 (97.3) | 188 (89.1) | 89 (90.8) | ||
| Uninsured | 9 (2.9) | 4 (1.8) | 7 (3.3) | 2 (2.0) | ||
| Child at high risk for complication from influenza [5] | <.001a | <.001 | ||||
| No | 272 (86.6) | 223 (98.7) | 194 (91.9) | 74 (75.5) | ||
| Yes | 42 (13.4) | 3 (1.3) | 17 (8.1) | 24 (24.5) | ||
| Previous influenza vaccination | .58 | .33 | ||||
| Yes | 303 (96.5) | 220 (97.3) | 202 (95.7) | 96 (98.0) | ||
| No | 11 (3.5) | 6 (2.7) | 9 (4.3) | 2 (2.0) | ||
| Caregiver’s English proficiency | .10 | .97a | ||||
| Excellent to good | 223 (71.0) | 143 (63.3) | 151 (71.6) | 69 (70.4) | ||
| Fair to poor | 84 (26.8) | 73 (32.3) | 55 (26.1) | 27 (27.6) | ||
| Not at all | 7 (2.2) | 10 (4.4) | 5 (2.4) | 2 (2.0) | ||
| Language in which caregiver prefers to receive text | .02 | .34 | ||||
| messages | ||||||
| Spanish | 136 (43.3) | 121 (53.5) | 94 (44.5) | 38 (38.8) | ||
| English | 178 (56.7) | 105 (46.5) | 117 (55.5) | 60 (61.2) | ||
| Caregiver’s education level | .22 | .71 | ||||
| Less than high school | 61 (19.4) | 32 (14.2) | 38 (18.0) | 21 (21.4) | ||
| High school only/GED/trade | 114 (36.3) | 81 (35.8) | 79 (37.4) | 33 (33.7) | ||
| Some college or more | 139 (44.3) | 113 (50.0) | 94 (44.6) | 44 (44.9) | ||
| Caregiver’s text message plan | >.99a | >.99a | ||||
| Unlimited | 24 (7.6) | 17 (7.5) | 16 (7.6) | 8 (8.2) | ||
| Limited or pay as you go | 289 (92.0) | 209 (92.5) | 194 (91.9) | 90 (91.8) | ||
| Don’t know | 1 (0.3) | 0 (0.0) | 1 (0.5) | 0 (0.0) | ||
| Frequency of text-message use by caregiver at baseline | .80a | .48a | ||||
| At least weekly | 310 (98.7) | 222 (98.2) | 209 (99.1) | 96 (98.0) | ||
| Every few weeks to months | 3 (1.0) | 3 (1.3) | 2 (0.9) | 1 (1.0) | ||
| Never receives texts | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (1.0) | ||
| Missing | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) | ||
Abbreviations: GED, general equivalency diploma; IIV, inactivated influenza vaccine; IIV3, trivalent IIV; IIV4, quadrivalent IIV; LAIV4, quadrivalent live attenuated influenza vaccine; SCHIP, State Children’s Health Insurance Program.
Fisher’s exact test.
Figure 2.
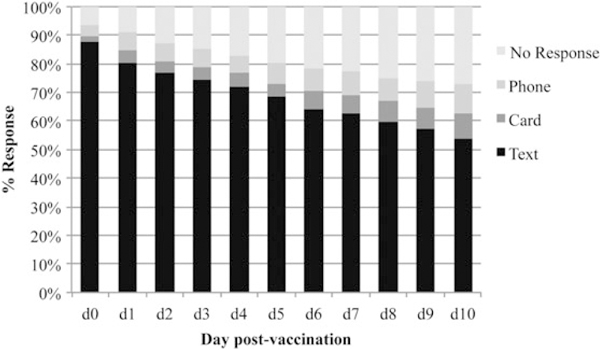
Source of fever data for enrolled children during the 2013–2014 influenza season.
Fever on d0 to d2 After Vaccination
For the 2013–2014 season, in both unadjusted and adjusted analyses, the frequency of temperature ≥ 100.4°F on d0 to d2 did not differ significantly in children who received LAIV4 from that in those who received IIV (3.8% vs 5.7%, respectively; adjusted relative risk [aRR] 0.60 [95% confidence interval (CI), 0.25–1.46]) (Table 2; Supplementary Table 2). Analyses were adjusted only for a priori–selected factors, because no additional factors reached a P value of <.1 (Supplementary Table 3).
Table 2.
Fever Frequency After Vaccination in Children Who Received LAIV Versus IIV (2013–2014 Season)
| Temperature > 100.4 F on d0-d2 |
|||
|---|---|---|---|
| Vaccine Type and Dose (n) | Frequency n (%) | RR (95% CI) | Adjusted RRa (95% CI) |
| LAIV4 vs IIV | |||
| LAIV4 (183) | 7(3.8) | 0.67 (0.28–1.62) | 0.60 (0.25–1.46) |
| IIV (264) | 15 (5.7) | Reference | Reference |
| LAIV4 vs IIV4 | |||
| LAIV4b (142) | 6 (4.2) | 0.60 (0.20–1.80) | 0.58 (0.19–1.72) |
| IIV4 (85) | 6(7.1) | Reference | Reference |
| IIV4 vs IIV3 | |||
| IIV4b (85) | 6(7.1) | 1.18 (0.35–4.02) | 1.02 (0.30–3.46) |
| IIV3 (67) | 4 (6.0) | Reference | Reference |
Children were included in the analysis if he or she (1) had a temperature of ≥100.4°F reported on d0, d1, or d2, even if the response was invalid or missing on other days, or (2) had valid temperature measurements (defined as temperature of ≥95°F) reported on all days (d0≥d2). Abbreviations: CI, confidence interval; d, day; IIV, inactivated influenza vaccine; IIV3, trivalent IIV; IIV4, quadrivalent IIV; LAIV, live attenuated influenza vaccine; LAIV4, quadrivalent live attenuated influenza vaccine; RR, relative risk.
Adjusted for age group (24–35, 36–47, or 48–59 months), history of previous influenza vaccination, concurrent 13-valent pneumococcal conjugate vaccination, based on historical association [9], and coadministration of the most common inactivated vaccines (Kinrix [diphtheria, pertussis acellular, tetanus, and polio vaccine] and hepatitis A).
Analyses directly comparing IIV3 versus IIV4 and LAIV4 versus IIV4 were limited to children who were ≥3 years of age because IIV4 vaccine was not available for those <3 years old at the study sites. Analyses also did not include the 5 children for whom IIV was given but the subtype was unclear. Hepatitis A and 13-valent pneumococcal conjugate coadministration was not included in the model because of problems with convergence.
For all sensitivity analyses, the aRRs were similar: (1) for information about fever reported on all days (d0–d2) (aRR, 0.65 [95% CI 0.27–1.59]); (2) for those who received influenza vaccine alone (aRR, 0.60 [95% CI, 0.15–2.40]); (3) when we excluded those who were given an antipyretic (aRR, 0.64 [95% CI, 0.22–1.88]); (4) when analysis was limited to ≥3-year-olds (aRR, 0.61 [95% CI, 0.23–1.64]); and (5) when analysis was limited to only text-message data (aRR, 0.57 [95% CI 0.22–1.46]). The aRRs were also similar when previous influenza vaccination was removed from the model because of the potential for multicollinearity (aRR, 0.58 [95% CI, 0.24–1.42]) and when race/ethnicity (0.72 [95% CI 0.29–1.75]) or the presence of a high-risk medical condition (0.56 [95% CI 0.23–1.36]) was added. We found no significant interactions between vaccine type and covariates for a temperature of ≥100.4°F on d0 to d2. Fever frequencies were low on all days (Supplementary Figure 1).
There were 4 moderate fevers (temperature ≥ 102.2°F) on d0 to d2 (LAIV4, n = 0; IIV3, n = 2; IIV4, n = 2). Because this occurrence was uncommon, no further analyses were conducted. There were no significant differences in the frequencies of fever in ≥3-year-olds who received IIV4 vs IIV3 or in those who received an IIV4 vs LAIV4 (Table 2).
Of the 7 children who received LAIV and had fever during d0 to d2, 4 had received LAIV simultaneously with at least 1 inactivated vaccine (hepatitis A [n= 1] or DTaP-IPV and MMR–varicella [n = 3]). Of the 15 children who received IIV and had a fever on d0 to d2, 8 had also received an inactivated vaccine (hepatitis A [n = 2], DTaP-IPV and MMR–varicella [n = 4], DTaP-IPV and individual MMR and varicella [n = 1], or DTaP, Haemophilus influenzae type b, PCV13, and hepatitis A [n= 1]).
Fever on d3 to d10 After Vaccination
Fewer families reported data for the d3 to d10 period. There were no significant between-group differences in fever frequencies on d3 to d10 on unadjusted or adjusted analyses for temperatures of ≥100.4°F (LAIV4 vs IIV, 10.3% vs 9.8%, respectively; aRR, 1.07 [95% CI, 0.55–2.08]) (Supplementary Table 4), and there were no significant interactions between vaccine types and covariates. There were also no significant differences for ≥3 year-olds who received IIV4 (6.3%) versus IIV3 (13.7%) (P = .21, Fisher’s exact test) or those who received IIV4 (6.3%) versus LAIV4 (12.7%) (P = .21, Fisher’s exact test). There were 10 fevers (temperature ≥ 102.2°F) on d3 to d10 (LAIV4 [n = 6]; IIV3 [n = 3]; IIV4 [n = 1]).
On d3 to d10, of the 14 fevers in children who received LAIV4, 4 occurred in those who received at least 1 other live vaccine (MMR–varicella and DTaP-IPV [n = 2]; MMR and DTaP-IPV [n = 1]; or MMR–varicella [n = 1]). Of the 19 children with a fever on d3 to d10 who received IIV, 6 also received a live vaccine (DTaP-IPV and MMR–varicella [n = 3]; DTaP-IPV, MMR, and varicella [n = 1]; MMR [n = 1]; or MMR and varicella [n = 1]).
Healthcare Utilization
There were no hospitalizations or febrile seizures noted for any participant between d0 and d10. Of the 22 children with a fever during d0 to d2, 2 had a medical visit. One child who received IIV4 alone had a d5 ambulatory care visit for an upper respiratory infection/allergy and a d10 pediatric emergency department (PED) visit for pharyngitis/asthma. The other child who received IIV3 and a hepatitis A vaccine had a d8 PED visit for gastroenteritis. In the whole sample, there were 9 PED visits of any kind during d0 to d10: 2 in the LAIV4 group (viral illness, laceration), 3 in the IIV3 group (pneumonia, vomiting, gastroenteritis), and 4 in the IIV4 group (blepharitis, thumb issue, diaper dermatitis, pharyngitis/asthma). There were 17 ambulatory care visits of any kind (LAIV4 group, n = 6; IIV3 group, n = 5; and IIV4 group, n = 6).
DISCUSSION
In this observational study conducted in the first season after the introduction of LAIV4 in the United States (2013–2014), we found no increase in the frequency of fever in 24- to 59-month-olds who received LAIV4 versus those who received the IIV3 or IIV4. Fevers after any type of influenza vaccine were mild and not common, and the numbers were similar to estimates after nonadjuvanted IIV in an analysis of randomized controlled trials (5.4%–7.6%) [22]. There were no hospitalizations or febrile seizures in the 10 days after vaccination for any vaccination type. These findings support the current recommendations for either type of influenza vaccine [23].
Understanding the AE profiles of different influenza vaccination types is useful for health care providers when providing anticipatory guidance to families. Non–medically attended fevers can affect parent perceptions of the vaccine, including its safety, and can lead to nonadherence to recommended vaccines [12, 13]. This may be particularly problematic for influenza vaccine, because coverage for it is lower nationally than that for other childhood vaccinations [24, 25], and parental concerns regarding adverse effects seem to play an important role in vaccine decision making [14]. Anticipatory guidance may ameliorate some of these concerns, but it is predicated on accurate information regarding the actual frequency of fever after a given vaccine.
Our study findings do not support our original hypothesis that fever would be more frequent after LAIV4 than after IIV, as noted previously by Belshe et al [2]. Reasons for the differences are not clear [2]. One potential explanation is that different viral strains might lead to different pyrogenicity profiles for IIV and LAIV. The strains used in the vaccine in the Belshe et al study were different than those used during our study. However, our finding of similar fever patterns over 2 seasons (pilot and full study) with different vaccine strains argues against this explanation.
Another possibility is that the Belshe et al study also included younger children aged 6 to 23 months. In our study, there were no significant relationships between age and fever; however, all the children were at least 24 months old. A previous study that assessed IIV4 found a slight increase in fever frequency in children <36 months old over that in 36- to 59-month-olds [26].Continued vaccine safety surveillance over future seasons might identify fever patterns [27]. In addition, in our study, the point estimates for fever between d3 and d10 were higher than those for fever during d0 to d2; however, the CIs on adjusted analyses were overlapping. In addition, the observation period was longer and response rates were lower, and some fevers might have been caused by simultaneous measles vaccination, which is known to cause fever in the 5 to 12 days after vaccination [28].
Our study results lend further support to the use of textmessage surveillance for vaccine adverse events in terms of feasibility and validity [9]. In this study, primary results were similar when analyses were limited only to data collected via text messaging and when using additional data collected via telephone and diary. The text-message responses were also greater overall and more timely. Only 39% of participants returned the paper diary, and the time from day of vaccination to return of the diary was prolonged. This delay would have made it difficult to collect samples or additional data proximal to a vaccine adverse event, if needed. Those with a higher text-message response rate also had a higher diary-return rate. Researchers in Australia also found response rates to text messages to be significantly higher and timelier than those to telephone interviews for AE reporting after influenza vaccination in pregnant women [29]. In our study, daily reporting rates dropped off in the later days of the 10-day period; additional studies should assess the optimal timing of text-message queries for prolonged data collection.
There were several limitations to this study. We were not powered to assess a less than 2-fold difference in fever frequencies in the LAIV4 and IIV groups on d0 to d2. However, the absolute differences in fever frequencies between vaccine types were very low (<3%), and in all the comparisons, the LAIV resulted in a lower rate. These small differences, even had we been powered to detect them as significant, may not be clinically relevant, given that the frequency of documented fevers was minimal. The study lacked power to assess differences in the subanalyses of fever frequency after IIV4 versus that after IIV3 or the effect of simultaneous vaccinations. In addition, the children were not randomly assigned to have a given vaccine type administered. Although potential confounders were assessed, there might have been residual confounding as a result of unmeasured variables. Also, because nearly all DTaP vaccines administered were given as a combination vaccine with IPV, we were unable to assess the potential effect of other DTaP-containing products. In addition, this study was conducted in a primarily Latino, urban population and among families of children aged 24 to 59 months. Not all the parents responded to our text messages, and it is possible that those who had children who were ill were more likely to not respond; however, we did not find differences in outcomes when we assessed text-message–only data and when we included data collected via diary and telephone. Finally, vaccine strains can change from year to year, although similar fever patterns were seen over the pilot and full study years when the vaccines strains differed.
CONCLUSIONS
Administration of an LAIV was not associated with increased postvaccination fever frequency in children aged 24 to 59 months during the 2013–2014 influenza season. Postvaccination fever frequencies with all influenza vaccine types were low. For the 2015–2016 influenza season, the ACIP recommended that LAIV4, IIV3, or IIV4 be used to prevent influenza in children aged ≥2 years who have no contraindications or need for precautions [6]. Our finding that the frequencies of fever were similar across these products may be informative to parents and providers when making decisions about influenza vaccination. Finally, the results of this study further support the use of text messaging for surveilling vaccine adverse events, because response rates to text messages were high relative to rates of response to other sources, and results were similar when analyses were limited to text-message data.
Supplementary Material
Acknowledgments
We thank Dr Frank DeStefano from the Centers for Disease Control and Prevention Immunization Safety Office for his technical contributions to the study, NewYork-Presbyterian Hospital for its support of the EzVac Immunization Information System, and the NewYork-Presbyterian Hospital Ambulatory Care Network and its staff and patients. We also thank Luis Alba, Ameriangel Roman Ereu, Zuleika Parra-Valencia, Wendy Gonzalez, and Solomon Torres for their help in this study.
Financial support. This study was supported through Clinical Immunization Safety Assessment Project Contract 200-2012-53665-0001 from the CDC.
Presented in part: 2015 Pediatric Academic Societies’ Annual Meeting in San Diego, CA (as a platform presentation), and 2014 Advisory Committee on Immunization Practices Meeting in Atlanta, GA.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. Dr Stockwell is a coinvestigator but receives no financial support for an unrelated investigator-initiated grant from the Pfizer Medical Education Group. Dr Iqbal currently works for Daiichi Sankyo, Inc., but not at the time of this study. The other authors have no reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Data
Supplementary materials are available at the Journal of the Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
References
- 1.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62. [PubMed] [Google Scholar]
- 2.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007; 356:685–96. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose CS, Wu X, Knuf M, Wutzler P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: a meta-analysis of 8 randomized controlled studies. Vaccine 2012; 30:886–92. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi S, Vertruyen A, Aristegui J, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–9. [DOI] [PubMed] [Google Scholar]
- 5.Grohskopf LA, Olsen SJ, Sokolow LZ, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 influenza season. MMWR Morb Mortal Wkly Rep 2015; 64:818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother 2012; 8:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petousis-Harris H, Poole T, Turner N, Reynolds G. Febrile events including convulsions following the administration of four brands of 2010 and 2011 inactivated seasonal influenza vaccine in NZ infants and children: the importance of routine active safety surveillance. Vaccine 2012; 30:4945–52. [DOI] [PubMed] [Google Scholar]
- 9.Stockwell MS, Broder K, LaRussa P, et al. Risk of fever after pediatric trivalent inactivated influenza vaccine and 13-valent pneumococcal conjugate vaccine. JAMA Pediatr 2014; 168:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson LA, Carste BA, Malais D, Froeschle J. Retrospective population-based assessment of medically attended injection site reactions, seizures, allergic responses and febrile episodes after acellular pertussis vaccine combined with diphtheria and tetanus toxoids. Pediatr Infect Dis J 2002; 21:781–6. [DOI] [PubMed] [Google Scholar]
- 11.Stockwell MS, Findley SE, Irigoyen M, Martinez RA, Sonnett M. Change in parental reasons for use of an urban pediatric emergency department in the past decade. Pediatr Emerg Care 2010; 26:181–5. [DOI] [PubMed] [Google Scholar]
- 12.Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Parental vaccine safety concerns in 2009. Pediatrics 2010; 125:654–9. [DOI] [PubMed] [Google Scholar]
- 13.Stockwell MS, Irigoyen M, Andres Martinez R, Findley SE. Failure to return: parental, practice, and social factors affecting missed immunization visits for urban children. Clin Pediatr (Phila) 2014; 53:420–7. [DOI] [PubMed] [Google Scholar]
- 14.Grant VJ, Le Saux N, Plint AC, et al. Factors influencing childhood influenza immunization. CMAJ 2003; 168:39–41. [PMC free article] [PubMed] [Google Scholar]
- 15.Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 2015; 33:459–64. [DOI] [PubMed] [Google Scholar]
- 16.Belshe RB, Ambrose CS, Yi T. Safety and efficacy of live attenuated influenza vaccine in children 2–7 years of age. Vaccine 2008; 26(Suppl 4):D10–6. [DOI] [PubMed] [Google Scholar]
- 17.Block SL, Falloon J, Hirschfield JA, et al. Immunogenicity and safety of a quadrivalent live attenuated influenza vaccine in children. Pediatr Infect Dis J 2012; 31:745–51. [DOI] [PubMed] [Google Scholar]
- 18.Rowhani-Rahbar A, Klein NP, Dekker CL, et al. Biologically plausible and evidence-based risk intervals in immunization safety research. Vaccine 2012; 31:271–7. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2012; 61:613–8. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013; 62:1–43. [PubMed] [Google Scholar]
- 21.Greenes DS, Fleisher GR. Accuracy of a noninvasive temporal artery thermometer for use in infants. Arch Pediatr Adolesc Med 2001; 155:376–81. [DOI] [PubMed] [Google Scholar]
- 22.Li-Kim-Moy J, Yin JK, Rashid H, et al. Systematic review of fever, febrile convulsions and serious adverse events following administration of inactivated trivalent influenza vaccines in children. Euro Surveill 2015; 20:pii:21159. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Seasonal influenza vaccination resources for health professionals. Available at: http://www.cdc.gov/flu/professionals/vaccination/index.htm. Accessed August 6, 2015.
- 24.Elam-Evans LD, Yankey D, Singleton JA, Kolasa M, Centers for Disease Control and Prevention. National, state, and selected local area vaccination coverage among children aged 19–35 months—United States, 2013. MMWR Morb Mortal Wkly Rep 2014; 63: 741–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2013–14 influenza season. Available at: http://www.cdc.gov/flu/fluvaxview/coverage-1314estimates.htm. Accessed May 7, 2015.
- 26.Domachowske JB, Pankow-Culot H, Bautista M, et al. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3–17 years. J Infect Dis 2013; 207:1878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmon DA, Pavia A, Gellin B. Editors’ introduction: vaccine safety throughout the product life cycle. Pediatrics 2011; 127 (Suppl 1):S1–4. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Possible side-effects from vaccines: MMR vaccine side-effects, MMRV vaccine side- effects. Available at: http://www.cdc.gov/vaccines/vac-gen/side-effects.htm#mmr. Accessed March 30, 2016.
- 29.Regan AK, Blyth CC, Tracey L, Mak DB, Richmond PC, Effler PV. Comparison of text-messaging to voice telephone interviews for active surveillance of adverse events following immunisation. Vaccine 2015; 33:3689–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


